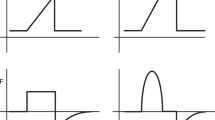Abstract
Recently, large cohort studies demonstrated that inadequate ventilation may contribute to an increase in perioperative morbidity and mortality in neonates. Various ventilation strategies were developed to improve lung ventilation with a “protective” and “open-lung” strategy to optimize functional residual capacity and prevent ventilation-induced lung injury. This chapter reviews the different ventilation modes that meet the ventilation requirements of the neonate according to the physiological characteristics of the respiratory system and the fluctuations in lung compliance. Hints will be provided for the selection and application of the best ventilation strategy that optimizes lung volume and guarantees adequate tissue oxygenation without augmenting lung stress and strain, two main contributors to lung injury, particularly in the immature lung. In addition, the potential benefits of noninvasive ventilation, particularly high flow nasal oxygenation, will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Recently, large cohort studies demonstrated that ventilation issues increased perioperative morbidity and mortality in neonates [1, 2]. Inadequate ventilation, manifested as either hypo- or hyperventilation, had respiratory and systemic consequences that may have contributed to morbidity and mortality [3, 4]. Strategies were developed to improve lung ventilation with a “protective” and “open-lung” strategy to optimize functional residual capacity (FRC) and prevent ventilation-induced lung injury (VILI) and bronchopulmonary dysplasia (BPD). Clinicians have since become aware of the potential harm of excessive hyperventilation in neonates because of large tidal volumes (Vt) that overdistend alveoli, increase wall shear stress, and liberate pro-inflammatory cytokines, which are the main features of the so-called VILI [5, 6]. Moreover, the resultant hypocapnia that results from extreme hyperventilation induces cerebral vasoconstriction and may promote the development of cystic periventricular leukomalacia [7]. Alternately, suboptimal Vt may result in poor gas exchange and hypercapnia that potentially increases the risk of intraventricular hemorrhage (IVH) [8]. Thus, several different ventilation modes are now available based on the application of volume guarantee modes to optimize tidal volume and meet the ventilation requirements of the neonate according to the physiological characteristics of the respiratory system and the fluctuations in lung compliance. Despite these new developments in ventilation, there is no evidence of the superiority of any one of these new ventilation strategies in terms of neonatal pulmonary and neurodevelopmental outcomes. Recently, there has been growing interest in the use of noninvasive ventilation in neonates, although evidence of its beneficial effects on both the lungs and the brain has been elusive. Whatever ventilation strategy is used, clinicians should be reminded that real-time pulmonary monitoring is essential to adapt the ventilation strategy to sudden changes in the mechanical properties of the lung that often occur during surgery. This chapter reviews the pulmonary indices and function in the neonate, describes the different ventilation modes available, and highlights the importance of using a protective and open-lung ventilation strategy.
Respiratory System
Three specific physiological characteristics distinguish the respiratory system in the neonate. First, the chest wall, which is made up of the intercostal muscles and ribs, is highly compliant in the neonate because the ribs are horizontally oriented and cartilaginous (non-ossified), giving the infant’s thorax a cylindrical shape of the rather than the elliptical shape seen in older children. Moreover, the effectiveness of the intercostal muscles to augment and support the chest wall and minimize chest wall distortion during inspiration is limited, contributing less to the developing tidal volume in neonates compared with older children. The main muscle that is responsible to develop the tidal volume in neonates is the diaphragm. However, in neonates, the diaphragm has less muscle mass, fewer high-endurance Type 1 twitch fibers, and inserts more horizontally than in older children [9]. Thus, in neonates with respiratory distress and increased oxygen requirements, when the diaphragm contracts, the lower rib cage moves inwards rather than downwards as seen in older children. In aggregate, the chest wall characteristics limit the ability of the neonate to increase alveolar ventilation efficiently and effectively in the face of stress. Thus, the neonate will be predisposed to early fatigue [10]. In the preterm neonate, these effects are even more extreme with a very low percentage of slow-twitch, high oxidative fibers that present as poorly developed ventilatory muscles, even less mature than those in term neonates [9].
The second important feature in the neonate that limits their ability to respond to increased ventilatory demand is the presence of the so-called “stiff lung.” This latter term relates to the increased static elastic recoil of the lung. The elastic recoil is determined by the elastic fibers within the lung and by the surface tension force generated at the air–liquid interface within the alveoli [11]. Surfactant reduces surface tension and maintains the patency of the terminal airways, independent of the alveolar diameter [12]. The high elastin/collagen ratio in neonates predisposes the lungs to collapse. Furthermore, the very compliant chest wall presents little resistance to hold the lungs open, resulting in a much smaller resting volume at end-expiration. The forces that favor the collapse of alveoli are opposed by those that prevent the lung from collapsing. The net effect of these opposing forces corresponds to the functional residual capacity (FRC) and represents the resting volume of the respiratory system. Consequently, even after externally delivered, effective lung surfactant, the FRC in the neonate is relatively small, and even smaller in the preterm neonate. In the latter age group, respiratory distress syndrome arises from a deficiency in surfactant, which causes the terminal airways to collapse. These changes may lead to atelectasis, loss of lung volume, and hypoxemia. This phenomenon is further exaggerated in the preterm neonate because collateral airflow does not occur at the level of the bronchoalveoli because the terminal airways are immature and alveolarization is disturbed [13, 14].
Finally, the neonatal respiratory system is challenged by the increased airway resistance that arises in the upper airways and larger conducting airways of the lungs. The resistance of the upper airways can be substantial in neonates, as the resistance to airflow in the narrow conducting airways down to the fifth bronchial division is inversely proportional to the fifth power of the reduction in the radius of the lumen as presented by the Fanning equation for turbulent flow (Reynold’s number >2100) (e.g., if the radius of the upper airway decreases by 50%, the resistance to gas flow increases by 32-fold). The radius of the airway may decrease substantially due to thickened mucous membranes arising from an inflammatory disorder, infection, or secretions. Thus, the radius of the airway is the most important determinant of the resistance of the upper airway. However, beyond the fifth bronchial division, the rapid increase in the cross-sectional area of the airways decreases the velocity of gas flow and resistance to flow. With the decrease in gas flow, the Reynold’s number decreases to <1800 and the flow becomes laminar. In laminar flow, the resistance is inversely proportional to the fourth power of the decrease in radius (Poiseuille’s equation for laminar flow). Furthermore, airway resistance decreases as the child grows in height [15, 16]. In neonates, airway resistance may also increase from airway closure and the loss in lung volume. One should be aware that when looking at the total respiratory system resistance, the tracheal tube combined with the resistance from the forces generated by the viscoelastic component of the lung account for more than two-thirds of the total resistance of the airway. In neonates, the use of tracheal tubes with small diameters increase the respiratory resistance, and the relatively high elastic recoil of the lung may further exacerbate the resistive component of the respiratory system [15]. Thus, when ventilating the lungs in a neonate, the peak pressure applied should overcome both the frictional forces that result from the airway resistances as well as those from both the tissue elastic recoil and the chest compliance. Many clinical conditions observed in neonates may alter the effectiveness of ventilation, jeopardizing our ability to maintain normocapnia and adequate oxygenation. Accordingly, positive pressure ventilation may become challenging in the presence of decreased lung compliance such as that seen with surfactant deficiency or congenital diaphragmatic hernia, which result in loss of lung volume. Other congenital or acquired diseases (tracheomalacia, bronchial infection, and inflammation) may increase resistance to airflow and require specific ventilation modes to avoid very high intratracheal pressures that may contribute to VILI.
Key Points
-
The specificity of the ventilatory muscles and the increased airway resistance contribute to respiratory fatigue in neonates.
-
The viscoelastic feature of the neonatal lung is the main determinant for optimal ventilation.
-
The surfactant plays a key role for alveolar stability and prevention of ventilation heterogeneity.
Ventilation Modes
A better knowledge of the neonatal respiratory pathophysiology as well as the advances obtained in ventilator technology have led to various complex ventilation modes, the usefulness of which has not yet been adequately proved primarily because of the lack of randomized trials in the neonates. Although the varied ventilation modes used routinely in the neonatal intensive care unit (NICU) have confusing terminology, many are simply not available on the anesthesia ventilators to deliver inhalation agents during surgery. However, the current ventilation modes can be distinguished based on whether they are volumetric (e.g., flow generator), barometric (the pressure generator), or a combination of both pressure and flow generators (dual modes). New ventilators in anesthetic workstations allow the child to trigger the onset of the ventilator cycle, which provides partially controlled or support ventilation modes.
