Abstract
Melanins are an attractive family of quinone-based molecules found in natural sources featuring mixed ionic-electronic transport, redox activity, metal binding affinity, biocompatibility, and biodegradability. In this review, we provide an overview of melanins’ chemical structure and physicochemical properties, with focus on eumelanin, the black-brown member of the family. We discuss aspects aspects related to eumelanin’s electrochemical and ionic-electronic behavior in presence of metallic cations. We conclude with considerations regarding the possibility of using eumelanin metal recovery from e-waste.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Electronics have dramatically impacted the everyday life, at the global level. The use of organic electronic materials extracted from natural sources (biosourced) opens new venues for electronics: less relying on critical chemical elements and eco-designed in terms of end-of-life scenarios, possibly including compostability (Santato and Alarco 2022). Organic electronic materials can sustain ionic and electronic transport, with implications in the concept itself of semiconductivity (Reali et al. 2021). Melanins are a family of biopigments relevant for sustainable organic electronics. Among melanins, eumelanin has been the member of the family most investigated by physicists, materials scientists and physical chemists (Reali et al. 2020, 2021). Eumelanin features a range of functional properties, such as ionic and electronic transport, redox activity, metal binding affinity, biocompatibility, and biodegradability (Di Mauro et al. 2017; Liu et al. 2004). These properties are relevant for optical, electrochemical, electronic, and metal recovery applications. This chapter will review the chemical, structural and physicochemical properties of eumelanin and its interactions with metal ions, focusing on recent developments and building on the state-of-the-art in this same field, we proposed in 2017 (Di Mauro et al. 2017).
2 Chemical Structure and Physicochemical Properties of Eumelanin
The term melanin indicates a set of indole/quinone-based natural pigments resulting from biochemical syntheses taking place in different living organisms (animals, plants, fungii, and bacteria) (Galeb et al. 2021; Xie et al. 2019; Cao et al. 2021).
Based on their chemical precursors, these pigments can be organized into five categories: eumelanin, pheomelanin, neuromelanin, pyomelanin, and allomelanin (Fig. 1).
Biosynthetic pathways of the structures of melanin: eumelanin, neuromelanin, pheomelanin, and the two nitrogen-free analogues, pyomelanin and allomelanin. Compilation of converging data for pathways from a wide range of literature sources with a historical timeline highlighting melanin discoveries. Adapted from ref. (Cao et al. 2021)
In all cases, except for allomelanin, the pigments are obtained through the oxidation/polymerization of the amino acid tyrosine molecules. Allomelanins are synthesized from phenolic compounds by fungi and plants through a process of biosynthesis (Singla et al. 2021).
Potential structures of natural melanins: (a) DHI eumelanin and (b) DHICA eumelanin; (c) Pheomelanin; (d) Neuromelanin; (e) Dimer of allomelanin; and (f) Two possible structures of pyomelanin. Adapted from ref. (Cao et al. 2021)
For any of these melanin categories, as given in Fig. 2, it is necessary to use rigorous extraction methods to isolate the melanin component from all the other components present in the medium where the biosynthesis took place.
The preparation processes and sampling techniques can drastically change the chemical composition of the extracted sample, thus affecting the physicochemical properties of the extracted samples. (Liu and Simon 2003; Madaras et al. 2010). For example, eumelanin extracted from the ink sac of cuttlefish using the “syringe” method brings about an ink including L-DOPA (L-3,4-dihydroxyphenylalanine), dopamine, and taurine. In contrast, the “milking” method bring about an ink including tyrosinase and epinephrine (Madaras et al. 2010; Derby 2014).
2.1 Eumelanin
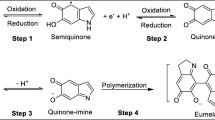
Within the melanin family of biopigments, eumelanin has been the most explored and studied, because of its application in various fields, such as electrochemical energy storage, bioelectronics and green electronics (João Paulin and Graeff 2021). Eumelanin is a natural biomacromolecule composed of two building blocks: 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole 2-carboxylic acid form (DHICA). The molecular ratio between DHI and DHICA building blocks affects the physicochemical properties of eumelanin (Terranova and Tamburri 2021). The presence of a quinone group in the building blocks, besides the presence of the carboxylic group in one of the two building blocks, is among the distinctive molecular features of eumelanin.
