Abstract
Starch is a really singular and remarkable natural biopolymer, central to human diet. To the variety of granular morphology and properties due to its different plant origins, food technological processes are able add new types and properties, generating opportunities for new products. High Hydrostatic Pressure (HHP) treatment is a trendy physical process that endows its products of a fresh and chemical-free label. This chapter focuses on corn starch HHP treatment, providing a comprehensive overview about the research development, including equipment technology, application and effects on starch, different methods of preparation and the more usual techniques for its characterization.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction
High pressure technology has experienced a distinct increase in the last decades, whether the number of industrial installations dedicated to food processing, or the annual production are considered. Many high pressure-treated food products of all types are currently in the market and the associated fresher, healthier, and chemicals-free labels make them widely appreciated by consumers.
High hydrostatic pressure (HHP) is an expanding technology that is quickly developing in worldwide food technology. However, the term “novel technology” is not really adequate, as the application of HHP to food is now over a century old, after the first food-related pressure studies (Bridgman, 1964). Pressure is a key property determining the states of matter, in a similar way to temperature. But, while thermal treatments’ effects include chemical reactions acceleration, pressure does not have this effect. Pressure in term of MPa (106 Pa) are usually employed as hydrostatic pressure units (1 Pascal, the SI derived pressure unit, equals 1 kg m- 1 s- 2, 1 MPa equals to 10 bar or 9.86923 atmospheres).
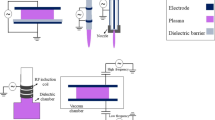
HHP treatments are currently applied to food because they are non-thermal and chemical-free processes, and so, they have a smaller energy consumption associated, while the undesired effect of added chemicals is avoided. Nevertheless, the instrumental investment required is significant. Figure 10.1 shows a scheme of a laboratory/pilot plant HHP processing equipment, while Fig. 10.2 depicts an industrial semi-continuous device. In both cases, samples/products are introduced into a steel chamber (of considerable thickness and weight, to withstand the pressures applied, several times those of the deepest abyssal marine trench). Pressure is supplied by different pumping systems and pressure intensifiers, which basically inject pressurizing fluid into the chamber (a pressure-transmitting fluid is required, as well as a suitable elastic container to separate external fluid from actual food, see below and Figs. 10.1 and 10.2 legend). Despite a large number of industrial applications of high pressure technology being directed to extend food self-life through microorganisms elimination, HHP offers many other effects of technological interest, such as enzyme activity modulation or inactivation, freezing-under-pressure transformations, or induction of structural and textural changes.
Cartoon showing a research food high hydrostatic pressure processing unit. HHP processing equipment, whether at a laboratory, pilot plant, or full industrial scale, is heavy, bulky and expensive. However, the total cost per food unit (weight or volume) is not too high, as there are considerably less energetic expenses. Additionally, water consumption is scarce and there are no wastes associated with the process
Laboratory equipment can be obtained at many different sizes and designs, but always the principles of Figs. 10.1 and 10.2 are followed: water (or a suitable food-compatible fluid) is injected by means of pumps and hydraulic pressure intensifiers into a metal cylindrical chamber, where the sample is pressurized. Steel is usually employed, although other alloys, such as copper-beryllium can be used in smaller vessels. Additives common for avoiding freezing problems are sometimes used (often the food-compatible propylene glycol). Freezing (which can be deleterious for the equipment) can take place upon decompression if work is carried out at temperatures a few degrees over the freezing point, as the adiabatic cooling can cause over 10 degrees temperature reduction. However, this fluid is not in contact with food. A flexible wall container, allowing pressure transmission, but still being a barrier for the external fluid, to avoid contamination, must be employed. This means plastic, although there is active research going on in developing biodegradable packaging materials fit for HHP use (e.g., Marcos et al., 2008). Even starch has been proposed as a source of biodegradable plastic packaging: starch-made packages could be used to pressurize starchy foods (e.g., Khan et al., 2017)
Cartoon showing an industrial semi-continuous food high hydrostatic pressure processing unit
HHP processing is basically a batch process: although equipment has been designed for industrially processing liquid foods, its working principle is essentially semi-continuous. Industrial equipment, as that shown in Fig. 10.2, loads a train of pre-packed food through one end of the pressure chamber. Then it is closed, pressure is built and held for a given time, and afterward, quickly released, for the load exiting through the other end. For industrial use, the processing time is short. The shorter the treatment duration, the cheaper it results. And it has been found that often a few minutes are enough to get the desired effect.
HHP laboratory equipment often allows thermal control, to choose the processing temperature. It can go from as high as 110 °C to as low as −20 °C. Measuring systems (often thermocouples) give information on temperature, even in different locations within the vessel. However, industrial scale equipment does not have these thermal control systems, and influx fixed temperature water is employed.
Starch gelatinization is a process in which hydrogen bonds among starch polysaccharide chains are interchanged with those of water. This water required for the gelatinization process acts as an internal pressure-transmitting medium
It must be noted that (actually, as well as with thermal processes) pressure treatments by themselves induce only reversible changes in matter: i.e., once the pressure is released and atmospheric conditions recovered, the changes that may have occurred are reverted. Even the adiabatic heating, a phenomenon by which temperature rises upon pressure increments (and that, depending on the physicochemical nature of each material, can increment temperature over 10 °C or more), are reverted, with a similar temperature reduction upon decompression (further details on adiabatic heating can be obtained in, for example, Otero et al., 2000).
Biological polymers are especially sensitive to high hydrostatic pressure. Meanwhile, small molecules, such as those mainly responsible for food taste, color, aroma or nutritional properties, are scarcely affected, at the pressure levels currently employed in food industry (up to 600 MPa) (Tauscher, 1995). This is due among other factors, to the existence of alternative conformations for biopolymers, energetically similar to the native ones. These conformations are often involved in the biomolecule physiological function. On the other hand, other biopolymers, such as cellulose, are scarcely affected by pressure.
The complex behavior of corn starch under pressure has been extensively studied (Douzalset al., 1998; Buckow et al., 2007, 2009; Oh et al., 2008; Kim et al., 2012; Okur et al., 2019, Rahman et al., 2020, Castro et al., 2020, Heydari et al., 2021). Starches from many biological origins have been investigated, since their pressure-behavior is depending on its origin (Belmiro et al., 2020; Conde et al., 2022; Leite et al., 2017). However, this chapter will refer primarily to corn (maize) starch, the most studied starch type, as a model for other sources.
In practice, and especially in such products as food, with a large number of highly concentrated ingredients, reversibility is impaired by many unspecific changes. Thermal processes add the disturbing effect of increased Brownian clashes among molecules in suspensions/solutions, and this gives rise to molecular drifts that alter structures, taking them often far from the original one, which impairs the recovery of the original conformation upon return to atmospheric pressure. Pressure treatments are freer from drift but (as well as thermal ones) cause easily the unspecific aggregation of structures altered by pressure.
All effects induced by changes of hydrostatic pressure are driven by the consequent volume changes associated: pressure increase occasions a volume reduction to minimize the free energy of the system, as described by Le Chatelier’s principle. Van’t Hoff equation describes the dependence of the equilibrium constant with pressure, in relation with the volume change in the process, within thermodynamic, equilibrium conditions. A similar relation can be found for non-equilibrium, kinetic processes, in which the reaction activation volume (volume difference with the activated intermediate state) relates pressure with the rate constant. For details of these equations see, for example: Heremans and Smeller (1998), Molina-García (2002) or Knorr et al. (2006).
The volume increments associated to a number of processes driven by pressure are known, but it must be noted that the volume to be considered is that of the global system, including hydration or solvation layers, which makes difficult to obtain accurate data and to extrapolate them for different treatment conditions. Moreover, unavoidable thermal fluctuations, as well as pressure (and temperature) dependent second order thermodynamic derivative properties, and molecular mobility related factors (viscosity, diffusivity), impair the practical use of volume changes to predict the effect of HHP on food systems. Additionally, pH and ionic dissociation, in general, are affected by HHP (Molina-García, 2002).
