Abstract
Lipoprotein(a) [Lp(a)] is an inherited and causal risk factor for atherosclerotic cardiovascular disease (ASCVD) and aortic valve stenosis. Use of stable isotope tracers and compartmental modelling has provided deeper understanding of the physiology and pathophysiology of Lp(a) metabolism in humans, and the mode of action of lipid-regulating agents. Kinetic data have demonstrated that newly synthesized Lp(a)-apolipoprotein (apo) B-100 and Lp(a)-apo(a) are secreted as a holoparticle, with tightly coupled apo(a) and apoB-100 residence times in the circulation. In subjects with normal or high Lp(a) concentrations, the plasma Lp(a) concentration is predominantly determined by the rate of production of Lp(a) particles, irrespective of apo(a) isoform size and background therapy with statins. Lp(a) particle catabolism may only play a modest role in determining Lp(a) concentration in subjects with normal Lp(a) concentration or larger apo(a) isoform size. Kinetic studies also show that niacin and cholesteryl ester transfer protein inhibitors lower plasma Lp(a) concentration by increasing the clearance or catabolism of apo(a), whereas apoB antisense oligonucleotides lower plasma Lp(a) concentration by decreasing hepatic production. Proprotein convertase subtilisin kexin type 9 inhibitors can lower plasma Lp(a) concentration by a dual mode of action involving both increased clearance and decreased production of apo(a), depending on background Lp(a) concentration and/or statin use. Further studies of RNA therapeutics targeted at apo(a), angiopoietin-like 3 and apoC-III on the metabolism of Lp(a) and other lipoproteins will further our understanding of these therapies in decreasing the development of ASCVD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Apolipoprotein(a)
- Atherosclerotic cardiovascular disease
- Fractional catabolic rate
- Lipoprotein(a)
- Production rate
- Proprotein convertase subtilisin kexin type 9
- Stable isotope
- Statins
-
Lipoprotein(a) [Lp(a)] is an inherited and causal risk factor for atherosclerotic cardiovascular disease (ASCVD) and aortic valve stenosis.
-
Use of stable isotope tracers and compartmental modelling has provided deeper understanding of the physiology and pathophysiology of Lp(a) metabolism in humans.
-
Plasma Lp(a) concentration is predominantly determined by the rate of production of Lp(a) particles, irrespective of apo(a) isoform size and background therapy with statins.
-
Niacin and cholesteryl ester transfer protein inhibitors lower plasma Lp(a) concentration by increasing the clearance or catabolism of apo(a).
-
ApoB antisense oligonucleotides lower plasma Lp(a) concentration by decreasing hepatic production.
-
Proprotein convertase subtilisin kexin type 9 inhibitors can lower plasma Lp(a) concentration by a dual mode of action involving both increased clearance and decreased production of apo(a),
-
Further studies should investigate nucleic acid-based inhibitors for apo(a), angiopoietin-like 3 and apoC-III inhibitors on the metabolism of Lp(a) and other lipoproteins.
Introduction
Lipoprotein(a) [Lp(a)] is one of the most important genetically determined risk factors for atherosclerotic cardiovascular disease (ASCVD) and aortic valve stenosis (Nordestgaard and Langsted 2016; Saleheen et al. 2017; Tsimikas et al. 2018; Cegla et al. 2009; Arsenault and Kamstrup 2022; Reyes-Soffer et al. 2022). Large clinical trials have consistently shown that patients with elevated Lp(a), even when treated with statins, are at an increased risk of ASCVD (Khera et al. 2014; Nicholls et al. 2010). The metabolic pathways governing the metabolism of Lp(a) have been extensively studied in cellular and animal model systems (McCormick and Schneider 2019; Boffa and Koschinsky 2022; Chemello et al. 2022a). However, only scare information is available on the metabolism of this lipoprotein in humans. Use of stable isotopically labelled tracers and compartmental modelling has greatly enhanced our understanding of Lp(a) metabolism (Chan et al. 2004; Barrett et al. 2006). In the present chapter, we review use of these techniques and its contribution to key knowledge of the physiology and pathophysiology of Lp(a) metabolism in humans. We focus on subjects with elevated Lp(a) and the mode of action of lipid-regulating agents.
Structure and Genetics of Lipoprotein(a) in Brief
Lp(a) is composed of one molecule of a highly polymorphic apolipoprotein(a) [apo(a)] particle covalently linked to one molecule of a low-density lipoprotein (LDL)-like particle containing apoB-100 by a single disulphide bond (Schmidt et al. 2016). Apo(a) is composed of 10 types of kringle 4 (KIV) domains related to plasminogen kringle 4, followed by a KV domain and an inactive protease-like domain. KIV2 exists in variable numbers (from 3 to >30), which gives rise to Lp(a) isoform size heterogeneity (Marcovina et al. 1996; Kronenberg and Utermann 2013).
The gene encoding apo(a), LPA, is located on the long arm of chromosome 6 at 6q2.6–2.7, adjacent to the human plasminogen gene. While the control of LPA expression is at present not well understood, certain factors, such as estrogen, hepatocyte nuclear factor 4α, interleukin-6 and tumour necrosis factor alpha, influence the expression of LPA (Kronenberg and Utermann 2013). Plasma Lp(a) concentration is largely controlled by the LPA gene locus. Up to 90% of its variation in Lp(a) concentration is attributable to genetic factors (Lamon-Fava et al. 1991; Austin et al. 1992; Boerwinkle et al. 1992), with approximately 30–70% explained by a variable number of KIV2 repeats in the LPA gene (Kronenberg and Utermann 2013). The unexplained genetic variance in Lp(a) concentration can be contributed by other genetic factors outside KIV2 repeat variation. Several single nucleotide polymorphisms (SNPs) in the LPA gene, such as rs3798220 (CT/CC) and rs10455872 (AG/GG), are strongly associated with an elevated Lp(a) concentration (Clarke et al. 2009). Genome-wide association studies (GWAS) have also identified many common genetic variants of small effect which can aggregately influence Lp(a) concentration (Coassin and Kronenberg 2022). Accordingly, a polygenic risk score for predicting Lp(a) concentration has recently been reported, explaining approximately 60–70% of the variance in Lp(a) levels in the EPIC-Norfolk and UK Biobank cohorts (Wu et al. 2021). APOE gene is one of the most important genetic factors modulating Lp(a) concentrations (Li et al. 2015; Zekavat et al. 2018; Mack et al. 2017; Chemello et al. 2022b); the ε2 allele is associated with reduced Lp(a) concentrations, whereas the ε4 allele is associated with increased Lp(a) concentrations compared with the ε3 allele (Moriarty et al. 2017). Several physiological states, such as kidney, thyroid and liver disease, and ancestry, also contribute to the variability in Lp(a) concentration (Enkhmaa and Berglund 2022).