The carboxylic group is essential to differentiate the structure of eumelanin from that of synthetic polydopamine, so explored in the last decade. The absence of the carboxylic group brings about planarity in the molecular structure of polydopamine. As opposed to that, eumelanin features a structure with twists in its carbon backbone, where it is possible to observed atropisomerism by adjacent DHICA moieties (Pezzella et al. 2002; d’Ischia et al. 2014). The existence of these stable conformational isomers is due to the sizeable torsional barrier along the inter-unit bond (Fig. 3). In agreement with that, in the ultraviolet (UV)-visible spectra, no intense absorption bands are observed above 400 nm for DHICA-based melanin structures whereas intense absorption bands are observed for the DHI-based melanin structures in the visible region (Micillo et al. 2016).
Supramolecular structures of DHI- and DHICA-melanins. (a) DHI melanin represented as planar oligomeric scaffolds, (b) DHICA melanin is made up of twisted linear oligomer structures featuring atropisomerism. Adapted from ref. (d’Ischia et al. 2014)
2.2 Eumelanin vs Other Melanins
The presence of the nitrogen atom also permits to differentiate among different melanin structures. Allomelanin and pyomelanin are nitrogen-free melanins whereas in eumelanin the nitrogen atom is part of a pyrrole ring and in pheomelanin it is part of a thiazine ring. Neuromelanin results from the combination of 5-S-cysteinyl-dopamine and dopaminochrome (Fig. 2d).
It is worth noticing that, in general, the presence of the nitrogen atom in aromatic heterocycles causes the non-bonding electron pair of the nitrogen to occupy a sp2 hybrid orbital. Therefore, the non-bonding electrons pair will be in orbitals with significant “s” character, close to the nucleus (and as such less prone to engage in chemical bondings). Despite reports on the participation of nitrogen atoms in chelation bondings, the effects of sp2 hybridization should be taken into consideration when studying melanin-metals interactions.
2.3 Chemical and Physical Disorder
Eumelanin features chemical disorder due to its two building blocks, several polymerization sites connecting the building blocks, and three redox states bringing about comproportionation and tautomeric equilibria (Fig. 4). The chemical disorder is associated to physical disorder, in the sense that several supramolecular organizations are possible considering the different chemical species available in the macromolecular eumelanin biopigment.
Molecular structures of DHI and DHICA, with R is –H in DHI and –COOH in DHICA. The redox forms of DHI and DHICA are: hydroquinone (H2Q), semiquinone (SQ), quinone (Q) and quinone imine (QI) (the tautomer of Q). The building blocks can polymerize into eumelanin oligomers and polymers at different sites of the monomers (shown as 2, 3, 4, 7 in the figure). Adapted from ref. (Di Mauro et al. 2017)
2.4 Functional Properties of Eumelanin
Beyond applications as UV–Vis absorbers, eumelanin features metal ion binding properties, radical scavenging activity against reactive oxygen species, antioxidant activity, and charge transfer/charge carrier (electronic and ionic) transport properties. In what follows, we will discuss charge transfer and electrochemical properties.
3 Electrochemical Properties of Eumelanin: Focus on Metal Ions
When dealing with redox-active materials and devices, electrochemistry helps to shed light on electronic properties and chemical changes in materials upon charge transfer processes (Zhu and Shi 2019). Most of natural redox active materials tend to be non-soluble in aqueous media and form unstable colloidal suspensions. In addition, their immobilization on the surfaces of working electrodes can be challenging.
3.1 Aspects of Redox Properties in Eumelanin, in Presence of Alkaline and Ammonium Ions
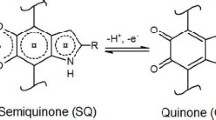
Eumelanin can feature proton-coupled electron transfer (PCET) (Costentin et al. 2010). Considering its quinone functionality (Fig. 4), it is reasonable to make the hypothesis that, during an electron transfer process in aqueous media, 2H+ and 2e− are simultaneously transferred.
For Sepia melanin (eumelanin extracted from the ink sac of cuttlefish) (Kim et al. 2014; Xu et al. 2017; Gouda et al. 2020), Kim et al. report values between −0.2 and 0.25 V vs. Ag/AgCl (Kim et al. 2014) whereas Xu et al.(Xu et al. 2017) report values of −0.06 V and 0.15 V vs. Ag/AgCl. These potentials are expected to depend on the pH value of the electrolyte (Gouda et al. 2020), although some authors report on potentials independent on pH (Serpentini et al. 2000). The interaction with alkaline metal ions produces shifts in the redox potentials, likely because the ions interact with the binding sites of eumelanin or intercalate within the supramolecular π–π stacks (Xu et al. 2017; Borghetti et al. 2010; Tian et al. 2019).
