Abstract
Over the past few decades, our pieces of knowledge about fatty liver syndromes and their association with metabolic syndrome have increased, and we are now at a milestone in the beginning for shifting from nonalcoholic fatty liver disease (NAFLD) to metabolic associated fatty liver disease (MAFLD). Recently, a consensus with well-established criteria for MAFLD diagnosis was made, which has been validated in different populations. To establish a diagnosis of NAFLD, it was formerly essential to rule out other chronic liver illnesses, such as “excessive” alcohol consumption. The problems associated with the diagnosis of NAFLD, the poor outcomes in therapy trials, and the inconsistencies and hazards inherent in a “negative” definition (nonalcoholic) vs. a positive one (metabolic) are projected to promote the suggested renaming of NAFLD to MAFLD. However, a premature shift in language would not necessarily solve the significant unmet requirements in this field and may be detrimental. The revised definition clarifies the difference among diagnostic criteria and inclusion criteria for research projects and clinical trials. Achieving consensus on the criteria for the diagnosis of MAFLD will help unify the nomenclature, increase the validity of clinical practice and clinical trials, improve clinical therapy, and advance in scientific liver research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nonalcoholic fatty liver disease
- Metabolic dysfunction-associated fatty liver disease
- Liver fibrosis
- Metabolic syndrome
- Type 2 diabetes mellitus
2.1 Introduction
In the last century, the burden of obesity, type 2 diabetes mellitus (T2DM), metabolic syndrome (MS), as well as its components has been rising [1]. The impact of these diseases is also reflected in the structure of liver parenchyma, by overloading the hepatocytes with fat leading to a high proportion of patients with liver fibrosis. Nonalcoholic fatty liver disease (NAFLD) has become one of the few pandemics that increase the risk of chronic liver disease by its subtypes, including nonalcoholic steatohepatitis (NASH) [2].
As a short history, fatty liver generally known as steatosis has been described since 1845 due to the work of Addison, who identified alcohol-induced liver histological abnormalities [3]. Lately, after one century, Connor identified the possibility for alcoholic or diabetic fatty liver disease to evolve into liver cirrhosis, while in 1964, Dianzani elucidated the etiology of steatosis [4, 5]. The words NASH and NAFLD were not coined until the 1980s by Ludwig et al. and Shaffner and Thaler, respectively [6, 7]. After several decades of study in this area, it is now common knowledge that NAFLD and NASH are caused by different pathogens, are ubiquitous in the overall population worldwide, impose substantial direct and indirect expenses, and lack a safe and efficient pharmacological therapy [8].
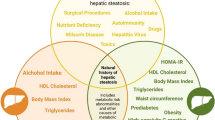
A panel of worldwide experts established an agreement in 2020 to reconsider the present concept of fatty liver disease, including renaming it as metabolic dysfunction-associated fatty liver disease (MAFLD) and establishing a simplified set of “positive” diagnostic criteria for both adults and children [9, 10]. MAFLD is diagnosed when a patient has hepatic steatosis, is overweight or obese, and has T2DM or two or more of the following: ethnicity-specific waist circumference cutoffs for central obesity; blood pressure ≥135/85 mmHg; plasma triglycerides ≥150 mg/dL; plasma HDL cholesterol <40 mg/dL for men and <50 mg/dL for women; fasting plasma glucose ≥100 mg/dL, 2-h post-load glucose ≥140 mg/dL, or hemoglobin A1c ≥5.7%; homeostasis model assessment of insulin resistance ≥2.5 and plasma high-sensitivity C-reactive protein >2 mg/L (Fig. 2.1, Table 2.1); or a specific drug treatment to counterbalance these metabolic disorders. This request garnered strong support from hepatologists throughout the world, hepatology scientific organizations, nursing and allied health leaders, pharmaceutical and regulatory science specialists, and patient associations. Notwithstanding, the new terminology has also generated criticism, emphasizing the necessity for a redefinition of NAFLD based on consensus [11].
