Abstract
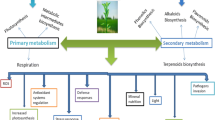
Secondary metabolites are natural defense elements that play a vital role in plant defense against adverse environmental conditions. The amounts of secondary metabolites increase significantly to adapt to harsh conditions. The increase in the synthesis and accumulation of plant secondary metabolites to cope with abiotic and biotic stress indicates a strong link between secondary metabolites and biotic and abiotic stress tolerance. In this chapter, the contribution of secondary metabolites to plant defense against biotic and abiotic stress factors such as salinity, drought, heat and cold, heavy metals, and UV is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
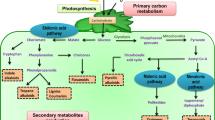
The lack of an immune system and active mobility of plants makes it very difficult for plants to resist environmental stresses (Meena et al. 2017). Therefore, plants develop a variety of defense responses, including secondary metabolites (SMs), to cope with the stresses that arise under variable growing conditions (Fig. 19.1). Although plant SMs do not directly affect plant metabolism and growth, they play an important role in the plant defense. Secondary metabolites are primary metabolite derivative compounds produced in plants when various physiological changes occur (Zandalinas et al. 2017). The SMs are substances produced by the plants to protect themselves in case of exposure to any stress condition (Isah 2019). Secondary metabolites play a role in alleviating biotic stresses such as fungi, viruses, and bacteria, as well as abiotic stresses (Jan et al. 2021). When plants are exposed to abiotic and biotic stress conditions, they can produce more than one hundred thousand SMs through different metabolic pathways (Meena et al. 2017). The biosynthesis and accumulation of SMs vary depending on abiotic and biotic stress. In plants, SMs are classified as N-containing compounds, phenolic compounds (phenolic acids, lignin, flavonoids, stilbenes, coumarins, tannins), terpenes (sterol, carotenoids, glycosides, volatiles), and S-containing compounds (glutathione, glucosinolates, phytoalexins, thionins, defensins, alliin) (Fang et al. 2011; Ashraf et al. 2018; Jamwal et al. 2018; Isah 2019). Various SMs are synthesized by alternative mechanisms in plants. Mono-, di-, and tetraterpenes, phytol, plastoquinone, and carotenoids are synthesized from the methylerythritol phosphate pathway; sterols, sesquiterpenes, and triterpenes are synthesized from the mevalonate pathway; phenolic compounds are synthesized from the shikimic acid and malonic acid pathway; and N-containing compounds are synthesized from the tricarboxylic acid pathway (Jamwal et al. 2018). Additionally, SMs have different chemical structures such as aliphatic (polyamine, isoprene, ethylene), aromatic (phenolic acid), hydroaromatic (terpenoid, jasmonic acid), and heterocyclic (flavonoid) that enable them to perform different functions (Edreva et al. 2008; Ahanger et al. 2020). Phenolics are compounds synthesized in plants during normal development or when the plant is exposed to biotic or abiotic stress (Xiao et al. 2019). Phenolics are aromatic plant SMs that have a phenyl ring attached to one or more hydroxyl groups (Mathew et al. 2015). In plants, it generally accumulates in the vacuoles located in the center of epidermal cells and in the cells under the epidermis in the leaves and shoots. Some phenolic compounds are covalently bound to the plant cell wall, while others are found on the outer surfaces of the cuticle or plant organs. However, in some tree species, flavonoids accumulate in the nucleus, and this causes the formation of a DNA-flavonoid complex, which protects against oxidative damage (Falcone Ferreyra et al. 2012). The most well-known property of phenolics is the scavenging of reactive oxygen species (ROS). Phenolic compounds such as esters, flavonoids, lignins, and tannins act as antioxidants under abiotic stress conditions (Selmar 2008). Terpenes consist only of isoprene units, whereas terpenoids have isoprene units as well as additional units such as ketone, heterocyclic, and hydroxyl. Terpenoids are one of the important secondary metabolites that play a role in defense against both abiotic and biotic stress factors in plants (Porres-Martinez et al. 2016). Due to their antioxidant activities, terpenes can prevent abiotic-induced oxidative stress (Blanch et al. 2009). Like carotenoids, terpenoids protect plants against photodamage and oxidative stress by promoting photorespiration (Bartwal et al. 2013). ROS formed due to photoinhibition are scavenged by antioxidants such as tocopherols and carotenoids (Goh et al. 2011). It is assumed that the isoprene units in the structure of terpenes can alleviate stress conditions, as large protein complexes increase the interaction between themselves or with membrane lipids, providing the stability of the membrane structure (Sharkey and Yeh 2001). Phytohormones stimulate the synthesis of volatile terpenes as signal compounds in plant defense and stress responses (Wani et al. 2016). Phytoalexins are one of the compounds that play a role in the defense mechanism in plants. Diterpenes and sesquiterpenes function as phytoalexins (Hwang and Sung 1989). Most plant SMs contain nitrogen (N) in their structure. Alkaloids, cyanogenic glycosides, glucosinolates, and nonprotein amino acids are mainly nitrogen-containing SMs. Alkaloids have antifungal, anti-insecticidal, and antibacterial effects (Singh 2018). The inhibitory effects of alkaloids on glycosidase metabolism deter herbivores and provide protection owing to their singlet reactive oxygen scavenging properties (Mithöfer and Boland 2012). Cyanogenic glycosides are involved in plant defense against both herbivores and phytopathogens, while glucosinolates are involved in plant defense against biotic agents (Ballhorn 2011). Nonprotein amino acids have different roles, including antiherbivore, antimicrobial, and allelochemical activities (Mcsweeney et al. 2008).
Studies have shown that the amount of plant SMs generally increases during abiotic and biotic stresses. This demonstrates the importance of accumulation of secondary metabolites in improving stress tolerance. In this chapter, the role of SMs against various stress factors such as salt, drought, cold, high temperature, heavy metals, UV, and pathogens to which plants are exposed has been discussed.
2 Abiotic Stress and Secondary Metabolites
2.1 Secondary Metabolites in the Salt Stress Tolerance of Plants
Salt stress is one of the important abiotic factors limiting the growth and development of plants worldwide (Yang et al. 2018). Salinity causes osmotic stress, which causes dehydration, oxidative stress, ionic stress, and physiological drought in plant cells. This situation causes a decrease in cytosolic and vacuolar volumes, a decrease in photosynthesis and growth rate, the inability of plants to take water despite the presence of water, and a decrease in nutrient uptake (Ashraf et al. 2015). Plants exposed to salt stress accumulate sugar alcohols such as sorbitol, mannitol, pinitol, and carbohydrates such as fructose, glucose, sucrose, trehalose, raffinose, and stachyose. These osmolytes stabilize the cell membranes against lipid peroxidation and maintain turgor pressure (Slama et al. 2015). Trehalose has an important role in the regulation of ionic balance and redox state. Additionally, raffinose and galactinol accumulations were detected in the intercellular space of plants exposed to stress (Yan S et al. 2022). It has been reported that raffinose and galactinol protect cells against oxidative damage by scavenging the hydroxyl radical (Nishizawa et al. 2008). In addition to their osmolyte function, amino acids have cellular functions such as scavenging ROS and controlling the transport of ions. For example, proline not only acts as an osmolyte in plants under salt stress but also protects plants against oxidative damage as a ROS scavenger with its antioxidant property. A positive correlation was found between proline accumulation and salinity tolerance in L. esculentum, M. sativa, and A. corniculatum plants. An increase in phenolic levels was detected in A. fragrantissima, C. annuum, and C. tinctorius exposed to salt stress (Navarro et al. 2006; Verma and Shukla 2015; Golkar and Taghizadeh 2018). In addition to proline, increases in the levels of amino acids such as phenylalanine, aspartic acid, valine, arginine, cysteine, citrulline, alanine, arginine, and glutamine were determined in plants under salt stress (Cao et al. 2017). Glycine betaine, which is an osmoprotectant and also acts as a ROS scavenger, reduces lipid peroxidation by improving the activities of glutathione S-transferase (GST) and glutathione peroxidase (GPX) enzymes. It protects the 3D structures of proteins by preventing protein carbonylation (Hoque et al. 2007; Hasanuzzaman et al. 2014).
Plants provide a maximum tolerance to salt stress by increasing or decreasing the secondary metabolite production (Golkar and Taghizadeh 2018). Corn, wheat, rice, and potato plants exposed to salt stress produce secondary compounds such as terpenoids, flavonoids, alkaloids, steroids, and phenolics (Jan et al. 2021). It has been reported that high alkaloid and phenolic concentrations in plants are effective in scavenging ROS caused by salt stress. They are compounds with high antioxidant properties (Chunthaburee et al. 2015). Again, the increase in flavonoid levels under stress shows that their antioxidant properties are effective in increasing tolerance to oxidative damage as a ROS scavenger. The amounts of chlorogenic acid, caffeic acid, ellagic acid, ferulic acid, gallic acid, syringic acid, vanillic acid, and p-coumaric acid increase under salinity conditions to maintain redox homeostasis (Razieh et al. 2021). Similarly, the increase in terpene accumulation in plants under salt stress showed that these compounds act as ROS scavengers with their antioxidant properties against oxidative stress (Dahham et al. 2015; Porres-Martinez et al. 2016). Salt stress increased the concentrations of various alkaloids and essential oils in Solanum nigrum, Catharanthus roseus, Rauvolfia tetraphylla, A. fragrantissima, Oryza sativa, and Datura innoxia (Said-Al and Omer 2011; Chunthaburee et al. 2015; Verma and Shukla 2015). Again, in S. nigrum seedlings exposed to salt stress, increases in the expression of flavonoid genes and genes related to carotenoids were determined (Ben Abdallah et al. 2016). Hyoscyamine 6β-hydroxylase is known to act as the factor responsible for alkaloid synthesis in plants exposed to salt stress (Schlesinger et al. 2019). Salinity stress in Matricaria chamomilla increased the concentration of various phenolics such as caffeic, chlorogenic, and protocatechuic acid (Kováčik et al. 2009). Oliveira et al. (2020) reported that the accumulation of cellulose, lignin, and matrix polysaccharides in stem and root tissues of maize (Zea mays) under salt stress and the expression of genes from phenylpropanoid biosynthesis and the activities of enzymes were stimulated. Another study reported that M. pulegium and N. sativa increased the amount of phenolic compounds under salinity stress (Oueslati et al. 2010). Similarly, in M. pulegium exposed to salt stress, an increase was detected in the amount of pulegone, but no change was observed in the amount of another terpene, neomenthol (Karray et al. 2009). Additionally, in a study conducted on H. annuus L. exposed to salt stress, significant increases in the polyamine content of the roots of the plant were observed (Chiapusio et al. 2016). Some SMs produced in different plants exposed to salt stress are detailed in Table 19.1.
Many natural and synthetic metabolites have been used exogenously to increase plant tolerance to salt stress as osmoregulatory and ROS scavengers. Various amino acids (proline, glycine betaine, etc.), hormones (salicylic acid, methyl jasmonate, benzyl aminopurine, melatonin), sugars (trehalose), polyamines (spermine, spermidine, putrescine), and vitamins are among these metabolites (Patel et al. 2020). These exogenous applications are suggested as a promising approach to increasing tolerance to salt stress. Another promising approach to increasing salt tolerance is the manipulation of genes involved in the synthesis of SMs with protective properties.
2.2 The Role of Secondary Metabolites in Reducing Drought Stress Damage in Plants
Drought stress, which is one of the stresses that mostly affect agricultural productivity in the world, has been reported by the World Health Organization as a factor that will cause 700 million people to migrate to different regions by 2030 (WHO 2020). Drought stress occurs when the amount of water available in the soil cannot meet the needs of the plants and when atmospheric. This situation causes physiological, morphological, biochemical, precipitation is insufficient ecological, and molecular changes in plants by affecting many metabolic processes, such as the growth and development of plants, amount of biomass, photosynthesis and transpiration rate, stomatal conductance, and cellular dehydration (Xu et al. 2010; Mashilo et al. 2017). Drought stress causes the production of ROS such as superoxide, hydroxyl and singlet oxide, and hydrogen peroxide, which are quite toxic and reactive in plants. Reactive oxygen species, on the other hand, cause disruption of cellular homeostasis by damaging protein, carbohydrate, lipid, and DNA (Anjum et al. 2017; Ibrahim et al. 2019). However, plants induce an enhanced SM production, phytohormonal response, and osmotic regulation to increase tolerance to drought stress (Larson 2018; Jogawat et al. 2021; Yadav et al. 2021a). Secondary metabolites reduce membrane lipid peroxidation with their antioxidant properties and serve as cell wall components to strengthen the cell wall against stress factors (Yang et al. 2018). Plants try to cope with drought conditions by increasing the amounts of SMs such as terpenes, saponins, alkaloids, and phenolics (anthocyanins and flavonoids) (Chen et al. 2011; Jaafar et al. 2012; Isah 2019). Generally, drought stress increases the concentrations of phenolic compounds by stimulating key genes in the phenylpropanoid pathway. Flavonoids, polyphenols, and terpenoids or isoprenoids are secondary compounds that are effective in scavenging increased ROS due to drought (Treml and Smejkal 2016). For example, increases in the levels of phenolic compounds were determined in H. brasiliense, P. sativum, Chrysanthemum sp., and Salix sp., which were exposed to drought stress (Dawid and Hille 2018; Hodaei et al. 2018; Larson 2018). In studies on cotton and potato, it has been determined that drought stress increases terpene and flavonoid synthesis (Payton et al. 2011; Zhang et al. 2014). In another study, it was determined that drought stress increased the amounts of glycosides, monoterpenes, terpenoids, and carotenoids in rosemary and grapes (Liu et al. 2014; Savoi et al. 2016). It has been reported that there is an increase in rosmarinic, ursolic, and oleanolic acid production in P. vulgaris and an increase in betulinic acid content in H. brasiliense (Chen et al. 2011; Jaafar et al. 2012). Razavizadeh and Komatsu (2018) found significant increases in the amounts of thymol, γ-terpinene, proline, and carbohydrates in seedlings exposed to mannitol-induced drought stress. Wang et al. (2019) found significant increases in the amounts of flavonoids, proanthocyanidins, and phenolics and in the activities of antioxidant enzymes in Matteuccia struthiopteris (L.) Todar. and A. multidentatum (Doll.) exposed to drought stress. Drought stress significantly increased the lignin and pectin content in the roots of soybean (Al-Hakimi 2006). Mazloom et al. (2020) reported that the treatment of lignin-based hydrogel reduced electrolyte leakage while increasing the water content and proline amount. The other groups of SMs produced in response to drought stress are N- and S-containing compounds. Increases in the amounts of alkaloids such as catharanthine, vindoline, capsaicinoid, vincristine, and vinblastine were determined in C. roseus under drought stress (Phimchan et al. 2012; Zhang et al. 2012). Cyanogenic glycosides act as antioxidants in water deficiency conditions, and the released hydrogen cyanide increases the amount of endogenous salicylic acid (SA) (Sun et al. 2018). Amino acids such as citrulline γ-aminobutyric acid, β-aminobutyric acid, β-alanine, and ornithine act as both antioxidants and osmolytes (Vranova et al. 2011). Glutathione and thionine are compounds that serve to scavenge ROS as powerful antioxidants. For some SMs produced in different plants exposed to drought stress, refer to Table 19.2. All these studies have shown that genes responsible for the biosynthesis of SMs can be used to increase drought tolerance. For example, overexpression of chalcone synthase in tobacco plant increased the amount of flavonoids (Zhao et al. 2019), and overexpression of the GH4CL7 gene in G. hirsutum and increase in lignin biosynthesis increased resistance to drought stress (Sun et al. 2020). In another study, it was determined that overexpression of IAA5, IAA6, and IAA19 genes in Arabidopsis thaliana stimulated glucosinolate accumulation (Salehin et al. 2019). It has been reported that overexpression of phosphoenolpyruvate carboxylase and an increase in anthocyanin biosynthesis in transgenic rice increase resistance to drought stress (He and Sheffield 2020). Therefore, manipulation and overexpression of genes related to the synthesis pathway of these metabolites to increase the amount of SMs have been suggested as one of the effective strategies to increase the resistance of plants to various stresses, including drought stress (Yadav et al. 2021b).
2.3 Cold Stress Alters Secondary Metabolism
Plants must grow at suitable temperatures to complete their growth and development. Both high and low temperatures require plants to cope with various challenges. A low temperature is one of the most detrimental stresses for plants living in temperate regions (Janská et al. 2010; Peng et al. 2015). Cold stress is divided into two classes: chilling stress, which is low temperatures between 0 and 15 °C without freezing (Chen L et al. 2020), and freezing stress, which is temperatures below 0 °C (Zhang et al. 2020). Freezing stress is more harmful to plants than chilling stress. It causes many damages such as chilling stress, a decrease in photosynthesis, osmotic damage, desiccation, oxidative stress, inhibition of protein synthesis and enzyme activities, a decrease in membrane permeability, ion leakage, and dehydration in cells and tissues (Ramakrishna and Ravishankar 2011). Low temperatures significantly affect plant growth and development by causing reduced root length, leaf loss, reduced leaf expansion, symptoms such as chlorosis and necrosis, and damage to reproductive organs such as pollen and pollen tube (Lyons 1973). Freezing stress causes the formation of ice crystals in both the intracellular and intercellular spaces. The ice crystal formation in the intercellular space causes dehydration and the withdrawal of water from the cell due to the decreasing water potential in the apoplastic space. Intracellular ice formation often leads to cell death, as it causes membrane rupture, changes in membrane permeability, mechanical deterioration in the protoplasm, and deformation of the cell wall (Levitt 1980; Steponkus 1984). The damage to the cell membrane of the cold is largely due to dehydration. A low temperature causes changes in the lipid content of the cell membrane, with an increase in phospholipid content and a decrease in the ratio of cerebrosides in general. Fatty acid saturation of the cell membrane of plant species and its sensitivity to cold are interrelated (Uemura and Steponkus 1999). Since the ratio of saturated fatty acids is higher in the membranes of plants that are sensitive to cold, membrane leaks occur as the membrane tends to pass from the liquid mosaic phase to the solid gel form during cold stress. Cold-tolerant plants has a lower transition temperature (the temperature at which it changes from the liquid mosaic phase to the gel phase) since it has higher unsaturated fatty acids (Wang et al. 2006). The organelle that is firstly and most severely affected by cold stress is chloroplasts (Liu et al. 2018). It causes changes in the structure of thylakoids and swelling (Kratsch and Wise 2000). Additionally, low temperatures also decrease photosynthesis due to the reduction of stomatal opening and the inhibition of CO2 exchange. However, since the photosynthetic apparatus captures more photons than necessary, PS II inhibits electron transport and causes photoinhibition, and photodamage occurs with the degradation of the D1 reaction center protein (Szilard et al. 2005; Yang et al. 2017). Under stress conditions, ROS are produced in the chloroplasts, mitochondria, and peroxisome and apoplastic regions of plants (Xie et al. 2019). The main reason for the formation of ROS in chloroplasts is stomatal closure, as well as restrictions in CO2 fixation due to disruptions in the electron transport chain (ETC) (Mignolet-Spruyt et al. 2016). Similarly, disruptions in the ETC in the mitochondria cause ROS production. ROS induce lipid peroxidation; deterioration of DNA, lipid, and carbohydrate structure; and inactivation of enzymes (Foyer and Noctor 2005). Plants increase the biosynthesis of different SMs to be protected from these damages. In a study conducted on O. basilicum L., it was determined that the application of 4 °C cold stress increased the amounts of camphor, bornyl acetate, eugenol, methyl chavicol, and methyl eugenol, as well as the the activity of superoxide dismutase (SOD) and GPX antioxidant enzymes (Rezaie et al. 2020). In the study, it was also determined that the total phenolic and flavonoid amounts were increased compared with the control group. These increases in the activities of antioxidant enzymes and the amounts of total phenolic and flavonoid levels have been associated with protection against ROS toxicity. This high increase in the amount of phenolic compounds was attributed to the increase in phenylalanine ammonia-lyase (PAL) activity. Phenolic compounds are SMs with the potential to scavenge ROS and prevent lipid peroxidation as electron and hydrogen atom donors (Huang et al. 2019). While flavonoids and phenolics serve as scavengers, unsaturated fatty acids also help increase tolerance to cold by improving cell membrane fluidity (Li J et al. 2019; Li Q 2020).
