Abstract
Pallidal deep brain stimulation is a well-known surgical treatment for cervical dystonia. The resolution of dystonia typically requires bilateral pallidal stimulation, but in some instances, unilateral stimulation has been successful. In such instances, generally, the stimulated hemisphere was contralateral to the dystonic sternocleidomastoid, but rarely it was ipsilateral. We sought for the physiological features that determine the basis for success and laterality of deep brain stimulation for cervical dystonia with prominent torticollis. We found that pallidal physiology such as high burst to tonic ratio and significant interhemispheric differences in the neuronal firing rate and regularity are critical determinants of successful treatment with unilateral deep brain stimulation. We also found that higher lateralized differences in pallidal physiological parameters predict more robust improvement. In three out of four patients, the stimulation of the hemisphere ipsilateral to the dystonic sternocleidomastoid muscle was effective. These patients did not have any structural brain abnormalities on clinically available imaging studies. One patient responded to the unilateral deep brain stimulation in the hemisphere contralateral to the dystonic sternocleidomastoid. This patient had a structural putamen lesion on brain MRI. These results provide objective parameters determining the success of pallidal deep brain stimulation for treatment of cervical dystonia. The results also depict differences in the pallidal physiology in patients where ipsilateral versus contralateral deep brain stimulation was effective.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Cervical dystonia (CD) is the most common form of isolated dystonia characterized by abnormal twisting and turning of the neck with or without head oscillations. Deep brain stimulation of globus pallidus internus (GPi-DBS) is a popular treatment for medically refractory CD. Traditionally, improvement in CD is described with bilateral GPi-DBS [1,2,3]; however, occasional literature reported improvement in CD with unilateral stimulation [4,5,6,7,8]. The unilateral GPi-DBS studies, mostly the case reports, focused on the treatment of torticollis due to dystonic sternocleidomastoid (SCM) contralateral to the stimulated hemisphere [4,5,6, 9]; but rarely ipsilateral stimulation was found effective [7, 8]. We asked what determines the efficacy of unilateral DBS in CD with prominent torticollis, and which factors determine the laterality of therapeutic DBS location. In order to address these overarching questions, our experiments examined:
-
1.
The differences in the physiology of pallidal neurons in therapeutic versus control hemisphere.
-
2.
How pallidal physiology differs in the patients that respond to therapeutic stimulation in the hemisphere contralateral to the dystonic SCM versus that ipsilateral to the dystonic SCM.
-
3.
Whether physiology of pallidal neurons that are located in the therapeutic hemisphere but outside of the volume of activated tissue differ from the physiology of the pallidal neurons in the control hemisphere.
Methods
Clinical Data and Outcomes
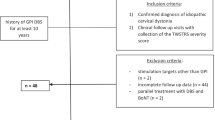
We analyzed the effects of unilateral GPi-DBS in four CD patients with prominent torticollis. Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) and the Tsui torticollis rating scale scores were used to measure the clinical outcome. Unilateral stimulation tests were carried out on each pair of contacts, followed by an evaluation of the patient several hours later. Then we choose a final program of unilateral stimulation for each patient (Table 1). Although in all patients the DBS electrodes were implanted in bilateral GPi, programming found effective treatment of their torticollis with only unilateral DBS stimulation; hence, only one hemisphere was stimulated. In three cases, the therapeutic DBS was ipsilateral to the dystonic SCM. In one patient, we found side contralateral to the dystonic SCM was effective. This patient characterized by putaminal stroke ipsilateral to the successfully stimulated hemisphere (Fig. 1).
