Abstract
For many years, synaptic transmission was considered as information transfer between presynaptic neuron and postsynaptic cell. At the synaptic level, it was thought that dendritic arbors were only receiving and integrating all information flow sent along to the soma, while axons were primarily responsible for point-to-point information transfer. However, it is important to highlight that dendritic spines play a crucial role as postsynaptic components in central nervous system (CNS) synapses, not only integrating and filtering signals to the soma but also facilitating diverse connections with axons from many different sources. The majority of excitatory connections from presynaptic axonal terminals occurs on postsynaptic spines, although a subset of GABAergic synapses also targets spine heads. Several studies have shown the vast heterogeneous morphological, biochemical, and functional features of dendritic spines related to synaptic processing. In this chapter (adding to the relevant data on the biophysics of spines described in Chap. 1 of this book), we address the up-to-date functional dendritic characteristics assessed through electrophysiological approaches, including backpropagating action potentials (bAPs) and synaptic potentials mediated in dendritic and spine compartmentalization, as well as describing the temporal and spatial dynamics of glutamate receptors in the spines related to synaptic plasticity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Dendritic Spine Electrophysiology
Dendritic spines, the subcellular formation characterized by tiny membrane protrusions (≤1 μm diameter) of dendrites, constitute the fundamental units of synaptic and information processing in the mammalian brain (Harris et al. 1992; Popovic et al. 2015b; Kwon et al. 2017). Most excitatory inputs on cortical pyramidal neurons are made on spines, rather than on dendritic shafts (Yuste 2010; Araya et al. 2014), but dendritic spines also modulate inhibitory synaptic inputs showing particularly high levels of plasticity (Chen et al. 2012; van Versendaal et al. 2012). Spines can individually detect temporal coincidence of synaptic activity, acting as basic functional units of neuronal integration (Yuste and Denk 1995; Sabatini et al. 2002; Noguchi et al. 2005; Yuste 2013). Additionally, spine shape and spine head size are correlated with changes in synaptic strength during synaptic plasticity (Yuste and Bonhoeffer 2001). While the complex and dynamic regional electrical properties of dendritic spines play an important role in determining the function and integration of neural circuits, the electrical behavior of spines is still not well understood and sometimes remains controversial.
For a long time, the limitations of available techniques prevented measuring voltage signals directly from the membrane of individual spines. Thus, the electrical properties of dendritic spines, including active conductances, were based on theoretical models (Harris and Stevens 1989; Koch and Zador 1993; Harris and Kater 1994; Johnston et al. 1996; Yuste and Tank 1996; Svoboda et al. 1996; Shepherd 1996; Yuste 2010) and based on channel densities and kinetics from recordings of dendritic patches. Whole-cell or cell-attached recordings of spine heads per se are still very difficult to perform in brain slices. However, advanced imaging techniques, including high voltage-sensitive dyes, low-affinity Ca2+ and Na+ indicators, two-photon Ca2+ imaging, and two-photon uncaging of glutamate, made it possible to detect membrane voltage signals from individual dendritic spines in brain slices (Holthoff et al. 2010; Popovic et al. 2015a, b; Miyazaki and Ross 2022). In addition, the intracellular voltage recordings from dendritic spines have been obtained by quantum-dot-coated nanopipettes in cells in culture and brain slices (Jayant et al. 2017), and patch-clamp recordings have been also performed in dissociated neurons and isolated spines (Priel et al. 2022). That is, the combination of advanced techniques in imaging and morphology with electrophysiology and spine models are providing valuable information about the spatiotemporal dynamics of biophysical properties of dendritic spines (Stuart et al. 1993; Stuart and Sakmann 1994; Denk et al. 1996; Mainen et al. 1999; Korngreen and Sakmann 2000; Oertner 2002; Nuriya et al. 2006; Araya et al. 2006).
3.1.1 Backpropagating Action Potentials and Synaptic Potentials in Dendritic Spine
Somatic action potentials (APs), after being generated in the axon initial segment, travel back through the dendritic tree as backpropagating action potentials (bAPs) (Häusser et al. 2000). Dendritic spines can regulate the synapses without attenuation of dendritic bAP (Popovic et al. 2015a; Cornejo et al. 2022), which can influence the processing of incoming synaptic inputs in the dendrites of most neurons (Stuart et al. 1997a, b; Xiong and Chen 2002). The coincidence and temporal sequence of bAP and excitatory postsynaptic potential (EPSP) within a dendritic spine determine the amount of Ca2+ entry through voltage-gated channels and/or ionotropic receptors, essential for synaptic plasticity (Yuste and Denk 1995; Schiller et al. 1998; Koester and Sakmann 1998; Yuste et al. 1999; Nevian and Sakmann 2004; Camiré and Topolnik 2014). The rapid time course of the bAP in spines may be a critical factor for the accurate regulation of spike timing-dependent synaptic plasticity within a very short time window, while the magnitude and time course of synaptically activated EPSP in dendritic spines determine how these synapses contribute to synaptic integration (Caporale and Dan 2008; Popovic et al. 2015a). Both synaptic integration and plasticity depend on spatiotemporal changes in the membrane voltage on the dendritic spine, and the ability to measure such modifications is improving.
Spine ionic conductance especially from voltage-gated sodium channels could play an important role in dynamically regulating bAP. Voltage-gated sodium channels have been found in spines (Caldwell et al. 2000; Araya et al. 2007; Bywalez et al. 2015; Miyazaki and Ross 2017). Calcium channel subunits (Caldwell et al. 2000; Bloodgood et al. 2009) and potassium channels (Kim et al. 2007; Kaufmann et al. 2013; Wang et al. 2014; Strobel et al. 2017; Tazerart et al. 2022) have also been localized in spines. These channels, together with functional N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptors (Yuste and Denk 1995; Schiller et al. 1998; Yuste et al. 1999; Kovalchuk et al. 2000; Makino and Malinow 2009; Park 2018), control synaptic potential. These relevant advances in detecting voltage changes in spine membrane are further discussed below.
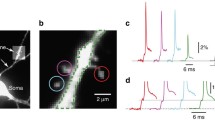
Voltage-sensitive dye recordings based on wide-field laser-excitation fluorescence microscopy permit monitoring of subthreshold electrical signal integration between individual dendritic spines and adjacent dendritic shafts in acute brain slice preparations (Popovic et al. 2015b; Weng et al. 2023). It is, then, possible to measure membrane potential (Vm) transients from individual dendritic spines by using intracellular high-sensitivity voltage-sensing dyes and high-resolution wide-field epi-fluorescence microscopy applied at optical magnification 10 times higher than that used to record membrane voltage from other neuronal parts (Cohen and Salzberg 1978; Weng et al. 2023). These measurements have a sufficient spatial and temporal resolution (approximately 1 μm and submillisecond range) for recording neuronal AP and synaptic potential signals (Popovic et al. 2015a). With this approach, the AP at the soma can be recorded by patch-clamp technique, while voltage-imaging signals of bAP at dendritic spines can be recorded at a frame rate of 2 kHz at high optical magnification, with a satisfactory signal-to-noise ratio (S/N) and insignificant interference of the scattered light or the photodynamic damage (Holthoff et al. 2010) (Fig. 3.1). It is also possible to detect the bAP invading spines with a rapid time course critical for regulating plasticity and very similar to the spikes recorded in the parent dendrite.
Optical recordings of backpropagating action potentials (bAPs) signals from individual dendritic spines of two neurons. (a, b) Upper micrographs show fluorescence images of dendritic branches with spines obtained with a CCD camera for voltage imaging. Lower micrographs show spiny dendrites reconstructed after deconvoluting stacks of spinning-disk confocal images. (c, d) Fluorescence intensity traces from locations 1 to 3 outlined in CCD images. Single-trial recordings and temporal averages of 4 (cell A) and 9 trials (cell B) are shown. Bottom traces are electrode recordings from the soma. The bAP signals, clearly recorded in spines, are absent from regions without spines (locations 2 and 3). (Reproduced from Holthoff et al. (2010), Figure 4, https://doi.org/10.1113/jphysiol.2009.184960, under CCC RightsLink® license 5516071490678)
There is an important functional implication for these data. The magnitude of the synaptic depolarization (EPSP) at the dendritic spine, usually difficult to determine, could provide crucial information about the influence of synaptic inputs on local voltage-dependent processes and cellular responses (Acker et al. 2016). The EPSP signals present an amplitude fivefold to tenfold smaller than an AP. Therefore, the optical resolution used for monitoring the dendritic spines’ bAP is insufficient for recording unitary subthreshold synaptic responses at the spatial scale of individual spines, considering an adequate sensitivity and spatiotemporal resolution with a suitable S/N to allow a precise quantitative analysis.
The combination of high-sensitivity voltage-sensing dyes with the 2-photon uncaging of glutamate could be a suitable strategy for recording dendritic spines (Popovic et al. 2015b; Weng et al. 2023). The increment of the sensitivity can be achieved by increasing excitation intensity from a laser at a required wavelength and reducing the photodynamic damage (Popovic et al. 2015b; Weng et al. 2023). Then, a subthreshold uncaging EPSP signal (uEPSP) can be evoked by stimulating one synapse on a dendritic spine using 2-photon uncaging of glutamate. For example, this procedure could be done by simultaneously recording optical signals along a small segment of a basal dendrite from a cortical layer V pyramidal neuron at a frame rate of 2 kHz (Popovic et al. 2015a, b) (Fig. 3.2) and from cortical layers II/III, V, and VI pyramidal neurons at a frame rate of 5 kHz (Weng et al. 2023). The optical recordings of local uEPSP signals evoked by two-photon glutamate uncaging were followed by a bAP signal evoked by depolarizing pulse delivered by the somatic patch electrode (Popovic et al. 2015a, b; Weng et al. 2023).
Recording subthreshold electrical events from individual dendritic spines. (a) Low-magnification fluorescence image of a basal dendrite labeled with a voltage-sensitive dye; z-stack of confocal images. Arrow: recorded spine. (b) High magnification confocal image of the same spine. Tip of iontophoretic electrode (labeled with the fluorescent dye) in the immediate vicinity of the spine head. (c) Single frame image of a spine in recording position obtained with a CCD camera for voltage imaging. (d) Traces shown on the left are evoked subthreshold excitatory postsynaptic potential (EPSP) recordings from the spine head (red) and its parent dendrite (green). Average of 16 trials. Bottom black traces are somatic electrode recording and the uncaging command pulse. Traces on the right are backpropagating action potentials (bAPs) signals from the same locations. Average of nine trials. (e) Left traces showing superimposed evoked subthreshold EPSP signals from the spine head and its parent dendrite calibrated in terms of membrane potential. Right traces represent bAP signals corrected for recording sensitivity difference. (f–j) Two-photon uncaging of glutamate. Same information as shown in (a–e). Red dot in h: position and approximate size of uncaging light spot. The evoked subthreshold EPSP and bAP recordings are average of 8 and 4 trials, respectively. (Adapted from Popovic et al. (2015b), Figure 5a–j, https://doi.org/10.1038/ncomms9436, under CC BY license, originally published by Springer Nature)
Also, the combined voltage imaging and glutamate uncaging using patterned illumination based on computer-generated holography allowed one-photon uncaging of glutamate on multiple spines to be carried out in parallel with voltage imaging from the parent dendrite and neighboring spines. In this procedure, somatosensory cortex in brain slices was used to perform electrical and optical recordings from cortical layer V pyramidal neurons filled with a voltage-sensitive dye. Subthreshold uEPSP and bAP signals were recorded at a frame rate of 2 or 5 kHz. Optical signals of a subthreshold uEPSP onto one spine and bAP evoked by depolarizing current pulse were able to be recorded from spine head and parent dendrite and also in two spines simultaneously, while somatic patch-clamp recordings of the subthreshold responses related to glutamate uncaging onto one and two spines were obtained (Tanese et al. 2017).
These approaches improved the accuracy required for spatiotemporal resolution to simultaneously record and reconstruct individual uEPSP and AP signals from the spine head and the parent dendrite at near physiological temperature (Popovic et al. 2015a, b; Tanese et al. 2017; Weng et al. 2023).
Recently, direct electrical recording from dendritic spines in culture and brain slices was achieved by using quantum-dot-coated nanopipettes (Jayant et al. 2017). The simultaneous electrical recordings on the soma and the spine of pyramidal cells from either primary hippocampal neuronal culture or neocortical slice preparations (Fig. 3.3) revealed that bAP invaded spines and was similar to a somatic AP. Also, the recorded spine bAP preceded the initial rise of EPSP measured in the soma (Fig. 3.4). Spontaneous EPSPs, with large amplitude (25–30 mV), fast rise time (1–2 ms), and half-width of approximately 5 ms were recorded as the nanopipette entered the spine head (Fig. 3.5) (Jayant et al. 2017). This possibility of direct measurement of the Vm at the spine level opens a way to explore the dynamics of local ionic conductances during synaptic integration and plasticity.