Stable Isotopic Tracer Methodologies
Plasma Lp(a) concentration in the circulation is determined by a balance between the rates of production and catabolism of Lp(a) particles. Stable isotope tracer studies using endogenous labelling of apolipoproteins with amino acid precursor molecules (isotopomers) and mathematical modelling have been employed to study Lp(a) kinetics (Barrett et al. 2006). This approach has provided better understanding of Lp(a) homeostasis and of the pathogenesis of elevated Lp(a), as well as the kinetic effects of statin and newer lipid-regulating agents, such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors.
Briefly, stable isotopically labelled amino acids (such as D3-leucine) are administered intravenously as a bolus or primed infusion with serial blood sampling over several days to assess the turnover of apo(a). Enrichment data (tracer/tracee ratio) are generated by gas- or liquid-chromatography mass spectrometry (GCMS or LCMS, respectively) analysis after separation of apo(a) from plasma (Chan et al. 2004). A novel LCMS method for quantification of apo(a) enrichment has recently been established by employing a synthetic peptide (LFLEPTQADIALLK) that targets the proteolytic domain of apo(a) following a standardized sample trypsin digestion procedure (Croyal et al. 2015, 2018). This method is more sensitive and less labour-intensive than the traditional approach based on immunoprecipitation and Western blotting. Enrichment data are then analysed via multicompartmental modelling, from which the fractional turnover rate of apo(a) in the circulation is derived. Fractional catabolic (or clearance) rate (FCR) refers to the fraction of trace lost from a defined plasma pool per day. From these primary kinetic data, together with the corresponding plasma pool size of apo(a), absolute transport rates in the circulation are calculated. We have detailed these methods elsewhere (Chan et al. 2004; Barrett et al. 2006). Figure 5.1 shows a multicompartmental model for the metabolism of Lp(a)-apo(a) and Lp(a)-apoB-100.
Compartmental model to describe Lp(a)-apo(a) and Lp(a)-apoB-100 tracer kinetics. Plasma leucine kinetics are described by a four-compartment model, which is connected to intrahepatic delay compartments (compartments 5 and 6) that accounts for the synthesis and secretion of Lp(a)-apo(a) and Lp(a)-apoB-100, with compartments 7 and 8 describing the plasma kinetics of Lp(a)-apo(a) and Lp(a)-apoB-100, respectively
Metabolism of Lipoprotein(a)
Synthesis, Assembly and Secretion
Apo(a) is exclusively synthesized by the liver (Schmidt et al. 2016). However, details of the assembly process have not been fully elucidated. The site of Lp(a) assembly may occur in hepatocytes, extracellularly in the space of Disse, or in the circulation (plasma space) (Hoover-Plow and Huang 2013; Youssef et al. 2022). Several pathways for Lp(a) assembly and secretion have been suggested. Apo(a) and apoB are assembled intrahepaticaly, forming an Lp(a) particle which is subsequently secreted into plasma. The Lp(a) particle may also be assembled in the circulation (e.g. on the hepatocyte surface) from its constituent protein; these are then independently secreted from the liver into plasma. There is also uncertainty concerning whether the kinetics in plasma of the two protein components of Lp(a) are coupled, and specifically whether apo(a) is recycled or cleared with apoB-100 as an Lp(a) holoparticle. Using stable isotope tracers and compartmental modelling, we demonstrated that in individuals with a wide range of plasma Lp(a) concentrations, Lp(a)-apoB-100 and Lp(a)-apo(a) have identical isotopic enrichment curves in plasma and similar FCRs (Watts et al. 2018). This finding was confirmed in another kinetic study of statin-treated patients (Ma et al. 2019a) (Fig. 5.2). Hence, these kinetic data generally support that newly synthesized Lp(a)-apoB-100 and Lp(a)-apo(a) are secreted as a holoparticle with tightly coupled apo(a) and apoB100 residence times in the circulation. However, it remains unclear whether the covalent binding of apo(a) to apoB-100 takes place in the liver or in the circulation.
Another outstanding issue concerning the assembly of Lp(a) particles is the extent to which the binding of apo(a) to triglyceride-rich lipoproteins (TRLs) contributes to the formation of Lp(a) particles in the circulation (Nassir et al. 1998; Ramos-Cáceres et al. 2022). Earlier radiolabelled kinetic studies suggest that apo(a) is unlikely to be adducted to a triglyceride-rich very low-density lipoprotein (VLDL) as a precursor of Lp(a) in the LDL/HDL density range (Krempler et al. 1980). In contrast, apo(a) can be associated with TRLs, such as chylomicrons and chylomicron remnants, after oral ingestion of a fatty meal (Bersot et al. 1986). This is supported by experimental evidence that the apoB-100-apo(a) complex within Lp(a) particles have a high affinity for TRL particles (Marcoux et al. 1997). A significant proportion of Lp(a) particles can bind non-covalently to TRLs in the hypertriglyceridemic state. Consistent with this, we and others have demonstrated a redistribution of a significant portion of apo(a) protein from Lp(a) to the TRL fraction, particularly in the postprandial state (Cohn et al. 1991; Ying et al. 2022). In a recent study of FH, we found that the impaired postprandial TRL-apo(a) response to a fat load was partially corrected by fish oil supplementation (Ying et al. 2022). The reduction in postprandial TRL-apo(a) with fish oil supplementation in response to the fat load was significantly associated with the corresponding reduction in postprandial triglyceride response. Hence, interaction with TRLs may influence the metabolism or catabolism of Lp(a). The underlying kinetic mechanism remains to be investigated employing stable isotopes and compartmental modelling.