Surgical Procedure and Physiological Data Collection
In each patient, two quadripolar DBS electrodes were implanted into bilateral GPi. The intervention was performed under local anesthesia. The Leksell stereotactic system was used, the intercommissural line and GPi coordinates were determined from the MR images. We recorded single-unit activity with tungsten microelectrodes (NeuroProbe, AlphaOmega) delineating the boundaries of the external and internal segments of the pallidum. To measure the motor evoked single-unit activity, the patients performed tasks such as shrugging shoulder or isometric contracting the SCM. The evoked activity was then recorded for off-line analysis. Once the ideal location of the electrode implantation was determined, we measured stimulation-induced side-effects using the current ranging from 1 to 5 mA. After implanting the DBS electrode, its location was radiologically confirmed. Subsequently, during an outpatient visit, the unilateral and bilateral stimulation tests were carried out on the different contacts with different parameters: duration, frequency, and amplitudes. The outcome was verified after 12 months of stimulation. Table 1 depicts the therapy parameters and DBS active electrode contact locations for all four patients.
Measuring the Volume of Tissue Activation
We used preoperative MRI and postoperative CT to determine the placement of electrodes by means of the Lead-DBS toolbox (https://www.lead-dbs.org/). We calculated the volume of activated tissue (VAT) and incorporated it in the patient’s own MRI using a known approach [10]. Then we classified GPi cells that were within the boundaries of VAT, i.e., “responsive neurons” and those outside of the region, i.e., “nonresponsive neurons.”
Data Analysis
We recorded spontaneous single-unit activity as the electrode advanced through GPe and GPi. Each cell was recorded for at least 20 s. Our inclusion criteria for additional offline analysis were that the neurons should have sufficiently long recordings containing at least 200 spikes. We used Spike 2 (CED, Cambridge, UK) for signal preprocessing and analysis. The signal was band-pass filtered between 300 Hz and 5000 Hz, and subsequently aligned for spike sorting. The amplitude threshold, at the value of 4-times standard deviation, was used to isolate spikes. Isolated single-units were then separated by K-means cluster selection in the principal component analysis (PCA) feature space based on several waveform parameters.
Statistical analysis with Mann–Whitney (MW), and Chi2 tests were performed with the Matlab Statistics toolbox and custom prepared algorithms. We computed instantaneous firing rate to measure the neuronal firing frequency. The coefficient of variance of interspike intervals (ISI) and asymmetry index (median ISI/mean ISI) measured the variability in the spike occurrence. The burst index, i.e., the number of ISI < 10 ms divided by the number of ISI > 10 ms, objectively characterized bursting behavior. The pause index, the number of ISI > 50 ms divided by the number of ISI < 50 ms, characterized pause behavior. We used the Poisson Surprise (PS) algorithm for burst detection. This algorithm assumes that the baseline firing rate follows the Poisson process with results equal to the mean firing rate of the sample spike train. The Poisson Surprise statistic is defined as S = −log(p), where p is the probability of more or the same number of N spikes occurring in the interval. Bursts are chosen to maximize the PS statistic with a surprise maximization algorithm [11]. Detected bursts for each isolated neuron were further used to determine the activity parameters such as burst percent (ratio of spikes in burst to the total number of spikes), inter- and preburst intervals, mean burst length, mean interspike interval within the burst, and mean value of spike-count within the burst. For grouping spike trains into specific patterns, we used hierarchical clustering [12].
Results
We asked how pallidal physiology differs in patients with CD (prominently torticollis) that respond to unilateral GPi-DBS. We also asked what determines the laterality of therapeutically successful side. The questions were addressed in four CD patients with unilateral GPi-DBS; three with DBS in GPi ipsilateral to the dystonic SCM; while in one with DBS in the GPi contralateral to the dystonic SCM. We measured the spontaneous activity of 265 pallidal neurons; 113 cells were localized in GPe while 152 cells were in GPi. Unsupervised machine learning separated the neuronal activity pattern into three types: burst, tonic, and pause (Fig. 2a). The hemisphere where DBS was therapeutic had relatively fewer GPi tonic neurons but more prevalent pause neurons (Fig. 2b, Chi2 = 7.51, p = 0.024). Such difference was not noteworthy in GPe (Fig. 2c, Chi2 = 5.8, p = 0.055).