Nanopipette dual somato-spine recordings. (a) Schematic describing the dual patch-nanopipette recording and associated signal transmission mechanisms to and from the spine. Action potentials (APs) originate in the axon hillock and propagate both forward (toward the postsynaptic cell) and backward into dendrites and spines. Backpropagating action potentials (bAPs) invade spines (inset) across the spine neck. Neurotransmitters released from the presynaptic axonal terminals activate receptors located on the spine head and cause spine head EPSPs (inset). Nanopipettes are labeled with QDs (CdSe/CdS/ZnS quantum dots) through adsorption (top right). (b) Schematic representation of dual somato-spine recordings in cultures and (c) slices. (d) Nanopipette “navigation” approach in slices toward a chosen target. (Adapted from Jayant et al. (2017), Figure 1a and Supplementary Figure 2 https://doi.org/10.1038/nnano.2016.268, under CCC RightsLink® license 5516540783944)
Nanopipette dual somato-spine recordings. (a, b) Dual somato-spine recordings in cultures (a, scale bar, 1.5 μm, panels (i)–(iii) and callout) and slices (b, scale bar, 20 μm left, 3 μm, inset middle). Dendritic and spine nanopipette recordings were restricted to within 100 μm from the soma to avoid space clamping and backpropagating action potential (bAP) attenuation. (c, f) Spine and presynaptic axon terminal recordings in culture (c, d) and slices (e, f). (c) bAPs invasion into spines. Somatic AP (bottom) and bAPs in the spine head (top) reveal that the nanopipette is capable of registering bAPs without the need for averaging. (d) Nanopipette recordings in the presynaptic axon terminal and postsynaptic soma. Note the characteristic 2–4 ms time delay Fig. 3.4 (continued) between the presynaptic AP peak and onset of the EPSP (inset: raw data, scale bar = 2 mV, 500 ms). (e) bAPs registration in spines probed in slices. (f) Putative presynaptic terminal recordings in slice preparations. Note the very short delay between the putative presynaptic AP peak and the EPSP rise measured at the soma. (g) Raw bAPs and somatic APs. Note the close fit in rise time and half-width indicating the bAPs invade the spine with nearly no loss in temporal structure. (Adapted from Jayant et al. (2017), Figure 5b–e, g–i https://doi.org/10.1038/nnano.2016.268, under CCC RightsLink® license 5516540783944)
Nanopipette synaptic potentials recorded in spine head. EPSPs in spines. Spontaneous break-in into the spine head (top) in cultures and corresponding somatic recordings (bottom). Note the large and fast-rising spontaneous EPSPs registered in the spine that are completely filtered from the soma. The gray-shaded region is zoomed in to show the typical rise and decay kinetics of the potential transient. (Adapted from Jayant et al. (2017), Figure 5j, https://doi.org/10.1038/nnano.2016.268, under CCC RightsLink® license 5516540783944)
Furthermore, the changes in Vm of spines during EPSP are associated with variations of intracellular concentration of Na+ and Ca2+ caused by changes in channels and receptor conductances. While the influx of Na+ is important for driving the synaptic potential and bAP, the Ca2+ influx is essential for synaptic plasticity processing. Compared to Na+ channels, Ca2+ channels are well described in terms of type, distribution, and function in dendritic spines (Bloodgood and Sabatini 2005).
Imaging studies of AP and synaptically evoked Ca2+ transients have revealed multiple classes of voltage-sensitive Ca2+ channels that contribute to Ca2+ influx in dendritic spines (Bloodgood and Sabatini 2007a). Ca2+ ion influx has been studied in some detail in a series of experiments, using two-photon Ca2+ imaging and two-photon uncaging of glutamate. For example, through a combination of conventional electrophysiological techniques with two-photon excitation laser scanning microscopy, Ca2+ imaging, and optogenetic, it was possible to investigate local Ca2+ transients in dendrites and spines (Nakamura et al. 1999; Camiré and Topolnik 2018). The bAPs in dendrites opened voltage-gated Ca2+ channels (Jaffe et al. 1992; Popovic et al. 2012) with consequent Ca2+ transients in dendrites and spines (Jaffe et al. 1992; Popovic et al. 2012). Moreover, using combined electrophysiology, two-photon Ca2+ imaging, and two-photon glutamate uncaging in layer II pyramidal cells from the rat medial entorhinal cortex in acute brain slices, it was possible to detect dendritic spine bAP-evoked Ca2+ transients by R- and T-type voltage-gated Ca2+ channel activation (Theis et al. 2018). These bAP-evoked Ca2+ transients did not affect the EPSP measured at the soma but induced a downscaling of NMDA receptor currents (Theis et al. 2018). Na+ conductance on spines of mice neocortical pyramidal neurons has also been detected in experiments using two-photon uncaged glutamate associated with application of tetrodotoxin (TTX), a voltage-gated Na+ channel blocker that significantly decreased the spine potentials (Araya et al. 2007). Interestingly, the Na+ K+-ATPase α3 isoform was found to be expressed in spines with excitatory synapses (Blom et al. 2011), which suggests the active maintenance of ionic gradients by the dendritic spine at the postsynaptic cell.
Recently, the time courses of synaptically induced changes in Ca2+ and Na+ concentrations in the dendritic spines of hippocampal CA1 pyramidal cells were studied in brain slices of mice. Na+ and Ca2+ transients in a spine were simultaneously measured in response to a minimal electrical stimulation, in order to generate normal neurotransmitter release (smaller than uncaging glutamate), combined with low-affinity linear Na+ and Ca2+ indicators and single photon laser fluorescence stimulation with a high-speed, sensitive CCD camera (Miyazaki and Ross 2022). The intracellular Na+ concentration in the spine increased following a single synaptic stimulation. The kinetics of this synaptically activated increment of intracellular Na+ concentration in the spines of hippocampal CA1 pyramidal cells of mice was consistent with previous measurements in rat pyramidal neuron spines (Miyazaki and Ross 2017, 2022). The rise time of the synaptically activated increment of intracellular Na+ concentration was around 7.0 ms, about 2 ms slower than the evoked AP in the axon initial segment (Filipis and Canepari 2021; Miyazaki and Ross 2022). That is, a Na+ current contributes to the fast EPSP in the spine (Palmer and Stuart 2009; Popovic et al. 2015b), that is slower than the duration of the Na+ current. Almost all synaptically activated Na+ currents were mediated by AMPA receptors with little Na+ entry through either voltage-gated Na+ channels or NMDA receptors (Grunditz et al. 2008; Miyazaki and Ross 2017, 2022). In fact, using a highly sensitive electron microscopic immunogold technique, no evidence was found for Na+ channels in the dendritic spines of hippocampal CA1 pyramidal cells (Lorincz and Nusser 2010).
Similar to Na+, the intracellular Ca2+ concentration in a spine, also, increased following a single synaptic stimulation. The synaptically activated Ca2+ transients exhibited fast rise time and half-decay time kinetics (about 7.7 ms and 26 ms, respectively) (Kovalchuk et al. 2000; Sabatini et al. 2002; Bloodgood and Sabatini 2005; Enoki et al. 2009; Miyazaki and Ross 2022). Resembling the Na+ transient, most of the Ca2+ entry was driven by the AMPA receptor-mediated EPSP in the dendritic spine (Miyazaki and Ross 2022). Therefore, processes that modulate AMPA receptor activation, such as desensitization or potentiation, might have a direct effect on Ca2+ signaling and synaptic potential in dendritic spines. In contrast, two-photon uncaging experiments (Hestrin et al. 1990; Spruston et al. 1995) and uncaging evoked signals by repetitive stimulation (Polsky et al. 2009) have demonstrated much larger Ca2+ entry into spines through NMDA receptors. However, the larger Ca2+ current through NMDA receptors may be attributed to the intense two-photon uncaging activation compared with synaptically evoked responses by minimal electrical stimulation. In addition, bAP-induced spine depolarization opens NMDA receptors by lifting the Mg2+ block (Schiller et al. 1998; Yuste et al. 1999; Nevian and Sakmann 2004), further enhancing the spine Vm. This increase in spine Vm may be elicited either by additional activation of voltage-gated Na+ channels during the coincidence of bAP and incoming excitatory synaptic input (Stuart and Häusser 2001) or by previous depolarization caused by AMPA receptors in the spine head (Grunditz et al. 2008). For example, using whole-cell recording in acute brain slices of mice coupled with two-photon Ca2+ imaging, Ca2+ transients in spines were driven mostly by the activity of NMDA receptors in GABAergic intercalated neurons, located between the basolateral (BLA, from which they receive glutamatergic inputs) and the central amygdala (CeA) (Royer et al. 1999; Strobel et al. 2015). Both bAP and evoked spine Ca2+ transients in this cell type attenuated rapidly with distance, a finding caused by voltage-gated K+ channels present in the spine head (probably containing Kv4 channels) (Kaufmann et al. 2013; Strobel et al. 2017). Other techniques showed different results (Bloodgood and Sabatini 2005; Araya et al. 2007; Bywalez et al. 2015), which suggests that neurons and spines may exhibit distinct characteristics depending on the brain area studied, its connectivity and function, and the spine morphology (Bloodgood and Sabatini 2007b; Araya et al. 2007; Bywalez et al. 2015). Synaptically activated potentials can also involve distinct receptors and channel compositions that contribute to the variation in Ca2+ transients.
To date, there is no direct information on dendritic spine ionic conductance obtained from direct electrical activity measurement by whole-cell or cell-attached recordings of dendritic spines in slice preparation. However, electrical recordings of isolated and in situ dendritic spines in cultured hippocampal neurons of adult mice were performed using patch-clamping, detecting backpropagating dendritic electrical oscillations and NMDA-mediated electrical activity (Priel et al. 2022). First, dendritic spines were isolated from the dissected hippocampus and then reconstituted using a lipid bilayer membrane system. Ca2+-dependent spontaneous current oscillations, with peak frequency at 1–2 Hz, and NMDA receptor single-channel currents were recorded. NMDA and Ca2+-dependent oscillations at diverse frequencies and amplitudes were similarly recorded in the isolated dendritic spines using patch-clamp technique in both voltage and current-clamping configuration. Voltage-dependent conductances, consistent with both Na+ and K+ currents, and AP were also recorded in the isolated dendritic spine. The cell-attached patch-clamp recordings of in situ dendritic spines identified NMDA-dependent EPSP-like intrinsic oscillations at diverse frequencies and amplitudes. Double patch-clamp recordings of a dendritic spine and its parent dendrite detected a NMDA-dependent activity with diverse frequencies and waveforms coupled with and propagated to the parent dendrite. While synapses between dissociated cells do not necessarily exhibit the same properties as those in the intact brain tissue, they are more easily accessible for imaging. Valuable information can also be obtained about the biophysical properties of the dendritic spines and their interaction with the adjacent dendritic segments and the soma (Priel et al. 2022).
Although most spines receive an excitatory input, GABA-mediated inhibitory postsynaptic potentials (IPSPs) participate in the control of neuronal excitability. Experiments using cell type-specific optical stimulation in combination with two-photon Ca2+ imaging showed that the dendritic spines’ heads of cortical pyramidal cells are targeted by somatostatin-expressing interneurons, compartmentalize GABAergic inhibition, limit both bAP and synaptically evoked Ca2+ entry, and regulate NMDAR-dependent synaptic integration. These inhibitory synapses on spines can participate in the control of electrical and biochemical signaling along dendritic segments, including Ca2+ signaling and synaptic plasticity (Chiu et al. 2013).
The conductance of different channels and receptors and the specific changes in the Vm of dendritic spines are now being revealed by the use of combined new technical approaches, evidencing spatiotemporal patterns of induced or spontaneous depolarizations of dendrites and spines during synaptic integration and plasticity.
3.2 Dendritic Spines as Dynamic Compartments
Since their discovery by Ramón y Cajal, it has been hypothesized that dendritic spines may be involved in the compartmentalization of synaptic signals (Yuste 2015). Such a compartmentalized microenvironment could modulate the functional properties of different synapses (Calabrese et al. 2006; Araya et al. 2014; Tønnesen and Nägerl 2016; Vallés and Barrantes 2021) affecting synaptic strength, cell information processing, circuits’ organization, and behavior (Yuste and Denk 1995; Sabatini et al. 2002; Noguchi et al. 2005; Araya 2014).
3.2.1 Biochemical Compartmentalization
Dendritic spines, with a wide diversity of sizes and shapes, have organized membrane-bound enzymes, lipids, and proteins into delimited regions or subcompartments of varying extension and composition (Honigmann and Pralle 2016). Compartmentalization is generated by the spine neck, which relatively isolates the spine head from the parent dendrite (Yuste and Denk 1995; Nevian and Sakmann 2004; Honigmann and Pralle 2016).
A series of experiments, including those using two-photon excitation laser scanning microscopy in combination with Ca2+ imaging, has revealed that spines function as isolated biochemical compartments, not only in excitatory synapses, but also in GABAergic synapses that directly target spine heads (Chen et al. 2011; Chiu et al. 2013; Camiré and Topolnik 2018).
Spines act as biochemical and highly differentiated subcellular compartments. They have the ability to dynamically control the membrane area available for specific domains and clustering of neurotransmitter receptors as well as the diffusion of signaling and regulatory molecules, such as Ca2+, kinases, second messengers, enzymes, scaffolding proteins, and cytoskeletal elements within a very small volume (See Fig. 1.13, Chap. 1 of this book). Thus, biochemical compartmentalization restricts and regulates biochemical processes in individual synapses to smaller regions, modulating synaptic output, integration, and plasticity (Yuste 2011; Tønnesen and Nägerl 2016). Various neurotransmitter receptors, including the cholinergic nicotinic receptor, for example, have been located in the raft-type lipid domains of the spine membrane (Brusés et al. 2001; Allen et al. 2007; Egawa et al. 2016). Also, spine membrane domains show temporal modifications that affect the timing of biochemical cascades following synaptic activation. These changes induce dynamic alterations in the spine head and neck width associated with the degree of the synaptic signals compartmentalization, thereby biochemically isolating synaptic inputs and enabling input-specific plasticity (Magee and Johnston 1997; Lee et al. 2009; Tønnesen et al. 2014). In addition, the dynamic changes of the spine neck length have been associated with the bidirectional modulation of the synaptic and the parent dendrite voltage, acting as an electrical compartment and modifying synaptic integration (Vanderklish and Edelman 2002; Araya et al. 2014; Cornejo et al. 2022). This spatiotemporal organization involving dendritic spines is crucial to coordinate the synaptic function in a variety of neurons throughout the CNS.