Clearance and Catabolism
It is well established that the liver is the main site of Lp(a) clearance and, to a much lesser extent, the kidney and the arterial wall (McCormick and Schneider 2019). The mechanisms of Lp(a) clearance from the circulation and the catalytic pathways involved remain uncertain, however. Several cellular receptors have been proposed to mediate the clearance of Lp(a) from the liver. These include LDL receptor and other members of the LDL-receptor family such as VLDL receptor, LDL receptor-related protein 1 (LRP1), megalin/gp330, scavenger receptor class B type 1 (SR-BI) and plasminogen receptor (McCormick and Schneider 2019).
The role of the LDL receptor in Lp(a) clearance remains controversial. Several experimental studies have demonstrated that LDL receptor can facilitate Lp(a) binding and uptake (Havekes et al. 1981; Reblin et al. 1997; Romagnuolo et al. 2015), and in mice overexpressing LDL receptor the clearance of Lp(a) particles is significantly increased (Hofmann et al. 1990). Very few kinetic studies have specifically investigated the metabolism of Lp(a) in patients with LDL receptor defects, such as familial hypercholesterolemia (FH). Using exogenous radiolabelled tracers, Rader et al. found that the clearance of Lp(a) did not differ significantly among homozygous FH patients, heterozygous FH parents and non-FH control subjects (Rader et al. 1995). Using endogenous stable isotope tracers, Croyal et al. reported that the FCRs of Lp(a)-apo(a) were similar in patients with PCSK9 gain-of-function mutations and control subjects (Croyal et al. 2020). Hence, defects in LDL receptor function do not appear to result in delayed clearance of Lp(a). In a study of healthy normolipidemic men, there was no significant association between the FCRs of apo(a) and LDL-apoB-100 (Chan et al. 2019). These kinetic findings suggest under physiological conditions that the LDL receptors may not play a major role in Lp(a) clearance. As discussed later, LDL receptor could play a role in Lp(a) clearance in a supraphysiological condition in which the activity of LDL receptors is markedly upregulated, such as in patients who are treated with a combination of statins and PCSK9 inhibitors (Watts et al. 2018).
Kinetic Determinants of Plasma Lipoprotein(a) Concentrations
Production Rate vs. Fractional Catabolic Rate
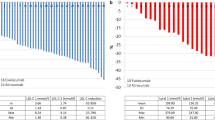
In a kinetic study of healthy normolipidemic men with a wide range of plasma Lp(a) concentration, Lp(a) particle concentration was significantly and positively associated with apo(a) production rate (PR) and inversely with apo(a) FCR (Chan et al. 2019). In another study of statin-treated subjects, plasma concentration of apo(a) was significantly and positively associated with apo(a) PR in patients with both normal and elevated Lp(a) concentrations (Ma et al. 2019b). However, there was no significant association between plasma apo(a) concentration and FCR in either of the groups. Hence, these observations reinforce the notion that plasma concentrations of Lp(a) are primarily determined by the rates of production and not clearance, irrespective of background statin use. Accordingly, plasma concentration and PR of apo(a) were significantly higher in statin-treated patients with elevated Lp(a) compared with those with normal Lp(a) (Fig. 5.3a, b). The FCR of apo(a) did not differ significantly between the groups (Fig. 5.3c). This finding suggests that elevated plasma Lp(a) concentration is a consequence of increased hepatic production of Lp(a) particles in patients with elevated Lp(a). In a constant-feeding study of healthy individuals, patients with high Lp(a) had increased apo(a) PR and reduced FCR compared with those without elevated Lp(a) concentration (Jenner et al. 2005). Plasma concentrations of Lp(a) were correlated significantly with both apo(a) PR and negatively with apo(a) FCR. These findings implicate a role of Lp(a) catabolism in determining Lp(a) plasma concentrations in the fed state.
Kinetic parameters of apo(a) in statin-treated subjects with (a) normal (<75 nmol/L), (b) high (75–145 nmol/L) and (c) very high apo(a) concentrations (>145 nmol/L). Data presented as mean ± SEM. Apo apolipoprotein, FCR fraction catabolic rate, PR production rate. *P < 0.001 compared with normal apo(a) group. †P < 0.001 compared with normal and moderate-high apo(a) group using ANOVA
Apo(a) Isoform Size
As discussed earlier, the plasma concentrations of Lp(a) is dependent on genetic variations in the number of KIV2 repeats (Marcovina and Koschinsky 1999; Kronenberg and Utermann 2013). Experimental data have suggested that the size of the apo(a) transcripts is inversely associated with hepatic LPA mRNA concentration (Wade et al. 1991; White et al. 1994) and by implication apo(a) production. Smaller apo(a) isoforms have been shown to have a shorter retention time in the endoplasmic reticulum and probably lesser intracellular apo(a) proteasome degradation, resulting in a more efficiently secretion from hepatocytes (White et al. 1994; Brunner et al. 1996; Lobentanz et al. 1998). On the other hand, Lp(a) with apo(a) isoforms of different sizes may have different binding affinities for the LDL receptor or other receptors (März et al. 1993). Lp(a) particles with larger isoform size have been shown to be more effectively removed via LDL receptor independent pathways.
In a study of healthy normolipidemic subjects, subjects with smaller apo(a) isoform sizes (≤22 KIV repeats) had significantly higher apo(a) concentration and PR, and lower apo(a) FCR than those with larger sizes (Chan et al. 2019). Plasma apo(a) concentration was significantly associated with apo(a) PR, but not with FCR in subjects with smaller apo(a) isoform size. In contrast, both apo(a) PR and FCR were significantly associated with plasma apo(a) concentrations in subjects with larger isoforms. Similar observations were observed in patients who were on statin (Ma et al. 2019c). Taken together, these findings again suggest that the plasma Lp(a) concentration is predominantly determined by the rate of production of Lp(a) particles, irrespective of apo(a) isoform size and background statin use. Lp(a) particle catabolism may only play a modest role in determining Lp(a) concentration in subjects with larger apo(a) isoform size. These observations also support the clinical use of agents that target the hepatic production and secretion of Lp(a) (Tsimikas 2017).