Pressure-Controlled Ventilation
This mode is the most popular ventilation strategy in neonates and is often recommended for use in clinical practice. The basic and most frequently applied mode in NICU is the time-cycled, pressure-limited ventilation (also called intermittent positive-pressure ventilation (IPPV)), and is also available with most anesthesia ventilators where it is termed pressure-controlled ventilation (PCV). The decelerating flow and the limited and constant inspiratory pressure that characterize this ventilation mode explain the reduced peak inspiratory pressure, which is accompanied by the reduced tracheal and alveolar pressures. This mode compensates for a potential leak around uncuffed tracheal tubes with leaks, still routinely used during anesthesia in neonates [17]. The combination of decelerating flow and constant airway pressure over time facilitates the equilibration of tracheal and alveolar pressures. Evidence suggests this mode improves ventilation distribution, and thereby gas exchange [18]. However, when applying PCV, three main factors are integral to determining the tidal volume: (i) the pressure gradient between the maximum inspiratory set pressure (peak inspiratory pressure (PIP)) and the positive end-expiratory pressure (PEEP), which defines the driving pressure, (ii) the inspiratory time (Ti) that may depend and/or determine the ratio between the inspiratory and expiratory times as well as the respiration rate, and (iii) the time constant to equilibrate the airways and alveoli pressures, which is a function of the total respiratory system compliance and resistance. Therefore, when setting the PCV mode, the operator should (i) select the Ti and expiratory times (Te) to determine the respiration rate close to the child’s physiological rate, (ii) adjust the peak inspiratory pressure (PIP) and the PEEP, (iii) select the inspiratory flow rate, and (iv) select the inspired concentration of oxygen. Although ventilating with a short or extended Ti does not affect the incidence of chronic pulmonary disease (CPD) in neonates, a short Ti may decrease the risk of an air leak and mortality [19]. The normal physiological Ti in neonates is between 0.35 and 0.5 s, which dictates the initial setting for Ti with a higher Te. This helps to establish the initial respiration rate. However, under anesthesia both Ti and PEEP can be increased in order to increase the mean airway pressure and thus tidal volume and to recruit alveoli [20]. At the same time, the higher Ti may also jeopardize venous return and cardiac output. Alternately, shortening Ti during weaning may help to synchronize the neonate’s respiration with the ventilator and is more advantageous in neonates. To overcome this limitation, synchronized ventilation with the neonate triggering the onset of ventilation is currently the common mode used in NICUs. Among the different triggering techniques that have been developed, flow triggering via a flow sensor interposed between the tracheal tube and the ventilator’s connection is the most sensitive [21] and is therefore routinely used in clinical practice. Synchronized intermittent mandatory ventilation (SIMV) pressure control mode and assist-control (AC) modes have been introduced in the intensive care settings to synchronize the respiratory rate with the child’s breathing (SIMV) or by assisting each breath using positive-pressure ventilation, but with a “control” of a minimum number of ventilator cycles (AC). Nonetheless, if the child breathes very frequently, the ventilator assists all of the triggered breaths by applying the initial Ti, which will decrease the Te and may lead to air trapping by reducing the time required to adequately exhale. This risk is also observed when using pressure support ventilation (PSV), a mode that has been introduced on almost all new anesthesia ventilators. In the PSV mode, the child’s breathing effort and the changes in the airflow together initiate and terminate the ventilator assistance. An important feature of this mode is that the child determines the Ti. Depending on the type of ventilator, expiration begins when the inspiratory flow level decreases to between 10 and 25% of the maximum inspiratory flow [22]. This mode is of interest to anesthesiologists. This ventilator mode has been used in older children to reduce the work of breathing (WOB) associated with spontaneous breathing and to counteract the greater resistance due to small tracheal tubes. Because of the greater risk of patient–ventilator asynchrony in the ill neonate whose respiration rate is very rapid, PSV may increase oxygen consumption and generate possible ineffective ventilatory efforts. Another novel technique called proportional assist ventilation (PAV) has been developed to prevent such phenomena, offering to further reduce the WOB. In this mode, the neonate controls the rate of lung inflation and thus the peak inspiratory pressure, which is proportional to the neonate’s efforts [23]. Since the pressure applied depends on the inspiratory flow generated by the neonate, this ventilation mode assumes that the neonate is not hypopneic and that there is no leak around the tracheal tube [20]. However, this ventilation mode, which is not available in anesthesia workstations, is known as the airway pressure release ventilation (APRV). This mode is based on the combination of a large continuous positive airway pressure and brief releases of the pressure, which are short enough to generate auto-positive end-expiratory pressure [24]. This mode generates large intratracheal pressures, but is advantageous when the neonate is breathing spontaneously throughout the APRV cycle. Although its effectiveness has not been elucidated [25], APRV has been associated with better cardiopulmonary interactions and improved lung perfusion in infants after cardiac surgery [26]. Of more widespread interest is the neurally adjusted ventilator assist (NAVA), which relies on the child’s respiratory control and diaphragmatic activity [27]. The inspiratory effort is detected via bipolar electrodes mounted on a nasogastric feeding tube and positioned at the level of the diaphragm. The level of ventilatory support is then proportional to the inspiratory effort. Even though this mode is unaffected by a leak around the tracheal tube, its utility is still not well-defined in neonates, especially in preterm infants with immature control of the ventilation. A very recent prospective crossover study demonstrated that NAVA is associated with improved patient–ventilator synchrony and a reduced peak airway pressure in comparison with PSV [28]. More recently, new modes of ventilation that were based on individualized variable ventilation experimentally promoted lung protection while providing adequate gas exchange both in normal and injured lungs [29, 30]. These new ventilation modalities based on the physiological variable ventilation in neonates could be beneficial during prolonged ventilation in the absence of a respiratory drive.
Volume-Controlled Ventilation
The major disadvantage of pressure-limited ventilation is that the tidal volume (Vt) varies, because of changes in the compliance of the lung and resistance of the airways, which occur frequently in neonates during both anesthesia and surgery. As a consequence, some anesthesiologists continue to advocate for volume-controlled ventilation (VCV), a strategy that is based on the traditional delivery of a fixed predetermined Vt at a preestablished rate. This mode does not, however, take into account the maximum inspiratory pressure needed to deliver the desired Vt. Furthermore, high tracheal pressures may be encountered during surgery, especially when the lung compliance decreases and/or airway resistance increases to deliver the predetermined Vt. These high driving pressures may be limited either by setting the pressure pop-off valve or by determining the level of Ti in order to limit excessive peak inspiratory pressures during mechanical ventilation of low compliant lungs. Nonetheless, this mode of ventilation is deceptively misleading, particularly in neonates, as the preset Vt is not delivered to the lungs since this mode does not compensate for the tidal volume lost to the compression of the gas within the ventilator circuit, the compliance of the breathing circuit and the leak around the uncuffed tracheal tubes. It is imperative that ventilation is monitored using a capnogram and oxygenation using an oximeter.
Volume-Targeted Ventilation
The increasing awareness of the usefulness of direct control of the PIP and the benefit of ventilation with a small, constant Vt led clinicians to develop dual-mode ventilation strategies to guarantee Vt with a limited pressure [31]. Several ventilators and ventilation modes have since been developed and are routinely used in the intensive care to deliver a target or guaranteed Vt with an adjustable high-pressure limit to autoregulate the maximum inspiratory pressure (within the maximum limit set) or the Ti to guarantee the target Vt. These modes are known as volume-targeted ventilation modes and include all of the modes that guarantee Vt by adjusting the inflating pressure in response to the exhaled Vt, which is compared with the target Vt [32]. There is evidence that volume-targeted ventilation reduces adverse outcomes including severe intraventricular hemorrhage (Grade 3 or 4), periventricular leukomalacia, and CPD when ventilating the lungs of preterm neonates compared with pressure-limited ventilation [33,34,35]. New anesthetic workstations now include ventilators with these modes, although these findings have not been documented during anesthesia and surgery, despite the theoretical advantage to deliver the Vt at a reduced intratracheal pressure, particularly when compliance and resistance vary during surgery.
Among the new ventilation modes developed, the volume guarantee (VG) ventilation mode is a pressure-limited, volume-targeted time or flow-cycled ventilator that has garnered the interest of clinicians. The driving pressure for this mode is based on the difference between the exhaled and predetermined Vts. The software analyzes each breath individually to maintain constant Vts. This mode can be combined with other standard ventilation modes often used in neonates such as SIMV, AC, or PSV. In addition to setting the maximum PIP to limit lung injury, both the desired Vt (exhaled Vt) and Ti are set, which determine the duration of insufflation. These ventilator characteristics are particularly appealing in preterm neonates who are weaned from spontaneous respiration since the inspiratory pressure is adjusted in real-time [31]. Other anesthesia workstations use the pressure-regulated volume control (PRVC) mode in which the gas flow rate is adjusted to generate an inspiratory pressure sufficient to deliver the targeted Vt [36]. Thus, this ventilation mode shares many similarities with pressure control modes in terms of the pressure and flow patterns but delivers the target Vt by adjusting the PIP based on the compliance of the lung. One study demonstrated that this ventilation mode is very effective in very low birth weight (VLBW) preterm infants as both the duration of mechanical ventilation and hemodynamic instability are less than those that occur with other ventilation modes [37, 38]. Furthermore, this mode is associated with lower peak intratracheal pressures and its impact on hemodynamics is less than with other modes [39]. When this mode is combined with other ventilator options that allow infants to breathe spontaneously with pressure support, it is called “auto mode,” which is currently built into some anesthesia workstations. To date, there have been no studies that document the advantages of this ventilation mode in neonates.