The cyclic voltammetries for eumelanin in presence of different alkaline and ammonium ions (Figs. 5a, b) show the presence of various redox signals. Voltammograms obtained with DHICA-melanin are better resolved compared to DHI-melanin, possibly because in DHICA-melanin, electron transfers, and ion exchanges are more localized than in DHI-melanin. Instead, voltammograms of DHI-melanin show higher values of the current owing to the more efficient π-π stacking in DHI-melanin concerning DHICA-melanin (Xu et al. 2017; Lucia et al. 2013).
Electrochemical behavior of eumelanin. DHICA-melanin (a) and DHI-melanin (b) in the presence of monovalent ions, ν = 5 mV/s, acid buffers. Adapted from ref. (Xu et al. 2017). (c) Mechanism proposed for the interaction/coordination of Mg2+ ions in the catechol groups in Sepia melanin during voltammetry cycles. States 1 and 3 represent the melanin in the direction of oxidation and reduction states, respectively. State 2 represents an intermediate state of a catechol group before forming a coordination bonding. (d) and (e) represent the cyclic voltammetries of Sepia melanin in the presence of an electrolyte based on Mg2+ ions, 0.5 M Mg(NO3)2. Adapted from ref. (Kim et al. 2014)
Tian et al. studied the mechanisms behind electron and ionic transfer in eumelanin pigments immersed in aqueous electrolytes including the monovalent cations Li+, Na+ and K+ (Tian et al. 2019). Their research succeeded in revealing structure-electron transfer (redox) property relationships, beyond the expected redox signals associated to the quinone/hydroquinone redox couple. In particular, redox processes were associated to specific interactions between metal ions and carboxylates pendant groups or aromatic amines. Further, the effect of the size of the metal ions was investigated.
On the one hand, there is a difference between natural and synthetic eumelanin, in terms of redox properties. For natural eumelanin, redox signals may vary from sample to sample due to the different ratios of DHI:DHICA. On the other hand, for synthetic eumelanin, redox signals are more reproducible. Here, however, heterogeneities can manifest if, for instance, during the polymerization process not all quinone groups are equally oxidized (Rózanowska et al. 1999; Mostert 2021; Panzella et al. 2013).
3.2 Redox Properties (Multivalent Ions)
Currently, multivalent ions (such as Mg2+, Ca2+, Zn2+, etc.) are investigated to replace Li-ions in energy storage devices with high energy densities. Eumelanin presents different binding sites to interact with these multivalent ions (Hong and Simon 2007). For divalent ions, catechol groups are responsible for chelation under an electrochemical regimen (Xu et al. 2017; Kim et al. 2014). In an interesting electrochemical fingerprinting study aiming at assessing structural changes in Sepia melanin-based electrodes during cycling, Kim et al. reported on porphyrin-like structures formed by tetramers of Sepia melanin building blocks (Kim et al. 2016).
4 The Effect of Metal Ions on the Electronic Transport of Eumelanin
In the 1970s, McGinness et al. observed a reversible electrical switching in hydrated eumelanin pellets (McGinness et al. 1974). This observation led to the description of the electrical behavior of eumelanin within the amorphous semiconductor model (McGinness et al. 1974; Davis and Mott 1970). More recent experimental results challenged such description proposing the possibility of predominant electronic transport in dry eumelanin and mixed ionic electronic transport in wet eumelanin (Mostert et al. 2012; McGinness et al. 1974; Davis and Mott 1970).
4.1 Monovalent Cations
Borghetti et al. studied the morphological and electrical properties of eumelanin after mixing with potassium-including salts (Borghetti et al. 2010). They both drop cast and electrodeposited eumelanin films on ITO and Au substrates from a mixture of potassium bromide and eumelanin-dimethyl sulfoxide (DMSO) solution, to study the effect of the salt on the formation of eumelanin aggregates. They propose that the interaction between potassium cations and nitrogen atoms in the pyrrole ring brings about additional electronic states in the valence band, as indicated by a transfer of spectral weight involving the HOMO level in XPS spectra, thus increasing the number of possible applications of the multifunctional eumelanin biomacromolecule.