Moreover, while protein pressure unfolding can be, under certain circumstances, related with relative ease, to the volume changes induced by pressure, the behavior of starch under HHP is even more difficult to predict, as starch gelatinization is a multi-step process, including irreversible stages.
Proteins undertake somehow similar phenomena when submitted to thermal treatments or to HHP: partial or total (reversible or irreversible) unfolding, dissociation of monomers, denaturation. However, the process and the resulting modified state are not quite the same, for the two perturbations (Heremans & Smeller, 1998). Starch behaves likewise, giving rise to similar but not identical modified products.
10.2 Modification Mechanism and Methodology
The result of HHP treatment of starch aqueous suspensions is its gelatinization, i.e., the well-known phenomenon in which water hydrogen bonds substitutes the intermolecular ones that keep the tight starch granular structure in its place (Muhr et al., 1982). Several observations follow: (1) Water is required for starch to gelatinize under pressure, in the same way that it is needed for thermal gelatinization. Pressure treatment at the levels usually employed for food processing, i.e., up to 600 MPa, in the absence of water (for example, suspending starch in ethanol), have no significant consequences on the granules (Molina-García, unpublished data). (2) No rupture of the starch amylose or amylopectin chains can be expected: the energy for breaking a covalent bond being much higher than that provided to the system by the previously mentioned pressure levels (Heremans & Smeller, 1998; Katopo et al., 2002). (3) Hydrostatic, isostatic pressure is always considered, i.e., a fluid is required to transmit pressure quasi-instantaneously and uniformly for all points in the vessel. For food uses, water is employed (with food-compatible additives in case the treatment involves temperatures close to freezing, to avoid mechanical damages in the pressure equipment). Pressure treatments in dry state cannot be considered hydrostatic: in them, pressure depends of directional vectors, scissor forces are generated and pressure has not a single and constant value over the whole pressure vessel (making possible, in this case, covalent bonds breaking).
Starch gelatinization is a well-known and exhaustively studied process, in which intermolecular hydrogen bonds are replaced by those with water. Gelatinization is essential for allowing accessibility of starch chains to digestive enzymes, as well as for most industrial uses of this product. It carries an associated crystallinity reduction, irreversible granule swelling and partial or total amylose chains release (Waigh et al., 1997). The complex and condensed starch structure is disentangled in a number of steps, some of them irreversible, which endows the whole process of irreversibility.
Figure 10.3 shows a cartoon focusing on two of the phenomena associated to starch pressure gelatinization: granule swelling and reorganization of polysaccharide chains. During gelatinization, starch basic polymeric chains gradually become released from the granules (causing them to swell), interacting with chains from other granules, which gives rise to gels, even when the whole granules are not completely dissolved, still keeping their individual character.
Cartoon showing two phenomena associated to starch pressure gelatinization: granule swelling and reorganization of polysaccharide chains. Starch granules incorporate water and polysaccharide chains become increasingly separated as gelatinization proceeds. Water-amylopectin hydrogen bonds substitutes previous bonds between amylopectin chains within the granule. Interaction among granules, mediated by the protruding amylopectin chains, increases viscosity, creates strong gels and, eventually, causes the complete granule disorganization. While in thermally gelatinized starch the exit of amylopectin from granules and its intergranular interaction is the main cause of viscosity increases, in HHP gelatinized starch viscosity is more dependent on granular swelling
Granule swelling and the interaction of amylose protruding chains from different granules causes large viscosity increases and gelification. However, pressure-treated starch granules preserve their individuality (Figure 10.4b, c) (as a difference with thermally gelatinized ones), with a scarce degree of amylose complete solubilization (Figure 10.4d). Pressure-treated granules can preserve a good part of their crystalline character, even after intense treatments (Katopo et al., 2002). A-crystalline pattern starch (proper of cereals starches) has been observed to change to B (more common in tuber starches), as a result of pressure-induced reorganizations (Yang et al., 2013). A consequence of the interactions among chains from different granules being less extended and part of the crystalline structure still retained in pressure-generated starches, is the weaker character of pressure gels (as compared to thermally induced ones).
Microscopic observations of corn starch granules: (A) Optical micrograph of native granules (untreated); (B) SEM micrograph of samples treated at 400 MPa, 40% w/v starch concentration, 35 min at 40 °C; (C) Optical micrograph of the same treatment as B, (D) Cryo-SEM micrograph of the same treatment as B with higher magnification. A small water content remaining in the starch cryo-SEM preparation allowed visualization of the amylopectin network protruding from granules, normally not visible in SEM micrographs. The bars correspond to 50 μm (A–C) or 20 μm (D)
Pressure has a synergic effect with temperature (Fig. 10.5) and the pressure level required for starch gelatinization is lower at higher temperatures. As well and in the same order as for thermal treatments, starch gelatinization pressures are depending on the biological origin of the starch (Knorr et al., 2006). Treatment time is also a parameter to be considered (Stolt et al., 2000). It has special relevance in industrial contexts where very short pressure processing times are favored. Although some of the effects of hydrostatic pressure are basically instantaneous, the kinetically controlled phases of starch gelatinization are reflected in a progress of the process in time. Figure 10.6 shows a comparison of DSC thermograms for starch treated at the same temperature and pressure but differing in duration. It can be appreciated that, while gelatinization temperature peak and onset are scarcely affected, the area of the gelatinization peak (proportional to the energy of thermally completing the gelatinization of the still ungelatinized starch fraction) is further reduced as treatment time increases.
Starch gelatinization pressure-temperature phase diagram: cartoon after several authors (Douzals et al., 1998; Bauer & Knorr, 2005; Knorr et al., 2006; Buckow et al., 2008). A synergy exists between temperature and pressure, so that starch gelatinization at a given pressure is facilitated by higher temperatures. Other factors of relevance are not considered here, such as time under pressure, starch/water ratio or water incubation previous to pressure treatment
Comparison of Differential Scanning Calorimetry thermograms for starch treated at the same temperature and pressure but differing in treatment duration. Thermal starch gelatinization is globally a two-step process, although its irreversible stages make the global process also irreversible (as shown by the curve wide from). The initial and final states can be seen to be clearly different as having a very distinct heat capacity (position of the baselines before and after gelatinization). In this experiment, corn starch suspensions were partially pressure gelatinized (400 MPa, 25 °C), for different times: 1–0 min, 2–15 min, 3–30 min, 4–60-min. The position of the gelatinization event in the temperature axis (onset and peak temperatures), related to the gelatinization mechanism, are not changing with time. But as time proceeds, the process enthalpy (proportional to the area over the curve), which is related to the number of intramolecular bonds replaced for those with water, is smaller, as a result of pressure gelatinization having advanced, and a smaller fraction of starch being still not gelatinized
Starch pressure gelatinization can be studied by different techniques, which can give information on the progress of the different steps of this process. Transient information (during pressure processing itself) is difficult to obtain in HHP treatments. But treated starch suspensions can be studied just after treatment (and before other processes -thermal, drying, retrogradation- have a chance to alter the HHP outcome) by techniques focusing on chemical groups interactions (RMN or FTIR), energetic balance of the thermal gelatinization still to be completed (DSC), crystallinity related ones, such as loss in birefringence, or molecular mobility and viscosity (Stolt et al., 2000).
Especially useful is DSC, as it has been shown that it can be employed to study starch in different gelatinization states, after drying to powder the resulting pressure-treated suspensions and later reconstituting with water (Teixeira et al., 2018). X-ray diffraction can also be performed in the dry state to obtain important information on crystallinity.
10.3 Starch Modifications by Pressure
10.3.1 Molecular Weight
Based on the gel permeation chromatography results, ultrahigh hydrostatic pressure treatments (690 MPa) did not change the molecular weight distribution of starch samples (Katopo et al., 2002). As already mentioned, hydrostatic pressure treatments average compression energy by means of the pressure transmitting medium. Without these media, pressure effects would be uneven. The average compression energy is not sufficient to break starch covalent bonds. However, in real foods, starch may coexist with amylolytic enzymes. These enzymes could degrade starch polysaccharide chains, once pressure gelatinization has facilitated its digestion.
