Abstract
The anatomical approach applied to the study of the heart has distant origins and has developed nowadays through the use of tomographic imaging such as the coronary CT angiography, allowing cardiovascular physicians to provide detailed in vivo anatomo-clinical diagnostic value, contributing significantly to changing the prognosis of patients with coronary artery disease.
Nowadays, CCT generates volumes with submillimetric, isotropic, cardiosynchronized voxels, which allow the morphological detailed analysis with multiplanar rendering, volumetric, and cine reconstructions, useful in evaluating not only the coronary stenosis but also plaque characteristics in order to plan interventional or surgical procedures.
The purpose of this chapter is to illustrate the intrinsic capabilities of the state-of-the-art CCT with a hint of the more recent applications that will have a role in the near future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Coronary computed tomography
- Coronary artery calcium score
- Coronary artery disease
- Atherosclerosis
- Chest pain
- Stenosis
- Ischemia
- Prognosis
- Risk stratification
- Risk assessment
- FFR-CT
- CT perfusion
1 Introduction
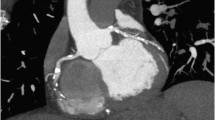
The execution and interpretation of CCT by highly experienced specialists allow to understand in depth the normal and pathological cardiac anatomy: the normal coronary tree (Fig. 1) and CAD; the morphological and functional characteristics of the cardiac chambers and valves; congenital anomalies; and the characteristic appearance of the heart linked to aging or pathogens.
Coronary CT angiography curved multiplanar reconstruction of the left anterior descending artery (LAD) showing a critical >70% luminal narrowing at the proximal segment due to a mixed plaque with spotty calcium (a). CT angiography dual-energy iodine color map in short-axis view showing the myocardial perfusion defect at the anteroseptal and anterior wall. Note the reduction of iodine content (iodine density: −0.9 mg/mL, −26%) with respect to the remote myocardium (b), Complete view on the heart (c)
Particular attention must be paid to the knowledge of the technical and technological part, which is an indispensable requirement in order to obtain sophisticated diagnoses. The planning of the acquisition phases without and with contrast medium, the use of three-dimensional cardiac-specific interpretation software, and the ability to identify and overcome image artifacts in the available image dataset [1, 2] provide the basis of the training process currently recommended to achieve the appropriate proficiency [3].
2 Coronary Anatomy Segmentation
The AHA recommended a schematic coronary tree segmentation classification that can be used to create a schematic CCT scoring system similarly to that used in conventional coronary angiography [4]. The coronary tree should be initially examined for the course and branching of the main coronary vessels and secondary branches following the 15- or 16-segment pattern.
The SCCT guidelines recommend an axial model of coronary segmentation, adapted for CCT [3]. This pattern varies from standard AHA segmentation in the following ways: an intermediate branch has been added as segment 17, and a left posterolateral branch is identified as segment 18.
Attention should initially be focused on the axial plane on the aortic root to confirm the normal origin of the coronaries. Any anomalies of origin and course and the relationship with surrounding structures such as the heart chambers, the aorta, the pulmonary artery, the cardiac veins, and the interventricular septum may require the use of evaluation plans other than the axial plane up to the need for unusual planes generated by the stretched centerline of the vessels.
3 Congenital Coronary Artery Anomalies
Coronary CT is considered an important and appropriate imaging modality for the evaluation of adult congenital heart disease, particularly of the coronary arteries. Congenital coronary anomalies are of great importance in clinical cardiology and cardiac surgery due to their association with myocardial ischemia and sudden death. These anomalies are detectable noninvasively by CCT and, according to various definitions, their prevalence ranges from 0.21% to 5.79%. The most commonly used classification is based solely on anatomical considerations [5, 6]. The working group of Anatomy and Pathology of the European Society of Cardiology has published a position statement (2016) in order to provide a classification linked to the mechanisms of coronary embryonic development and to congenital coronary anomalies [7].
The high spatial resolution of CCT allows us to evaluate the intrinsic mechanisms of the coronary artery anomalies generating dysfunction (stenosis) and clinical or prognostic relevance.
The correct knowledge of the normal coronary origin and course and the coronary anomalies related to ischemia represents a key role in the operative planning, and highly detailed anatomical images are crucial to define the surgical indication. Consequently, CCT allows us to verify the surgical result by highlighting all anatomical details of the surgical techniques employed [8].
Angelini P. (2019) pointed out that among all coronary anomalies, the intramural course of an anomalous coronary artery from the opposite sinus of Valsalva (ACAOS-IM) can cause coronary insufficiency (i.e., myocardial ischemia) in young adults involved in strenuous exertion [9].
The main cause of ischemia in these patients is generally the narrowing of the initial segment of the coronary artery as it enters or exits the aortic wall, at an intramural course by compression in between the inner and outer layers of the aortic tunica media [10, 11]. This morphologic variation during the cardiac cycle of the coronary morphology at the level of the intramural course is usually seen by IVUS, but nowadays a retrospective CCT acquisition permits a noninvasive evaluation of systolic to diastolic variations in terms of both morphology and degree of stenosis (Fig. 2).