As discussed earlier, APOE genotype can influence the concentration of Lp(a) (Moriarty et al. 2017; Croyal et al. 2020; Chemello et al. 2022a). However, the effect of APOE genotype, particularly the presence of apoE2 and apoE4, on Lp(a) concentrations is known to be affected by the size of apo(a) (Klausen et al. 1996; Blanchard et al. 2021). Accordingly, the effect of apoE genotype on the metabolism of Lp(a) in subjects with large and small apo(a) isoform merits further investigation.
Mechanisms of Action of Lipid-Regulating Agents on Lipoprotein(a) Metabolism
A major challenge in managing patients with elevated Lp(a) is a lack of effective and specific treatment for lowering Lp(a) concentrations (Tsimikas 2017; Tsimikas et al. 2018; Reyes-Soffer et al. 2022; Schwartz and Ballantyne 2022). Diet and lifestyle interventions, such as weight loss or physical activity, do not seem to influence Lp(a) concentrations. Lipoprotein apheresis is the only FDA approved treatment for elevated Lp(a). Currently, there are no approved pharmacologic therapies that specifically target Lp(a) concentrations (Cegla et al. 2009; Tsimikas 2017). The kinetic effect of several established and newer therapies, including statins, niacin, PCSK9 inhibitors, cholesteryl ester transfer protein (CETP) inhibitors and apoB antisense oligonucleotides (ASO), on Lp(a) metabolism are discussed below and in Table 5.1, with specific reference to the mechanisms of action.
Statins
The value of statins in lowering LDL-cholesterol is well recognized. Statins competitively inhibit HMG CoA reductase, thereby decreasing cholesterol biosynthesis, reciprocally upregulating hepatic LDL receptors and enhancing clearance of LDL and other apoB-100-containing particles, including TRLs (Ginsberg 2006). Given the structural similarities between LDL and Lp(a), one would speculate that statins could lower Lp(a) concentration by increasing the clearance of Lp(a). However, the effect of statins on Lp(a) levels is conflicting: some studies show a neutral role (Wang et al. 2021; de Boer et al. 2022), while others report a decrease (Takagi and Umemoto 2012) or increase of plasma Lp(a) levels (Tsimikas et al. 2020). It appears that the influence of stains on Lp(a) level may depend on the type of statins; atorvastatin and rosuvastatin increase Lp(a) levels whereas pitavastatin has no impact or may tend to decrease plasma Lp(a) concentrations (Tsimikas et al. 2020). The statin-induced increase in Lp(a) level is supported by experimental evidence in HepG2 cells showing a higher LPA mRNA level in response to atorvastatin (Tsimikas et al. 2020). In a study of healthy normolipidemic subjects, atorvastatin (80 mg daily) did not significantly alter the FCR or PR of apo(a) (Watts et al. 2017). This finding does not support a role of LDL receptor in the regulation of apo(a) FCR under physiological condition. However, it remains unclear whether statin has a potential impact on Lp(a) metabolism in subjects with high Lp(a) concentration. There is also evidence showing that statins increase Lp(a) levels exclusively in patients with a small size apo(a) defined as ≤22 KIV repeats (Yahya et al. 2019). The precise mechanisms of action of this effect on Lp(a) metabolism remain to be investigated.
Niacin
Niacin is one of few agents that can significantly lower plasma Lp(a) concentrations. Experimental data suggest that niacin decreases the expression of LPA mRNA (Chennamsetty et al. 2012). This is consistent with a kinetic study showing that niacin lowered Lp(a) concentration by decreasing the production of apo(a) in non-diabetic, obese and hypertriglyceridemic men (Croyal et al. 2015). The lowering of the PR of apo(a) by niacin was confirmed in another postprandial kinetic study in statin-treated patients with type 2 diabetes (Ooi et al. 2015). In this study, extended-release niacin (1–2 g/day) significantly decreased plasma Lp(a) concentration and the production rates of apo(a), with greater treatment effect in individuals with elevated Lp(a) concentration. This is consistent with another study showing that extended-release niacin was more effective in lowering Lp(a) level in subjects with small apo(a) isoform than those with large isoform (Artemeva et al. 2015).
PCSK9 Inhibitors
Inhibition of PCSK9 in combination with statins and/or ezetimibe provides a highly effective approach for lowering LDL-cholesterol concentrations in patients with hypercholesterolemia (Duprez et al. 2020; Ying et al. 2021; Ferri et al. 2020). Monoclonal antibodies (mAbs) targeting PCSK9, such as evolocumab and alirocumab, have been consistently known to significantly lower plasma LDL-cholesterol and the incidence of ASCVD outcomes (Sabatine et al. 2017; Schwartz et al. 2018; Deedwania et al. 2021). PCSK9mAbs can similarly lower plasma Lp(a) concentration. The effectiveness of PCSK9 mAbs in reducing ASCVD events is also found to be most pronounced in patients with high Lp(a) and that the reduction in Lp(a) could also partly mediate the cardiovascular benefit of PCSK9 mAbs (Bittner et al. 2020; Schwartz et al. 2021).
In a kinetic study of healthy normolipidemic men, evolocumab monotherapy significantly decreased plasma Lp(a) concentration chiefly by reducing the PR of apo(a) with no effect on the corresponding FCR (Watts et al. 2018). This effect is consistent with a tracer study conducted in non-human primates in which alirocumab decreased the PR of apo(a) (Croyal et al. 2018). The mechanistic effect of evolocumab may involve reduced hepatic production of Lp(a) by decreasing the assembly of Lp(a) particles through the reduction of apo(a) binding with LDL on the surface of hepatocytes (Lambert et al. 2017). This speculation is supported by in vitro studies showing that PCSK9 induces Lp(a) intracellular assembly and secretion, whereas PCSK9 mAbs reduce the extracellular release of Lp(a) (Villard et al. 2016).