Another high-pressure food technological process, not to be mistaken with HHP, is high pressure homogenization. In it, sample solutions or suspensions are forced through small perforations at high pressure. This gives rise to extreme scissor forces and can degrade starch granule reducing the size of polysaccharide chains (Apostolides & Mandala, 2020).
10.3.2 Structure and Morphology
Gelatinization implies starch structural alterations, such as a progressive degradation of the granule crystalline regions, based on the double helix arrangement of amylopectin, from initial distortions caused by incipient hydration to a later water intrusion in the crystalline core, for its subsequent complete hydration (Lund, 1984; Oh et al., 2008). Starch crystallinity and micro- and macro-structural properties alterations are closely related to thermal properties (Lemos et al., 2018; Rahman et al., 2020).
The progress of gelatinization is visibly evidenced by changes in the morphology of starch granule. Corn starch granules have polyhedral shape, irregular sizes, and a relatively smooth surface. Granules contain specific channels and cavities penetrating its interior, connected to open surface pores (Fannon et al., 1993; van de Velde et al., 2002). These granule morphological parameters show only slight changes, as observed by scanning electron microscopy (SEM), after moderate HHP treatments (300 MPa during 15–30 min, Rahman et al., 2020, or 400 MPa at 38 °C for 35 min, Deladino et al., 2015), when size is slightly increased, and surface gets faceted and rougher. At the latter conditions, the alteration of pores connecting surface and granule inner core is observed by confocal laser scanning microscopy, which also allowed observing the increased granule size, due to a limited swelling. A higher level of penetration of FITC dye was attributed to these pores and to the presence of voids and fractures due to incomplete HHP-induced gelatinization (Teixeira et al., 2015).
At these intermediate pressure levels, a narrowing in the profile of native starch pore size (from 8 to 60 nm, with a maximum number of pores between 8 and 12 nm, to pore diameters ranging from 4 to 10 nm), accompanied of an increased of the total pore volume, is reported, as determined by mercury intrusion. Moreover, when using the nitrogen adsorption/desorption technique, HHP treated starch showed a decrease in pore size distribution and changes in the distribution curve shape, with an increase in the number of smallest pores. The BET surface area increased from 0.277 m2/g for native starch to 0.407 m2/g for the HHP treated starch, showing an increase of 47% due to HHP treatment (Deladino et al., 2015). These results were attributed to granule reorganization induced by high pressure, which may enhance the connection between external pores with interior channels that were not accessible in untreated starch.
Higher pressures can alter granular surface, creating cracks, flattening granules, which collapse at its center, and completely disintegrating granules (Błaszczak et al., 2005; Douzals et al., 1998; Heydari et al., 2021; Rahman et al., 2020). After Okur et al. (2019), morphological changes develop slowly, in lengthy treatments, at pressures over 500 MPa.
The observation of the gelatinization process by light microscopy photographs of I2 solution-dyed starch treated at 400 MPa (at 40 °C for 15 min), showed a high level of disorder at the center of the granule while the classic non-gelatinized granular ordering of native starch was intact at the external layers. However, at more drastic conditions (700 MPa, at 35 °C, for 25 min) the erosion of the surface and the granule integrity loss and the formation of a gel network, are the features appreciated (Teixeira et al., 2018) and might be associated to the hydration of the amorphous phase and/or melting of crystallinity (Wang et al., 2008). Rahman et al. (2020) also observed by SEM that maize starch granules were completely disintegrated and gelatinized, showing large starch granules and lamellar structure when treated at 500 MPa for 15 and 30 min.
Starch crystallinity is based on the double helix arrangement of amylopectin. X-ray diffraction provides information on these crystalline domains. It allows the classification of native different biological origin starches as A-type (cereals: rice, corn), B-type (tubers: potato) and C-type (tapioca, pea) starches (Cheetham & Tao, 1998; Hibi et al., 1993; Le Bail et al., 1999; Zobel, 1988). Starch crystalline helices are more compact in A than in B-type, which has a more hydrated core. C-type is often considered as a mixture of both A and B-types (Teixeira et al., 2018).
Li et al. (2011) reported a weakening of diffraction peaks with increasing pressure, related to the destruction of crystalline structure in gelatinization. B-type starches resist pressure more than A- or C-type starches (Katopo et al., 2002; Oh et al., 2008; Rahman et al., 2020; Rubens et al., 1999; Stute et al., 1996). A-type could have more scattered and flexible branching amylopectin structures than B-type, and can be rearranged under pressure, generating channels letting water molecules in, which triggers the pressure-induced transformation from A to B-type (Katopo et al., 2002; Yang et al., 2016; Teixeira et al., 2018). Actually, Teixeira et al., (2018) found that peaks characteristics from A-type and B-type patterns coexist after HHP treatment, evidencing the partial gelatinization transformation. Similarly, Rahman et al. (2020) detected the decrease of intensity of A-type peaks and the appearance of peaks typical of B-type pattern in treatments at 500 MPa and attributed the transformation of the diffraction pattern from A-type to B-type to the disruption of the crystalline regions of maize starch granules.
The crystallinity fraction (CF) is closely related to the molecular structure and contents of both of amylopectin and amylose (including chain length, branching and polydispersity). It is also related to starch interesting properties, such as pasting and starch digestibility (Irani et al., 2017). CF is reduced after HHP treatments, as follows from crystalline structure weakening and destruction (Heydari et al., 2021). Native corn starch CF, 24%, as derived from X-ray diffraction data (Teixeira et al., 2015), is reduced by nearly a 50% by HHP treatments at moderate pressures (400 MPa, 30 °C, 15 or 35 min), while higher pressures (700 MPa) leave a residual CF of 2.8%, only a 11.7% of the native crystallinity (Teixeira et al., 2018). Other authors also found a similar loss of native crystalline structure and molecular order (Katopo et al., 2002). At the same type, the B-type crystallite increased, more drastically at the more elevated pressures (in good agreement with the GD obtained from DSC measurements) (Teixeira et al., 2018). This A and B-type patterns mixture resulting from HHP treatment was suggested to be induced by recrystallization (Choi et al., 2009), just after pressure-gelatinization. Different starch/water ratio (SWR) are not correlated to significant modifications in peak positions and intensities, or CF (Teixeira et al., 2018).
In samples submitted to very high pressure (700 MPa) or long treatment times at 400 MPa, an additional X-ray diffraction pattern, V, was detected. V-diffraction pattern reflects the presence of complexes of amylose with lipids and similar molecules (Cheetham & Tao, 1998; Shi et al., 2017). The preservation of starch structure after harsher pressure treatments (9 min at 650 MPa), although its crystallinity decreased, has been justified resorting to V-pattern, in high amylose samples also containing lipids, whose complexes would impair swelling stabilizing the granular structure (Katopo et al., 2002; Teixeira et al., 2018; Wang et al., 2008). Peaks characteristic of V-pattern (Shamai et al., 2003) have intensities increasing with treatment time and SWR at moderate pressures (Teixeira et al., 2018). More extreme conditions (700 MPa, 25 min, 30 °C), gave rise to a high intensity for this peak. This implies also a reduced amylose leach, as it is complexed in the granule (Teixeira et al., 2018). Similar results were obtained by Le Bail et al. (1999).
Addition of hydrocolloids before pressure treatments does not alter starch crystalline fraction. However, V-type polymorph X-ray peak intensity increases, in correlation with lower amylose concentration in solution. The formation of the V-crystalline complex would be favored, as hydrocolloids, linear polysaccharides, could contribute to amylose stabilization, leading to lower amylose release of HHP treated starch (Katopo et al., 2002; Teixeira et al., 2018).
HHP affects starch component chemical bonding, and this can reflect on FTIR spectra (Cui & Zhu, 2019). HHP treatment respects corn starch FTIR spectra major peaks but there is a clear effect on peak intensity. Broadbands (3100–3700 cm−1) are related to starch OH− stretching (Jiang et al., 2011). Corn starch OH − stretching band width and intensity was reportedly reduced after HHP treatments at 500 MPa, while unaffected at low (100 MPa) or medium (300 MPa) pressures, although there are significant changes in the fingerprint area. Moreover, the asymmetric C–H stretching band also shows peak lowering and narrowing, reflecting changes in conformation and crystallinity of amylose-amylopectin (Kizil et al., 2002; Rahman et al., 2020). Other peaks, related to firmly bound water and characteristic of the anhydrous glucose ring C-O stretch, had also their intensity reduced after pressure treatment (Fang et al., 2002).
