Abstract
Vaccines have been a life-saviour in combating different kinds of infectious diseases. Although they are considered as one of the biggest achievements in medical science, successful solutions are yet to be achieved for a number of infectious diseases. A number of vaccines have been developed, but they are mostly inefficient due to shortcomings such as low immunogenicity, high toxicity, inability to boost the immune system and short-term stability. This raises an urgent need to develop an effective and long-term solution. In this scenario, nanoparticles (NPs) have shown promising prospects in various niches of biomedicine. Nanostructures designed from organic compounds or inorganic metal nanoparticles are emerging as a reliable alternative as vaccines and as vaccine carriers, with the ability to boost the host’s immune responses and cell-specific targeting. A small number of nanovaccines have been authorized for human use, and many more are being researched in clinical or preclinical trials. There are various different types of nanoparticles that could be used to deliver vaccination antigens and to preserve antigen from degradation. In this chapter, the prospects of nanovaccines have been clearly discussed, along with the advantages, disadvantages and the therapeutic approach in neurotherapy, cancer immunotherapy, infectious diseases, hepatitis B virus (HBV), human papillomavirus (HPV) and veterinary sciences. In the coming years, nanovaccines can act as a breakthrough in the prevention of avian flu outbreaks, combating life-threatening viral outbreaks, repair neurological damages and probably cure certain types of cancers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
16.1 Introduction
Vaccines have been a life-saviour ever since its introduction in the 1900s. With the rise of deadly epidemics around the world, the need for the development of vaccines has increased significantly. Hence, by the end of 1940s, successful vaccines against pertussis, diphtheria, tetanus and smallpox had given a glimpse of hope to combat against the major epidemics and in a few years resulted in constriction and elimination of respective viral breakouts (Pollard & Bijker, 2021). Subsequently, specialized research in the field of vaccines and vaccination had acquired a substantial importance. Vaccines against polio, influenza, measles, etc., have also been developed, paving way for more advanced and effective vaccines in the future. However, as the history stands witness, vaccine development is recognized as a tedious process that consumes almost more than a decade for successful completion of all stages of development (Han, 2015). The reason for this long tenure is the phases of clinical trials that are followed by the exploratory phase, which is considered the pioneering phase of development. In the beginning, preclinical assessment of vaccine development is accomplished, during which the vaccine is tested on cell culture systems proceeding to tissue cultures and then to animal models for assessing immunogenicity and safety of it. Depending on the results achieved after animal model trials, further human trials are performed. This includes similar assessment for safety and immunogenicity starting from smaller groups of people and gradually moving towards larger groups. The entire process is summed up in three phases of human clinical trials for approval.
Even though the development of vaccines is the need of the hour, researchers often encounter challenges, especially in case of trials on the international level due to the differences in rules prevailing in different countries. Apart from this, finding progressive trial sites is also a challenging task. Lack of infrastructure, finding the correct dosage that can suffice efficacy globally and lack of skilled personnel may also emerge as notable limitations against successful vaccination development process (Drury et al., 2019).
Vaccines have been one of the most significant achievements in the history of medical science. Vaccination had emerged as one of the most effective methods to combat life-threatening infectious diseases. Although over the years scientists constantly strive towards developing more and more vaccines, most of them are inefficient in the process. This is because most of the vaccines that have already been designed are characterized with lower immunogenicity (Kim et al., 2014). Besides, the existing vaccines have also been reported to have higher toxicity and lower in vivo stability (Kheirollahpour et al., 2020). In most cases, vaccination requires multiple follow-up administration of doses and cold chains. Moreover, the vaccines previously developed were futile in providing prolonged immune responses against the causative agent. Hence, currently, scientists are approaching adopting nanovaccines as a better and more advanced alternative to the existing conventional vaccines.
Thus, this chapter discusses nanovaccines and the types of nanovaccines that are currently being considered in vaccinology. Brief discussion has been provided regarding the importance of green nanotechnology in the field of vaccinology and their benefits as an advanced future alternative.
16.2 Nanovaccine
Nanoparticle (NP)-based vaccines ranging in the size between 10 and 1000 nm have shown exceptional physicochemical properties and a potential for enabling drug delivery. Particles like biocompatible nanoparticles, exosomes and liposomes in the above-mentioned range can be considered effective vaccine delivery systems. Such nanovaccines offer more stability and are able to enhance the bioavailability of antigens, messenger ribonucleic acid (mRNA) and deoxyribonucleic acid (DNA) in an encapsulated form (Pardi et al., 2018; Sharma et al., 2020). Nanovaccines have the potential to overcome the limitations of subunit vaccines due to their customized surface chemistry, adjustable size, serum stability and enhanced controlling capacity (Yıldız & Ünver, 2022; Zhao et al., 2014). NP-based vaccines are capable of entrapping antigens or enabling surface attachment, thereby facilitating subcellular trafficking. Thus, these can induce Th1 type immunity in the host organism, which can effectively combat against intracellular pathogenic viral species such as Trypanosoma, Mycobacterium, Leishmania and human immunodeficiency virus (HIV) (Fries et al., 2021; Petkar et al., 2021).
16.2.1 Green Nanotechnology in Vaccines
The implementation of nanotechnology in vaccines has paved a pathway for the implementation of advanced therapeutic measures. Currently, for the preparation of vaccines, scientists have been preferring green synthesis methods with the help of biocompatible compounds for reduced toxicity and better bioavailability (Shah et al., 2015). Green synthesis involves the implementation of plant derivatives, proteins, microbes, etc. Such compounds act not only as reducing agents in the nanoparticle synthesis but also as capping agents in most cases. Specific capping agents for metal or nonmetal nanoparticles are selected, which not only enhance their biomedical properties but also adhere to their additive properties (Schröfel et al., 2014; Singh et al., 2016). These, when implemented in the case of nanovaccines, specific biological components are used. Thus, nanovaccines made up of liposomes or viral protein components carry antigenic components that thereby help in successfully enhancing the immunity in the hosts. The green synthesis method of metallic nanoparticles has been represented diagrammatically in Fig. 16.1.
16.2.2 Factors Determining the Successful Development of Nanovaccines
For the successful development of vaccines, three important factors need to be given primary importance: (i) a good antigen for the activation of immunity, (ii) an immune adjuvant for the costimulation of the innate immunity and (iii) a carrier system for receiving the antigen-presenting cells (APC) (Facciolà et al., 2019). Any vaccine is developed keeping these factors in mind. However, for the preparation of nanovaccine, the properties of nanoparticles should be considered alongside. The size, charge, distribution and shape of the nanoparticle play an important role in the development of nanovaccines. This is because studies conducted on several cell lines have shown some to be highly specific to allowing only microscale particles. Apart from this, the size of nanoparticles also plays an important role in determining the immune responses. As per the study conducted by Hirosue et al. (2010), the type 1 CD4+ and CD8+ cells are stimulated by nanoparticles that belong to the range below 500 nm, whereas the type 2 CD4+ cells can be stimulated for antibody production by particles of size greater than 500 nm. Hence, the physicochemical properties of the nanoparticles used for vaccine development play a crucial role in determining the efficacy of the nanovaccines.