The incidence of MAFLD is growing, even among nonobese persons, and affects 50% of the world’s overweight and obese adult population. This rise is found worldwide, mostly in low- and low-middle-income nations in Africa, Asia, and South America, and it constitutes a significant worldwide burden on healthcare expenses [12, 13]. Lifestyle modifications and a balanced diet remain the mainstay of the therapeutic management of these individuals, as there are no currently authorized drugs [12]. The majority of patients with fatty liver disease are previously identified and then managed in clinical settings by primary care physicians (PCPs). There is unambiguous evidence of the health-promoting effects of primary care and its involvement in sickness and mortality prevention [14]. In addition, in contrast to specialty care, the provision of primary care as a healthcare service for all populations is more egalitarian. In this setting, primary care is crucial and may thus aid or hinder the delivery of effective treatment for chronic diseases. To offer effective and high-quality treatment, PCPs must include new information, abilities, and positive attitudes toward care that emphasize system transformation and participatory patient and primary care team connections.
2.2 NAFLD Roadblocks
NAFLD, which includes the complete range of alcohol-like liver disorders present in nonalcoholics, was originally regarded as “the hepatic manifestation of the MS” [15]. However, this outmoded notion is at best unsatisfactory, and accumulating data suggests that the relationship between NAFLD and MS is complementary and bidirectional [1]. In the absence of competing causes of (steatogenic) liver disease, NAFLD is diagnosed noninvasively (through biomarkers and/or imaging modalities). Compared to these highly sensitive and specific biomarkers, conventional ultrasonography (US) retains a significant role since it is inexpensive, repeatable, widely available, and cost-efficient for excluding focal liver disease, with a semiquantitative assessment of liver structure changes including steatogenic and focal lesion diagnosing [16].
However, liver biopsy (LB) is the gold standard for diagnosis providing a definitive characterization of the fundamental histological lesions: steatosis, ballooning, inflammation, and fibrosis, allowing differentiation between the more indolent, uncomplicated steatosis and the more rapidly progressive NASH forms [17]. According to the categorization, NAFLD is considered “primary” when it is coupled with MS or is seen as a precursor to its occurrence. “Secondary” types of NAFLD are many and include, among others, illnesses resulting from dietary abnormalities, consequences of abdominal surgery, drug use, occupational exposure to chemical solvents, and (rarely) metabolic disorders [18,19,20,21]. In addition, NAFLD is frequently caused by common viral infections (viral associated fatty liver disease—VAFLD) and recurrent endocrine problems. These secondary types of NAFLD must be recognized from the main NAFLD because, for example, VAFLD caused by HIV infection has a poorer prognosis than primary NAFLD and NAFLD caused by hypothyroidism has a particular pathophysiology that, in theory, may be entirely reversed by thyroid replacement treatment [22].
NAFLD is notoriously underdiagnosed in primary care, data from current literature derived mostly from US studies indicating a prevalence of 2% and 5%, respectively, well below the anticipated population prevalence of 25–30% [23, 24]. NAFLD is not detected even in the presence of MS comorbidities and US or imaging testing findings of hepatic steatosis. The reasons for these “missed” diagnostics are complicated, with survey research indicating that NAFLD is not a priority in primary care and that there is a significant knowledge gap in NAFLD diagnosis and therapy [25]. These occurrences result in a significant gap between existing standards and actual clinical practice. However, the problems that led to a change in nomenclature to eliminate the confusing factor are the following: (1) in routine primary care settings, adherence to NAFLD clinical practice recommendations appears to be problematic for reasons other than a lack of understanding; (2) the intricacy of the diagnostic criteria for NAFLD poses a considerable hurdle for PCPs to initiate screening or active case diagnosis; (3) simplifying the diagnostic criteria for fatty liver disease acceptable for a busy primary care setting is essential for expanding therapy into primary care settings; and (4) with the time necessary to collect a complete and accurate alcohol history, patient care may be misdirected as a result of this dichotomization into alcoholic and nonalcoholic [26]. In addition, the limited availability and use of sensitive direct alcohol markers in primary, secondary, and tertiary care settings in various regions of the world render interviews or questionnaires the only method for distinguishing between alcoholic and nonalcoholic fatty liver disease.