However, as combination therapy with high-dose atorvastatin, evolocumab reduced the plasma concentration of Lp(a) chiefly by a significant increase in the FCR of apo(a) (Watts et al. 2018). The PR of Lp(a) was not significantly altered with the combination. Similar results were also found in another kinetic study in healthy individuals receiving alirocumab treatment (Reyes-Soffer et al. 2017). However, the increase in apo(a) FCR in the latter study was not statistically significant, probably owing to greater variability in study subject characteristics (e.g. mixed race and gender). The mechanistic effect of evolocumab in combination with atorvastatin may involve supraphysiological upregulation of the activity of LDL receptors and decreased competition of Lp(a) with very low concentrations of LDLs for clearance by these receptors. This mechanism suggests that the LDL receptor likely plays a significant role in mediating Lp(a) clearance only when its expression is markedly upregulated and when LDL plasma levels are substantially lowered, allowing decreased competition between LDL and Lp(a) for receptor-mediated uptake in the liver.
The mechanism of action of PCSK9 inhibition has recently been studied in statin-treated patients with high Lp(a). Using stable isotopes, PCSK9 inhibition with alirocumab-lowered plasma Lp(a) concentration by increasing apo(a) FCR in patients with elevated Lp(a) receiving maximally tolerated statin therapy (Watts et al. 2020). However, in patients with very high-Lp(a) concentration, alirocumab significantly lowered plasma Lp(a) concentration by a dual mode of action involving both increased clearance and decreased production of apo(a) (Ying et al. 2022). Taken together, the mechanistic action of PCSK9 mAbs on the PR and FCR of apo(a) appears to be dependent on background statin use and Lp(a) concentration at baseline.
Unlike evolocumab or alirocumab, small interfering RNA on PCSK9 mRNA transcript (e.g. Inclisiran) is a new approach to targeting PCSK9 intracellularly (German and Shapiro 2020; Smith and White 2022). This novel agent was shown to inhibit hepatic synthesis of the PCSK9 protein, and lower apoB-100-containing lipoproteins, including Lp(a) (Ray et al. 2020; Raal et al. 2020). This implies that the effect of PCSK9 inhibition on Lp(a) is irrespective of mode of inhibition of PCSK9 (intracellular or extracellular. However, the mechanisms of action of inclisiran on Lp(a) metabolism remain to be elucidated.
CETP Inhibitors
CETP plays an important role in lipoprotein metabolism, primarily by its ability to facilitate transfer of esterified cholesterol from high-density lipoproteins (HDL) to apoB-containing lipoproteins (Tall 1993). Treatment with CETP inhibitors, either alone or in combination with statin, can lower Lp(a) concentrations up to 30% (Schmidt et al. 2021). In a kinetic study of patients with hypercholesterolaemia (Thomas et al. 2017), CETP inhibition with anacetrapib lowered Lp(a) concentration by reducing the PR of apo(a) with no effect on the corresponding FCR. However, there is no clear explanation for the reduction in apo(a) PR with anacetrapib which merits further investigation. Despite these metabolic changes, CETP inhibitors did overall not have clinically meaningful effects in large clinical trials. While several CETP inhibitors, including torcetrapid, evacetrapid, dalcetrapid and anacetrapid, have fallen after disappointing clinical trial outcomes (Berberich et al. 2017; Schwartz et al. 2012; Lincoff et al. 2017; Schmidt et al. 2021), two clinical trials with a newer CETP inhibitor obicetrapib (TA-8995; 10 mg) has been shown to increase HDL-cholesterol by 160%, and reduce LDL-cholesterol, apoB and Lp(a) levels approximately by 50–60%, 30–50% and 25–50%, respectively, in patients treated with atorvastatin or rosuvastatin (Hovingh et al. 2015; Ray 2022). The mechanisms of action of this agent on Lp(a) and other lipoproteins merit investigation.
ApoB Antisense Oligonucleotides
Mipomersen is an antisense oligonucleotide (ASO) directed to liver mRNA of apoB that inhibits apoB synthesis (Parham and Goldberg 2019). Accordingly, mipomersen has been shown to significantly lower plasma concentrations of apoB-containing lipoproteins including LDL and Lp(a). In a kinetic study of healthy volunteers, treatment with mipomersen caused a significant decrease of plasma Lp(a) levels that was associated with a significant increase in the FCR of Lp(a), with no effect on corresponding apo(a) PR (Nandakumar et al. 2018). These results were unexpected because inhibition of apoB synthesis with mipomersen would reduce the availability of apoB100 substrate for the assembly of hepatic apoB with apo(a) to form an Lp(a). It is noteworthy that mipomersen also did not reduce VLDL apoB secretion in the same subjects studied (Reyes-Soffer et al. 2016). These observations appear to support the presence of spare apoB pool in the liver that would be utilized for the assembly of Lp(a) in order to maintain hepatic homeostasis for apoB. However, this speculation requires further investigation. In the same study, the increase in Lp(a) FCR observed was similar to the 30% increase in the FCR of LDL apoB100, supporting a role for the LDL receptors or related receptors in the clearance of Lp(a) particles.
Other Therapies
Lipoprotein apheresis effectively lowers Lp(a) and LDL levels by approximately 60–70%. Kinetic studies, using stable isotope methods, have shown inconsistent findings when comparing Lp(a) and LDL FCRs in patients undergoing apheresis (Parhofer et al. 1999; Armstrong et al. 1989; Kroon et al. 2000). In studying the rebound of Lp(a) and LDL particle concentration following lipoprotein apheresis (Ma et al. 2019c), the clearance of Lp(a) is significantly slower than that of LDL-apoB in patients with elevated Lp(a) and ASCVD. These findings suggest that the clearance pathways for Lp(a) differ from those of LDL-apoB.
Selective thyroid hormone receptor (THR) agonists (such as Resmetirom) can effectively lower plasma Lp(a) concentrations (Hovingh et al. 2022). Activation of THR has been shown to increase LDL receptor expression, resulting in reduced circulating LDL particles (Erion et al. 2007). Whether the lowering effect of Lp(a) is mediated by upregulating the activity of LDL receptor remains unclear and merits further investigation.
Administration of aspirin has been shown to lower Lp(a) levels in patients with high-Lp(a) concentrations irrespectively of apo(a) isoform size (Akaike et al. 2002). This observation is supported by experimental data in HepG2 cells that aspirin reduced Lp(a) production in H2G cell via the reduction of apo(a) gene transcriptional activity with suppression of apo(a) mRNA expression (Kagawa et al. 1999). However, no kinetic studies have yet specified investigated the effect of aspirin on Lp(a) metabolism in humans.