16.2.3 Nanocarriers for Vaccine Delivery
In the hunt for developing effective solutions that can combat against some of the major life-threatening viruses in the world, nanotechnology has been highly successful. Considering the wide range of applications of nanotechnology, it was also implemented in the field of vaccinology. Implementing nanotechnology in vaccinology has resulted in upgraded forms of vaccine development overcoming several underlying issues (Kallon et al., 2021). The advanced form of treatment has been reported to have higher immunogenicity, prolonged stability and better efficacy. The type of nanostructures involved plays a vital role, as the intrinsic properties of the nanocarriers become a deciding factor in the therapeutic approach. Various nanocarriers used for the synthesis of nanovaccines have been depicted in Fig. 16.2.
16.2.3.1 Organic Nanocarriers
16.2.3.1.1 Exosome Vaccines
Exosome vaccines have played an important role in cancer immunotherapy. Exosomes used for the treatment of cancer have shown promising results in activating anticancer immune response. Cells that are detected as malignant secrete an increased number of exosomes that are infused with tumour-inducing antigens. According to the study conducted by Chen et al. (2018), proteinaceous checkpoints such as programmed death-ligand 1 (PD-L1) have been detected on the surface of exosomes that are freshly budding from the malignant cells. These after interacting with the programmed death-1 (PD-1) receptors present on the cytotoxic T cells do not allow the destruction of the tumour cells. However, as per the present research, the tumour-derived exosomes (TEXs) and the dendritic cell (DC)-derived exosomes (DEXs) have shown promising results in the development of anticancer vaccines used for therapeutic purposes (Naseri et al., 2020).
To develop exosome vaccines, scientists are using reverse engineering technology to find cures for cancers. As per reports, TEXs have shown reversal ability to tumorigenesis and immunosuppressing ability. TEX vaccines under preclinical trials have shown significant results in stimulating immune responses against cancer causing elements in breast cancer, metastatic carcinoma and leukemic models (Naseri et al., 2020). Besides, several immune-cell derived exosomes artificially engineered with neoantigens may have therapeutic anticancerous properties (Shenoda & Ajit, 2016).
16.2.3.1.2 Liposome Vaccines
Due to their high immunogenicity (Zahednezhad et al., 2019), liposomes are being used as potential drug delivery systems and gene transporters. Liposomes are recognized as spherical in shape, lipid bilayers, with an internal cavity that serves as carriers of biologically active compounds (van der Koog et al., 2022). Measuring between 50 and 1000 nm, liposomes may have one or more than one concentric membrane. The biophysical and physicochemical properties of liposomes are flexible (Kim & Jeong, 2021), which make them useful for drug or vaccine delivery systems, fabrication, biosensors and diagnosis (Nakhaei et al., 2021). Hence, in the field of biomedicine, liposomes are being incorporated as nanovaccines in the form of nanostructures (Vijayan et al., 2019; Moon et al., 2011). Implementing liposomes in the nano form enhances their intrinsic properties and also makes them more bioavailable with an increased efficiency.
Liposome vaccines have reported comparatively lower reactogenicity. These flexible and biodegradable components act as adjuvants that strengthen the vaccine’s immune response. This occurs as the antigen gets embedded inside the aqueous core or absorbed on the membrane for transportation. However, there are several factors that determine the proficiency of the liposomes as a reliable vaccine. In this, the lamellarity of the liposome, size, bilayer fluidity, surface charge and immunostimulatory lipid addition play a crucial role as, either singly or jointly, they determine the cumulative function of liposomes as a vaccine.
16.2.3.1.3 Virus-Like Particles (VLPs)
Nanoparticles formed by the self-assembly of protein capsid devoid of the genetic material, categorized as virus-like particles (VLPs), have been effectively implemented in the development of viral vaccines (Cappelli et al., 2022; Kushnir et al., 2012). Derived from different types of viruses through different cellular systems (plant, yeast, baculovirus, Escherichia coli, etc.) and cell-free systems, the VLPs can range in size between 20 and 800 nm (Lin et al., 2022; Pushko et al., 2013). VLPs naturally mimic the size of the viruses and have a repetitive structural order. These particles have high immunity boosting ability without the negative property of inducing viral infection. Thus, self-assembly of such rapidly processing nanoparticles induces a faster and a long-term immune response in the host, even when there is an absence of adjuvant (Jeong & Seong, 2017). Considering such factors, scientists have been striving towards developing VLP-based vaccines. The ones that are already available in the market have been derived by self-assembly of viral proteins extracted from the virus against which the vaccine is being developed. Several VLP-based vaccines are currently under the various trial phases, while researchers are constantly developing more (Khan et al., 2022; Kushnir et al., 2012).
VLP-based vaccines have been under intensive research for the last few decades. In 1986, the very first VLP-based vaccine, with the ability of stimulating the activation of CD8+ and CD4+ T cells, was designed against the highly pathogenic hepatitis B. It also paved way for future alternatives for viral infections like hepatitis E virus (HEV) and human papillomavirus. VLP-based vaccine for hepatitis B contains surface antigen of hepatitis B (HBsAg) that is formed through recombinant yeast cells. Such yeast-based VLPs also comprise non-glycosylated, hydrophobic S-proteins, lipids (derived from the host cell) and disulphide bonds for structure stabilization (Souri et al., 2022; Roldão et al., 2010). Thus, it can be recognized as a lipoprotein having a hydrophobic fluid core surrounded by a rigid lipid layer that is meant for absorbing the protein moiety (Zhao et al., 2013).
Another ribonucleic acid (RNA)-based viral infection of hepatitis E has been recognized as one of the leading chronic infections and can be fatal, especially in the first trimester of pregnancy (Yang et al., 2018). Extensive studies have been done, henceforth, on hepatitis E virus, its seroprevalence, core structure and viral potential, and several new vaccines were designed with different protein composition. In this regard, hepatitis E-based VLPs have also been designed by self-assembling core protein, ORF2. The model was the first of its kind designed at that time, and thus, the potential of the vaccine had thereafter been thoroughly assessed and were then subjected to necessary clinical trials (Cid & Bolívar, 2021). Besides the direct administration, Li et al. (2005) used the p239 protein and successfully developed hepatitis E-based VLP in bacteria through recombinant DNA technology (RDT). Successful prevention against the viral infection was obtained in both the cases. A similar study was conducted by Go et al. (2021) where the HEV VLP–based vaccines were designed by expressing the HEV-3 capsid protein, p239 of pigs or swine.