High-Frequency Ventilation
High-frequency ventilation (HFV) rapidly became a strategic and favorite ventilation mode in neonates with chronic and severe lung disease as ventilation is ensured by applying small tidal volumes at a MAP (mean airway pressure) that is higher than with conventional ventilation. This strategy is very effective in infants with severe respiratory failure since HFV improves the gas exchange by optimizing the lung volume while ventilating at lower proximal airway pressures, thereby avoiding lung damage [40]. The principle upon which HFV is based on the natural “resonant” frequency of the lung and that less pressure is required to move the gas into and out of the lungs at its resonant frequency, which is around 10 Hz (1 Hz = 60 bpm) in neonates and even greater in preterm infants. HFV improves the gas exchange by enhancing both the convection and the diffusion of respiratory gases. Different ventilators delivering HFV are available without evidence that one type of HFV ventilator is superior to another. The first ventilation modality was high-frequency jet ventilation (HFJV), a technique that has become well-established in anesthesia. This mode delivers quick bursts of gas (with very short Ti) at very high frequency (up to 600/min) combined with a constant gas flow that determines the level of PEEP. This technique required a specific tracheal tube but failed to prove its effectiveness in clinical practice as the neurological and respiratory outcomes in neonates were conflicting [41,42,43]. Another modality that used HFV is known as high-frequency flow interruption (HFFI), consisting of a continuous gas flow, which is delivered by a high-pressure gas source that is interrupted at a high frequency (up to 20 Hz) [44]. This technique also failed to become widely adopted as it offered no advantages in pulmonary outcomes and yielded a greater incidence of air leaks in preterm babies compared with traditional ventilation modes [45, 46]. The most frequently used ventilation mode currently is high-frequency oscillatory ventilation (HFOV). This mode is based on the presence of an electromagnetically driven piston or vibrating diaphragms that generate biphasic pressure waveforms at very high frequencies (up to 15 Hz). Thus, HFOV has both an active inspiratory phase and an active expiratory phase (by inducing a negative proximal airway pressure during exhalation). When using HFOV it is important to adjust the I/E ratio to avoid gas trapping that may occur as a result of the active exhalation part [22]. HFOV provides very small oscillatory tidal volumes that are superimposed on an adjustable MAP. HFOV offers a particular advantage when a large tidal volume strategy is required to maintain the FRC as HFOV maintains FRC with a smaller MAP compared with other modes of ventilation. Nevertheless, a recent meta-analysis failed to demonstrate any benefit of HFOV over conventional ventilation modes when it is used as a primary or rescue mode to ventilate infants with acute pulmonary dysfunction [47]. The incidence of chronic lung disease in preterm babies may be less if the lungs are ventilated with HFOV but the evidence is weak [47]. Nonetheless, HFOV may still have a great clinical benefit in the operating room, particularly to ventilate low compliant lungs in neonates with congenital diaphragmatic hernia or severe respiratory distress.
Continuous Positive Airway Pressure and Noninvasive Ventilation
Many neonates may benefit from noninvasive respiratory support that applies continuous positive airway pressure (CPAP) and/or the delivery of noninvasive ventilation (NIV). Nasal CPAP maintains the FRC by recruiting and maintaining a patent airway and inflated lungs [48]. It also decreases the WOB and reduces the frequency of apnea of prematurity [49]. Therefore, nasal CPAP has become a standard ventilation strategy to support the lungs in recently extubated preterm infants, as an alternative to tracheal intubation and ventilation, to support those experiencing significant apnea of prematurity and for those with respiratory distress soon after birth. Some newer systems also provide a phasic positive increase in pressure (pressure support or pressure-controlled) in addition to the CPAP and can be synchronized (SNIMV, synchronized nasal intermittent mandatory ventilation) or not (NIMV). During the past decade, the use of NIV in neonatal intensive care units for acute respiratory failure has been expanding and the factors that predict the successful use of NIV have recently been identified [50]. While meta-analyses have failed to demonstrate the benefit of NIV in the presence of respiratory distress syndrome [51], its ability to prevent extubation failure in neonates is well established [52]. Accordingly, the use of NIV to protect against the risk of reintubation during the first 72 h is the current evidence-based indication for NIV in neonates [48]. For this purpose, NIV is started after the minimal PEEP level is set to ~6 cmH2O and the PIP to 10–12 cmH2O.
CPAP can be delivered by one of two techniques: (i) A continuous flow and a device that varies the exhalation either by modifying the expiratory orifice size or by immersing the distal end into a water reservoir to a specific level, the depth of which defines the level of CPAP. This latter is known as bubble CPAP—the bubbles creating pressure oscillations that are transmitted to the airway opening. It has been suggested that this phenomenon may improve gas exchange by facilitating diffusion [53]. (ii) A variable flow that allows changes in the level of CPAP using nasal prongs, which redirect the exhaled gas out of the expiratory limb. The WOB with the variable-flow CPAP is less than that with the bubble CPAP [54]. Another system that is based on the variable-flow setting is the bi-level CPAP or BiPAP. BiPAP allows the child to trigger the inspiratory phase and to breathe between two levels of positive pressure, with some systems including an abdominal wall sensor to synchronize inspiration with the child’s efforts. The BiPAP system may be better at improving oxygenation and ventilation than the CPAP system in low birth weight (LBW) infants [55].
The application and successful use of CPAP and NIV rely on the airway interfaces and their ability to guarantee comfort and optimize the delivery of pressure to the airway. Of all the interfaces that provide CPAP, the bi-nasal prongs are superior [56]. Although bi-nasal prongs are associated with a greater incidence of nasal trauma in infants [22], leaks remain a major concern in NIV. Such leaks may reduce alveolar ventilation, impair child–ventilator synchrony, and increase nasal resistance.
Nasal High-Flow Oxygenation
In the last decade, nasal high-flow oxygenation (NHFO) has gained widespread popularity in several populations, particularly in neonates, to improve oxygenation and/or as an alternative to noninvasive ventilation. This method is based on the delivery of a mixture of air and oxygen via a nasal cannula at a high gas flow, matching or exceeding the child’s peak inspiratory flow. Compared with conventional low-flow (2 L/min) nasal cannulae, NHFO delivers greater gas flow rates, at 1–4 L/kg/min in neonates. Such large gas flows necessitate the prior heating and humidification of the gas mixture since the delivery of a cold, dry gas would desiccate the mucosa of the nasal cavity and the lower airways, leading to trauma and airway edema, as well as impairing mucociliary flow. The net result is to retain secretions [57]. Thus, this technique is also referred to as heated, humidified nasal high-flow, and accordingly, current devices integrate a heater–humidifier to deliver a gas mixture at a temperature of 37 °C saturated with water. The choice of the prongs (made of soft silicone) is of importance in neonates as a sufficient leak (20–50% of the nares’ internal diameter) should be allowed around to cannulae to both facilitate expiration as well as to prevent the accumulation of excessive end-expiratory pressures generated by the continuous flow [58].
Several mechanisms have been proposed to explain the improved oxygenation with NHFO. First, since the gas flows delivered by the NHFO match or exceed the peak inspiratory flow, and the nasopharyngeal dead space is constantly washed out by the gas mixture, room air entrainment and CO2 rebreathing are reduced. Consequently, the partial pressure of oxygen in the nasopharyngeal space is closer to the set FiO2 during NHFO compared with low-flow oxygenation, resulting in a greater diffusion gradient for oxygen between the upper airway and the alveoli. Secondly, NHFO creates positive airway pressure that increases airway patency and end-expiratory lung volume [59]. This pressure correlates linearly with the flow [58, 60, 61] and depends on the child’s weight as well as the size of the leak around the prongs [62]. Unlike CPAP or noninvasive ventilation, NHFO generates pressures that vary during both inspiration and expiration. Since airway pressures depend on both the direction and the magnitude of the ventilation flow relative to the delivered flow, pressures are minimized at end-inspiration and maximized at mid-expiration, with end-expiratory pressures decrease in between the two readings [63]. In preterm infants, gas flows of 2–8 L/min delivered by three different NHFO devices resulted in end-expiratory pharyngeal pressures of 2–6 cmH2O, with a mean increase of 0.5 cmH2O for each 1 L/min increase over 2 L/min [62, 64], albeit with large variance. Although there is no clear consensus, mouth opening did not affect the pharyngeal pressures in neonates and infants, unlike in adults [60, 62, 65, 66].