Anomalous origin of the right coronary artery (RCA) from the left sinus of Valsalva with interarterial course and “slit-like ostium” (a) showing oval shape during diastole (b) and compression during systole (d). Hybrid SPECT/CCT volume rendering (e) and bull’s eye (c) showing the segmental ischemia and its relationship with the anomalous coronary origin. The conventional angiography (f) confirms the “slit-like ostium”
In recent years, CCT has also acquired a role of increasing importance in the diagnosis and preoperative planning of congenital heart defects [12], allowing the study of the coronary tree together with structural abnormalities. In adult GUCH undergoing multiple surgical procedures during their life, the role of CCT becomes crucial in defining the relationship between cardiac structures, the coronary distribution, and the anterior chest wall in order to plan the surgical approach and avoid complications. In this scenario, the development of tenacious cardio-sternal adhesions represents an element of considerable bleeding risk during the chest reopening phase. Therefore, CCT imaging becomes a fundamental aid in guiding the reopening procedure allowing the adoption of the strategy with less surgical risk [13].
Another field of application is represented by the group of congenital anomalies concerning systemic and pulmonary venous returns. In particular, for the correct preoperative diagnostic definition of partial and total anomalous pulmonary venous connections, echocardiography is often insufficient to guide surgical planning, and a second-level imaging examination such as CCT becomes essential. Especially in the total and mixed forms of anomalous pulmonary venous connection, the questions that CCT must answer are the anatomy of the vein confluence and the course and draining site of the venous collector [14].
The choice of the best surgical technique is the consequence of a perfect and exhaustive preoperative anatomical definition.
4 Coronary Atherosclerosis
The SCCT recommends performing preliminary non-contrast CT examination for coronary artery and other cardiac structural calcifications [3, 15].
Calcified lesions are usually quantified using the “Agatston score” [16, 17].
The SCCT and the STR have produced a consensus document regarding the prognostic value of CACS [18] because the coronary calcium quantification has been shown to be the best predictor of future cardiovascular events in the general population, in the elderly, and in the diabetics.
After intravenous injection of contrast agent, CCT can visualize the coronary artery lumen and the lesions involved in the stenosis [19, 20].
Atherosclerotic lesions should be considered in relationship to their segmental position to determine the overall myocardium risk [21, 22]. The impact of luminal plaque should be evaluated in terms of resultant maximal diameter stenosis [3]. CCT can visualize the coronary wall alterations related to CAD and plaque remodeling, and it can differentiate the calcified and noncalcified components of the plaque (mixed) [18].
Maurovich-Horvat et al. [23] proposed a qualitative assessment of plaque features related to histopathologic findings. Plaque attenuation pattern-based classification has been proposed distinguishing noncalcified plaque with or without “napkin-ring” sign.
SCOT-Heart Study [22] assessed the association between coronary plaque features and clinical outcome defining four types of adverse plaque: positive remodeling, low-attenuation plaque, spotty calcification, and “napkin-ring” sign. These specific plaque features are detectable and should be annotated because of their prognostic significance [24, 25].
The qualitative and quantitative grading of the coronary stenosis severity and the plaque features along the vessel are the main information to be reported [26, 27]. The SCOT-Heart study modified SCCT Guidelines stenosis grading defining as normal coronary segment with or without nonobstructive plaque; moreover, obstructive disease was defined as >70% stenosis in one or more epicardial vessels or 50% stenosis in the LM.
On the basis of clinical trials [21, 22], the SCCT, the ACR, and the NASCI have evaluated the clinical utility and the relevance of CCT findings in the context of suspected stable CAD and in patients with acute chest pain. In order to describe a standardized reporting system for patients undergoing CCT, CAD-RADS (Coronary Artery Disease Reporting and Data System) was proposed with the aim to improve the communication between interpreting and referring physicians, facilitate research, and offer mechanisms to contribute to peer review and quality assurance, ultimately resulting in improvements to the quality of care [28].
The current European Guidelines advocate the use of CCT in patients with suspected CAD with a Class I recommendation (Level of Evidence B) due to its diagnostic and prognostic performance [29]. CCT is considered a first-line tool for all patients presenting with chest pain of suspected cardiac origin and the most cost-effective imaging-based strategy [30].
From the 64-slice CCT systems which have a temporal resolution of 175 ms, nowadays the temporal resolution has increased up to 66 ms; the spatial resolution reaches isotropic dimensions of ~0.2–0.3 mm, which allow a good assessment of significant coronary artery stenosis and plaque characterization [31].
The last-generation CCT scanners allow a spatial resolution of up to 0.1 mm combined with photon counting technology [32].
A strength of CCT is the exclusion of the presence of CAD or the identification of patients with nonobstructive CAD, in order to restratify the clinical risk stratification (an intermediate risk of hard events and may represent the target population) [33, 34].
The prognosis related to CAD is related to the presence, extent, and severity of the lesions. The anatomical coronary evaluation can nowadays be supported by myocardial CT perfusion, FFR-CT, and high-risk plaque feature quantification in order to refine and improve risk assessment for future cardiac events [35,36,37,38].
5 CCT Prognostic Value
Several longitudinal studies demonstrated that CCT holds important prognostic value in both patients with known and suspected CAD [39,40,41,42,43,44].