Sun et al. (2021) detected increases in the amount of free fatty acids, lysophosphatidylcholines, and lysophosphatidylethanolamine, which are biomarkers of freezing damage, in cold stress-tolerant and cold stress-sensitive A. arguta. These accumulations indicate membrane damage caused by cold stress. It was determined that the amounts of phenolic compounds, such as hydroxytyrosol, tyrosol, and oleuropein, and the enzyme activities of PAL and polyphenol oxidase increased in the leaves of olive trees exposed to –7 °C (Ortega-García and Peragon 2009). The authors associated polyphenol oxidase and oleuropein with the antioxidant defense system. Additionally, it was determined that the accumulation of anthocyanin and flavonoid in A. thaliana, Petunia hybrid, and Z. mays plants exposed to cold stress (Janas et al. 2002; Yang et al. 2018); the total phenol concentrations and particularly the genistein amount in the roots of Glycine max plant (Janas et al. 2002); and the chlorogenic acid production in M. domestica tree were increased. It has been reported that low-temperature stress also increases the synthesis of phenolic compounds, which participate in the structuring of the cell wall and serve in the biosynthesis of lignin and suberin (Griffith and Yaish 2004). However, Krol et al. (2015) reported that long-term cold stress decreases the amount of phenolic compounds, and this may be related to the slowing down of some elements of the secondary metabolism. More phenolic reduction was found in the cold-sensitive V. vinifera cultivar than the cold-tolerant cultivar. However, it was also determined that the total level of phenolic compounds and antioxidant activity in the cold-resistant cultivar were higher than that in the susceptible cultivar. Glycosylated terpenoids are SMs that play a role in increasing tolerance to cold stress (Yeshi et al. 2022). Zhou et al. (2017) found an increase in the amount of nerolidol in frost-damaged tea and suggested that this increase is a response to cold stress. Zhao et al. (2020) reported that increases in the level of glycosylated sesquiterpene and nerolidol glucoside, which have antioxidant and ROS scavenging ability, in the tea under cold stress may be effective in increasing tolerance to cold stress. Additionally, with the effect of cold stress, terpenoids, such as β-phellandrene, (E)-β-ocimene, δ-elemene, α-humulene, β-caryophyllene, withanolide A, withaferin A, and nerolidol glucoside, and increases in the concentration of phenolics, such as pelargonidin, anthocyanins, anthocyanidins, genistein, and daidzein, and alkaloids, such as vindoline, were detected (Janas et al. 2002; Dutta et al. 2007; Copolovici et al. 2012; Mir et al. 2015; Jeon et al. 2018; Zhao et al. 2020).
Data obtained from studies on different plants indicate that polyamines are also effective in increasing tolerance to cold stress. It is stated that the polyamine levels of plants such as T. aestivum, M. sativa, and P. antiscorbutica increase considerably under cold stress, and this increase in the amount of polyamine may be related to cold tolerance (Akula and Ravishankar 2011; Kovacs et al. 2011). In a similar study, it was reported that putrescine and polyamines of spermine and melatonin synthesized in the D. carota plant protect against apoptosis caused by cold stress (Lei et al. 2004). In another study, it was stated that the plant S. tuberosum produces polyamine to eliminate the harmful effects of ROS formed by the effect of cold stress (Kou et al. 2018). It increases tolerance to cold by preventing cytolysis by binding to phospholipids in the cell membranes of polyamines (Li and He 2012). Some other SMs under cold stress are mentioned in Table 19.3.
Another response used by plants against cold stress is carbohydrate metabolism. Carbohydrates serve to retain water in cells, stabilize cell membranes, and scavenge ROS. While the decrease in temperature decreased the water potential and starch amount, it increased the amount of soluble sugar, sucrose, mannitol, and osmotin (PR-5 protein) (Antognozzi et al. 1993; D’Angeli and Altamura 2007; Eris et al. 2007). Amino acids such as betaine, arginine, and proline also act as osmoprotectants in increasing tolerance to cold stress (Meilong et al. 2020).
With the activation of genes responsible for the synthesis of SMs, the tolerance level and adaptation of plants to various stress conditions can be achieved (Jan et al. 2021). Determining the genetic responses of plants to stress is one of the important research areas for developing cold stress-tolerant plants. GOLS1, GOLS3, GR-RBP3, HYDROLASE22, RHL41, CAU1, PME41, DREB26, and CRK45 are necessary genes for increasing the tolerance of Camellia sinensis to cold stress (Samarina et al. 2020). In the study of O. basilicum L., it was determined that cold stress (4 °C) increased the amounts of methyl eugenol and methyl chavicol and that these metabolites were in a positive correlation with the expression levels of eugenol synthase 1 (EGS1) and eugenol O-methyl transferase (EOMT) genes. Therefore, EGS1 and EOMT genes have been proposed as candidate genes for genetic manipulation of the phenylpropanoid biosynthesis pathway in increasing the cold tolerance of O. basilicum (Rezaie et al. 2020). It was determined that cold stress increased the levels of steroidal alkaloids, glycoalkaloids, phenolic acids, and flavonoids in S. viarum, a medicinal plant (Patel et al. 2022). It has been reported that there is a correlation between the transcription levels of genes involved in the biosynthesis of glycoalkaloids and flavonoids and the amounts of these metabolites. Recent research confirmed the expression and posttranslational modifications of genes that control the production of SMs to increase plant tolerance to such stresses.
2.4 Production of Plant Secondary Metabolites Under Heat Stress
In the last five decades, increases in CO2 and other greenhouse gases because of human activities have caused the world to warm by approximately 0.85 °C (Bein et al. 2020). This degree may seem small, but it is not. Even an increase of 1 °C is the beginning of the road to disaster. When an increase of 2 °C is reached, the temperatures normally seen once every decade will begin to be seen every 2 years. When it reaches 1.5 °C, this temperature increase will be seen almost every 5 years. Similar results will be valid for excessive precipitation and drought. Therefore, heat stress is one of the important factors affecting the growth and development of plants now and in the future. High temperatures cause deterioration of membrane integrity of plants, a decrease in photosynthesis rate, and premature aging of plants. Seed germination inhibition, growth reduction, and excessive ROS production are among their main adverse effects (Hasanuzzaman et al. 2013). Heat stress induces the production of alkaloid and phenolic compounds in various plant species (Ramakrishna and Ravishankar 2011). It has been stated that F. vesca, S. officinarum, and L. sativa plants exposed to heat stress produce high amounts of phenolic acids, antioxidants, flavones, and anthocyanins (Wu et al. 2007). α-Tocopherol and plastoquinone, which are synthesized in high amounts in L. esculentum plant under heat stress, facilitate photosynthesis by acting as antioxidants and electron carriers (Havaux 2020). Similarly, the continuous synthesis and emission of terpenes are effective in countering the damage caused by heat stress (Korankye et al. 2017). With the effect of heat stress, increases in the concentrations of terpenoids such as β-phellandrene, 2-carene, α-phellandrene, limonene in S. lycopersicum and α-caryophyllene, and β-farnesene in D. carota were determined. Additionally, a high amount of flavonoid production has been reported in the O. basilicum plant, which is exposed to high-temperature stress (Al-Huqail et al. 2020). Isoprenes synthesized from the mevalonate pathway in plants help to heal the photosynthetic apparatus damaged by the effect of heat shock and to improve thermotolerance (Li and Sharkey 2013). Similarly, increases in the concentration of isoprene terpenoids were detected in Q. rubra exposed to heat stress (Hanson and Sharkey 2001). Carotenoids and phenolic compounds such as flavonoid, lignin, and tannin show antioxidant properties under heat stress, scavenge ROS, and protect against oxidative damage (Sehgal et al. 2016). There has been an increase in the amount of anthocyanin, coumaric acid, and caffeic acid phenolics in D. carota with the effect of heat stress (Commisso et al. 2016). Alterations in the amount of different SMs under heat stress are mentioned in Table 19.4. Under heat stress, the synthesis of SMs generally increases, leading to the protection of cellular structures from oxidative damage (Sehgal et al. 2016), but there are also reports emphasizing a decrease in the concentration of SMs in plants under heat stress. Temperature is an important environmental factor affecting anthocyanin metabolism in plants. In some studies, it has been reported that high temperatures inhibit the expression of genes that control anthocyanin synthesis and the accumulation of activators (Wang et al. 2016; Rehman et al. 2017). It has been reported that there is a decrease in the levels of anthocyanins and carotenoids in many species such as V. vinifera and Brassicaceae, due to partial pigment degradation and reduced gene transcription in plants under the high-temperature stress (Yang et al. 2018). Again, in a recent study, Liu et al. (2019) reported that a high temperature decreased the amount of anthocyanin in the S. tuberosum. Authors pointed out that the reason for this decrease was the directing of the flow to lignin and chlorogenic acid biosynthesis of isoprene, a more beneficial metabolite with antioxidant and thermotolerance properties (Wahid et al. 2012; Austen et al. 2019).
Plant survival strategies against high temperatures include osmoprotectants such as chaperones, proline, glycine betaine, sugars, and polyamines (Sakamoto and Murata 2002; Gepstein et al. 2005; Chen et al. 2007). In the study conducted on V. aconitifolia exposed to 42 °C for 7 days, increases in the amount of proline and total sugar and the activities of antioxidant enzymes were shown as evidence of thermotolerance (Harsh et al. 2016).
2.5 Plant Secondary Metabolites Produced in Response to Heavy Metal Stress
Heavy metal stress is one of the main abiotic stress factors that prevent metabolic processes in plants due to reasons such as contamination of soil, air, and water, high bioaccumulation, toxicity, and lowering the quality of natural products produced by plants (Keunen et al. 2016; Sahay and Gupta 2017). Heavy metal stress causes changes in the conformation of chloroplasts in plants and increases the efficiency of various signaling (ethylene and jasmonic acid) pathways that stimulate aging (Keunen et al. 2016). They produce ROS and damage DNA, RNA, and protein by causing oxidative stress (Kumar and Sharma 2018). They decrease the amount of chlorophyll a and b due to the inhibition of enzymes involved in the biosynthesis of pigments (Rai et al. 2016). For example, it has been reported that Pb stress decreases the amount of photosynthetic pigments even in B. juncea, which is used in heavy metal phytoremediation (Chandra and Kang 2016). Plants protect themselves from the toxicity of metals by various mechanisms. These mechanisms include antioxidant defense, binding to the cell wall or deposition in the vacuole, returning the metal ions in free form or complex form to the rhizosphere, synthesis of low molecular weight organic acids, accumulation of osmoprotectants, chelate formation with sulfur donor phytochelatins and metallothioneins, and production of SMs such as isoprenoids, phenolics, flavonoids, and carotenes (Dalvi and Bhalerao 2013; Umar et al. 2013; Khare et al. 2020). All these studies indicate that SMs can be an effective strategy for reducing the toxicity of heavy metals (Table 19.5). Since the cell wall is the first barrier that metals encounter, cell wall components protect the protoplast by binding to metals. For example, the functional groups of lignin bind more than one metal ion to itself. Phenolic compounds such as lignin, quercetin, coumaric acid, catechin, ferulic acid, and myricetin protect the cell against metal stress by contributing to the increase in cell wall thickness (Guo et al. 2008; Krzesłowska 2011). The peroxidase (POX) oxidizes monolignols to radicals that combine with the lignin polymer. These radicals then combine to form the lignin polymer and thus contribute to the strengthening of the cell wall (Wang et al. 2013). Moreover, phenols, alkaloids, and saponins can prevent the harmful effects of metal toxicity by forming stable complexes with different metals or by chelation with metals (Berni et al. 2019; Nobahar et al. 2021). Plants exposed to metal stress secrete root exudate, which includes metabolites such as phenolics, amino acids and derivatives, sugar and organic acids, and proteins, and mucilage into the soil. These metabolites chelate metals in the rhizosphere and apoplast, preventing them from entering the symplast and reducing the toxicity in the cytoplasm (Nigam et al. 2001). Histidine and nicotinamide are amino acids that play an important role in the chelation of heavy metals. Nicotianamine, a free amino acid, can bind metals such as iron (Fe), copper (Cu), and nickel (Ni) (Higuchi et al. 1999). Histidine, which is chelator-like nicotinamide, also forms a complex with zinc (Zn) and Ni, reducing heavy metal toxicity (Salt et al. 1999; Richau et al. 2009). Proline, another amino acid, acts as an osmoregulator in the regulation of the water balance disorder that occurs during heavy metal stress. It also detoxifies •OH and 1O2 and increases the activities of intracellular antioxidant enzymes (Mourato et al. 2012). Organic acids such as malate, malonate, oxalate, tartrate, citrate, and aconitate reduce toxicity by forming chelates with metals in the cytosol (Anjitha et al. 2021). Metallothioneins, which are rich in cysteine, reduce metal toxicity by binding to metals with the thiol group of cysteine (Zhou and Goldsbrough 1994). They also increase tolerance to oxidative stress by acting in ROS detoxification. Glutathione is an important ROS and methylglyoxal (a cytotoxic compound) scavenger and an antioxidant effective in the chelation of metals (Saito et al. 2011). Additionally, phytochelatins, a cysteine-rich polypeptide family that plays an important role in reducing metal toxicity, are also synthesized from glutathione (Yang et al. 2005). Like metallothioneins, the heavy metal is accumulated in the vacuole by forming a complex with the heavy metal with the thiol groups they have, and its free circulation in the cytosol is limited (Sanit`a Di Toppi and Gabbrielli 1999). Anthocyanins (cyanidin, delphinidin, petunidin, etc.) with adjacent hydroxyl groups have strong metal-chelating effects (Tang and Giusti 2020). Janeeshma et al. (2020) found a high accumulation of anthocyanins in maize under Zn stress. Cyanidin gained electrons and formed a complex with zinc, increasing the tolerance to high Zn stress. The phenolic and flavonoid compounds in G. pseudochina plants chelate Zn and Cd metals. It has also been reported that Cinchona alkaloids can form complexes with different metals, such as Fe, lead (Pb), Cu, and cobalt (Co), and that phenolic compounds such as catechin and juglone can form complexes with Fe (Chobot and Hadacek 2010). It has been determined that tannins extracted from plant seeds can chelate metals such as Zn, Fe, and Cu (Karamac 2009). Many studies conducted to date show that various SMs synthesized under heavy metal stress play an active role in reducing the damage of heavy metals in the cytoplasm by forming chelates with metals.
Antioxidants such as tocopherol, carotenoids, glutathione, ascorbate, and phenolic compounds, such as coumarin, tannin, lignin, anthocyanin, and flavonoids, act as ROS scavengers in plants exposed to heavy metal stress (Maleki et al. 2017). Phenolic compounds and flavonoids, which act as antioxidant compounds due to their hydrogen atom or electron-donating abilities, can directly scavenge ROS (Okem et al. 2015). Phenolic compounds and flavonoids with redox properties act as antioxidants and ROS scavengers and can chelate metals (Rice-Evans and Paganga 1996). For example, it was determined that increased phenolic compounds in P. vulgaris exposed to Pb stress scavenge ROS and reduce lipid peroxidation and oxidative damage (Neelofer et al. 2010). The increase in phenolic, flavonoid, and anthocyanin concentrations in Salvia sclarea increased its tolerance to Cd metal (Dobrikova et al. 2021). These metabolites have been reported to act as ROS scavengers. It has been determined that Cu metal increases the production of phenolic and lignin compounds in P. ginseng and W. somnifera plants (Khatun et al. 2008), and Cu2+ and Cd2+ metals stimulate the biosynthesis of betalain, shikonin, and digitalin (Trejo et al. 2001). Zn2+ metal increased lepidine production in L. sativa plant (Saba et al. 2000). Thomas et al. (2011) reported that Cd and Co metals stimulated diosgenin accumulation in T. foenum-graecum. AgNO3 and CdCl2 increased the concentration of scopolamine and hyoscyamine, and Pb increased the synthesis of phenolic compounds (Winkel-Shirley 2001). The downregulation of the hyoscyamine 6β-hydroxylase enzyme responsible for the synthesis of scopolamine by silver ions increased the amount of scopolamine (Pitta et al. 2000). Winkel-Shirley (2001) reported that plants grown on aluminum-containing soils have a high flavonoid content, and this may help reduce damage caused by oxidative stress.
2.6 UV Stress Affects the Production of Secondary Metabolites in Plants
Light is an important abiotic factor that can affect plant growth, production, and quality of SMs. The responses of different plant species to UV stress differ depending on the signal transmission mechanism, the amount and intensity of light, and the effect of gene expression (Parikrama and Esyanti 2014). UV-B radiation causes the formation of ROS such as H2O2 in plants, damaging DNA and chloroplasts, specifically photosystem II (Del Valle et al. 2020). Plants can adapt to UV changes by accumulating various SMs such as terpenoids, flavonoids (flavonols, anthocyanins, catechins, etc.), hydroxycinnamic acids, phenylpropanoids, tannins, cyanogenic glycosides, α-tocopherol, glucosinolates, carotenoids, and alkaloids (Morales et al. 2010; Jan et al. 2021). Ferulic acid, caffeic acid, and p-coumaric acid are the most effective phenolics for reducing the harmful effects of UV. Most phenols, such as hydrocinnamic acids, p-coumaric acid, and ferulic acid, help in cell wall formation and represent the beginning of lignification (Antonova et al. 2012). Some other SMs in different plants exposed to UV stress and their defense effects are presented in Table 19.6.
SMs accumulated in the epidermal layers of the cells of plants exposed to UV-B stress protect the underlying sensitive tissues against the harmful effects of stress. However, Zhao et al. (2013) reported that long-term exposure to UV-B stress may decrease the protectiveness of these metabolites due to less photosynthate production. The photosensitive and highly stable cellular components absorb excess UV-B and prevent photodamage. UV-B and UV-C stimulate flavonoid synthesis and synthesis of compounds synthesized from the phenylpropanoid pathway (Warren et al. 2003). Flavonoids and phenylpropanoid derivatives, deposited in the epidermal cells, significantly inhibit the effect of UV stress as a UV-absorbing sunscreen (Mazza et al. 2000). In addition to flavonoids, compounds such as carotenoids and anthocyanins accumulate in the upper epidermis of the leaves and form UV-B blocks as UV absorbers and prevent the formation of ROS (Hideg et al. 2013). Flavonoids alleviate photoinhibition and photooxidative damage by eliminating the harmful effects of ROS, owing to their radiation absorption (UV-absorbing) properties (Jordan 2002). UV-B stress increases the concentration of flavonoid content in H. vulgare, P. incarnata, P. quadrangularis, P. edulis, and K. pinnata, polyamines in C. sativus, and flavonols in P. abies (Antognoni et al. 2007). As it is known, the cell wall of plants is the largest carbon source in the biosphere. The cell wall consists of polysaccharides such as cellulose and hemicellulose, as well as pectin, lignin, structural proteins, and other compounds. Some studies have shown that while UV-B increases the level of phenolic compounds in the structure of the cell wall, it causes relaxation in the cell wall with the release of -CH4 from pectin (Ruhland et al. 2005; Messenger et al. 2009). In another study, Cuzzuol et al. (2020) determined that UV-B increased polyphenols such as flavonoids and lignins and the total antioxidant capacity in sun-tolerant Paubrasilia echinata. However, it was found that there was an increase in lignin content despite the decrease in hemicelluloses in sun-resistant ecotypes. The increases in lignin and flavonoid content strengthen the cell wall and increase mechanical resistance, thus reducing the UV-B transfer from the leaf surface to the mesophyll and increasing tolerance to stress (Cuzzuol et al. 2020). Additionally, epidermal cuticle configurations are capable of scattering some of the UV radiation, although small reflectivity may be required for UV scattering.