3.2.2 Electrical Compartmentalization
Electrical compartmentalization relates to the ability of dendritic spines to process synaptic inputs, modulating the amplitude, kinetics, and integration of synaptic potentials into the spine head (Tsay and Yuste 2004). Spine heads exhibit voltage-gated ion channels and neurotransmitter receptors (Kim et al. 2007; Araya et al. 2007; Chung et al. 2009), which modulate the voltage inputs. In addition, the geometry of the spine neck influences voltage propagation into the adjacent dendritic shaft, acting as a high resistance pathway. The process of compartmentalization is influenced by membrane passive electrotonic and active properties (Koch and Segev 2000; Sjöström et al. 2008). Then, the geometry and passive properties of dendritic spines, such as axial resistance, membrane capacitance, and diameter, are crucial factors to understand how a spine modulates synaptic potentials and alters synaptic strength (Spruston et al. 1994), input integration (Yuste 2010), and, consequently, neural circuits (Yuste 2010; Kwon et al. 2017). As opposed to the well-established biochemical compartments, the electrical compartmentalization of dendritic spines is a matter of debate.
The spine electrical compartmentalization has been investigated with various experimental techniques in vitro and in vivo. For example, the strategy of combining a high-sensitivity organic voltage-sensitivity dye with two-photon uncaging of glutamate (Popovic et al. 2015b) detected subthreshold uEPSP in dendritic spines of layer V pyramidal neurons (Popovic et al. 2015a, b). The subthreshold uEPSP presented no significant attenuation along the spine neck, which exhibited low electrical resistance relative to the input impedance of the parent dendrite (mean value of 27 ± 6 MΩ) (Popovic et al. 2015b). These data suggested that spine synapses were not electrically isolated from the parent dendrites and may exhibit the same electrical behavior as those contacts made directly on dendrites (Popovic et al. 2014, 2015b). The low resistance of the spine neck relative to the impedance of the parent dendrite is similar to previous theoretical predictions and diffusional resistance measurements (Wilson 1984; Koch and Zador 1993; Svoboda et al. 1996; Bloodgood and Sabatini 2005; Tønnesen et al. 2014; Takasaki and Sabatini 2014).
Experiments using simultaneous Na+ and Ca2+ imaging with single-spine resolution in pyramidal neurons in rat hippocampal slices detected a rapid diffusion of Na+ out of the spine head through the spine neck to the parent dendrite, also suggesting that the neck offers low resistance for ionic diffusion (Miyazaki and Ross 2017). Moreover, the amplitude of EPSP and Ca2+ transients evoked by 2-photon glutamate uncaging also showed no correlation with the neck geometry, supporting the lack of a functional impact of spine shape on electrical compartmentalization (Takasaki and Sabatini 2014; Bywalez et al. 2015). With that assumption in mind, constricted spine neck diameter would rather serve to isolate metabolic events by reducing diffusion of activated molecules to neighboring synapses, without significantly influencing the transfer of synaptic charge to the postsynaptic dendrite (Harris and Stevens 1988, 1989).
Alternatively, other experiments have demonstrated that dendritic spines may constitute fundamental electric compartments (Tsay and Yuste 2004; Harnett et al. 2012; Yuste 2013; Acker et al. 2016; Jayant et al. 2017; Kwon et al. 2017; Strobel et al. 2017; Cornejo et al. 2022). For example, experiments using combined dendritic patch electrophysiology, Ca2+ imaging, and glutamate uncaging in spines at apical dendrites of rat hippocampal CA1 pyramidal neurons demonstrated that, when spine neck resistance was high (mean value of 514 ± 44 MΩ) (Harnett et al. 2012), passive amplification of the spine EPSP up to 50-fold would occur compared to an unitary dendritic EPSP. This amplification was very much dependent on the spine and neck structure in different neurons (Harnett et al. 2012).
Another study used two-photon voltage-sensitive organic dye recording with two-photon glutamate uncaging to measure the amplitude and duration of an uEPSP in single spines from the basal dendrites of layer V pyramidal neurons in acute brain slices of mice (Acker et al. 2016). Data from this study showed high spine neck resistance with a mean value of 179 ± 25 MΩ (ranging from 23 to 420 MΩ), suggesting the existence of a substantial electrical compartmentalization in spines.
Study using a genetically encoded voltage indicator and two-photon glutamate uncaging to identify electrical properties of dendritic spines in cultured hippocampal neurons showed that the invading bAP was unchanged. However, the spine neck resistance had values ranging from 15 to 332 MΩ (mean value: 101 ± 95 MΩ) reducing the synaptic and intrinsic voltage-gated currents that reach the adjacent dendritic shaft. Thus, the spines could compartmentalize voltage and attenuate the synaptic inputs by about twofold as they drive through the spine neck to the parent dendrite. Spines with longer neck attenuated uEPSP by fourfold as they propagate to the parent dendrites and exhibited an estimated neck resistance of 332 MΩ (Kwon et al. 2017).
Intracellular recordings of dendritic spines with long and narrow necks from neurons in brain slices using quantum-dot-coated glass nanopipettes revealed neck resistances ranging from 250 to 536 MΩ (mean: 425 ± 102 MΩ) (Jayant et al. 2017). The EPSPs recorded were large in the spine head (mean 26 mV), but strongly attenuated at the soma (0.5–1 mV), and the estimated neck resistance was large enough to generate significant local voltage compartmentalization in spines (Jayant et al. 2017) (Fig. 3.6).
Nanopipette recordings in spines reveal electrical compartmentalization. (a) Electrical equivalent circuit of the passive dendritic spine. Erest, leak reversal potential; Rm, spine head passive membrane resistance; Cm, spine head passive membrane capacitance; Esyn, synaptic reversal potential; gsyn, synaptic conductance; Rpore, pore resistance; Rseal, seal resistance; Rneck, neck resistance; Re, pipette resistance; Ce, pipette capacitance; Rdendrite, dendritic resistance; Rm(d), dendrite passive membrane resistance; Cm(d), dendrite passive membrane capacitance; Ed, dendrite reversal potential. (b, c) Typical rise and decay kinetics for spontaneous PSPs recorded in the spine head. Notice the millisecond resolution rise and decay indicative of an extremely small RC time constant. The data shown in (b) and (c) are not deconvolved. (d) Raw EPSPs in spines from slices (marked stars) are larger than somatic EPSPs (∼0.5 mV flickers). Note the sudden registration of EPSPs in the soma (gray bar, bottom) that signify concomitant inputs from other spines. (e) Deconvolved Rneck (left), EPSP amplitudes (middle), and RMPs (right) in the spine head. The range of deconvolved EPSP amplitudes is in accordance with the spontaneous EPSP. The range for Rneck indicates that EPSPs that invade a low input resistance dendrite will be heavily attenuated. (Adapted from Jayant et al. (2017), (a–c) Supplementary Figure 12 and (d, e) Figure 5k, l, https://doi.org/10.1038/nnano.2016.268, under CCC RightsLink® license 5516540783944)
Two-photon Ca2+ imaging coupled with whole-cell recording in amygdaloid GABAergic intercalated neurons in acute brain slices showed that Ca2+ enters the spine head mediated by the activity of NMDA receptors and with little spread of the Ca2+ signal. That finding also suggested that a dendritic spine head can act as an isolated electrical compartment (Strobel et al. 2017). High spine neck resistances, associated with longer and thinner spine necks, would be expected to increase the voltage in the spine head during a given synaptic input, reducing the driving force for synaptic current flow (Koch and Zador 1993).
Interestingly, the size of the spine head has also been correlated with the area of the postsynaptic density (PSD), a characteristic specialization of the postsynaptic cell membrane (Harris and Stevens 1989; Trommald and Hulleberg 1997; Arellano 2007), and with the amplitude of the generated EPSP (Matsuzaki et al. 2001; Noguchi et al. 2011). Varied EPSPs’ amplitudes have been observed in different experiments, which likely related to a spectrum of spine neck resistances correlated with distinct neck lengths, even in the same dendritic shaft and neuron type (Miyazaki and Ross 2022). Not only does EPSP modulation by spines have important repercussions for synaptic plasticity but also NMDA receptor activation and intra-spine Ca2+ levels activate a variety of signaling pathways that can modify actin filament assembly and contributing to activity-dependent alterations in spine morphology (Tada and Sheng 2006; Araya 2014; Yasuda 2017).
Finally, using in vivo two-photon microscopy and a genetically encoded voltage indicator, it was possible to measure the Vm of basal dendrites and their spines while performing simultaneous somatic whole-cell recordings in layers II/III pyramidal neurons of the somatosensory cortex of mice. Recordings were obtained during spontaneous activity and sensory stimulation (Cornejo et al. 2022) (Fig. 3.7). The estimated spine neck resistance was 226.6 ± 128.8 MΩ (mean ± SD), ranging from approximately 0 to 530.8 MΩ, similar to that seen in previous publications (Harnett et al. 2012; Tønnesen et al. 2014; Jayant et al. 2017). Spines and dendrites were depolarized together during AP, the dendritic potentials propagated into spines, and there was no attenuation of dendritic potentials or AP but synaptic potentials were significantly reduced. During subthreshold and resting potentials, spines could be activated independently, even in the absence of dendritic or somatic activity.
Two-photon optogenetics and voltage imaging showing the voltage compartmentalization in dendritic spines in vivo. (a) Experimental design. (b) Construct and representative fluorescence changes in soma (light green, raw fluorescence; black, 10-Hz low-pass filtered) during 500-ms stimulation trials (red, 100 mW power). (c) Representative soma (top) and peak fluorescence response (bottom) during stimulation trials (×10, 500 ms, 100 mW). The dotted circle shows the stimulation area (scale bar, 10 μm). (d) Representative in vivo voltage-clamp recordings during optogenetic stimulation of proximal dendrites (100 mW, 100 ms) are shown on the left. Peak currents are shown on the right; −22.7 ± 11.3 pA (mean ± SD), 10 trials (n = 7 cells and 4 animals). (e) Representative peak fluorescence changes during optogenetic activation of dendritic shafts are shown on the left (stimulation ROIs are indicated by white dotted circles; 10 trials, 100 ms, 100 mW; color bar same as in (c); scale bar, 5 μm). Peak fluorescence changes in stimulated dendritic shaft (dendrite stim), adjacent dendritic spine (spine), and unstimulated dendritic shaft (dendrite no stim) are shown on the right (n = 34 dendrites and 9 animals). (f) Same as (e) during optogenetic activation of spines (n = 35 spines and 12 animals; scale bar, 5 μm). ****p < 0.0001. (g) Simplified electrical model. (h) Resistance ratio (Rn to Rden) of spine-only events during spontaneous (Spon, n = 116), sensory stimuli (Sen, n = 181), and optogenetic spine stimulation (Opto, n = 35). (i) Values of spine neck resistance for stimulated spines; median = 213.7 MΩ (n = 35 spines). In (d), (h), and (i), boxes and whiskers represent median (line), 25th to 75th percentiles (box), range (whiskers), and mean as a “+.” *postASAP: postsynaptic accelerated sensor of APs. (Reproduced from Cornejo et al. (2022), Figure 4, https://doi.org/10.1126/science.abg0501, under CCC RightsLink® license 1338950-1)
These findings, including the spine-independent depolarization during spontaneous and evoked activity, contribute to our understanding of dendritic spine biophysics, showing that dendritic spines serve not only for biochemical but also for electrical compartmentalization of synaptic inputs essential for synaptic integration, information processing, and plasticity (London and Häusser 2005; Yuste 2011; Cornejo et al. 2022).
3.3 Spatiotemporal Dynamics of Dendritic Spines Related to Synaptic Plasticity
Dendritic spines are known to be the site of long-term synaptic plasticity (Matsuzaki et al. 2004; Lai and Ip 2013). In fact, the activity-dependent morphological changes in the head and/or neck of spines (Matsuzaki et al. 2004; Tønnesen et al. 2014; Araya et al. 2014) have been correlated with modifications in synaptic strength in cortical pyramidal neurons involving biochemical and electrical mechanisms (Araya et al. 2006; Araya 2014).
Studies using signal imaging techniques based on fluorescence resonance energy transfer (FRET) in combination with photostimulation of single synapses revealed the temporal sequence of biochemical signaling cascades and their spatial spreading from stimulated spines (Nishiyama and Yasuda 2015). Dendritic spine plasticity directly modifies the synaptic strength of neuronal networks through changes in protein synthesis, spine cytoskeleton structure, spine morphology, and density of receptors at the synaptic membrane (Nakahata and Yasuda 2018). These modifications guide rearrangements of connections between presynaptic and postsynaptic elements and consequently promote a shift of synaptic efficacy, either potentiating or depressing synaptic transmission. Over the long term, two processes involving various molecular mechanisms promote synaptic strength or weakness, respectively: long-term potentiation (LTP) and long-term depression (LTD) (Citri and Malenka 2008). Together, spine synaptic plasticity allows the establishment and reorganization of connectivity within neuronal circuits during normal brain development to adulthood and aging. It also may be involved in degenerative processes and in a large spectrum of brain disorders that involve deficits in information processing, such as schizophrenia, Alzheimer’s disease, autism spectrum disorder, and epilepsy (Penzes et al. 2011; Dickstein et al. 2013; Nishiyama 2019; Runge et al. 2020; Rossini et al. 2021; Zaccard et al. 2023).
Before describing the dynamics of receptors in the spines during LTP and LTD, it is important to consider a fundamental synaptic component that influences spine synaptic plasticity and the formation and stabilization of new dendritic spines, that is, the extracellular matrix.