In a meta-analysis including 29,243 patients (median follow-up of 25 months), adverse cardiovascular events among patients with normal findings on CCT were demonstrated to be rare (annual MACE rate of 0.21%) [45].
Nonobstructive (<50% stenosis) or obstructive (≥50% stenosis) CAD was demonstrated to predict increasing future MACE (annualized event rates of 1.24–6.21%, respectively, p < 0.05) [45]. Most of the key answers on the prognostic utility of CCT are derived from the CONFIRM registry that includes more than 32,000 consecutive adults with suspected CAD who underwent ≥64-slice CCT at 12 centers in 6 countries between 2005 and 2009, investigating the link between cardiovascular risk factors, symptoms, coronary atherosclerotic plaque burden, and outcome. Some studies from this registry have demonstrated that the presence, extent, and severity of CAD on CCT result in increased future risk to the patient, across age, gender, and other several clinical sub-analyses [46,47,48].
A very low annual event rate for those with normal CCT findings has been consistently demonstrated, which is comparable to the background event rate among healthy low-risk individuals (<1%). In risk-adjusted analysis, both per-patient nonobstructive (hazard ratio [HR]: 1.60; 95% confidence interval [CI]: 1.18–2.16; p = 0.002) and obstructive (>50% stenosis) (HR: 2.60; 95% CI: 1.94–3.49; p < 0.0001) CAD conferred increased risk of mortality compared with patients without evidence of CAD [46]. The clinical importance of nonobstructive CAD and its strong relationship with all-cause mortality were evidenced. Moreover, the total coronary plaque burden has emerged as an important predictor of outcomes (Fig. 3).
The CT screening can be used cost effectively to reduce morbidity and mortality from CHD in symptomatic patients [47].
Moreover, as the definition of clinically significant atherosclerosis includes asymptomatic disease, the identification of individuals at risk requires a screening strategy and CCT seems to express adequate characteristics to be used for this purpose in this area also in order to evaluate the drug therapy efficacy (Fig. 4) [48].
6 CCT Plaque Characterization
Some features of the coronary plaque seen at CCT have been demonstrated to be correlated with the risk of rupture and subsequent risk of ACS [35]. These high-risk features include the low-attenuation plaque, positive remodeling, spotty calcification, “napkin-ring” sign, and dishomogeneity of the plaque components [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51].
Low-attenuation plaque features (<60 HU) and “napkin-ring” sign were the most powerful MACE predictors (HR 4.96; 95% CI: 2.0–12.2 and HR 3.85; 95% CI: 1.7–8.6; p < 0.0001) in a study by Feuchtner et al. (mean follow-up of 7.8 years) [52].
Adverse features such as positive remodeling, low-attenuation plaque, or “napkin ring” were demonstrated to be associated with increased risk of death, MI, or hospitalization for unstable angina at 2 years (HR 2.73, 95% CI 1.89–3.93) [53].
Accordingly, the low-attenuation plaque burden (i.e., % plaque to vessel volume) was demonstrated to be the strongest predictor of fatal or nonfatal MI irrespective of cardiovascular risk score, CACS, or coronary artery area stenosis (HR 1.60, 95% CI: 1.10–2.34 per doubling; p = 0.014) [54].
7 Myocardial CT Perfusion and FFR-CT
The new-generation CT scanners permit both the static (single-phase) and the dynamic (multiphase) myocardial CTP acquisition. The qualitative and quantitative evaluation with the assessment of perfusion parameters of ischemia, such as the myocardial blood flow and volume [55], is evaluable together with coronary anatomy evaluation. CFD algorithms could enable prediction of changes in coronary flow and pressure for the noninvasive estimation of FFR (FFR-CT) [55, 56].
The inability of the traditional ICA to assess the functional significance of coronary stenosis and determine the need of revascularization [52] has led to the development of techniques that are able to assess the functional severity of coronary stenoses. FFR was introduced to the clinical setting in the mid-1990s and was established as a crucial enhancement to ICA for clinical decision-making in CAD [53] based on a linear relationship between flow and pressure. The FFR was initially presented as a pressure-derived assessment index of the impairment of coronary flow due to the presence of arterial stenoses. When the FFR value is close to 1, a normal coronary physiology is assumed with no need for revascularization. The well-accepted FFR cutoff value has been set to 0.75, under which myocardial ischemia occurs with an overall accuracy that reaches 97% [57]. However, there is a so-called gray zone which ranges from 0.75 to 0.80, at which the clinician has to assess every parameter in order to decide on a possible revascularization procedure. Due to the noninvasive nature of CCT, the application of CFD algorithms on CCT-derived 3D arterial models has received wide clinical interest regarding the noninvasive FFR assessment. According to this approach, hemodynamic factors such as flow and pressure are not known a priori, so lumped parameter models regarding the cardiac output, the resistance of the coronary microcirculation, and the pressure of the systemic circulation are coupled with the flow domain of the aortic root and the epicardial arteries, where the governing equations of flow dynamics are solved and can consequently provide FFR calculations. DISCOVER-FLOW, DeFACTO, and HeartFlow NXT studies compared their computational FFR results to the measured FFR values, producing promising results and making the method a valuable tool in the clinical settings [58,59,60].