4.2 Multivalent Cations
Mostert et al. reported that the modulation of the proton concentration in eumelanin by the chelation of the transition metal ion Cu2+ induces a modulation of the conductivity (Mostert et al. 2020). The modulation of the proton conductivity was characterized using Electron Paramagnetic Resonance (EPR). For this study, CuCl2·2H2O was added to a eumelanin solution (eumelanin powder obtained from D–L, dopa, dissolved in water and NH3) and then processed in thin film form. Cu2+ was chelated by hydroquinone/quinone moieties of melanin. The presence of Cu2+ increases its conductivity due to the increase in proton concentration in melanin. Afterwards, Organic Electrochemical Transistors (OECTs) were fabricated with the melanin-based copper-including quasi solid-electrolyte as the ionic gating medium and PEDOT:PSS as the transistor channel material. The enhancement of the proton conductivity in melanin is relevant for proton-to-electron transducing devices.
5 On the Interactions Between Eumelanin and Metal Electrodes
Wünsche et al. reported on the interaction between gold electrodes and hydrated eumelanin films deposited on gold electrode-pre-patterned SiO2 substrates (Wünsche et al. 2013). They showed that interaction between Au and eumelanin under electrical bias in wet environment results in the formation of Au-eumelanin nanoaggregates and dendrites. These dendrites can bridge one electrode to the other after the bias, leading to a dramatic increase of the current. The formation of the bridging dendrites following the dissolution of the Au electrodes was attributed to both the metal-binding properties of phenolic hydroxyl groups in eumelanin and Cl− (present low amounts of in eumelanin). It is worth mentioning that eumelanin also features reducing properties that can bring, chemically (i.e., in absence of electrical bias, gold cations to metallic gold. Di Mauro et al. (2016) later studied in detail the chemical and structural changes occurring at interfaces between metal electrodes (Pd, Cu, Fe, Ni and Au) and hydrated films of eumelanin, under bias (Di Mauro et al. 2016, 2019).
6 On the Possibility to Use Eumelanin in Metal Extraction from E-Waste
The field of electronics has profoundly modified the life quality of everyone, from information and communication technologies to education and industrial production (Patwa et al. 2021). Unfortunately, planned obsolescence and rapid upgrading of consumer electronics have led to the dramatic accumulation of waste electrical and electronic equipment (WEEE). Globally, about 50 million tons of electronic-waste (e-waste) are produced per year, with detrimental effects on human health and the environment (Dar et al. 2020; Tchounwou et al. 2012).
The composition of e-waste includes organic and inorganic materials such plastics, flame-retardants, and metals (Du et al. 2023). The global recycling rate for e-waste is about 20% (31% in Europe and North America, 12% in Eastern and South Eastern Asia, 5% in Central and Southern Asia, 4% Sub-Saharan Africa and 1% Latin America) (Boubellouta and Kusch-Brandt 2022). A relevant portion of e-waste is exported, sometimes illegally, to sub-Saharan and South-East Asian countries.
Precious metals, e.g., gold, palladium and platinum, have high economic value by considering their primary application in the electrical and electronics industry (Rafiee et al. 2021; Gunarathne et al. 2022). Recovering precious metals from e-waste represents an important economic opportunity.
There are several physicochemical and biological methods to extract metals from e-waste.
6.1 Hydrometallurgy
In hydrometallurgy, liquid chemistry based on the use of different chemicals (cyanide, thiourea, thiosulfates and acids) is employed to extract metals. After leaching (where solutions are used to solubilize the metal-containing materials by converting them into soluble salts), metals are usually further processed for purification and extraction (Gaydardjiev 1998; Whitworth et al. 2022). Cyanide ions (CN−) and aqua regia are used to recover gold in hydrometallurgy (La Brooy et al. 1994; Syed 2012).
6.2 Biohydrometallurgy
The biohydrometallurgical method is based on the use of different bacteria including chemolithoautotrophic bacteria, heterotrophic bacteria and fungii, such as Aspergillus Niger and Penicillum simplicissimum (Esmaeili et al. 2022), to transform insoluble metal oxides/sulfides into soluble metal ions for their subsequent recovery (Gu et al. 2018). There are different types of bacteria that have been used for bioleaching, such as mesophilic (temperature range 25–35 °C) and thermophilic (50 °C) (Gu et al. 2018; Kaksonen et al. 2017). A frequently used microorganism in sulfide ores bioleaching is Acidithiobacillus ferrooxidans (Watling 2006).
Bioleaching has been used in metal mining from the decades (Ji et al. 2022). Recently, this low cost and environmentally friendly method has been employed to extract heavy metals from ash and sewage sludge for bioremediation purposes (Gu et al. 2018).