RMN T2 transverse relaxation time studies suggest that longer HHP treatments at elevated temperatures have a complex effect, in terms of hydration and starch structural changes. T2 increases after HHP treatments, meanwhile heat-induced gelatinization is associated to T2 decreases (Okur et al., 2019; Ozel et al., 2017). Other heat and HHP-induced starch gelatinization differences can also contribute to the reverse effect on T2, such as the shear forces associated to stirring, usually absent in HHP treatments (BeMiller & Huber, 2015). The apparition of B-type crystals in pressure-induced gelatinization implies reduced double helix dissociation (while heat gelatinization causes intense double helix dissociation) (Pei-Ling et al., 2010), which produced less swollen starch granules due to reduced amylose leaching (Yang et al., 2016).
10.3.3 Swelling and Solubility
Gelatinization gives rise to hydration of starch amorphous regions, causing granular swelling. A second step of this process implies further granule weakening and swelling, release of amylose chains and interaction among these from different granules, with loss of granule identity, in the way to gel formation: a dispersion of free amylose and amylopectin including granule remnants (BeMiller & Whistler, 1996; Lund, 1984; Oh et al., 2008). HHP gelatinized starch shows differences from the heat-induced product, including less amylose release, and reduced starch granule swelling and disintegration (Knorr et al., 2006; Stute et al., 1996).
Granule birefringence under polarized light is closely associated to swelling and loss of internal structure. Its characteristic Maltese cross is due to amylopectin double helix radial orientation in starch crystalline regions (Castro et al., 2020; Deng et al., 2014). While potato starch retains birefringence after a strong pressure treatment (600 MPa, 30 min), corn and tapioca starches loss this property totally or partially at these pressures. At lower pressures (150–300 MPa), birefringence was still shown. However, at intermedium values (400–450 MPa), birefringence reduction was noted, while it was partially maintained (Castro et al., 2020; Oh et al., 2008; Teixeira et al., 2015). In a similar way to other starch properties related to structure, or to its thermal parameters, swelling behavior after HHP depends on starch biological origin and treatment parameters (Oh et al., 2008). Some authors consider it dependent on granule size and its distribution, and on the amylose content (Heydari et al., 2021; Oh et al., 2008).
The swelling power of HHP treated starches has been reported as higher than in native starch (Heydari et al., 2021) and, contrariwise, lower than in untreated starch (Okur et al., 2019). Oh et al. (2008) found an 18% swelling degree after pressurization at 400 MPa for corn starch. In a similar way, Deladino et al. (2017) found that HHP almost doubled the swelling power of corn starch treated at 400 MPa (at 38 °C, for 30 min), over a thermal gelatinization value around 8%. However, Rahman et al. (2020) found that both solubility and swelling power of maize starch were significantly decreased with increasing pressure (0.1 to 500 MPa) in comparison with native starch. Corn starch swelling at 600 MPa was under 50% (Oh et al., 2008; Stute et al., 1996). Some starches including corn, have a reduced swelling associated to HHP treatment, perhaps related to a lower thermal drift effect, as compared to thermal gelatinization. On its hand, waxy corn starch was not found to swell considerably at intermediate pressures (400 MPa), while achieving 100% swelling at 600 MPa. Potato starch was much less affected by swelling (Oh et al., 2008). Some authors (Li et al., 2012), consider swelling as caused by amylopectin, as amylose would be scarcely solubilized, as most granules would be still intact. A limited granular swelling is usually taking place after HHP treatments (Stolt et al., 1999; Heydari et al., 2021).
Some authors (Katopo et al., 2002; Teixeira et al., 2018), observed (by scanning electron microscopy) a retention of granular identity in corn starch, even after HHP treatment at 690 MPa. Lower amylose release and less water binding could justify this granular preservation (Douzals et al., 1998), while amylose–lipid complexes could have a role in restricting swelling (Katopo et al., 2002; Liu et al., 2016).
Amylose solubilization, taking place at the more advanced gelatinization phases, can also be affected by HHP treatments, either increasing or limiting it, through the amylose-amylopectin interaction (Liu et al., 2016). Different balances of inter-associative forces within starch amorphous and crystalline domains, amylose/amylopectin ratio and their branching characteristics, together with the role of other minor starch components may be the cause of these solubilization differences (Kumoro et al., 2012; Liu et al., 2016). Corn starch gelatinized under HHP conserve most of its granule independence and the solubilization of amylose is limited (Douzals et al., 1998; Knorr et al., 2006; Stolt et al., 2000; Teixeira et al., 2018). Birefringence reveals only some granules gelatinized and swollen at moderate pressures and temperatures (400 MPa, 30 °C), while harsher treatments (700 MPa) affect most granules, whose integrity is lost, while a gel network can be detected (Oh et al., 2008; Teixeira et al., 2018).
The main differences of HHP gelatinized starches with thermally treated ones can be summarized in a smaller amount of amylose released from the granules, a limited granule swelling and starch granules appearing intact or just partially disintegrated (Knorr et al., 2006; Teixeira et al., 2018).
10.3.4 Thermal Properties
Thermal parameters are a suitable measurement of the gelatinization progress. A DSC experiment with native starch produces a single endotherm, providing the SWR under 30%. At higher SWR values, gelatinization, basically a reaction with water, is incomplete, and additional thermal transitions are obtained. Meanwhile, higher water contents ensures a complete gelatinization, practically independent of the actual ratio (Wang et al., 2014, 2016). Some concerns about the availability of water for starch gelatinization in HHP treatments have been addressed elsewhere (Teixeira et al., 2018).
The endotherm is roughly symmetrical, though not completely, reflecting the nonreversible character of gelatinization. The temperature at which it takes place: onset temperature (To) (the endotherm starts, usually considered the gelatinization temperature) is a measurement of the stability of the initial starch state. Tpeak and Tendset, reflect starch heterogeneity, which widens the transition. The area under the thermogram, the endotherm enthalpy (ΔH), corresponds to the energy exchange in the process. Both enthalpy and temperatures are characteristic of each starch type.
A DSC thermogram of previously treated starch informs of the extent of the gelatinization undergone (the gelatinization degree, GD) and the remaining intramolecular bonds, still not replaced by hydration (Oh et al., 2008). After a given HHP (or thermal) treatment, the ΔH is reduced, indicating partial gelatinization. If the process is complete, no endotherm will be observed. For corn, depending on authors, varieties and other process parameters, this takes place between 500 and 700 MPa (Teixeira et al., 2018). This enthalpy decrease reflects the energy employed in breaking the intramolecular hydrogen bonds in helical chains of the crystalline regions and, therefore, the progress in the crystallinity and internal granule order destruction by the different treatments leading ultimately to gelatinization (Castro et al., 2020).
While the decrease of enthalpy upon treatments inducing gelatinization is widely accepted, in dependence of treatment pressure, duration and starch biological origin (Stolt et al., 2000; Bauer & Knorr, 2005; Kawai et al., 2007a, b; Teixeira et al., 2018; Cui & Zhu, 2019), this is not the same with gelatinization temperature. Many factors related to starch origin, amylose/amylopectin composition, crystalline degree, etc., are involved, and results differ for different cases and authors. The actual extent of gelatinization may be also a crucial factor. Justification of this temperature drift can be based in the destabilization of crystalline structure by partial gelatinization (which would contribute to To decrease), or in the preferential gelatinization of the smaller (less resistant) granules, leaving intact the larger and more stable ones (which would cause To increase) (Castro et al., 2020; Teixeira et al., 2018). According to some authors, starches with higher crystallinity degrees would exhibit higher ΔH and To (Heydari et al., 2021).
