Abstract
Headache is one of the most common neurological presentations of COVID-19 disease. SARS-CoV-2 causes headache at a higher rate than other respiratory viral infection agents. Hypoxia, systemic inflammatory process, postviral immune-mediated complications, and vascular complications are blamed on pathophysiology.
COVID-19-related headache is characterized by a mild severity, usually bilateral frontal and temporal localized, sudden and gradual onset, longer than 72 hours, intermittent, evening-preferred, resistant to analgesics, aggravated by exercise and coughing, and relieved by sleep. Headache features observed in the early phase of the disease in mild-to-moderate COVID-19 patients were investigated more than in severe COVID-19 patients.
It was reported that headache-related COVID-19 secondary complications such as cough, systemic viral infection, hypoxia, psychiatric disorders, encephalopathy, viral meningitis/encephalitis, reversible vasoconstriction syndrome, subarachnoid hemorrhage, cerebral venous sinus thrombosis, metabolic disorders, mucormycosis, pituitary apoplexy, vasculitis, optic neuritis, and facial paralysis. However, the relationship of some of the secondary complications with COVID-19 is still unclear. Another overlooked point is that headache may develop as a side effect of drugs used to treat the disease.
Headache secondary to COVID-19 complications should be suspected in the presence of male gender, older age, acute onset, progressive and severe headache, a thunderclap headache, epileptic seizure, impaired consciousness, focal neurologic deficit, and comorbid disease. It should not be ignored that headache may be more than an isolated symptom in COVID-19-positive patients. Diagnostic algorithms should be carefully selected to ensure appropriate treatment protocols in these patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
The clinical presentation of COVID-19 is heterogeneous with a wide range of symptoms and diseases observed and can continue even months after the onset [1]. Neurological manifestation of COVID-19 can be scrutinized in three categories: central nervous system (CNS) (headache, dizziness, encephalopathy, seizure, cerebrovascular disease), peripheral nervous system (PNS) (anosmia, ageusia, visual impairment, neuropathic pain, Guillain-Barré syndrome, and its variants), and skeletal muscle damage [2]. Neurological complications during the viral invasion may occur due to hypoxia, systemic inflammatory process, postviral immune-mediated complications, and vascular complications [3].
Headache is deemed the most common and sometimes the first neurological presentation of the disease [1, 4]. It is estimated that there is a fivefold increase in headaches in the regions affected by the COVID-19 pandemic [5]. Headache frequencies are variable and were observed at 25.2% in a meta-analysis of 104,751 infected patients [6]. Headache in early stages can be attributed to excessive secretion of pro-inflammatory mediators and cytokines such as interleukin 1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α) [7]. The release of these pro-inflammatory mediators and cytokines may activate perivascular trigeminal nerve terminations during COVID-19 infection [8]. Headache may also occur due to overstimulation of the trigeminovascular system with inflammation in the endothelial cells of the vessel wall due to the direct invasion of peripheral trigeminal nerve terminations in the nasal cavity. Overactivation of those angiotensin-converting enzyme 2 (ACE2) receptors, nitric oxide (NO), and calcitonin gene-related peptide (CGRP) release resulting from hypoxia, cortical spreading depression, and disruption of the blood-brain barrier (BBB) are other possible pathophysiological mechanisms [9].
Headache, anosmia, and ageusia generally appear in the early stages of the symptomatic phase (within 1–2 days on average) and are more common in less severe cases [10]. Anosmia-ageusia with headache in the acute phase is the primary indicator of COVID-19; it is observed at a rate of 74.5% in COVID-19-positive patients [11]. This relationship of anosmia and ageusia with headache and their emergence in similar periods suggests that there may be common underlying pathophysiological mechanisms. It has been hypothesized that SARS-CoV-2 has neurotrophic properties and can flood peripheral nerve endings and penetrate the central nervous system by transsynaptic paths. The anatomical structure of the trigeminal nerve branches is thought to provide a path between the nasal epithelium and the central nervous system. This may explain the anosmia complaints seen in COVID-19 [12]. Another issue suggestive of the neurotropism of COVID-19 is the frequency of phonophobia in headache patients [10]. Researchers reported phonophobia in up to 67% of COVID-19-positive patients with headache, particularly in patients with hyposmia/anosmia and hypogeusia/ageusia [10, 11, 13].
6.2 Primary Headache Subtypes Observed in COVID-19 Patients
Headache was the most widespread symptom, along with anosmia in a meta-analysis of 1420 mild-to-moderate COVID-19 patients. The prevalence of pain was higher in women, young people, and those with a previous history of headache [14]. Headache attributed to COVID-19 was observed less frequently in men and older patients [13, 15]. Genetic, hormonal, and psychosocial dissimilarities can explain potential causes for disparity between the sexes range [16].