The DISCOVER-FLOW study exhibited a good correlation between FFR-CT and FFR (r = 0.68) with the respective diagnostic accuracy, sensitivity, specificity, positive predictive value, and negative predictive value for predicting hemodynamically significant stenoses (FFR ≤0.8) being 84, 88, 82, 74, and 92% [60]. Furthermore, when compared to cases of ≥50% stenosis detected solely by CCT, FFR-CT showed superior discrimination (AUC: 0.90 vs. 0.75, p = 0.001). In the DeFACTO study, stable CAD patients underwent CCT, FFR-CT, and invasive coronary angiography with FFR measurement [58]. The per patient diagnostic accuracy, sensitivity, specificity, positive predictive value, and negative predictive value for predicting an FFR ≤0.8 were 73, 90, 54, 67, and 84%, respectively. Good correlation was also found between the two methods (r = 0.68). The most recent HeartFlow NXT further validated FFR-CT, by making use of updated proprietary software which included refined mathematical models and further increased automation, image quality assessment, and better image segmentation [59]. Diagnostic accuracy, sensitivity, specificity, positive predictive value, and negative predictive value for predicting an FFR ≤0.8 were 81, 86, 79, 65, and 93%, respectively, on a per-patient basis and 86, 84, 86, 61, and 95%, respectively, on a per-vessel basis. Finally, good correlation was found between FFR-CT and FFR (r = 0.82). The PLATFORM study focused on the clinical outcomes of FFR by CCT-guided diagnostic strategies compared to the common care in CAD-suspected patients, providing insight on the clinical utilization of FFR-CT [61]. Following the findings of the PLATFORM trial, the PROMISE study concluded that if ICA is performed only in patients with FFR-CT ≤0.8, then selected ICA with obstructive stenosis could decrease by 44% and the total number of patients with ICA requiring appropriate revascularization would increase by 24% [57]. Sensitivity and specificity have been shown to vary through different cohorts (DISCOVER-FLOW, DeFACTO, NXT [58,59,60], Kim et al. [62], Renker et al. [63], Coenen et al. [64], Kruk et al. [65], Ko et al. [66]) due to differences in sample sizes and study population characteristics [67]. Nowadays, there are four approaches in noninvasive, in silico CCT-derived FFR estimation: full-order model computations, reduced-order/steady-state modeling, hybrid models, and deep machine learning algorithms, including commercially available solutions and technologies still in progress [68]. These techniques are applied to a patient-specific anatomic coronary artery 3D model, obtained via a preliminary segmentation and contouring of the vessels. The full-order approaches require a complete model of the entire coronary tree, and an additional physiology model of the coronary microcirculation fluid dynamics (derived from patient-specific boundary conditions), from which a coronary blood flow model is computed. This process is computationally demanding, requiring off-site supercomputers in core laboratories. In order to simplify the processes, lean models have been introduced, which are either segment specific and/or rely on a generalized (nonpatient-specific) hemodynamic model. For these reasons, CCT together with CTP or FFR-CT potentially should be the method to combinedly evaluate CAD phenotype and ischemic functional significance of the stenosis. Initial evidence on the prognostic value and improvement in risk stratification of CTP has been shown in the CORE-320 trial demonstrating that a combined approach with coronary CCT and CTP enables similar prediction of 2-year major adverse cardiac events and event-free survival, when compared to invasive coronary angiography and SPECT combined [55]. Moreover, stress dynamic CTP has incremental predictive value for future major adverse cardiac events over clinical risk factors and detection of coronary stenosis at CCT [56, 69,70,71]. On the other side, FFR-CT, besides an improved accuracy for the detection of hemodynamically relevant lesions, may have favorable clinical outcomes, similar quality of life, and lower costs and radiation exposure, when compared with usual care over 1-year follow-up [69, 72, 73]. However, despite efforts to create an artificial score similar to the useful value of FFR but through noninvasive imaging of the CCT to decide which stenosis should be revascularized, the inherent limitation of this method remains its inability to discriminate whether a stenosis is severe, that is, if it is flow limiting, around the value 0.8, which is exactly in the gray zone around the value of 0.8, which corresponds to the uncertainty value of the CCT (i.e., stenosis between 50 and 70%). So, these models may have a clinical role as their performance improves along with technology. On the other hand, motion or beam hardening artifacts that may occur during CTP acquisition can create erroneous signals of ischemia; therefore, in the near future, the technology will improve its performance in this regard.
8 Dual-Energy CT and Multi-Energy CT
Rapid advances in CT hardware and software technology have occurred and have been applied to last-generation scanners DECT and multi-energy CT imaging [74,75,76].
Four different technical approaches have been used to develop DECT technology: 1) two X-ray tubes operating at two different energy levels (70–80–90/140–150 kVp); 2) fast switching of kVp between low- and high-energy spectra; 3) two temporally sequential scans (not applied in cardiac imaging); and 4) multilayer detector for spectral separation [74].
DECT systems allow the signal differentiation of different materials by evaluating the attenuation characteristics at two different energy levels of the photons.