Finally, irrespective of the gas flows being between 2 and 8 L/min, NHFO decreased the WOB as evidenced by transcutaneous diaphragmatic electromyography and respiratory inductance plethysmography [67, 68]. Decreased airway resistance due to the splinting effect of positive pressure, decreased minute ventilation due to dead space washout, and decreased metabolic demand due to the heating and humidification are all plausible mechanisms that individually or in aggregate decrease the WOB.
In spontaneously breathing neonates [66] and preterm infants [62], NHFO improves oxygenation and elimination of CO2, although it failed to prevent hypercapnia in apneic children [69, 70]. Nonetheless, during the past decade, NHFO has been increasingly used for apneic oxygenation during intubation and airway procedures to prevent or delay oxygen desaturation [69,70,71,72]. In neonates and infants aged 0–6 months, NHFO increased the mean time to desaturate to an SpO2 of 92% from 109 s to 196 s [70]. The improved oxygenation during NHFO is due to pharyngeal dead space washout, greater achievable FiO2, airway splinting effect, and maintenance of the FRC. However, the failure to augment CO2 elimination may be explained in part by the structural properties of the neonatal airways. A computer simulation suggested that a key mechanism that clears CO2 during NHFO is via cardiogenic oscillations augmenting alveolar ventilation [73]. However, in the apneic infant and child, cardiac oscillations are not augmented because airway resistance is increased and this might explain why NHFO failed to clear CO2 from the lungs [74]. This peculiarity may explain the similarity in oxygenation during apnea after administering 100% oxygen at 2 L/kg/min via NHFO and that via traditional low-flow cannulae at 0.2 L/kg/min [70].
In summary, NHFO may be considered an effective strategy to oxygenate the infant during airway procedures by prolonging the time available to intubate the trachea, particularly in the neonate, those with a difficult intubation [17] and those with limited oxygen reserves [70, 75]. It is essential to recognize that despite oxygenating the child, NHFO incompletely clears CO2 during apnea. Alternately, maintaining oxygenation via a traditional low-flow cannula is a suitable and cost-effective method to routinely implement in clinical practice.
Key Points
-
There is a shift from controlled mechanical ventilation to combined modes with spontaneous ventilation to improve alveolar ventilation and cardiopulmonary interactions.
-
The pressure-regulated volume control mode meets the specific characteristics of neonatal respiratory physiology.
-
There is still no evidence for the advantage of one given mode over the other as clinical trials in neonates are sparse with large inhomogeneity.
Application of Ventilation Modes in the Operating Theatre
Despite the great advances in the development of new anesthetic ventilators, which include a variety of modes of ventilation widely used in the operating room and intensive care setting, the benefits of these modes of ventilation during general anesthesia to improve clinical outcomes have not been forthcoming. In neonates, these ventilation modes contribute to “lung protective ventilation” by optimizing the distribution of ventilation in the presence of alveolar instability. Alveolar instability refers to the presence of both collapsed and overdistended alveoli in the lungs. The repetitive alveoli recruitment and collapse generated by positive pressure ventilation with each breath may cause excessive dynamic shear stress on the alveolar walls, while overdistensing adjacent alveoli produces a dynamic strain with each breath. As a consequence, a new concept was recently developed that promotes “specific tidal volume,” which is determined by the driving pressure determined by the inspiratory pressure over the PEEP level set on the ventilator. The goal is to optimize gas exchange while decreasing lung stress and strain. Thus, the most appropriate ventilation mode would be the one that maintains the smallest driving pressure as possible to meet the “lung protective ventilation” concept.
Therefore, the traditional mandatory VCV strategy is far from the ideal strategy to ventilate the lungs in the neonate because it connotes a constant flow that generates large driving pressures and fails to account for the compressible volume of the breathing circuit and the potential gas leak around uncuffed tracheal tubes. The constant flow characterizing the VCV mode induces large PIPs with less time for equilibration between the airway pressure (Paw) and the alveolar pressure (Palv), known as the time-constant. Moreover, the compressible volume is an important issue in the neonate and it is important to understand whether the ventilator corrects for this compressible volume in the case of a fixed Vt setting. Most modern ventilators available in anesthesia currently correct for the compressible volume when the ventilator is checked during the machine check. If there is a change of circuits between infants, it is important to recheck the compressible volume as the neonate’s Vt may be of a similar order of magnitude as the compressible volume. It is also important to ensure that the tidal volumes displayed on the workstation monitor are accurate by performing the pre-use anesthesia workstation testing (which compensates for circuit compliance) with the circuit tubing in either the compressed or expanded state, depending on how the circuit will be used during anesthesia [76]. For a ventilator that predates software that corrects for compressible volume or limits compensation if the pressure exceeds 30 cmH2O [77], it is essential to account for the compressible volume when setting the Vt. For example, if the compressible volume reaches 1 mL/cmH2O, and the delivered Vt is 7 mL/kg in a 4 kg neonate, the ventilator may generate a PIP of 25 cmH2O during ventilation, yielding a compressible volume of 25 mL. The preset Vt should be adjusted to almost 13 mL/kg since 50% or more may be lost due to the compressible volume (not taking into account the dead space and the potential leak). Thus, the use of the VCV mode requires that the overpressure valve be set to protect the lung from any dangerous increase in peak inspiratory pressure that may result from changes in lung compliance during the surgery. In neonates, particularly those with less compliant lungs, we believe that PCV is the preferred Te mode of ventilation in neonates.
The decelerating flow pattern that characterizes the PCV mode offers a limited and constant inspiratory pressure with a plateau pressure that is reached much faster, but at a lower PIP and thus driving pressure than VCV. This mode improves the distribution of ventilation and decreases the intrapulmonary shunt, thereby improving oxygenation. Furthermore, the PCV mode compensates for the presence of a gas leak around tracheal tubes. Although PCV better satisfies the criteria required by the protective-ventilation strategy, Vt will fluctuate in this mode, particularly if lung compliance decreases or respiratory resistance increases during surgery. During PCV, Vt depends on three components: (i) the time constant, (ii) the pressure gradient between the maximal set peak pressure and the PEEP level, and (iii) Ti, which is determined by the respiration rate and the I/E ratio. The time constant is characterized by the mechanical properties of the respiratory system, which include the total respiratory system compliance (Crs) and resistance (Rrs). Application of the time constant concept to the inspiratory phase implies that Ti is set to allow sufficient time to equilibrate the pressures between the airways and alveoli. Accordingly, if Rrs increases and/or Crs decreases, the time to equilibration will increase. Further, it is important to allow sufficient time to complete deflation, as the expiratory flow has an exponential decelerating profile that can take up to 3 to 4 time constants of the respiratory system to completely deflate (see Fig. 6.1).
Flow vs time curve demonstrating the “time constant concept” with decelerating flows. Ti should be of sufficient duration to permit the inspiratory flow to return to 0. Similarly, Te should be of sufficient duration to preclude intrinsic PEEP. Ti Inspiratory time, Te Expiratory time, PEEP Positive end-expiratory pressure
Finally, it is important to note that anesthetic ventilators that are currently available vary in the maximum insufflation flow they can generate. Thus, the Vt generated by a given pressure level may vary from one ventilator to another [78]. Considering Ti is short and the respiratory rate is rapid in neonates, it is important to note that the physiological Ti does not exceed 0.5 s. However, in an anesthetized neonate, who is paralyzed with a muscle relaxant, one may exceed this Ti to recruit alveoli. At the same time, increasing the Ti may also impact hemodynamic variables and lead to asynchrony if the PSV mode is selected. Therefore, the initial ventilator settings in PCV mode should include the pressure gradient established with a PEEP of 5 cmH2O and a positive inspiratory pressure over PEEP of 10 cmH2O. The respiration rate will be determined by initially selecting a Ti up to 0.6 s. The choice of both the Ti and the pressure gradient will depend on the compliance and the resistance of the respiratory system and the blood gas analysis. It is also important to consider Te, particularly in neonates with obstructive lung disease, such as bronchopulmonary dysplasia, where the expiratory time constant should permit sufficient time to exhale to minimize auto-PEEP and hyperinflation.