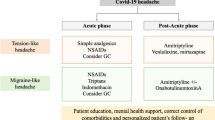
The type of headache observed in the acute period in patients with COVID-19 is generally tension-type headache (TTH) (17.1–64%) [10, 11, 13]. Migraine (14–36.3%) [10, 11, 13, 17], primary cough headache (5.4%) [10], headache secondary to systemic viral infection [17], cluster headache [18], and trigeminal and occipital neuralgia [1] are other types of headaches in COVID-19. Migraine-like and TTH-like pain have also been reported in some studies (Table 6.1) [10, 19]. Pain is generally mild-to-moderate severity (47–75.2%) [10, 11, 13], bilaterally located (88.9–94%) [10, 13], and pulsatile (50.9–58.7) [10, 11, 13]. The mean onset time of headache is 1.7 days; pain continues for an average of 2–4 days [13], but 15% of patients had persistent headache lasting at least 15 days. In patients whose headache is not steady, attacks proceed for an average of 120 minutes [10]. 44.3–64% of patients have a previous history of headaches [10, 11, 20]. The pain intensity was severe in 23.4–53% of patients [10, 11, 13] and very intense in 2.3% [11], and pain influenced 52.8% of daily activities [21].
6.3 Other Primary Headache Subtypes
6.3.1 Primary Cough Headache
The authors have classified headaches due to COVID-19 in two stages of the disease. In phase I (the influenza-like phase), it is seen as acute headache attributed to systemic viral infection, primary cough headache, tension-type headache, and headache attributed to heterophoria. In phase II (the cytokine storm phase), headache attributed to hypoxia and new-onset headache occur (Table 6.1) [15]. Cough headaches are too common in men and people over 40. Cough triggers headaches in COVID-19 patients who have previously had migraine or tension-type headaches. Secondary outcomes of cytokine release syndrome (CRS) in COVID-19 may be responsible for the underlying pathophysiologies and boost other inflammatory markers, such as D-dimer and CGRP, which play a position in headache [20]. CGRP is a neuropeptide with an essential position in migraine pathophysiology and has a suspicious connection to transient receptor potential (TRP) channels [22]. Possible viral activation of TRP channels is implicated in anosmia, cough, and gastrointestinal disturbances in patients with COVID-19. This activation then results in the release of CGRP in some patients. Releasing of CGRP is thought to facilitate the T cell response toward a more pro-inflammatory state characterized by Th17 and IL-17 [20]. In patients with cough-triggered headache, possible intracranial lesions such as middle cranial fossa or posterior fossa tumors, midbrain cyst, reversible cerebral vasoconstriction syndrome, subdural hematoma, cerebral aneurysms, Arnold Chiari malformation type 1, spontaneous intracranial hypotension, carotid or vertebrobasilar diseases, basilar impression, and platybasia must be ruled out [23]. However, it led to diagnostic limitations as COVID-19 protocols for imaging have increased the magnetic resonance imaging (MRI) threshold in the studies [24]. Although there is not enough data, the headache types of some patients may be “headache attributed to increased cerebrospinal fluid (CSF) pressure” or “Headache attributed to low CSF pressure,” according to the International Classification of Headache Disorders, 3rd edition (ICHD-3) criteria (Table 6.1) [23]. Considering the hypoxic, toxic, and metabolic disorders that COVID-19 may cause in patients, headache in these patients should be evaluated in terms of “headache attributed to intracranial hypertension secondary to metabolic, toxic, or hormonal cause” (Table 6.1) [20, 23, 25].
6.3.2 New Daily Persistent Headache (NPDH)
NDPH is a primary headache belonging to the chronic daily headache (CDH) group, characterized as a marked and clearly remembered beginning, becoming continuous, and lasting pain within 24 hours and permanent for over 3 months [23]. Various triggers associated with NDPH have been reported, such as most commonly recent infection episodes or stressful life events [26]. Persistent headache that develops during or after acute infection and persists after the resolution of the disease remains relatively common, suggesting that infection triggers the development of NDPH [27]. 12.6–39.1% of COVID-19 patients have new-onset headaches [7,8,9], and 10% have headaches lasting longer than 3 months [21]. Persistent headaches at rates of 60.7% after an acute episode of COVID-19 have been reported, and some of these also met the NPDH diagnostic criteria (Table 6.1) [19].