6.3 Pyrometallurgy
Pyrometallurgy is based on the thermal treatment of materials, such as minerals and ores, to obtain precious metals (Harvey et al. 2022; Zhu et al. 2022). Compared to hydrometallurgy, it involves partial or complete conversion of chemical compounds into their elemental form. For example, pyrite is converted into pyrrhotite and elemental sulphur (Whitworth et al. 2022). During the pyrometallurgy process, different oxides and reducing agents are being used. For instance, to extract platinum-based metals (PBMs), lead oxides are used (Kim et al. 2013; Peng et al. 2017).
6.4 A Possible Perspective on the Interaction of Melanin with Metals for e-Waste Recovery
There are number of physicochemical mechanisms that can be exploited for the extraction of metals with melanin (Di Mauro et al. 2017; Meredith and Sarna 2006; Pilas et al. 1988; Hong et al. 2007).
We propose that in the future natural chelating agents could be used to promote metal recovery in urban mining, to recover precious, critical or strategic metals (Electronic Waste: Recycling and Reprocessing for a Sustainable Future, Maria E. Holuszko (Editor), Amit Kumar (Editor), Denise C. R. Espinosa (Editor) ISBN: 978-3-527-34,490-1).
Literature reports that, melanin extracted from squid ink features high adsorption tendency for lead (Xue et al. 2009). Systematic studies are needed to shed light on the effect of the source of melanin and its molecular and supramolecular structures (as well as possible presence of other chemical compounds in the natural or synthetic melanin material) on its binding affinity to metals, for metal recovery purposes. For instance, eumelanin prepared by L-DOPA can remove 95% of initial lead present in the investigated sample, a percentage dramatically higher than that one observed with eumelanin extracted from human hair (Sono et al. 2012). Results reported by Darwish et al. show that synthetic melanin nanoparticles (5,6-diacetoxy indole precursor that is hydrolyzed in situ into dihydroxy indole (DHI)) adsorb different metal ions; here highest adsorption values from 50 ppm solutions were observed for Co2+, Ni2+ and Zn2+ and lowest for Cu2+, Cd2+ and Pb2+ (Darwish et al. 2021).
Well beyond eumelanin, we wish to encourage the research community active in the field of metal recovery and water and soil remediation to explore, at large scale, the use of biosourced organic chelating agents considering their abundance, low cost, biodegradability and, possibly, biocompatibility.
Abbreviations
- DHI:
-
5,6-dihydroxyindole
- DHICA:
-
5,6-dihydroxyindole 2-carboxylic acid
- HOMO:
-
Highest Occupied Molecular Orbital
- LUMO:
-
Lowest Unoccupied Molecular Orbital
- OECT:
-
Organic ElectroChemical Transistor
- PEDOT:PSS:
-
Poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate)
- WEEE (e-waste):
-
Waste electrical and electronic equipment
- XPS:
-
X-ray Photoelectron Spectroscopy
References
Borghetti P, Goldoni A, Castellarin-Cudia C et al (2010) Effects of potassium on the supramolecular structure and electronic properties of eumelanin thin films. Langmuir 26:19007–19013. https://doi.org/10.1021/la102973u
Boubellouta B, Kusch-Brandt S (2022) Driving factors of e-waste recycling rate in 30 European countries: new evidence using a panel quantile regression of the EKC hypothesis coupled with the STIRPAT model. Environ Dev Sustain:1–28. https://doi.org/10.1007/s10668-022-02356-w
Cao W, Zhou X, McCallum NC et al (2021) Unraveling the structure and function of melanin through synthesis. J Am Chem Soc 143:2622–2637. https://doi.org/10.1021/jacs.0c12322
Costentin C, Robert M, Savéant JM (2010) Concerted proton-electron transfers: electrochemical and related approaches. Acc Chem Res 43:1019–1029. https://doi.org/10.1021/ar9002812
d’Ischia M, Napolitano A, Ball V et al (2014) Polydopamine and eumelanin: from structure–property relationships to a unified tailoring strategy. Acc Chem Res 47:3541–3550. https://doi.org/10.1021/ar500273y