2.3 NAFLD and the Metabolic Syndrome: Common Pathophysiology Pathway and Bidirectional Interplay
Insulin resistance is the shared pathophysiological common denominator between NAFLD and MS, as previously stated. In the medical literature, the “chicken-and-egg” issue regarding the temporal relationship of NAFLD and MS was finally resolved by recent evidence demonstrating that NAFLD is both the cause and the result of MS [27, 28]. Nonetheless, it became evident immediately that tackling the major pathogenic causes of NASH would not necessarily improve disease outcomes. Insulin sensitizers did not restore or even exacerbate mitochondrial defects in NASH, but pharmaceutical treatments, such as vitamin E, acting via pathways other than insulin sensitization led to histological improvement in at least some individuals [29]. Therefore, in blatant contradiction to pathogenesis studies, there are a few questions that arise. Firstly, is the treatment of insulin resistance never adequate to successfully cure NASH in the vast majority of patients? This is likely the outcome of a variety of pathogenic pathways that interact to cause varying degrees of liver damage in particular patients. Based on this premise, therapy should be individualized for each patient. However, it is not clear how this may be achieved. Determining the role of each pathophysiological process in the development of NAFLD/NASH in an individual patient remains a scientific and clinical practice obstacle. Second, should people with NAFLD who do not have MS and those with lean NAFLD be treated similarly to those who are obese? In the absence of supporting data, should men and women be treated equally? Regardless of whether NAFLD is the cause or outcome of MS (which is both, as we now know), it is essential to recognize that these two conditions are synergistic HCC risk factors [29, 30]. HCC is the most prevalent primary liver cancer and the fourth leading cause of cancer-related death [31].
In their landmark publication, Bellentani et al. correctly noted the limits of a “negative” definition of NAFLD and NASH as opposed to a “positive” one, i.e., “metabolic,” raising concerns from an established pipeline of prior investigations [32]. In accord, Fouad et al. identified that the reference to alcohol in the phrase “nonalcoholic” posed concerns of trivialization, stigmatization, and disregard by health authorities [33]. Recently, the factors that can influence clinical trials regarding NASH have been debated, for example their differentiation according to the fibrotic status of the patient (cirrhotic or non-cirrhotic), the differentiation between clinical and preclinical studies, but also the homogeneity of the studies regarding the histological diagnosis of NASH by LB, not emphasizing the extrahepatic manifestations or rather the patient’s comorbidities [34]. Recent studies emphasize a rapid progression of the degree of liver fibrosis in patients with NASH in patients with metabolic comorbidities, who are at a high risk of developing cirrhosis. Therefore, a change in nomenclature is necessary to establish the risk of individual mortality and morbidity.