HHP treated (600 MPa) corn starch To was reported to increase over that of native starch (Katopo et al., 2002; Oh et al., 2008). However, Rahman et al. (2020) found a significant decrease at treatments at 300 MPa (with a widened To range), while at 500 MPa, no endotherm was reported. Similar results were observed in other biological origin starches (Guo et al., 2015; Heydari et al., 2021; Li & Zhu, 2018). The decrease in To has been ascribed to granule structure disruption and crystallinity reductions (Błaszczak et al., 2005). Other authors report virtually no change (or only a slight reduction) in To for potato varieties starch (Cui & Zhu, 2019) and corn at moderate pressures (300–400 MPa), while at 700 MPa, To could be seen to decrease (Teixeira et al., 2018; Heydari et al., 2021).
The degree of starch gelatinization measured by DSC increased accordingly with the intensity (pressure level and exposure time) of the HHP treatment. The data from several studies is summarized in Table 10.1, which shows that HHP treatment may have a range of effects on starch, including inducing gelatinization, altering crystalline structure, rheology and improving the thermal stability.
Achieving only a partial gelatinization degree through HHP treatment is not always a failure: partially gelatinized starch shows mixed properties: granular identity and independence, but incipient interactions among the partially degraded granules and with other molecules. HHP provides more homogeneous and better-defined partially gelatinized products, with a specific crystallinity degree, in a process easier to monitorize (Heydari et al., 2021; Knorr et al., 2006; Teixeira et al., 2018).
The presence of hydrocolloids did not introduce significant differences in these parameters (Teixeira et al., 2018; Tester & Sommerville, 2003). However, for thermal gelatinization, a possible stabilization of the granular structure by hydrocolloid has been reported (Biliaderis et al., 1997). On the other hand, enthalpy may be reduced by bacterial cellulose fibrils (Díaz-Calderón et al., 2018).
10.3.5 Pasting Properties
Several studies support the fact that the application of pressure imparts structural changes on starch, restricting the leaching of amylose and amylopectin, increasing pasting temperature and reducing viscosity (Ahmed & Thomas, 2017; Heydari et al., 2021; Hoover et al., 2010; Liu et al., 2016). Pasting temperature agrees with the temperature at which gelatinization of starch begins. Li and Zhu (2018) reported that the pasting temperature decreased at 600 MPa for corn starch, whereas the peak pasting temperature was increased, agreeing with the result previously informed by Oh et al. (2008) for normal and waxy corn starch.
Regarding viscosity, Oh et al. (2008) found that the initial viscosity for waxy corn starch increased from 5.7 MPa s1 before pressure treatment to 3530 MPa s1 after pressure treatment at 600 MPa. BeMiller (2019) also reported that the viscosity increased in association to the increase in swollen starch granules and its maximum values corresponded to the formation of complexes among different granules.
10.3.6 In Vitro Digestibility
Starch can be divided into three groups according to its digestion rate: resistant starch (RS), rapidly digestible starch (RDS), and slowly digestible starch (SDS). Digestion of SDS occurs at a lower rate than RDS, so SDS raises blood-glucose levels more slowly than RDS, being helpful in preventing hyperglycemia-related diseases (Okur et al., 2019). Accordingly, SDS would be the more appropriate type of dietary starch, since its digestion in the small intestine is retarded and it would reduce postprandial blood glucose levels (Huang et al., 2020). Shen et al. (2018) studied the effect of high-pressure treatment on the internal structure of high amylose corn starch and its influence on digestion. They found that HHP treatment at 200 and 400 MPa led to a lower digestion rate compared to native starch. But HHP treatment at 600, 800 and 1000 MPa resulted in a significantly enhanced digestion rate. In the same way, HHP caused a significant decrease at 400 and 500 MPa for RS content of corn starch, while SDS content increased significantly (Okur et al., 2019). Papathanasiou et al. (2015) measured the available glucose content after the enzymatic digestion of starch type A, B, C and resistant starch was examined. The pressurized starch suspensions were treated, achieving different gelatinization degrees (from 25% to 100%) and they released less amount of glucose after enzymatic digestion in comparison to the equivalent thermally treated samples. Among the studies samples, resistant and waxy corn starch exhibited the lowest levels of released glucose after enzymatic digestion. So, they could be used to produce starch-based low-glucose foods. Besides, the effect of freeze-drying on their available glucose content after enzymatic digestion was not significant, meaning that treated starches tolerated one of the typical industrial procedures. Meanwhile, pressure-gelatinized (600 MPa, 15 min) samples of normal and waxy corn starches showed a rise in resistant starch content and relative crystallinity degree with the increase in storage time. These retrograded starches also showed restricted starch swelling power and lower pasting viscosities.
Moreover, different moisture contents (30–70%) digestion rates of high amylose corn starch did not change significantly at pressures in the range 200–800 MPa but did at 1000 MPa, suggesting that applied pressure plays an important role rather than moisture content in altering starch digestion (Shen et al., 2018).
According to these studies, treatments at low pressure would lead to the reordering of crystalline structure or molecular chains, to make the internal structure of starch granules more organized than its native state. However, a high-pressure application can destroy the internal arrangement, giving rise to a less-organized structure, which enhances the susceptibility of starch molecules to enzymatic hydrolysis.
10.4 Applications
Many of the structural changes that HHP provokes on starch granules positively impact its functional and nutritional properties, diversifying its uses in food, medical or pharmaceutical applications. Figure 10.7 reviews some potential applications of HHP treated corn starches.
10.5 Previous and Recent Trends in Feasibility and Utilization of Modified Starch
HHP induces modifications on biopolymers and represents a workable opportunity to avoid chemical or genetically modified ingredients in health and food-related industries, attending to consumers’ claims on chemical-free, clean, and/or green technologies.
High hydrostatic pressure commercialized products are mainly sterilized or pasteurized stuff, such as processed meat, fish, juices, fruit-based preparations and ready-to-eat products in general, products where the microbial safety is important (restricts microbial spoilage in fruit juices and milk). Also, it has been adopted in emulsion processing (reduction in droplet size contributes to emulsion increased stability) and pharmaceuticals (O/W emulsions employed in drug delivery to increase bioavailability) (Aganovic et al., 2021; Dumay et al., 2013; Raghunathan et al., 2021). Figure 10.8 summarizes the regulation institutions and the current legal status of HHP worldwide.
10.6 Case Studies
Several combinations of pressure and temperature have been performed while studying the effect of HHP on corn carrying properties (Deladino et al., 2015, 2017; Teixeira et al., 2015, 2018). The different treatments were applied on 10 g/100 mL corn starch aqueous suspensions. The crystalline fraction (CF) was calculated from X-ray diffraction (XRD) patterns as the ratio between the absorption peaks and the total diffractogram area, expressed as a percentage (%) (Teixeira et al., 2015). The degree of gelatinization of starches was determined by DSC (Tp: peak temperature (starch gelatinization temperature); ΔG: enthalpy of gelatinization on dry basis). Table 10.2 summarizes the most important effects of HHP treatments on carrying ability of corn starches and/or their potential for the design of new ingredients for food formulations with nutritional and functional added value.
High pressure caused partial granule gelatinization and increased the granule specific surface area (Table 10.2). This increase in surface was due to the generation of pores, which favored the adsorption of the yerba mate hydrosoluble polyphenols (Deladino et al., 2015). Alkali treatment also increased the number of pores in the granules whereas the combined procedures (HHP + alkali) led to a central disruption and an enlarged deep pore number (Fig. 10.9). Accordingly, the high pore volume found in alkali-treated starches created more binding sites to load high amounts of zinc and magnesium (Teixeira et al., 2018). Also, the decrease in temperature and enthalpy of gelatinization, associated to high pressure granular starch suspensions, was moderated in alkali-treated samples by the creation of new charged interactions.
Confocal laser scanning micrographs of corn starch granules: (a) Native granules (untreated), (b) Alkali-treated starch, (c) Samples treated at 400 MPa, 10% w/v starch concentration and 35 min at 40 °C, and (d) HHP treatment as in C + alkali. The bars correspond to 10 μm. Large and deep pores can be seen developing in pressure-treated samples (c, d), which can be of interest for incorporating binding agents (e.g., minerals, nutrients)
The X-ray diffractometry A pattern is associated mainly with cereal starches in the native granular forms, the C pattern is a mixture of both A and B types, the last one being more resistant to amylolytic digestion (Liu et al., 2016).