Antioxidants are compounds that protect against oxidative stress caused by various stress factors. Metabolites such as ascorbic acid, phenolic compounds, carotenoids, glutathione, flavonoids, and α-tocopherol, which are nonenzymatic antioxidants, serve to scavenge ROS species and prevent lipid peroxidation (Miret and Munné-Bosch 2015). Moreover, phenolic acids such as hydrocinnamic acid, anthocyanins, stilbenes, and various other phenylpropanoid pathway compounds also have a high antioxidant activity (Agati and Tattini 2010). For example, the concentration of phenolics, a compound with antioxidant properties, increases with the effect of UV-B stress. However, these antioxidant capacities of SMs vary not only from their concentrations but also from their biochemical structures and the cellular regions (cell walls, vacuoles of epidermal and mesophyll cells, chloroplasts, trichomes) where they are synthesized and accumulated. For example, monohydroxylated B-ringed flavonoids containing a single -OH group absorb more UV-B than dihydroxy B-ringed flavonoids containing two -OH groups (Agati and Tattini 2010). It was found that flavonoids with the catechol group in the B ring showed better antioxidant properties (Agati et al. 2009). It has also been stated that light increases the biosynthesis of terpenoid indole alkaloids in C. roseus (Liu et al. 2018). Increases in the amount of carbonic acid, a diterpene, were also determined in R. officinalis under UV-B stress (Luis et al. 2007). It has been reported that carbonic acid, an antioxidant, prevents the deterioration of the structure of the cell membrane by preventing lipid peroxidation against UV-B stress (Munne-Bosch and Alegre 2002). Carotenoids, which act as photosynthetic pigments, were also increased in plants exposed to UV stress (Sankari et al. 2017). It protects the thylakoid membrane lipids of carotenoids and some terpenoids against high light damage. Xanthophylls and tetraterpene carotenoids increase photosynthesis by preventing photooxidative damage in the photosynthetic apparatus (Jahns and Holzwarth 2012; Pattanaik and Lindberg 2015).
Carotenoids act as ROS scavengers during stress and protect thylakoid membranes and proteins. They prevent free radical chain reactions by reacting with the products formed because of lipid peroxidation and protect the photosynthetic apparatus (Niyogi et al. 2001; Swapnil et al. 2021). UV stress causes anthocyanin accumulation in P. avium, M. domestica, P. frutescens, D. carota, and F. vesca (Winkel-Shirley 2001; Ramakrishna and Ravishankar 2011). In a study investigating the effects of anthocyanins accumulated in the mesophyll and epidermis against UV stress, it was determined that the antioxidant activity of anthocyanins accumulated in the mesophile against oxidative damage was more effective than their sunscreen properties (Kytridis and Manetas 2006). In plants exposed to UV-B stress, an increase in alkaloid biosynthesis was determined by the effect of the tryptophan decarboxylase enzyme and WRKY6 factor (Mehrotra et al. 2018). In a similar study with P. brachyceras leaves under UV stress, alkaloid increases were detected due to the increase in the expression of genes encoding the enzyme that produces tryptamine, the indole precursor of alkaloid synthesis (Nascimento et al. 2015).
3 Plant Secondary Metabolite Synthesis Under Biotic Stress
Nematodes, fungi, viruses, insects, viroids, and bacteria cause serious damage by affecting the growth and development of plants. Plants have developed various defense mechanisms against pathogens. Phytochemicals with antimicrobial effects, such as phenolics, flavonoids, coumarins, terpenoids, lignins, alkaloids, stilbenes, and glucosinolates, are important metabolites of defense responses in plants. The first barrier against pathogens in plant defense is the cuticle and cell walls (Berto et al. 1999). The accumulation of cutins or waxes increases resistance to the pathogen (Xu et al. 2022). Cuticles, which are rich in cutin, prevent the germination of spores of fungi and mycelial growth due to their hydrophobic properties. Additionally, triterpenoids, the main components of cuticular wax, confer chemical resistance to fungal pathogens. It has been determined that 16-hentriacontanone (palmitone), which is the main component of the cuticular wax of A. squamosa, shows resistance and antifungal activity (Shanker et al. 2007). The SMs associated with defense in plants are generally divided into two groups: phytoanticipins and phytoalexins (Mansfield 1999). Phytoalexins and phytoanticipins are SMs with antimicrobial properties against insect, microorganism, and herbivorous attacks (Morant et al. 2008). Phytoanticipins such as saponin, glucosinolates, and cyanogenic glucosides are low molecular weight antimicrobial compounds, which exist in plants before infection or also occur after infection.
Phytoanticipins can accumulate in dead cells or be excreted into the rhizosphere. The inactive forms are stored in the vacuole. When necessary, they are hydrolyzed and become active, that is, toxic. For example, quinone, catechol, and protocatechuic acid have inhibited the germination of the spores of C. circinans and B. cinerea. Glycosides and glucosinolates are synthesized in healthy tissues before infection but are activated when tissue damage occurs. Although found at higher levels in healthy plants, saponins with surfactant properties are glycosides that impair the integrity and function of the membrane by binding to the sterols in the cell membranes of some pathogens (Tiku 2020). It has been determined that avenacins localized in the roots of the oat plant prevent G. graminis var. tritici infection (Osbourn 1996). However, it was determined that 26-desglucoavenacosides A and B, which are active forms of avenacosides localized in the leaves and shoots of the oat plant, have antifungal properties (Gus-Mayer et al. 1994a, b; Osbourn et al. 1994). Since some saponins bind to proteins and inhibit proteinases, they impair digestion in the guts of insects (Amtul and Shakoori 2014). Newman (2014) reported that saponins isolated from B. vulgaris leaves have a deterrent activity against P. xylostella. α-Tomatine, which is the main saponin of tomato and is found at high levels in the flowers, leaves, and fruits of the tomato plant, provided a high resistance against fungi such as F. oxysporum f. sp. lycopersici and V. albo-atrum (Smith and MacHardy 1982; Pegg and Woodward 1986). However, α-tomatine has been reported to be active at a certain pH. For example, since A. solani lowers the pH at the infection site, α-tomatine becomes inactive, so the pathogen cell membrane cannot break down, and the antifungal effect disappears (Roddick and Drysdale 1984). Cyanogenic glycosides containing nitrogen are degraded by hydrolytic enzymes such as β-glycosidases and hydroxy nitrile lyases, released by plants after infection to produce hydrogen cyanide, which is highly toxic to pathogens (animals, insects, etc.) (Poulton and Li 1994; Tiku 2020). Hydrogen cyanide binds to and inhibits cytochrome oxidase to stop electron transport, damaging the respiratory system of predators. However, plants protect themselves from the toxic effects of hydrogen cyanide with detoxification enzymes (Miller and Conn 1980). Glucosinolates, which are S-containing glycosides found in members of the Brassicaceae, are converted by myrosinase (a thioglucosidase) into different products such as nitrile, thiocyanate, and isothiocyanate, which are highly toxic to many pathogens. The toxic effects of these degradation products on pathogens such as Alternaria sp., P. parasitica, L. maculans, and M. brassicicola have been determined, and it has also been reported that they can be used as fungicides against other plant pests such as grains (Mari et al. 1993; Angus et al. 1994). Benzoxazinoids, another phytoanticipins, are predominantly found in grains such as wheat, rye, and corn and in some dicot plants with antimicrobial properties. In response to tissue damage caused by pathogen attack, they are hydrolyzed by β-glycosidase to produce toxic BX-Glcs aglycones (Korte et al. 2015; Del Cueto et al. 2018). Various compounds synthesized from the phenylpropanoid pathway exhibit antifungal properties by inhibiting spore germination and serve as phytoanticipins. For example, caffeic acid, p-coumaric acid, ferulic acid, and methoxycinnamic acid induce resistance to A. flavus Link and A. parasiticus Speare (Sobolev et al. 2006). The protective effects of hesperidin against P. digitatum, kaempferide triglycoside and hydroxyacetophenone against F. oxysporum, and sakuranetin against M. grisea infection have been determined (Marchesini et al. 1996). Phytoalexins, which include substances synthesized through the terpenoid and phenylalanine pathway, are low molecular weight antimicrobial compounds and get accumulated in plants after infection and inhibit the growth of bacteria and fungi (Jeandet et al. 2013) and inhibit spore growth and growth of hyphae to pathogenic fungi. They are thus considered defense compounds against diseases caused by pathogens. However, the amount and rate of accumulation of phytoalexins affect the development of pathogens (Duke 2018). Stilbenoids are metabolites derived from the amino acid phenylalanine. p-Coumaryl-CoA and malonyl-CoA enable the production of resveratrol (3,5,4′-trihydroxy-trans-stilbene) and various flavonoids in plants. However, the activity of the stilbene synthase enzyme is required for the synthesis of these two compounds. Because of this, using a single biosynthetic gene, it is possible to obtain a phytoalexin of the stilbene type, which is an important compound for defense against fungal infection in noninfected plants. Stilbenes, which are also considered phytoalexins, have a strong antifungal activity because they accumulate in the necessary concentrations to prevent fungal infection in plants (Morales et al. 2000). An example of this is the accumulation of pinosylvin and pinosylvin 3-O-methyl ether against C. versicolor and G. trabeum infection in conifers (Schultz et al. 1992). Resveratrol, a stilbene analog and first isolated from V. grandiflorum in 1940, is a compound with many activities such as antibacterial, antiviral, antioxidant, and antitumor (Jeandet et al. 1995; Song et al. 2021). It was determined that resveratrol inhibits the penetration and spore germination of V. inaequalis in apples (Schulze et al. 2005). It has also been reported that B. cinerea and P. viticola also reduce sporangia germination (Pezet et al. 2004). Song et al. (2021) reported that resveratrol derivatives formed due to modifications such as the removal of phenolic hydroxyl groups and ester formation in the structure of resveratrol inhibit tobacco mosaic virus (TMV).
Phenols are the most well-known and common defense compounds against insects, various bacteria, and fungi (Uleberg et al. 2012). Phenol derivatives and tannins prevent the proliferation of bacteria by increasing membrane damage and permeability and inactivating metabolism (Khameneh et al. 2019). Flavonoids, tannins, isoflavonoids, anthocyanins, lignins, phytoalexins, and furanocoumarins are important phenolic compounds that act as defense compounds against pests (Rani and Jyothsna 2010). Phenolics and flavonoids inhibit pathogens by disrupting their structures by causing lipid peroxidation in the cell membrane and mitochondrial membrane in fungi (VanEtten et al. 1994). Phenylpropanoids and flavonoids have phenolic hydroxyl groups that form ionic and hydrogen bonds with peptides and protons, causing the denaturation of proteins and enzymes, thus inhibiting the physiological activities of pathogens, including the reproductive system (Morrissey and Lou 2009). Phenolic compounds also show antibacterial properties by inhibiting enzymes such as NADH reductase and ATP synthase (Rempe et al. 2017). Flavonoids not only inhibit bacterial cell wall proteins and DNA synthesis but also cause inactivation of metabolism (Bouarab-chibane et al. 2019). Additionally, phenolics and flavonoids such as chlorogenic acid synthesized from the phenylpropanoid pathway increase the activities of defense enzymes and activate the SA signaling pathway (Jiao et al. 2018). p-Coumaric acid increases the activity of antioxidant enzymes and regulates the PR genes and phenylpropanoid pathway (Yuan et al. 2019). It has been reported that N-hydroxypipecolic acid, a secondary metabolite, can induce systemic acquired resistance (SAR) during pathogen infection (Yildiz et al. 2021). Polyphenols form covalent bonds with SH, OH, or free amino groups of some proteins of phytopathogens, causing the degradation of the 3D structures of proteins and thus inactivation (Zaynab et al. 2018). Polyphenols such as catechins have been reported to be effective in defense by changing plasma membrane permeability and oxygen production in different bacterial species such as P. aeruginosa, S. marcescens, B. bronchiseptica, B. subtilis, and S. aureus (Wang et al. 2018). It was determined that the increase in resveratrol O-methyltransferase and resveratrol synthase 3 enzymes in soybean exposed to R. solani inhibited the growth of the fungus (Zernova et al. 2014). Hydroxycinnamic derivatives, oleuropein derivatives, flavonol monoglucoside, and tyrosol derivatives were found to be effective in the defense against Fusicladium oleagineum, which causes leaf spot disease in olive trees (Talhaoui et al. 2015). Significant differences in endogenous phenolic levels were detected in plants exposed to fungal infections by L. angustifolius (Verma and Shukla 2015). It was determined that the amount of phenolics such as kaempferol, quercetin, caffeic acid, and chlorogenic acid increased in plants against virus infection (Parr and Bolwell 2000). Other SMs that act as defense compounds against fungi and insects are alkaloids such as caffeine, cocaine, morphine, and nicotine (Ogbanna and Opara 2017). Cyanogenic glycosides, another N-containing compound, are also important SMs with toxic properties, which play a role in the defense against herbivores and insects (Santisree et al. 2020). The nicotine found in tobacco leaves binds to the receptors of nicotinic acetylcholine, blocks endogenous neurotransmitters, and causes paralysis and even death in insects (Dewey and Xie 2013). It has been reported that dhurrin is highly effective in deterring insects in S. bicolor by its effective hydrolysis and subsequent release of cyanide (Krothapalli et al. 2013). Since terpenoids have repellent properties against herbivores, they prevent larvae feeding and reduce egg laying (Maffei 2010). It has been determined that latex, which is secreted from the roots of the dandelion plant and is in the terpene group, protects the plant against M. melolontha larvae (Huber et al. 2016). Studies on some SMs found to be effective in the development of disease resistance to pathogens in plants are presented in Table 19.7.
In addition to these phytochemicals, SA, jasmonic acid (JA), and ethylene (ET) are critical in regulating defense responses. The JA, SA, ET, and methyl jasmonate are signal molecules that take part in the fight against pathogens and stimulate the antioxidant system and secondary metabolite. Defense against biotrophic pathogens is mediated by an SA-dependent pathway in plants, whereas neurotropic pathogens usually induce a defense system mediated by JA and ET (Fig. 19.2). Insect or pathogen attacks cause the accumulation of endogenic hormones such as SA, JA, and ET, which will activate the defense mechanisms in plants. Specific plant hormones such as SA, JA, and ET, on the other hand, are effective in the formation of hypersensitive response and SAR, by acting as stimulants in the synthesis of antioxidants that are effective in creating resistance to pathogens and harmful insects, with various SMs, phenolics, phytoalexins, and pathogen-related proteins (PR) (Jumali et al. 2011) (Fig. 19.2).
SA, JA, and ET signal transduction pathway and disease resistance (ET ethylene, EREBPS ethylene-responsive element-binding protein, EDR1 enhanced disease resistance 1, ERF1 ethylene response factor, HR hypersensitive response, ISR induced systemic resistance, JA jasmonic acid, MAPK mitogen-activated protein kinase, NPR1 nonexpressor of pathogenesis-related genes 1, PAL phenylalanine ammonia-lyase, PDF1.2 plant defensin 1.2, PR pathogen-related proteins, SA salicylic acid, SAR systemic acquired resistance)
PR proteins, with 17 families identified in different plant species, are considered markers in SAR (Van Loon et al. 2006). Chitinases belong to the PR-3, PR-4, PR-8, and PR-11 classes and catalyze the hydrolysis of chitin, which is a component of the fungal cell wall and helps in the development of resistance to the pathogen. The hydrolysis of glucan, another structural component of the fungal cell wall, is catalyzed by glucanase, a PR-2 class protein (Van Loon et al. 1994). PR proteins show the following different functions: PR1 (antifungal), PR2 (β-1,3-glucanases), PR3 (chitinases), PR4 (class I and II chitinases), PR5 (thaumatin-like proteins), PR9 (peroxidases), PR12 (defensins), and PR13 (thionins) (Van Loon and Van Strien 1999). Studies have shown that genetically modified potato plants expressing tobacco PR-5 osmotin are more resistant to P. infestans, F. solani, and R. solani (Rivero et al. 2012). More resistance to C. arachidicola and A. flavus is developed in peanuts due to the overexpression of the rice chitinase gene (Prasad et al. 2013). Besides, defensin and thionine serve as effective defense responses against various phytopathogens in antimicrobial proteins rich in small cysteine (Kaur et al. 2011).
Investigation of metabolite pathways specific to a plant species, determination of biosynthetic genes, and transfer of the gene responsible for the synthesis of the metabolite to the plant that does not contain this metabolite have allowed the development of plants resistant to pathogens. The many SMs such as isoflavonoids, hydrocinnamic acid amides, terpenes, camalexin, and alkaloids besides stilbenes or genes encoding the enzymes involved in the synthesis of these metabolites can be transferred to other plants and in this way a resistance against various diseases (Muroi et al. 2012; Rook 2016). The genomic sequence of the beta-amyrin synthase enzyme involved in the biosynthesis of saponins, following the cloning from A. strigosa, and expressed transgenically in turf plants has developed resistance to fungal pathogens such as F. culmorum, S. nodorum, and G. graminis (Silva et al. 2018). Tobacco plants overexpressing heterologous phenylalanine ammonia-lyase (PAL) transgenes have been observed to show resistance to C. nicotianae and P. parasitica fungal pathogens (Way et al. 2002). It has been reported that it acts as a repellent against M. sexta in transgenic tobacco plants containing volatile isoprene, thereby preventing the feeding of this herbivore (Laothawornkitkul et al. 2008).
Another strategy is to increase the resistance in transgenic plants formed by transferring genes encoding polyamines such as spermine, spermidine, and putrescine, which serve to increase resistance or tolerance to biotic stresses. Hazarika and Rajam (2011) have reported that when they transferred a gene that is effective in polyamine synthesis to tomato plants, disease resistance developed in tomato plants against wilt disease caused by F. oxysporum and early blight caused by A. solani together with an increase in polyamine synthesis.
The limitations of the traditional breeding methods such as time loss and high cost have led to the development of plant tissue culture techniques such as in vitro protoplast fusion, secondary metabolite production, and haploid technology. The protoplast fusion is based on the combination of the nuclei and cytoplasm of two separate protoplasts through chemical or electrical means. The plant resulting from this combination is called somatic hybrid (Lakhani et al. 2016; Tiwari 2018). In the control of plant diseases caused by some fungi, Trichoderma species, known as biocontrol agents and distributed in many parts of the world, have been used. These fungal species increase antagonistic properties by producing bioactive substances in the fight against plant diseases and stimulate SAR in plants with their hyperparasitism (Shah and Afiya 2019). The studies at a molecular genetic level have also focused on increasing the proteinase or chitinase activities acting on the pathogen cell walls or by increasing the copy number of suitable genes or combining these genes with strong promoters (Pcbh1, ech42) to increase the biocontrol ability of Trichoderma. The protoplast fusion is a good tool in the improvement of Trichoderma species and the development of hybrid strains in other filamentous fungi. It has been reported that this technique is useful for developing superior hybrid strains and enhancing the antagonistic activity of Trichoderma spp. against various fungal pathogens such as F. oxysporum, M. phaseolina, R. solani, and S. rolfsii (Lakhani et al. 2016).