After being able to successfully contain and prevent HBV infection, scientists had thereafter shifted to the HPV, which was not only widespread but also life-threatening. Hence, VLP-based vaccine against human papillomavirus (HPV) was approved in 2006 for successful human implementation (Cutts et al., 2007). For this, the role of the virus was intensively studied. HPV belonged to the group of oncogenic viruses, which was responsible for causing lesions in several skin layers and the mucous membranes, which may or may not be malignant. In the present days, HPV is specifically characterized with cervical malignancy and can be categorized into typical and atypical cancer types. The former is associated with malignancies in the vagina, anus, vulva, oropharynx and penis, whereas the latter is associated with cancer detected in the prostate, rectum or bladder region. This has been restricted up to a certain extent after the advent of the VLP-based vaccines. Studies reveal that aligned vaccination has been able to reduce the widespread cases of cervical cancer approximately 90% (Kavanagh et al., 2017). The HPV vaccines are also designed by self-assembly of 72 pentameric components of the viral capsid (L1 protein) in yeast cells via RDT. Following such mechanism, three VLP-based HPV vaccines have been approved by the US Food and Drug Administration (FDA): The first vaccine was developed in 2006 when a tetravalent vaccine was developed, the second bivalent vaccine was developed in 2009, and a third nonavalent vaccine was approved in 2014 (Brown et al., 2021; Chaturvedi et al., 2011).
16.2.3.1.4 Self-Assembled Proteins
Self-assembly means a procedure of forming organized structures from an unorganized system of components that were already existing. The process may occur without any external interference but by means of local or internal interaction (Chung et al., 2015). When the organized structures are formed of disorganized proteins, those are known as self-assembly of proteins. Although similar to the VLPs, self-assembled proteins differ in the fact that it is not composed of the viral components. Self-assembling proteins such as those of ferritin and major vault protein (MVP) have shown promising therapeutic approaches. Ferritin could form consecutive spheres measuring up to 10 nm, which when combined with the influenza hemagglutinin (HA) protein produced eight trimeric spikes of HA, which showed an enhanced immune response compared with conventional vaccines (Iyer et al., 2022; Kanekiyo et al., 2013).
The major vault protein is an intracellular, ubiquitously occurring protein; self-assembling from 96 copies results in the formation of a nanoparticle mimicking the shape of a barrel of the dimension of 70 nm × 40 nm. Antigens are first attached to the active domain of the MVP, which is then wrapped inside the self-assembled structure. These proteins have shown successful results in delivering the major outer membrane protein (MOMP) of Chlamydia sp. for the active stimulation of the mucosal immunity (Reddy, 2022; Champion et al., 2009).
16.2.3.2 Inorganic Nanoparticles as Vaccines
16.2.3.2.1 Calcium Phosphate Nanoparticle
One of the prime reasons why calcium phosphate nanoparticles have been explored for their vaccine-carrying capacity is because of their biodegradable nature and structural attributes (Temchura et al., 2014). Calcium phosphate nanoparticles have a unique feature of naturally releasing the encapsulated components into the cell even if deprived of any external factor. This occurs due to the change in pH encountered by the nanoparticles after being phagocytized inside the cell (from neutral pH outside the cell to acidic pH inside the cell). In addition, calcium nanoparticles are also preferred because of their biocompatibility and stability. The proficiency of calcium phosphate nanoparticle as stable nanocarriers was proven by Morgan et al. (2008) when a suitably modified carbon phosphate nanoparticle with a size of 80 nm carrying genetic components was tested in liver both in vivo and in vitro. In both the cases, the nanoparticles were able to prevent the encapsulated genetic components from being degraded by deoxyribonuclease (DNase) in both the conditions provided. This motivated the scientists to research its vaccine-carrying potential as well. Thus, several trials, to estimate the potential of the nanoparticles as adjuvants for immune responses in the mucosal region and for DNA vaccines, have also been successfully performed (Zhao et al., 2021; Knuschke et al., 2014).
16.2.3.2.2 Gold Nanoparticle
Due to their intrinsic therapeutic properties, gold nanoparticles are recognized as an important component in the field of nanotechnology. The versatility of the gold nanoparticles resides in the flexibility of their shape as they can be moulded into cubical shape and spherical or cylindrical rod shape according to their applicability. Besides shape, the length of gold nanoparticles ranging in size between 1 and 150 nm also plays a role in regulating the augmentation of the immune responses through various cytokine pathways (Cai et al., 2022; Niikura et al., 2013). Although gold nanoparticles have been used in several occasions in the biomedical field, it has also been proven as effective carriers for antigen conjugates of influenza virus and for HIV (Facciolà et al., 2017). Xu et al. (2012) experimented with three different components used as surface coatings on gold nanorods. The compounds used are polyethyleneimine (PEI), cetyltrimethylammonium bromide (CTAB) and poly(diallyldimethylammonium chloride) (PDDAC), among which gold-based PEI or PDDAC nanorods showed the successful activation of APCs, along with T-cell proliferation, leading to an increase in both humoral immunity and cellular immunity. Thus, gold-based nanoparticles as vaccine carriers showed better efficacy as compared to treatments with naked HIV-1 envelope plasmid DNA.
Several experiments have been performed by scientists to test the extent of therapeutic potential of gold nanoparticles. In a separate study, a specific ovalbumin 323–339 peptide–D-glucose complex was designed based on gold glyconanoparticles carrying a tetrasaccharide epitope of the Streptococcus pneumoniae type 14 capsular polysaccharide (Pn14PS) (Colombo et al., 2018). The complex when administered on mice induced the activation of Th cell by generating more cytokine and anti-Pn14PS IgG antibodies and thereby showed an enhanced immune response.
16.2.3.2.3 Carbon Nanoparticle
Since carbon is an essential cellular component, several scientists have researched its applicability as vaccines. Extensive research has been done to assess its application as a suitable antiviral agent in the form of a nanoparticle, either as a carrier or as an adjuvant (Serrano-Aroca et al., 2021). Carbon nanoparticles may have a wide variation in the physiochemical properties owing to the differences in the structure of the nanoparticles. This also regulates the antigen-carrying capacity of the nanoparticles and boosts their immune responses via specific surface modifications (Liu et al., 2014). Although carbon nanoparticles of different shapes have been designed, they in the form of nanotubes have garnered a distinct attention. Carbon nanotubes (CNTs) are made up of graphene sheets that are rolled into hollow cylindrical shapes. CNTs can either be single-walled, known as single-walled carbon nanotubes (SWCNTs), or be multiwalled by forming concentric sheets (with more than one sheet, up to 50 sheets), known as multiwalled carbon nanotubes (MWCNT) (Rozhina et al., 2021; De Volder et al., 2013). Where the former can measure between 0.5 and 1.5 nm, the latter may measure nearly 100 nm. The size of the carbon nanotubes is important to consider as the functionality and applicability of the carbon nanotubes vary accordingly.