DECT can improve the diagnostic performance of CT in myocardial perfusion and scar imaging by improving iodine contrast-to-noise ratio (CNR) (Fig. 5) [76].
Coronary CT angiography curved multiplanar reconstruction of the left anterior descending artery (LAD) showing a critical >70% luminal narrowing at the proximal segment due to a mixed plaque with spotty calcium (a). CT angiography dual-energy iodine color map in short-axis view showing the myocardial perfusion defect at the anteroseptal and anterior wall. Note the reduction of iodine content (iodine density: −0.9 mg/mL, −26%) with respect to the remote myocardium (b)
DECT allows the quantification of iodine distribution within the myocardium through the direct correlation with myocardial blood flow, thus being useful for differentiating between normal, ischemic, and necrotic myocardium providing color-coded iodine images. In this way, a measurement of myocardial per-voxel iodine concentration expressed in mg/mL is provided, improving accuracy over the standard visual analysis.
Furthermore, DECT acquisition can reduce artifacts such as beam hardening and blooming artifacts usually present in single-energy CT acquisitions, without increasing the radiation dose [75, 76].
More recently, new energy-sensitive PCD has been developed allowing to directly count the number of incident photons and measure their photon energies separately. Multi-energy CT with PCD can provide more spectral information than DECT systems, but they are the subject of ongoing research and their commercialization is only now starting. A recent preclinical experimental model demonstrated the feasibility and accuracy of PCDs with respect to MRI and histology as the gold standard for quantitative separation of blood pool, scar, and remote myocardium using a simultaneous protocol of multi-contrast agents [77].
9 Conclusions
CCT has been shown to provide diagnostic and prognostic information regarding CAD and ischemia. CCT, if used with the most advanced technology by expert operators, is able to offer at the same time the accurate anatomical evaluation of the heart and coronaries and the phenotype of the coronary plaque, quantify the atherosclerotic plaque burden, simulate the coronary flow alterations, and guide revascularization. CCT is therefore a useful tool to stratify the risk of CAD in the population as suggested by international guidelines and to study the pathophysiology of human atherosclerosis with a noninvasive method that is well accepted by patients. The certain exclusion of CAD, the main prerogative of CCT, and the characterization of the nonobstructive disease are certainly a necessary aid to guide preventive therapy and modify the risk of events. The evaluation of CAD using advanced imaging and with the help of radiomics, machine learning, and deep learning features [78] is being proposed as an integrated system that generates new knowledge to be used in the near future.
Abbreviations
- ACR:
-
American College of Radiology
- ACS:
-
Acute coronary syndrome
- AHA:
-
American Heart Association
- CACS:
-
Coronary artery calcium score
- CAD:
-
Coronary artery disease
- CCT:
-
Coronary CT angiography
- CFD:
-
Computational fluid dynamics
- CT:
-
Computed tomography
- CTP:
-
CT perfusion
- DECT:
-
Dual-energy CT
- FFR-CT:
-
CT-derived fractional flow reserve
- GUCH:
-
Grown-up congenital heart disease
- ICA:
-
Invasive coronary angiography
- IVUS:
-
Intravascular ultrasound
- NASCI:
-
North American Society for Cardiovascular Imaging
- PCD:
-
Photon counting detector
- SCCT:
-
Society of Cardiovascular Computed Tomography
- STR:
-
Society of Thoracic Radiology
References
Kramer CM, Budoff MJ, Fayad ZA, et al. ACCF/AHA 2007 clinical competence statement on vascular imaging with computed tomography and magnetic resonance. A report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. J Am Coll Cardiol. 2007;50:1097–114.
Beller GA, Bonow RO, Fuster V. ACCF 2008 recommendations for training in adult cardiovascular medicine core cardiology training (COCATS 3) (revision of the 2002 COCATS Training Statement). J Am Coll Cardiol. 2008;51:335–8.
Leipsic J, Abbara S, Achenbach S, et al. SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography guidelines committee. J Cardiovasc Comput Tomogr. 2014;8:342–58.
Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(4 suppl):5–40.
Angelini P, Villason S, Chan AV Jr, Diez JG. Normal and anomalous coronary arteries in humans. In: Angelini P, editor. Coronary artery anomalies: a comprehensive approach. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 27–150.
Angelini P. Coronary artery anomalies—current clinical issues: definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex Heart Inst J. 2002;29(4):271–8. Review
Pérez-Pomares JM, de la Pompa JL, Franco D, et al. Congenital coronary artery anomalies: a bridge from embryology to anatomy and pathophysiology—a position statement of the development, anatomy, and pathology ESC Working Group. Cardiovasc Res. 2016;109:204–16. https://doi.org/10.1093/cvr/cvv251.
Mery CM, De Leòn LE, Molossi S, Sexson-Tejtel SK, Agrawal H, Krishnamurthy R, Masand P, Qureshi AM, McKenzie ED, Fraser CD, jr. Outcomes of surgical intervention for anomalous aortic origin of a coronary artery: a large contemporary prospective cohort study. J Thorac Cardiovasc Surg. 2018;155(1):305–19.