The fact that all samples with hydrocolloids showed higher intensities of V-type polymorph peak was correlated with a lower amylose concentration in the supernatants, compared to the HHP treated sample without gum, indicating that the formation of the V-crystalline complex would be favored by hydrocolloid presence under pressure (Teixeira et al., 2018). Hydrocolloids, being linear polysaccharides, would contribute to the lixiviated amylose stabilization, leading to lower amylose contents in the supernatants of HHP treated samples.
10.7 Research Gaps and Scope of Future Studies
Research on HHP-modified starch future trends would be surely bond to both the developments taking place in HHP-treated food products, as well as the market and consumer requirements for starch as a main food component, not forgetting the newest consumer acceptance trends.
An always rich source of new ideas and developments lies on the study of starch biopolymer within foods, either as a natural component or as an added ingredient (with nutritional, textural or other purposes). The complexity of HHP effects on starch gets multiplied when research is focused in complex mixtures, with a limited water availability and, moreover, when thermal or other simultaneous processes (may be homogenization, blending, ultrasounds, etc.) are to be considered.
Other source for new products to be studied come from the introduction of different plants in diet, bringing associated different starch types that will have to be characterized and whose potential applications should be tuned with the more interesting technological approaches. Starch can be used also as a binder, texture creator, encapsulating agent and for many other structural derived purposes. Combinations of the already known products and their properties with novel ones, would multiply the richness that this product can endow to food production.
A present trend in consumers is the search for less energy-rich food ingredients that still preserve the textural and flavor properties of traditional foods. All type of research studies on HHP starch dwelling on the generation of resistant starch, its characterization, and the progress in the knowledge of how it behaves in real nutrition, will find an immediate echo, both in industry and consumers.
Lastly, food properties that are currently carried out by proteins, especially animal origin ones, are growingly being replaced by different molecules, that could mimic meat properties in its interactions or, in a very particular case, substitute the remarkable gluten properties that give rise to the wide bread products family. To modify starch in ways in which the sought binding, elasticity, gas retaining properties of meat proteins and gluten can be substituted, is very surely to be one of the trendier food processing lines in the near future, and HHP-treated starch can have a very promising role in the achievement of this goal.
10.8 Conclusions
HHP is a chemical free tool to achieve modifications on starch. The broadened uses of this technology in food industry would counteract the drawback of the high cost of the equipment, since nowadays companies can rent their pressure equipment, increasing the accessibility of this technology for obtaining new starch-based materials. The main advantage of this treatment is the possibility of obtaining tailor-made ingredients, by modifying the operating conditions (time, pressure) and the incorporation of other ingredients during the process. Many factors have a strong influence on starch response to HPP, principally the biological origin, but also a range of treatment parameters, such as pressure levels, process temperature, treatment duration and starch to water ratio.
The differences between HHP and thermally gelatinized corn starches can be summarized in a smaller amount of amylose released from granules, a limited granule swelling and the partial preservation of granular integrity. The presence of hydrocolloids does not have a significant influence on HPP induced corn starch gelatinization, though may give rise to crystallinity changes implying a limited release of amylose.
The possibilities of achieving only a partial gelatinization degree though HHP treatment may be of practical interest, as partially gelatinized corn starch shows a mix of properties: granular identity and independence are preserved, but incipient interactions among granules and with other molecules are formed. HHP would yield more homogeneous, better-defined partially gelatinized products with a specific crystallinity degree than the thermal treatment, in an easily controllable process.
HHP treatments are a valuable resource for increasing the range of starch derived formulations for applications so different as transport of molecules of nutritional interest, controllable behavior within complex food products and gelatinization versatility.
References
Aganovic, K., Hertel, C., Vogel, R. F., Johne, R., Schlüter, O., Schwarzenbolz, U., et al. (2021). Aspects of high hydrostatic pressure food processing: Perspectives on technology and food safety. Comprehensive Reviews in Food Science and Food Safety, 20(4), 3225–3266.
Ahmed, J., & Thomas, L. (2017). Pasting properties of starch: Effect of particle size, hydrocolloids and high pressure. In Glass transition and phase transitions in food and biological materials (Vol. 427). Wiley.
Apostolidis, E., & Mandala, I. (2020). Modification of resistant starch nanoparticles using high-pressure homogenization treatment. Food Hydrocolloids, 103, 105677.
Bauer, B. A., & Knorr, D. (2005). The impact of pressure, temperature and treatment time on starches: pressure-induced starch gelatinisation as pressure time temperature indicator for high hydrostatic pressure processing. Journal of Food Engineering, 68(3), 329–334.
Belmiro, R. H., Tribst, A. A. L., & Cristianini, M. (2020). Effects of high pressure processing on common beans (Phaseolus Vulgaris L.): cotyledon structure, starch characteristics, and phytates and tannins contents. Starch-Stärke, 72(3–4), 1900212.
BeMiller, J. N. (2019). Corn starch modification. In Corn (pp. 537–549). AACC International Press.
BeMiller, J. N., & Huber, K. C. (2015). Physical modification of food starch functionalities. Annual Review of Food Science and Technology, 6, 19–69.
BeMiller, J. N., & Whistler, R. L. (1996). Carbohydrates. Marcel Dekker.
Biliaderis, C. G., Arvanitoyannis, I., Izydorczyk, M. S., & Prokopowich, D. J. (1997). Effect of hydrocolloids on gelatinization and structure formation in concentrated waxy maize and wheat starch gels. Starch-Stärke, 49(7–8), 278–283.
Błaszczak, W., Valverde, S., & Fornal, J. (2005). Effect of high pressure on the structure of potato starch. Carbohydrate Polymers, 59(3), 377–383.
Błaszczak, W., Buciński, A., & Górecki, A. R. (2015). In vitro release of theophylline from starch-based matrices prepared via high hydrostatic pressure treatment and autoclaving. Carbohydrate Polymers, 117, 25–33.
Bridgman, P. W. (1964). The coagulation of albumen by pressure. In Collected Experimental Papers (Vol. II, pp. 735–736). Harvard University Press.
Buckow, R., Heinz, V., & Knorr, D. (2007). High pressure phase transition kinetics of maize starch. Journal of Food Engineering, 81(2), 469–475.
Buckow, R., Jankowiak, L., Knorr, D., & Versteeg, C. (2009). Pressure-temperature phase diagrams of maize starches with different amylose contents. Journal of Agricultural and Food Chemistry, 57(24), 11510–11516.
Cappa, C., Barbosa-Cánovas, G. V., Lucisano, M., & Mariotti, M. (2016). Effect of high pressure processing on the baking aptitude of corn starch and rice flour. LWT, 73, 20–27.
Castro, L. M., Alexandre, E. M., Saraiva, J. A., & Pintado, M. (2020). Impact of high pressure on starch properties: A review. Food Hydrocolloids, 106, 105877.
Cheetham, N. W., & Tao, L. (1998). Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydrate Polymers, 36(4), 277–284.
Choi, H. S., Kim, H. S., Park, C. S., Kim, B. Y., & Baik, M. Y. (2009). Ultra high pressure (UHP)-assisted acetylation of corn starch. Carbohydrate Polymers, 78(4), 862–868.
Conde, L. A., Kebede, B., Leong, S. Y., & Oey, I. (2022). Effect of High Hydrostatic Pressure Processing on Starch Properties of Cassava Flour. Applied Sciences, 12(19), 10043.
Cui, R., & Zhu, F. (2019). Physicochemical properties and bioactive compounds of different varieties of sweetpotato flour treated with high hydrostatic pressure. Food Chemistry, 299, 125129.
Deladino, L., Teixeira, A. S., Navarro, A. S., Alvarez, I., Molina-García, A. D., & Martino, M. (2015). Corn starch systems as carriers for yerba mate (Ilex paraguariensis) antioxidants. Food and Bioproducts Processing, 94, 463–472.
Deladino, L., Teixeira, A. S., Plou, F. J., Navarro, A. S., & Molina-García, A. D. (2017). Effect of High Hydrostatic Pressure, alkaline and combined treatments on corn starch granules metal binding: Structure, swelling behavior and thermal properties assessment. Food and Bioproducts Processing, 102, 241–249.