(a) Hierarchical clustering algorithm to separate 265 pallidal cells into burst, tonic and pause neurons. The firing patterns in inset describe an example of the three neuron subtypes. In the inset, the neuronal spikes are plotted as a time-series signal (x-axis). The distribution of GPi neurons (b) and GPe neurons (c) into burst, tonic and pause subtypes. Comparison is done between control (nonstimulated) and therapeutic (stimulated) hemispheres
Subsequent analysis compared the physiological parameters of pallidal neurons between two hemispheres (Table 2). The GPi neurons in the therapeutic hemisphere had significantly lower median firing rate 50 (34–80) spikes/s compared to control hemisphere 67 (47–84) spikes/s (MW, p = 0.01). The GPi neurons on the therapeutic hemisphere were less regular, had more pause, and had less burst behavior compared to the control hemisphere. Less regularity was depicted by the smaller coefficient of variation (Table 2). The pause index measures the pause behavior, while the burst index and preburst interval is a gauge of burst behavior. The pause and burst indices of GPe and GPi neurons were notably different between therapeutic and control hemispheres. Such differences in GPe were however opposite compared to GPi. The GPe on the therapeutically effective side had less pause and more burst behavior compared to the control hemisphere (Table 2).
The analyzed group had three patients where the therapeutic side was ipsilateral to the dystonic SCM muscle. One patient had therapeutic response to the stimulation of the hemisphere that was contralateral to the dystonic SCM muscle. We compared the physiological differences in control and therapeutic hemisphere in those who had contralateral versus ipsilateral pallidal stimulation. The instantaneous firing rate was significantly lower in therapeutic GPi (Table 3) in three patients where the therapeutic hemisphere was ipsilateral to the dystonic SCM. In addition, the firing pattern was characterized by more pause-burst behavior in therapeutic GPi, that is significantly lower asymmetry index and higher pause index and preburst interval. At the same time, the firing rate was significantly higher in the therapeutic side GPe, while pause index and preburst interval were significantly lower.
The subsequent analysis separated GPi neurons that were within the volume of activated tissue (VAT), modeled according to the therapeutic DBS parameters. We found 28 GPi neurons within the boundary of therapeutic VAT, 53 GPi cells were outside of this region. There were significantly larger proportion of pause neurons and less prevalent tonic cells within the volume of activated tissue compared to outside of this region (Chi2 = 6.85, p = 0.03) (Fig. 3). There was no difference in the firing rate of pallidal neurons inside [46 (32–59) spikes/s] versus outside [47 (34–79) spikes/s] the volume of activated GPi. However, the cells within the volume of activated tissue were more bursty (asymmetry index: inside = 0.61 (0.54–0.64); outside = 0.63 (0.58–0.71), burst spike percent: inside = 29% (20–41); outside = 24% (15–33)).
(a) Example of neuronal coordinates plotted on the patient specific MR map. Each symbol depicts the location of the pallidal neuron, while sphere depicts the volume of activated tissue (VAT). (b) Distribution of burst, tonic and pause neurons in the region activated by electrical field (VAT in) and outside of the electrical field (VAT out)
We also separately characterized the case with the most effective outcome (70%). The GPi neurons in the therapeutic hemisphere of this patient had significantly lower median firing rate 44 spikes/s compared to the control hemisphere 66 spikes/s (MW, p = 0.006), and a higher median pause index 0.12 compared to the control hemisphere 0.04 (MW, p = 0.001). The similar characteristics we observed in nine neck sensitive cells: median firing rate 45 spikes/s and median pause index 0.11. We did not find significant differences between therapeutic and control hemisphere single-unit activity in GPe.
We did not find any lateralized differences in the GPi single-unit physiology in one patient who had therapeutic improvement after stimulation of GPi on the side contralateral to the dystonic sternocleidomastoid. However, the differences were present in GPe, and they were comparable to the other cohort in which firing rate was higher on therapeutic hemisphere, and firing also had more irregularity (Table 3).