The extracellular matrix, together with pre- and postsynaptic elements and glial processes, form the so-called tetrapartite synapse (Dityatev and Schachner 2003; Dityatev et al. 2006; Dityatev and Rusakov 2011; Song and Dityatev 2018). The fully developed extracellular matrix has the ability to stabilize neuronal connectivity, restraining morphological changes of dendritic spines and decreasing cortical plasticity in the adult brain. In fact, as the circuitry reaches its adult form, spine motility diminishes and connectivity stabilizes (Oray et al. 2004). A prominent component of the matrix, chondroitin sulfate proteoglycans (CSPGs), may play an important role in limiting cortical plasticity, restraining the structural remodeling of neurons, and inducing inhibition of synaptic transmission (Oray et al. 2004).
Degradation of CSPGs proved to affect ionic conductances, control receptors, and channel localization at the cell membrane (Gundelfinger et al. 2010; Dityatev et al. 2010), induce rapid structural changes of dendritic spines by increasing the size of the spine head, and enhance spine mobility thereby restoring plasticity (de Vivo et al. 2013). CSPGs modulate structural plasticity by preventing integrin activation and signaling at synaptic sites (Orlando et al. 2012).
Several other molecules of the extracellular matrix have been involved in the regulation of synaptic plasticity. For example, hyaluronan, the backbone of extracellular matrix, was found to regulate hippocampal synaptic plasticity by modulating postsynaptic L-type Ca2+ channels (Kochlamazashvili et al. 2010). Another important glycoprotein, Reelin, is secreted into the extracellular matrix by neurons, such as GABAergic cells. Reelin regulates the composition of NMDA receptors, the increment of AMPA responses by increasing the number of AMPA receptors on the postsynaptic membrane, and robustly enhances LTP (Qiu et al. 2006; Campo et al. 2009; Hellwig et al. 2011). Reelin strongly induces spine remodeling, regulating spine size and stability, and the number of synaptic contacts per spine (Beffert et al. 2006; Pujadas et al. 2010). Reelin interacts with integrins to regulate AMPA receptor internalization and cell membrane trafficking of NMDA receptor subunits (Dityatev et al. 2010; McGeachie et al. 2011), important for receptor dynamics during synaptic plasticity.
Furthermore, in mature neurons, integrins are involved in homeostatic synaptic scaling and interact with matrix metalloproteinases, promoting spine volume changes induced by long-term plasticity (Nagy et al. 2006; Wang et al. 2008). Cell adhesion molecules (CAMs) mediate extracellular matrix and PSD signaling that may influence the actin cytoskeleton network of dendritic spines, and consequently, spine shape (Huntley et al. 2002; Washbourne et al. 2004; Koleske 2013). Spine remodeling is, also, controlled by autocrine signaling of brain-derived neurotrophic factor (BDNF), crucial for LTP (Harward et al. 2016; Hedrick et al. 2016) and for proteolytic cleavage of extracellular matrix (Nakahata and Yasuda 2018). The increased intracellular Ca2+ concentration, mediated by NMDA receptors or by voltage-gated Ca2+ channels, induces lysosomal fusion with the plasma membrane, releasing the protease Cathepsin B to the extracellular matrix, which cleaves and inhibits the endogenous inhibitor of the matrix metalloprotease 9. The disinhibited matrix metalloprotease 9 cleaves the CAMs and the extracellular matrix, facilitating the structural remodeling of spines (Nakahata and Yasuda 2018). Thus, the extracellular matrix that fills perisynaptic spaces surrounding dendritic spines plays a crucial role in remodeling neural circuits, affecting long-term synaptic plasticity and receptor trafficking (Dityatev et al. 2010; Orlando et al. 2012).
3.3.1 Dynamics of Receptors in Spines During Long-Term Potentiation
The most studied form of spine structural plasticity is LTP (Nakahata and Yasuda 2018). The typical protocol for inducing LTP involves brief and high-frequency stimulation (for review, see Nicoll 2017), and paired pre- and postsynaptic stimulation, known as spike timing-dependent plasticity (STDP) (Markram et al. 1997; Debanne et al. 1998; Bi and Poo 1998; Sjöström et al. 2008; Feldman 2012). However, there are protocols applying lower-frequency stimulations to induce LTP mediated by metabotropic glutamate receptor (mGluR) and protein kinase A activation (Lanté et al. 2006). Importantly, LTP is input-specific at the level of individual synapses, that is, only stimulated spines may trigger LTP-induced synaptic modifications (Magee and Johnston 1997; Lee et al. 2009; Tønnesen et al. 2014). Input-specific LTP induction can be performed by two-photon glutamate uncaging. For example, using an uncaged glutamate-LTP protocol to study the visual cortex of anesthetized rats in vivo, it was possible to demonstrate the induction of individual spine enlargements (Noguchi et al. 2019) reinforcing the LTP-synaptic specificity (Harvey and Svoboda 2007). Although LTP can also be mediated by presynaptic mechanisms, here we focus on the NMDA receptor-dependent form of LTP (Luscher and Malenka 2012).
Multiple molecular and cellular mechanisms contribute to induction, expression, and maintenance of NMDA-dependent form of LTP. Ca2+ represents a key factor for long-term synaptic plasticity (Mateos-Aparicio and Rodríguez-Moreno 2020). During postsynaptic depolarization, Ca2+ entry through activated NMDA receptors results in a local increase in dendritic excitability and an increase in synaptic strength (Luscher and Malenka 2012). Activation of NMDA receptors with subsequent increase of Ca2+ influx is a well-known mechanism involved in the early phase of LTP, which lasts approximately 60 min (Becker and Tetzlaff 2021). Experimental approaches using chemical and selective activation of NMDA receptors were able to induce synaptic potentiation, that was blocked by a competitive antagonist, D-2-amino-5-phosphonopentanoate (AP5) (Lu et al. 2001). Following NMDA receptors agonist washout of cultured hippocampal neurons, synaptic potentiation was still enhanced, revealing the contribution of AMPA receptors in the maintenance of postsynaptic excitatory response potentiation (Lu et al. 2001). Additionally, NMDA-dependent LTP could be blocked by MK-801, a competitive NMDAR antagonist, and BAPTA, a Ca2+ chelator, highly supporting the involvement of NMDA and AMPA receptor activation and Ca2+ influx in LTP expression (Lu et al. 2001) (Fig. 3.8).
LTP induction. (a) Up: The illustration demonstrates that brief stimulation of postsynaptic NMDA receptors with glycine may induce LTP. Bottom: Representative traces of mEPSC. Glycine-induced LTD increases mEPSC amplitude and frequency measured by whole-cell patch clamp in hippocampal neurons of primary dissociated cultures. (b) Averaged mEPSCs demonstrating before, during, and following the application of glycine. (c) Pretreatment with AP5, MK-801, and BAPTA blocked LTP, decreasing both mEPSC amplitude and frequency. (Adapted from Lu et al. (2001), https://doi.org/10.1016/S0896-6273(01)00194-5, (a) Figure 1A,D,F,G and (b, c) Figure 2E,F, under CCC RightsLink® license 5516571126317)
LTP induced by STDP at CA3-CA1 hippocampal synapses and some synapses of neocortical layers II/III pyramidal cells are dependent on NMDA receptors (Nishiyama et al. 2000; Froemke et al. 2005). NMDA receptor-mediated Ca2+ accumulation in a spine is also associated with actin polymerization-dependent shrinkage of the activated spine neck and an increase in synaptic strength, as demonstrated during the timing-dependent LTP triggered by two-photon glutamate uncaging at the presynaptic site paired with postsynaptic spikes in layer V pyramidal neurons from juvenile mice (Tazerart et al. 2020) (Fig. 3.9a, c).
Induction of t-LTP and t-LTD in single dendritic spines. (a) Experimental protocol for t-LTP induction in single dendritic spines (sp). (b) Representative experiment where a spine was activated with t-LTP pre- and post-pairing protocol of +13 ms. Traces correspond to average of ten uncaging EPSPs (uEPSPs) recorded in the soma and generated by 2P uncaging before (control, black trace) and after t-LTP induction (red trace) over the indicated spine (red dot). (c) Experimental protocol for t-LTD induction in single dendritic spines. (d) Representative experiment where a spine was activated with t-LTD post–pre pairing of −15 ms. Traces correspond to average of 10 uEPSPs recorded at the soma and generated by 2P uncaging before (control, black trace) and after Fig. 3.9 (continued) t-LTD induction (red trace) over indicated spine (red dot). (e) Biochemical signaling pathways of the STDP induced by NMDA receptors. N and A, NMDA, and AMPA receptors, respectively. (Figure and legend adapted from (a–d) Tazerart et al. (2020), https://doi.org/10.1038/s41467-020-17861-7, Figures 1a,b and 2a,b, respectively, under CC BY license, originally published by Springer Nature; (e) Feldman (2012), https://doi.org/10.1016/j.neuron.2012.08.001, Figure 4A, under CCC RightsLink® license 5516580280377)
Furthermore, during synaptic potentiation, increased Ca2+ influx leads to activation of biochemical cascades through Ca2+-calmodulin-dependent protein kinase II (CaMKII), followed by phosphorylation of AMPA receptors and insertion of new receptors in the postsynaptic membrane sites. CaMKII antagonists block the synaptic potentiation, preventing exocytosis of new receptors at the postsynaptic membrane (Matsuzaki et al. 2004; Lee et al. 2009; Makino and Malinow 2009; Lisman et al. 2012; Jaafari et al. 2013; Penn et al. 2017). In vivo studies also revealed that CaMKII is crucial for LTP, learning, memory, and long-lasting spine enlargements (Miller et al. 2002; Matsuzaki et al. 2004; Buard et al. 2010). Indeed, the enlargement of dendritic spines associated with increase in AMPA receptor-mediated currents (Matsuzaki et al. 2004) and the expression of new AMPA receptors in the spine membrane at the stimulated synapse (Tanaka and Hirano 2012; Vitureira and Goda 2013) contribute to the enhancement of synaptic transmission during LTP (Park 2018) and are associated with larger PSDs (Chater and Goda 2014). Those changes were dependent on NMDA receptors, Ca2+ entry, CaMKII, and actin polymerization (Matsuzaki et al. 2004; Lee et al. 2009; Makino and Malinow 2009; Lisman et al. 2012; Jaafari et al. 2013; Penn et al. 2017). Also, the enlargement of individual spines precedes the increase in AMPA receptors at the postsynaptic site at spines (Chater and Goda 2014).
A protocol of glycine-induced LTP in dissociated cortical and hippocampal neuronal cultures also showed an increase of AMPA receptors expression in the spine membrane by exocytosis from intracellular pools. It begins within a few minutes after the LTP-inducing stimulation and is associated with a long-lasting potentiation of AMPA receptor-mediated excitatory postsynaptic current (EPSC) (Lu et al. 2001; Jaafari et al. 2013; Vitureira and Goda 2013; Henley and Wilkinson 2016). These new AMPA receptors permeable to Ca2+ (CP-AMPA receptors) are GluA2-lacking receptors and most of them contain the GluA1 subunit (Lu et al. 2001; Clem and Barth 2006; Jaafari et al. 2013), whose reversible phosphorylation plays an important role in long-term plasticity (Lee et al. 2010). The reduction of AMPA receptors diffusion in the spine membrane directly impairs hippocampal-dependent fear learning (Penn et al. 2017). There is strong evidence that synaptic CP-AMPA receptors have a crucial role in vivo and in vitro, tuning neuronal excitability to induce plasticity. However, how and to what extent CP-AMPA receptors are incorporated during LTP remains an area of research (Henley and Wilkinson 2016) (Fig. 3.10a, b).
AMPA receptors trafficking in synaptic plasticity. (a) Under basal conditions, synaptic AMPA receptors (AMPAR) are predominantly calcium impermeable and are anchored to postsynaptic density protein 95 (PSD95) and the actin cytoskeleton. They undergo both lateral diffusion in the membrane and rapid constitutive recycling. Furthermore, under certain conditions, Ca2+-permeable AMPARs (CP-AMPARs) can be observed extrasynaptically. (b) During long-term potentiation (LTP) induction, activation of protein kinase A (PKA), which is anchored at synapses by the scaffold protein AKAP150, leads to phosphorylation (indicated by the P) of GluA1 at S845 in both spines and dendrites, promoting its surface expression and leading to the appearance of synaptic CP-AMPAR. In addition, enhanced binding of protein interacting with C kinase 1 (PICK1) to GluA2-containing Ca2+-impermeable AMPARs (CI-AMPAR) in intracellular vesicles restricts their recycling to the cell surface, thus further enhancing the ratio of CP-AMPAR to CI-AMPAR. (c) During activity deprivation to induce synaptic homeostatic scaling, calcineurin (CaN) activity is reduced, favoring PKA-mediated phosphorylation of GluA1 subunits at S845, and promoting surface expression of GluA1-containing AMPAR and their synaptic incorporation. Furthermore, induction of homeostatic scaling via NMDA receptor (NMDAR) blockade promotes the local translation of GluA1 in dendrites, and an increase in surface expression of GluA1-containing CP-AMPAR. By contrast, (d) long-term depression (LTD) is associated with dephosphorylation of GluA1 at S845, reducing the extrasynaptic pool of GluA1-containing CP-AMPARs, which then undergo lysosomal degradation. (Reproduced from Henley and Wilkinson (2016), https://doi.org/10.1038/nrn.2016.37, Figure 4, under CCC RightsLink® license 5516911470380)
Besides AMPA receptors, NMDA receptors also exhibit gating and trafficking during LTP. This includes the rapid delivery of functional receptors and an increase in receptor clusters on the membrane of dendrites and dendritic spines thereby modulating neuronal excitability (Lan et al. 2001; Grosshans et al. 2002; Lau and Zukin 2007; Lau et al. 2010). The modifications in NMDA receptors are induced by protein kinase C (PKC) and tyrosine kinase Src (Lan et al. 2001; Grosshans et al. 2002; Lau and Zukin 2007; Lau et al. 2010) (Fig. 3.11a). Therefore, both AMPA and NMDA receptors contribute to synaptic potentiation. Increase of both components of EPSP (AMPA and NMDA) with a prominent potentiation of AMPA over the NMDA was reported during early and late LTP (Dozmorov et al. 2006), as well as, for the delayed NMDA-dependent LTP (Watt et al. 2004) (Fig. 3.12).