Lomitapide is a small molecule that inhibits lipid transfer by direct binding to microsomal triglyceride transfer protein (MTP) in the liver and intestine (Berberich and Hegele 2017). By inhibiting MTP in hepatocytes and enterocyte, lomitapide reduces VLDL assembly and secretion, and lowers plasma levels of all apoB-1containing lipoproteins, including VLDL, LDL and Lp(a) independent of LDL receptor (Cuchel and Rader 2013; Harada-Shiba et al. 2017). Accordingly, lomitapide is specifically approved for lowering LDL-cholesterol in homozygous FH (Berberich et al. 2017). Kinetic studies showed a marked reduction in the production of LDL-apoB-100 (Cuchel et al. 2007). Whether lomitapide reduces Lp(a) concentrations by decreasing apo(a) PR remains to be investigated.
Conclusions and Future Perspectives
Lp(a) is associated with an increased risk of ASCVD, even in patients on intensive lipid-lowering therapy. However, elevated Lp(a) remains an under-recognized, under-treated and under-researched condition with an extremely high risk of ASCVD. This atherogenic disorder has received little attention due to a significant knowledge gap in understanding Lp(a) pathophysiology. Stable isotope tracer methods provide unique information of the dynamics of Lp(a) particles in the circulation. The interferences from these studies are important for understanding the metabolism of Lp(a) and for developing new therapies. Knowledge of the mode of action of therapeutic interventions is also important for informing shared-decision making and improving adherences to therapies. Future research is still needed to understand whole body metabolism of Lp(a), including the stability of the covalent bonding between apo(a) and apoB-100, the potential recycling of apo(a) in the circulation, the possible formation of Lp(a) complexes with TRLs, and the relative roles of hepatic and renal receptors in the clearance of Lp(a) particles. The precise modes of action of CETP inhibitors, apoB ASO and THR agonists on the metabolism of Lp(a), particularly in patients with high Lp(a), also merit further clarification.
While several therapeutic interventions can lower plasma Lp(a) concentrations (Korneva et al. 2021), it is uncertain that it would mitigate the adverse effects of elevated Lp(a) on ASCVD. Nevertheless, some of the cardiovascular benefit of PCSK9 mAbs in clinical outcome trials are known to be mediated by the lowering of Lp(a) independently of the concurrent reduction in LDL cholesterol. More aggressive treatment strategies involve use of multiple lipid-regulating agents to treat elevated Lp(a). This approach harnesses the complementary mechanisms of action of the different agents. Possible combinations include PCSK9 inhibitor with niacin, CETP inhibitor or THR agonist. Inhibiting hepatic apo(a) synthesis with nucleic acid therapeutics has emerged as a potent approach to reduce plasma Lp(a) levels up to 90% which is not affected by LPA gene variants and isoform size (Karwatowska-Prokopczuk et al. 2021). The effect of this novel and specific agent on the metabolism of Lp(a) and other apoB-containing lipoproteins warrants investigation. Further studies are required to characterize the mode of action of newer lipid-regulating agents on the metabolism TRLs and Lp(a). These include inhibitors of angiopoietin-like protein 3 (ANGPTL3) and apoC-III (antibodies and/or nucleic acid-based ASO therapies) (Ward et al. 2022).
Conflicts of Interest
GFW has received honoraria for lectures and advisory boards or research grants from Amgen, Arrowhead, AstraZeneca, Esperion, Kowa, Novartis, Regeneron and Sanofi. DCC has nothing to declare.
References
Akaike M, Azuma H, Kagawa A, Matsumoto K, Hayashi I, Tamura K, Nishiuchi T, Iuchi T, Takamori N, Aihara K, Yoshida T, Kanagawa Y, Matsumoto T. Effect of aspirin treatment on serum concentrations of lipoprotein(a) in patients with atherosclerotic diseases. Clin Chem. 2002;48:1454–9.
Armstrong VW, Schleef J, Thiery J, Muche R, Schuff-Werner P, Eisenhauer T, Seidel D. Effect of HELP-LDL-apheresis on serum concentrations of human lipoprotein(a): kinetic analysis of the post-treatment return to baseline levels. Eur J Clin Investig. 1989;19:235–40.
Arsenault BJ, Kamstrup PR. Lipoprotein(a) and cardiovascular and valvular diseases: a genetic epidemiological perspective. Atherosclerosis. 2022;349:7–16.
Artemeva NV, Safarova MS, Ezhov MV, Afanasieva OI, Dmitrieva OA, Pokrovsky SN. Lowering of lipoprotein(a) level under niacin treatment is dependent on apolipoprotein(a) phenotype. Atheroscler Suppl. 2015;18:53–8.
Austin MA, Sandholzer C, Selby JV, Newman B, Krauss RM, Utermann G. Lipoprotein(a) in women twins: heritability and relationship to apolipoprotein(a) phenotypes. Am J Hum Genet. 1992;51:829–40.
Barrett PH, Chan DC, Watts GF. Thematic review series: patient-oriented research. Design and analysis of lipoprotein tracer kinetics studies in humans. J Lipid Res. 2006;47:1607–19.
Berberich AJ, Hegele RA. Lomitapide for the treatment of hypercholesterolemia. Expert Opin Pharmacother. 2017; 18:1261–8.
Bersot TP, Innerarity TL, Pitas RE, Rall SC Jr, Weisgraber KH, Mahley RW. Fat feeding in humans induces lipoproteins of density less than 1.006 that are enriched in apolipoprotein [a] and that cause lipid accumulation in macrophages. J Clin Invest. 1986;77:622–30.
Bittner VA, Szarek M, Aylward PE, Bhatt DL, Diaz R, Edelberg JM, Fras Z, Goodman SG, Halvorsen S, Hanotin C, Harrington RA, Jukema JW, Loizeau V, Moriarty PM, Moryusef A, Pordy R, Roe MT, Sinnaeve P, Tsimikas S, Vogel R, White HD, Zahger D, Zeiher AM, Steg PG, Schwartz GG. Effect of alirocumab on lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J Am Coll Cardiol. 2020;75:133–44.