Hence, in 2003, Pantarotto et al. conducted an experiment with the viral peptides of hand-foot-mouth disease (Hoang et al., 2019). In this study, the enveloped viral proteins were covalently attached to the carbon nanotubes. It was observed that the structure of the epitope continued to maintain its immunogenicity even after getting attached to the carbon nanotubes, and the viral protein–CNT complexes were able to successfully release IgG for neutralizing the infection.
In an experiment performed by Hassan et al. in 2016, multiwalled antigen conjugates of different lengths and varied surface charges were used, and their applicability was tested. The results showed a boost in the cellular uptake level with reduced levels of negative changes in the multiwalled carbon nanotubes, which also resulted in enhanced immunity. Hence, it was proved that the surface properties of the carbon nanotubes played a role in regulating their efficacy as vaccine carriers both in vivo and in vitro.
16.2.3.2.4 Silica Nanoparticles (SiNPs)
Silica nanoparticles (SiNPs) are primarily preferred in therapeutic approaches where specific targeting of tumours is needed. However, recent researches have also discovered their excellent applicability as vaccine carriers or as drug delivery agents (Yu et al., 2015). The most striking feature of the silica nanoparticles is that they can stimulate immune response both in vivo and in vitro without any antigenic conjugation (Hou et al., 2022; Wibowo et al., 2014). With necessary modification, silica nanoparticles have proven to be efficient in cellular interaction, improving cell recognition, biomolecule absorption and uptake of necessary cellular components. Silica nanoparticles with versatile applications were also developed so as to enhance the effectiveness of the particles. Thus, mesoporous silica nanoparticles (MSNs) ranging in size between 50 and 200 nm were developed, which could serve both as adjuvants and as nanocarriers for antigen delivery (Ahmed et al., 2022; Chen et al., 2012). To understand their potential, Mody et al. in 2016 tested SiNPs in the form of vesicles against bovine viral diarrhoea virus (BVDV), which resulted in restricted release of the BVDV antigen that was a codon-optimized E2 peptide, thereby preventing the viral infection.
16.3 Biomedical Applications of Nanovaccines
Vaccines have emerged as a medical necessity for not only combating against life-threatening viral infections and diseases but also preventing them. Over the years, scientists have strived towards developing prophylactic vaccines that have successfully helped in almost eradicating diseases like tetanus and smallpox and are continuously striving towards eliminating viral infections like hepatitis (Soriano et al., 2022). However, with the increase in the number of life-threatening diseases, the need for advanced vaccines is on the rise. Thus, therapeutic vaccines that can not only provide life-long immunity against specific diseases but also modulate immune responses are the need of the hour. To obtain reliable solutions, scientists are now shifting towards nanovaccines for an advanced approach against viral diseases.
Nanoparticles are being highly implemented in several sectors of biomedicine. Besides being used in bioimaging, antibiotics, etc., its recent addition as an alternative for conventional vaccines has been praiseworthy. The extent of applications of nanovaccines is still being studied, and the potential of nanoparticle vaccines as an immune system stimulant is being stressed on. Nanovaccines are being recognized for their ability to enhance both the adaptive immune system and modulating innate immunity (Luo et al., 2017). These are easily retained in the lymph nodes, show better loading of antigen and require lesser dosage in terms of both the frequency and the amount to be administered. With the help of suitable nanoparticles loaded with different foreign antigens, overcoming immunotolerance has also been possible, which thereby diminishes the need for booster doses. Thus, their potential as a therapeutic agent has been appreciated for preventing cancer, tuberculosis, influenza, acquired immunodeficiency syndrome (AIDS) and malaria (Bhardwaj et al., 2020).
Owing to the specific antigen-targeting ability, nanoparticles have proven to be a significant therapeutic approach in the treatment of cancer. Nanocarriers like polymers, liposomes and nanospheres have shown successful results in the delivery of vaccine antigen, thereby stabilizing the anti-tumour T cells. At present, nanovaccines are highly implemented as drug delivery agents in chemotherapies for tumour cell targeting. Current researches are including formulations with dendritic cells, which have proven to be efficient as APCs for the treatment of cancer. These dendritic formulations function by providing selective delivery meant only for specific antigens targeting lymphoid organs, thereby progressing the time period for cytotoxic T-cell immune response. Besides, scientists are also focusing on formulating nanovaccines in combination with T cells for the improvement of adoptive T-cell therapy (Xiao et al., 2021; Fan & Moon, 2015; Park et al., 2013).
The efficacy of nanovaccines has not been limited to humans, but it has also been implemented in animals. The application of nanotechnology in veterinary sciences has been a breakthrough in improving the health of several animals due to the development of effective vaccines and targeted drug delivery. For instance, VGX Animal Health, Inc. has devised ways for inducing both cellular and humoral immune response that has led to the formulation of vaccines meant either for oral ingestion or for intradermal and intramuscular injection. Such drugs are often administered in the form of biobullets available with polyethylene glycol (PEG) coating and are used in the vaccination against Brucella abortus in bisons (Malek-Khatabi et al., 2022) or as cancer therapy in dogs (Zhou et al., 2022).
With myriad changes in both biotic and abiotic factors on the earth, sudden advents of noble viruses that may take the shape of epidemic and pandemic are on the rise. Thus, effective vaccination to combat different types of infectious diseases is the need of the hour. Since nanoparticles exhibit successful antiviral properties (Gurunathan et al., 2020), green synthesized nanoparticles are optimally tested for their antiviral efficacy. For instance, in a study conducted by Neuhaus et al. in 2014 included double-adjuvant vaccine with HAC1 (H1N1 influenza hemagglutinin influenza antigen), c-di-GMP (bis-(3′,5′)-cyclic dimeric guanosine monophosphate, mucosal vaccine adjuvant) and silica nanoparticle where the latter acted as the drug delivery system. Vaccine administration was done in the lower lungs of mice model where effective antigen response was observed along with the reactivation of both systemic and local immune response in order to protect against the influenza virus.
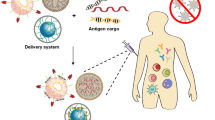
Even today malaria is one of the dreaded diseases, which is responsible of deaths globally. To combat against the high rise of malaria, a vaccine was introduced which arrests the parasite, Plasmodium falciparum, in its sexual stage. Among the many tested, Pfs25 was recognized as a promising one (Yadav et al., 2018). However, in order to obtain an immune response over an extended period of time, the combination with gold nanoparticles has yielded promising results. In the study conducted by Kumar et al. (2015) the use of codon-harmonized recombinant Pfs25 (CHrPfs25) with gold nanoparticles acting as delivery agents resulted in enhanced immunogenicity, thereby providing a promising therapeutic approach against malaria. Biomedical applications of nanovaccines are illustrated in Fig. 16.3.
