Abstract
Respiratory viral infections are pervasive in humans. For many, viral-induced pathogenesis is mild and largely an annoyance to daily life. However, for some, the severity of disease can be life-threatening, such as that seen with recent outbreaks of SARS-CoV-2 and influenza virus. This spectrum of disease presentation is underpinned by an inflammatory lung pathology, and many of the symptoms accompanying respiratory viral infections, including cough, an itchy or irritated throat, perceptions of difficulty breathing and excessive mucous and airflow limitations, suggest that viral-induced pulmonary inflammation alters the activity in neural pathways that ordinarily regulate bronchopulmonary function. In this chapter, we explore novel ways in which respiratory viral infections may impact bronchopulmonary nerves, focussing on the sensory fibres of the vagus nerves and their brain connections. We review work from our group and others that offers compelling evidence that vagal neuroinflammation may be an important and unrecognised component of respiratory viral pathogenesis and an important consideration for future advances in clinical management of patients with severe respiratory viral disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 An Overview of the Neurobiology of Airway Sensation
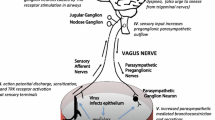
The respiratory tract is densely innervated by sensory neurons, many of which arise from the vagus nerves (Fig. 1) (Mazzone et al. 2020; Lee and Yu 2014; Mazzone and Undem 2016). The cell bodies of vagal sensory neurons reside in two distinct collections of cells known as the nodose (inferior) and jugular (superior) ganglia. These ganglia are located bilaterally, extrinsic to the respiratory system, at the cranial end of the vagus nerves (Mazzone and Undem 2016). The nodose and jugular ganglia differ in embryonic origin, and consequently the sensory neurons residing within each set of ganglia display differing patterns of gene and protein expression, resulting in distinct anatomical and functional airway sensory neuron phenotypes (Mazzone and Undem 2016; Wang et al. 2017; Carr and Undem 2003). Peripherally, although some overlap of the location of nodose and jugular neuron terminal fields exists, nodose sensory neuron terminals are more prevalent within the intrapulmonary airways and lungs, whereas jugular neurons have terminations mostly in the large extrapulmonary airways and larynx. The central projections of nodose and jugular neurons are quite distinct. Nodose airway sensory fibres project mainly to the nucleus of the solitary tract, whereas jugular airway sensory fibres terminate in and around the paratrigeminal nucleus in the medulla. Collectively, these two broad classes of sensory neurons serve to detect a wide range of physical, chemical or thermal stimuli that can occur in the airways and lungs and send this information about the airway environment to the central nervous system to regulate a range of respiratory and autonomic processes important for normal homeostatic functioning and pulmonary defence (Lee and Yu 2014; Carr and Undem 2003).
Mechanisms involved in the pathogenesis of respiratory viral infections. (a) A viral infection of the respiratory epithelium initiates an inflammatory response which serves to limit the extent of viral propagation and promote viral clearance. (b) Respiratory epithelial cells are juxtaposed to vagal sensory nerve terminals, which may be activated or sensitised by respiratory viruses and/or the resultant products of inflammation. (c) Severe respiratory viral infections have been shown to cause inflammation within the vagal sensory ganglia, characterised by inflammatory cell infiltration and altered neuronal gene expression. (d) Neuroinflammation may extend to the brainstem and brain where alterations in glial cell activity, inflammatory gene expression and synaptic function have been reported. Collectively, alterations in normal function within these diverse sites of inflammatory pathology are thought to contribute to acute and long-term symptoms associated with respiratory viral infections. Abbreviations: CNS central nervous system; DAMPs damage-associated molecular patterns; IFNAR1 interferon alpha and beta receptor subunit 1; TLR4 toll-like receptor 4. (Created with BioRender.com)
Vagal sensory nerve fibres terminate in proximity to epithelial cells, mucosal glands, vasculature and/or airway smooth muscle in the conducting airways, respiratory airways and lung parenchyma (Lee and Yu 2014; Carr and Undem 2003; Brouns et al. 2021). A major functional subclass of airway sensory neurons serve as ‘physiological’ receptors, monitoring lung inflation, deflation and airway smooth muscle tone. These sensory neurons are typically myelinated fast-conducting A-fibres, derived from the nodose vagal ganglia, and play an important role in optimising breathing, airway patency and gas exchange (Mazzone and Undem 2016; Carr and Undem 2003; Kollarik et al. 2010; Mazzone 2004). This class of sensory neuron is not the focus of this chapter and has been described in detail elsewhere (Mazzone and Undem 2016; Wang et al. 2017; Carr and Undem 2003; Kollarik et al. 2010; Kupari et al. 2019). A second major functional class of airway sensory neurons, often called nociceptors, are derived from both the nodose and jugular ganglia and mainly consist of slower conducting C-fibres (although Aδ-fibre nociceptors exist in some species) (Mazzone and Undem 2016; Carr and Undem 2003). Nociceptors are so named as they are specialised to sense noxious and potentially harmful stimuli that could impact the airways and compromise normal pulmonary functions (Mazzone and Undem 2016; Carr and Undem 2003). They are especially important as the respiratory tract is an open system, exposed to the environment via the air that is inhaled which can contain pathogens and irritant stimuli (e.g. smoke or other chemicals) that can cause airway damage (Mazzone and Undem 2016; Carr and Undem 2003). Additionally, the initial anatomical conduits for foodstuffs and air are shared with potential for airway damage via the aspiration of consumed foods and liquids or refluxed gastric contents. Many nociceptors are positioned in or close to the airway epithelial barrier allowing for constant monitoring of the presence of these potentially damaging stimuli (Mazzone and Undem 2016; Carr and Undem 2003). Collectively, the two broad functional classes of vagal sensory neurons play an important role in pulmonary homeostasis and defence by providing feedback from the respiratory environment to the brainstem driving a variety of responses including the Hering-Breuer inflation and deflation reflexes, reflex changes in bronchomotor tone and cough (Lee and Yu 2014; Carr and Undem 2003; Brouns et al. 2021).