PSV mode has become popular in routine practice in pediatric anesthesia as it maintains diaphragmatic activity during general anesthesia, which decreases ventilation–perfusion mismatch. New ventilators include a flow trigger that is very sensitive to minimal variations in flow rate (similar to those observed with intensive care ventilators) and can therefore be applied in neonates since a minimal WOB is required to initiate an inspiratory effort [79]. The pressure support is based on a decelerating flow, which generates a fixed insufflation pressure; thus, Vt may vary with the neonate’s inspiratory efforts, the level of pressure support, and the mechanical characteristics of the lung as well. Currently, few modern anesthetic ventilators have cycling adaptation (transition from inspiration and expiration). In the case of ventilators with fixed cycling, insufflation will stop when the flow is less than 25% of the maximum inspiratory flow. This limitation may have a negative impact on the neonate with obstructive disease for which cycling should occur later [80]. Although studies using the PSV mode in neonates have not been forthcoming, this mode can be applied during anesthesia in clinical practice to compensate for the increase in the WOB, which is particularly great in neonates. For instance, the application of pressure support of 5 cmH2O in addition to PEEP at induction will maintain patent airways, thus offsetting the WOB and optimizing the gas exchange during inhalation inductions. During the maintenance phase of anesthesia, more pressure support may be necessary (up to 10 cmH2O) to overcome the resistance from the tracheal tube and the anesthesia circuit, as well as to guarantee an optimal Vt for gas exchange [81]. After anesthesia, the PSV mode allows for a smoother recovery, emergence, and weaning from the ventilator. In all cases, it is important to set a minimum respiration rate to deliver breaths in a pressure control mode even during an apnea. In such a case, PCV adapts Ti to avoid asynchrony with the ventilator that may increase the WOB. Lastly, some anesthetic ventilators allow changes in the pressure slope (the time to achieve pressure support). By increasing this time (and thereby decreasing the pressure slope), we can limit the auto-trigger activated by the cardiac activity, which is frequently observed in neonates at low trigger threshold [82]. To avoid this phenomenon, it is also possible to increase the trigger threshold with the risk of increasing the WOB.
Recently, the PRVC mode with auto mode (auto-flow) was introduced in new anesthetic ventilators in the operating theatre. VCV with a decelerating flow in combination with synchronization with a pressure support of the spontaneous ventilation offers the advantages of pressure modes with a guaranteed minimum Vt. Theoretically, this mode overcomes the inconvenience of both PCV and VCV and may offer an enormous advantage in anesthetized neonates, particularly when lung compliance decreases abruptly during surgery (i.e., laparoscopy, or abdominal or thoracic surgery). To determine the initial guaranteed volume, the child’s lungs should initially be managed using pressure control and extract the tidal volume that ensures adequate alveolar ventilation. Thereafter, the determined tidal volume can be considered as the targeted tidal volume in PRVC mode while adapting the ventilator setting according to PCV mode and by applying a maximum inspiratory pressure of 30 cmH2O to protect the lungs from overpressure. Nonetheless, no data have been forthcoming regarding the use of this ventilation mode in the operating theatre, particularly in neonates and hence although this mode meets the physiological characteristics of the lung during surgery, its usehas remained anecdotal and based on the experience of different clinicians.
Key Points
-
Alveolar instability promotes ventilation heterogeneity with excessive shear stress and enhancement of dynamic strain at each breath.
-
Ventilation modes with decelerating flows provide a low driving pressure and thus exert less lung stress and strain.
-
Allowing spontaneous ventilation with pressure support as soon as possible improves ventilation homogeneity and cardiorespiratory hemodynamic interactions.
Ventilation Strategy in the Operating Theatre
Maintaining adequate ventilation during sedation or anesthesia is of particular importance in neonates, who are vulnerable to hypoxemia. Inadequate bag and mask ventilation during induction of anesthesia may result in insufficient alveolar ventilation, hypoxemia, hypercapnia, and gastric inflation and regurgitation of gastric contents with subsequent pulmonary aspiration. Furthermore, the accumulation of air within the stomach may further compromise respiratory function and gas exchange in neonates whose functional residual capacity is less than its closing volume. While the use of an accessory circuit such as a modified T-piece breathing system (i.e., Jackson Rees) continues to be advocated as the best system to ensure both adequate ventilation and maintenance of FRC [83, 84], it is important to monitor airway pressure during manual ventilation to avoid both excessive peak inflation pressure and gastric air insufflation. The development of low resistance circle systems rendered their use in routine practice popular [85, 86], although they may be less effective in applying a continuous positive airway pressure (CPAP) at end-expiration to maintain upper airway patency and FRC. In this context, applying gentle mask ventilation with CPAP to maintain a mean airway pressure around 5–10 cmH2O becomes an increasingly popular ventilation strategy at induction of anesthesia, even in the presence of a full stomach to avoid airway collapse and to ensure adequate oxygenation [87, 88]. Alternatively, using PSV at induction of anesthesia will ensure an adequate CPAP level to maintain the FRC and a low-pressure support to optimize alveolar ventilation. This so-called controlled induction technique meets the criteria for an “open-lung strategy,” which should be considered at all stages when ventilating a neonate’s lungs in the operating theatre.
The open-lung strategy primarily addresses concerns about atelectasis and the resultant ventilation inhomogeneity observed during general anesthesia, which can significantly impair pulmonary gas exchange. First, the physiological characteristics of the chest wall (large compliance) and the lung (increased static elastic recoil pressure) in neonates promote airway closure and a decrease in FRC. The decreased ventilation associated with general anesthetics and inactivation of the intercostal muscle activity associated with the cranial shift of the diaphragm are also responsible, in part, for the lungs collapsing and atelectasis formation. The latter is enhanced by the resorption of alveolar gas when an excessive FiO2 is used. Thus, this “open-lung strategy” requires that recruitment maneuvers should be regularly performed, particularly after the loss of positive end-expiratory level (at zero PEEP level). Such recruitment can be achieved by applying a vital capacity maneuver (or twice the Vt, but limiting the maximum inspiratory pressure to 30 cmH2O in normal lungs) after induction, after disconnection and suction, and thereafter every 30 min during the anesthetic procedure [89]. However, a minimum PEEP level of 5 cmH2O is required to maintain the recruitment of the distal airways [90], while high concentrations of oxygen should be avoided as tolerated. Nonetheless, an increased PEEP may be required in the presence of poorly compliant and atelectatic lungs to maintain adequate alveolar recruitment.
Beyond this open-lung strategy, it is crucial to apply “protective ventilation” in an attempt to protect against VILI, which has been associated with an increase in lung stress and strain [91]. Ventilation with small Vt at optimal FRC is therefore essential in the operating theatre as well. Optimizing the level of PEEP will increase the lung volume, while adapting Ti and Te will guarantee adequate lung inflation and deflation, respectively, especially if Ti/Te is adjustable based on estimations of the time constants. This “protective ventilation” strategy should account for the continuous changes in respiratory compliance of the neonate undergoing surgery and the ventilation inhomogeneity.
This ventilation strategy may lead to mild hypercapnia, 6–6.5 kPa (61–67 cmH2O), which is regarded as safe in the absence of high intracerebral pressure and pulmonary hypertension. Furthermore, mild hypercapnia improves both the cerebral oxygen saturation and the subcutaneous tissue oxygenation [92]. Fetal hemoglobin has a greater affinity for oxygen compared with adult hemoglobin, thus explaining the leftward shift of the oxyhemoglobin dissociation curve in the neonate and the resultant reduced P50. Further reduction in the P50 may occur with hyperventilation (Bohr effect), thereby further decreasing tissue oxygen delivery [93]. Moreover, hyperventilation and/or the application of large tidal volumes may lead to hypocapnia and thus, cerebral vasoconstriction, a major risk factor that predisposes to cerebral ischemia and possible neurocognitive impairment in young infants [4].
Therefore, in an attempt to meet the physiological requirements stated above, it is advisable to always consider the pressure-regulated volume control mode. This mode delivers a constant tidal volume with the smallest inspiratory flow and driving pressure and prevents perturbations in the carbon dioxide tension. Studies demonstrated a greater incidence of cerebral insults in infants with extreme carbon dioxide, partial pressures less than 4.6 kPa (47 cmH2O) or greater than 6.6 kPa (67 cmH2O) [94,95,96]. The required tidal volume can be determined first by setting the pressure control mode with a pressure gradient between a PEEP level of 5 cmH2O and a peak inspiratory pressure of 10 cmH2O as well as a Ti of 0.6 s. These variables will dictate the respiration rate and the primary tidal volume. Then, based on the end-tidal carbon dioxide tension, the driving pressure should be adjusted (<13 cmH2O) to obtain the targeted values. In a second step, the resultant tidal volume will be maintained as ventilation is switched to PRVC without changing other settings.