Secondary metabolite production is another way to obtain pathogen-resistant plants using various methods in tissue cultures. SMs, such as alkaloids, phenols, flavonoids, lignins, organic acids, peptides, steroids and derivatives, tannins, terpenes, and vitamins, may be produced using cell culture techniques. These substances may be produced in vitro using a cell and tissue culture technique. Another method used in the production of SMs is elicitor application. Elicitors are stimulants that allow the plant to protect itself by producing antimicrobial substances in case of stress conditions (Narayani and Srivastava 2017). The elicitors that act as signals bind to elicitor-specific receptors on the cell membrane of the plant, and the signal is detected, activating the transduction cascade, inducing the expression of the relevant genes and transcription factors and the synthesis of the SMs (Halder et al. 2019). Oligogalacturonic acids in the plant cell wall stimulate the synthesis of phytoalexin, whereas chitin in the fungus stimulates the synthesis of phenolic compounds (Gadzovska et al. 2015). When elicitors such as SA and methyl jasmonate are used as stimulants, they induce defense against pathogens by stimulating stilbene and gymnemic acid biosynthesis (Chodisetti et al. 2015; Xu et al. 2015). It was determined that phytohormone applications such as abscisic acid, gibberellin, and ET increased the amounts of phenolic compounds (Liang et al. 2013). Tashackori et al. (2018) in their study, in which P. indica used the cell wall as an elicitor, found that it caused significant increases in the amounts of cinnamic acid, ferulic acid, SA, myricetin, kaempferol, diosmin, and flavonoids lignins and lignans in Linum album cell cultures. Significant increases were detected in the amounts of PAL, anthocyanin, carotenoid, flavonoid, phenolic, and antioxidant capacity in pepper seedlings treated with proline. The increase in the PAL activity induced by proline increased the amounts of flavonoids and anthocyanins, thus increasing the tolerance of pepper against P. capsici infection (Koç 2017, 2022). Kumar et al. (2008) have reported that an application of toxins created by different plant pathogens to the cultures produced with cell suspensions, somatic embryos, and organogenic and embryogenic calluses may allow the pathogen-resistant plants to be developed. It has been determined that when compounds belonging to P. megasperma are applied to a soybean plant in the cell cultures, they produce a secondary metabolite called glycolide, and similarly, when a compound obtained from the pathogen P. aphanidermatum is applied, it produces various SMs such as ajmaline, tabersonine, and catharanthine (Razdan 2003).
4 Conclusion
The biosynthetic mechanisms of SMs, one of the most important defense strategies developed by plants for survival, are regulated by various stress factors. Many studies have shown that abiotic and biotic stresses cause changes in the levels of phenolic compounds, terpenes, alkaloids, flavonoids, antioxidants, osmoregulators, carotenoids, anthocyanins, glucosinolates, and phytohormones in plants. Stress tolerance in plants can be increased by manipulating the biosynthesis and accumulation of SMs. For this, it is important to identify the genes encoding the enzymes of the secondary metabolite pathways, such as the mevalonate (MVA) and methylerythritol phosphate and (MEP) pathways for terpenoids and carotenoids, the shikimic acid and tricarboxylic acid pathways for alkaloids, and the malonic acid and the shikimic acid pathways for phenolics. Another effective option for increasing the production of SMs is elicitor application. Additionally, different strategies can be combined to produce a high amount of desired – targeted – compounds.
References
Agati G, Tattini M (2010) Multiple functional roles of flavonoids in photoprotection. New Phytol 186:786–793
Agati G, Stefano G, Biricolti S, Tattini M (2009) Mesophyll distribution of antioxidant flavonoid glycosides in Ligustrum vulgare leaves under contrasting sunlight irradiance. Ann Bot 104:853–861
Ahanger MA, Bhat JA, Siddiqui MH, Rinklebe J, Ahmad P (2020) Integration of silicon and secondary metabolites in plants: a significant association in stress tolerance. J Exp Bot 71:6758–6774
Ahmad T, Cawood M, Iqbal Q, Ariño A, Batool A, Tariq RMS, Azam M, Akhtar S (2019) Phytochemicals in Daucus carota and their health benefits. Foods 8:424. https://doi.org/10.3390/foods8090424
Ahmed SA, Baig MMV (2014) Biotic elicitor enhanced production of psoralen in suspension cultures of Psoralea corylifolia L. Saudi J Biol Sci 21:499–504
Akula R, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731
Alcázar R, Altabella T, Marco F, Bortolotti C, Reymond M, Koncz C, Carrasco P, Tiburcio AF (2010) Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249
Alcázar R, Bueno M, Tiburcio AF (2020) Polyamines: small amines with large effects on plant abiotic stress tolerance. Cells 9:2373. https://doi.org/10.3390/cells9112373
Alhaithloul HAS (2019) Impact of combined heat and drought stress on the potential growth responses of the desert grass Artemisia sieberi alba: relation to biochemical and molecular adaptation. Plants 8:416. https://doi.org/10.3390/plants8100416
Al-Hakimi AMA (2006) Counteraction of drought stress on soybean plants by seed soaking in salicylic acid. Int J Botany 2:421–426
Al-Huqail A, El-Dakak RM, Sanad MN, Badr RH, Ibrahim MM, Soliman D, Khan F (2020) Effects of climate temperature and water stress on plant growth and accumulation of antioxidant compounds in sweet basil (Ocimum basilicum L.) leafy vegetable. Scientifica 2020:3808909. https://doi.org/10.1155/2020/3808909
Al-Sammarraie ON, Alsharafa KY, Al-Limoun MO, Khleifat KM, Al-Sarayreh SA, Al-Shuneigat JM, Kalaji HM (2020) Effect of various abiotic stressors on some biochemical indices of Lepidium sativum plants. Sci Rep 10:1–10
Amtul JS, Shakoori AR (2014) Potential of azadirachtin and neem (Azadirachta indica) based saponins as biopesticides for in vitro insect pests cellulase (beta-1, 4-endoglucanase) enzyme inhibition and in vivo repellency on Tribolium castaneum. Br Biotechnol J 4:904–917
Andrade AWL, Figueiredo DDR, Islam MT, Nunes AMV, da Conceição MK, da Conceição MK, Uddin SJ, Shilpi JA, Rouf R, de Carvalho Melo Cavalcante AA (2019) Toxicological evaluation of the biflavonoid, agathisflavone in albino Swiss mice. Biomed Pharmacother 110:68–73
Angus JF, Gardner PA, Kirkegaard JA, Desmarchelier JM (1994) Biofumigation: isothiocyanates released from Brassica roots inhibit growth of the take-all fungus. Plant Soil 162:107–112
Anjitha KS, Sameena PP, Puthur JT (2021) Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress 2:100038. https://doi.org/10.1016/j.stress.2021.100038
Anjum SA, Ashraf U, Tanveer M, Khan I, Hussain S, Shahzad B, Zohaib A, Abbas F, Saleem MF, Ali I (2017) Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front Plant Sci 8:69. https://doi.org/10.3389/fpls.2017.00069
Antognoni F, Zheng S, Pagnucco C, Baraldi R, Poli F, Biondi S (2007) Induction of flavonoid production by UV-B radiation in Passiflora quadrangularis callus cultures. Fitoterapia 78:345–352
Antognozzi E, Famiani F, Proietti P, Pannelli G, Alfei B (1993, September) Frost resistance of some olive cultivars during the winter. In: II international symposium on olive growing, vol 356, pp 152–155
Antonova GF, Varaksina TN, Zheleznichenko TV, Stasova VV (2012) Changes in phenolic acids during maturation and lignification of Scots pine xylem. Russ J Dev Biol 43:199–208
Araújo M, Prada J, Mariz-Ponte N, Santos C, Pereira JA, Pinto DC, Silva AMS, Dias MC (2021) Antioxidant adjustments of olive trees (Olea europaea) under field stress conditions. Plants 10:684. https://doi.org/10.3390/plants10040684
Arrowsmith S, Egan TP, Meekins JF, Powers D, Metcalfe M (2012) Effects of salt stress on capsaicin content, growth, and fluorescence in a Jalapeño cultivar of Capsicum annuum (Solanaceae). Bios 83:1–7
Ashraf MA, Iqbal M, Hussain I, Rasheed R (2015) Physiological and biochemical approaches for salinity tolerance: managing salt tolerance in plants. In: Wani SH, Hossain MA (eds) Molecular and genomic perspectives. Taylor & Francis, New york, p 79
Ashraf MA, Iqbal M, Rasheed R, Hussain I, Riaz M, Arif MS (2018) Environmental stress and secondary metabolites in plants: an overview. In: Ahmad P, Ahanger MA, Singh VP, Tripathi DK, Alam P, Alyemeni MN (eds) Plant metabolites and regulation under environmental stress. Elseiver, United Kingdom, pp 153–167
Austen N, Walker HJ, Lake JA, Phoenix GK, Cameron DD (2019) The regulation of plant secondary metabolism in response to abiotic stress: interactions between heat shock and elevated CO2. Front Plant Sci 10:1463. https://doi.org/10.3389/fpls.2019.01463
Badmus UO, Crestani G, O'Connell RD, Cunningham N, Jansen MA (2022) UV-B induced accumulation of tocopherol in Arabidopsis thaliana is not dependent on individual UV photoreceptors. Plant Stress 5:100105. https://doi.org/10.1016/j.stress.2022.100105
Ballhorn DJ (2011) Constraints of simultaneous resistance to a fungal pathogen and an insect herbivore in lima bean (Phaseolus lunatus L.). J Chem Ecol 37:141–144
Bartwal A, Mall R, Lohani P, Guru SK, Arora S (2013) Role of secondary metabolites and brassinosteroids in plant defense against environmental stresses. J Plant Growth Regul 32:216–232
Behn H, Albert A, Marx F, Noga G, Ulbrich A (2010) Ultraviolet-B and photosynthetically active radiation interactively affect yield and pattern of monoterpenes in leaves of peppermint (Mentha x piperita L). J Agric Food Chem 58:7361–7367
Bein T, Karagiannidis C, Quintel M (2020) Climate change, global warming, and intensive care. Intensive Care Med 46:485–487
Ben Abdallah S, Aung B, Amyot L, Lalin I, Lachâal M, Karray- Bouraoui N, Hannoufa A (2016) Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol Plant 38:72. https://doi.org/10.1007/s11738-016-2096-8
Benjamin JJ, Lucini L, Jothiramshekar S, Parida A (2019) Metabolomic insights into themechanisms underlying tolerance to salinity in different halophytes. Plant Physiol Biochem 135:528–545
Berberich T, Sagor GHM, Kusano T (2015) Polyamines in plant stress response. In: Kusano T, Suzuki H (eds) Polyamines. Springer, Tokyo, pp 155–168
Berni R, Luyckx M, Xu X, Legay S, Sergeant K, Hausman JF, Lutts S, Cai G, Guerriero G (2019) Reactive oxygen species and heavy metal stress in plants: impact on the cell wall and secondary metabolism. Environ Exp Bot 161:98–106
Berto P, Comménil P, Belingheri L, Dehorter B (1999) Occurrence of a lipase in spores of Alternaria brassicicola with a crucial role in the infection of caulif lower leaves. FEMS Microbiol Lett 180:183–189
Blanch JS, Peñuelas J, Sardans J, Llusià J (2009) Drought, warming and soil fertilization effects on leaf volatile terpene concentrations in Pinus halepensis and Quercus ilex. Acta Physiol Plant 31:207–218
Bouarab-Chibane L, Forquet V, Lantéri P, Clément Y, Léonard-Akkari L, Oulahal N, Degreave P, Bordes C (2019) Antibacterial properties of polyphenols: characterization and QSAR (Quantitative structure–activity relationship) models. Front Microbiol 10:829. https://doi.org/10.3389/fmicb.2019.00829
Brown GD (2010) The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules 15:7603–7698
Cai Z, Kastell A, Speiser C, Smetanska I (2013) Enhanced resveratrol production in Vitis vinifera cell suspension cultures by heavy metals without loss of cell viability. Appl Biochem Biotechnol 171:330–340
Cakir R, Cebi U (2010) The effect of irrigation scheduling and water stress on the maturity and chemical composition of virginia tobacco leaf. Field Crop Res 119:269–276
Cao D, Lutz A, Hill CB, Callahan DL, Roessner U (2017) A quantitative profiling method of phytohormones and other metabolites applied to barley roots subjected to salinity stress. Front Plant Sci 7:2070. https://doi.org/10.3389/fpls.2016.02070
Carvalho IS, Cavaco T, Carvalho LM, Duque P (2010) Effect of photoperiod on flavonoid pathway activity in sweet potato (Ipomoea batatas (L.) Lam.) leaves. Food Chem 118:384–390
Chandra R, Kang H (2016) Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. Forest Sci Technol 12:55–61
Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S (2007) Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J Expt Bot 58:4245–4255
Chen Y, Guo Q, Liu L, Liao L, Zhu Z (2011) Influence of fertilization and drought stress on the growth and production of secondary metabolites in Prunella vulgaris L. J Med Plant Res 5:1749–1755
Chen Y, Zhang X, Guo Q, Liu L, Li C, Cao L, Qin Q, Zhao M, Wang W (2018) Effects of UV- B radiation on the content of bioactive components and the antioxidant activity of Prunella vulgaris L. Spica during development. Molecules 23:989. https://doi.org/10.3390/molecules23050989
Chen L, Hu W, Mishra N, Wei J, Shen G (2020) AKR2A interacts with KCS1 to improve VLCFAs contents and chilling tolerance of Arabidopsis thaliana. Plant J 103:1–43
Chen S, Li X, Liu X, Wang N, An Q, Ye XM, Zhao ZT, Zhao M, Han Y, Ouyang KH, Wang WJ (2020) Investigation of chemical composition, antioxidant activity, and the effects of alfalfa flavonoids on growth performance. Oxid Med Cell Longev 2020:8569237
Chobot V, Hadacek F (2010) Iron and its complexation by phenolic cellular metabolites: from oxidative stress to chemical weapons. Plant Signal Behav 5(1):4–8. https://doi.org/10.4161/psb.5.1.10197
Chiapusio G, Gonzalez L, Reigosa-Roger MJ, Pellissier F (2016) Changes in polyamines, proline and protein contents in radish seedlings could serve as indicators of allelopathic stress induced by 2-benzoxazolinone and p-hydroxybenzoic acid. J Allelochem Interact 2:39–49
Chodisetti B, Rao K, Gandi S, Giri A (2015) Gymnemic acid enhancement in the suspension cultures of Gymnema sylvestre by using the signaling molecules-Methyl jasmonate and salicylic acid. Vitro Cell Dev Biol Plant 51:88–92
Chunthaburee S, Sanitchon J, Pattanagul W, Theerakulpisut P (2015) Effects of salt stress after late booting stage on yield and antioxidant capacity in pigmented rice grains and alleviation of the saltinduced yield reduction by exogenous spermidine. Plant Prod Sci 18:32–42
Civelek C, Yıldırım E (2019) Effects of exegenous glycine betaine treatments on growth and some physiological characteristics of tomato under salt stress condition. Atatürk Üniv Ziraat Fak Derg 50:153–158
Commisso M, Toffali K, Strazzer P, Stocchero M, Ceoldo S, Baldan B, Levi M, Guzzo F (2016) Impact of phenylpropanoid compounds on heat stress tolerance in carrot cell cultures. Front Plant Sci 7:1439. https://doi.org/10.3389/fpls.2016.01439
Copolovici L, Kannaste A, Pazouki L, Niinemets U (2012) Emissions of green leaf volatiles and terpenoids from Solanum lycopersicum are quantitatively related to the severity of cld and heat shock treatments. J Plant Physiol 169:664–672
Cretton S, Dorsaz S, Azzollini A, Favre-Godal Q, Marcourt L, Ebrahimi SN, Voinesco F, Michellod E, Sanglard D, Gindro K, Wofender JL, Cuendet M, Christen P (2016) Antifungal quinoline alkaloids from Waltheria indica. J Nat Prod 79:300–307
Cuzzuol GRF, Gama VN, Zanetti LV, Werner ET, Pezzopane JEM (2020) UV-B effects on growth, photosynthesis, total antioxidant potential and cell wall components of shade-tolerant and sun-tolerant ecotypes of Paubrasilia echinata. Flora 271:151679
D’Angeli S, Altamura MM (2007) Osmotin induces cold protection in olive trees by affecting programmed cell death and cytoskeleton organization. Planta 225:1147–1163
Da Silva Magedans YV, Matsuura HN, Tasca RAJC, Wairich A, de Oliveira Junkes CF, de Costa F, Fett-Neto AG (2017) Accumulation of the antioxidant alkaloid brachycerine from Psychotria brachyceras Müll. Arg. is increased by heat and contributes to oxidative stress mitigation. Environ Exp Bot 143:185–193
Dahham SS, Tabana YM, Iqbal MA, Ahamed MB, Ezzat MO, Majid AS, Majid AM (2015) The anticancer, antioxidant and antimicrobial properties of the sesquiterpene-caryophyllene from the essential oil of Aquilaria crassna. Molecules 20:11808–11829
Dal Belo CA, Lucho APDB, Vinadé L, Rocha L, Seibert França H, Marangoni S, Rodrigues-Simioni L (2013) In vitro antiophidian mechanisms of Hypericum brasiliense choisy standardized extract: quercetin-dependent neuroprotection. BioMed Res Int. https://doi.org/10.1155/2013/943520
Dalvi AA, Bhalerao SA (2013) Response of plants towards heavy metal toxicity: an overview of avoidance, tolerance and uptake mechanism. Ann Plant Sci 2:362–368
Dawid C, Hille K (2018) Functional metabolomics-A useful tool to characterize stress-induced metabolome alterations opening new avenues towards tailoring food crop quality. Agronomy 8:138. https://doi.org/10.3390/agronomy8080138
De Silva HCC, Asaeda T (2017) Effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J Plant Interact 12:228–236
Del Cueto J, Møller BL, Dicenta F, Sánchez-Pérez R (2018) β-Glucosidase activity in almond seeds. Plant Physiol Biochem 126:163–172
Del Valle JC, Buide ML, Whittall JB, Valladares F, Narbona E (2020) UV radiation increases phenolic compound protection but decreases reproduction in Silene littorea. PLoS ONE 15:1–18
Dewey RE, Xie J (2013) Molecular genetics of alkaloid biosynthesis in Nicotiana tabacum. Phytochemistry 94:10–27
Di Toppi LS, Gabbrielli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Dobrikova A, Apostolova EL, Han´c A, Yotsova E, Borisova P, Sperdouli I, SDI A, Moustakas M (2021) Cadmium toxicity in Salvia sclarea L. an integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotoxicol Environ Saf 209:111851. https://doi.org/10.1016/j.ecoenv.2020.111851
Dubey S, Gupta A, Khare A, Jain G, Bose S, Rani V (2018) Long-and short-term protective responses of rice seedling to combat Cr (VI) toxicity. Environ Sci Pollut Re 25:36163–36172