6.3.3 External-Pressure Headache/Personal Protection Equipment (PPE) Headache
Ong et al. described a new type of PPE-related headache in staff working in COVID-19 services (Table 6.1). This survey-based study found de novo PPE-related headaches in 81% of the participants. It was also stated that preexisting headaches, especially migraines, were exacerbated in some patients [28]. It was confined that this was due to prolonged mask usage that touches the scalp or using a double mask. Some masks may cause breathing difficulties, especially during periods of exertion [29]. Furthermore, factors such as mechanical squeeze triggered by PPE, hypoxemia, and hypercarbia are other factors responsible for the pathogenesis of headache [28]. Considering the mechanical pressure and compression caused by PPE, occasionally headaches in these patients are classified as “external-pressure headache” according to ICHD-3 criteria (Table 6.1) [23]. However, patients were not evaluated for this concern in studies. For PPEs, it has been suggested that it can initiate neural activity by stimulating trigeminal and occipital nerve terminations through compression and peripheral sensation [28]. In a study, the higher frequency of allodynia in patients with worsening migraine seems to support this [29]. When we consider the role of trigeminovascular system activation in the pathophysiology of migraine, we can think that PPEs may lead to migraine activation [30]. Besides, it has been reported that the use of disinfectants in patients with osmophobia is one of the reasons for deteriorating migraine attacks [29].
6.4 Secondary Headache Subtypes Observed in COVID-19 Patients
6.4.1 Systemic Viral Infection and Headache
While 78.5–85.2% of the patients reported pain different from the pain they had experienced before [10, 11, 13, 21], 12.6–25.7% developed de novo pain [10, 13]. It is emphasized that headache is 1.7–2.2 times more common in COVID-19 patients than other non-COVID-19 viral infections (other respiratory viral infections) [6]. Thus, some studies have investigated the characteristics of headache attributed to COVID-19. The most striking features of headache are these: usually on bilateral frontal and temporal localization; sudden and gradual onset; lasting longer than 72 h; an intermittent, mild, and vague type of pain; mainly during night period; resistant to analgesics; exacerbated by exercise and coughing; relieved by sleep; and high recurrence rate limited to the active phase of COVID-19 [9, 24]. Another study evaluated the distinctive variants of headache attributed to COVID-19, bilateral localization, duration over 72 h, male gender, analgesic resistance, gastrointestinal symptoms, and presence of anosmia/ageusia; factors that increase the hazard of headache due to COVID-19 infection were emphasized [31]. No relationship between headache and Valsalva maneuvers was found except for exercise and coughing [17]. Pain may be accompanied by nausea and vomiting, photophobia, phonophobia, osmophobia, and allodynia, but trigeminal autonomic symptoms are infrequent [10, 11, 13, 17]. This different pain phenotype attributed to COVID-19 may correspond to the diagnosis of acute or chronic headache attributed to systemic viral infection, according to the ICHD-3 criteria (Table 6.1) [17, 23]. The precise mechanisms of headache attributed to systemic infection have not yet been thoroughly studied. Nevertheless, it may occur due to fever and exogenous or endogenous pyrogens, direct effects of microorganisms, and activation of various immunoinflammatory mediators [32].
6.4.2 Hypoxia/Hypercapnia and Headache
In severe cases of COVID-19, patients have severe dyspnea and hypoxemia and require an intensive care unit. Cardiovascular diseases, hypertension, diabetes mellitus, chronic lung disorder, systemic malignancies, and chronic renal failure are comorbid conditions monitored [33]. Headache secondary to hypoxemia developing in alveolar tissues may occur during COVID-19 infection (headache attributed to hypoxia and/or hypercapnia) (Table 6.1) [23, 34]. Hypoxia to ischemia and ischemia may also lead to headache by increasing free radicals [34, 35]. However, headache characteristics of patients have not been adequately questioned in studies. This may be because most studies have focused on COVID-19 symptoms in general, and the frequency of headaches has been underestimated because of respiratory symptoms that cause patients to be hospitalized most frequently [10].
6.4.3 Psychiatric Disorders and Headache
The lack of treatment, uncertainty in the process, fear of death, social isolation, and economic problems, especially in the initial phases of the COVID-19 pandemic, have led to an increased incidence of psychiatric disorders such as panic, anxiety, and depression [36]. Perlis et al. found that the prevalence of these symptoms among COVID-19 patients was 52.4%, and the existence of headache was more highly associated with moderate or severe depressive symptoms [37]. In another study, researchers reported that the probability of anxiety disorder and depression increased 2.2–4 times in patients with headache, especially in individuals with migraine and chronic TTH, compared to healthy controls [38]. Individuals with migraine may experience intense anxiety and are more likely to commit suicide [39]. Although it has not been studied in detail, headache types in these patients may fit classifications as “headache attributed to depressive disorder,” “headache attributed to panic disorder,” and “headache attributed to generalized anxiety disorder” according to the ICHD-3 criteria (Table 6.1) [23]. It is unclear whether the psychiatric symptoms are due to the psychosocial problems caused by the pandemic or to the direct effects of the virus on the central nervous system [40]. Mental stress, intense anxiety, and lifestyle modifications are likely causes of headaches [41]. In addition, the ongoing neuroinflammation and cytokine release in COVID-19 can affect the metabolism of neurotransmitters such as serotonin, dopamine, and glutamate through synthesis, release, and reuptake [42]. As a result, any neurotransmitter deterioration may lead to psychiatric symptoms. Disease severity, hospitalization, previous mental disorders, female gender, infected individuals in the family, post-infection physical pain, and raised inflammatory markers play a vital role in developing depression and anxiety in COVID-19 patients [43, 44].