Dar AA, Chen J, Shad A et al (2020) A combined experimental and computational study on the oxidative degradation of bromophenols by Fe(VI) and the formation of self-coupling products. Environ Pollut 258:113678. https://doi.org/10.1016/J.ENVPOL.2019.113678
Darwish ER, Kalil H, Alqahtani W et al (2021) Fast and reliable synthesis of melanin nanoparticles with fine-tuned metal adsorption capacities for studying heavy metal ions uptake. Nanotechnol Sci Appl 14:101–111. https://doi.org/10.2147/NSA.S296722
Davis EA, Mott NF (1970) Conduction in non-crystalline systems V. Conductivity, optical absorption and photoconductivity in amorphous semiconductors. Philos Mag 22:0903–0922. https://doi.org/10.1080/14786437008221061
Derby C (2014) Cephalopod ink: production, chemistry, functions and applications. Mar Drugs 12:2700–2730. https://doi.org/10.3390/md12052700
Di Mauro E, Carpentier O, Yáñez Sánchez SI, Ignoumba Ignoumba N, Lalancette-Jean M, Lefebvre J, Zhang S, Graeff CFO, Cicoirab F, Santato C (2016) Resistive switching controlled by the hydration level in thin films of the biopigment eumelanin. J Mater Chem C 4:9544–9553. https://doi.org/10.1039/C6TC02793H
Di Mauro E, Xu R, Soliveri G, Santato C (2017) Natural melanin pigments and their interfaces with metal ions and oxides: emerging concepts and technologies. MRS Commun 7:141–151. https://doi.org/10.1557/mrc.2017.33
Di Mauro E, Hebrard E, Boulahia Y et al (2019) On the interfaces between organic bio-sourced materials and metals for sustainable electronics: the eumelanin case. Jpn J Appl Phys 58:051014. https://doi.org/10.7567/1347-4065/ab1061
Du J, Waite TD, Biesheuvel PM, Tang W (2023) Recent advances and prospects in electrochemical coupling technologies for metal recovery from water. J Hazard Mater 442:130023. https://doi.org/10.1016/J.JHAZMAT.2022.130023
Esmaeili A, Arshadi M, Yaghmaei P, PEDS (2022) Simultaneous leaching of Cu, Al, and Ni from computer printed circuit boards using Penicillium simplicissimum. Resour Conserv Recycl 177:105976. https://doi.org/10.1016/j.resconrec.2021.105976
Galeb HA, Wilkinson EL, Stowell AF et al (2021) Melanins as sustainable resources for advanced biotechnological applications. Glob Challenges 5:2000102. https://doi.org/10.1002/gch2.202000102
Gaydardjiev SS (1998) Hydrometallurgy of precious metals: effects on the environment. In: Mineral processing and the environment. Springer, Netherlands, pp 257–280
Gouda A, Soavi F, Santato C (2020) Eumelanin electrodes in buffered aqueous media at different pH values. Electrochim Acta 347:136250. https://doi.org/10.1016/j.electacta.2020.136250
Gu T, Rastegar SO, Mousavi SM et al (2018) Advances in bioleaching for recovery of metals and bioremediation of fuel ash and sewage sludge. Bioresour Technol 261:428–440. https://doi.org/10.1016/J.BIORTECH.2018.04.033
Gunarathne V, Rajapaksha AU, Vithanage M et al (2022) Hydrometallurgical processes for heavy metals recovery from industrial sludges. Crit Rev Environ Sci Technol 52:1022–1062. https://doi.org/10.1080/10643389.2020.1847949
Harvey J-P, Courchesne W, Vo MD et al (2022) Greener reactants, renewable energies and environmental impact mitigation strategies in pyrometallurgical processes: a review. MRS Energy Sustain 2022:1–36. https://doi.org/10.1557/S43581-022-00042-Y
Hong L, Liu Y, Simon JD (2007) Binding of metal ions to melanin and their effects on the aerobic reactivity¶. Photochem Photobiol 80:477–481. https://doi.org/10.1111/j.1751-1097.2004.tb00117.x
Hong L, Simon JD (2007) Current understanding of the binding sites, capacity, affinity, and biological significance of metals in melanin. J Phys Chem B 111:7938–7947. https://doi.org/10.1021/jp071439h
Ji X, Yang M, Wan A et al (2022) Bioleaching of typical electronic waste–printed circuit boards (WPCBs): a short review. Int J Environ Res Public Health 19:7508. https://doi.org/10.3390/ijerph19127508
João Paulin SV, CF OG, Hamedi MM, Paulin JV (2021) From nature to organic (bio)electronics: a review on melanin-inspired materials. J Mater Chem C 9:14514–14531. https://doi.org/10.1039/D1TC03029A