Discussion
Unilateral DBS was shown effective in selected cases of CD. Previous literature had reported that DBS on the side contralateral to the dystonic SCM (i.e., ipsilateral to the side of torticollis) is effective [4,5,6, 9]. On the contrary, a few reports suggested improvement after stimulating the GPi ipsilateral to the dystonic SCM [7, 8]. Physiological rationale for the choice of stimulated side remains unclear. The current study asks what determines the efficacy of unilateral DBS in CD and which factors determine the laterality of therapeutic DBS location. The study addressed three questions.
-
1.
The differences in the physiology of pallidal neurons in therapeutic versus control hemisphere.
-
2.
How pallidal physiology differs in the patients that respond to therapeutic stimulation in the hemisphere contralateral to the dystonic SCM versus that ipsilateral to the dystonic SCM.
-
3.
Whether physiology of pallidal neurons that are located in the therapeutic hemisphere but outside of the volume of activated tissue differ from the physiology of the pallidal neurons in the control hemisphere.
We recently showed that the pallidal activity featuring lower firing rate, burst index, and alpha oscillation score is associated with an excellent clinical outcome [13]. This study showed similar physiological trends when comparing the pallidal activity between two hemispheres, except for additional differences in the firing irregularity [14, 15]. The fundamental difference between our previous study and current data is that participants in the previous study required bilateral DBS for therapeutic response. In the current study, we found successful treatment with unilateral GPi DBS. The differences in cases where unilateral DBS was effective had much robust differences between two hemispheres; lower firing rate and burst index and frequent pause behavior of GPi cells in the contralateral side to the direction of torticollis.
Three parameters determined the therapeutic efficacy of unilateral DBS in patients with prominent torticollis – reduced firing rate, increased firing variability, higher proportions of pause, and less tonic neurons. These parameters are consistent with those thought to be the predictors of DBS efficacy within the pallidum [13]. The results depicted physiological characteristics alone do not determine whether stimulated location will be efficacious, but robust lateralized differences in the firing characteristics are also important. More polarized differences predict higher chances for unilateral DBS to succeed.
In the three CD cases, the effective DBS side was ipsilateral to the dystonic SCM. These patients did not have any imaging evidence of structural brain deficits. In contrast, the patient with putamen lesion responded to the DBS therapy in the hemisphere DBS in the opposite direction as dystonic SCM. In this patient, the activity of right and left GPi was comparable, but lateralization was found in the activity of GPe, again contralateral to the dystonic SCM. The interpretation of the efficacy of DBS on the unilateral side of the dystonic SCM is based on data from a single patient, such limitation must be viewed with caution. Nevertheless, the results provide important differences in the network dysregulation in those where focal lesions in the basal ganglia lead to dystonia, as opposed to those with no structural neurological deficits.
In summary, our study looked for the pallidal physiological features of patients with cervical dystonia that respond to unilateral DBS. We found that high burst to tonic ratio and interhemispheric differences in the neuronal firing rate and regularity are the critical determinants of the successful unilateral DBS therapy.
References
Krack P, Volkmann J, Tinkhauser G, Deuschl G. Deep brain stimulation in movement disorders: from experimental surgery to evidence-based therapy. Mov Disord. 2019;34:1795–810.
Moro E, Piboolnurak P, Arenovich T, Hung SW, Poon Y-Y, Lozano AM. Pallidal stimulation in cervical dystonia: clinical implications of acute changes in stimulation parameters. Eur J Neurol. 2009;16:506–12.
Walsh RA, Sidiropoulos C, Lozano AM, et al. Bilateral pallidal stimulation in cervical dystonia: blinded evidence of benefit beyond 5 years. Brain. 2013;136:761–9.