Sensory neuron transduction of stimuli and conduction of action potentials is mediated by a suite of ionotropic receptors, G protein-coupled receptors and voltage-gated ion channels, expressed on the peripheral nerve terminals and axons (Fig. 1) (Lee and Yu 2014; Mazzone and Undem 2016; Wang et al. 2017; Kollarik et al. 2010). Some stimuli directly activate channels and receptors expressed by airway sensory neurons, whilst others activate sensory neurons via intermediary molecules produced from resident or infiltrating cells, including immune cells. The unique complement of these receptors and channels confers sensory neurons with their subtype functional specificity, allowing for the encoding of different modalities of stimulation (Lee and Yu 2014; Mazzone and Undem 2016; Wang et al. 2017; Kollarik et al. 2010). Nociceptors are often characterised by the expression of one or more of the transient receptor potential (TRP) family of ionotropic sensory transduction channels (Mazzone and Undem 2016; Kollarik et al. 2010; Mazzone 2004; De Logu et al. 2016). Of the TRP family, TRPV1 and TRPA1 channels are abundantly expressed on airway nociceptors and have been shown to play a key role in response to noxious thermal stimuli, exogenous chemicals (including components of smoke or natural products in plants such as capsaicin from chilli peppers) and endogenous inflammatory mediators (Mazzone and Undem 2016; De Logu et al. 2016; Caceres et al. 2009). Interestingly, the activation of these channels has been implicated as playing an important role in respiratory diseases. For example, the ablation of TRPA1 in a murine asthma model showed a reduction in bronchial hyper-responsiveness and inhibition of infiltrating eosinophils and levels of interleukin (IL)-5 (De Logu et al. 2016; Caceres et al. 2009).
Additional to these channels, nociceptors express a variety of receptors for inflammatory mediators and pathogen-associated molecular patterns including receptors for interferon alpha (IFNAR1, IFNAR2) (Wang et al. 2017; Patil et al. 2020), tumour necrosis factor (TNFR2) (Wang et al. 2017) and toll-like receptor 3 (Wang et al. 2017). Activation of each of these transduction receptors and channels results in the modification of membrane excitability and depolarisation. Altered membrane voltage leads to the activation of voltage-gated sodium channels (e.g. NaV1.7, NaV1.8 and NaV1.9), initiating centrally directed propagation of action potentials. Some nociceptors can also release neuropeptides including substance P, calcitonin gene-related peptide, neurokinin A and vasoactive intestinal peptide locally in the airway tissues (Lee and Yu 2014; Mazzone and Undem 2016; Carr and Undem 2003; Kollarik et al. 2010). Neuropeptides can interact directly and indirectly on structural cells to alter pulmonary physiology and on immune cells to modulate inflammation and are therefore important mediators regulating a variety of pulmonary pathologies. Calcitonin gene-related peptide, for example, is a potent vasodilator and has been shown to inhibit dendritic cell maturation by modulating the antigen presentation, negatively affecting T cell activation (Assas et al. 2014; Rochlitzer et al. 2011).