Kaksonen AH, Morris C, Wylie J et al (2017) Continuous flow 70 °C archaeal bioreactor for iron oxidation and jarosite precipitation. Hydrometallurgy 168:40–48. https://doi.org/10.1016/J.HYDROMET.2016.08.015
Kim YJ, Khetan A, Wu W et al (2016) Evidence of porphyrin-like structures in natural melanin pigments using electrochemical fingerprinting. Adv Mater 28:3173–3180. https://doi.org/10.1002/adma.201504650
Kim BS, Lee JC, Jeong J et al (2013) A novel process for extracting precious metals from spent Mobile phone PCBs and automobile catalysts. Mater Trans 54:1045–1048. https://doi.org/10.2320/MATERTRANS.M2013051
Kim E, Liu Y, Leverage WT et al (2014) Context-dependent redox properties of natural phenolic materials. Biomacromolecules 15:1653–1662. https://doi.org/10.1021/bm500026x
Kim YJ, Wu W, Chun S-E et al (2014) Catechol-mediated reversible binding of multivalent cations in eumelanin half-cells. Adv Mater 26:6572–6579. https://doi.org/10.1002/adma.201402295
La Brooy SR, Linge HG, Walker GS (1994) Review of gold extraction from ores. Miner Eng 7:1213–1241. https://doi.org/10.1016/0892-6875(94)90114-7
Liu Y, Hong L, Kempf VR et al (2004) Ion-exchange and adsorption of Fe(III) by Sepia melanin. Pigment Cell Res 17:262–269. https://doi.org/10.1111/J.1600-0749.2004.00140.X
Liu Y, Simon JD (2003) The effect of preparation procedures on the morphology of melanin from the ink sac of Sepia officinalis. Pigment Cell Res 16:72–80. https://doi.org/10.1034/J.1600-0749.2003.00009.X
Madaras F, Gerber JP, Peddie F, Kokkinn MJ (2010) The effect of sampling methods on the apparent constituents of ink from the squid Sepioteuthis australis. J Chem Ecol 36:1171–1179. https://doi.org/10.1007/s10886-010-9869-0
McGinness J, Corry P, Proctor P (1974) Amorphous semiconductor switching in Melanins. Science 183:853–855. https://doi.org/10.1126/SCIENCE.183.4127.853
Meredith P, Sarna T (2006) The physical and chemical properties of eumelanin. Pigment Cell Res 19:572–594. https://doi.org/10.1111/J.1600-0749.2006.00345.X
Micillo R, Panzella L, Koike K et al (2016) “Fifty shades” of black and red or how carboxyl groups fine tune eumelanin and pheomelanin properties. Int J Mol Sci 17:746. https://doi.org/10.3390/IJMS17050746
Mostert AB (2021) Melanin, the what, the why and the how: an introductory review for materials scientists interested in flexible and versatile polymers. Polymers 13:1670. https://doi.org/10.3390/polym13101670
Mostert AB, Powell BJ, Pratt FL et al (2012) Role of semiconductivity and ion transport in the electrical conduction of melanin. Proc Natl Acad Sci 109:8943–8947. https://doi.org/10.1073/pnas.1119948109
Mostert AB, Rienecker SB, Sheliakina M et al (2020) Engineering proton conductivity in melanin using metal doping. J Mater Chem B 8:8050–8060. https://doi.org/10.1039/D0TB01390K
Panzella L, Gentile G, D’Errico G et al (2013) Atypical structural and π-electron features of a Melanin polymer that lead to superior free-radical-scavenging properties. Angew Chem Int Ed 52:12684–12687. https://doi.org/10.1002/anie.201305747
Patwa N, Sivarajah U, Seetharaman A et al (2021) Towards a circular economy: an emerging economies context. J Bus Res 122:725–735. https://doi.org/10.1016/J.JBUSRES.2020.05.015
Peng Z, Li Z, Lin X et al (2017) Pyrometallurgical recovery of platinum group metals from spent catalysts. JOM 69:1553–1562. https://doi.org/10.1007/s11837-017-2450-3
Pezzella A, Vogna D, Prota G (2002) Atropoisomeric melanin intermediates by oxidation of the melanogenic precursor 5,6-dihydroxyindole-2-carboxylic acid under biomimetic conditions. Tetrahedron 58:3681–3687. https://doi.org/10.1016/S0040-4020(02)00335-6
Pilas B, Sarna T, Kalyanaraman B, Swartz HM (1988) The effect of melanin on iron associated decomposition of hydrogen peroxide. Free Radic Biol Med 4:285–293. https://doi.org/10.1016/0891-5849(88)90049-4
Rafiee P, Ghassa S, Moosakazemi F et al (2021) Recovery of a critical metal from electronic wastes: germanium extraction with organic acid. J Clean Prod 315:128223. https://doi.org/10.1016/J.JCLEPRO.2021.128223