Another common psychiatric disorder in recovering COVID-19 patients is posttraumatic stress disorder (PTSD) [44]. PTSD is characterized by severe anxiety, flashbacks, nightmares, and emotional numbness following a typically traumatic event, such as death, grave violence, sexual or physical attack, or a fight. Fear of pain is hypothesized to contribute significantly to the development of chronic pain. Augmented anxiety, fear, and stress increase perceived pain intensity and decrease pain tolerance [45]. PTSD seems more in those with severe respiratory distress than those without respiratory symptoms [46]. This shows that hypoxemia may also have an effect in addition to the aforementioned pathophysiological mechanisms that can cause psychiatric symptoms [47]. The incidence of PTSD among COVID-19 patients ranged from 20% to 30% [48]. A study is demonstrated that young age, gender, obesity, severe respiratory symptoms, and comorbid psychiatric condition lead to hospitalization and intensive care unit care as risk factors [49]. Persistent symptoms such as headache, anosmia, fatigue, shortness of breath, sleep problems, chest ache, cough, and mental health problems that can be observed after COVID-19 substantially impact the quality of life caused by PTSD [50]. Therefore, patients who develop a headache after COVID-19 should also be evaluated in terms of “headache attributed to posttraumatic stress disorder” according to ICHD-3 criteria (Table 6.1) [23].
6.4.4 Substance Withdrawal and Headache
Illicit substances, particularly opioids, seriously impair lung function by reducing immune function [51]. COVID-19 patients with comorbid substance abuse disorder had a higher rate of hospitalization and death than patients without a history of substance abuse [52]. Isolation practices and restrictions, especially in the pandemic’s early stages, led to an inability to obtain addictive substances in this group of patients and the subsequent emergence of alarming withdrawal symptoms in these patients [53]. COVID-19-positive patients with headache and a history of substance use should be evaluated in terms of “headache attributed to substance withdrawal” according to the ICHD-3 criteria (Table 6.1). However, there is insufficient information in the literature.
6.4.5 Encephalopathy and Headache
Encephalopathy is a change in one or more brain functions such as altered consciousness, confusion, seizures, and acute focal disorders caused by systemic disease and is typically reversible. A study detected headache and encephalopathy in 40% of COVID-19 patients, but the particulars and diagnostic criteria are not disclosed [4]. Saniasiaya and Kulasegarah suggested that headache may occur due to mechanisms such as inflammation in neural nerves, hypoxia, hypercoagulopathy, and re-exposure of the brain to the pathogen [54]. Although encephalopathy is reversible, it can be prolonged in severe COVID-19 patients and is a risk factor for poor outcomes. In patients with encephalopathy, there is no evidence of brain inflammation shown on imaging or CSF. Patients who developed unexplained encephalopathic features showed leptomeningeal enhancement on brain MRI (62%), perfusion abnormalities on MRI (100%), and ischemic cerebrovascular attack (23%) [55].
Posterior reversible leukoencephalopathy (PRES) is a potential neurological complication associated with COVID-19 characterized by headache, altered consciousness, visual impairment, and seizures (according to ICHD-3 criteria, “headache attributed to hypertensive encephalopathy”) (Table 6.1). The cytokine storm of COVID-19 can cause endothelial dysfunction leading to increased permeability of the BBB and sensitization to blood pressure changes [56]. Renal failure may be another explanation because renal failure is a familiar complication associated with critically ill patients with COVID-19 [57]. Compared with other etiologies, there are higher bleeding rates in neuroimaging cases of PRES due to COVID-19 [56].
6.4.6 Viral Meningitis/Encephalitis and Headache
Intracranial infections in COVID-19 patients are reported very seldom. Clinically, it may present as encephalitis, meningoencephalitis, or meningitis. According to ICHD-3 criteria, “headache attributed to viral meningitis or encephalitis” should be suspected when symptoms of fever, neck stiffness, light sensitivity, and nausea and/or vomiting are observed in COVID-19 patients (Table 6.1). Meningitis and/or encephalitis diagnosis can be confirmed through histology studies or findings of inflammatory cells in the CSF. The genetic material of SARS-CoV-2 was found in the CSF of a patient with clinical meningoencephalitis [58]. This suggests that COVID-19 may lead to a direct central nervous system infection. Underlying pathophysiological mechanisms are developing cytokine release after SARS-CoV-2 infection and secondary of this disruption of the BBB and injury to the brain parenchyma [59].