Angelini P. Imaging approaches for coronary artery anomalies: purpose and techniques. Curr Cardiol Rep. 2019;21(9):101.
Angelini P, Uribe C. Anatomic spectrum of left coronary artery anomalies and associated mechanisms of coronary insufficiency. Catheter Cardiovasc Interv. 2018;92:313–21.
Angelini P, Uribe C, Monge J, et al. Origin of the right coronary artery from the opposite sinus of Valsalva in adults: characterization by intravascular ultrasonography at baseline and after stent angio-plasty. Catheter Cardiovasc Interv. 2015;86:199–208.
Ou P, Celermajer DS, Calcagni G, Brunelle F, Bonnet D, Sidi D. Three-dimensional CT-scanning: a new diagnostic modality in congenital heart disease. Heart 2007; 93(8): 908–13.).
Hamid IU, Digney R, Soo L, Leung S, Graham AN. Incidence and outcome of re-entry injury in redo cardiac surgery: benefits of preoperative planning. Eur J Cardiothorac Surg. 2015;47(5):819–23.
Shi G, Zhu Z, Chen J, Ou Y, Hong H, Nie Z, Zhang H, Liu X, Zheng J, Sun Q, Liu J, Chen H, Zhuang J. Total anomalous pulmonary venous connection: the current management strategies INA pediatric cohort of 768 patients. Circulation. 2017;135(1):48–58.
Mantini C, Maffei E, Toia P, Ricci F, Seitun S, Clemente A, Malagò R, Runza G, La Grutta L, Midiri M, Cotroneo AR, Forte E, Cademartiri F. Influence of image reconstruction parameters on cardiovascular risk reclassification by Computed Tomography Coronary Artery Calcium Score. Eur J Radiol. 2018;101:1–7. https://doi.org/10.1016/j.ejrad.2018.01.005.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32.
Yaghoubi S, Tang W, Wang S, et al. Offline assessment of atherosclerotic coronary calcium from electron beam tomograms. Am J Card Imaging. 1995;9:231–6.
Hecht et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: a report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Thorac Imaging 2017;32(5).
Maffei E, Martini C, Arcadi T, Clemente A, Seitun S, Zuccarelli A, Torri T, Mollet NR, Rossi A, Catalano O, Messalli G, Cademartiri F. Plaque imaging with CT coronary angiography: effect of intra-vascular attenuation on plaque type classification. World J Radiol. 2012;4(6):265–72. https://doi.org/10.4329/wjr.v4.i6.265.
Maffei E, Martini C, Rossi A, Mollet N, Lario C, Castiglione Morelli M, Clemente A, Gentile G, Arcadi T, Seitun S, Catalano O, Aldrovandi A, Cademartiri F. Diagnostic accuracy of second-generation dual-source computed tomography coronary angiography with iterative reconstructions: a real-world experience. Radiol Med. 2012;117(5):725–38. https://doi.org/10.1007/s11547-011-0754-x. Epub 2011 Nov 17
Douglas PS, Hoffmann U, Patel MR, et al. PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–300.
SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;6736:60291–4.
Maurovich-Horvat P, Schlett CL, Alkadhi H, et al. The napkin-ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. J Am Coll Cardiol Img. 2012;5:1243–52.
Maffei E, Nieman K, Martini C, Catalano O, Seitun S, Arcadi T, Malagò R, Rossi A, Clemente A, Mollet NR, Cademartiri F. Classification of noncalcified coronary atherosclerotic plaque components on CT coronary angiography: impact of vascular attenuation and density thresholds. Radiol Med. 2012;117(2):230–41. https://doi.org/10.1007/s11547-011-0744-z.
Maffei E, Seitun S, Martini C, Aldrovandi A, Arcadi T, Clemente A, Messalli G, Malagò R, Weustink A, Mollet N, Nieman K, Ardissino D, de Feyter P, Krestin G, Cademartiri F. Prognostic value of CT coronary angiography: focus on obstructive vs. nonobstructive disease and on the presence of left main disease. Radiol Med. 2011;116(1):15–31. https://doi.org/10.1007/s11547-010-0592-2.
Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–36.
Cheng V, Gutstein A, Wolak A, et al. Moving beyond binary grading of coronary arterial stenoses on coronary computed tomographic angiography: insights for the imager and referring clinician. J Am Coll Cardiol Img. 2008;1:460–71.
Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) Coronary Artery Disease—reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2016;10(4):269–81. https://doi.org/10.1016/j.jcct.2016.04.005. Epub 2016 Jun 15
Knuuti J, Wijns W, Saraste A, et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77.
National Institute for Health and Clinical Excellence. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin (update). CG95. London: National Institute for Health and Clinical Excellence; 2016. http://www.nice.org.uk/guidance/CG95.
Maffei E, Martini C, De Crescenzo S, et al. Low dose CT of the heart: a quantum leap into a new era of cardiovascular imaging. Radiol Med. 2010;115:1179–207.
Boussel L, Coulon P, Thran A, Roessl E, Martens G, Sigovan M, Douek P. Photon counting spectral CT component analysis of coronary artery atherosclerotic plaque samples. Br J Radiol. 2014;87(1040):20130798.