NMDA receptors trafficking in synaptic plasticity. (a) In the hippocampal CA1 of adult animals, high-frequency stimulation (HFS) and long-term potentiation (LTP) promote rapid insertion of NR2A-containing NMDA receptors and an increase in NMDA field excitatory postsynaptic potentials (fEPSPs) at CA1 synapses through a protein kinase C (PKC)- and Src-dependent pathway (blue box). HFS-induced activation of mGluR5 can also induce LTP with NMDA receptors trafficking (red box). (b) Long-term depression (LTD) of synaptic NMDA EPSCs (LTDNMDAR) occurs by retrieval of NMDA receptors from synaptic sites. Low-frequency stimulation (LFS) of Schaffer collaterals can induce metabotropic glutamate receptors (mGluR)-dependent LTDNMDAR or NMDA-dependent LTD. (mGluR)-dependent LTD is associated with enhanced internalization of NMDA receptors (red box). NMDA-dependent LTD promotes actin depolymerization and lateral diffusion of NMDA receptors away from the synapse site (blue boxes). LFS of connected pairs of CA3 pyramidal neurons induces LTDNMDAR by stimulating dynamin-dependent internalization of NMDA receptors (green box). (Adapted from Lau and Zukin (2007), https://doi.org/10.1038/nrn2153, Figure 5, under CCC RightsLink® license 5516591094919)
AMPA and NMDA components of the LTP response. (a) Representative traces of mEPSCs before (control), immediately after LTP induction (early LTP), and >2 h after LTP induction (late LTP). At early LTP, mEPSC amplitude and frequency increase significantly. At times >2 h after LTP induction (late LTP), mEPSC frequency returned to control levels. (b) Average mEPSC waveforms from control (gray) and early LTP (black) showing that the rapid AMPA component of the mEPSC was potentiated (AMPA-LTP), whereas the slowly decaying NMDA component was unaffected. (c) Average mEPSC waveforms from early LTP (gray) and late LTP (black). During late LTP, potentiation of the NMDA component (NMDA-LTP) was now also evident. (d) AMPA and NMDA components of mEPSCs at early and late LTP. (Up) Increased AMPA component of mEPSCs at both periods, and a delayed potentiation of NMDA component of mEPSCs only at late LTP. (Bottom) Time course of AMPA (open boxes) and NMDA (filled triangles) mEPSC amplitudes, with a prominent contribution of AMPA component. (Adapted from Watt et al. (2004), https://doi.org/10.1038/nn1220, (a–c) Figures 1 and (d) 6a,b under CCC RightsLink® license 5516591392907)
At later stages of LTP, structural modifications of spines are triggered to support synaptic strength enhancement. Spine structural modifications are essential to synaptic plasticity (Fig. 3.13) (Harvey and Svoboda 2007; Noguchi et al. 2019), since it is highly correlated with the molecular reorganization and functional expression of receptors, such as AMPA and NMDA in excitatory synapses (Cao and Harris 2012; Baltaci et al. 2019). Increased NMDA-mediated synaptic transmission not only can modify dendritic spine morphology but also can induce new spine formation, stabilizing recently formed spines and enlarging already-existing spines (Lai and Ip 2013). Indeed, an increase in synaptic strength by LTP triggers the pruning and replacement of nonactivated spines by new ones together with a selective stabilization of activated synapses (De Roo et al. 2008) and the rapid formation of new spines that can be blocked by NMDA receptors antagonists (Toni et al. 1999; Ruddy et al. 2015).
In vitro and in vivo enlargement of spines under LTP. (a) (Up) LTP induction in vitro leads to rapid expansion of spine heads. Images before (23 min) and after (25 min) LTP induction. At time = 0, the LTP protocol (30 uncaging pulses at 0.5 Hz, 4-ms pulse duration, postsynaptic potential 0 mV) was applied to the spine marked by a circle (LTP spine). A triangle marks a tested nearby spine. (Middle) uEPSCs, averaged across all cells, in response to test stimuli before (23 min; gray) and after (40 min; black) the LTP protocol. (Bottom) Time course of the changes in uEPSC amplitude and spine volume at the LTP spine (filled circles; n 5 7) and at nearby spines (open triangles; uEPSC, n 5 7; Vol, n 5 31). The arrow marks the LTP protocol. (b) (Up) Induction of spine enlargement in the visual cortex in vivo using two-photon glutamate uncaging. Time-lapse images show an expansion of stimulated spines. (Bottom) Spine’s volume increased after LTP stimulation. Spine (i) in cyan; and spine (ii) in magenta. (Adapted from (a) Harvey and Svoboda (2007), https://doi.org/10.1038/nature06416, Figure 1a,b under CCC RightsLink® license 5517380739551; and (b) from Noguchi et al. (2019), https://doi.org/10.1038/s41598-019-50445-0, Figure 1b,c under CC BY license, originally published by Springer Nature)
NMDA receptor-dependent enlargement of spines can be reversed by low-frequency stimulation (LFS) via a phosphatase-dependent mechanism, a well-known mechanism of LTD (Luscher and Malenka 2012).
Therefore, spine dynamics may constitute the basis of long-term molecular reorganization correlated with long-term memory and cognition. Strengthening synaptic connections is believed to be a common mechanism of both processes, and abnormal spine profiles can be correlated with altered synaptic transmission in various neurological and neuropsychiatric conditions (Kasai et al. 2010; Lai and Ip 2013).
3.3.2 Dynamics of Receptors in Spines During Long-Term Depression
LTD has been considered as a parallel and opposite process of LTP, associated with a reduction of functional synapses and, consequently, reduction in reactivity to afferent stimulation (Luscher and Malenka 2012). There are several protocols for LTD induction, including a low-frequency stimulation (LFS: 0.5–3 Hz), chemical induction (He et al. 2011) and paired pre- and postsynaptic stimulation (STDP) (Markram et al. 1997; Debanne et al. 1998; Bi and Poo 1998; Sjöström et al. 2001; Feldman 2012) or mediated by metabotropic glutamate receptors (mGluR) (Hasegawa et al. 2015).
The NMDA receptor-mediated LTD involves the activation of NMDA receptors followed by a small but lasting elevation in postsynaptic Ca2+ concentration, and activation of a serine-threonine protein phosphatase cascade that act to modulate the spine actin cytoskeleton (Stein and Zito 2019). For example, NMDA receptor-mediated moderated intracellular Ca2+ signals in spines and parent dendrites were observed in the timing-dependent LTD triggered by two-photon glutamate uncaging using the post- and pre-protocol in a single spine of layer V pyramidal neurons in juvenile mice (Tazerart et al. 2020) (Fig. 3.9b, c). The LTD induced by STDP at CA3-CA1 hippocampal synapses and some synapses on neocortical layers II/III pyramidal cells is dependent on NMDA receptors (Nishiyama et al. 2000; Froemke et al. 2005; Feldman 2012) (Nishiyama et al. 2000; Froemke et al. 2005). The LTD induced by STDP at several synapses in layers II/III and V of somatosensory and visual cortex and in cortical synapses onto striatal medium spiny neurons is dependent on mGluR- and endocannabinoids receptors (CB1) (Nevian and Sakmann 2006; Feldman 2012).
Following Ca2+ entry through activated NMDA receptors, LTD requires the activation of protein phosphatase 1 (PP1), which is essential for the functional process of LTD but not for spine structural modifications. Cofilin, a family of actin-binding proteins that trigger a rapid depolymerization of actin microfilaments, mediates spine structural modifications, that is, spine shrinkage induced by LFS (Zhou et al. 2004). In addition, the activation of NMDA receptors with Ca2+ entry and subsequent activation of calcineurin and AP2 are required for both functional and structural processes of LTD (Zhou et al. 2004) and for triggering AMPA receptors endocytosis at the dendritic spine (Vitureira and Goda 2013). The NMDA receptor-induced internalization of AMPA receptors can be inhibited by preventing the increment of intracellular Ca2+ concentration or by specific calcineurin inhibitors (Carroll et al. 2001). The removal of extrasynaptic CP-AMPA receptors and replacement by Ca2+-impermeable AMPA receptors (CI-AMPA receptors) is associated with LTD also in mGluR-induced LTD (Bellone and Lüscher 2005; Henley and Wilkinson 2016). During another form of plasticity, in which the neuron self-tunes its synapses, called the homeostatic synaptic scaling (Fig. 3.10a, c) (Turrigiano 2008), the recruitment of CP-AMPA receptors remains controversial and it may be dependent on the synapse, developmental stage and mode of induction. Overall, the activity-dependent removal of CP-AMPA receptors can define a time frame in which memories can be erased (Henley and Wilkinson 2016) (Fig. 3.10a, d).
NMDAR trafficking is also implicated in LTD following NMDA receptor activation (Carroll et al. 2001; Lau and Zukin 2007) (Fig 3.11b). In the hippocampus, for example, NMDAR trafficking requires actin depolymerization (Morishita et al. 2005) for the lateral diffusion of NMDA receptors between synaptic and extrasynaptic sites or dynamin-dependent internalization (Montgomery et al. 2005). Notably, activity-dependent alterations in NMDA synaptic strength influence metaplasticity, experience-dependent plasticity, and structural remodeling of local circuitry (Philpot et al. 2003; Lau and Zukin 2007).
Live imaging of dendritic spines after stimulation by low-frequency uncaging glutamate demonstrates that the stimulus of LTD leads to spine shrinkage specifically of the stimulated spine but not of neighboring spines. Like LTP-induced spine enlargement, spine shrinkage induced by LTD is also synapse-specific (Oh et al. 2013). Volume reduction was observed in both large and small spines after pharmacological manipulations in combination with two-photon glutamate uncaging. Shrinkage of small dendritic spines required activation of NMDA receptors, whereas shrinkage of large spines required signaling through both NMDA and metabotropic glutamate receptors mGluR and the second messenger inositol 1,4,5-trisphosphate receptors (IP3Rs) (Oh et al. 2013). LTD induction in dendritic spines followed or not by spine shrinkage is highly correlated with synaptic weakness (Fig. 3.14). However, these mechanisms also play a vital role in the neural circuit plasticity that underlies learning. Indeed, the formation and stabilization of new dendritic spines as new circuits are formed during learning is only possible due to the elimination of preexisting spines (Yang et al. 2008; De Roo et al. 2008) (see also Chap. 7 in this book).
LTD induction and structural plasticity of spines in vitro and in vivo. (a) Low-frequency stimulation (LFS) induces long-term depression. (b) (Up) In vitro shrinkage of spines at different time points before and after stimulation. (Bottom) Quantitative measure of spine head diameter demonstrating a decrease of spine volume after LTD induction. (c) (Up) In vivo shrinkage of spines after LTD induction, showing a decrease of spine volume in the stimulated spine. (Bottom) The magenta, yellow, and white circles indicate spine S1, n1, and n2 traces, respectively. (Adapted from (a) and (b): Zhou et al. (2004), https://doi.org/10.1016/j.neuron.2004.11.011, (a, b Bottom) Figure 2A,B and (b Up) Figure 1C,D, under CCC RightsLink® license 5518550626563; (c) from Noguchi et al. (2019), https://doi.org/10.1038/s41598-019-50445-0, Figure 3A,B under CC BY license, originally published by Springer Nature)
3.4 Concluding Remarks
For many years, the electrical properties of dendritic spines, such as membrane voltage and ionic conductances, were not able to be directly measured due to technical limitations usually difficult to overcome. However, several important biophysical properties of spines have been unveiled recently. Advancements result from the ongoing development of novel and integrated technologies, as described in this chapter.
We highlighted some important achievements for detecting the electrical properties of dendritic spines. First, we discussed the direct measurement of the Vm (bAPs and synaptic potentials) at the spine head, composed by a rich variety of ion channels and receptors and whose dynamics and trafficking are influenced by intracellular signaling and extracellular matrix during synaptic activation and plasticity. By comparing the modifications of the Vm of spines to the parent dendrites and soma, we can now have a better understanding about signal processing and integration. Second, we described the possibility to measure the spine neck resistance, which is associated with the electrical compartmentalization of spines. Spine neck resistance is crucial for tuning the amplitude and kinetics of synaptic signals along the dendrite and controls the spatiotemporal dynamics of information during the synaptic integration and plasticity. Although the electrical role of dendritic spines remains an open issue, these constant advances in technology are providing valuable evidence on the intrinsic properties of dendritic spines for the biochemical and electrical compartmentalization and associated molecular and structural modifications during synaptic plasticity that contribute actively to neuronal behavior.