Duke SE (2018) Differences in active defense responses of two Gossypium barbadense L. cultivars resistant to Fusarium oxysporum f. sp. vasinfectum Race 4. J Agric Food Chem 66:12961–12966. https://doi.org/10.1021/acs.jafc.8b05381
Dutta A, Sen J, Deswal R (2007) Downregulation of terpenoid indole alkaloid biosynthetic pathway by low temperature and cloning of a AP2 type C-repeat binding factor (CBF) from Catharanthus roseus (L). G Don Plant Cell Rep 26:1869–1878
Edreva A, Velikova V, Tsonev T, Dagnon S, Gürel A, Aktaş L, Gesheva E (2008) Stress-protective role of secondary metabolites: diversity of functions and mechanisms. Gen Appl Plant Physiol 34:67–78
Eirini S, Paschalina C, Ioannis T, Kortessa DT (2017) Effect of drought and salinity on volatile organic compounds and other secondary metabolites of Citrus aurantium leaves. Nat Prod Commun 12:1934578X1701200213
El Mihyaoui A, Esteves da Silva JC, Charfi S, Candela Castillo ME, Lamarti A, Arnao MB (2022) Chamomile (Matricaria chamomilla L.): a review of ethnomedicinal use, phytochemistry and pharmacological uses. Life 12:47. https://doi.org/10.3390/molecules22122128
Ellenberger J, Siefen N, Krefting P, Schulze Lutum JB, Pfarr D, Remmel M, Schröder L, Röhlen-Schmittgen S (2020) Effect of UV radiation and salt stress on the accumulation of economically relevant secondary metabolites in bell pepper plants. Agronomy 10:142. https://doi.org/10.3390/agronomy10010142
El-Naggar MH, Elgam A, Abdel Bar F, Badria FA (2017) Antimicrobial and antiquorum-sensing activity of Ricinus communis extracts and ricinine derivatives. Nat Prod Res 15:1–7
Elsharkawy ER, Alghanem SM, Elmorsy E (2021) Effect of habitat variations on the chemical composition, antioxidant, and antimicrobial activities of Achillea fragrantissima (Forssk) Sch. Bip. Biotechnol Rep 29:e00581. https://doi.org/10.1016/j.btre.2020.e00581
Emendack Y, Burke J, Laza H, Sanchez J, Hayes C (2018) Abiotic stress effects on sorghum leaf dhurrin and soluble sugar contents throughout plant development. Crop Sci 58:1706–1716
Eris A, Gulen H, Barut E, Cansev A (2007) Annual patterns of total soluble sugars and proteins related to coldhardiness in olive (Olea europaea L.‘Gemlik’). J Hortic Sci Biotechnol 82:597–604
Falcone Ferreyra ML, Rius SP, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3:222. https://doi.org/10.3389/fpls.2012.00222
Fang X, Yang CQ, Wei YK, Ma QX, Yang L, Chen XY (2011) Genomics grand for diversified plant secondary metabolites. Plant Div Res 33:53–64
Fang H, Zhang F, Zhang C, Wang D, Shen S, He F, Ning Y (2022) Function of hydroxycinnamoyl transferases for the biosynthesis of phenolamides in rice resistance to Magnaporthe oryzae. JGG 49:776–786
Fatima S, Mujib A, Tonk D (2015) NaCl amendment improves vinblastine and vincristine synthesis in Catharanthus roseus: a case of stress signalling as evidenced by antioxidant enzymes activities. PCTOC 121:445–458
Foyer CH, Noctor G (2005) Oxidant and antioxidant signaling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 29:1056–1071
Fu H, Yu H, Li T, Zhang X (2018) Influence of cadmium stress on root exudates of high cadmium accumulating rice line (Oryza sativa L.). Ecotoxicol Environ Saf 150:168–175
Gadzovska Simic S, Tusevski O, Maury S, Delaunay A, Lainé E, Joseph C, Hagege D (2015) Polysaccharide elicitors enhance phenylpropanoid and naphtodianthrone production in cell suspension cultures of Hypericum perforatum. Plant Cell Tissue Organ Cult 122:649–663
Gan RY, Kong KW, Li HB, Wu K, Ge YY, Chan CL, Shi XM, Corke H (2018) Separation, identification, and bioactivities of the main gallotannins of red sword bean (Canavalia gladiata) coats. Front Chem 6:39. https://doi.org/10.3389/fchem.2018.00039
Gao SQ, Chen M, Xu ZS, Zhao CP, Li L, Xu HJ, Tang YM, Zhao X, Ma YZ (2011) The soybean GmbZIP1 transcription factor enhances multiple abiotic stress tolerances in transgenic plants. Plant Mol Biol 75:537–553
Gao J, Chen B, Lin H, Liu Y, Wei Y, Chen F, Li W (2020) Identification and characterization of the glutathione S-Transferase (GST) family in radish reveals a likely role in anthocyanin biosynthesis and heavy metal stress tolerance. Gene 743:144484. https://doi.org/10.1016/j.gene.2020.144484
Gepstein S, Grover A, Blumwald E (2005) Producing biopharmaceuticals in the desert: building an abiotic stress tolerance in plants for salt, heat and drought. In: Knablein J, Muller RH (eds) Modern biopharmaceuticals. Wiley-VCH Verlag GmbH & Co, Weinhaum, pp 967–994
Ghasemi S, Kumleh HH, Kordrostami M (2019) Changes in the expression of some genes involved in the biosynthesis of secondary metabolites in Cuminum cyminum L. under UV stress. Protoplasma 256:279–290
Goh CH, Ko SM, Koh S, Kim YJ, Bae HJ (2011) Photosynthesis and environments: photoinhibition and repair mechanisms in plants. J Plant Biol 55:93–101
Golkar P, Taghizadeh M (2018) In vitro evaluation of phenolic and osmolite compounds, ionic content, and antioxidant activity in safflower (Carthamus tinctorius L.) under salinity stress. Plant Cell Tissue Organ Cult 134:357–368
Griffith M, Yaish MW (2004) Antifreeze proteins in overwintering plants: a tale of two activities. Trends Plant Sci 9:399–405
Gromkowska-Kępka KJ, Markiewicz-Żukowska R, Nowakowski P, Naliwajko SK, Moskwa J, Puścion-Jakubik A, Bielecka J, Grabia M, Mielcarek K, Soroczynska J, Socha K (2021) Chemical composition and protective effect of young barley (Hordeum vulgare L.) dietary supplements extracts on UV-treated human skin fibroblasts in vitro studies. Antioxidants 10:1402. https://doi.org/10.3390/antiox10091402
Guo X, Zhang S, Shan XQ (2008) Adsorption of metal ions on lignin. J Hazard Mater 151:134–142
Guo X, Chang B, Zu Y, Tang Z (2014) The impacts of increased nitrate supply on Catharanthus roseus growth and alkaloid accumulations under ultraviolet-B stress. J Plant Interact 9:640–646
Guo Q, Li X, Niu L, Jameson PE, Zhou W (2021) Transcription-associated metabolomic adjustments in maize occur during combined drought and cold stress. Plant Physiol 186:677–695
Gus-Mayer S, Brunner H, Schneider-Poetsch HAW, Lottspeich F, Eckerskorn C, Grimm R, Rüdiger W (1994a) The amino acid sequence previously attributed to a protein kinase or a TCP1-related molecular chaperone and co-purified with phytochrome is a β-glucosidase. FEBS Lett 347:51–54
Gus-Mayer S, Brunner H, Schneider-Poetsch HAW, Rüdiger W (1994b) Avenacosidase from oat: purification, sequence analysis and biochemical characterization of a new member of the BGA family of P-glucosidases. Plant Mol Biol 26:909–921
Gutsch A, Sergeant K, Keunen E, Prinsen E, Guerriero G, Renaut J, Hausman JF, Cuypers A (2019) Does long-term cadmium exposure influence the composition of pectic polysaccharides in the cell wall of Medicago sativa stems? BMC Plant Biol 19:271
Halder M, Sarkar S, Jha S (2019) Elicitation: a biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng Life Sci 19:880–895
Hamid N, Bakhari N, Jawad F (2010) Physiological responses of Phaseolus vulgaris to different lead concentrations. Pak J Bot 42:239–246
Hanson DT, Sharkey TD (2001) Effect of growth conditions on isoprene emission and other thermotolerance-enhancing compounds. Plant Cell Environ 24:929–936
Harsh A, Sharma YK, Joshi U, Rampuria S, Singh G, Kumar S, Sharma R (2016) Effect of short-term heat stress on total sugars, proline and some antioxidant enzymes in moth bean (Vigna aconitifolia). Ann Agric Sci 61:57–64
Hasanuzzaman M, Nahar K, Alam MM, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684
Hasanuzzaman M, Alam MM, Nahar K, Ahamed KU, Fujita M (2014) Exogenous salicylic acid alleviates salt stress-induced oxidative damage in Brassica napus by enhancing the antioxidant defense and glyoxalase systems. Aust J Crop Sci 8:631–639
Havaux M (2020) Plastoquinone in and beyond photosynthesis. Trends Plant Sci 25:1252–1265
Hazarika P, Rajam MV (2011) Biotic and abiotic stress tolerance in transgenic tomatoes by constitutive expression of S-adenosylmethionine decarboxylase gene. Physiol Mol Biol Plants 17:115–128
He X, Sheffield J (2020) Lagged compound occurrence of droughts and pluvials globally over the past seven decades. Geophys Res Lett 47:e2020GL087924
Hernandez-Cumplido J, Giusti MM, Zhou Y, Kyryczenko-Roth V, Chen YH, Rodriguez-Saona C (2018) Testing the ‘plant domestication-reduced defense’hypothesis in blueberries: the role of herbivore identity. Arthropod Plant Inte 12:483–493
Hideg É, Jansen MA, Strid Å (2013) UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends Plant Sci 18(2):107–115
Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa NK, Mori S (1999) Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol 119:471–479
Hodaei M, Rahimmalek M, Arzani A, Talebi M (2018) The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind Crops Prod 120:295–304
Hoque MA, Banu MNA, Okuma E, Amako K, Nakamura Y, Shimoishi Y, Murata Y (2007) Exogenous proline and glycinebetaine increase NaCl-induced ascorbate–glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J Plant Physiol 164:1457–1468
Hornyák M, Dziurka M, Kula-Maximenko M, Pastuszak J, Szczerba A, Szklarczyk M, Płażek A (2022) Photosynthetic efficiency, growth and secondary metabolism of common buckwheat (Fagopyrum esculentum Moench) in different controlled-environment production systems. Sci Rep 12:1–13
Hosseini MS, Samsampour D, Ebrahimi M, Abadía J, Khanahmadi M (2018) Effect of drought stress on growth parameters, osmolyte contents, antioxidant enzymes and glycyrrhizin synthesis in licorice (Glycyrrhiza glabra L.) grown in the field. Phytochemistry 156:124–134
Huang X, Yao J, Zhao Y, Xie D, Jiang X, Xu Z (2016) Efficient rutin and quercetin biosynthesis through flavonoids-related gene expression in Fagopyrum tataricum Gaertn. hairy root cultures with UV-B irradiation. Front Plant Sci 7:63. https://doi.org/10.3389/fpls.2016.00063
Huang H, Ullah F, Zho, DX, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800
Huber M, Epping J, Schulze Gronover C, Fricke J, Aziz Z, Brillatz T, Swyers M, Köllner TG, Vogel H, Hammerbacher A, Triebwasser-Freese D (2016) A latex metabolite benefits plant fitness under root herbivore attack. PLOS Biol 14:1002332. https://doi.org/10.1371/journal.pbio.1002332
Hussien HA, Salem H, Mekki BED (2015) Ascorbate-glutathione-α-tocopherol triad enhances antioxidant systems in cotton plants grown under drought stress. Int J Chem Tech Res 8:1463–1472
Hwang BK, Sung NK (1989) Effect of metalaxyl on capsidiol production in stems of pepper plants infected with Phytophthora capsici. Plant Dis 73:748–751
Ibrahim W, Zhu YM, Chen Y, Qiu CW, Zhu S, Wu F (2019) Genotypic differences in leaf secondary metabolism, plant hormones and yield under alone and combined stress of drought and salinity in cotton genotypes. Physiol Plant 165:343–355
Isah T (2019) Stress and defense responses in plant secondary metabolites production. Biol Res 52:39
Itkin M, Heinig U, Tzfadia O, Bhide AJ, Shinde B, Cardenas PD, Bocopza SE, Unger T, Malitsky S, Finkers R, Tıkunov Y, Bovy A, Chikate Y, Singh P, Rogachev I, Beekwilder J, Giri AP, Aharoni S (2013) Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science 341:175–179
Jaafar HZ, Ibrahim MH, Fakri NFM (2012) Impact of soil field water capacity on secondary metabolites, phenylalanine ammonia-lyase (PAL), maliondialdehyde (MDA) and photosynthetic responses of Malaysian Kacip Fatimah (Labisia pumila Benth). Molecules 17:7305–7322
Jahan MS, Guo S, Baloch AR, Sun J, Shu S, Wang Y, Ahammed GJ, Kabir K, Roy R (2020) Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol Environ Saf 197:110593. https://doi.org/10.1016/j.ecoenv.2020.110593
Jahan MS, Guo S, Sun J, Shu S, Wang Y, Abou El-Yazied A, Hasan MM (2021) Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol Biochem 167:309–320
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta Bioenerg 1817:182–193
Jain A, Singh A, Singh S, Singh HB (2015) Phenols enhancement effect of microbial consortium in pea plants restrains Sclerotinia sclerotiorum. Biol Control 89:23–32
Jamshidi M, Ghanati F (2017) Taxanes content and cytotoxicity of hazel cells extract after elicitation with silver nanoparticles. Plant Physiol Biochem 110:178–184
Jamwal K, Bhattacharya S, Puri S (2018) Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J Appl Res Med Aromat Plants 9:26–38
Jan R, Asaf S, Numan M, Kim KM (2021) Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 11:968. https://doi.org/10.3390/agronomy11050968
Janas KM, Cvikrová M, Pałagiewicz A, Szafranska K, Posmyk MM (2002) Constitutive elevated accumulation of phenylpropanoids in soybean roots at low temperature. Plant Sci 163:369–373
Janská A, Marsík P, Zelenková S, Ovesná J (2010) Cold stress and acclimation - what is important for metabolic adjustment? Plant Biol (Stuttg) 12:395-405. https://doi.org/10.1111/j.14388677.2009.00299.x
Janeeshma E, Rajan VK, Puthur JT (2020) Spectral variations associated with anthocyanin accumulation; an apt tool to evaluate zinc stress in Zea mays L. Chem Ecol 37:32–49
Jeandet P, Bessis R, Sbaghi M, Meunier P (1995) Production of the phytoalexin resveratrol by grapes as a response to Botrytis attack under natural conditions. J Phytopathol 143:135–139
Jeandet P, Clément C, Courot E, Cordelier S (2013) Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int J Mol Sci 14:14136–14170
Jeon J, Kim JK, Wu Q, Park SU (2018) Effects of cold stress on transcripts and metabolites in tartary buckwheat (Fagopyrum tataricum). Environ Exp Bot 155:488–496
Jiao W, Li X, Wang X, Cao J, Jiang W (2018) Chlorogenic acid induces resistance against Penicillium expansum in peach fruit by activating the salicylic acid signaling pathway. Food Chem 260:274–282
Jogawat A, Yadav B, Lakra N, Singh AK, Narayan OP (2021) Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: a review. Physiol Plant 23:101040. https://doi.org/10.1111/ppl.13328
Jordan BR (2002) Molecular response of plant cells to UV-B stress. Funct Plant Biol 29:909–916
Jumali SS, Said IM, Ismail I, Zainal Z (2011) Genes induced by high concentration of salicylic acid in ‘Mitragyna speciosa’. Aust J Crop Sci 5:296–303
Kandziora-Ciupa M, Ciepał R, Nadgórska-Socha A, Barczyk G (2013) A comparative study of heavy metal accumulation and antioxidant responses in Vaccinium myrtillus L. leaves in polluted and non-polluted areas. Environ Sci Pollut Res 20:4920–4932
Kanno Y, Hanada A, Chiba Y, Ichikawa T, Nakazawa M, Matsui M, Koshiba T, Kamiya Y, Seo M (2012) Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc Natl Acad Sci 109:9653–9658
Karamać M (2009) Chelation of Cu (II), Zn (II), and Fe (II) by tannin constituents of selected edible nuts. Int J Mol Sci 10(12):5485–5497
Karray-Bouraoui N, Rabhi M, Neffati M, Baldan B, Ranieri A, Marzouk B, Lachaâl M, Smaoui A (2009) Salt effect on yield and composition of shoot essential oil and trichome morphology and density on leaves of Mentha pulegium. Ind Crop Prod 30:338–343
Kaur J, Sagaram US, Shah D (2011) Can plant defensins be used to engineer durable commercially useful fungal resistance in crop plants? Fungal Biol Rev 25(3):128–135
Keunen E, Schellingen K, Vangronsveld J, Cuypers A (2016) Ethylene and metal stress: small molecule, big impact. Front Plant Sci 7:23. https://doi.org/10.3389/fpls.2016.00023
Khameneh B, Iranshahy M, Soheili V, Fazly Bazzaz BS (2019) Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control 8:1–28
Khare S, Singh NB, Singh A, Hussain I, Niharika K, Yadav V et al (2020) Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J Plant Biol 63:203–216
Khatun S, Ali MB, Hahn EJ, Paek KY (2008) Copper toxicity in Withania somnifera: growth and antioxidant enzymes responses of in vitro grown plants. EEB 64:279–285
Kim SI, Ahn YJ (2017) Larvicidal activity of lignans and alkaloid identified in Zanthoxylum piperitum bark toward insecticide-susceptible and wild Culex pipiens pallens and Aedes aegypti. Parasit Vectors 10:1–10
King AJ, Brown GD, Gilday AD, Larson TR, Grahama IA (2014) Production of bioactive diterpenoids in the euphorbiaceae depends on evolutionarily conserved gene clusters. Plant Cell 26:3286–3298
Kısa D, Elmastaş M, Öztürk L, Kayır Ö (2016) Responses of the phenolic compounds of Zea mays under heavy metal stress. Appl Biol Chem 59:813–820
Klein FRS, Reis A, Kleinowski AM, Telles RT, Amarante LD, Peters JA, Braga EJB (2018) UV-B radiation as an elicitor of secondary metabolite production in plants of the genus Alternanthera. Acta Bot Bras 32:615–623
Koç E (2017) Alleviation of Phytophthora capsici-induced oxidative stress by foliarly applied proline in Capsicum annuum L. Arch Biol Sci 69:733–742
Koç E (2022) Physiological responses of resistant and susceptible pepper plants to exogenous proline application under Phytophthora capsici stress. Acta Bot Croat 81:89–100
Koç E, Işlek C, Üstün AS (2010) Effect of cold on protein, proline, phenolic compounds and chlorophyll content of two pepper (Capsicum annuum L.) varieties. Gazi Univ J Sci 23:1–6
Korankye EA, Lada RR, Asiedu S, Caldwell C (2017) Plant senescence: the role of volatile terpene compounds (VTCs). Am J Plant Sci 8:3120–3139
Korte AR, Yandeau-Nelson MD, Nikolau BJ, Lee YJ (2015) Subcellular-level resolution MALDI-MS imaging of maize leaf metabolites by MALDI-linear ion trap-Orbitrap mass spectrometer. Anal Bioanal Chem 407:2301–2309
Kou S, Chen L, Tu W, Scossa F, Wang Y, Liu J, Fernie AE, Song B, Xie C (2018) The arginine decarboxylase gene ADC 1, associated to the putrescine pathway, plays an important role in potato cold-acclimated freezing tolerance as revealed by transcriptome and metabolome analyses. Plant 96:1283–1298
Kováčik J, Klejdus B, Hedbavny J, Stork F, Backor M (2009) Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil 320:231. https://doi.org/10.1007/s11104-009-9889-0
Kovacs Z, Simon-Sarkadi L, Szucs A, Kocsy G (2011) Differential effects of cold, osmotic stress and abscisic acid on polyamine accumulation in wheat. Amino Acids 38:623–631