2 Pathogenesis of Respiratory Viruses
Pathogen-dependent respiratory diseases in humans are mostly caused by viruses, which notably include influenza A virus (IAV), human coronaviruses (hCoV), respiratory syncytial virus (RSV), human metapneumovirus (hMPV) and rhinovirus (Lee 2017; Schmidt and Varga 2018; Rey-Jurado et al. 2020; Allie and Randall 2017; Gillim-Ross and Subbarao 2006; Johansson and Kirsebom 2021). Viral respiratory tract infections are extremely frequent across the life span and can range in severity from subclinical presentation with no symptomatology, mild cold-like symptoms, to severe symptoms such as pneumonia leading to acute respiratory distress syndrome (ARDS) or even death (Lee 2017; Schmidt and Varga 2018; Rey-Jurado et al. 2020; Allie and Randall 2017; Gillim-Ross and Subbarao 2006; Johansson and Kirsebom 2021). The disease severity is dependent on the host immune response, patient age, any pre-existing patient comorbidities and type/strain of virus in question.
Respiratory viruses commonly infect epithelial cells in the upper (nose, pharynx and/or larynx) and lower (trachea, bronchi, bronchioles and alveoli) respiratory tracts (Fig. 1) (Allie and Randall 2017; Braciale et al. 2012; Wong et al. 2019). Epithelial infection is an important mode of viral replication and a primary trigger for the initiation of the immune response. Viral-induced inflammation is also facilitated early in the infection by alveolar macrophages, whilst resident and infiltrating dendritic cells play an important role in both innate host defence and the co-ordination of innate and adaptive immune responses. Epithelial and resident immune cells in the respiratory tract can detect viral pathogens through a variety of host cell pathogen recognition receptors including toll-like receptors, retinoic acid-inducible gene 1-like receptors, cytosolic DNA sensors and nucleotide oligomerisation domain-like receptors (Allie and Randall 2017; Braciale et al. 2012; Wong et al. 2019). These pathogen recognition receptors detect both pathogen-associated molecular patterns expressed by invading viruses and damage-associated molecular patterns released by infected, injured and necrotic/apoptotic cells (Allie and Randall 2017; Braciale et al. 2012; Wong et al. 2019).
Early inflammation is orchestrated through the secretion of a first wave of tissue damage-associated molecular patterns and inflammatory cytokines including ATP, HMGB1, IL-33, IFNγ, IFNβ, IL-6, TNF, IL-12, IL-23 and IL-1β (Allie and Randall 2017; Braciale et al. 2012; Wong et al. 2019; Troy and Bosco 2016; Yoo et al. 2013). In addition to effects on resident respiratory tract cells, these mediators can attract other innate immune cells such as cytotoxic T-cells, natural killer cells and innate lymphoid cells to the infected region. These cells release additional cytokines exerting cytotoxic effects on infected cells. The second wave of cytokines will also attract circulating neutrophils and monocytes, thereby further amplifying the immune response (Allie and Randall 2017; Braciale et al. 2012; Wong et al. 2019; Troy and Bosco 2016; Yoo et al. 2013). In addition, cytokines promote dendritic cell maturation. Matured dendritic cells are antigen-presenting cells that migrate to local lymph nodes to prime the adaptive responses (Allie and Randall 2017; Braciale et al. 2012; Wong et al. 2019). In the lymph nodes, naïve CD4+ T-cells and CD4+ effector cells are activated and proliferate and differentiate. CD8+ T-cells differentiate to cytotoxic and memory CD8+ T-cells releasing potent cytokines and aid in apoptosis and clearance of infected cells (Allie and Randall 2017; Braciale et al. 2012; Wong et al. 2019; Troy and Bosco 2016; Yoo et al. 2013).
Host inflammatory responses following viral infection are ideally regulated to effectively clear virus and virally infected cells from the respiratory tract and promote tissue repair (Allie and Randall 2017; Braciale et al. 2012; Wong et al. 2019; Troy and Bosco 2016; Yoo et al. 2013). However, during some respiratory infections an uncontrolled immune response is observed with an exacerbated cytokine response, commonly referred to as a ‘cytokine storm’. The uncontrolled immune response has cytotoxic properties, augmenting the tissue injury in the pulmonary system by increasing apoptosis and necrosis to the alveolar epithelium (Matthay and Zemans 2011; Matthay et al. 2019). A resultant excessive activation of neutrophils is central to this pathology, progressing affected patients towards the development of ARDS and the release of toxic mediators that increase paracellular epithelial permeability and promote severe vascular damage (Matthay and Zemans 2011; Matthay et al. 2019). This immunopathology contributes significantly to disease severity and patient morbidity typically includes symptoms such as excessive coughing and severe dyspnoea, suggestive of profound, yet poorly described impacts on pulmonary sensory nerves.