References
Acker CD, Hoyos E, Loew LM (2016) EPSPs measured in proximal dendritic spines of cortical pyramidal neurons. eNeuro 3(2):ENEURO.0050-15.2016. https://doi.org/10.1523/ENEURO.0050-15.2016
Allen JA, Halverson-Tamboli RA, Rasenick MM (2007) Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci 8(2):128–140. https://doi.org/10.1038/nrn2059
Araya R (2014) Input transformation by dendritic spines of pyramidal neurons. Front Neuroanat 8:141. https://doi.org/10.3389/fnana.2014.00141
Araya R, Jiang J, Eisenthal KB, Yuste R (2006) The spine neck filters membrane potentials. Proc Natl Acad Sci U S A 103(47):17961–17966. https://doi.org/10.1073/pnas.0608755103
Araya R, Nikolenko V, Eisenthal KB, Yuste R (2007) Sodium channels amplify spine potentials. Proc Natl Acad Sci U S A 104(30):12347–12352. https://doi.org/10.1073/pnas.0705282104
Araya R, Vogels TP, Yuste R (2014) Activity-dependent dendritic spine neck changes are correlated with synaptic strength. Proc Natl Acad Sci U S A 111(28):E2895–E2904. https://doi.org/10.1073/pnas.1321869111
Arellano JI (2007) Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci 1(1):131–143. https://doi.org/10.3389/neuro.01.1.1.010.2007
Baltaci SB, Mogulkoc R, Baltaci AK (2019) Molecular mechanisms of early and late LTP. Neurochem Res 44(2):281–296. https://doi.org/10.1007/s11064-018-2695-4
Becker MFP, Tetzlaff C (2021) The biophysical basis underlying the maintenance of early phase long-term potentiation. PLoS Comput Biol 17(3):e1008813. https://doi.org/10.1371/journal.pcbi.1008813
Beffert U, Durudas A, Weeber EJ, Stolt PC, Giehl KM, Sweatt JD, Hammer RE, Herz J (2006) Functional dissection of Reelin signaling by site-directed disruption of Disabled-1 adaptor binding to apolipoprotein E receptor 2: distinct roles in development and synaptic plasticity. J Neurosci 26(7):2041–2052. https://doi.org/10.1523/JNEUROSCI.4566-05.2006
Bellone C, Lüscher C (2005) mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci 21(5):1280–1288. https://doi.org/10.1111/j.1460-9568.2005.03979.x
Bi G, Poo M (1998) Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18(24):10464–10472. https://doi.org/10.1523/JNEUROSCI.18-24-10464.1998
Blom H, Rönnlund D, Scott L, Spicarova Z, Widengren J, Bondar A, Aperia A, Brismar H (2011) Spatial distribution of Na+-K+-ATPase in dendritic spines dissected by nanoscale superresolution STED microscopy. BMC Neurosci 12(1):16. https://doi.org/10.1186/1471-2202-12-16
Bloodgood BL, Sabatini BL (2005) Neuronal activity regulates diffusion across the neck of dendritic spines. Science 310(5749):866–869. https://doi.org/10.1126/science.1114816
Bloodgood BL, Sabatini BL (2007a) Ca2+ signaling in dendritic spines. Curr Opin Neurobiol 17(3):345–351. https://doi.org/10.1016/j.conb.2007.04.003
Bloodgood BL, Sabatini BL (2007b) Nonlinear regulation of unitary synaptic signals by CaV2.3 voltage-sensitive calcium channels located in dendritic spines. Neuron 53(2):249–260. https://doi.org/10.1016/j.neuron.2006.12.017
Bloodgood BL, Giessel AJ, Sabatini BL (2009) Biphasic synaptic Ca influx arising from compartmentalized electrical signals in dendritic spines. PLoS Biol 7(9):e1000190. https://doi.org/10.1371/journal.pbio.1000190
Brusés JL, Chauvet N, Rutishauser U (2001) Membrane lipid rafts are necessary for the maintenance of the α7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons. J Neurosci 21(2):504–512. https://doi.org/10.1523/JNEUROSCI.21-02-00504.2001
Buard I, Coultrap SJ, Freund RK, Lee YS, Dell’Acqua ML, Silva AJ, Bayer KU (2010) CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. Neurosci 30(24):8214–8220. https://doi.org/10.1523/JNEUROSCI.1469-10.2010
Bywalez WG, Patirniche D, Rupprecht V, Stemmler M, Herz AVM, Pálfi D, Rózsa B, Egger V (2015) Local postsynaptic voltage-gated sodium channel activation in dendritic spines of olfactory bulb granule cells. Neuron 85(3):590–601. https://doi.org/10.1016/j.neuron.2014.12.051
Calabrese B, Wilson MS, Halpain S (2006) Development and regulation of dendritic spine synapses. Physiology 21(1):38–47. https://doi.org/10.1152/physiol.00042.2005
Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR (2000) Sodium channel Na(v)1.6 is localized at nodes of ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A 97(10):5616–5620. https://doi.org/10.1073/pnas.090034797
Camiré O, Topolnik L (2014) Dendritic calcium nonlinearities switch the direction of synaptic plasticity in fast-spiking interneurons. J Neurosci 34(11):3864–3877. https://doi.org/10.1523/JNEUROSCI.2253-13.2014
Camiré O, Topolnik L (2018) Two-photon calcium imaging in neuronal dendrites in brain slices. J Vis Exp (133):56776. https://doi.org/10.3791/56776
Campo CG, Sinagra M, Verrier D, Manzoni OJ, Chavis P (2009) Reelin secreted by GABAergic neurons regulates glutamate receptor homeostasis. PLoS One 4(5):e5505. https://doi.org/10.1371/journal.pone.0005505
Cao G, Harris KM (2012) Developmental regulation of the late phase of long-term potentiation (L-LTP) and metaplasticity in hippocampal area CA1 of the rat. J Neurophysiol 107(3):902–912. https://doi.org/10.1152/jn.00780.2011
Caporale N, Dan Y (2008) Spike timing–dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci 31(1):25–46. https://doi.org/10.1146/annurev.neuro.31.060407.125639
Carroll RC, Beattie EC, von Zastrow M, Malenka RC (2001) Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci 2(5):315–324. https://doi.org/10.1038/35072500
Chater TE, Goda Y (2014) The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front Cell Neurosci 8:401. https://doi.org/10.3389/fncel.2014.00401
Chen X, Leischner U, Rochefort NL, Nelken I, Konnerth A (2011) Functional mapping of single spines in cortical neurons in vivo. Nature 475(7357):501–505. https://doi.org/10.1038/nature10193
Chen JL, Villa KL, Cha JW, So PTC, Kubota Y, Nedivi E (2012) Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron 74(2):361–373. https://doi.org/10.1016/j.neuron.2012.02.030
Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GCR, Higley MJ (2013) Compartmentalization of GABAergic inhibition by dendritic spines. Science 340(6133):759–762. https://doi.org/10.1126/science.1234274
Chung HJ, Qian X, Ehlers M, Jan YN, Jan LY (2009) Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci U S A 106(2):629–634. https://doi.org/10.1073/pnas.0811615106
Citri A, Malenka RC (2008) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33(1):18–41. https://doi.org/10.1038/sj.npp.1301559
Clem RL, Barth A (2006) Pathway-specific trafficking of native AMPARs by in vivo experience. Neuron 49(5):663–670. https://doi.org/10.1016/j.neuron.2006.01.019
Cohen LB, Salzberg BM (1978) Optical measurement of membrane potential. Rev Physiol Biochem Pharmacol 83:35–88. https://doi.org/10.1007/3-540-08907-1_2
Cornejo VH, Ofer N, Yuste R (2022) Voltage compartmentalization in dendritic spines in vivo. Science 375(6576):82–86. https://doi.org/10.1126/science.abg0501
De Roo M, Klauser P, Muller D (2008) LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol 6(9):e219. https://doi.org/10.1371/journal.pbio.0060219
de Vivo L, Landi S, Panniello M, Baroncelli L, Chierzi S, Mariotti L, Spolidoro M, Pizzorusso T, Maffei L, Ratto GM (2013) Extracellular matrix inhibits structural and functional plasticity of dendritic spines in the adult visual cortex. Nat Commun 4(1):1484. https://doi.org/10.1038/ncomms2491
Debanne D, Gähwiler BH, Thompson SM (1998) Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. J Physiol 507(Pt 1):237–247. https://doi.org/10.1111/j.1469-7793.1998.237bu.x
Denk W, Yuste R, Svoboda K, Tank DW (1996) Imaging calcium dynamics in dendritic spines. Curr Opin Neurobiol 6(3):372–378. https://doi.org/10.1016/s0959-4388(96)80122-x
Dickstein DL, Weaver CM, Luebke JI, Hof PR (2013) Dendritic spine changes associated with normal aging. Neuroscience 251:21–32. https://doi.org/10.1016/j.neuroscience.2012.09.077
Dityatev A, Rusakov DA (2011) Molecular signals of plasticity at the tetrapartite synapse. Curr Opin Neurobiol 21(2):353–359. https://doi.org/10.1016/j.conb.2010.12.006
Dityatev A, Schachner M (2003) Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci 4(6):456–468. https://doi.org/10.1038/nrn1115
Dityatev A, Frischknecht R, Seidenbecher CI (2006) Extracellular matrix and synaptic functions. In: Gundelfinger ED, Seidenbecher CI, Schraven B (eds) Cell communication in nervous and immune system. Springer, Berlin Heidelberg, pp 69–97
Dityatev A, Schachner M, Sonderegger P (2010) The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci 11(11):735–746. https://doi.org/10.1038/nrn2898
Dozmorov M, Li R, Abbas A-K, Hellberg F, Farre C, Huang F-S, Jilderos B, Wigström H (2006) Contribution of AMPA and NMDA receptors to early and late phases of LTP in hippocampal slices. Neurosci Res 55(2):182–188. https://doi.org/10.1016/j.neures.2006.03.001
Egawa J, Pearn ML, Lemkuil BP, Patel PM, Head BP (2016) Membrane lipid rafts and neurobiology: age-related changes in membrane lipids and loss of neuronal function. J Physiol 594(16):4565–4579. https://doi.org/10.1113/JP270590
Enoki R, Hu Y, Hamilton D, Fine A (2009) Expression of long-term plasticity at individual synapses in hippocampus is graded, bidirectional, and mainly presynaptic: optical quantal analysis. Neuron 62(2):242–253. https://doi.org/10.1016/j.neuron.2009.02.026
Feldman DE (2012) The spike-timing dependence of plasticity. Neuron 75(4):556–571. https://doi.org/10.1016/j.neuron.2012.08.001
Filipis L, Canepari M (2021) Optical measurement of physiological sodium currents in the axon initial segment. J Physiol 599(1):49–66. https://doi.org/10.1113/JP280554
Froemke RC, Poo M-M, Dan Y (2005) Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature 434(7030):221–225. https://doi.org/10.1038/nature03366
Grosshans DR, Clayton DA, Coultrap SJ, Browning MD (2002) LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci 5(1):27–33. https://doi.org/10.1038/nn779
Grunditz Å, Holbro N, Tian L, Zuo Y, Oertner TG (2008) Spine neck plasticity controls postsynaptic calcium signals through electrical compartmentalization. J Neurosci 28(50):13457–13466. https://doi.org/10.1523/JNEUROSCI.2702-08.2008
Gundelfinger ED, Frischknecht R, Choquet D, Heine M (2010) Converting juvenile into adult plasticity: a role for the brain’s extracellular matrix: converting juvenile into adult plasticity. Eur J Neurosci 31(12):2156–2165. https://doi.org/10.1111/j.1460-9568.2010.07253.x
Harnett MT, Makara JK, Spruston N, Kath WL, Magee JC (2012) Synaptic amplification by dendritic spines enhances input cooperativity. Nature 491(7425):599–602. https://doi.org/10.1038/nature11554
Harris KM, Kater SB (1994) Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci 17:341–371. https://doi.org/10.1146/annurev.ne.17.030194.002013
Harris K, Stevens J (1988) Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. J Neurosci 8(12):4455–4469. https://doi.org/10.1523/JNEUROSCI.08-12-04455.1988
Harris K, Stevens J (1989) Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci 9(8):2982–2997. https://doi.org/10.1523/JNEUROSCI.09-08-02982.1989
Harris KM, Jensen FE, Tsao B (1992) Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci 12(7):2685–2705. https://doi.org/10.1523/JNEUROSCI.12-07-02685.1992
Harvey CD, Svoboda K (2007) Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature 450(7173):1195–1200. https://doi.org/10.1038/nature06416
Harward SC, Hedrick NG, Hall CE, Parra-Bueno P, Milner TA, Pan E, Laviv T, Hempstead BL, Yasuda R, McNamara JO (2016) Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature 538(7623):99–103. https://doi.org/10.1038/nature19766
Hasegawa S, Sakuragi S, Tominaga-Yoshino K, Ogura A (2015) Dendritic spine dynamics leading to spine elimination after repeated inductions of LTD. Sci Rep 5:7707. https://doi.org/10.1038/srep07707
Häusser M, Spruston N, Stuart GJ (2000) Diversity and dynamics of dendritic signaling. Science 290(5492):739–744. https://doi.org/10.1126/science.290.5492.739
He K, Lee A, Song L, Kanold PO, Lee H-K (2011) AMPA receptor subunit GluR1 (GluA1) serine-845 site is involved in synaptic depression but not in spine shrinkage associated with chemical long-term depression. J Neurophysiol 105(4):1897–1907. https://doi.org/10.1152/jn.00913.2010
Hedrick NG, Harward SC, Hall CE, Murakoshi H, McNamara JO, Yasuda R (2016) Rho GTPase complementation underlies BDNF-dependent homo- and heterosynaptic plasticity. Nature 538(7623):104–108. https://doi.org/10.1038/nature19784
Hellwig S, Hack I, Kowalski J, Brunne B, Jarowyj J, Unger A, Bock HH, Junghans D, Frotscher M (2011) Role for Reelin in neurotransmitter release. J Neurosci 31(7):2352–2360. https://doi.org/10.1523/JNEUROSCI.3984-10.2011