Kratsch HA, Wise RR (2000) The ultrastructure of chilling stress. Plant Cell Environ 23:337–350
Krol A, Amarowicz R, Weidner S (2015) The effects of cold stress on the phenolic compounds and antioxidant capacity of grapevine (Vitis vinifera L.) leaves. J Plant Physiol 189:97–104
Krothapalli K, Buescher EM, Li X, Brown E, Chapple C, Dilkes BP, Tuinstra MR (2013) Forward genetics by genome sequencing reveals that rapid cyanide release deters insect herbivory of Sorghum bicolor. Genetics 195:309–318
Krzesłowska M (2011) The cell wall in plant cell response to trace metals: polysaccharide remodeling and its role in defense strategy. Acta Physiol Plant 33:35–51
Kumar I, Sharma RK (2018) Production of secondary metabolites in plants under abiotic stress: an overview. Significances Bioeng Biosci 2:196–200
Kumar JV, Kumari BR, Sujatha G, Castano E (2008) Production of plants resistant to Alternaria carthami via organogenesis and somatic embryogenesis of safflower cv. NARI-6 treated with fungal culture filtrates. Plant Cell Tissue Organ Cult 93:85–96
Kundrátová K, Bartas M, Pečinka P, Hejna O, Rychlá A, Čurn V, Červeň J (2021) Transcriptomic and proteomic analysis of drought stress response in opium poppy plants during the first week of germination. Plants 10:1878. https://doi.org/10.3390/plants10091878
Kytridis V, Manetas Y (2006) Mesophyll versus epidermal anthocyanins as potential in vivo antioxidants: evidence linking the putative antioxidant role to the proximity of oxy-radical source. J Exp Bot 57:2203–2210
Lakhani H, Vakharia D, Makhlouf A, Eissa R, Hassan M (2016) Influence of protoplast fusion in Trichoderma spp. on controlling some soil borne diseases. J Plant Pathol Microbiol 7:2. https://doi.org/10.4172/2157-7471.1000370
Laothawornkitkul J, Paul ND, Vickers CE, Possell M, Taylor JE, Mullineaux PM, Hewitt CN (2008) Isoprene emissions influence herbivore feeding decisions. Plant Cell Environ 31:1410–1415
Larson R (2018) Reaction mechanisms in environmental organic chemistry. Routledge, UK
Lee HA, Kim S, Kim S, Choi D (2017) Expansion of sesquiterpene biosynthetic gene clusters in pepper confers nonhost resistance to the Irish potato famine pathogen. New Phytol 215:1132–1143
Lee EB, Kim JH, An CW, Kim YJ, Noh YJ, Kim SJ, Kim JE, Shrestha AC, Ham HN, Lemm JY, Jo HK, Kim DS, Moon KH, Lee JH, Jeong KO, Kim DK (2018) Longevity and stress resistant property of 6-Gingerol from Zingiber officinale Roscoe in Caenorhabditis elegans. Biomol Ther 26:568. https://doi.org/10.4062/biomolther.2017.215
Lei XY, Zhu RY, Zhang GY, Dai YR (2004) Attenuation of cold induced apoptosis by exogenous melatonin in carrot suspension cells: the possible involvement of polyamines. J Pineal Res 36:126–131
Leng X, Jia H, Sun X, Shangguan L, Mu Q, Wang B, Fang J (2015) Comparative transcriptome analysis of grapevine in response to copper stress. Sci Rep 5:1–17
Levitt J (1980) Chilling, freezing, and high temperature stresses. In: Responses of plants to environmental stresses. Academic, New York, pp 23–283
Li YD, He JG (2012) Advance in metabolism and response to stress of polyamines in plant. Acta Agric Bor Sin 27:240–245
Li Z, Sharkey TD (2013) Molecular and pathway controls on biogenic volatile organic compound emissions. In: Niinemets Ü, Monson RK (eds) Biology, controls and model of tree volatile organic compound emission. Springer, London, pp 119–151
Li J, Yang P, Yang Q, Gong X, Ma H, Dang K, Chen G, Gao X, Feng B (2019) Analysis of flavonoid metabolites in buckwheat leaves using UPLC-ESI-MS/MS. Molecules 24:1310
Li Z, Hou J, Zhang Y, Zeng W, Cheng B, Hassan MJ, Zhang Y, Pu Q, Peng Y (2020) Spermine regulates water balance associated with Ca2+-dependent aquaporins (TrTIP2-1, TrTIP2-2, and TrPIP2-7) expression in plants under water stress. Plant Cell Physiol 61:1576–1589
Li Q, Xiang C, Xu L, Cui J, Fu S, Chen B, Yang S, Wang P, Xie Y, Wei M, Wang Z (2020) SMRT sequencing of a full-length transcriptome reveals transcript variants involved in C18 unsaturated fatty acid biosynthesis and metabolism pathways at chilling temperature in Pennisetum giganteum. BMC Genom 21:3. https://doi.org/10.1186/s12864-019-6441-3
Li Y, Leveau A, Zhao Q, Feng Q, Lu H, Miao J, Osbourn A (2021) Subtelomeric assembly of a multi-gene pathway for antimicrobial defense compounds in cereals. Nat Commun 12:1–13
Liang Z, Ma Y, Xu T, Cui B, Liu Y, Guo Z, Yang D (2013) Effects of abscisic acid, gibberellin, ethylene and their interactions on production of phenolic acids in Salvia miltiorrhiza bunge hairy roots. PLoS One 8:e72806. https://doi.org/10.1371/journal.pone.0072806
Lim JH, Park KJ, Kim BK, Jeong JW, Kim HJE (2012) ect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem 135:1065–1070
Lin Y, Qasim M, Hussain M, Akutse KS, Avery PB, Dash CK, Wang L (2017) The herbivore-induced plant volatiles methyl salicylate and menthol positively affect growth and pathogenicity of entomopathogenic fungi. Sci Rep 7:1–11
Liu F, Xu G, Wu X, Ding Q, Zheng J, Zhang R, Gao Y (2014) Effect of drought stress and re-watering on emissions of volatile organic compounds from Rosmarinus officinalis. J Zhejiang Univ 31:264–271
Liu Y, Meng Q, Duan X, Zhang Z, Li D (2017) Effects of PEG-induced drought stress on regulation of indole alkaloid biosynthesis in Catharanthus roseus. J Plant Interact 12:87–91
Liu X, Zhou Y, Xiao J, Bao F (2018) Effects of chilling on the structure, function and development of chloroplasts. Front Plant Sci 9:1715. https://doi.org/10.3389/fpls.2018.01715
Liu Y, Lin-Wang K, Espley RV, Wang L, Li Y, Liu Z, Zhou P, Zeng L, Zhang X, Zhan J, Allen AC (2019) StMYB44 negatively regulates anthocyanin biosynthesis at high temperatures in tuber flesh of potato. J Exp Bot 70:3809–3824
Llorens MJA, Vacas S (2017) Effect of drought stress on essential oil composition of Thymus vulgaris L. (Chemotype 1, 8-cineole) from wild populations of Eastern Iberian Peninsula. J Essent Oil Res 29:145–155
Luis JC, Pérez RM, González FV (2007) UV-B radiation effects on foliar concentrations of rosmarinic and carnosic acids in rosemary plants. Food Chem 101:1211–1215
Luvisi A, Aprile A, Sabella E, Vergine M, Nicolì F, Nutricati E, Miceli A, Negro C, Bellis D (2017) L. Xylella fastidiosa subsp. pauca (CoDiRO strain) infection in four olive (Olea europaea L.) cultivars: profile of phenolic compounds in leaves and progression of leaf scorch symptoms. Phytopathol Mediterr 56:259–273
Lyons JM (1973) Chilling injury in plants. Annu Rev Plant Physiol Plant Mol Biol 24:445–466
Ma Y, Dias MC, Freitas H (2020) Drought and salinity stress responses and microbe-induced tolerance in plants. Front Plant Sci 11:591911. https://doi.org/10.3389/fpls.2020.591911
Ma H, Xin C, Xu Y, Wang D, Lin X, Chen Z (2021) Effect of salt stress on secondary metabolites of cotton and biological characteristics and detoxification enzyme activity of cotton spider mites. Crop Prot 141:105498. https://doi.org/10.1016/j.cropro.2020.105498
Maffei ME (2010) Sites of synthesis, biochemistry and functional role of plant volatiles. S Afr J Bot 76:612–631
Maleki M, Ghorbanpour M, Kariman K (2017) Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress. Plant Gene 11:247–254
Mandhania S, Sangwan RS, Siwach SS, Pundir SR, Sangwan O, Janu A (2018) Role of biochemical constituents and minerals against cotton leaf curl disease in cotton. J Environ Biol 39:221–227
Mannucci A, Santin M, Vanhaelewyn L, Sciampagna MC, Miras-Moreno MB, Zhang L, Lucini L, Quartacci MK, DVD S, Castagna A, Ranieri A (2022) Foliar and root comparative metabolomics and phenolic profiling of micro-tom tomato (Solanum lycopersicum L.) plants associated with a gene expression analysis in response to short daily uv treatments. Plants 1(14):1829. https://doi.org/10.3390/plants11141829
Manquián-Cerda K, Escudey M, Zúñiga G, Arancibia-Miranda N, Molina M, Cruces E (2016) Effect of cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets grown in vitro. Ecotoxicol Environ Saf 133:316–326
Mansfield JW (1999) Antimicrobial compounds and resistance: the role of phytoalexins and antianticipins. In: Slusarenko AJ, Fraser RSS, Van Loon LC (eds) Mechanisms of resistance to plant diseases. Springer, Dordrecht, pp 325–370
Marchesini G, Fabbri A, Bianchi G, Brizi M, Zoli M (1996) Zinc supplementation and amino acid-nitrogen metabolism in patients with advanced cirrhosis. Hepatol 23:1084–1092
Marchive C, Léon C, Kappel C, Coutos-Thévenot P, Corio-Costet MF, Delrot S, Lauvergeat V (2013) Over-expression of VvWRKY1 in grapevines induces expression of jasmonic acid pathway-related genes and confers higher tolerance to the downy mildew. PLoS One 8:e54185. https://doi.org/10.1371/journal.pone.0054185
Mari M, Lori R, Leoni O, Marchi A (1993) In vitro activity of glucosinolate-derived isothiocyanates against postharvest fruit pathogens. Ann Appl Biol 123:155–164
Martínez-Ballesta M, Moreno-Fernández DA, Castejón D, Ochando C, Morandini PA, Carvajal M (2015) The impact of the absence of aliphatic glucosinolates on water transport under salt stress in Arabidopsis thaliana. Front Plant Sci 6:524. https://doi.org/10.3389/fpls.2015.00524
Martínez J, Fuentes R, Farías K, Lizana C, Alfaro JF, Fuentes L, Calabrase N, Bigot S, Quinet M, Lutts S (2020) Effects of salt stress on fruit antioxidant capacity of wild (Solanum chilense) and domesticated (Solanum lycopersicum var. cerasiforme) tomatoes. Agronomy 10:1481
Mashilo J, Odindo AO, Shimelis HA, Musenge P, Tesfay SZ, Magwaza LS (2017) Drought tolerance of selected bottle gourd [Lagenaria siceraria (Molina) Standl.] landraces assessed by leaf gas exchange and photosynthetic efficiency. Plant Physiol Biochem 120:75–87
Masondo NA, Aremu AO, Finnie JF, Van Staden J (2014) Plant growth regulator induced phytochemical and antioxidant variations in micropropagated and acclimatized Eucomis autumnalis subspecies autumnalis (Asparagaceae). Acta Physiol Plant 36:2467–2479
Mathew S, Abraham TE, Zakaria ZA (2015) Reactivity of phenolic compounds towards free radicals under in vitro conditions. JFST 52(9):5790–5798
Mazloom N, Khorassani R, Zohury GH, Emami H, Whalen J (2020) Lignin-based hydrogel alleviates drrought stress in maize. Environ Exp Bot 175:104055
Mazza CA, Boccalandro HE, Giordano CV, Battista D, Scopel AL, Ballaré CL (2000) Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops. Plant Physiol 122:117–126
McSweeney C, Collins E, Blackall L, Seawright A (2008) A review of anti-nutritive factors limiting potential use of Acacia angustissima as a ruminant feed. Anim Feed Sci Technol 147:158–171
Meena KK, Sorty AM, Bitla UM, Choudhary K, Gupta P, Pareek A, Singh DP, Prabha R, Sahu PK, Gupta VK, Singh HB (2017) Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Front Plant Sci 8:172. https://doi.org/10.3389/fpls.2017.00172
Mehrotra S, Mishra S, Srivastava V (2018) Hairy root cultures for monoterpene indole alkaloid pathway: investigation and biotechnological production. In: Srivastava V, Mehrotra S, Mishra S (eds) Hairy roots. Springer, Singapore, pp 95–121
Meier S, Alvear M, Borie F, Aguilera P, Ginocchio R, Cornejo P (2012) Influence of copper on root exudate patterns in some metallophytes and agricultural plants. Ecotoxicol Environ Saf 75:8–15
Meilong X, Qian T, Yi W, Zemin W, Guangzhao X, Kirabi EG, Li S, Li Z (2020) Transcriptomic analysis (of) grapevine LEA gene family in response to osmotic and cold stress, and functional analyses of VamDHN3 gene. Plant Cell Physiol 61:775–786
Messenger DJ, Mcleod AR, Fry SC (2009) The role of ultraviolet radiance, photosensitizers, reactive oxygen species and ester groups in mechanisms of methane formation from pectin. Plant Cell Environ 32:1–9
Mignolet-Spruyt L, Xu E, Idanheimo N, Hoeberichts FA, Muhlenbock P, Brosche M, Van Breusegem F, Kangasjarvi J (2016) Spreading the news: subcellular and organellar reactive oxygen species production and signalling. J Exp Bot 67:3831–3844
Miller JM, Conn EE (1980) Metabolism of hydrogen cyanide by higher plants. Plant Physiol 65:1199–1202
Minh LT, Khang DT, Ha PT, Tuyen PT, Minh TN, Quan NV, Xuan TD (2016) Effects of salinity stress on growth and phenolics of rice (Oryza sativa L.). Int Lett Natt Sci 57:1–10
Mir BA, Mir SA, Khazir J, Tonfack LB, Cowan DA, Vyas D, Koul S (2015) Cold stress affects antioxidative response and accumulation of medicinally important withanolides in Withania somnifera (L.) Dunal. Ind Crop Prod 74:1008–1016
Miret JA, Munné-Bosch S (2015) Redox signaling and stress tolerance in plants: a focus on vitamin E. Ann N Y Acad Sci 1340:29–38
Mithöfer A, Boland W (2012) Plant defense against herbivores: chemical aspects. Ann Rev Plant Biol 63:431–450
Mølmann JA, Steindal AL, Bengtsson GB, Seljåsen R, Lea P, Skaret J, Johansen TJ (2015) Effects of temperature and photoperiod on sensory quality and contents of glucosinolates, flavonols and vitamin C in broccoli florets. Food Chem 172:47–55
Morales M, Ros Barcelo A, Pedreno MA (2000) Plant stilbenes: recent advances in their chemistry and biology. Adv Plant Physiol 3:39–70
Morales LO, Tegelberg R, Brosché M, Keinänen M, Lindfors A, Aphalo PJ (2010) Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol 30:923–934
Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sánchez-Pérez R, Møller BL, Bak S (2008) β-Glucosidases as detonators of plant chemical defense. Phytochem 69:1795–1813
Moreira-Rodríguez M, Nair V, Benavides J, Cisneros-Zevallos L, Jacobo-Velázquez DA (2017) UVA, UVB light, and methyl jasmonate, alone or combined, redirect the biosynthesis of glucosinolates, phenolics, carotenoids, and chlorophylls in broccoli sprouts. Int J Mol Sci 18:2330. https://doi.org/10.3390/ijms18112330
Morrissey J, Lou GM (2009) Iron uptake and transport in plants: the good, the bad, and the ionome. Chem Rev 109:4553–4567
Mourato M, Reis R, Martins LL (2012) Characterization of plant antioxidative system in response to abiotic stresses: a focus on heavy metal toxicity. In: Montanaro G, Dichio B (eds) Advances in selected plant physiology aspects. InTech, Vienna, pp 23–44
Munne-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in plants. Crit Rev Plant Sci 21:31–57
Muroi A, Matsui K, Shimoda T, Kihara H, Ozawa R, Ishihara A, Nishihara M, Arimura GI (2012) Acquired immunity of transgenic torenia plants overexpressing agmatine coumaroyl transferase to pathogens and herbivore pests. Sci Rep 2:1–7
Muthulakshmi SM, Gurulakshmi SG, Rajathi S (2013) Effect of salt stress on physiological and biochemical characteristics in Solanum nigrum L. Int J Sci Res 4:567–571
Nahar K, Hasanuzzaman M, Alam MM, Rahman A, Suzuki T, Fujita M (2016) Polyamine and nitric oxide crosstalk: antagonistic effects on cadmium toxicity in mung bean plants through upregulating the metal detoxification, antioxidant defense and methylglyoxal detoxification systems. Ecotoxicol Environ Saf 126:245–255
Narayani M, Srivastava S (2017) Elicitation: a stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem Rev 16:1227–1252
Nascimento LBS, Leal-Costa MV, Menezes EA, Lopes VR, Muzitano MF, Costa SS, Tavares ES (2015) Ultraviolet-B radiation effects on phenolic profile and flavonoid content of Kalanchoe pinnata. J Photochem Photobiol B 148:73–81
Navarro JM, Flores P, Garrido C, Martinez V (2006) Changes in the contents of antioxidant compounds in pepper fruits at ripening stages, as affected by salinity. Food Chem 96:66–73
Nazari MR, Abdossi V, Hargalani FZ et al (2022) Antioxidant potential and essential oil properties of Hypericum perforatum L. assessed by application of selenite and nano-selenium. Sci Rep 12:6156. https://doi.org/10.1038/s41598-022-10109-y
Neelofer H, Nosheen B, Faiza J (2010) Physiological responses of Phaseolus vulgaris to different lead concentrations. Pak J Bot 42:239–246
Newman K (2014) Feeding and oviposition preferences of the diamondback moth Plutella xylostella (Lepidoptera: Plutellidae) on six Brassicaceae host plant species. M.Sc Thesis
Nigam R, Srivastava S, Prakash S, Srivastava MM (2001) Cadmium mobilisation and plant availability–the impact of organic acids commonly exuded from roots. Plant Soil 230:107–113
Nishizawa T, Okamoto H, Takemura T, Sugimoto N, Matsui I, Shimizu A (2008) Aerosol retrieval from two-wavelength backscatter and one-wavelength polarization lidar measurement taken during the MR01K02 cruise of the R/V Mirai and evaluation of a global aerosol transport model. J Geophys Res Atmos 113:(D21). https://doi.org/10.1029/2007JD009640
Niyogi KK, Shih C, Soon Chow W, Pogson BJ, DellaPenna D, Björkman O (2001) Photoprotection in a zeaxanthin-and lutein-deficient double mutant of Arabidopsis. Photosynt Res 67:139–145
Nobahar A, Carlier JD, Miguel MG, Costa MC (2021) A review of plant metabolites with metal interaction capacity: a green approach for industrial applications. Biometals 34:1–33
Nocchi N, Duarte HM, Pereira RC, Konno TUP, Soares AR (2020) Effects of UV-B radiation on secondary metabolite production, antioxidant activity, photosynthesis and herbivory interactions in Nymphoides humboldtiana (Menyanthaceae). J Photochem Photobiol B Biol 212:112021. https://doi.org/10.1016/j.jphotobiol.2020.112021
Ogbanna MJ, Opara EU (2017) Pathogen penetration into the host plant tissue challenges and obstacles- an overview. Sch J Agric Vet Sci 4:175–185
Okem A, Stirk WA, Street RA, Southway C, Finnie JF, Van Staden J (2015) Effects of Cd and Al stress on secondary metabolites, antioxidant and antibacterial activity of Hypoxis hemerocallidea Fisch. & CA Mey. Plant Physiol Biochem 97:147–155
Oliveira DM, Mota TR, Salatta FV, Sinzker RC, Konˇcitíková R, Kopeˇcný D, Simister R, Silva M, Goeminne G, Morreel K, Gutierrez JRA, Tryfona T, Marchiosi R, Dupree P, Del Rio DC, Boerjan W, McQueen-Mason SSJ, Gomez LD, Ferrarese-Filho O, dos Santos WD (2020) Cell wall remodeling under salt stress: insights into changes in polysaccharides, feruloylation, lignification, and phenolic metabolism in maize. Plant Cell Environ 43:2172–2191