2.4 NAFLD-MAFLD: A Debate Near the End
An expert group from as many as 22 nations developed the word MAFLD in an effort to combine ideas about the inaccuracy and potential detrimental implications of using the term “NAFLD” that had gathered over the previous few decades [9,10,11,12]. This idea has quickly garnered support across Latin America, North Africa, and the Middle East, suggesting a consensus that the justifications for discarding the existing nomenclature exceed those for preserving it [35, 36]. The diagnostic criteria for MAFLD exceed the inconveniences that were previously encountered for the diagnosis of NAFLD; for example, NAFLD in diabetic patients will follow the same path as in metabolically healthy obese patients. Similarly, it is unknown whether persons with altered metabolic derangements would be susceptible to the same risk of developing hepatic and extrahepatic problems as are usually associated with overt diabetes [9]. NAFLD and its subtypes nonalcoholic fatty liver (NAFL) and NASH were more thoroughly characterized than MAFLD from a histological standpoint, and characterizing liver histology remains a milestone in our ability to predict the clinical consequences of illness. Nevertheless, physicians and patients will welcome the option of noninvasively identifying MAFLD, considering the numerous critiques that may be linked to LB [27, 37]. Experts developed a set of diagnostic criteria to establish the diagnosis of MAFLD-associated cirrhosis, hence removing the phrase cryptogenic cirrhosis among dysmetabolic individuals. Considering that fatty changes could disappear over time, the committee proposed that patients with existing cirrhosis, despite the absence of histopathologic proof of steatohepatitis, should then be deemed to have MAFLD-related cirrhosis if at least one of the following requirements is met: past or present evidence of dysmetabolic features that meet the criteria for the diagnosis of MAFLD (as described above) that have at least one of the following criteria in their medical history: prior histopathology-proven MAFLD or confirmation of liver steatosis using imaging modalities [9,10,11,12]. In this light, it is essential to note the 1999 pivotal research in which Caldwell, based on his series of 70 cases, was the first to argue that “NASH plays an under-recognized role in many patients with cryptogenic cirrhosis, the majority of whom are older, T2DM-positive, and obese females” [38]. MAFLD takes a step further to accurately describe NAFLD individuals; however, it is unlikely to be the ultimate answer to all unmet clinical requirements. In addition, the unique concept of MAFLD integrates the insights acquired on the alarming interplay between NAFLD and MS, a relationship that impairs liver histology, accelerates fibrosis advancement, raises the chance of developing HCC, and diminishes the life expectancy of NAFLD patients.
2.5 Pro Arguments for MAFLD: Improve Disease Awareness
Decades of effort have been expended to raise the knowledge of NAFLD; nevertheless, a recent study demonstrates that switching from NAFLD to MAFLD boosted awareness of the illness among primary care providers and physicians of other specialties. Two further investigations have demonstrated that the new label MAFLD has increased patient awareness [35, 39]. Despite moderate acceptance, this illustrates the efficacy of the MAFLD criteria in the context of ordinary clinical care and suggests that the results are generalizable. Utilizing the MAFLD criteria more broadly could result in even larger gains in the care of MAFLD patients if this momentum is capitalized on [40] (Fig. 2.2).
The existing MAFLD care strategy can be reduced based on a transformational shift from NAFLD to MAFLD: better allocation of resources to diagnose more patients (expanding access and coverage), improved identification of patients at risk of disease progression and accelerated treatment initiation (linkage to care), reduction in complications among high-risk populations, and reduction in the long-term medical costs of complications, such as those associated with advanced liver disease, extrahepatic cholestasis, and extrahepatic cholestasis (optimizing referral pathway).
Unfortunately, the fact that current NAFLD diagnosis is centered on the rejection of other liver diseases poses a substantial barrier to the holistic management of patients with liver diseases, as well as the advancement of research into the interplay among fatty liver disease and other liver diseases. This may result in misclassification, underreporting, and suboptimal care for these patients, particularly in light of increasing evidence that patients with MAFLD and other consequent liver diseases, such as chronic viral hepatitis, alcohol intake, or autoimmune hepatitis, have a more severe liver injury than those with each disease alone [41,42,43]. An international committee of experts has emphasized the need of including MAFLD in the hepatitis C eradication campaign. Notably, numerous recent studies have indicated that in patients with simultaneous chronic hepatitis B or chronic hepatitis C, the MAFLD criteria are superior to the previous NAFLD criteria for detecting individuals with more severe liver damage, such as steatosis, fibrosis, and increased liver enzymes. On the other hand, the transition to MAFLD will permit the establishment of a multidisciplinary clinic with contributions from primary care, hepatology, endocrinology, and cardiology to improve both liver-related and cardiometabolic health [44].