Blanchard V, Chemello K, Hollstein T, Hong-Fong CC, Schumann F, Grenkowitz T, Nativel B, Coassin S, Croyal M, Kassner U, Lamina C, Steinhagen-Thiessen E, Lambert G. The size of apolipoprotein (a) is an independent determinant of the reduction in lipoprotein (a) induced by PCSK9 inhibitors. Cardiovasc Res. 2021;26:cvab247.
Boerwinkle E, Leffert CC, Lin J, Lackner C, Chiesa G, Hobbs HH. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J Clin Invest. 1992;90:52–60.
Boffa MB, Koschinsky ML. Understanding the ins and outs of lipoprotein (a) metabolism. Curr Opin Lipidol. 2022;33:185–92.
Brunner C, Lobentanz EM, Pethö-Schramm A, Ernst A, Kang C, Dieplinger H, Müller HJ, Utermann G. The number of identical kringle IV repeats in apolipoprotein(a) affects its processing and secretion by HepG2 cells. J Biol Chem. 1996;271:32403–10.
Cegla J, Neely RDG, France M, Ferns G, Byrne CD, Halcox J, Datta D, Capps N, Shoulders C, Qureshi N, Rees A, Main L, Cramb R, Viljoen A, Payne J, Soran H. HEART UK consensus statement on lipoprotein(a): a call to action. Atherosclerosis. 2009;29:62–70.
Chan DC, Barrett PH, Watts GF. Lipoprotein transport in the metabolic syndrome: methodological aspects of stable isotope kinetic studies. Clin Sci (Lond). 2004;107:221–32.
Chan DC, Watts GF, Coll B, Wasserman SM, Marcovina SM, Barrett PHR. Lipoprotein(a) particle production as a determinant of plasma Lipoprotein(a) concentration across varying apolipoprotein(a) isoform sizes and background cholesterol-lowering therapy. J Am Heart Assoc. 2019;8:e011781.
Chemello K, Blom DJ, Marais AD, Lambert G, Blanchard V. Genetic and mechanistic insights into the modulation of circulating lipoprotein(a) concentration by apolipoprotein E isoforms. Curr Atheroscler Rep. 2022a;24:399–405.
Chemello K, Chan DC, Lambert G, Watts GF. Recent advances in demystifying the metabolism of lipoprotein(a). Atherosclerosis. 2022b;349:82–91.
Chennamsetty I, Kostner KM, Claudel T, Vinod M, Frank S, Weiss TS, Trauner M, Kostner GM. Nicotinic acid inhibits hepatic APOA gene expression: studies in humans and in transgenic mice. J Lipid Res. 2012;53:2405–12.
Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, Parish S, Barlera S, Franzosi MG, Rust S, Bennett D. Genetic variants associated with Lp (a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–28.
Coassin S, Kronenberg F. Lipoprotein(a) beyond the kringle IV repeat polymorphism: the complexity of genetic variation in the LPA gene. Atherosclerosis. 2022;349:17–35.
Cohn JS, Lam CW, Sullivan DR, Hensley WJ. Plasma lipoprotein distribution of apolipoprotein(a) in the fed and fasted states. Atherosclerosis. 1991;90:59–66.
Croyal M, Ouguerram K, Passard M, Ferchaud-Roucher V, Chétiveaux M, Billon-Crossouard S, de Gouville AC, Lambert G, Krempf M, Nobécourt E. Effects of extended-release nicotinic acid on apolipoprotein (a) kinetics in hypertriglyceridemic patients. Arterioscler Thromb Vasc Biol. 2015;35:2042–7.
Croyal M, Tran TT, Blanchard RH, Le Bail JC, Villard EF, Poirier B, Aguesse A, Billon-Crossouard S, Ramin-Mangata S, Blanchard V, Nativel B, Chemello K, Khantalin I, Thedrez A, Janiak P, Krempf M, Boixel C, Lambert G, Guillot E. PCSK9 inhibition with alirocumab reduces lipoprotein(a) levels in nonhuman primates by lowering apolipoprotein(a) production rate. Clin Sci (Lond). 2018;132:1075–83.
Croyal M, Blanchard V, Ouguerram K, Chétiveaux M, Cabioch L, Moyon T, Billon-Crossouard S, Aguesse A, Bernardeau K, Le May C, Flet L, Lambert G, Hadjadj S, Cariou B, Krempf M, Nobécourt-Dupuy E. VLDL (very-low-density lipoprotein)-apo E (apolipoprotein E) may influence Lp(a) (lipoprotein [a]) synthesis or assembly. Arterioscler Thromb Vasc Biol. 2020;40:819–29.
Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–56.
Cuchel M, Rader DJ. Microsomal transfer protein inhibition in humans. Curr Opin Lipidol. 2013;24:246–50.
de Boer LM, Oorthuys AOJ, Wiegman A, Langendam MW, Kroon J, Spijker R, Zwinderman AH, Hutten BA. Statin therapy and lipoprotein(a) levels: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;29:779–92.
Deedwania P, Murphy SA, Scheen A, Badariene J, Pineda AL, Honarpour N, Keech AC, Sever PS, Pedersen TR, Sabatine MS, Giugliano RP. Efficacy and safety of PCSK9 inhibition with evolocumab in reducing cardiovascular events in patients with metabolic syndrome receiving statin therapy: secondary analysis from the FOURIER randomized clinical trial. JAMA Cardiol. 2021;6:139–47.
Duprez DA, Handelsman Y, Koren M. Cardiovascular outcomes and proprotein convertase subtilisin/kexin type 9 inhibitors: current data and future prospects. Vasc Health Risk Manag. 2020;16:403–18.
Enkhmaa B, Berglund L. Non-genetic influences on lipoprotein(a) concentrations. Atherosclerosis. 2022;349:53–62.
Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD, Linemeyer DL. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci U S A. 2007;104:15490–4595.
Ferri N, Grego MF, Corsini A, Ruscica M. Proprotein convertase subtilisin/kexin type 9: an update on the cardiovascular outcome studies. Eur Heart J Suppl. 2020;22:E64–7.