Titrating the inspired oxygen fraction (FiO2) in preterm and term neonates is not straightforward. It is important to avoid excessive inspired concentrations as the resulting oxidative stress contributes to potential major organ injuries including effects in the lung, brain, and eyes [97]. In addition, given the large affinity of fetal hemoglobin for O2 and the shape of the oxyhemoglobin dissociation curve, an arterial oxygen saturation (SaO2) >92% may not accurately correlate with the arterial partial pressure of oxygen (PaO2). Hence, small fluctuations in the SaO2 may reflect very large fluctuations in the PaO2 [98]. Knowing the particular harm that an excess of oxygen may cause in preterm and full-term neonates, the inspired oxygen fraction should be titrated to target a SaO2 between ~90 and ~94% [99, 100]. Setting a minimum FiO2 after induction of anesthesia would also benefit identifying the loss in lung volume and decrease in FRC, which are at the root of intraoperative hypoxemia in infants. Thus, when SaO2 decreases in a neonate or infant, ventilation/perfusion mismatch should be suspected. A recruitment maneuver at any FiO2 will re-establish an acceptable SaO2, which may be maintained with an adequate level of PEEP. Hence, it is prudent to maintain the FiO2 at ~30–35% during anesthesia in neonates to identify intraoperative alveolar closure as soon as it occurs and initiate a recruitment maneuver.
Key Points
-
Hypoxia and/or hypocapnia are the major burden during mechanical ventilation.
-
Pressure regulated volume control is the most appropriate mode in neonate as the inspiratory decelerating flow will adapt continuously to the changing respiratory compliance.
-
The inspired oxygen fraction should be titrated to avoid hyperoxia and to detect early onset of ventilation/perfusion mismatch.
Monitoring of Ventilation
Although real-time pulmonary monitoring is essential to interpret the changes in lung physiology that occur during mechanical ventilation in neonates, it is crucial to associate the information obtained from different waveforms displayed by the ventilators and the output on gas exchange and tissue oxygenation. Applying a protective open-lung ventilation strategy requires adaptation of the ventilator settings according to this real-time pulmonary monitoring. Most ventilators available in the operating theatre display continuous waveforms of pressure, volume, flow, and loops, as well as automatically derived respiratory mechanical variables. The classical pulmonary waveforms are represented by pressure, volume, and flow displayed versus time. The pressure and flow curves are specific for the ventilation mode used and thus, while displaying the pressure curve is essential during VCV (since pressure is the dependent variable), it is equally important to focus on the flow versus time curve in the PCV mode since the effectiveness of alveolar ventilation depends on the greatest extent on the flow waveform. This allows the anesthesiologist to detect: (i) an interruption in the inspiratory waveform, indicating insufficient time to equilibrate the alveolar and airway pressures, with the risk being inadequate lung inflation, and (ii) an incomplete deflation of the lung with the risk of auto-PEEP, overdistension of the lung, and an enhanced risk of barotrauma. Thus, in terms of the flow curve, it is essential to adjust both Ti and Te (either by changing the ratio or by decreasing the respiratory rate) to let the waveform reach the zero-flow state before transitioning to the next insufflation or exsufflation [101].
The pressure–volume and flow–volume loops afford an insight into the respiratory mechanics during mechanical ventilation, namely the respiratory system compliance and resistance. The flow–volume loop is very useful to detect changes in the inspiratory or expiratory resistances. For instance, increases in airway resistance are obvious in the flow–volume curve with a decrease in the expiratory flow peak, which is expressed by a concave expiration loop. Moreover, an incomplete flow–volume loop indicates an air leak, which can occur in neonates with uncuffed tracheal tubes are present. The dynamic pressure–volume (P–V) loop, which is displayed by the ventilator, describes the mechanical behavior of the respiratory system during inflation and deflation and includes the resistive and convective acceleration components of flow. Thus, the dynamic P–V curve provides essential information to track the dynamic trends of the respiratory system compliance (defined by the slope of the loop), as well as tidal volume. Although some information can be obtained from the curve to help determine the “best” PEEP, the beginning of the dynamic inspiration provides evidence on lung recruitment from tidal ventilation, independent of PEEP [102], particularly during PCV when the pressure remains constant. Conversely, when ventilating with a constant flow such as under VCV, the P–V loop may detect overdistension of the lung as evidenced by a change in the slope of the inspiratory P–V curve, namely the upper inflection point. The lower inflection point at the lower part of the loop corresponds to the beginning of an alveolar recruitment. This may provide insight into the importance of airway closure.
The automatically derived values displayed by the ventilator should be interpreted with some caution as the absolute values are global parameters including the whole respiratory system as well as the equipment (circuit, tracheal tube, filter, and so on) [103]. These values are often obtained using the interruption technique, which is based on the ratio of the pressure decrease due to the interruption of the inspiratory flow and that before the interruption. It is important to note that >40% of the values are related to the equipment itself and thus, clinicians should not rely on these absolute values to interpret physiological changes in the respiratory system itself.
An indirect monitor of ventilation recently introduced is near-infrared spectroscopy (NIRS), a noninvasive technique for monitoring tissue oxygenation and perfusion [104, 105]. NIRS indirectly reflects ventilation via perturbations in the cerebral perfusion. Oxygenation may be a consequence of a significant cardiorespiratory interaction due to the presence of large intrathoracic pressures, which decrease the total venous return and thus, cardiac output. The cerebrovascular reactivity to carbon dioxide, particularly in preterm and full-term neonates, is another major factor affecting cerebral perfusion and may indirectly indicate an inappropriate ventilator setting.
Key Points
-
Flow versus time curve and pressure–volume loop are essential to adapt the ventilation strategy.
-
The near-infrared spectroscopy should be considered as an important indirect monitoring of mechanical ventilation.
-
A decrease in oxygen regional saturation may be due to a decrease in cerebral perfusion as a consequence of decrease in cardiac output or vasoconstriction due to hypocapnia.
Conclusion
There is growing evidence of the benefit of applying an open and protective lung strategy in neonates. Over the past two decades, technological advances have introduced several new ventilation modes, which have undoubtedly advanced neonatal ventilation. Although the superiority of one ventilation mode over another in terms of neonatal pulmonary and neural outcomes has not been established, there is some evidence that pressure-regulated volume control may provide the most appropriate ventilation mode. However, the remaining challenge is to determine how best to mechanically ventilate the lungs based on respiratory physiology of the preterm and full-term neonate to optimize lung volume and guarantee adequate tissue oxygenation without augmenting lung stress and strain, which may induce lung injury (particularly in the immature lung) and lead to serious hemodynamic consequences with subsequent adverse neurological and metabolic outcomes.
References
Habre W, Disma N, Virag K, et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): a prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir Med. 2017;5:412–25.
Christensen RE, Haydar B, Voepel-Lewis TD. Pediatric cardiopulmonary arrest in the postanesthesia care unit, rare but preventable: analysis of data from wake up safe, the pediatric anesthesia quality improvement initiative. Anesth Analg. 2017;124:1231–6.
Bhananker SM, Ramamoorthy C, Geiduschek JM, et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg. 2007;105:344–50.
McCann ME, Lee JK, Inder T. Beyond anesthesia toxicity: anesthetic considerations to lessen the risk of neonatal neurological injury. Anesth Analg. 2019;129:1354–64.
Moloney ED, Griffiths MJ. Protective ventilation of patients with acute respiratory distress syndrome. Br J Anaesth. 2004;92:261–70.
Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323.
Wiswell TE, Graziani LJ, Kornhauser MS, et al. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high-frequency jet ventilation. Pediatrics. 1996;98:918–24.
Kaiser JR, Gauss CH, Pont MM, Williams DK. Hypercapnia during the first 3 days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. J Perinatol. 2006;26:279–85.
Keens TG, Bryan AC, Levison H, Ianuzzo CD. Developmental pattern of muscle fiber types in human ventilatory muscles. J Appl Physiol Respir Environ Exerc Physiol. 1978;44:909–13.
Muller N, Volgyesi G, Becker L, Bryan MH, Bryan AC. Diaphragmatic muscle tone. J Appl Physiol. 1979;47:279–84.
Nicolai T. The physiological basis of respiratory support. Paediatr Respir Rev. 2006;7:97–102.
Macklem PT, Proctor DF, Hogg JC. The stability of peripheral airways. Respir Physiol. 1970;8:191–203.
Hjalmarson O, Sandberg K. Abnormal lung function in healthy preterm infants. Am J Respir Crit Care Med. 2002;165:83–7.
Menkes H, Gardiner A, Gamsu G, Lempert J, Macklem PT. Influence of surface forces on collateral ventilation. J Appl Physiol. 1971;31:544–9.
Lanteri CJ, Sly PD. Changes in respiratory mechanics with age. J Appl Physiol. 1993;74:369–78.