2.6 Conclusion
The MAFLD definition’s revolutionary simplification of the diagnosis and evaluation may facilitate the implementation of effective fatty liver disease management, prevent overdiagnosis and overtreatment, and reduce underdiagnosis by PCPs. Thereby, this modification will enable PCPs to continue contributing to the health and well-being of patients in the community, based on accessibility, equity, and respect for the patient’s individuality, with a possible decrease in morbidity and mortality due to fatty liver worldwide.
References
Muzica CM, Sfarti C, Trifan A, Zenovia S, Cuciureanu T, Nastasa R, Huiban L, Cojocariu C, Singeap AM, Girleanu I, Chiriac S, Stanciu C. Nonalcoholic fatty liver disease and type 2 diabetes mellitus: a bidirectional relationship. Can J Gastroenterol Hepatol. 2020;28:6638306. https://doi.org/10.1155/2020/6638306.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. https://doi.org/10.1002/hep.28431. Epub 2016 Feb 22.
Addison T. Observations on fatty degeneration of the liver. Guys Hosp Rep. 1836;1:485.
Connor CL. Fatty infiltration of the liver and the development of cirrhosis in diabetes and chronic alcoholism. Am J Pathol. 1938;14(3):347–364.9.
Dianzani MU. Sulla patogenesi dell’accumulo del grasso nella steatosi epatica. Rass Med Sarda. 1964;66:67–90. Italian.
Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8.
Schaffner F, Thaler H. Nonalcoholic fatty liver disease. Prog Liver Dis. 1986;8:283–98.
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. https://doi.org/10.1038/nrgastro.2017.109. Epub 2017 Sep 20.
Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–9.
Eslam M, Alkhouri N, Vajro P, et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol Hepatol. 2021;6:864–73.
Younossi ZM, Rinella ME, Sanyal A, et al. From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology. 2020;73(3):1194–8. https://doi.org/10.1002/hep.31420.
Eslam M, Fan J-G, Mendez-Sanchez N. Non-alcoholic fatty liver disease in non-obese individuals: the impact of metabolic health. Lancet Gastroenterol Hepatol. 2020;5:713–5.
Ye Q, Zou B, Yeo YH, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:739–52.
Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005;83(3):457–502.
Diehl AM, Goodman Z, Ishak KG. Alcohol-like liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95:1056–62.
Zenovia S, Stanciu C, Sfarti C, Singeap A-M, Cojocariu C, Girleanu I, Dimache M, Chiriac S, Muzica CM, Nastasa R, Huiban L, Cuciureanu T, Trifan A. Vibration-controlled transient elastography and controlled attenuation parameter for the diagnosis of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Diagnostics. 2021;11:787. https://doi.org/10.3390/diagnostics11050787.
Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906.
Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015;47:181–90.
Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G, Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:936–44.
Guaraldi G, Lonardo A, Ballestri S, Zona S, Stentarelli C, Orlando G, Carli F, Carulli L, Roverato A, Loria P. Human immunodeficiency virus is the major determinant of steatosis and hepatitis C virus of insulin resistance in virus-associated fatty liver disease. Arch Med Res. 2011;42:690–7.
Lonardo A, Mantovani A, Lugari S, Targher G. NAFLD in some common endocrine diseases: prevalence, pathophysiology, and principles of diagnosis and management. Int J Mol Sci. 2019;20:2841.
Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Aliment Pharmacol Ther. 2015;41:368–78.
Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16(1):130.
Loomba R, Wong R, Fraysse J, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther. 2020;51:1149–59.
Standing HC, Jarvis H, Orr J, et al. GPs' experiences and perceptions of early detection of liver disease: a qualitative study in primary care. Br J Gen Pract. 2018;68(676):e743–9.
Eslam M, Sanyal AJ, George J. Toward more accurate nomenclature for fatty liver diseases. Gastroenterology. 2019;157(3):590–3.
Lonardo A, Carani C, Carulli N, Loria P. Chicken or egg turned into head or belly. J Hepatol. 2006;45:454–6.
Lonardo A. Renaming NAFLD to MAFLD: could the LDE system assist in this transition? J Clin Med. 2021;10(3):492. https://doi.org/10.3390/jcm10030492.