3 Neurotropism of Respiratory Viruses
Severe respiratory tract infections have been shown to result in systemic inflammation and cause extrapulmonary disorders, including neurological clinical manifestations such as encephalopathies characterised by seizures, memory loss, confusion, personality changes, depression, anxiety and ‘brain fog’, encephalitis and syndromes including Guillain-Barre syndrome. These neurological manifestations can be induced by neurotropic viruses with less severe symptoms usually caused by non-neurotropic viruses (Ruisanchez-Nieva et al. 2017; Ryabkova et al. 2021; Glaser et al. 2012; Mao et al. 2020; Zubair et al. 2020; Frankl et al. 2021; Bohmwald et al. 2018; Desforges et al. 2019).
Respiratory viruses including strains of RSV, hMPV, IAV and hCoV are known to be neurotropic and could conceivably invade the nervous system at the site of airway infection (Bohmwald et al. 2018; Desforges et al. 2014, 2019; Koyuncu et al. 2013; Dey et al. 2021). In support of this assertion, following intranasal inoculation of rodents with influenza virus, viral antigen has been recovered from trigeminal and vagal ganglia in the absence of any systemic infection (Shinya et al. 2000; Matsuda et al. 2004). hMPV has been shown to infect and persist in local neuronal processes in the airway wall following epithelial replication (Liu et al. 2009). These data suggest a capacity of sensory neurons to uptake and potentially transport some respiratory viral strains. As the nose is typically the first site of viral infection, transport of virus to the brain via the olfactory nerves has been widely postulated (Bohmwald et al. 2018; Desforges et al. 2019; Koyuncu et al. 2013; Dey et al. 2021) and recent observations with SARS-CoV-2 suggest this is a possible mechanism for the neurological sequalae in patients with COVID-19 (Meinhardt et al. 2021; Douaud et al. 2022). However, little is known about the specific viral and neuronal entry factors that allow for neuronal infection. In the case of SARS-CoV-2, sensory neurons do not appreciably express the most common entry factor needed for viral uptake (ACE2) (Shiers et al. 2020), suggesting non-traditional factors may be important for viral interactions with neurons. Whether this extends to other neurotropic respiratory viruses is not entirely clear. After entry occurs at the nerve endings, the mobility of the virus along axons to the CNS is dependent on anterograde or retrograde transport using neuronal motor proteins, including dynein and kinesins (Bohmwald et al. 2018; Desforges et al. 2019; Koyuncu et al. 2013; Dey et al. 2021).
Viruses may impact the nervous system via means other than direct infection of neurons. Another possible route of entry is through the haematogenous route where the virus reaches peripheral ganglia and/or crosses either the blood-brain barrier or the blood-cerebrospinal fluid barrier in the choroid plexus, or by infection of leukocytes diffusing to the CNS (Bohmwald et al. 2018; Desforges et al. 2014, 2019; Koyuncu et al. 2013; Dey et al. 2021). This mode of action is not restricted to the intact native virus but can also occur for isolated viral proteins. For example, shed spike proteins from SARS-CoV-2 have been detected in the CNS driving inflammatory responses independent of any detectable replication competent virus (Rhea et al. 2021). Most IAV strains circulating seasonally in humans are non-neurotropic and cannot replicate in the CNS giving less severe neurological clinical manifestations (Bohmwald et al. 2018; Desforges et al. 2019; Koyuncu et al. 2013). However, through blood-borne routes viruses can still cause secondary effects on the CNS, including via systemic inflammation and the release of neurotropic factors produced by inflammation (Bohmwald et al. 2018; Desforges et al. 2019).
4 Evidence for Viral-Mediated Neuroinflammation
4.1 Impact of Respiratory Viral Infection on Vagal Sensory Neurons
Pulmonary vagal sensory neurons have not been studied extensively during respiratory viral infections. Kaelberer et al. (2020) showed lipopolysaccharide-induced pulmonary inflammation, mimicking a bacterial infection, resulting in transcriptional changes in neurons of vagal sensory ganglia innervating the pulmonary system. These transcriptional changes were characterised by an upregulation in gene expression associated with innate immune responses, more specifically genes Lrg1 and C3 that could indicate the maturing of resident or infiltrating ganglia immune cells, or alternatively the changing of satellite cells towards an active immune cell type (Kaelberer et al. 2020). Transcriptomic changes in vagal sensory ganglia, including the pulmonary vagal sensory neurons themselves, were also observed during severe respiratory IAV infection in a murine model (Verzele et al. 2021). Notably in this study, the sensory neuron transcriptional alterations induced by active viral infection were mimicked by both the administration of cytokines and the inoculation of the lung of healthy mice with viral-inactivated lung homogenates from previously infected animals (Verzele et al. 2021). This suggests that the sensory neuron impacts of respiratory viral infection can occur independently of neuronal viral infection but instead are dependent on mediators of pulmonary inflammation. Consistent with this, the upregulated genes were associated with defence and pro-inflammatory responses, including signalling downstream of interferon production (Verzele et al. 2021).