Biomedical applications of nanovaccine. (Image created by BioRender.com)
16.4 Advantages and Disadvantages of Nanovaccines
With researchers constantly striving towards developing a better alternative, nanovaccines are emerging as a suitable option. Specifically, nanovaccines are being observed to have an upper hand over traditional vaccines for several reasons. For instance, the administration of traditional vaccines requires the whole body to partake, whereas, in case of nanovaccines, specific infection-encountered areas and source of origination are targeted. Besides, nanosystems ensure proper maintenance of the antigen integrity by increasing stability and preventing degradation. This is possible by increasing the rate of solubility of such compounds that are hydrophobic in nature, thereby enduring better administration. Moreover, nanovaccines also require a reduced dose as compared to traditional vaccine doses (Stammers et al., 2013; Shahbazi & Santos, 2015). One of the distinguished advantages of nanovaccines is their complementing size with that of the cellular components, which facilitates better penetration via endocytosis. Nanovaccines are also capable of targeted drug delivery if coated with antibodies meant for targeting cell-specific receptors and enhance antigen absorption by APCs. Nanovaccines have the ability of cross-presenting antigens with the help of major histocompatibility complex class I (MHC I), thereby activating both the cell’s immune system and humoral immunity and thus displaying better efficacy than conventional vaccines (Hirosue et al., 2010; Baljon & Wilson, 2022). Some other notable advantages of the nanovaccine are that the administration of the nanovaccine requires neither a booster dose nor a continuous maintenance of the cold chain. Nanovaccines are capable of creating active targets and are known to have increased longevity in the bloodstream due to greater stability (Gheibi Hayat & Darroudi, 2019).
However, there are several limitations related to the production and use of nanovaccine such as chemical composition, capping agent used and nanoparticle morphology. Toxicity is recognized as one of the major limitations of nanoparticles. It is an unwanted side effect that needs to be minimised if not completely eliminated, for an enhanced efficacy of nanoparticles. The size of the nanoparticle and the zeta potential determine the toxicity of the nanovaccines (Rasmussen et al., 2020). Hence, nanovaccines with lower toxicity and higher stability are preferred for human use. Besides toxicity, nanoparticles inside the body do have access to tissues they pass through. Furthermore, achieving a sterile laboratory condition during scaling-up is another challenge that the nano-industry is currently facing and needs to be solved for future productions at the industrial level (Shahbazi & Santos, 2015).
16.5 Conclusions, Outlook and Future Aspects
Implementation of nanotechnology in vaccinology offers a promising solution to several medical issues. The new generation of vaccines is developed using nanoparticles as potential drug delivery agents and immunostimulants with fewer side effects. The use of nanoparticles in vaccine development enhances the antigen uptake from the antigen-presenting cells. The efficacy of nanoparticle-based nanovaccines is primarily determined by the size, shape and charge of the particles used, which consecutively affects the adjuvanticity, immune system stimulation, antigenicity and inflammatory responses inside the host.
With every progressing day, newer preclinical reports are obtained, which suggest that cationic liposomes and poly(lactic-co-glycolic acid) (PLGA) yield better efficacy in terms of nanovaccines that have also shown future possibility of overcoming drawbacks related to subunit vaccines and live-attenuated vaccines. Nanovaccines are designed based on the physicochemical properties and optimized according to the purpose and target of the cell or organ inside the host. These have gained due attention for their enhanced bioavailability, biocompatibility and biodegradability. These cost-effective formulations of nanovaccines have shown potential therapeutic results against malaria, influenza virus, hepatitis B, HIV and cancer, thereby paving way for future breakthrough. Besides, these are comparatively safer than conventional vaccines as the nanovaccines have low toxicity and higher stability in the bloodstream. However, an extensive study about the physiochemical properties and the binding or functioning inside the host environment is still required. This is because nanovaccines are still a new addition to both the fields of nanotechnology and vaccinology, and a vast amount of studies is still required to assess probable vaccine components in order to eradicate existing life-threatening diseases. Nanovaccines as potential drug delivery agents have provided several ground-breaking therapeutic approaches in both humans and animals; however, an in-depth research regarding feasible alternatives in the form of nanovaccines, along with their preclinical trials, to eliminate risk factors should be continued.
References
Ahmed, H., Gomte, S. S., Prabakaran, A., Agrawal, M., & Alexander, A. (2022). Biomedical applications of mesoporous silica nanoparticles as a drug delivery carrier. Journal of Drug Delivery Science and Technology, 76, 103729.
Baljon, J. J., & Wilson, J. T. (2022). Bioinspired vaccines to enhance MHC class-I antigen cross-presentation. Current Opinion in Immunology, 77, 102215.
Bhardwaj, P., Bhatia, E., Sharma, S., Ahamad, N., & Banerjee, R. (2020). Advancements in prophylactic and therapeutic nanovaccines. Acta Biomaterialia, 108, 1–21.
Brown, D. R., Joura, E. A., Yen, G. P., Kothari, S., Luxembourg, A., Saah, A., Walia, A., Perez, G., Khoury, H., Badgley, D., & Stanley, M. (2021). Systematic literature review of cross-protective effect of HPV vaccines based on data from randomized clinical trials and real-world evidence. Vaccine, 39(16), 2224–2236.
Cai, F., Li, S., Huang, H., Iqbal, J., Wang, C., & Jiang, X. (2022). Green synthesis of gold nanoparticles for immune response regulation: Mechanisms, applications, and perspectives. Journal of Biomedical Materials Research Part A, 110(2), 424–442.
Cappelli, L., Cinelli, P., Giusti, F., Ferlenghi, I., Utrio-Lanfaloni, S., Wahome, N., Bottomley, M. J., Maione, D., & Cozzi, R. (2022). Self-assembling protein nanoparticles and virus like particles correctly display β-barrel from meningococcal factor H-binding protein through genetic fusion. PLoS One, 17(9), e0273322.
Champion, C. I., Kickhoefer, V. A., Liu, G., Moniz, R. J., Freed, A. S., Bergmann, L. L., Vaccari, D., Raval-Fernandes, S., Chan, A. M., Rome, L. H., & Kelly, K. A. (2009). A vault nanoparticle vaccine induces protective mucosal immunity. PLoS One, 4(4), e5409.
Chaturvedi, A. K., Engels, E. A., Pfeiffer, R. M., Hernandez, B. Y., Xiao, W., Kim, E., Jiang, B., Goodman, M. T., Sibug-Saber, M., Cozen, W., & Liu, L. (2011). Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of Clinical Oncology, 29(32), 4294.
Chen, K., Zhang, J., & Gu, H. (2012). Dissolution from inside: A unique degradation behaviour of core–shell magnetic mesoporous silica nanoparticles and the effect of polyethyleneimine coating. Journal of Materials Chemistry, 22(41), 22005–22012.