Adamson PD, Williams MC, Dweck MR, et al. Guiding therapy by coronary CT angiography improves outcomes in patients with stable chest pain. J Am Coll Cardiol. 2019;74:2058–70.
Chow BJ, Small G, Yam Y, et al. Prognostic and therapeutic implications of statin and aspirin therapy in individuals with nonobstructive coronary artery disease: results from the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter registry) registry. Arterioscler Thromb Vasc Biol. 2015;35:981–9.
Rosa GM, Bauckneht M, Masoero G, et al. The vulnerable coronary plaque: update on imaging technologies. Thromb Haemost. 2013;110:706–22.
Seitun S, De Lorenzi C, Cademartiri F, et al. CT myocardial perfusion imaging: a new frontier in cardiac imaging. Biomed Res Int. 2018;2018:7295460.
Cademartiri F, Seitun S, Clemente A, et al. Myocardial blood flow quantification for evaluation of coronary artery disease by computed tomography. Cardiovasc Diagn Ther. 2017;7:129–50.
Seitun S, Castiglione Morelli M, Budaj I, et al. Stress computed tomography myocardial perfusion imaging: a new topic in cardiology. Rev Esp Cardiol (Engl Ed). 2016;69:188–200.
Maffei E, Seitun S, Palumbo A, et al. Prognostic value of Morise clinical score, calcium score and computed tomography coronary angiography in patients with suspected or known coronary artery disease. Radiol Med. 2011;116:1188–202.
Cademartiri F, Seitun S, Romano M, et al. Prognostic value of 64-slice coronary angiography in diabetes mellitus patients with known or suspected coronary artery disease compared with a nondiabetic population. Radiol Med. 2008;113:627–43. https://doi.org/10.1007/s11547-008-0268-3.
Maffei E, Seitun S, Martini C, et al. Prognostic value of computed tomography coronary angiography in patients with chest pain of suspected cardiac origin. Radiol Med. 2011;116:690–705. https://doi.org/10.1007/s11547-011-0647-z.
Aldrovandi A, Maffei E, Palumbo A, et al. Prognostic value of computed tomography coronary angiography in patients with suspected coronary artery disease: a 24-month follow-up study. Eur Radiol. 2009;19:1653–60. https://doi.org/10.1007/s00330-009-1344-3.
Van Werkhoven JM, Cademartiri F, Seitun S, et al. Diabetes: prognostic value of CT coronary angiography—comparison with a nondiabetic population. Radiology. 2010;256(1):83–92.
Andreini D, Pontone G, Mushtaq S, et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc Imaging. 2012;5:690–701.
Jiang B, Wang J, Lv X, Cai W. Prognostic value of cardiac computed tomography angiography in patients with suspected coronary artery disease: a meta-analysis. Cardiology. 2014;128:304–12.
Min JK, Dunning A, Lin FY, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings: results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 Patients Without Known Coronary Artery Disease. J Am Coll Cardiol. 2011;58:849–60.
Rubin GD. Emerging and evolving roles for CT in screening for coronary heart disease. J Am Coll Radiol. 2013;10(12):943–8.
Lee JH, Han D, Hartaigh BÓ, et al. Influence of symptom typicality for predicting MACE in patients without obstructive coronary artery disease: from the CONFIRM registry (Coronary Computed Tomography Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry). Clin Cardiol. 2018;41:586–93.
Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgözoğlu L, Lewis EF. Atherosclerosis Nat Rev Dis Primers. 2019;5(1):56.
Blanke P, Naoum C, Ahmadi A, et al. Long-term prognostic utility of coronary CT angiography in stable patients with diabetes mellitus. JACC Cardiovasc Imaging. 2016;9:1280–8.
Nadjiri J, Hausleiter J, Jähnichen C, et al. Incremental prognostic value of quantitative plaque assessment in coronary CT angiography during 5 years of follow up. J Cardiovasc Comput Tomogr. 2016;10:97–104.
Feuchtner G, Kerber J, Burghard P, et al. The high-risk criteria low-attenuation plaque < 60 HU and the napkin-ring sign are the most powerful predictors of MACE: a long-term follow-up study. Eur Heart J Cardiovasc Imaging. 2017;18:772–9.
Seitun S, Clemente A, De Lorenzi C, Benenati S, Chiappino D, Mantini C, Sakellarios AI, Cademartiri F, Bezante GP, Porto I. Cardiac CT perfusion and FFRCTA: pathophysiological features in ischemic heart disease. Cardiovasc Diagn Ther. 2020;10(6):1954–78. https://doi.org/10.21037/cdt-20-414.
Sakellarios A, Correia J, Kyriakidis S et al. A cloud-based platform for the non-invasive management of coronary artery disease. Enterprise Inf Syst. 2020. ([Epub ahead of print]). https://doi.org/10.1080/17517575.2020.1746975.
Chen MY, Rochitte CE, Arbab-Zadeh A, et al. Prognostic value of combined CT angiography and myocardial perfusion imaging versus invasive coronary angiography and nuclear stress perfusion imaging in the prediction of major adverse cardiovascular events: the CORE320 multicenter study. Radiology. 2017;284:55–65.