Tissue injury is commonly associated with inflammatory cell influx and/or proliferation into the nerves that innervate the injured tissue. During respiratory IAV infection, inflammatory cells are increased in the vagal sensory ganglia (Fig. 2) (Verzele et al. 2021), a further sign of vagal neuroinflammation. The importance of inflammatory cell recruitment is still unknown; however, these immune cells could potentially play a role in inducing or facilitating molecular, structural and functional changes in sensory neurons. The ganglionic signalling mechanisms that lead to the increase in inflammatory cells are also unknown. One possible mediator is the tissue alarmin HMGB1, which can be released from injured or activated cells (Fig. 2) (Mazzone et al. 2021). We previously demonstrated the mobilisation of neuronal HMGB1 following respiratory infection with IAV or pneumovirus, and subsequent changes in sensory neuron structure and function dependent upon the HMGB1 receptor RAGE (Mazzone et al. 2021). Notably, respiratory infection resulted in the increase in neurite outgrowth in vagal sensory neurons, indicating neuronal sprouting and growth, a common feature in airway pathologies (Mazzone et al. 2021; Shapiro et al. 2021; Drake et al. 2021). Additionally, HMGB1 increased the excitability of the vagal sensory neurons, via a mechanism dependent on RAGE expression (Mazzone et al. 2021). HMGB1 has a well-known role in early initiation of inflammatory cell influx and activation (Yang et al. 2021; Magna and Pisetsky 2014) and thus neuronally released HMGB1 may be pivotal in establishing vagal neuroinflammation. How HMGB1 is mobilised from neurons is not clear, although increased action potential traffic along the axon may be involved (Yang et al. 2021).
Vagal neuroinflammation accompanying respiratory viral infection. Top panel micrographs show immune cell infiltration into the vagal sensory vagal ganglia following influenza A (IAV) infection. Immunohistochemistry for immune cells MHCII (major histocompatibility class II – red) and the pan-neuronal marker MAP2 (microtubule-associated protein 2 – green) following either Mock (saline) or IAV inoculation of the lungs. The bottom panel micrographs show inoculation of the lungs with IAV inducing translocation of the danger-associated molecular pattern, and HMGB1 (red) from the nucleus to the cytoplasm in vagal ganglia sensory neurons. Neurons are outlined by dotted lines and arrowheads point to nuclei, visualised by DAPI (blue). Scale bar in top and bottom panels represent 75 μm and 25 μm, respectively
Collectively, the sensory neuron impacts of respiratory viral infection may underpin the development of a sensory hypersensitivity, resulting in increased coughing and other defensive reflexes that are beneficial for the clearance of the airways and potentially for transmission of the virus to new hosts. Persistence of the hypersensitivity beyond the period of active viral infection, perhaps due to a sustained neuroinflammatory state, is hypothesised to be responsible for the long-lasting respiratory and neurological symptoms commonly associated with respiratory viral infection (Undem et al. 2015).
4.2 Impact of Respiratory Viral Infection on Vagal Sensory Pathways in the Brain
The impact of respiratory viral infections on the CNS is an underexplored area, although it has received more attention recently after increasing reports of long-term neurological complications seen in patients with SARS-CoV-2 infections. In general, viral-induced neurological manifestations have been noted after respiratory infections with both neurotropic and non-neurotropic viruses, perhaps indicating that the impacts on the CNS are not simply dependent on neuroinvasion (Fig. 1) (Hosseini et al. 2018).
Studies of asthma and allergic inflammation in the pulmonary system have offered insight into the impact of non-pathogenic lung diseases on vagal sensory processing pathways in the CNS (Bonham et al. 2006). At the level of the nucleus of the solitary tract, alterations in synaptic efficacy between primary vagal afferent and second-order neurons have been noted (Bonham et al. 2006; Chen et al. 2001; Klein et al. 2016), thought to reflect alterations in the release of neurotransmitters from primary afferent terminals and/or changes in the intrinsic excitability of nucleus of the solitary tract neurons (Bonham et al. 2006). For example, in allergen sensitised rats, respiratory allergen challenge changed the membrane properties of the second-order neurons in the nucleus of the solitary tract, increasing membrane depolarisation and potentially modulating the output of vagal efferent nerves (Chen et al. 2001). Effects on nucleus of the solitary tract processing of vagal inputs have also been seen in models of cigarette smoke exposure and lung fibrosis (Litvin et al. 2018; Mutoh et al. 2000), suggesting that either an excessive level of vagal sensory neuronal activation in lung disease or accompanying systemic inflammation results in a centrally sensitised state that impacts vagal sensory processing.