Ortega-García F, Peragón J (2009) The response of phenylalanine ammonia-lyase, polyphenol oxidase and phenols to cold stress in the olive tree (Olea europaea L. cv. Picual). J Sci Food Agric 89:1565–1573
Osbourn AE (1996) Preformed antimicrobial compounds and plant defense against fungal attack. Plant Cell 8:1821–1831
Osbourn AE, Clarke BR, Lunness P, Scott PR, Daniels MJ (1994) An oat species lacking avenacin is susceptible to infection by Gaeumannomyces graminis var. tritici. Physiol Mol Plant Pathol 45:457–467
Oueslati S, Karray-Bouraoui N, Attia H, Rabhi M, Ksouri R, Lachaal M (2010) Physiological and antioxidant responses of Mentha pulegium (Pennyroyal) to salt stress. Acta Physiol Plant 32:289–296
Ozfidan-Konakci C, Yildiztugay E, Yildiztugay A, Kucukoduk M (2019) Cold stress in soybean (Glycine max L.) roots: exogenous gallic acid promotes water status and increases antioxidant activities. Bot Serb 43:59–71
Pandey N, Pandey-Rai S (2014) Short term UV-B radiation-mediated transcriptional responses and altered secondary metabolism of in vitro propagated plantlets of Artemisia annua L. Plant Cell Tissue Organ Cult 116:371–385
Parikrama R, Esyanti RR (2014) Effect of UV elicitation on callus growth, alkaloid and terpenoid contents in Eurycoma longifolia Jack. Int J Adv Chem Eng Biol Sci 1:12–15
Parr AJ, Bolwell GP (2000) Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J Sci Food Agric 80:985–1012
Patel MK, Mishra A, Jaiswar S, Jha B (2020) Metabolic profiling and scavenging activities of developing circumscissile fruit of psyllium (Plantago ovata Forssk.) reveal variation in primary and secondary metabolites. BMC Pant Biol 20(1):1–15
Patel P, Prasad A, Srivastava D, Niranjan A, Saxena G, Singh SS, Misra P, Chakrabarty D (2022) Genotype-dependent and temperature-induced modulation of secondary metabolites, antioxidative defense and gene expression profile in Solanum viarum Dunal. Environ Exp Bot 194:104686. https://doi.org/10.1016/j.envexpbot.2021.104686
Pattanaik B, Lindberg P (2015) Terpenoids and their biosynthesis in cyanobacteria. Life 5:269–293
Pavić V, Jakovljević M, Molnar M, Jokić S (2019) Extraction of carnosic acid and carnosol from sage (Salvia officinalis L.) leaves by supercritical fluid extraction and their antioxidant and antibacterial activity. Plants 8:16. https://doi.org/10.3390/plants8010016
Payton P, Kottapalli KR, Kebede H, Mahan JR, Wright RJ, Allen RD (2011) Examining the drought stress transcriptome in cotton leaf and root tissue. Biotechnol Lett 33:821–828
Pazcel EMM, Wannaz ED, Pignata ML, Salazar MJ (2018) Tagetes minuta L. Variability in terms of lead phytoextraction from polluted soils: is historical exposure a determining factor? Environ Process 5:243–259
Pegg GF, Woodward S (1986) Synthesis and metabolism of α-tomatine in tomato isolines in relation to resistance to Verticillium albo-atrum. Physiol Mol Plant Pathol 28:187–201
Peng X, Teng L, Yan X (2015) The cold responsive mechanism of the paper mulberry: decreased photosynthesis capacity and increased starch accumulation. BMC Genomics 16:898. https://doi.org/10.1186/s12864-015-2047-6
Petrulova V, Vilkova M, Kovalikova Z, Sajko M, Repcak M (2020) Ethylene Induction of non-enzymatic metabolic antioxidants in Matricaria chamomilla. Molecules 25:5720
Pezet R, Gindro K, Viret O, Richter H (2004) Effect of resveratrol, viniferins and pterostilbene on Plasmopara viticola zoospore mobility and disease development. Vitis 43:145–148
Phimchan P, Techawongstien S, Chanthai S, Bosland PW (2012) Impact of drought stress on the accumulation of capsaicinoids in capsicum cultivars with different initial capsaicinoid levels. HortScience 47:1204–1209
Pitta-Alvarez SI, Spollansky TC, Giulietti AM (2000) The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzyme Microb Technol 26:252–258
Plengmuankhae W, Tantitadapitak C (2015) Low temperature and water dehydration increase the levels of asiaticoside and madecassoside in Centella asiatica (L.) Urban. South Afr J Bot 97:196–203
Porres-Martinez M, Gonzalez-Burgos E, Carretero ME, Gomez-Serranillos MP (2016) In vitro neuroprotective potential of the monoterpenes alpha-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z Nat C J Biosci 71:191–199
Porto DD, Matsuura HN, Henriques AT, Rosa LMG, Fett JP, Fett-Neto AG (2020) The alkaloid brachycerine contributes to protection against acute UV-B damage in Psychotria. Ind Crops Prod 147:112216. https://doi.org/10.1016/j.indcrop.2020.112216
Poulton JE, Li CP (1994) Tissue leve1 compartmentation of(R)-amygdalin and amygdalin hydrolase prevents large-scale Cyanogenesis in undamaged Prunus seeds. Plant Physiol 104:29–35
Prasad K, Bhatnagar-Mathur P, Waliyar F, Sharma KK (2013) Overexpression of a chitinase gene in transgenic peanut confers enhanced resistance to major soil borne and foliar fungal pathogens. J Plant Biochem Biot 22:222–233
Radwan A, Kleinwächter M, Selmar D (2017) Impact of drought stress on specialised metabolism: biosynthesis and the expression of monoterpene synthases in sage (Salvia officinalis). Phytochemistry 141:20–26
Rai R, Meena RP, Smita SS, Shukla A, Rai SK, Pandey-Rai S (2011) UV-B and UV-C pre-treatments induce physiological changes and artemisinin biosynthesis in Artemisia annua L. an antimalarial plant. J Photochem Photobiol B: Biol 105:216–225
Rai R, Agrawal M, Agrawal SB (2016) Impact of heavy metals on physiological processes of plants: with special reference to photosynthetic system. In: Singh A, Prasad SM, Singh RP (eds) Plant Responses to Xenobiotics. Springer, Singapore, pp 127–140
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731
Rani PU, Jyothsna Y (2010) Biochemical and enzymatic changes in rice plants a mechanism of defense. Acta Physiol Plant 32:695–701
Razavizadeh R, Komatsu S (2018) Changes in essential oil and physiological parameters of callus and seedlings of Carum copticum L. under in vitro drought stress. J Food Meas Charact 12:1581–1592
Razdan MK (2003) Introduction to plant tissue culture, 2nd edn. Science Publishers/Oxford and IBH publishing, Enfield/Plymouth
Razieh K, Arzani A, Mirmohammady Maibody SAM (2021) Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front Plant Sci 12:646221. https://doi.org/10.3389/fpls.2021.646221
Raziq A, Din AMU, Anwar S, Wang Y, Jahan MS, He M, Ling CG, Sun J, Shu S, Guo S (2022) Exogenous spermidine modulates polyamine metabolism and improves stress responsive mechanisms to protect tomato seedlings against salt stress. Plant Physiol Biochem 187:1–10
Regvar M, Bukovnik U, Likar M, Kreft I (2012) UV-B radiation affects flavonoids and fungal colonisation in Fagopyrum esculentum and F. tataricum. Open Life Sci 7:275–283
Rehman RNU, You Y, Zhang L, Goudia BD, Khan AR, Li P, Ma F (2017) High temperature induced anthocyanin inhibition and active degradation in Malus profusion. Front Plant Sci 8:1401. https://doi.org/10.3389/fpls.2017.01401
Rempe CS, Burris KP, Lenaghan SC, Stewart CN Jr (2017) The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front Microbiol 8:422. https://doi.org/10.3389/fmicb.2017.00422
Rezaie R, Abdollahi Mandoulakani B, Fattahi M (2020) Cold stress changes antioxidant defense system, phenylpropanoid contents and expression of genes involved in their biosynthesis in Ocimum basilicum L. Sci Rep 10:1–10
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure – antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Richau KH, Kozhevnikova AD, Seregin IV, Vooij R, Koevoets PL, Smith JAC, Ivanov VB, Schat H (2009) Chelation by histidine inhibits the vacuolar sequestration of nickel in roots of the hyperaccumulator Thlaspi caerulescens. New Phytol 183:106–116
Rivero M, Furman N, Mencacci N, Picca P, Toum L, Lentz E, Bravo-Almonacid F, Mentaberry A (2012) Stacking of antimicrobial genes in potato transgenic plants confers increased resistance to bacterial and fungal pathogens. J Biotechnol 157:334–343
Rivero J, Álvarez D, Flors V, Azcón-Aguilar C, Pozo MJ (2018) Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol 220:1322–1336
Roddick JG, Drysdale RB (1984) Destabilization of liposome membranes by the steroidal glycoalkaloid a-tomatine. Phytochemistry 23:543–547
Rook F (2016) Metabolic engineering of chemical defence pathways in plant disease control. In: Collinge DB (ed) Plant pathogen resistance biotechnology. Wiley, London, pp 71–89
Ruhland CT, Xiong FS, Clark WD, Day TA (2005) The influence of ultraviolet-B radiance on growth, hydroxycinnamic acids and flavonoids of Deschampsia antarctica during springtime ozone depletion in Antarctica. Photochem Photobiol 81:1086–1093
Rysiak A, Dresler S, Hanaka A, Hawrylak-Nowak B, Strzemski M, Kováčik J, Sova I, Latalski M, Wójciak M (2021) High temperature alters secondary metabolites and photosynthetic efficiency in Heracleum sosnowskyi. Int J Mol Sci 22:4756. https://doi.org/10.3390/ijms22094756
Saba-Pande D, Iqbal M, Srivastava PS (2000) Effect of ZnSO4 and CuSO4 on regeneration and lepidine content in Lepidium Sativum L. Biol Plant 43:253–256
Sahay S, Gupta M (2017) An update on nitric oxide and its benign role in plant responses under metal stress. Nitric Oxide 67:39–52
Said-Al A, Omer EA (2011) Medicinal and aromatic plants production under salt stress. A review. Herba Pol 57(2):72–87
Saito R, Yamamoto H, Makino A, Sugimoto T, Miyake C (2011) Methylglyoxal functions as Hill oxidant and stimulates the photoreductin of O2 at photosystem I: a symptom of plant diabetes. Plant Cell Environ 34:1454–1464
Sakamoto A, Murata N (2002) The role of glycine betaine in the protection of plants from stress: clues from transgenic plants. Plant Cell Environ 25:163–171
Salehin M, Li B, Tang M, Katz E, Song L, Ecker JR, Kliebenstein DJ, Estelle M (2019) Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat Commun 10:4021
Salt DE, Prince RC, Baker AJM, Raskin I, Pickering IJ (1999) Zinc legands in the metal hyperaccumulator Thalspi caerulescens as determined using X-absorption spectroscopy. In: Glaze WH (ed) Environmental science and technology. ACS Publication, America, pp 713–717
Samarina LS, Bobrovskikh AV, Doroshkov AV, Malyukova LS, Matskiv AO, Rakhmangulov RS, Koninskaya NG, Malyarovskaya VI, Tong W, Xia E, Manakhova KA, Ryndine AV, Orlov YL (2020) Comparative expression analysis of stress-inducible candidate genes in response to cold and drought in tea plant [Camellia sinensis (L.) Kuntze]. Front Genet 11:1613. https://doi.org/10.3389/fgene.2020.611283
Sancho-Knapik D, Sanz MÁ, Peguero-Pina JJ, Niinemets Ü, Gil-Pelegrín E (2017) Changes of secondary metabolites in Pinus sylvestris L. needles under increasing soil water deficit. Ann For Sci 74:1–10
Sankari M, Hridya H, Sneha P, Doss CGP, Ramamoorthy S (2017) Effect of UV radiation and its implications on carotenoid pathway in Bixa orellana L. J Photochem Photobiol B:Biol 176:136–144
Santisree P, Jalli LCL, Bhatnagar‐Mathur P, Sharma KK (2020) Emerging roles of salicylic acid and jasmonates in plant abiotic stress responses. In: Roychoudhury A, Tripathi DK (eds) Protective chemical agents in the amelioration of plant abiotic stress: biochemical and molecular perspectives. John Wiley & Sons Ltd, pp. 342–373
Sarker U, Oba S (2018) Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol 18:1–15
Sarri E, Termentzi A, Abraham EM, Papadopoulos GK, Baira E, Machera K, Loukas V, Komaitis F, Tani E (2021) Salinity stress alters the secondary metabolic profile of M. sativa, M. arborea and their hybrid (Alborea). Int J Mol Sci 22:4882. https://doi.org/10.3390/ijms22094882
Savoi S, Wong DC, Arapitsas P, Miculan M, Bucchetti B, Peterlunger E, Fait A, Mattivi F, Castellarin SD (2016) Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol 16:67. https://doi.org/10.1186/s12870-016-0760-1
Scagel CF, Lee J, Mitchell JN (2019) Salinity from NaCl changes the nutrient and polyphenolic composition of basil leaves. Ind Crop Prod 127:119–128
Schlesinger D, Rikanati RD, Volis S, Faigenboim A, Vendramin V, Cattonaro F, Hooper M, Oren E, Taylor M, Sitrit Y, Inbar M (2019) Alkaloid chemodiversity in Mandragora spp. is associated with loss-of-functionality of MoH6H, a hyoscyamine 6β-hydroxylase gene. Plant Sci 283:301–310
Schultz TP, Boldin WD, Fisher TH, Nicholas DD, McMurtrey KD, Pobanz K (1992) Structure-fungicidal properties of some 3- and 4-hydroxylated stilbenes and bibenzyl analogues. Phytochemistry 31:3801–3806
Schulze K, Schreiber L, Szankowski I (2005) Inhibiting effects of resveratrol and its glucoside piceid against Venturia inaequalis, the causal agent of apple scab. J Agric Food Chem 53:356–362
Sebastian A, Kumari R, Kiran BR, Prasad MNV (2018) Ultraviolet B induced bioactive changes of enzymatic and non-enzymatic antioxidants and lipids in Trigonella foenum-graecum L. (Fenugreek). Euro Biotech J 2:64–71
Sehgal A, Sita K, Nayyar H (2016) Heat stress in plants: sensing and defense mechanisms. J Plant Sci Res 32:195–210
Selmar D (2008) Potential of salt and drought stress to increase pharmaceutical significant secondary compounds in plants. Landbauforsch Volkenrode 58:139–144
Selmar D, Kleinwächter M (2013) Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind Crops Prod 42:558–566
Selmar D, Kleinwächter M, Abouzeid S, Yahyazadeh M, Nowak M (2017) The impact of drought stress on the quality of spice and medicinal plants. In: Ghorbanpour M, Varma A (eds) Medicinal plants and environmental challenges. Springer, Cham, pp 159–175
Shah MM, Afiya H (2019) Introductory chapter: identification and isolation of Trichoderma spp.-their significance in agriculture, human health, industrial and environmental application. In: Shah MM, Sharif U, Buhari TR (eds) Trichoderma-The most widely used fungicide. IntechOpen. https://doi.org/10.5772/intechopen.83528
Shang Y, Ma YS, Zhou Y, Zhang HM, Duan LX, Chen HM (2014) Biosynthesis, regulation, and domestication of bitterness in cucumber. Science 346:1084–1088
Shanker KS, Kanjilal S, Rao BVSK, Kishore KH, Misra S, Prasad RBN (2007) Isolation and antimicrobial evaluation of isomeric hydroxy ketones in leaf cuticular waxes of Annona squamosa. Phytochem Anal 18:7–12
Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Biol 52:407–436
Shen S, Peng M, Fang H, Wang Z, Zhou S, Jing X, Zhang M, Yang C, Guo H, Li Y, Lei L, Shi Y, Sun Y, Liu X, Xu C, Tohge T, Yuan M, Fernie AR, Ning Y, Wang GL, Luo J (2021) An oryza-specific hydroxycinnamoyl tyramine gene cluster contributes to enhanced disease resistance. Sci Bull 66:2369–2380
Shi J, Fu XZ, Peng T, Huang XS, Fan QJ, Liu JH (2010) Spermine pre-treatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol 30:914–922
Shirazi Z, Aalami A, Tohidfar M, Sohani MM (2019) Triterpenoid gene expression and phytochemical content in Iranian licorice under salinity stress. Protoplasma 256:827–837
Silva MS, Arraes FBM, de Araújo CM, Grossi-de-Sa M, Fernandez D, de Souza CE, Cardoso MH, Franco OL, Grossi-de-Sa MF (2018) Potential biotechnological assets related to plant immunity modulation applicable in engineering disease-resistant crops. Plant Sci 270:72–84
Singh SK (2018) Explorations of plant’s chemodiversity: role of nitrogen-containing secondary metabolites in plant defense. In: Singh A, Singh IK (eds) Molecular aspects of plant-pathogen interaction. Springer, Singapore, pp 309–332
Slama I, Abdelly C, Bouchereau A, Flowers T, Savouré A (2015) Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot 115:433–447
Smith CA, MacHardy WE (1982) The significance of tomatine in the host response of susceptible and resistant tomato isolines infected with two races of Fusarium oxysporum f. sp. lycopersici. Phytopathology 72:415–419
Soares MS, da Silva DF, Forim MR, Fernandes JB, Silva DB VPC, Machado MA (2015) Quantification and localization of hesperidin and rutin in Citrus sinensis grafted on C. limonia after Xylella fastidiosa infection by HPLC-UV and MALDI imaging mass spectrometry. Phytochem 115:161–170
Soares MS, Silva DF, Amaral JC, Silva MM, Forim MR, Rodrigues-Filho E, Silva MF, Fernandes JB, Machado MA, de Souza AA, Martins JHG (2020) Rapid differentiation of graft Citrus Sinensis with and without Xylella fastidiosa infection by mass spectrometry. Rapid Commun Mass Spectrom 34:e8745. https://doi.org/10.1002/rcm.8745
Sobolev VS, Horn BW, Potter TL, Deyrup ST, Gloer JB (2006) Production of stilbenoids and phenolic acids by the peanut plant at early stages of growth. J Agric Food Chem 54:3505–3511
Song P, Yu X, Yang W, Wang Q (2021) Natural phytoalexin stilbene compound resveratrol and its derivatives as anti-tobacco mosaic virus and anti-phytopathogenic fungus agents. Sci Rep 11:1–10
Sosa MC, Salazar MJ, Zygadlo JA, Wannaz ED (2016) Effects of Pb in Tagetes minuta L. (Asteraceae) leaves and its relationship with volatile compounds. Ind Crops Prod 82:37–43
Spochacz M, Chowański S, Szymczak M, Lelario F, Bufo SA, Adamski Z (2018) Sublethal effects of Solanum nigrum fruit extract and its pure glycoalkaloids on the physiology of Tenebrio molitor (Mealworm). Toxins 10:504. https://doi.org/10.3390/toxins10120504
Steponkus PL (1984) Role of the Plasma Membrane in Freezing Injury and Cold Acclimation. Annu Rev Plant Physiol 38:543–584
Stevenson PC, Nicolson SW, Wright GA (2017) Plant secondary metabolites in nectar: impacts on pollinators and ecological functions. Funct Ecol 31:65–75
Sun Z, Zhang K, Chen C, Wu Y, Tang Y, Georgiev MI, Zhang X, Lin M, Zhou M (2018) Biosynthesis and regulation of cyanogenic glycoside production in forage plants. Appl Microbiol Biotechnol 102:9–16
Sun Y, Liao J, Zou X, Xu X, Yang J, Chen HY, Ruan H (2020) Coherent responses of terrestrial C: N stoichiometry to drought across plants, soil, and microorganisms in forests and grasslands. Agric For Meteorol 292:108104. https://doi.org/10.1016/j.agrformet.2020.108104