Deng, Y., Jin, Y., Luo, Y., Zhong, Y., Yue, J., Song, X., & Zhao, Y. (2014). Impact of continuous or cycle high hydrostatic pressure on the ultrastructure and digestibility of rice starch granules. Journal of Cereal Science, 60(2), 302–310.
Díaz-Calderón, P., MacNaughtan, B., Hill, S., Foster, T., Enrione, J., & Mitchell, J. (2018). Changes in gelatinisation and pasting properties of various starches (wheat, maize and waxy maize) by the addition of bacterial cellulose fibrils. Food Hydrocolloids, 80, 274–280.
Douzals, J. P., Perrier Cornet, J. M., Gervais, P., & Coquille, J. C. (1998). High-pressure gelatinization of wheat starch and properties of pressure-induced gels. Journal of Agricultural and Food Chemistry, 46(12), 4824–4829.
Dumay, E., Chevalier-Lucia, D., Picart-Palmade, L., Benzaria, A., Gràcia-Julià, A., & Blayo, C. (2013). Technological aspects and potential applications of (ultra) high-pressure homogenisation. Trends in Food Science & Technology, 31(1), 13–26.
Fang, J. M., Fowler, P. A., Tomkinson, J., & Hill, C. A. S. (2002). The preparation and characterisation of a series of chemically modified potato starches. Carbohydrate Polymers, 47(3), 245–252.
Fannon, J. E., Shull, J. M., & BeMILLER, J. N. (1993). Interior channels of starch granules. Cereal Chemistry, 70, 611–611.
Fernández, P. P., Sanz, P. D., Martino, M. N., & García, A. M. (2008). Partially-gelatinised starches by high hydrostatic pressure as oligoelement carriers. Spanish Journal of Agricultural Research, 1, 129–137.
Guo, Z., Zeng, S., Zhang, Y., Lu, X., Tian, Y., & Zheng, B. (2015). The effects of ultra-high pressure on the structural, rheological and retrogradation properties of lotus seed starch. Food Hydrocolloids, 44, 285–291.
Heremans, K., & Smeller, L. (1998). Protein structure and dynamics at high pressure. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology, 1386(2), 353–370.
Heydari, A., Razavi, S. M. A., Hesarinejad, M. A., & Farahnaky, A. (2021). New insights into physical, morphological, thermal, and pasting properties of HHP-treated starches: Effect of starch type and industry-scale concentration. Starch-Stärke, 73(7–8), 2000179.
Hibi, Y. O. S. H. I. K. O., Matsumoto, T. A. D. A. S. H. I., & Hagiwara, S. H. I. G. E. K. O. (1993). Effect of high pressure on the crystalline structure of various starch granules. Cereal Chemistry, 70, 671–671.
Hoover, R., Hughes, T., Chung, H. J., & Liu, Q. (2010). Composition, molecular structure, properties, and modification of pulse starches: A review. Food Research International, 43(2), 399–413.
Huang, H. W., Hsu, C. P., & Wang, C. Y. (2020). Healthy expectations of high hydrostatic pressure treatment in food processing industry. Journal of Food and Drug Analysis, 28(1), 1–13.
Irani, M., Abdel-Aal, E. S. M., Razavi, S. M., Hucl, P., & Patterson, C. A. (2017). Thermal and functional properties of hairless canary seed (Phalaris canariensis L.) starch in comparison with wheat starch. Cereal Chemistry, 94(2), 341–348.
Jiang, Q., Gao, W., Li, X., & Zhang, J. (2011). Characteristics of native and enzymatically hydrolyzed Zea mays L., Fritillaria ussuriensis Maxim. and Dioscorea opposita Thunb. starches. Food Hydrocolloids, 25(3), 521–528.
Katopo, H., Song, Y., & Jane, J. L. (2002). Effect and mechanism of ultrahigh hydrostatic pressure on the structure and properties of starches. Carbohydrate Polymers, 47(3), 233–244.
Kawai, K., Fukami, K., & Yamamoto, K. (2007a). Effects of treatment pressure, holding time, and starch content on gelatinization and retrogradation properties of potato starch–water mixtures treated with high hydrostatic pressure. Carbohydrate Polymers, 69(3), 590–596.
Kawai, K., Fukami, K., & Yamamoto, K. (2007b). State diagram of potato starch–water mixtures treated with high hydrostatic pressure. Carbohydrate Polymers, 67(4), 530–535.
Khan, B., Bilal Khan Niazi, M., Samin, G., & Jahan, Z. (2017). Thermoplastic starch: A possible biodegradable food packaging material—A review. Journal of Food Process Engineering, 40(3), e12447.
Kim, H. S., Kim, B. Y., & Baik, M. Y. (2012). Application of ultra high pressure (UHP) in starch chemistry. Critical Reviews in Food Science and Nutrition, 52(2), 123–141.
Kizil, R., Irudayaraj, J., & Seetharaman, K. (2002). Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. Journal of Agricultural and Food Chemistry, 50(14), 3912–3918.
Knorr, D., Heinz, V., & Buckow, R. (2006). High pressure application for food biopolymers. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1764(3), 619–631.
Kumoro, A. C., Retnowati, D. S., & Budiyati, C. S. (2012). Water solubility, swelling and gelatinization properties of raw and ginger oil modified gadung (Dioscorea hispida Dennst) flour. Research Journal of Applied Sciences, Engineering and Technology, 4(1), 2854–2860.
Larrea-Wachtendorff, D., Di Nobile, G., & Ferrari, G. (2020). Effects of processing conditions and glycerol concentration on rheological and texture properties of starch-based hydrogels produced by high pressure processing (HPP). International Journal of Biological Macromolecules, 159, 590–597.
Larrea-Wachtendorff, D., Sousa, I., & Ferrari, G. (2021). Starch-Based Hydrogels Produced by High-Pressure Processing (HPP): Effect of the Starch Source and Processing Time. Food Engineering Reviews, 13(3), 622–633.
Le Bail, P., Bizot, H., Ollivon, M., Keller, G., Bourgaux, C., & Buleon, A. (1999). Monitoring the crystallization of amylose–lipid complexes during maize starch melting by synchrotron x-ray diffraction. Biopolymers: Original Research on Biomolecules, 50(1), 99–110.
Leite, T. S., de Jesus, A. L. T., Schmiele, M., Tribst, A. A., & Cristianini, M. (2017). High pressure processing (HPP) of pea starch: Effect on the gelatinization properties. LWT-Food Science and Technology, 76, 361–369.
Lemos, P. V. F., Barbosa, L. S., Ramos, I. G., Coelho, R. E., & Druzian, J. I. (2018). The important role of crystallinity and amylose ratio in thermal stability of starches. Journal of Thermal Analysis and Calorimetry, 131(3), 2555–2567.
Li, G., & Zhu, F. (2018). Effect of high pressure on rheological and thermal properties of quinoa and maize starches. Food Chemistry, 241, 380–386.
Li, W., Zhang, F., Liu, P., Bai, Y., Gao, L., & Shen, Q. (2011). Effect of high hydrostatic pressure on physicochemical, thermal and morphological properties of mung bean (Vigna radiata L.) starch. Journal of Food Engineering, 103(4), 388–393.
Li, W., Bai, Y., Mousaa, S. A., Zhang, Q., & Shen, Q. (2012). Effect of high hydrostatic pressure on physicochemical and structural properties of rice starch. Food and Bioprocess Technology, 5(6), 2233–2241.
Li, W., Tian, X., Wang, P., Saleh, A. S., Luo, Q., Zheng, J., Ouyang, S., & Zhang, G. (2016). Recrystallization characteristics of high hydrostatic pressure gelatinized normal and waxy corn starch. International Journal of Biological Macromolecules, 83, 171–177.
Liu, H., Wang, L., Cao, R., Fan, H., & Wang, M. (2016). In vitro digestibility and changes in physicochemical and structural properties of common buckwheat starch affected by high hydrostatic pressure. Carbohydrate Polymers, 144, 1–8.