Although peripheral inflammation is the likely initiator of CNS sensitisation, central processes also undoubtedly become involved which notably includes the recruitment and activation of CNS glial cells and the development of a central neuroinflammatory state. During respiratory viral infections or following exposure to either bleomycin or inhaled diesel exhaust particles, brainstem microglia cells and astrocytes have been shown to be activated, along with an upregulation of brainstem inflammatory gene expression (Undem et al. 2015; Litvin et al. 2018; Chen et al. 2021). These CNS support cells are important for the brain homeostasis and neuroprotective functions but can contribute to a CNS inflammatory state that alters the processing of incoming sensory inputs (Siracusa et al. 2019; Bachiller et al. 2018). For example, the activation of microglia leads to the production of pro-inflammatory cytokines that then affect neuronal morphology, synaptic structure and function leading to an imbalance in normal excitatory and inhibitory neurotransmission (Bohmwald et al. 2018; Hosseini et al. 2018). These inflammatory events are a hallmark feature of central sensitisation.
Similar events can occur beyond the brainstem, in higher brain regions that may contribute to complex sensory, motor or cognitive processing. For example, during severe respiratory viral infections, microglia and astrocyte activation and an accompanying pro-inflammatory state have been reported in a variety of brain regions including the hypothalamus, hippocampus and substantia nigra impacting the function of each region (Hosseini et al. 2018; Wang et al. 2018; Jang et al. 2009, 2012). Indeed, respiratory infection with highly pathogenic strains of IAV resulted in CNS viral infection of both neurons and microglia, induction of pro-inflammatory cytokines, prolonged microgliosis which coincided with a loss of dopaminergic neurons in the substantia nigra as well as aggregation of alpha-synuclein in the hippocampus, brainstem and cortex, suggesting that certain respiratory viruses could be important aetiological agents in the development of neurodegenerative diseases including Parkinson’s and Alzheimer’s disease (Bohmwald et al. 2018; Magna and Pisetsky 2014; Siracusa et al. 2019; Bachiller et al. 2018; Jang et al. 2009, 2012). How these higher brain CNS neuropathologies induced by respiratory viral infections impact vagal sensory processing circuits in the brain, important for pulmonary interoception, has not been investigated.
5 The Clinical Significance of Respiratory Viral-Induced Neuropathy
Respiratory viral infections represent a significant burden to the human health as they are extremely prevalent and can cause both acute and long-term clinical manifestations. The neurological impacts of respiratory viral infections are especially difficult to treat or manage. For example, cough is one of the most common symptoms associated with respiratory viral infection (Bohmwald et al. 2018), and whilst it may resolve spontaneously for many patients, soon after viral clearance and natural resolution of the disease, cough can persist for some people for many weeks or months. Post-viral cough syndrome is poorly understood, and there are no effective therapies for treatment, but may reflect the consequences of the neuroinflammatory state established during viral infection. Similarly, other persistent neurological symptoms have been reported following viral infections of the lungs. A significant population of patients infected with SARS-CoV-2 continue to experience chronic fatigue, cognitive impairment, pain and other symptoms as part of the long COVID syndrome, consistent with long-term changes in the functioning of the peripheral and central nervous system (Bohmwald et al. 2018; Song et al. 2021; Buckley et al. 2021). Similar symptomatology may occur following IAV infections. Again, the mechanism behind these long-lasting manifestations has yet to be determined and treatment options are limited. A deeper understanding of the neuroinflammatory events that accompany respiratory viral infections, including their persistence, responsiveness to standard therapies and the resultant changes that are imparted on neural function, may offer new avenues for therapeutic intervention and effective management of these challenging and debilitating symptoms.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- ARDS:
-
Acute respiratory disease syndrome
- ATP:
-
Adenosine 5′-triphosphate
- CD4+ cells:
-
Cluster of differentiation 4 (T helper cells)
- CD8+ cells:
-
Cluster of differentiation 8 (cytotoxic T cells)
- CNS:
-
Central nervous system
- DAMPs:
-
Damage-associated molecular patterns
- hCoV:
-
Human coronavirus
- HMGB1:
-
High mobility group box protein 1
- hMPV:
-
Human metapneumovirus
- IAV:
-
Influenza A virus
- IFNAR1:
-
Interferon alpha receptor, type 1
- IFNAR2:
-
Interferon alpha receptor, type 2
- IFNb:
-
Interferon beta
- IFNγ:
-
Interferon gamma
- IL-12:
-
Interleukin 12
- IL-1b:
-
Interleukin 1 beta
- IL-23:
-
Interleukin 23
- IL-33:
-
Interleukin 33
- IL-5:
-
Interleukin 5
- RAGE:
-
Receptor for advanced glycation end-products
- RSV:
-
Respiratory syncytial virus
- SARS-CoV-2:
-
Severe acute respiratory syndrome-coronavirus 2
- TLR:
-
Toll-like receptor
- TNF:
-
Tumour necrosis factor
- TRPA1:
-
Transient receptor potential, ankyrin 1
- TRPV1:
-
Transient receptor potential, vanilloid 1
References
Allie SR, Randall TD. Pulmonary immunity to viruses. Clin Sci (Lond). 2017;131(14):1737–62.