İşlekel S, Zileli M, Zileli B. Unilateral pallidal stimulation in cervical dystonia. Stereotact Funct Neurosurg. 1999;72:248–52.
Torres CV, Moro E, Dostrovsky JO, Hutchison WD, Poon Y-YW, Hodaie M. Unilateral pallidal deep brain stimulation in a patient with cervical dystonia and tremor: case report. J Neurosurg. 2010;113:1230–3.
Chang JW. Unilateral globus pallidus internus stimulation improves delayed onset post-traumatic cervical dystonia with an ipsilateral focal basal ganglia lesion. J Neurol Neurosurg Psychiatry. 2002;73:588–90.
Escamilla-Sevilla F, Mínguez-Castellanos A, Arjona-Morón V, et al. Unilateral pallidal stimulation for segmental cervical and truncal dystonia: which side? Mov Disord. 2002;17:1383–5.
Harries AM, Sandhu M, Spacey SD, Aly MM, Honey CR. Unilateral pallidal deep brain stimulation in a patient with dystonia secondary to episodic ataxia type 2. Stereotact Funct Neurosurg. 2013;91:233–5.
Sobstyl M, Ząbek M, Dzierzęcki S, Górecki W. Unilateral pallidal stimulation in a patient with truncal dystonia. Clin Neurol Neurosurg. 2012;114:1320–1.
Mädler B, Coenen VA. Explaining clinical effects of deep brain stimulation through simplified target-specific modeling of the volume of activated tissue. Am J Neuroradiol. 2012;33:1072–80.
Cotterill E, Charlesworth P, Thomas CW, Paulsen O, Eglen SJ. A comparison of computational methods for detecting bursts in neuronal spike trains and their application to human stem cell-derived neuronal networks. J Neurophysiol. 2016;116:306–21.
Myrov V, Sedov A, Belova E. Neural activity clusterization for estimation of firing pattern. J Neurosci Methods. 2019;311:164–9.
Sedov A, Popov V, Gamaleya A, et al. Pallidal neuron activity determines responsiveness to deep brain stimulation in cervical dystonia. Clin Neurophysiol. 2021;132:3190–6.
Sedov A, Usova S, Semenova U, et al. The role of pallidum in the neural integrator model of cervical dystonia. Neurobiol Dis. 2019;125:45–54.
Sedov A, Semenova U, Usova S, et al. Chapter 20 – Implications of asymmetric neural activity patterns in the basal ganglia outflow in the integrative neural network model for cervical dystonia. In: Ramat S, Shaikh AG, editors. Progress in brain research. Elsevier; 2019. p. 261–8.
Financial Disclosure and Conflict of Interest
The authors have no conflicts of interests. Shaikh serves on speaker bureau for Acorda Pharmaceuticals. Jinnah is consultant for Retrophin Inc., CoA Therapeutics, and Cavion Therapeutics.
Funding
The study was funded by the Russian Science Foundation (project 18-15-00009, Sedov): MER data collection and analysis. The study was partly supported by the Russian Science Foundation (project 23-25-00406, Semenova): Lead-DBS analysis. Shaikh was supported by the Career Development Grant from the American Academy of Neurology, George C. Cotzias Memorial Fellowship, Network Models in Dystonia grant from the Dystonia Medical Research Foundation, Department of VA Merit Review (I01CX002086), and philanthropic funds to the Department of Neurology at University Hospitals (Penni and Stephen Weinberg Chair in Brain Health and Woll Fund).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sedov, A. et al. (2023). Does Pallidal Physiology Determine the Success of Unilateral Deep Brain Stimulation in Cervical Dystonia?. In: Shaikh, A., Sadnicka, A. (eds) Basic and Translational Applications of the Network Theory for Dystonia. Advances in Neurobiology, vol 31. Springer, Cham. https://doi.org/10.1007/978-3-031-26220-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-26220-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-26219-7
Online ISBN: 978-3-031-26220-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)