Another recently reported secondary infection is rhino-orbital mucormycosis, a deadly angio-invasive disease caused by mold fungi such as Rhizopus, Mucor, Rhizomucor, and Cunninghamella and usually affecting immunocompromised patients. Diabetes mellitus (DM) and corticosteroids appear to be risk elements, because there was DM in 80% of cases. Also, 76.3% of mucormycosis and COVID-19 patients used corticosteroids as treatment [60]. One study suggested that patients with both head and face pain should be suspected of mucormycosis [61]. This type of pain can be examined under the subtitle “headache attributed to acute rhinosinusitis” according to ICHD-3 criteria (Table 6.1).
6.4.7 Subarachnoid Hemorrhage/Reversible Vasoconstriction Syndrome and Headache
Reversible vasoconstriction syndrome (RVCS) is one of the rare neurological complications of COVID-19. COVID-19 does not directly cause global or segmental vasoconstriction. However, downregulation of ACE2 receptors leads to overactivation of the classical renin-angiotensin axis, resulting in vasoconstriction and/or sympathetic hypertonia of cerebral vessels [62]. RCVS can lead to complications such as seizures, ischemic stroke, intracerebral hemorrhage, PRES, and convexity subarachnoid hemorrhage (cSAH). Recurrent thunderclap headaches are typical for RCVS. In a study, it was emphasized that even in the mild course of COVID-19, especially in young patients, recurrent headaches and thunder headaches should be a warning that it is a typical symptom of RCVS [63]. This headache is evaluated under the subtitle “headache attributed to reversible cerebral vasoconstriction syndrome” according to ICHD-3 criteria (Table 6.1).
Intracranial hemorrhage is infrequent in patients with COVID-19 [64]. It was observed as the second common finding in neuroimaging studies in patients with neurological involvement with COVID-19 [65]. Intracranial hemorrhage is commonly associated with risk factors such as arterial hypertension and anticoagulation therapy [64]. A higher incidence of aneurysmal SAH is suspected in patients with COVID-19 [65]. Acute onset of thunderclap headache is typical for SAH, which is classified as an “Acute headache attributed to non-traumatic subarachnoid hemorrhage” according to the ICHD-3 criteria (Table 6.1). The pain has been reported to be holocephalic and progressive in a few cases [63, 65]. It may present a complication of RVCS, especially in patients younger than 60 years of age, and is usually localized at the level of convexity.
6.4.8 Stroke/Cerebral Venous Sinus Thrombosis and Headache
The most common types of acute stroke are ischemic and hemorrhagic. Ischemic stroke is probably the most common type of stroke [66]. Cerebral venous sinus thrombosis (CVST) is 0.08% in hospitalized patients infected with COVID-19, accounting for 4.2% of all cerebrovascular events in the context of COVID-19 infection. CVST evolves within 1–8 weeks after respiratory or systemic symptoms of SARS-CoV-2 infection [67]. Systemic inflammation, cytokine release, direct immune-mediated postinfectious mechanism, and endotheliitis may cause arterial and venous thrombosis in COVID-19 patients [56]. All CVST cases have been reported in individuals with headache (headache attributed to cerebral venous thrombosis according to ICHD-3) (Table 6.1) [23, 68]. With the presence of headache, seizures, focal neurological signs, encephalopathy, or mental status change in the acute and subacute stage of COVID-19, CVST should be suspected, and intracerebral vascular imaging (e.g., CT or MR venography) should be performed. As CVST is one of the few causes of cerebral hemorrhage needing anticoagulation therapy, diagnostic confidence is essential to initiate proper treatment.
6.4.9 Pituitary Apoplexy and Headache
Pituitary apoplexy is a rare disorder resulting from ischemia or bleeding in a pituitary adenoma or, infrequently, a physiologically enlarged pituitary gland. It is observed in roughly 2–12% of all patients with pituitary adenoma. Pituitary apoplexy usually presents with a sudden onset of severe headache. Rapidly progressive visual disturbances, nausea, pituitary hormone lacks, and intracranial hypertension symptoms can follow headaches. Pituitary apoplexy has been reported in a few cases of COVID-19 [69, 70]. Taneja et al. also described ischemic pituitary apoplexy in a 74-year-old female patient who presented with acute and new-onset, very painful (the most excruciating pain in her life) headache accompanied by nausea and vomiting [70]. The acute-onset, severe headache described in these cases is classified as a “headache attributed to pituitary apoplexy” according to the ICHD-3 criteria (Table 6.1). SARS-CoV-2 can precipitate pituitary infarction and/or hemorrhage, combined with overstimulation of the pituitary gland, and influence the coagulation cascade by provoking thrombocytopenia and platelet dysfunction in the setting of acute infection [71].