Chen, G., Huang, A. C., Zhang, W., Zhang, G., Wu, M., Xu, W., Yu, Z., Yang, J., Wang, B., Sun, H., & Xia, H. (2018). Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature, 560(7718), 382–386.
Chung, E. J., Mlinar, L. B., Sugimoto, M. J., Nord, K., Roman, B. B., & Tirrell, M. (2015). In vivo biodistribution and clearance of peptide amphiphile micelles. Nanomedicine: Nanotechnology, Biology and Medicine, 11(2), 479–487.
Cid, R., & Bolívar, J. (2021). Platforms for production of protein-based vaccines: From classical to next-generation strategies. Biomolecules, 11(8), 1072.
Colombo, C., Pitirollo, O., & Lay, L. (2018). Recent advances in the synthesis of glycoconjugates for vaccine development. Molecules, 23(7), 1712.
Cutts, F. T., Franceschi, S., Goldie, S., Castellsague, X. D., De Sanjose, S., Garnett, G., Edmunds, W. J., Claeys, P., Goldenthal, K. L., Harper, D. M., & Markowitz, L. (2007). Human papillomavirus and HPV vaccines: A review. Bulletin of the World Health Organization, 85, 719–726.
De Volder, M. F., Tawfick, S. H., Baughman, R. H., & Hart, A. J. (2013). Carbon nanotubes: Present and future commercial applications. Science, 339(6119), 535–539.
Drury, G., Jolliffe, S., & Mukhopadhyay, T. K. (2019). Process mapping of vaccines: Understanding the limitations in current response to emerging epidemic threats. Vaccine, 37(17), 2415–2421.
Facciolà, A., Venanzi Rullo, E., Ceccarelli, M., D’aleo, F., Di Rosa, M., Pinzone, M. R., Condorelli, F., Visalli, G., Picerno, I., Fisichella, R., & Nunnari, G. (2017). Kaposi’s sarcoma in HIV-infected patients in the era of new antiretrovirals. European Review for Medical and Pharmacological Sciences, 21(24), 5868–5869.
Facciolà, A., Visalli, G., Laganà, P., La Fauci, V., Squeri, R., Pellicanò, G. F., Nunnari, G., Trovato, M., & Di Pietro, A. (2019). The new era of vaccines: The “nanovaccinology”. European Review for Medical and Pharmacological Sciences, 23(16), 7163–7182.
Fan, Y., & Moon, J. J. (2015). Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccine, 3(3), 662–685.
Fries, C. N., Curvino, E. J., Chen, J. L., Permar, S. R., Fouda, G. G., & Collier, J. H. (2021). Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nature Nanotechnology, 16(4), 1–14.
Gheibi Hayat, S. M., & Darroudi, M. (2019). Nanovaccine: A novel approach in immunization. Journal of Cellular Physiology, 234(8), 12530–12536.
Go, H. J., Park, B. J., Ahn, H. S., Kim, D. H., Kim, D. Y., Kim, J. H., Lee, J. B., Park, S. Y., Song, C. S., Lee, S. W., & Choi, Y. K. (2021). Pigs immunized with the virus-like particle vaccine are protected against the hepatitis E-3 virus. Vaccine, 9(11), 1265.
Gurunathan, S., Qasim, M., Choi, Y., Do, J. T., Park, C., Hong, K., Kim, J. H., & Song, H. (2020). Antiviral potential of nanoparticles—Can nanoparticles fight against coronaviruses? Nanomaterials, 10(9), 1645.
Han, S. (2015). Clinical vaccine development. Clinical and Experimental Vaccine Research, 4(1), 46–53.
Hassan, H. A., Smyth, L., Rubio, N., Ratnasothy, K., Wang, J. T. W., Bansal, S. S., Summers, H. D., Diebold, S. S., Lombardi, G., & Al-Jamal, K. T. (2016). Carbon nanotubes’ surface chemistry determines their potency as vaccine nanocarriers in vitro and in vivo. Journal of Controlled Release, 225, 205–216.
Hirosue, S., Kourtis, I. C., van der Vlies, A. J., Hubbell, J. A., & Swartz, M. A. (2010). Antigen delivery to dendritic cells by poly (propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and T cell activation. Vaccine, 28(50), 7897–7906.
Hoang, C. Q., Nguyen, T. T. T., Ho, N. X., Nguyen, H. D., Nguyen, A. B., Nguyen, T. H. T., Phan, H. C., & Phan, L. T. (2019). Transmission and serotype features of hand foot mouth disease in household contacts in Dong Thap, Vietnam. BMC Infectious Diseases, 19(1), 1–10.
Hou, F., Teng, Z., Ru, J., Liu, H., Li, J., Zhang, Y., Sun, S., & Guo, H. (2022). Flower-like mesoporous silica nanoparticles as an antigen delivery platform to promote systemic immune response. Nanomedicine: Nanotechnology, Biology and Medicine, 42, 102541.
Iyer, S., Yadav, R., Agarwal, S., Tripathi, S., & Agarwal, R. (2022). Bioengineering strategies for developing vaccines against respiratory viral diseases. Clinical Microbiology Reviews, 35(1), e00123–e00121.
Jeong, H., & Seong, B. L. (2017). Exploiting virus-like particles as innovative vaccines against emerging viral infections. Journal of Microbiology, 55(3), 220–230.
Kallon, S., Samir, S., & Goonetilleke, N. (2021). Vaccines: Underlying principles of design and testing. Clinical Pharmacology & Therapeutics, 109(4), 987–999.
Kanekiyo, M., Wei, C. J., Yassine, H. M., McTamney, P. M., Boyington, J. C., Whittle, J. R., Rao, S. S., Kong, W. P., Wang, L., & Nabel, G. J. (2013). Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature, 499(7456), 102–106.
Kavanagh, K., Pollock, K. G., Cuschieri, K., Palmer, T., Cameron, R. L., Watt, C., Bhatia, R., Moore, C., Cubie, H., Cruickshank, M., & Robertson, C. (2017). Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: A 7-year cross-sectional study. The Lancet Infectious Diseases, 17(12), 1293–1302.
Khan, W. H., Ahmad, R., Khan, N., & Ansari, M. A. (2022). SARS-CoV-2 variants associated challenges in the ongoing vaccination of COVID-19.
Kheirollahpour, M., Mehrabi, M., Dounighi, N. M., Mohammadi, M., & Masoudi, A. (2020). Nanoparticles and vaccine development. Pharmaceutical Nanotechnology, 8(1), 6–21.
Kim, E. M., & Jeong, H. J. (2021). Liposomes: Biomedical applications. Chonnam Medical Journal, 57(1), 27.