Liu, Z., Fu, Y., Zhang, F., Zhao, Q., Xue, Y., Hu, J., & Shen, Q. (2022). Comparison of the molecular structure of heat and pressure-treated corn starch based on experimental data and molecular dynamics simulation. Food Hydrocolloids, 125, 107371.
Lund, D. (1984). Influence of time, temperature, moisture, ingredients and processing conditions on starch gelatinization. CRC Critical Reviews in Food Science and Nutrition, 20, 249–273.
Ma, Z., Hu, X., & Boye, J. I. (2018). Research advances on the formation mechanism of resistant starch type III: A review. Critical Reviews in Food Science and Nutrition, 60(2), 276–297.
Marcos, B., Aymerich, T., Monfort, J. M., & Garriga, M. (2008). High-pressure processing and antimicrobial biodegradable packaging to control Listeria monocytogenes during storage of cooked ham. Food Microbiology, 25(1), 177–182.
Molina-García, A. D. (2002). The effect of hydrostatic pressure on biological systems. Biotechnology and Genetic Engineering Reviews, 19(1), 3–54.
Muhr, A. H., Wetton, R. E., & Blanshard, J. M. V. (1982). Effect of hydrostatic pressure on starch gelatinisation, as determined by DTA. Carbohydrate Polymers, 2(2), 91–102.
Oh, H. E., Pinder, D. N., Hemar, Y., Anema, S. G., & Wong, M. (2008). Effect of high-pressure treatment on various starch-in-water suspensions. Food Hydrocolloids, 22(1), 150–155.
Okur, I., Ozel, B., Oztop, M. H., & Alpas, H. (2019). Effect of high hydrostatic pressure in physicochemical properties and in vitro digestibility of cornstarch by nuclear magnetic resonance relaxometry. Journal of Food Process Engineering, 42(6), e13168.
Otero, L., Molina-García, A. D., & Sanz, P. D. (2000). Thermal effect in foods during quasi-adiabatic pressure treatments. Innovative Food Science & Emerging Technologies, 1(2), 119–126.
Ozel, B., Dag, D., Kilercioglu, M., Sumnu, S. G., & Oztop, M. H. (2017). NMR relaxometry as a tool to understand the effect of microwave heating on starch-water interactions and gelatinization behavior. LWT-Food Science and Technology, 83, 10–17.
Papathanasiou, M. M., Reineke, K., Gogou, E., Taoukis, P. S., & Knorr, D. (2015). Impact of high pressure treatment on the available glucose content of various starch types: a case study on wheat, tapioca, potato, corn, waxy corn and resistant starch (RS3). Innovative Food Science & Emerging Technologies, 30, 24–30.
Pei-Ling, L., Xiao-Song, H., & Qun, S. (2010). Effect of high hydrostatic pressure on starches: A review. Starch-Stärke, 62(12), 615–628.
Raghunathan, R., Pandiselvam, R., Kothakota, A., & Khaneghah, A. M. (2021). The application of emerging non-thermal technologies for the modification of cereal starches. LWT, 138, 110795.
Rahman, M. H., Mu, T. H., Zhang, M., Ma, M. M., & Sun, H. N. (2020). Comparative study of the effects of high hydrostatic pressure on physicochemical, thermal, and structural properties of maize, potato, and sweet potato starches. Journal of Food Processing and Preservation, 44(11), e14852.
Rubens, P., Snauwaert, J., Heremans, K., & Stute, R. (1999). In situ observation of pressure-induced gelation of starches studied with FTIR in the diamond anvil cell. Carbohydrate Polymers, 39(3), 231–235.
Shamai, H. K., Bianco-Peled, H., & Simoni, E. (2003). Polymorphism of resistant starch type III. Carbohydrate Polymers, 54, 363–369.
Shen, X., Shang, W., Strappe, P., Chen, L., Li, X., Zhou, Z., & Blanchard, C. (2018). Manipulation of the internal structure of high amylose maize starch by high pressure treatment and its diverse influence on digestion. Food Hydrocolloids, 77, 40–48.
Shi, L., Fu, X., Tan, C. P., Huang, Q., & Zhang, B. (2017). Encapsulation of ethylene gas into granular cold-water-soluble starch: Structure and release kinetics. Journal of Agricultural and Food Chemistry, 65(10), 2189–2197.
Stolt, M., Stoforos, N. G., Taoukis, P. S., & Autio, K. (1999). Evaluation and modelling of rheological properties of high pressure treated waxy maize starch dispersions. Journal of Food Engineering, 40(4), 293–298.
Stolt, M., Oinonen, S., & Autio, K. (2000). Effect of high pressure on the physical properties of barley starch. Innovative Food Science & Emerging Technologies, 1(3), 167–175.
Stute, R., Klingler, R. W., Boguslawski, S., Eshtiaghi, M. N., & Knorr, D. (1996). Effects of high pressures treatment on starches. Starch-Stärke, 48(11–12), 399–408.
Tauscher, B. (1995). Pasteurization of food by hydrostatic high pressure: Chemical aspects. Zeitschrift für Lebensmittel-Untersuchung und Forschung, 200(1), 3–13.
Teixeira, A. S., Navarro, A. S., Molina-García, A. D., Martino, M., & Deladino, L. (2015). Corn starch systems as carriers for yerba mate (Ilex paraguariensis) antioxidants: Effect of mineral addition. Food and Bioproducts Processing, 94, 39–49.
Teixeira, A. S., Deladino, L., Garcia, M. A., Zaritzky, N. E., Sanz, P. D., & Molina-García, A. D. (2018). Microstructure analysis of high pressure induced gelatinization of maize starch in the presence of hydrocolloids. Food and Bioproducts Processing, 112, 119–130.
Tester, R. F., & Sommerville, M. D. (2003). The effects of non-starch polysaccharides on the extent of gelatinisation, swelling and α-amylase hydrolysis of maize and wheat starches. Food Hydrocolloids, 17(1), 41–54.
van de Velde, F., van Riel, J., & Tromp, R. H. (2002). Visualisation of starch granule morphologies using confocal scanning laser microscopy (CSLM). Journal of the Science of Food and Agriculture, 82(13), 1528–1536.
Waigh, T. A., Hopkinson, I., Donald, A. M., Butler, M. F., Heidelbach, F., & Riekel, C. (1997). Analysis of the native structure of starch granules with X-ray microfocus diffraction. Macromolecules, 30(13), 3813–3820.
Wang, B., Li, D., Wang, L. J., Chiu, Y. L., Chen, X. D., & Mao, Z. H. (2008). Effect of high-pressure homogenization on the structure and thermal properties of maize starch. Journal of Food Engineering, 87(3), 436–444.
Wang, S., Li, C., Yu, J., Copeland, L., & Wang, S. (2014). Phase transition and swelling behaviour of different starch granules over a wide range of water content. LWT-Food Science and Technology, 59(2), 597–604.
Wang, S., Zhang, X., Wang, S., & Copeland, L. (2016). Changes of multi-scale structure during mimicked DSC heating reveal the nature of starch gelatinization. Scientific Reports, 6(1), 1–9.
Yang, Z., Gu, Q., & Hemar, Y. (2013). In situ study of maize starch gelatinization under ultra-high hydrostatic pressure using X-ray diffraction. Carbohydrate Polymers, 97(1), 235–238.
Yang, Z., Swedlund, P., Hemar, Y., Mo, G., Wei, Y., Li, Z., & Wu, Z. (2016). Effect of high hydrostatic pressure on the supramolecular structure of corn starch with different amylose contents. International Journal of Biological Macromolecules, 85, 604–614.
Zobel, H. F., Young, S. N., & Rocca, L. A. (1988). Starch gelatinization: An X-ray diffraction study. Cereal Chemistry, 65(6), 443–446.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Deladino, L., Schneider-Teixeira, A., Molina-García, A.D. (2023). High Hydrostatic Pressure Treatment of Starch. In: Sharanagat, V.S., Saxena, D.C., Kumar, K., Kumar, Y. (eds) Starch: Advances in Modifications, Technologies and Applications. Springer, Cham. https://doi.org/10.1007/978-3-031-35843-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-35843-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35842-5
Online ISBN: 978-3-031-35843-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)