Sun S, Fang J, Lin M, Hu C, Qi X, Chen J, Zhong Y, Muhammed A, Li Z, Li Y (2021) Comparative metabolomic and transcriptomic studies reveal key metabolism pathways contributing to freezing tolerance under cold stress in kiwifruit. Front Plant Sci 12:628969. https://doi.org/10.3389/fpls.2021.628969
Sutulienė R, Ragelienė L, Samuolienė G, Brazaitytė A, Urbutis M, Miliauskienė J (2021) The Response of antioxidant system of drought-stressed green pea (Pisum sativum L.) affected by watering and foliar spray with silica nanoparticles. Horticulturae 8:35. https://doi.org/10.3390/horticulturae8010035
Suzuki K, Nomura I, Ninomiya M, Tanaka K, Koketsu M (2018) Synthesis and antimicrobial activity of b-carboline derivatives with N2-alkyl modifications. Bioorg Med Chem Lett 28:2976–2978
Swapnil P, Meena M, Singh SK, Dhuldhaj UP, Marwal A (2021) Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr Plant Biol 21:00203. https://doi.org/10.1016/j.cpb.2021.100203
Sytar O, Mbarki S, Zivcak M, Brestic M (2018) The involvement of different secondary metabolites in salinity tolerance of crops. In: Kumar V, Wani SH, Suprasanna P, Tran LSP (eds) Salinity responses and tolerance in plants. Springer, Berlin/Heidelberg, pp 21–48
Szilard A, Sass L, Hideg E, Vass I (2005) Photoinactivation of photosystem II by flashing light. Photosynth Res 84:15–20
Takshak S, Agrawal SB (2016) The role of supplemental ultraviolet-B radiation in altering the metabolite profile, essential oil content and composition, and free radical scavenging activities of Coleus forskohlii, an indigenous medicinal plant. Environ Sci Poll Res 23:7324–7337
Talhaoui N, Taamalli A, Gómez-Caravaca AM, Fernández-Gutiérrez A, Segura-Carretero A (2015) Phenolic compounds in olive leaves: analytical determination, biotic and abiotic influence, and health benefits. Food Res Int 77:92–108
Tang P, Giusti MM (2020) Metal chelates of petunidin derivatives exhibit enhanced color and stability. Foods 9:1426. https://doi.org/10.3390/foods9101426
Tashackori H, Sharifi M, Chashmi NA, Behmanesh M, Safaie N (2018) Piriformospora indica cell wall modulates gene expression and metabolite profile in Linum album hairy roots. Planta 248:1289–1306
Thomas JE, Bandara M, Driedger D, Lee EL (2011) Fenugreek in western Canada. Am J Plant Sci Biotech 5:44
Tian F, Wang W, Liang C, Wang X, Wang G, Wang W (2017) Overaccumulation of glycine betaine makes the function of the thylakoid membrane better in wheat under salt stress. Crop J 5:73–82
Tiedeken EJ, Stout JC, Stevenson PC, Wright GA (2014) Bumblebees are not deterred by ecologically relevant concentrations of nectar toxins. J Exp Biol 217:1620–1625
Tiku AR (2018) Antimicrobial compounds and their role in plant defense. In: Singh A, Singh I (eds) Molecular aspects of plant-pathogen interaction. Springer, Singapore, pp 283–307
Tiku AR (2020) Antimicrobial compounds (phytoanticipins and phytoalexins) and their role in plant defense. In: Mérillon JM, Ramawat K (eds) Co-evolution of secondary metabolites, Reference series in phytochemistry. Springer, Cham, pp 845–868
Tiwari DN (2018) Current status and future prospective of use of biotechnology in plant disease management. IJAER 4:238–245
Torun H, Eroğlu E, Yalçin V, Elmas U (2021) Physicochemical and antioxidant responses of St. John’s Wort (Hypericum perforatum L.) under drought stress. Düzce Üniv Bil Teknol Derg 9:40–50
Trdá L, Janda M, Macková D, Pospíchalová R, Dobrev PI, Burketová L, Matušinsky P (2019) Dual mode of the saponin aescin in plant protection: antifungal agent and plant defense elicitor. Front Plant Sci 10:1448. https://doi.org/10.3389/fpls.2019.01448
Trejo-Tapia G, Jimenez-Aparicio A, Rodriguez-Monroy M, De Jesus-Sanchez A, Gutierrez Lopez G (2001) Influence of cobalt and other microelements on the production of betalains and the growth of suspension cultures of Beta vulgaris. Plant Cell Tissue Org Cult 67:19–23
Treml J, Šmejkal K (2016) Flavonoids as potent scavengers of hydroxyl radicals. Compr Rev Food Sci Food Saf 15:720–738
UdDin I, Bano A, Masood S (2015) Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotoxicol Environ Saf 113:271–278
Uemura M, Steponkus PL (1999) Cold acclimation in plants: relationship between the lipid composition and the cryostability of the plasma membrane. J Plant Res 112:245
Uleberg E, Rohloff J, Jaakola L, Trôst K, Junttila O, Häggman H, Martinussen I (2012) Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J Agric Food Chem 60:10406–10414
Umar S, Gauba N, Anjum NA, Siddiqi TO (2013) Arsenic toxicity in garden cress (Lepidium sativum Linn.) significance of potassium nutrition. Environ Sci Pollut Res Int 20:6039–6049
Valifard M, Mohsenzadeh S, Kholdebarin B, Rowshan V, Niazi A, Moghadam AE (2019) ect of salt stress on terpenoid biosynthesis in Salvia mirzayanii: from gene to metabolite. J Hortic Sci Biotechnol 94:389–399
Van Loon LC, Van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Molecular Plant Pathol 55:85–97
Van Loon LC, Pierpoint WS, Boller TH, Conejero V (1994) Recommendations for naming plant pathogenesis-related proteins. Plant Mol Biol Rep 12:245–264
Van Loon LC, Rep M, Pieterse CM (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44:135–162
VanEtten HD, Mansfield JW, Bailey JA, Farmer EE (1994) Two classes of plant antibiotics: phytoalexins versus ‘phytoanticipins’. Plant Cell 6:1191–1192
Vashisth D, Kumar R, Rastogi S, Patel VK, Kalra A, Gupta MM, Gupta AK, Shasany AK (2018) Transcriptome changes induced by abiotic stresses in Artemisia annua. Sci Rep 8:3423. https://doi.org/10.1038/s41598-018-21598-1
Vaughan MM, Christensen S, Schmelz EA, Huffaker A, Mcauslane HJ, Alborn HT, Romero M, Allen HR, Teal PEA (2015) Accumulation of terpenoid phytoalexins in maize roots is associated with drought tolerance. Plant Cell Environ 38:2195–2207
Verma N, Shukla S (2015) Impact of various factors responsible for fluctuation in plant secondary metabolites. J Appl Res Med Aromatic Plants 2:105–113
Vickers CE, Gershenzon J, Lerdau MT, Loreto F (2009) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5:283–291
Vidal C, Ruiz A, Ortiz J, Larama G, Perez R, Santander C, Ferreira PAA, Cornejo P (2020) Antioxidant responses of phenolic compounds and immobilization of copper in Imperata cylindrica, a plant with potential use for bioremediation of Cu contaminated environments. Plants 9:1397. https://doi.org/10.3390/plants9101397
Viret O, Spring JL, Gindro K (2018) Stilbenes: biomarkers of grapevine resistance to fungal diseases. Oeno One 52:235–241
Vranova V, Rejsek K, Skene KR, Formanek P (2011) Non-protein amino acids: plant, soil and ecosystem interactions. Plant Soil 342:31–48
Wahid A, Farooq M, Hussain I, Rasheed R, Galani S (2012) Responses and management of heat stress in plants. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York/Dordrecht/Heidelberg/London, pp 135–157
Wallis CM, Wallingford AK, Chen J (2013) Grapevine rootstock effects on scion sap phenolic levels, resistance to Xylella fastidiosa infection, and progression of Pierce's disease. Front Plant Sci 4:502. https://doi.org/10.3389/fpls.2013.00502
Wang X, Li W, Li M, Welti R (2006) Profiling lipid changes in plant response to low temperatures. Physiol Plant 126:90–96
Wang DH, Du F, Liu HY, Liang ZS (2010) Drought stress increases iridoid glycosides biosynthesis in the roots of Scrophularia ningpoensis seedlings. J Med Plant Res 4:2691–2699
Wang Y, Chantreau M, Sibout R, Hawkins S (2013) Plant cell wall lignification and monolignol metabolism. Front Plant Sci 4:220. https://doi.org/10.3389/fpls.2013.00220
Wang Q, Eneji AE, Kong X, Wang K, Dong H (2015) Salt stress effects on secondary metabolites of cotton in relation to gene expression responsible for aphid development. PloS One 10:e0129541. https://doi.org/10.1371/journal.pone.0129541
Wang N, Zhang Z, Jiang S, Xu H, Wang Y, Feng S, Chen X (2016) Synergistic effects of light and temperature on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult 127:1–11
Wang L, Zeng B, Liu Z, Liao Z, Zhong Q, Gu L, Fang X (2018) Green tea polyphenols modulate colonic microbiota diversity and lipid metabolism in high-fat diet treated HFA mice. J Food Sci 83(3):864–873
Wang Y, Gao S, He X, Li Y, Li P, Zhang Y, Chen W (2019) Growth, secondary metabolites and enzyme activity responses of two edible fern species to drought stress and rehydration in Northeast China. Agronomy 9:137. https://doi.org/10.3390/agronomy9030137
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4:162–176
Wani KI, Choudhary S, Zehra A, Naeem M, Weathers P, Aftab T (2021) Enhancing artemisinin content in and delivery from Artemisia annua: a review of alternative, classical, and transgenic approaches. Planta 254:1–15
Warren JM, Bassman JH, Fellman JK, Mattinson DS, Eigenbrode S (2003) Ultraviolet-B radiation alters phenolic salicylate and flavonoid composition of Populus trichocarpa leaves. Tree Physiol 23:527–535
Way HM, Kazan K, Mitter N, Goulter KG, Birch RG, Manners JM (2002) Constitutive expression of a phenylalanine ammonia-lyase gene from Stylosanthes humilis in transgenic tobacco leads to enhanced disease resistance but impaired plant growth. Physiol Mol Plant Pathol 60:275–282
WHO (2020). https://www.who.int/health-topics/drought#tab=tab_1
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology and biotechnology. Plant Physiol 126:485–493
Wittayathanarattana T, Wanichananan P, Supaibulwatana K, Goto E (2022) Enhancement of bioactive compounds in baby leaf Amaranthus tricolor L. using short-term application of UV-B irradiation. Plant Physiol Biochem 182:202–215
Wittek B, Carnat G, Tison JL, Gypens N (2020) Response of dimethylsulfoniopropionate (DMSP) and dimethylsulfoxide (DMSO) cell quotas to salinity and temperature shifts in the sea-ice diatom Fragilariopsis cylindrus. Polar Biol 43:483–494
Wu GJ, Chen TG, Chang HC, Chiu WT, Chang CC, Chen RM (2007) Nitric oxide from both exogenous and endogenous sources activates mitochondria-dependent events and induces insults to human chondrocytes. J Cell Biochem 101:1520–1531
Xiang Q, Rathinasabapathi B (2022) Differential tolerance to heat stress of young leaves compared to mature leaves of whole plants relate to differential transcriptomes involved in metabolic adaptations to stress. AoB Plants 14(4):plac024. https://doi.org/10.1093/aobpla/plac024
Xiao L, Carrillo J, Siemann E, Ding J (2019) Herbivore-specific induction of indirect and direct defensive responses in leaves and roots. AoB Plants 11:plz003. https://doi.org/10.1093/aobpla/plz003
Xie X, He Z, Chen N, Tang Z, Wang Q, Cai Y (2019) The roles of environmental factors in regulation of oxidative stress in plant. Biomed Res Int 2019:9732325. https://doi.org/10.1155/2019/9732325
Xu Z, Zhou G, Shimizu H (2010) Plant responses to drought and rewatering. Plant Signal Behav 5:649–654
Xu A, Zhan JC, Huang WD (2015) Effects of ultraviolet C, methyl jasmonate and salicylic acid, alone or in combination, on stilbene biosynthesis in cell suspension cultures of Vitis vinifera L. cv. Cabernet Sauvignon. Plant Cell Tissue Organ Cult 122:197–211
Xu Z, Zhou J, Ren T, Du H, Liu H, Li Y, Zhang C (2020) Salt stress decreases seedling growth and developmentbut increases quercetin and kaempferol content in Apocynum venetum. Plant Biol 22:813–821
Xu X, Chen Y, Li B, Zhang Z, Qin G, Chen T, Tian S (2022) Molecular mechanisms underlying multi-level defense responses of horticultural crops to fungal pathogens. Hortic Res 9. https://doi.org/10.1093/hr/uhac066
Yadav B, Jogawat A, Lal SK, Lakra N, Mehta S, Shabek N, Narayan OP (2021a) Plant mineral transport systems and the potential for crop improvement. Planta 253:1–30
Yadav B, Jogawat A, Rahman MS, Narayan OP (2021b) Secondary metabolites in the drought stress tolerance of crop plants: a review. Gene Rep 23:101040. https://doi.org/10.1016/j.genrep.2021.101040
Yan H, Xie N, Zhong C, Su A, Hui X, Zhang X, Jin Z, Li Z, Feng J, He J (2018) Aphicidal activities of Amaryllidaceae alkaloids from bulbs of Lycoris radiata against Aphis citricola. J Ind Crop Prod 123:372–378
Yan F, Qu D, Chen X, Zeng H, Li X, Hu CY (2022) Metabolomics reveals 5-aminolevulinic acid improved the ability of tea Leaves (Camellia sinensis L.) against cold stress. Metabolites 12:392. https://doi.org/10.3390/metabo12050392
Yan S, Liu Q, Li W, Yan J, Fernie AR (2022) Raffinose family oligosaccharides: crucial regulators of plant development and stress responses. Crit Rev Plant Sci 41(4):286–303
Yang XE, Jin XF, Feng Y, Islam E (2005) Molecular mechanisms and genetic basis of heavy metal tolerance/hyperaccumulation in plants. J Integr Plant Biol 47:1025–1035
Yang YJ, Chang W, Huang W, Zhang SB, Hu H (2017) The effects of chilling-light stress on photosystems I and II in three Paphiopedilum species. Bot Stud 58:1–12
Yang L, Wen KS, Ruan X, Zhao YX, Wei F, Wang Q (2018) Response of plant secondary metabolites to environmental factors. Molecules 23:762. https://doi.org/10.3390/molecules23040762
Yang HL, Wang TJ, Yu XH, Yang Y, Wang CF, Yang QH, Wang XH (2020) Enhanced sugar accumulation and regulated plant hormone signalling genes contribute to cold tolerance in hypoploid Saccharum spontaneum. BMC Genom 21:507
Yeshi K, Crayn D, Ritmejerytė E, Wangchuk P (2022) Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 27:313. https://doi.org/10.3390/molecules27010313
Yildiz I, Mantz M, Hartmann M, Zeier T, Kessel J, Thurow J, Gatz J, Petzsch P, Köhrer K, Zeiger J (2021) The mobile SAR signal N-hydroxypipecolic acid induces NPR1-dependent transcriptional reprogramming and immune priming. Plant Physiol 186:1679–1705
Yuan S, Li W, Li Q, Wang L, Cao J, Wiang J (2019) Defense responses, induced by p-coumaric acid and methyl p-coumarate, of jujube (Ziziphus jujuba Mill.) fruit against black spot rot caused by Alternaria alternata. J Agric Food Chem 67:2801–2810
Zafari S, Sharifi M, Chashmi NA, Mur LA (2016) Modulation of Pb-induced stress in Prosopis shoots through an interconnected network of signaling molecules, phenolic compounds and amino acids. Plant Physiol Biochem 99:11–20
Zahra H, Naser K, Masoud M, Saeed M (2012) Enhancement of compatible solute and secondary metabolites production in Plantago ovata Forsk. by salinity stress. J Med Plant Res 6:3495–3500
Zandalinas SI, Sales C, Beltrán J, Gómez-Cadenas A, Arbona V (2017) Activation of secondary metabolism in citrus plants is associated to sensitivity to combined drought and high temperatures. Front Plant Sci 7:1954. https://doi.org/10.3389/fpls.2016.01954
Zaynab M, Fatima M, Abbas S, Sharif Y, Umair M, Zafar MH, Bahadar K (2018) Role of secondary metabolites in plant defense against pathogens. Microb Pathol 124:198–202
Zechmann B (2014) Compartment-specifc importance of glutathione during abiotic and biotic stress. Front Plant Sci 5:566. https://doi.org/10.3389/fpls.2014.00566
Zernova OV, Lygin AV, Pawlowski ML, Hill CB, Hartman GL, Widholm JM, Lozovaya VV (2014) Regulation of plant immunity through modulation of phytoalexin synthesis. Molecules 19:7480–7496
Zhang J, Zhang Y, Du Y, Chen S, Tang H (2011) Dynamic metabonomic responses of tobacco (Nicotiana tabacum) plants to salt stress. J Proteome Res 10:1904–1914
Zhang N, Wen Q, Feng H, Cao R, Zhou X, Tagn J, Wu N (2012) Effects of water stress and nitrogen nutrition on regulation of Catharanthus roseus alkaloids metabolism. Chin J Chinese Materia Medica 37:1346–1352
Zhang N, Liu B, Ma C, Zhang G, Chang J, Si H, Wang D (2014) Transcriptome characterization and sequencing-based identification of drought-responsive genes in potato. Mol Biol Rep 41:505–517
Zhang Z, Zhu L, Song A, Wang H, Chen S, Jiang J, Chen F (2020) Chrysanthemum (Chrysanthemum morifolium) CmICE2 conferred freezing tolerance in Arabidopsis. Plant Physiol Bioch 146:31–41
Zhao N, Wang G, Norris A, Chen X, Chen F (2013) Studying plant secondary metabolism in the age of genomics. Crit Rev Plant Sci 32:369–382
Zhao M, Jin L, Hu B, Yao H, Gao Y, Wang R, Li F, Guo J, Li K (2019) Overexpression of chalcone synthase gene improves flavonoid accumulation and drought tolerance in tobacco. https://doi.org/10.20944/preprints201906.0103.v1
Zhao M, Zhang N, Gao T, Jin J, Jing T, Wang J, Wu Y, Wan X, Schwab W, Song C (2020) Sesquiterpene glucosylation mediated by glucosyltransferase UGT91Q2 is involved in the modulation of cold stress tolerance in tea plants. New Phytol 226:362–372
Zheng Y, Zhao Z, Fan L, Meng S, Song C, Qiu L, Xu P, Chen J (2017) Dietary supplementation with rutin has pro-/anti-inflammatory effects in the liver of juvenile GIFT tilapia, Oreochromis niloticus. Fish Shellfish Immunol 64:49–55
Zhou J, Goldsbrough PB (1994) Functional homologs of fungal metallothionein genes from Arabidopsis. Plant Cell 6:875–884
Zhou F, Pichersky E (2020) The complete functional characterisation of the terpene synthase family in tomato. New Phytol 226:1341–1360
Zhou Y, Zeng L, Liu X, Gui J, Mei X, Fu X, Dong F, Tang J, Zhang L, Yang Z (2017) Formation of (E)-nerolidol in tea (Camellia sinensis) leaves exposed to multiple stresses during tea manufacturing. Food Chem 231:78–86
Zhu W, Yang B, Komatsu S, Lu X, Li X, Tian J (2015) Binary stress induces an increase in indole alkaloid biosynthesis in Catharanthus roseus. Front Plant Sci 6:582. https://doi.org/10.3389/fpls.2015.00582
Zhu Y, Chen Y, Zhang X, Xie G, Qin M (2020) Copper stress-induced changes in biomass accumulation, antioxidant activity and flavonoid contents in Belamcanda chinensis calli. Plant Cell Tiss Organ Cult 142:299–311
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Koç, E., Karayiğit, B. (2023). Plant Secondary Metabolites in Stress Tolerance. In: Hasanuzzaman, M. (eds) Climate-Resilient Agriculture, Vol 1. Springer, Cham. https://doi.org/10.1007/978-3-031-37424-1_19
Download citation
DOI: https://doi.org/10.1007/978-3-031-37424-1_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-37423-4
Online ISBN: 978-3-031-37424-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)