Henley JM, Wilkinson KA (2016) Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci 17(6):337–350. https://doi.org/10.1038/nrn.2016.37
Hestrin S, Nicoll RA, Perkel DJ, Sah P (1990) Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol 422:203–225. https://doi.org/10.1113/jphysiol.1990.sp017980
Holthoff K, Zecevic D, Konnerth A (2010) Rapid time course of action potentials in spines and remote dendrites of mouse visual cortex neurons: optical recording of APs in dendritic spines. J Physiol 588(7):1085–1096. https://doi.org/10.1113/jphysiol.2009.184960
Honigmann A, Pralle A (2016) Compartmentalization of the cell membrane. J Mol Biol 428(24):4739–4748. https://doi.org/10.1016/j.jmb.2016.09.022
Huntley GW, Gil O, Bozdagi O (2002) The cadherin family of cell adhesion molecules: multiple roles in synaptic plasticity. Neuroscientist 8(3):221–233
Jaafari N, Konopacki FA, Owen TF, Kantamneni S, Rubin P, Craig TJ, Wilkinson KA, Henley JM (2013) SUMOylation is required for glycine-induced increases in AMPA receptor surface expression (ChemLTP) in hippocampal neurons. PLoS One 8(1):e52345. https://doi.org/10.1371/journal.pone.0052345
Jaffe DB, Johnston D, Lasser-Ross N, Lisman JE, Miyakawa H, Ross WN (1992) The spread of Na+ spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature 357(6375):244–246. https://doi.org/10.1038/357244a0
Jayant K, Hirtz JJ, Plante IJ-L, Tsai DM, De Boer WDAM, Semonche A, Peterka DS, Owen JS, Sahin O, Shepard KL, Yuste R (2017) Targeted intracellular voltage recordings from dendritic spines using quantum-dot-coated nanopipettes. Nat Nanotechnol 12(4):335–342. https://doi.org/10.1038/nnano.2016.268
Johnston D, Magee JC, Colbert CM, Christie BR (1996) Active properties of neuronal dendrites. Annu Rev Neurosci 19:165–186. https://doi.org/10.1146/annurev.ne.19.030196.001121
Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J (2010) Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci 33(3):121–129. https://doi.org/10.1016/j.tins.2010.01.001
Kaufmann WA, Matsui K, Jeromin A, Nerbonne JM, Ferraguti F (2013) Kv4.2 potassium channels segregate to extrasynaptic domains and influence intrasynaptic NMDA receptor NR2B subunit expression. Brain Struct Funct 218(5):1115–1132. https://doi.org/10.1007/s00429-012-0450-1
Kim J, Jung S-C, Clemens AM, Petralia RS, Hoffman DA (2007) Regulation of dendritic excitability by activity-dependent trafficking of the A-type K+ channel subunit Kv4.2 in hippocampal neurons. Neuron 54(6):933–947. https://doi.org/10.1016/j.neuron.2007.05.026
Koch C, Segev I (2000) The role of single neurons in information processing. Nat Neurosci 3(S11):1171–1177. https://doi.org/10.1038/81444
Koch C, Zador A (1993) The function of dendritic spines: devices subserving biochemical rather than electrical compartmentalization. J Neurosci 13(2):413–422. https://doi.org/10.1523/JNEUROSCI.13-02-00413.1993
Kochlamazashvili G, Henneberger C, Bukalo O, Dvoretskova E, Senkov O, Lievens PM-J, Westenbroek R, Engel AK, Catterall WA, Rusakov DA, Schachner M, Dityatev A (2010) The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca2+ channels. Neuron 67(1):116–128. https://doi.org/10.1016/j.neuron.2010.05.030
Koester HJ, Sakmann B (1998) Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating action potentials and subthreshold excitatory postsynaptic potentials. Proc Natl Acad Sci U S A 95(16):9596–9601. https://doi.org/10.1073/pnas.95.16.9596
Koleske AJ (2013) Molecular mechanisms of dendrite stability. Nat Rev Neurosci 14(8):536–550. https://doi.org/10.1038/nrn3486
Korngreen A, Sakmann B (2000) Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. J Physiol 525(Pt 3):621–639. https://doi.org/10.1111/j.1469-7793.2000.00621.x
Kovalchuk Y, Eilers J, Lisman J, Konnerth A (2000) NMDA receptor-mediated subthreshold Ca2+ signals in spines of hippocampal neurons. J Neurosci 20(5):1791–1799. https://doi.org/10.1523/JNEUROSCI.20-05-01791.2000
Kwon T, Sakamoto M, Peterka DS, Yuste R (2017) Attenuation of synaptic potentials in dendritic spines. Cell Rep 20(5):1100–1110. https://doi.org/10.1016/j.celrep.2017.07.012
Lai K-O, Ip NY (2013) Structural plasticity of dendritic spines: the underlying mechanisms and its dysregulation in brain disorders. Biochim Biophys Acta (BBA) – Mol Basis Dis 1832(12):2257–2263. https://doi.org/10.1016/j.bbadis.2013.08.012
Lan J, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MVL, Zukin RS (2001) Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci 4(4):382–390. https://doi.org/10.1038/86028
Lanté F, de Jésus Ferreira M-C, Guiramand J, Récasens M, Vignes M (2006) Low-frequency stimulation induces a new form of LTP, metabotropic glutamate (mGlu5) receptor- and PKA-dependent, in the CA1 area of the rat hippocampus. Hippocampus 16(4):345–360. https://doi.org/10.1002/hipo.20146
Lau CG, Zukin RS (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8(6):413–426. https://doi.org/10.1038/nrn2153
Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MVL, Zukin RS (2010) SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci 30(1):242–254. https://doi.org/10.1523/JNEUROSCI.4933-08.2010
Lee S-JR, Escobedo-Lozoya Y, Szatmari EM, Yasuda R (2009) Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 458(7236):299–304. https://doi.org/10.1038/nature07842
Lee H-K, Takamiya K, He K, Song L, Huganir RL (2010) Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J Neurophysiol 103(1):479–489. https://doi.org/10.1152/jn.00835.2009
Lisman J, Yasuda R, Raghavachari S (2012) Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci 13(3):169–182. https://doi.org/10.1038/nrn3192
London M, Häusser M (2005) Dendritic computation. Annu Rev Neurosci 28:503–532
Lorincz A, Nusser Z (2010) Molecular identity of dendritic voltage-gated sodium channels. Science 328(5980):906–909. https://doi.org/10.1126/science.1187958
Lu W-Y, Man H-Y, Ju W, Trimble WS, MacDonald JF, Wang YT (2001) Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29(1):243–254. https://doi.org/10.1016/S0896-6273(01)00194-5
Luscher C, Malenka RC (2012) NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol 4(6):a005710. https://doi.org/10.1101/cshperspect.a005710
Magee JC, Johnston D (1997) A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science 275(5297):209–213. https://doi.org/10.1126/science.275.5297.209
Mainen ZF, Maletic-Savatic M, Shi SH, Hayashi Y, Malinow R, Svoboda K (1999) Two-photon imaging in living brain slices. Methods 18(2):231–239. https://doi.org/10.1006/meth.1999.0776
Makino H, Malinow R (2009) AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron 64(3):381–390. https://doi.org/10.1016/j.neuron.2009.08.035
Markram H, Lübke J, Frotscher M, Sakmann B (1997) Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275(5297):213–215. https://doi.org/10.1126/science.275.5297.213
Mateos-Aparicio P, Rodríguez-Moreno A (2020) Calcium dynamics and synaptic plasticity. Adv Exp Med Biol 1131:965–984. https://doi.org/10.1007/978-3-030-12457-1_38
Matsuzaki M, Ellis-Davies GCR, Nemoto T, Miyashita Y, Iino M, Kasai H (2001) Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci 4(11):1086–1092. https://doi.org/10.1038/nn736
Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429(6993):761–766. https://doi.org/10.1038/nature02617
McGeachie AB, Cingolani LA, Goda Y (2011) A stabilising influence: integrins in regulation of synaptic plasticity. Neurosci Res 70(1):24–29. https://doi.org/10.1016/j.neures.2011.02.006
Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M (2002) Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation Neuron 36(3):507–519. https://doi.org/10.1016/s0896-6273(02)00978-9
Miyazaki K, Ross WN (2017) Sodium dynamics in pyramidal neuron dendritic spines: synaptically evoked entry predominantly through AMPA receptors and removal by diffusion. J Neurosci 37(41):9964–9976. https://doi.org/10.1523/JNEUROSCI.1758-17.2017
Miyazaki K, Ross WN (2022) Fast synaptically activated calcium and sodium kinetics in hippocampal pyramidal neuron dendritic spines. eNeuro 9(6):ENEURO.0396-22.2022. https://doi.org/10.1523/ENEURO.0396-22.2022
Montgomery JM, Selcher JC, Hanson JE, Madison DV (2005) Dynamin-dependent NMDAR endocytosis during LTD and its dependence on synaptic state. BMC Neurosci 6:48. https://doi.org/10.1186/1471-2202-6-48
Morishita W, Marie H, Malenka RC (2005) Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat Neurosci 8(8):1043–1050. https://doi.org/10.1038/nn1506
Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW (2006) Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci 26(7):1923–1934. https://doi.org/10.1523/JNEUROSCI.4359-05.2006
Nakahata Y, Yasuda R (2018) Plasticity of spine structure: local signaling, translation and cytoskeletal reorganization. Front Synaptic Neurosci 10:29. https://doi.org/10.3389/fnsyn.2018.00029
Nakamura T, Barbara J-G, Nakamura K, Ross WN (1999) Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron 24(3):727–737. https://doi.org/10.1016/S0896-6273(00)81125-3
Nevian T, Sakmann B (2004) Single spine Ca2+ signals evoked by coincident EPSPs and backpropagating action potentials in spiny stellate cells of layer 4 in the juvenile rat somatosensory barrel cortex. J Neurosci 24(7):1689–1699. https://doi.org/10.1523/JNEUROSCI.3332-03.2004
Nevian T, Sakmann B (2006) Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci 26(43):11001–11013. https://doi.org/10.1523/JNEUROSCI.1749-06.2006
Nicoll RA (2017) A brief history of long-term potentiation. Neuron 93(2):281–290. https://doi.org/10.1016/j.neuron.2016.12.015
Nishiyama J (2019) Plasticity of dendritic spines: molecular function and dysfunction in neurodevelopmental disorders. Psychiatry Clin Neurosci 73(9):541–550. https://doi.org/10.1111/pcn.12899
Nishiyama J, Yasuda R (2015) Biochemical computation for spine structural plasticity. Neuron 87(1):63–75. https://doi.org/10.1016/j.neuron.2015.05.043
Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K (2000) Calcium stores regulate the polarity and input specificity of synaptic modification. Nature 408(6812):584–588. https://doi.org/10.1038/35046067
Noguchi J, Matsuzaki M, Ellis-Davies GCR, Kasai H (2005) Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron 46(4):609–622. https://doi.org/10.1016/j.neuron.2005.03.015
Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GCR, Kitamura K, Kano M, Matsuzaki M, Kasai H (2011) In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J Physiol 589(Pt 10):2447–2457. https://doi.org/10.1113/jphysiol.2011.207100
Noguchi J, Nagaoka A, Hayama T, Ucar H, Yagishita S, Takahashi N, Kasai H (2019) Bidirectional in vivo structural dendritic spine plasticity revealed by two-photon glutamate uncaging in the mouse neocortex. Sci Rep 9(1):13922. https://doi.org/10.1038/s41598-019-50445-0
Nuriya M, Jiang J, Nemet B, Eisenthal KB, Yuste R (2006) Imaging membrane potential in dendritic spines. Proc Natl Acad Sci U S A 103(3):786–790. https://doi.org/10.1073/pnas.0510092103
Oertner TG (2002) Functional imaging of single synapses in brain slices. Exp Physiol 87(6):733–736. https://doi.org/10.1113/eph8702482
Oh WC, Hill TC, Zito K (2013) Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proc Natl Acad Sci U S A 110(4):E305–E312. https://doi.org/10.1073/pnas.1214705110
Oray S, Majewska A, Sur M (2004) Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron 44(6):1021–1030. https://doi.org/10.1016/j.neuron.2004.12.001
Orlando C, Ster J, Gerber U, Fawcett JW, Raineteau O (2012) Perisynaptic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J Neurosci 32(50):18009–18017. https://doi.org/10.1523/JNEUROSCI.2406-12.2012
Palmer LM, Stuart GJ (2009) Membrane potential changes in dendritic spines during action potentials and synaptic input. J Neurosci 29(21):6897–6903. https://doi.org/10.1523/JNEUROSCI.5847-08.2009
Park M (2018) AMPA receptor trafficking for postsynaptic potentiation. Front Cell Neurosci 12:361. https://doi.org/10.3389/fncel.2018.00361
Penn AC, Zhang CL, Georges F, Royer L, Breillat C, Hosy E, Petersen JD, Humeau Y, Choquet D (2017) Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature 549(7672):384–388. https://doi.org/10.1038/nature23658
Penzes P, Cahill ME, Jones KA, VanLeeuwen J-E, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14(3):285–293. https://doi.org/10.1038/nn.2741
Philpot BD, Espinosa JS, Bear MF (2003) Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci 23(13):5583–5588. https://doi.org/10.1523/JNEUROSCI.23-13-05583.2003
Polsky A, Mel B, Schiller J (2009) Encoding and decoding bursts by NMDA spikes in basal dendrites of layer 5 pyramidal neurons. J Neurosci 29(38):11891–11903. https://doi.org/10.1523/JNEUROSCI.5250-08.2009