Kim, M. G., Park, J. Y., Shon, Y., Kim, G., Shim, G., & Oh, Y. K. (2014). Nanotechnology and vaccine development. Asian Journal of Pharmaceutical Sciences, 9(5), 227–235.
Knuschke, T., Bayer, W., Rotan, O., Sokolova, V., Wadwa, M., Kirschning, C. J., Hansen, W., Dittmer, U., Epple, M., Buer, J., & Westendorf, A. M. (2014). Prophylactic and therapeutic vaccination with a nanoparticle-based peptide vaccine induces efficient protective immunity during acute and chronic retroviral infection. Nanomedicine: Nanotechnology, Biology and Medicine, 10(8), 1787–1798.
Kumar, R., Ray, P. C., Datta, D., Bansal, G. P., Angov, E., & Kumar, N. (2015). Nanovaccines for malaria using Plasmodium falciparum antigen Pfs25 attached gold nanoparticles. Vaccine, 33(39), 5064–5071.
Kushnir, N., Streatfield, S. J., & Yusibov, V. (2012). Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine, 31(1), 58–83.
Li, S. W., Zhang, J., He, Z. Q., Gu, Y., Liu, R. S., Lin, J., Chen, Y. X., Ng, M. H., & Xia, N. S. (2005). Mutational analysis of essential interactions involved in the assembly of hepatitis E virus capsid. Journal of Biological Chemistry, 280(5), 3400–3406.
Lin, X., Li, W., Wen, Y., Su, L., & Zhang, X. (2022). Aggregation-induced emission (AIE)-based nanocomposites for intracellular biological process monitoring and photodynamic therapy. Biomaterials, 287, 121603.
Liu, Y., Xu, Y., Tian, Y., Chen, C., Wang, C., & Jiang, X. (2014). Functional nanomaterials can optimize the efficacy of vaccines. Small, 10(22), 4505–4520.
Luo, M., Samandi, L. Z., Wang, Z., Chen, Z. J., & Gao, J. (2017). Synthetic nanovaccines for immunotherapy. Journal of Controlled Release, 263, 200–210.
Malek-Khatabi, A., Tabandeh, Z., Nouri, A., Mozayan, E., Sartorius, R., Rahimi, S., & Jamaledin, R. (2022). Long-term vaccine delivery and immunological responses using biodegradable polymer-based carriers. ACS Applied Bio Materials, 5, 5015.
Moon, J. J., Suh, H., Bershteyn, A., Stephan, M. T., Liu, H., Huang, B., Sohail, M., Luo, S., Ho Um, S., Khant, H., & Goodwin, J. T. (2011). Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nature Materials, 10(3), 243–251.
Morgan, T. T., Muddana, H. S., Altinoglu, E. I., Rouse, S. M., Tabakovic, A., Tabouillot, T., Russin, T. J., Shanmugavelandy, S. S., Butler, P. J., Eklund, P. C., & Yun, J. K. (2008). Encapsulation of organic molecules in calcium phosphate nanocomposite particles for intracellular imaging and drug delivery. Nano Letters, 8(12), 4108–4115.
Nakhaei, P., Margiana, R., Bokov, D. O., Abdelbasset, W. K., Jadidi Kouhbanani, M. A., Varma, R. S., Marofi, F., Jarahian, M., & Beheshtkhoo, N. (2021). Liposomes: Structure, biomedical applications, and stability parameters with emphasis on cholesterol. Frontiers in Bioengineering and Biotechnology, 9, 748.
Naseri, M., Bozorgmehr, M., Zöller, M., Ranaei Pirmardan, E., & Madjd, Z. (2020). Tumor-derived exosomes: The next generation of promising cell-free vaccines in cancer immunotherapy. Oncoimmunology, 9(1), 1779991.
Neuhaus, V., Chichester, J. A., Ebensen, T., Schwarz, K., Hartman, C. E., Shoji, Y., Guzmán, C. A., Yusibov, V., Sewald, K., & Braun, A. (2014). A new adjuvanted nanoparticle-based H1N1 influenza vaccine induced antigen-specific local mucosal and systemic immune responses after administration into the lung. Vaccine, 32(26), 3216–3222.
Niikura, K., Matsunaga, T., Suzuki, T., Kobayashi, S., Yamaguchi, H., Orba, Y., Kawaguchi, A., Hasegawa, H., Kajino, K., Ninomiya, T., & Ijiro, K. (2013). Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano, 7(5), 3926–3938.
Pantarotto, D., Partidos, C. D., Graff, R., Hoebeke, J., Briand, J. P., Prato, M., & Bianco, A. (2003). Synthesis, structural characterization, and immunological properties of carbon nanotubes functionalized with peptides. Journal of the American Chemical Society, 125(20), 6160–6164.
Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines—a new era in vaccinology. Nature Reviews Drug Discovery, 17(4), 261–279.
Park, Y. M., Lee, S. J., Kim, Y. S., Lee, M. H., Cha, G. S., Jung, I. D., Kang, T. H., & Han, H. D. (2013). Nanoparticle-based vaccine delivery for cancer immunotherapy. Immune Network, 13(5), 177–183.
Petkar, K. C., Patil, S. M., Chavhan, S. S., Kaneko, K., Sawant, K. K., Kunda, N. K., & Saleem, I. Y. (2021). An overview of nanocarrier-based adjuvants for vaccine delivery. Pharmaceutics, 13(4), 455.
Pollard, A. J., & Bijker, E. M. (2021). A guide to vaccinology: From basic principles to new developments. Nature Reviews Immunology, 21(2), 83–100.
Pushko, P., Pumpens, P., & Grens, E. (2013). Development of virus-like particle technology from small highly symmetric to large complex virus-like particle structures. Intervirology, 56(3), 141–165.
Rasmussen, M. K., Pedersen, J. N., & Marie, R. (2020). Size and surface charge characterization of nanoparticles with a salt gradient. Nature Communications, 11(1), 1–8.
Reddy, B. M. (2022). Nanomedicine and Nanovaccinology tools in targeted drug delivery. Nanovaccinology as Targeted Therapeutics, 21–52.
Roldão, A., Mellado, M. C. M., Castilho, L. R., Carrondo, M. J., & Alves, P. M. (2010). Virus-like particles in vaccine development. Expert Review of Vaccines, 9(10), 1149–1176.
Rozhina, E., Batasheva, S., Miftakhova, R., Yan, X., Vikulina, A., Volodkin, D., & Fakhrullin, R. (2021). Comparative cytotoxicity of kaolinite, halloysite, multiwalled carbon nanotubes and graphene oxide. Applied Clay Science, 205, 106041.
Schröfel, A., Kratošová, G., Šafařík, I., Šafaříková, M., Raška, I., & Shor, L. M. (2014). Applications of biosynthesized metallic nanoparticles–a review. Acta Biomaterialia, 10(10), 4023–4042.