Meinel FG, Pugliese F, Schoepf UJ, et al. Prognostic value of stress dynamic myocardial perfusion CT in a multicenter population with known or suspected coronary artery disease. AJR Am J Roentgenol. 2017;208:761–9.
Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–8.
Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012;308(12):1237–45.
Norgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol. 2014;63:1145–55.
Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58:1989–97.
Douglas PS, De Bruyne B, Pontone G, Patel MR, Norgaard BL, Byrne RA, et al. 1-year outcomes of FFRCT-guided care in patients with suspected coronary disease: the PLATFORM study. J Am Coll Cardiol. 2016;68:435–45.
Kim HL, Kim YJ, Lee SP, et al. Incremental prognostic value of sequential imaging of single-photon emission computed tomography and coronary computed tomography angiography in patients with suspected coronary artery disease. Eur Heart J Cardiovasc Imaging. 2014;15:878–85.
Renker M, Schoepf UJ, Wang R, et al. Comparison of diagnostic value of a novel noninvasive coronary computed tomography angiography method versus standard coronary angiography for assessing fractional flow reserve. Am J Cardiol. 2014;114(9):1303–8.
Coenen A, Lubbers MM, Kurata A, et al. Fractional flow reserve computed from noninvasive CT angiography data: diagnostic performance of an on-site clinician-operated computational fluid dynamics algorithm. Radiology. 2015;274:674–83.
Kruk M, Wardziak Ł, Demkow M, et al. Workstation-based calculation of CTA-based FFR for intermediate stenosis. JACC Cardiovasc Imaging. 2016;9:690–9.
Ko BS, Cameron JD, Munnur RK, et al. Noninvasive CT-derived FFR based on structural and fluid analysis: a comparison with invasive FFR for detection of functionally significant stenosis. JACC Cardiovasc Imaging. 2017;10:663–73.
Papafaklis MI, Mavrogiannis MC, Siogkas PK, Lakkas LS, Katsouras, Fotiadis DI, Michalis LK. Functional assessment of lesion severity without using the pressure wire: coronary imaging and blood flow simulation. Expert Rev Cardiovasc Ther. 2017;15(11):863–77.
Tesche C, De Cecco CN, Albrecht MH, Duguay TM, Bayer RR 2nd, Litwin SE, Steinberg DH, Schoepf UJ. Coronary CT angiography-derived fractional flow reserve. Radiology. 2017;285(1):17–33.
Seitun S, Clemente A, De Lorenzi C et al. Cardiac CT perfusion and FFRCTA: pathophysiological features in ischemic heart disease. Cardiovasc Diagn Ther. 2020. https://doi.org/10.21037/cdt-20-414.
Nakamura S, Kitagawa K, Goto Y, et al. Incremental prognostic value of myocardial blood flow quantified with stress dynamic computed tomography perfusion imaging. JACC Cardiovasc Imaging. 2019;12(7 Pt 2):1379–87.
van Assen M, De Cecco CN, Eid M, et al. Prognostic value of CT myocardial perfusion imaging and CT-derived fractional flow reserve for major adverse cardiac events in patients with coronary artery disease. J Cardiovasc Comput Tomogr. 2019;13:26–33.
Bilbey N, Blanke P, Naoum C, et al. Potential impact of clinical use of noninvasive FFRCT on radiation dose exposure and downstream clinical event rate. Clin Imaging. 2016;40:1055–60.
Douglas PS, De Bruyne B, Pontone G, et al. PLATFORM investigators. 1-Year outcomes of FFRCT-guided care in patients with suspected coronary disease: the PLATFORM study. J Am Coll Cardiol. 2016;68:435–45.
McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology. 2015;276:637–53. https://doi.org/10.1148/radiol.2015142631.
Danad I, Fayad ZA, Willemink MJ, et al. New applications of cardiac computed tomography: dual-energy, spectral, and molecular CT imaging. JACC Cardiovasc Imaging. 2015;8:710–23. https://doi.org/10.1016/j.jcmg.2015.03.005.
Danad I, Hartaigh Ó, B, Min JK. Dual-energy computed tomography for detection of coronary artery disease. Expert Rev Cardiovasc Ther. 2015;13:1345–56. https://doi.org/10.1586/14779072.2015.1102055.
Symons R, Cork TE, Lakshmanan MN, et al. Dual-contrast agent photon-counting computed tomography of the heart: initial experience. Int J Cardiovasc Imaging. 2017;33:1253–61. https://doi.org/10.1007/s10554-017-1104-4.
Kolossváry M, De Cecco CN, Feuchtner G, Maurovich-Horvat P. Advanced atherosclerosis imaging by CT: radiomics, machine learning and deep learning. J Cardiovasc Comput Tomogr. 2019;13:274–80.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Clemente, A. (2023). Computed Tomography Cardiac Imaging: Coronary Artery Disease and Ischemia. In: Concistrè, G. (eds) Ischemic Heart Disease. Springer, Cham. https://doi.org/10.1007/978-3-031-25879-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-25879-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25878-7
Online ISBN: 978-3-031-25879-4
eBook Packages: MedicineMedicine (R0)