Sly PD, Hayden MJ, Petak F, Hantos Z. Measurement of low-frequency respiratory impedance in infants. Am J Respir Crit Care Med. 1996;154:161–6.
Engelhardt T, Virag K, Veyckemans F, Habre W, Network AGotESoACT. Airway management in paediatric anaesthesia in Europe-insights from APRICOT (Anaesthesia Practice In Children Observational Trial): a prospective multicentre observational study in 261 hospitals in Europe. Br J Anaesth. 2018;121:66–75.
Munoz J, Guerrero JE, Escalante JL, Palomino R, De La Calle B. Pressure-controlled ventilation versus controlled mechanical ventilation with decelerating inspiratory flow. Crit Care Med. 1993;21:1143–8.
Kamlin CO, Davis PG. Long versus short inspiratory times in neonates receiving mechanical ventilation. Cochrane Database Syst Rev. 2004:CD004503.
Keszler M. State of the art in conventional mechanical ventilation. J Perinatol. 2009;29:262–75.
Dimitriou G, Greenough A, Cherian S. Comparison of airway pressure and airflow triggering systems using a single type of neonatal ventilator. Acta Paediatr. 2001;90:445–7.
Greenough A, Donn SM. Matching ventilatory support strategies to respiratory pathophysiology. Clin Perinatol. 2007;34(35-53):v–vi.
Schulze A, Rieger-Fackeldey E, Gerhardt T, Claure N, Everett R, Bancalari E. Randomized crossover comparison of proportional assist ventilation and patient-triggered ventilation in extremely low birth weight infants with evolving chronic lung disease. Neonatology. 2007;92:1–7.
Bein T, Wrigge H. Airway pressure release ventilation (APRV): do good things come to those who can wait? J Thorac Dis. 2018;10:667–9.
Jain SV, Kollisch-Singule M, Sadowitz B, et al. The 30-year evolution of airway pressure release ventilation (APRV). Intensive Care Med Exp. 2016;4:11.
Walsh MA, Merat M, La Rotta G, et al. Airway pressure release ventilation improves pulmonary blood flow in infants after cardiac surgery. Crit Care Med. 2011;39:2599–604.
Sinderby C, Beck J, Spahija J, et al. Inspiratory muscle unloading by neurally adjusted ventilatory assist during maximal inspiratory efforts in healthy subjects. Chest. 2007;131:711–7.
Breatnach C, Conlon NP, Stack M, Healy M, O’Hare BP. A prospective crossover comparison of neurally adjusted ventilatory assist and pressure-support ventilation in a pediatric and neonatal intensive care unit population. Pediatr Crit Care Med. 2010;11(1):7–11.
Walesa M, Bayat S, Albu G, Baudat A, Petak F, Habre W. Comparison between neurally-assisted, controlled, and physiologically variable ventilation in healthy rabbits. Br J Anaesth. 2018;121:918–27.
Fodor GH, Bayat S, Albu G, et al. Variable ventilation is equally effective as conventional pressure control ventilation for optimizing lung function in a rabbit model of ARDS. Front Physiol. 2019;10:803.
Keszler M, Abubakar KM. Volume guarantee ventilation. Clin Perinatol. 2007;34(107-16):vii.
Singh J, Sinha SK, Clarke P, Byrne S, Donn SM. Mechanical ventilation of very low birth weight infants: is volume or pressure a better target variable? J Pediatr. 2006;149:308–13.
Klingenberg C, Wheeler KI, McCallion N, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation in the neonate. Cochrane Database Syst Rev. 2017;(10):CD003666.pub4.
Wheeler KI, Schmolzer GM, Morley CJ, Davis PG. High-frequency ventilation with the Drager Babylog 8000plus: measuring the delivered frequency. Acta Paediatr. 100:67–70.
Peng W, Zhu H, Shi H, Liu E. Volume-targeted ventilation is more suitable than pressure-limited ventilation for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2014;99:F158–65.
Singh J, Sinha SK, Donn SM. Volume-targeted ventilation of newborns. Clin Perinatol. 2007;34, 93:–105, vii.
Piotrowski A, Sobala W, Kawczynski P. Patient-initiated, pressure-regulated, volume-controlled ventilation compared with intermittent mandatory ventilation in neonates: a prospective, randomised study. Intensive Care Med. 1997;23:975–81.
D’Angio CT, Chess PR, Kovacs SJ, et al. Pressure-regulated volume control ventilation vs synchronized intermittent mandatory ventilation for very low-birth-weight infants: a randomized controlled trial. Arch Pediatr Adolesc Med. 2005;159:868–75.
Kocis KC, Dekeon MK, Rosen HK, et al. Pressure-regulated volume control vs volume control ventilation in infants after surgery for congenital heart disease. Pediatr Cardiol. 2001;22:233–7.
Lampland AL, Mammel MC. The role of high-frequency ventilation in neonates: evidence-based recommendations. Clin Perinatol. 2007;34:129–44. viii
Wiswell TE, Graziani LJ, Kornhauser MS, et al. High-frequency jet ventilation in the early management of respiratory distress syndrome is associated with a greater risk for adverse outcomes. Pediatrics. 1996;98:1035–43.
Carlo WA, Siner B, Chatburn RL, Robertson S, Martin RJ. Early randomized intervention with high-frequency jet ventilation in respiratory distress syndrome. J Pediatr. 1990;117:765–70.
Keszler M, Modanlou HD, Brudno DS, et al. Multicenter controlled clinical trial of high-frequency jet ventilation in preterm infants with uncomplicated respiratory distress syndrome. Pediatrics. 1997;100:593–9.
Cronin JH. High frequency ventilator therapy for newborns. J Intensive Care Med. 1994;9:71–85.
Thome U, Kossel H, Lipowsky G, et al. Randomized comparison of high-frequency ventilation with high-rate intermittent positive pressure ventilation in preterm infants with respiratory failure. J Pediatr. 1999;135:39–46.
Craft AP, Bhandari V, Finer NN. The sy-fi study: a randomized prospective trial of synchronized intermittent mandatory ventilation versus a high-frequency flow interrupter in infants less than 1000 g. J Perinatol. 2003;23:14–9.
Cools F, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2015:CD000104.
Courtney SE, Barrington KJ. Continuous positive airway pressure and noninvasive ventilation. Clin Perinatol. 2007;34:73–92, vi.
Lemyre B, Davis PG, De Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database System Rev. 2017;292:CD003212. https://doi.org/10.1002/14651858.CD003212.pub3.
Bernet V, Hug MI, Frey B. Predictive factors for the success of noninvasive mask ventilation in infants and children with acute respiratory failure. Pediatr Crit Care Med. 2005;6:660–4.
Ho JJ, Subramaniam P, Sivakaanthan A, Davis PG. Early versus delayed continuous positive airway pressure (CPAP) for respiratory distress in preterm infants. Cochrane Database System Rev. 2020;10(10):CD002975. https://doi.org/10.1002/14651858.CD002975.pub2.
Subramaniam P, Ho JJ, Davis PG. Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database System Rev. 2016;(6):CD001243. https://doi.org/10.1002/14651858.CD001243.pub3.
Pillow JJ, Travadi JN. Bubble CPAP: is the noise important? An in vitro study. Pediatr Res. 2005;57:826–30.
Liptsen E, Aghai ZH, Pyon KH, et al. Work of breathing during nasal continuous positive airway pressure in preterm infants: a comparison of bubble vs variable-flow devices. J Perinatol. 2005;25:453–8.
Migliori C, Motta M, Angeli A, Chirico G. Nasal bilevel vs. continuous positive airway pressure in preterm infants. Pediatr Pulmonol. 2005;40:426–30.
De Paoli AG, Davis PG, Faber B, Morley CJ. Devices and pressure sources for administration of nasal continuous positive airway pressure (NCPAP) in preterm neonates. Cochrane Database Syst Rev. 2008:CD002977.
Cuquemelle E, Pham T, Papon JF, Louis B, Danin PE, Brochard L. Heated and humidified high-flow oxygen therapy reduces discomfort during hypoxemic respiratory failure. Respir Care. 2012;57:1571–7.
Lampland AL, Plumm B, Meyers PA, Worwa CT, Mammel MC. Observational study of humidified high-flow nasal cannula compared with nasal continuous positive airway pressure. J Pediatr. 2009;154:177–82.
Hough JL, Pham TM, Schibler A. Physiologic effect of high-flow nasal cannula in infants with bronchiolitis. Pediatr Crit Care Med. 2014;15:e214–9.
Kubicka ZJ, Limauro J, Darnall RA. Heated, humidified high-flow nasal cannula therapy: yet another way to deliver continuous positive airway pressure? Pediatrics. 2008;121:82–8.