Assas BM, Pennock JI, Miyan JA. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front Neurosci. 2014;8:23.
Bachiller S, et al. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci. 2018;12:488.
Bohmwald K, et al. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386.
Bonham AC, et al. Plasticity in the nucleus tractus solitarius and its influence on lung and airway reflexes. J Appl Physiol. 2006;101(1):322–7.
Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12(4):295–305.
Brouns I, et al. The pulmonary neuroepithelial body microenvironment: a multifunctional unit in the airway epithelium. In: The pulmonary neuroepithelial body microenvironment : a multifunctional unit in the airway epithelium. Springer International Publishing, Cham; 2021. p. 1–65.
Buckley BJR, et al. Prevalence and clinical outcomes of myocarditis and pericarditis in 718,365 COVID-19 patients. Eur J Clin Investig. 2021;51(11):e13679.
Caceres AI, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106(22):9099–104.
Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology. 2003;8(3):291–301.
Chen CY, et al. Extended allergen exposure in asthmatic monkeys induces neuroplasticity in nucleus tractus solitarius. J Allergy Clin Immunol. 2001;108(4):557–62.
Chen Z, et al. Glial activation and inflammation in the NTS in a rat model after exposure to diesel exhaust particles. Environ Toxicol Pharmacol. 2021;83:103584.
De Logu F, et al. TRP functions in the broncho-pulmonary system. Semin Immunopathol. 2016;38(3):321–9.
Desforges M, et al. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75–96.
Desforges M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):14.
Dey J, et al. Neuroinvasion of SARS-CoV-2 may play a role in the breakdown of the respiratory center of the brain. J Med Virol. 2021;93(3):1296–303.
Douaud G, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank, vol. 604. medRxiv; 2022. p. 697.
Drake MG, et al. Airway sensory nerve plasticity in asthma and chronic cough. Front Physiol. 2021;12:720538.
Frankl S, et al. Influenza-associated neurologic complications in hospitalized children. J Pediatr. 2021;239:24–31.e1.
Gillim-Ross L, Subbarao K. Emerging respiratory viruses: challenges and vaccine strategies. Clin Microbiol Rev. 2006;19(4):614–36.
Glaser CA, et al. A population-based study of neurologic manifestations of severe influenza A(H1N1)pdm09 in California. Clin Infect Dis. 2012;55(4):514–20.
Hosseini S, et al. Long-term Neuroinflammation induced by influenza A virus infection and the impact on hippocampal neuron morphology and function. J Neurosci. 2018;38(12):3060–80.
Jang H, et al. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A. 2009;106(33):14063–8.
Jang H, et al. Inflammatory effects of highly pathogenic H5N1 influenza virus infection in the CNS of mice. J Neurosci. 2012;32(5):1545–59.
Johansson C, Kirsebom FCM. Neutrophils in respiratory viral infections. Mucosal Immunol. 2021;14(4):815–27.
Kaelberer MM, Caceres AI, Jordt SE. Activation of a nerve injury transcriptional signature in airway-innervating sensory neurons after lipopolysaccharide-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L953–l964.
Klein B, et al. Allergy enhances neurogenesis and modulates microglial activation in the hippocampus. Front Cell Neurosci. 2016;10:169.
Kollarik M, Ru F, Brozmanova M. Vagal afferent nerves with the properties of nociceptors. Auton Neurosci. 2010;153(1–2):12–20.
Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. 2013;13(4):379–93.
Kupari J, et al. An atlas of vagal sensory neurons and their molecular specialization. Cell Rep. 2019;27(8):2508–2523.e4.
Lee KY. Pneumonia, acute respiratory distress syndrome, and early immune-modulator therapy. Int J Mol Sci. 2017;18(2):388.
Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol. 2014;4(1):287–324.
Litvin DG, et al. Lung-injury depresses glutamatergic synaptic transmission in the nucleus tractus solitarii via discrete age-dependent mechanisms in neonatal rats. Brain Behav Immun. 2018;70:398–422.
Liu Y, et al. Human metapneumovirus establishes persistent infection in the lungs of mice and is reactivated by glucocorticoid treatment. J Virol. 2009;83(13):6837–48.
Magna M, Pisetsky DS. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol Med. 2014;20(1):138–46.
Mao L, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–90.