6.4.10 Metabolic or Systemic Disorders and Headache
Headache in SARS-CoV-2 infection may occur as a result of more general indirect mechanisms that are not specific to the disease, including hypoxia, dehydration, systemic inflammation, and metabolic disorders [20]. This situation can be evaluated under the subtitle of “headache attributed to other metabolic or systemic disorder” according to the ICHD-3 criteria (Table 6.1). Systemic metabolic changes, such as fluid and electrolyte imbalance, hormonal dysfunction, and accumulation of toxic metabolites, reduce cerebral perfusion. It may cause some nonspecific neurological presentations of the disease such as headache, confusion, and agitation [25]. The autopsy examinations of brain tissue performed in patients who died from acute COVID-19 determined that they had serious diseases and that most had systemic and metabolic disorders that were not specific to pathology before death [72].
6.4.11 Other Complications and Headache
CNS angiitis can occur due to some conditions such as inflammatory, infectious, malignant, or toxic. Headache is the dominant symptom in the patients, but CNS angiitis has no specific features [23]. CNS angiitis secondary to COVID-19 was reported in a 28-year-old man who presented with intense headache, dysarthria, and deviation of lip rhyme to the left (headache attributed to secondary angiitis of the central nervous system according to ICHD-3) (Table 6.1) [23, 73].
In addition, the prevalence of Bell’s palsy in patients infected with SARS-CoV-2 was found to be 0.08%, and recurrence was 8.6% in those diagnosed with Bell’s palsy before infection [77]. In one case, left retro-auricular pain and taste perversion with facial paralysis on the left side in a patient infected with SARS-CoV-2 have been reported (headache or facial pain attributed to disorder of the cranium, neck, eyes, ears, nose, sinuses, teeth, mouth, or other facial or cervical structure according to ICHD-3 appendix criteria) (Table 6.1) [23, 74].
Another point that is neglected when evaluating headaches secondary to COVID-19 complications is that, although rare, headache can be seen as a side effect of drugs used in the treatment (headache attributed to the occasional use of non-headache medication according to ICHD-3) (Table 6.1). Headache can be observed in patients at varying rates as a side effect of drugs such as ivermectin, azithromycin, remdesivir, posaconazole, and tocilizumab, which are used to manage complications and treat the infection [75].
Finally, cases of optic neuritis have been reported in some COVID-19-positive patients. 92.2% of optic neuritis patients have eye pain that worsens with eye movement (painful optic neuritis according to ICHD-3) (Table 6.1). However, there is no evidence of human coronaviruses’ etiological role in acute monosymptomatic optic neuritis [76].
6.5 Conclusion
Headache is one of the most common neurological complications of the COVID-19 disease and may cause different pain phenotypes in patients in acute, subacute, and chronic phases. Headache, anosmia, and ageusia usually occur in the early stages of the symptomatic phase of mild-moderate COVID-19. A systemic inflammatory process, hypoxia, vascular complications, and postviral immune-mediated complications can cause headaches in COVID-19-positive patients. The features of headache attributed to COVID-19 headache are usually bilateral frontal and temporal localization; showing sudden and gradual onset; lasting longer than 72 h; having an intermittent, mild, and vague type of pain; showing evening preference; being resistant to analgesics; exacerbated by exercise and coughing; relieved by sleep; and showing high recurrence rate limited to the active phase of COVID-19. Besides this, SARS-CoV-2 can also trigger some primary headache subtypes such as TTH and migraine. In the studies, while the headaches observed in the early period of the COVID-19 infection were well examined, headaches secondary to complications of COVID-19 observed in the later period of the disease have not been adequately investigated. Reasons for this are the following:
-
1.
Preceding severe symptoms such as pulmonary and cardiac in patients than headache complaints
-
2.
Inability of patients with encephalopathy to express headache, if any
-
3.
Being the high threshold for advanced examinations such as neuroimaging and CSF analysis to minimize the risk of transmission, especially in the early period of the pandemic
Although some secondary headache subtypes can observe together with COVID-19 complications, the relationship between some complications of COVID-19 and headache is still not proven. Secondary headache disorders attributed to COVID-19 complications should be suspected in patients of the male gender, older age, acute onset, progressive and severe headache, a thunderclap headache, epileptic seizure, impaired consciousness, focal neurological deficit, and history of concomitant comorbid disease. In these patients, detailed medical history, neurological examination, and neuroimaging methods should be designed for the suspected diagnosis. Psychiatric complications of the disease should not be overlooked. Psychosocial rehabilitation should be provided by furnishing necessary consultations with appropriate individuals. In patients with severe COVID-19 infection, although the risk of headache secondary to COVID-19 complications is higher, it should be kept in mind that even mild-moderate COVID-19 disease may present with severe symptoms, and a complete neurological evaluation should be performed in all patients.
References
Köseoğlu Toksoy C, et al. Neurological symptoms and findings in COVID-19: a prospective clinical study. Neurol Res. 2022;44(1):1–6.
Ling MWM (2020) Neurological manifestations of Hospitalized Patients with COVID-19 in Wuhan. China: a retrospective case series study.
Boldrini M, et al. How COVID-19 affects the brain. JAMA Psychiat. 2021;78(6):682–3.