Serrano-Aroca, Á., Takayama, K., Tuñón-Molina, A., Seyran, M., Hassan, S. S., Pal Choudhury, P., Uversky, V. N., Lundstrom, K., Adadi, P., Palù, G., & Aljabali, A. A. (2021). Carbon-based nanomaterials: Promising antiviral agents to combat COVID-19 in the microbial-resistant era. ACS Nano, 15(5), 8069–8086.
Shah, M., Fawcett, D., Sharma, S., Tripathy, S. K., & Poinern, G. E. J. (2015). Green synthesis of metallic nanoparticles via biological entities. Materials, 8(11), 7278–7308.
Shahbazi, M. A., & Santos, H. A. (2015). Revolutionary impact of nanovaccines on immunotherapy. New Horizons in Translational Medicine, 2(2), 44–50.
Sharma, J., Shepardson, K., Johns, L. L., Wellham, J., Avera, J., Schwarz, B., Rynda-Apple, A., & Douglas, T. (2020). A self-adjuvanted, modular, antigenic VLP for rapid response to influenza virus variability. ACS Applied Materials & Interfaces, 12(16), 18211–18224.
Shenoda, B. B., & Ajit, S. K. (2016). Modulation of immune responses by exosomes derived from antigen-presenting cells. Clinical Medicine Insights: Pathology, 9, CPath-S39925.
Singh, P., Kim, Y. J., Zhang, D., & Yang, D. C. (2016). Biological synthesis of nanoparticles from plants and microorganisms. Trends in Biotechnology, 34(7), 588–599.
Soriano, V., Edagwa, B., Mendoza, C. D., Barreiro, P., Corral, O., Treviño, A., & Gendelman, H. E. (2022). Ultra-long-acting antivirals as chemical vaccines to prevent viral diseases. Future Microbiology, (0), 887.
Souri, M., Chiani, M., Farhangi, A., Mehrabi, M. R., Nourouzian, D., Raahemifar, K., & Soltani, M. (2022). Anti-COVID-19 nanomaterials: Directions to improve prevention, diagnosis, and treatment. Nanomaterials, 12(5), 783.
Stammers, T. S., Erden, Y. J., & Hunt, G. (2013). The promise and challenge of nanovaccines and the question of global equity. Nanotechnology Perceptions, 9, 16.
Temchura, V. V., Kozlova, D., Sokolova, V., Überla, K., & Epple, M. (2014). Targeting and activation of antigen-specific B-cells by calcium phosphate nanoparticles loaded with protein antigen. Biomaterials, 35(23), 6098–6105.
van der Koog, L., Gandek, T. B., & Nagelkerke, A. (2022). Liposomes and extracellular vesicles as drug delivery systems: A comparison of composition, pharmacokinetics, and functionalization. Advanced Healthcare Materials, 11(5), 2100639.
Vijayan, V., Mohapatra, A., Uthaman, S., & Park, I. K. (2019). Recent advances in nanovaccines using biomimetic immunomodulatory materials. Pharmaceutics, 11(10), 534.
Wibowo, N., Chuan, Y. P., Seth, A., Cordoba, Y., Lua, L. H., & Middelberg, A. P. (2014). Co-administration of non-carrier nanoparticles boosts antigen immune response without requiring protein conjugation. Vaccine, 32(29), 3664–3669.
Xiao, P., Wang, J., Fang, L., Zhao, Z., Sun, X., Liu, X., Cao, H., Zhang, P., Wang, D., & Li, Y. (2021). Nanovaccine-mediated cell selective delivery of neoantigens potentiating adoptive dendritic cell transfer for personalized immunization. Advanced Functional Materials, 31(36), 2104068.
Xu, L., Liu, Y., Chen, Z., Li, W., Liu, Y., Wang, L., Liu, Y., Wu, X., Ji, Y., Zhao, Y., & Ma, L. (2012). Surface-engineered gold nanorods: Promising DNA vaccine adjuvant for HIV-1 treatment. Nano Letters, 12(4), 2003–2012.
Yadav, H. K., Dibi, M., Mohammad, A., & Srouji, A. E. (2018). Nanovaccines formulation and applications-a review. Journal of Drug Delivery Science and Technology, 44, 380–387.
Yang, C., Yu, W., Bi, Y., Long, F., Li, Y., Wei, D., Hao, X., Situ, J., Zhao, Y., & Huang, F. (2018). Increased oestradiol in hepatitis E virus-infected pregnant women promotes viral replication. Journal of Viral Hepatitis, 25(6), 742–751.
Yıldız, Ş., & Ünver, Y. (2022). Nanomaterial-based vaccination to ameliorate the challenges of traditional vaccination, icaens. In 3rd international conference on applied engineering and natural sciences.
Yu, Z., Xu, Q., Dong, C., Lee, S. S., Gao, L., Li, Y., D’Ortenzio, M., & Wu, J. (2015). Self-assembling peptide nanofibrous hydrogel as a versatile drug delivery platform. Current Pharmaceutical Design, 21(29), 4342–4354.
Zahednezhad, F., Saadat, M., Valizadeh, H., Zakeri-Milani, P., & Baradaran, B. (2019). Liposome and immune system interplay: Challenges and potentials. Journal of Controlled Release, 305, 194–209.
Zhao, Q., Li, S., Yu, H., Xia, N., & Modis, Y. (2013). Virus-like particle-based human vaccines: Quality assessment based on structural and functional properties. Trends in Biotechnology, 31(11), 654–663.
Zhao, L., Seth, A., Wibowo, N., Zhao, C. X., Mitter, N., Yu, C., & Middelberg, A. P. (2014). Nanoparticle vaccines. Vaccine, 32(3), 327–337.
Zhao, P., Wang, C., Ding, J., Zhao, C., Xia, Y., Hu, Y., Zhang, L., Zhou, Y., Zhao, J., & Fang, R. (2021). Evaluation of immunoprotective effects of recombinant protein and DNA vaccine based on Eimeria tenella surface antigen 16 and 22 in vivo. Parasitology Research, 120(5), 1861–1871.
Zhou, J., Miyamoto, Y., Ihara, S., Kroll, A. V., Nieskens, N., Tran, V. N., Hanson, E. M., Fang, R. H., Zhang, L., & Eckmann, L. (2022). Codelivery of antigens and adjuvant in polymeric nanoparticles coated with native parasite membranes induces protective mucosal immunity against Giardia lamblia. The Journal of Infectious Diseases, 226, 319.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dey, S., Singh, G.B. (2023). Green Nanotechnology Approaches in Vaccinology: Advantages and Disadvantages in Biomedical Sciences. In: Pal, K. (eds) Nanovaccinology. Springer, Cham. https://doi.org/10.1007/978-3-031-35395-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-35395-6_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35394-9
Online ISBN: 978-3-031-35395-6
eBook Packages: MedicineMedicine (R0)