Matsuda K, et al. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet Pathol. 2004;41(2):101–7.
Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–63.
Matthay MA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18.
Mazzone SB. Sensory regulation of the cough reflex. Pulm Pharmacol Ther. 2004;17(6):361–8.
Mazzone SB, Undem BJ. Vagal afferent innervation of the airways in health and disease. Physiol Rev. 2016;96(3):975–1024.
Mazzone SB, et al. Transcriptional profiling of individual airway projecting vagal sensory neurons. Mol Neurobiol. 2020;57(2):949–63.
Mazzone SB, et al. Modulation of vagal sensory neurons via high mobility group Box-1 and receptor for advanced glycation end products: implications for respiratory viral infections. Front Physiol. 2021;12:744812.
Meinhardt J, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24(2):168–75.
Mutoh T, Joad JP, Bonham AC. Chronic passive cigarette smoke exposure augments bronchopulmonary C-fibre inputs to nucleus tractus solitarii neurones and reflex output in young guinea-pigs. J Physiol. 2000;523 Pt 1(Pt 1):223–33.
Patil MJ, et al. Acute activation of bronchopulmonary vagal nociceptors by type I interferons. J Physiol. 2020;598(23):5541–54.
Rey-Jurado E, et al. Contribution of NKT cells to the immune response and pathogenesis triggered by respiratory viruses. Virulence. 2020;11(1):580–93.
Rhea EM, et al. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2021;24(3):368–78.
Rochlitzer S, et al. The neuropeptide calcitonin gene-related peptide affects allergic airway inflammation by modulating dendritic cell function. Clin Exp Allergy. 2011;41(11):1609–21.
Ruisanchez-Nieva A, et al. Influenza-associated seizures in healthy adults: report of 3 cases. Epilepsy Behav Case Reports. 2017;8:12–3.
Ryabkova VA, Churilov LP, Shoenfeld Y. Influenza infection, SARS, MERS and COVID-19: Cytokine storm - The common denominator and the lessons to be learned. Clin Immunol (Orlando, Fla.). 2021;223:108652.
Schmidt ME, Varga SM. The CD8 T cell response to respiratory virus infections. Front Immunol. 2018;9:678.
Shapiro CO, et al. Airway sensory nerve density is increased in chronic cough. Am J Respir Crit Care Med. 2021;203(3):348–55.
Shiers S, et al. ACE2 and SCARF expression in human dorsal root ganglion nociceptors: implications for SARS-CoV-2 virus neurological effects. Pain. 2020;161(11):2494–501.
Shinya K, et al. Avian influenza virus intranasally inoculated infects the central nervous system of mice through the general visceral afferent nerve. Arch Virol. 2000;145(1):187–95.
Siracusa R, Fusco R, Cuzzocrea S. Astrocytes: role and functions in brain pathologies. Front Pharmacol. 2019;10:1114.
Song WJ, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med. 2021;9(5):533–44.
Troy NM, Bosco A. Respiratory viral infections and host responses; insights from genomics. Respir Res. 2016;17(1):156.
Undem BJ, et al. Neural dysfunction following respiratory viral infection as a cause of chronic cough hypersensitivity. Pulm Pharmacol Ther. 2015;33:52–6.
Verzele NAJ, et al. The impact of influenza pulmonary infection and inflammation on vagal bronchopulmonary sensory neurons. FASEB J. 2021;35(3):e21320.
Wang J, et al. Distinct and common expression of receptors for inflammatory mediators in vagal nodose versus jugular capsaicin-sensitive/TRPV1-positive neurons detected by low input RNA sequencing. PLoS One. 2017;12(10):e0185985.
Wang H, et al. Increased hypothalamic microglial activation after viral-induced pneumococcal lung infection is associated with excess serum amyloid A production. J Neuroinflammation. 2018;15(1):200.
Wong JJM, et al. Insights into the immuno-pathogenesis of acute respiratory distress syndrome. Ann Transl Med. 2019;7(19):504.
Yang H, et al. HMGB1 released from nociceptors mediates inflammation. Proc Natl Acad Sci U S A. 2021;118(33):e2102034118.
Yoo JK, et al. Viral infection of the lung: host response and sequelae. J Allergy Clin Immunol. 2013;132(6):1263–76. quiz 1277
Zubair AS, et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–27.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Verzele, N.A.J., Short, K.R., Mazzone, S.B., McGovern, A.E. (2023). Vagal Neuroinflammation Accompanying Respiratory Viral Infection: An Overview of Mechanisms and Possible Clinical Significance. In: Brierley, S.M., Spencer, N.J. (eds) Visceral Pain. Springer, Cham. https://doi.org/10.1007/978-3-031-25702-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-25702-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25701-8
Online ISBN: 978-3-031-25702-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)