Mao L, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–90.
Lippi G, et al. Headache is an important symptom in patients with coronavirus disease 2019 (COVID-19). Diagnosi. 2020;7(4):409–11.
Soares FHC, et al. Prevalence and characteristics of new-onset pain in COVID-19 survivours, a controlled study. Eur J Pain. 2021;25(6):1342–54.
Oguz-Akarsu E, et al. Insight into pain syndromes in acute phase of mild-to-moderate COVID-19: frequency, clinical characteristics, and associated factors. Eur J Pain. 2022;26(2):492–504.
Rocha-Filho S, et al. Headache associated with COVID-19: frequency, characteristics and association with anosmia and ageusia. Cephalalgia. 2020;40(13):1443–51.
Mutiawati E, et al. Global prevalence and pathogenesis of headache in COVID-19: a systematic review and meta-analysis. F1000Res. 2020;9
Patarca-Montero R. Medical Etiology, assessment, and treatment of chronic fatigue and malaise: clinical differentiation and intervention; what does the research say? CRC Press; 2004.
Edvinsson L, et al. Does inflammation have a role in migraine? Nat Rev Neurol. 2019;15(8):483–90.
Bolay H, et al. COVID-19 is a real headache! Headache. 2020;60(7):1415–21.
Uygun Ö, et al. Headache characteristics in COVID-19 pandemic-a survey study. J Headache Pain. 2020;21(1):1–10.
Lechien JR, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288(3):335–44.
Belvis R. Headaches during COVID-19: my clinical case and review of the literature. Headache. 2020;60(7):1422–6.
Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–66.
Membrilla JA, et al. Headache as a cardinal symptom of coronavirus disease 2019: a cross-sectional study. Headache. 2020;60(10):2176–91.
Porta-Etessam J, et al. Spectrum of headaches associated with SARS-CoV-2 infection: study of healthcare professionals. Headache. 2020;60(8):1697–704.
Caronna E, et al. Headache: a striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 2020;40(13):1410–21.
Bobker SM, Robbins MS. COVID-19 and headache: a primer for trainees. Headache. 2020;60(8):1806–11.
Rocha-Filho S, et al. Headache, anosmia, ageusia and other neurological symptoms in COVID-19: a cross-sectional study. J Headache Pain. 2022;23(1):1–11.
Benemei S, Dussor G. TRP channels and migraine: recent developments and new therapeutic opportunities. Pharmaceuticals. 2019;12(2):54.
Olesen J, et al. The international classification of headache disorders, 3rd edition (ICHD-3). Cephalalgia. 2018;38:1–121.
Sharma M, Menon B. Headache incidence and characteristics in COVID-19 patients: a hospital-based study. Ann Indian Acad Neurol. 2022;25(1):88.
Shen Q, et al. COVID-19: systemic pathology and its implications for therapy. Int J Biol Sci. 2022;18(1):386.
Robbins MS, et al. Clinical and prognostic subforms of new daily-persistent headache. Neurology. 2010;74(17):1358–64.
Peng K-P, Wang S-J. Update of new daily persistent headache. Curr Pain Headache Rep. 2022:1–6.
Ong JJY, et al. Headaches associated with personal protective equipment–a cross-sectional study among frontline healthcare workers during COVID-19. Headache. 2020;60(5):864–77.
Yuksel H, et al. The impacts of masks and disinfectants on migraine patients in the COVID-19 pandemic. J Clin Neurosci. 2022;
Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154:S44–53.
Zubair AS, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–27.
Arnold M. Headache classification committee of the international headache society (IHS) the international classification of headache disorders. Cephalalgia. 2018;38(1):1–211.
Berlin, A. D., et al. (2020) "Covid-19." The New England Journal of Medicine [Internet].[acceso: 25/05/2020]. Disponible en: Disponible en: https://doi.org/10.1056/NEJMcp2009575.
Abboud H, et al. COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020;140:49–53.
Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Tsamakis K, et al. COVID-19 related stress exacerbates common physical and mental pathologies and affects treatment. Exp Ther Med. 2020;20(1):159–62.
Perlis RH, et al. Association of acute symptoms of COVID-19 and symptoms of depression in adults. JAMA Netw Open. 2021;4(3):e213223.
Baskin SM, Smitherman TA. Migraine and psychiatric disorders: comorbidities, mechanisms, and clinical applications. Neurol Sci. 2009;30(1):61–5.
Amoozegar F. Depression comorbidity in migraine. Int Rev Psychiatry. 2017;29(5):504–15.
Adiguzel A, et al. Investigation of the relationship between headache and anxiety during the late COVID-19 pandemic period: a prospective case-control study. Dicle Tıp Dergisi. 2022;49(1):92–101.
Garg RK. Spectrum of neurological manifestations in Covid-19: a review. Neurol India. 2020;68(3):560.