Reali M, Gouda A, Bellemare J et al (2020) Electronic transport in the biopigment sepia melanin. ACS Appl Bio Mater 3:5244–5252. https://doi.org/10.1021/acsabm.0c00373
Reali M, Saini P, Santato C (2021) Electronic and protonic transport in bio-sourced materials: a new perspective on semiconductivity. Mater Adv 2:15–31. https://doi.org/10.1039/d0ma00579g
Rózanowska M, Sarna T, Land EJ, Truscott TG (1999) Free radical scavenging properties of melanin: interaction of eu- and pheo-melanin models with reducing and oxidising radicals. Free Radic Biol Med 26:518–525. https://doi.org/10.1016/S0891-5849(98)00234-2
Santato C, Alarco PJ (2022) The global challenge of electronics: managing the present and preparing the future. Adv Mater Technol 7:2101265. https://doi.org/10.1002/admt.202101265
Serpentini CL, Gauchet C, De Montauzon D et al (2000) First electrochemical investigation of the redox properties of DOPA–melanins by means of a carbon paste electrode. Electrochim Acta 45:1663–1668. https://doi.org/10.1016/S0013-4686(99)00388-6
Singla S, Htut KZ, Zhu R et al (2021) Isolation and characterization of Allomelanin from pathogenic black knot fungus–a sustainable source of melanin. ACS Omega 6:35514–35522. https://doi.org/10.1021/acsomega.1c05030
Sono K, Lye D, Moore CA et al (2012) Melanin-based coatings as lead-binding agents. Bioinorg Chem Appl 2012:361803. https://doi.org/10.1155/2012/361803
Syed S (2012) Recovery of gold from secondary sources—a review. Hydrometallurgy 115–116:30–51. https://doi.org/10.1016/J.HYDROMET.2011.12.012
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. EXS 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6/COVER
Terranova ML, Tamburri E (2021) Understanding the way eumelanin works: a unique example of properties and skills driven by molecular heterogeneity. Polymer 229:123952. https://doi.org/10.1016/j.polymer.2021.123952
Tian Z, Hwang W, Kim YJ (2019) Mechanistic understanding of monovalent cation transport in eumelanin pigments. J Mater Chem B 7:6355–6361. https://doi.org/10.1039/C9TB01211G
Watling HR (2006) The bioleaching of sulphide minerals with emphasis on copper sulphides–a review. Hydrometallurgy 84:81–108. https://doi.org/10.1016/J.HYDROMET.2006.05.001
Whitworth AJ, Vaughan J, Southam G et al (2022) Review on metal extraction technologies suitable for critical metal recovery from mining and processing wastes. Miner Eng 182:107537. https://doi.org/10.1016/j.mineng.2022.107537
Wünsche J, Cardenas L, Rosei F et al (2013) In situ formation of dendrites in eumelanin thin films between gold electrodes. Adv Funct Mater 23:5591–5598. https://doi.org/10.1002/adfm.201300715
Xie W, Pakdel E, Liang Y et al (2019) Natural eumelanin and its derivatives as multifunctional materials for bioinspired applications: a review. Biomacromolecules 20:4312–4331. https://doi.org/10.1021/acs.biomac.9b01413
Xu R, Prontera CT, Di Mauro E et al (2017) An electrochemical study of natural and chemically controlled eumelanin. APL Mater 5:126108. https://doi.org/10.1063/1.5000161
Xue C, Chen S, Wang J et al (2009) Adsorption of Pb(II) and Cd(II) by squid ommastrephes bartrami melanin. Bioinorg Chem Appl 2009:901563. https://doi.org/10.1155/2009/901563
Zhu X, Shi L (2019) Electrochemistry. In: Nano-inspired biosensors for protein assay with clinical applications. Elsevier, Amsterdam, pp 209–236
Zhu X, Yang J, Yang Y et al (2022) Pyrometallurgical process and multipollutant co-conversion for secondary aluminum dross: a review. J Mater Res Technol 21:1196–1211. https://doi.org/10.1016/J.JMRT.2022.09.089
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Matos-Peralta, Y., Gao, Z., Dar, A.A., Santato, C. (2023). Exploiting Melanin-Metal Interactions for Emerging Technologies. In: Gosset, G. (eds) Melanins: Functions, Biotechnological Production, and Applications. Springer, Cham. https://doi.org/10.1007/978-3-031-27799-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-27799-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-27798-6
Online ISBN: 978-3-031-27799-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)