Lonardo A, Bellentani S, Ratziu V, Loria P. Insulin resistance in nonalcoholic steatohepatitis: necessary but not sufficient—death of a dogma from analysis of therapeutic studies? Expert Rev Gastroenterol Hepatol. 2011;5:279–89.
Wainwright P, Byrne CD. Bidirectional relationships and disconnects between NAFLD and features of the metabolic syndrome. Int J Mol Sci. 2016;17:367.
Lonardo A, Ballestri S, Chow PKH, Suzuki A. Sex disparity in hepatocellular carcinoma owing to NAFLD and non-NAFLD etiology. Epidemiological findings and patho-mechanisms. Hepatoma Res. 2020;6:83.
Bellentani S, Tiribelli C. Is it time to change NAFLD and NASH nomenclature? Lancet Gastroenterol Hepatol. 2017;2:547–8.
Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What’s in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020;40:1254–61.
Ratziu V, Friedman SL. Why do so many NASH trials fail? Gastroenterology. 2020;18:S0016-5085(20)30680-6.
Mendez-Sanchez N, Arrese M, Gadano A, Oliveira CP, Fassio E, Arab JP, Chávez-Tapia NC, Dirchwolf M, Torre A, Ridruejo E, et al. The Latin American association for the study of the liver (ALEH) position statement on the redefinition of fatty liver disease. Lancet Gastroenterol Hepatol. 2021;6:65–72.
Shiha G, Alswat K, Al Khatry M, Sharara AI, Örmeci N, Waked I, Benazzouz M, Al-Ali F, Hamed AE, Hamoudi W, et al. Nomenclature and definition of metabolic-associated fatty liver disease: a consensus from the Middle East and north Africa. Lancet Gastroenterol Hepatol. 2021;6:57–64.
Polyzos SA, Kang ES, Tsochatzis EA, Kechagias S, Ekstedt M, Xanthakos S, Lonardo A, Mantovani A, Tilg H, Côté I, et al. Commentary: nonalcoholic or metabolic dysfunction-associated fatty liver disease? The epidemic of the 21st century in search of the most appropriate name. Metabolism. 2020;113:154413.
Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–9.
Abdel Alem SGY, AbdAlla M, Said E, Fouad Y. Capturing patient experience: a qualitative study of change from NAFLD to MAFLD real-time feedback. J Hepatol. 2021;74(5):1261–2.
Eslam M, George J. MAFLD: now is the time to capitalize on the momentum. J Hepatol. 2020;74(5):1262–3.
Fouad Y, Saad Z, Raheem EA, et al. Clinical validity of the diagnostic criteria for metabolic-associated fatty liver disease: a real-world experience. medRxiv. 2020;8(20):20176214. https://doi.org/10.1101/2020.08.20.20176214.
Gaber Y, AbdAllah M, Salama A, Sayed M, Abdel Alem S, Nafady S. Metabolic-associated fatty liver disease and autoimmune hepatitis: an overlooked interaction. Expert Rev Gastroent. 2021;15:1–9.
Choi HS, Brouwer WP, Zanjir WM, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology. 2020;71(2):539–48.
Eslam M, Ahmed A, Després J-P, et al. Incorporating fatty liver disease in multidisciplinary care and novel clinical trial designs for patients with metabolic diseases. Lancet Gastroenterol Hepatol. 2021;9(6):P743–53. https://doi.org/10.1016/S2468-1253(21)00132-1.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Zenovia, S., Girleanu, I. (2023). Nonalcoholic Fatty Liver Disease Versus Metabolic Associated Fatty Liver Disease. In: Trifan, A., Stanciu, C., Muzica, C. (eds) Essentials of Non-Alcoholic Fatty Liver Disease. Springer, Cham. https://doi.org/10.1007/978-3-031-33548-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-33548-8_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33547-1
Online ISBN: 978-3-031-33548-8
eBook Packages: MedicineMedicine (R0)