Miller AH, et al. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30(4):297–306.
Parker C, et al. Depression, anxiety, and acute stress disorder among patients hospitalized with COVID-19: a prospective cohort study. J Acad Consult Liaison Psychiatry. 2021;62(2):211–9.
Mazza MG, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600.
Michaelides A, Zis P. Depression, anxiety and acute pain: links and management challenges. Postgrad Med. 2019;131(7):438–44.
Chamberlain SR, et al. Post-traumatic stress disorder symptoms in COVID-19 survivors: online population survey. BJPsych open. 2021;7:2.
de Medeiros C, et al. The psychiatric impact of the novel coronavirus outbreak. Psychiatry Res. 2020;286:112902.
Nakamura ZM, et al. Neuropsychiatric complications of COVID-19. Curr Psychiatry Rep. 2021;23(5):1–9.
Halpin SJ, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013–22.
Malik P, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)—a systematic review and meta-analysis. J Med Virol. 2022;94(1):253–62.
Tomashefski JF Jr, Felo JA. The pulmonary pathology of illicit drug and substance abuse. Curr Diagn Pathol. 2004;10(5):413–26.
Wang QQ, et al. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2021;26(1):30–9.
Bhola S, et al. Neurological toll of COVID-19. Neurol Sci. 2022:1–16.
Saniasiaya J, Kulasegarah J. Dizziness and COVID-19. Ear Nose Throat J. 2021;100(1):29–30.
Helms J, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–70.
Sharifian-Dorche M, et al. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J Neurol Sci. 2020;417:117085.
Abdelzaher A, et al. Neuroimaging findings in hospitalized patients with COVID-19. Egypt J Radiol Nucl Med. 2022;53(1):1–11.
Moriguchi T, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–8.
Ahmad I, Rathore FA. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020;77:8–12.
Singh AK, et al. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr Clin Res Rev. 2021;15(4):102146.
Desai EJ, et al. Epidemiology, clinical features and management of rhino orbital mucormycosis in post COVID 19 patients. Indian J Otolaryngol Head Neck Surg. 2022;74(1):103–7.
Vieira C, et al. Downregulation of membrane-bound angiotensin converting enzyme 2 (ACE2) receptor has a pivotal role in COVID-19 immunopathology. Curr Drug Targets. 2021;22(3):254–81.
Scheer M, et al. Case report of a fulminant non-aneurysmal convexity subarachnoid hemorrhage after COVID-19. Interdiscip Neurosurg. 2022;27:101437.
Dogra S, et al. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29(8):104984.
Cezar-Junior AB, et al. Subarachnoid hemorrhage and COVID-19: association or coincidence? Medicine. 2020;99:51.
Sheraton M, et al. A review of neurological complications of COVID-19. Cureus. 2020;12:5.
Baldini T, et al. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur J Neurol. 2021;28(10):3478–90.
Dehnavi AZ, et al. Clinical, laboratory and imaging characteristics of hospitalized COVID-19 patients with neurologic involvement; a cross-sectional study. Arch Acad Emerg Med. 2022;10:1.
Chan JL, et al. Pituitary apoplexy associated with acute COVID-19 infection and pregnancy. Pituitary. 2020;23(6):716–20.
Taneja C, et al. Rapidly progressive pituitary apoplexy in a patient with COVID-19 disease treated with endoscopic endonasal surgery. J Neurol Surg Rep. 2022;83(01):e8–e12.
Frara S, et al. COVID-19 and the pituitary. Pituitary. 2021;24(3):465–81.
Matschke J, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19(11):919–29.
de Sousa GC, et al. Vasculitis-related stroke in young as a presenting feature of novel coronavirus disease (COVID19)–case report. J Clin Neurosci. 2020;79:169–71.
Goh Y, et al. Pearls and oy-sters: facial nerve palsy as a neurological manifestation of Covid-19 infection. Neurology. 2020;
Kumar A, et al. Neuropsychiatric manifestation of the drugs used in the treatment of SARS-2-CoV-2019 (COVID-19) infection and their management: an overview and practice implications. Asian J Psychiatr. 2022;103101
Dessau RB, et al. Coronaviruses in spinal fluid of patients with acute monosymptomatic optic neuritis. Acta Neurol Scand. 1999;100(2):88–91.
Tamaki A, et al. Incidence of bell palsy in patients with COVID-19. JAMA Otolaryngol Head Neck Surg. 2021;147(8):767–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Genç, H., Uludüz, D. (2023). Secondary Headache Disorders Attributed to COVID-19 Complications. In: Özge, A., Uludüz, D., Bolay, H., Karadaş, Ö. (eds) Headache Disorders in Pandemic Conditions . Headache. Springer, Cham. https://doi.org/10.1007/978-3-031-26309-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-26309-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-26308-8
Online ISBN: 978-3-031-26309-5
eBook Packages: MedicineMedicine (R0)