Spence KL, Murphy D, Kilian C, McGonigle R, Kilani RA. High-flow nasal cannula as a device to provide continuous positive airway pressure in infants. J Perinatol. 2007;27:772–5.
Liew Z, Fenton AC, Harigopal S, Gopalakaje S, Brodlie M, O’Brien CJ. Physiological effects of high-flow nasal cannula therapy in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2020;105:87–93.
Parke RL, McGuinness SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care. 2013;58:1621–4.
Collins CL, Holberton JR, Konig K. Comparison of the pharyngeal pressure provided by two heated, humidified high-flow nasal cannulae devices in premature infants. J Paediatr Child Health. 2013;49:554–6.
Wilkinson DJ, Andersen CC, Smith K, Holberton J. Pharyngeal pressure with high-flow nasal cannulae in premature infants. J Perinatol. 2008;28:42–7.
Mazmanyan P, Darakchyan M, Pinkham MI, Tatkov S. Mechanisms of nasal high flow therapy in newborns. J Appl Physiol. 1985;2020(128):822–9.
Jeffreys E, Hunt KA, Dassios T, Greenough A. Diaphragm electromyography results at different high flow nasal cannula flow rates. Eur J Pediatr. 2019;178:1237–42.
Hough JL, Shearman AD, Jardine L, Schibler A. Nasal high flow in preterm infants: a dose-finding study. Pediatr Pulmonol. 2020;55:616–23.
Riva T, Pedersen TH, Seiler S, et al. Transnasal humidified rapid insufflation ventilatory exchange for oxygenation of children during apnoea: a prospective randomised controlled trial. Br J Anaesth. 2018;120:592–9.
Humphreys S, Lee-Archer P, Reyne G, Long D, Williams T, Schibler A. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth. 2017;118:232–8.
Lyons C, Callaghan M. Apnoeic oxygenation with high-flow nasal oxygen for laryngeal surgery: a case series. Anaesthesia. 2017;72:1379–87.
Gustafsson IM, Lodenius A, Tunelli J, Ullman J, Jonsson FM. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE)—a physiological study. Br J Anaesth. 2017;118:610–7.
Laviola M, Das A, Chikhani M, Bates DG, Hardman JG. Computer simulation clarifies mechanisms of carbon dioxide clearance during apnoea. Br J Anaesth. 2019;122:395–401.
Else SDN, Kovatsis PG. A narrative review of oxygenation during pediatric intubation and airway procedures. Anesth Analg. 2020;130:831–40.
Patel R, Lenczyk M, Hannallah RS, McGill WA. Age and the onset of desaturation in apnoeic children. Can J Anaesth. 1994;41:771–4.
Glenski TA, Diehl C, Clopton RG, Friesen RH. Breathing circuit compliance and accuracy of displayed tidal volume during pressure-controlled ventilation of infants: a quality improvement project. Pediatr Anesth. 2017;27:935–41.
Jaber S, Langlais N, Fumagalli B, et al. Performance studies of 6 new anesthesia ventilators: bench tests. Ann Fr Anesth Reanim. 2000;19:16–22.
Stayer SA, Bent ST, Skjonsby BS, Frolov A, Andropoulos DB. Pressure control ventilation: three anesthesia ventilators compared using an infant lung model. Anesth Analg. 2000;91:1145–50.
Aslanian P, El Atrous S, Isabey D, et al. Effects of flow triggering on breathing effort during partial ventilatory support. Am J Respir Crit Care Med. 1998;157:135–43.
Tassaux D, Michotte JB, Gainnier M, Gratadour P, Fonseca S, Jolliet P. Expiratory trigger setting in pressure support ventilation: from mathematical model to bedside. Crit Care Med. 2004;32:1844–50.
von Goedecke A, Brimacombe J, Hormann C, Jeske HC, Kleinsasser A, Keller C. Pressure support ventilation versus continuous positive airway pressure ventilation with the ProSeal laryngeal mask airway: a randomized crossover study of anesthetized pediatric patients. Anesth Analg. 2005;100:357–60.
Odin I, Nathan N. What are the changes in paediatric anaesthesia practice afforded by new anaesthetic ventilators? Ann Fr Anesth Reanim. 2006;25:417–23.
Nakae Y, Miyabe M, Sonoda H, Tamiya K, Namiki A. Comparison of the Jackson-Rees circuit, the pediatric circle, and the MERA F breathing system for pediatric anesthesia. Anesth Analg. 1996;83:488–92.
Von Ungern-Sternberg BS, Saudan S, Regli A, Schaub E, Erb TO, Habre W. Should the use of modified Jackson Rees T-piece breathing system be abandoned in preschool children? Paediatr Anaesth. 2007;17:654–60.
Spears RS, Yeh A, Fisher DM, Zwaas MS. The "educated hand": can anesthesiologists assess changes in neonatal pulmonary compliance manually? Anesthesiology. 1991;75:693–6.
Schily M, Koumoukelis H, Lerman J, Creighton RE. Can pediatric anesthesiologists detect an occluded tracheal tube in neonates? Anesth Analg. 2001;93:66–70.
Weiss M, Gerber AC. Induction of anaesthesia and intubation in children with a full stomach. Time to rethink! Anaesthesist. 2007;56:1210–6.
Eich C, Timmermann A, Russo SG, et al. A controlled rapid-sequence induction technique for infants may reduce unsafe actions and stress. Acta Anaesthesiol Scand. 2009;53:1167–72.
Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna G. Prevention of atelectasis during general anaesthesia. Lancet. 1995;345:1387–91.
von Ungern-Sternberg BS, Regli A, Schibler A, Hammer J, Frei FJ, Erb TO. The impact of positive end-expiratory pressure on functional residual capacity and ventilation homogeneity impairment in anesthetized children exposed to high levels of inspired oxygen. Anesth Analg. 2007;104:1364–8.
Tobin MJ. Advances in mechanical ventilation. N Engl J Med. 2001;344:1986–96.
Akca O, Liem E, Suleman MI, Doufas AG, Galandiuk S, Sessler DI. Effect of intra-operative end-tidal carbon dioxide partial pressure on tissue oxygenation. Anaesthesia. 2003;58:536–42.
Oski FA. Clinical implications of the oxyhemoglobin dissociation curve in the neonatal period. Crit Care Med. 1979;7:412–8.
Brown MK, Poeltler DM, Hassen KO, et al. Incidence of hypocapnia, hypercapnia, and acidosis and the associated risk of adverse events in preterm neonates. Respir Care. 2018;63:943–9.
Klingenberg C, Wheeler KI, McCallion N, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst Rev. 2017;(10):CD003666.
Zhou W, Liu W. Hypercapnia and hypocapnia in neonates. World J Pediatr. 2008;4:192–6.
Habre W, Petak F. Perioperative use of oxygen: variabilities across age. Br J Anaesth. 2014;113 Suppl 2:ii26–36.
Bucher HU, Fanconi S, Baeckert P, Duc G. Hyperoxemia in newborn infants: detection by pulse oximetry. Pediatrics. 1989;84:226–30.
Sola A, Golombek SG, Montes Bueno MT, et al. Safe oxygen saturation targeting and monitoring in preterm infants: can we avoid hypoxia and hyperoxia? Acta Paediatr. 2014;103:1009–18.
Kayton A, Timoney P, Vargo L, Perez JA. A review of oxygen physiology and appropriate management of oxygen levels in premature neonates. Adv Neonatal Care. 2018;19(2):98–104.
Becker MA, Donn SM. Real-time pulmonary graphic monitoring. Clin Perinatol. 2007;34(1-17):v.
Adams AB, Cakar N, Marini JJ. Static and dynamic pressure-volume curves reflect different aspects of respiratory system mechanics in experimental acute respiratory distress syndrome. Respir Care. 2001;46:686–93.
Babik B, Petak F, Asztalos T, Deak ZI, Bogats G, Hantos Z. Components of respiratory resistance monitored in mechanically ventilated patients. Eur Respir J. 2002;20:1538–44.
Aly S, El-Dib M, Lu Z, El Tatawy S, Mohamed M, Aly H. Factors affecting cerebrovascular reactivity to CO2 in premature infants. J Perinat Med. 2019;47:979–85.
Milan A, Freato F, Vanzo V, Chiandetti L, Zaramella P. Influence of ventilation mode on neonatal cerebral blood flow and volume. Early Hum Dev. 2009;85:415–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Balogh, A., Habre, W. (2023). Neonatal Ventilation Strategies and Their Practical Application. In: Lerman, J. (eds) Neonatal Anesthesia. Springer, Cham. https://doi.org/10.1007/978-3-031-25358-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-25358-4_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25357-7
Online ISBN: 978-3-031-25358-4
eBook Packages: MedicineMedicine (R0)