Popovic M, Gao X, Zecevic D (2012) Voltage-sensitive dye recording from axons, dendrites and dendritic spines of individual neurons in brain slices. J Vis Exp (69):4261. https://doi.org/10.3791/4261
Popovic MA, Gao X, Carnevale NT, Zecevic D (2014) Cortical dendritic spine heads are not electrically isolated by the spine neck from membrane potential signals in parent dendrites. Cereb Cortex 24(2):385–395. https://doi.org/10.1093/cercor/bhs320
Popovic M, Vogt K, Holthoff K, Konnerth A, Salzberg BM, Grinvald A, Antic SD, Canepari M, Zecevic D (2015a) Imaging submillisecond membrane potential changes from individual regions of single axons, dendrites and spines. In: Canepari M, Zecevic D, Bernus O (eds) Membrane potential imaging in the nervous system and heart. Springer International Publishing, Cham, pp 57–101
Popovic MA, Carnevale N, Rozsa B, Zecevic D (2015b) Electrical behaviour of dendritic spines as revealed by voltage imaging. Nat Commun 6(1):8436. https://doi.org/10.1038/ncomms9436
Priel A, Dai X-Q, Chen X-Z, Scarinci N, Cantero MR, Cantiello HF (2022) Electrical recordings from dendritic spines of adult mouse hippocampus and effect of the actin cytoskeleton. Front Mol Neurosci 15:769725. https://doi.org/10.3389/fnmol.2022.769725
Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, Rossi D, de Lecea L, Martínez A, Delgado-García JM, Soriano E (2010) Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J Neurosci 30(13):4636–4649. https://doi.org/10.1523/JNEUROSCI.5284-09.2010
Qiu S, Zhao LF, Korwek KM, Weeber EJ (2006) Differential Reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci 26(50):12943–12955. https://doi.org/10.1523/JNEUROSCI.2561-06.2006
Rossini L, De Santis D, Mauceri RR, Tesoriero C, Bentivoglio M, Maderna E, Maiorana A, Deleo F, de Curtis M, Tringali G, Cossu M, Tumminelli G, Bramerio M, Spreafico R, Tassi L, Garbelli R (2021) Dendritic pathology, spine loss and synaptic reorganization in human cortex from epilepsy patients. Brain 144(1):251–265. https://doi.org/10.1093/brain/awaa387
Royer S, Martina M, Paré D (1999) An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci 19(23):10575–10583. https://doi.org/10.1523/JNEUROSCI.19-23-10575.1999
Ruddy RM, Chen Y, Milenkovic M, Ramsey AJ (2015) Differential effects of NMDA receptor antagonism on spine density. Synapse 69(1):52–56. https://doi.org/10.1002/syn.21784
Runge K, Cardoso C, de Chevigny A (2020) Dendritic spine plasticity: function and mechanisms. Front Synaptic Neurosci 12:36. https://doi.org/10.3389/fnsyn.2020.00036
Sabatini BL, Oertner TG, Svoboda K (2002) The life cycle of Ca2+ ions in dendritic spines. Neuron 33(3):439–452. https://doi.org/10.1016/S0896-6273(02)00573-1
Schiller J, Schiller Y, Clapham DE (1998) NMDA receptors amplify calcium influx into dendritic spines during associative pre- and postsynaptic activation. Nat Neurosci 1(2):114–118. https://doi.org/10.1038/363
Shepherd GM (1996) The dendritic spine: a multifunctional integrative unit. J Neurophysiol 75(6):2197–2210. https://doi.org/10.1152/jn.1996.75.6.2197
Sjöström PJ, Turrigiano GG, Nelson SB (2001) Rate, timing, and cooperativity jointly determine cortical synaptic plasticity. Neuron 32(6):1149–1164. https://doi.org/10.1016/S0896-6273(01)00542-6
Sjöström PJ, Rancz EA, Roth A, Häusser M (2008) Dendritic excitability and synaptic plasticity. Physiol Rev 88(2):769–840. https://doi.org/10.1152/physrev.00016.2007
Song I, Dityatev A (2018) Crosstalk between glia, extracellular matrix and neurons. Brain Res Bull 136:101–108. https://doi.org/10.1016/j.brainresbull.2017.03.003
Spruston N, Jaffe DB, Johnston D (1994) Dendritic attenuation of synaptic potentials and currents: the role of passive membrane properties. Trends Neurosci 17(4):161–166. https://doi.org/10.1016/0166-2236(94)90094-9
Spruston N, Jonas P, Sakmann B (1995) Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol 482(2):325–352. https://doi.org/10.1113/jphysiol.1995.sp020521
Stein IS, Zito K (2019) Dendritic spine elimination: molecular mechanisms and implications. Neuroscientist 25(1):27–47. https://doi.org/10.1177/1073858418769644
Strobel C, Marek R, Gooch HM, Sullivan RKP, Sah P (2015) Prefrontal and auditory input to intercalated neurons of the amygdala. Cell Rep 10(9):1435–1442. https://doi.org/10.1016/j.celrep.2015.02.008
Strobel C, Sullivan RKP, Stratton P, Sah P (2017) Calcium signalling in medial intercalated cell dendrites and spines: dendritic properties of medial ITC neurons. J Physiol 595(16):5653–5669. https://doi.org/10.1113/JP274261
Stuart GJ, Häusser M (2001) Dendritic coincidence detection of EPSPs and action potentials. Nat Neurosci 4(1):63–71. https://doi.org/10.1038/82910
Stuart GJ, Sakmann B (1994) Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature 367(6458):69–72. https://doi.org/10.1038/367069a0
Stuart GJ, Dodt HU, Sakmann B (1993) Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch 423(5–6):511–518. https://doi.org/10.1007/BF00374949
Stuart G, Schiller J, Sakmann B (1997a) Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol 505(3):617–632. https://doi.org/10.1111/j.1469-7793.1997.617ba.x
Stuart G, Spruston N, Sakmann B, Häusser M (1997b) Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci 20(3):125–131. https://doi.org/10.1016/S0166-2236(96)10075-8
Svoboda K, Tank DW, Denk W (1996) Direct measurement of coupling between dendritic spines and shafts. Science 272(5262):716–719. https://doi.org/10.1126/science.272.5262.716
Tada T, Sheng M (2006) Molecular mechanisms of dendritic spine morphogenesis. Curr Opin Neurobiol 16(1):95–101. https://doi.org/10.1016/j.conb.2005.12.001
Takasaki K, Sabatini BL (2014) Super-resolution 2-photon microscopy reveals that the morphology of each dendritic spine correlates with diffusive but not synaptic properties. Front Neuroanat 8:29. https://doi.org/10.3389/fnana.2014.00029
Tanaka H, Hirano T (2012) Visualization of subunit-specific delivery of glutamate receptors to postsynaptic membrane during hippocampal long-term potentiation. Cell Rep 1(4):291–298. https://doi.org/10.1016/j.celrep.2012.02.004
Tanese D, Weng J-Y, Zampini V, De Sars V, Canepari M, Rozsa B, Emiliani V, Zecevic D (2017) Imaging membrane potential changes from dendritic spines using computer-generated holography. Neurophotonics 4(3):031211. https://doi.org/10.1117/1.NPh.4.3.031211
Tazerart S, Mitchell DE, Miranda-Rottmann S, Araya R (2020) A spike-timing-dependent plasticity rule for dendritic spines. Nat Commun 11(1):4276. https://doi.org/10.1038/s41467-020-17861-7
Tazerart S, Blanchard MG, Miranda-Rottmann S, Mitchell DE, Navea Pina B, Thomas CI, Kamasawa N, Araya R (2022) Selective activation of BK channels in small-headed dendritic spines suppresses excitatory postsynaptic potentials. J Physiol 600(9):2165–2187. https://doi.org/10.1113/JP282303
Theis A-K, Rózsa B, Katona G, Schmitz D, Johenning FW (2018) Voltage gated calcium channel activation by backpropagating action potentials downregulates NMDAR function. Front Cell Neurosci 12:109. https://doi.org/10.3389/fncel.2018.00109
Toni N, Buchs P-A, Nikonenko I, Bron CR, Muller D (1999) LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402(6760):421–425. https://doi.org/10.1038/46574
Tønnesen J, Nägerl UV (2016) Dendritic spines as tunable regulators of synaptic signals. Front Psych 7:101. https://doi.org/10.3389/fpsyt.2016.00101
Tønnesen J, Katona G, Rózsa B, Nägerl UV (2014) Spine neck plasticity regulates compartmentalization of synapses. Nat Neurosci 17(5):678–685. https://doi.org/10.1038/nn.3682
Trommald M, Hulleberg G (1997) Dimensions and density of dendritic spines from rat dentate granule cells based on reconstructions from serial electron micrographs. J Comp Neurol 377(1):15–28. https://doi.org/10.1002/(SICI)1096-9861(19970106)377:1<15::AID-CNE3>3.0.CO;2-M
Tsay D, Yuste R (2004) On the electrical function of dendritic spines. Trends Neurosci 27(2):77–83. https://doi.org/10.1016/j.tins.2003.11.008
Turrigiano GG (2008) The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135(3):422–435. https://doi.org/10.1016/j.cell.2008.10.008
Vallés AS, Barrantes FJ (2021) Nanoscale sub-compartmentalization of the dendritic spine compartment. Biomol Ther 11(11):1697. https://doi.org/10.3390/biom11111697
van Versendaal D, Rajendran R, Saiepour MH, Klooster J, Smit-Rigter L, Sommeijer J-P, De Zeeuw CI, Hofer SB, Heimel JA, Levelt CN (2012) Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron 74(2):374–383. https://doi.org/10.1016/j.neuron.2012.03.015
Vanderklish PW, Edelman GM (2002) Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A 99(3):1639–1644. https://doi.org/10.1073/pnas.032681099
Vitureira N, Goda Y (2013) Cell biology in neuroscience: the interplay between Hebbian and homeostatic synaptic plasticity. J Cell Biol 203(2):175–186. https://doi.org/10.1083/jcb.201306030
Wang X, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q, Huntley GW (2008) Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci U S A 105(49):19520–19525. https://doi.org/10.1073/pnas.0807248105
Wang K, Lin MT, Adelman JP, Maylie J (2014) Distinct Ca2+ sources in dendritic spines of hippocampal CA1 neurons couple to SK and Kv4 channels. Neuron 81(2):379–387. https://doi.org/10.1016/j.neuron.2013.11.004
Washbourne P, Dityatev A, Scheiffele P, Biederer T, Weiner JA, Christopherson KS, El-Husseini A (2004) Cell adhesion molecules in synapse formation: figure 1. J Neurosci 24(42):9244–9249. https://doi.org/10.1523/JNEUROSCI.3339-04.2004
Watt AJ, Sjöström PJ, Häusser M, Nelson SB, Turrigiano GG (2004) A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nat Neurosci 7(5):518–524. https://doi.org/10.1038/nn1220
Weng J-Y, Ceballos C, Zecevic D (2023) Contribution of individual excitatory synapses on dendritic spines to electrical signalling. bioRxiv:2022.02.15.480607. https://doi.org/10.1101/2022.02.15.480607
Wilson CJ (1984) Passive cable properties of dendritic spines and spiny neurons. J Neurosci 4(1):281–297. https://doi.org/10.1523/JNEUROSCI.04-01-00281.1984
Xiong W, Chen WR (2002) Dynamic gating of spike propagation in the mitral cell lateral dendrites. Neuron 34(1):115–126. https://doi.org/10.1016/S0896-6273(02)00628-1
Yang Y, Wang X, Frerking M, Zhou Q (2008) Spine expansion and stabilization associated with long-term potentiation. J Neurosci 28(22):5740–5751. https://doi.org/10.1523/JNEUROSCI.3998-07.2008
Yasuda R (2017) Biophysics of biochemical signaling in dendritic spines: implications in synaptic plasticity. Biophys J 113(10):2152–2159. https://doi.org/10.1016/j.bpj.2017.07.029
Yuste R (2010) Dendritic spines. MIT Press, Cambridge, MA
Yuste R (2011) Dendritic spines and distributed circuits. Neuron 71(5):772–781. https://doi.org/10.1016/j.neuron.2011.07.024
Yuste R (2013) Electrical compartmentalization in dendritic spines. Annu Rev Neurosci 36(1):429–449. https://doi.org/10.1146/annurev-neuro-062111-150455
Yuste R (2015) The discovery of dendritic spines by Cajal. Front Neuroanat 9:18. https://doi.org/10.3389/fnana.2015.00018
Yuste R, Bonhoeffer T (2001) Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci 24(1):1071–1089. https://doi.org/10.1146/annurev.neuro.24.1.1071
Yuste R, Denk W (1995) Dendritic spines as basic functional units of neuronal integration. Nature 375(6533):682–684. https://doi.org/10.1038/375682a0
Yuste R, Tank DW (1996) Dendritic integration in mammalian neurons, a century after Cajal. Neuron 16(4):701–716. https://doi.org/10.1016/S0896-6273(00)80091-4
Yuste R, Majewska A, Cash SS, Denk W (1999) Mechanisms of calcium influx into hippocampal spines: heterogeneity among spines, coincidence detection by NMDA receptors, and optical quantal analysis. J Neurosci 19(6):1976–1987. https://doi.org/10.1523/JNEUROSCI.19-06-01976.1999
Zaccard CR, Gippo I, Song A, Geula C, Penzes P (2023) Dendritic spinule-mediated structural synaptic plasticity: implications for development, aging, and psychiatric disease. Front Mol Neurosci 16:1059730. https://doi.org/10.3389/fnmol.2023.1059730
Zhou Q, Homma KJ, Poo M (2004) Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44(5):749–757. https://doi.org/10.1016/j.neuron.2004.11.011
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Righes Marafiga, J., Calcagnotto, M.E. (2023). Electrophysiology of Dendritic Spines: Information Processing, Dynamic Compartmentalization, and Synaptic Plasticity. In: Rasia-Filho, A.A., Calcagnotto, M.E., von Bohlen und Halbach, O. (eds) Dendritic Spines. Advances in Neurobiology, vol 34. Springer, Cham. https://doi.org/10.1007/978-3-031-36159-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-36159-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36158-6
Online ISBN: 978-3-031-36159-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)