German CA, Shapiro MD. Small interfering RNA therapeutic inclisiran: a new approach to targeting PCSK9. BioDrugs. 2020;34:1–9.
Ginsberg HN. Efficacy and mechanisms of action of statins in the treatment of diabetic dyslipidemia. J Clin Endocrinol Metab. 2006;91:383–92.
Harada-Shiba M, Ikewaki K, Nohara A, Otsubo Y, Yanagi K, Yoshida M, Chang Q, Foulds P. Efficacy and safety of lomitapide in Japanese patients with homozygous familial hypercholesterolemia. J Atheroscler Thromb. 2017;24:402–11.
Havekes L, Vermeer BJ, Brugman T, Emeis J. Binding of LP(a) to the low density lipoprotein receptor of human fibroblasts. FEBS Lett. 1981;132:169–73.
Hofmann SL, Eaton DL, Brown MS, McConathy WJ, Goldstein JL, Hammer RE. Overexpression of human low density lipoprotein receptors leads to accelerated catabolism of Lp(a) lipoprotein in transgenic mice. J Clin Invest. 1990;85:1542–7.
Hoover-Plow J, Huang M. Lipoprotein(a) metabolism: potential sites for therapeutic targets. Metabolism. 2013;62:479–91.
Hovingh GK, Kastelein JJ, van Deventer SJ, Round P, Ford J, Saleheen D, Rader DJ, Brewer HB, Barter PJ. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2015;386:452–60.
Hovingh GK, Klausen IC, Heggen E, McCarty K, Zhou R, Isaac BF, Taub R, Langslet G, Kastelein JJP. Resmetirom (MGL-3196) in patients with heterozygous familial hypercholesterolemia. J Am Coll Cardiol. 2022;79:1220–2.
Jenner JL, Seman LJ, Millar JS, Lamon-Fava S, Welty FK, Dolnikowski GG, Marcovina SM, Lichtenstein AH, Barrett PHR, Schaefer EJ. The metabolism of apolipoproteins(a) and B-100 within plasma lipoprotein(a) in human beings. Metabolism. 2005;54:361–9.
Kagawa A, Azuma H, Akaike M, Kanagawa Y, Matsumoto T. Aspirin reduces apolipoprotein(a) (apo(a)) production in human hepatocytes by suppression of apo(a) gene transcription. J Biol Chem. 1999;274:34111–5.
Karwatowska-Prokopczuk E, Clouet-Foraison N, Xia S, Viney NJ, Witztum JL, Marcovina SM, Tsimikas S. Prevalence and influence of LPA gene variants and isoform size on the Lp(a)-lowering effect of pelacarsen. Atherosclerosis. 2021;324:102–8.
Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, Mora S. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). Circulation. 2014;129:635–42.
Klausen IC, Gerdes LU, Hansen PS, Lemming L, Gerdes C, Faergeman O. Effects of apoE gene polymorphism on Lp(a) concentrations depend on the size of apo(a): a study of 466 white men. J Mol Med (Berl). 1996;74:685–90.
Korneva VA, Kuznetsova TY, Julius U. Modern approaches to lower lipoprotein(a) concentrations and consequences for cardiovascular diseases. Biomedicine. 2021;9:1271.
Krempler F, Kostner GM, Bolzano K, Sandhofer F. Turnover of lipoprotein (a) in man. J Clin Invest. 1980;65:1483–90.
Kronenberg F, Utermann G. Lipoprotein(a): resurrected by genetics. J Intern Med. 2013;273:6–30.
Kroon AA, van’t Hof MA, Demacker PNM, Stalenhoef AFH. The rebound of lipoproteins after LDL-apheresis. Kinetics and estimation of mean lipoprotein levels. Atherosclerosis. 2000;152:519–26.
Lambert G, Thedrez A, Croyal M, Ramin-Mangata S, Couret D, Diotel N, Nobécourt-Dupuy E, Krempf M, LeBail JC, Poirier B, Blankenstein J, Villard EF, Guillot E. The complexity of lipoprotein (a) lowering by PCSK9 monoclonal antibodies. Clin Sci (Lond). 2017;131:261–8.
Lamon-Fava S, Jimenez D, Christian JC, Fabsitz RR, Reed T, Carmelli D, Castelli WP, Ordovas JM, Wilson PW, Schaefer EJ. The NHLBI Twin Study: heritability of apolipoprotein A-I, B, and low density lipoprotein subclasses and concordance for lipoprotein(a). Atherosclerosis. 1991;91:97–106.
Li J, Lange LA, Sabourin J, Duan Q, Valdar W, Willis MS, Li Y, Wilson JG, Lange EM. Genome- and exome-wide association study of serum lipoprotein (a) in the Jackson Heart Study. J Hum Genet. 2015;60:755–61.
Lincoff AM, Nicholls SJ, Riesmeyer JS, Barter PJ, Brewer HB, Fox KAA, Gibson CM, Granger C, Menon V, Montalescot G, Rader D, Tall AR, McErlean E, Wolski K, Ruotolo G, Vangerow B, Weerakkody G, Goodman SG, Conde D, McGuire DK, Nicolau JC, Leiva-Pons JL, Pesant Y, Li W, Kandath D, Kouz S, Tahirkheli N, Mason D, Nissen SE. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933–42.
Lobentanz E-M, Krasznai K, Gruber A, Brunner C, Müller H-J, Sattler J, Kraft H-G, Utermann G, Dieplinger H. Intracellular metabolism of human apolipoprotein(a) in stably transfected Hep G2 cells. Biochemistry. 1998;37:5417–25.
Ma L, Chan DC, Ooi EMM, Barrett PHR, Watts GF. Fractional turnover of apolipoprotein(a) and apolipoprotein B-100 within plasma lipoprotein(a) particles in statin-treated patients with elevated and normal Lp(a) concentration. Metabolism. 2019a;96:8–11.
Ma L, Chan DC, Ooi EMM, Marcovina SM, Barrett PHR, Watts GF. Apolipoprotein(a) kinetics in statin-treated patients with elevated plasma lipoprotein(a) concentration. J Clin Endocrinol Metab. 2019b;104:6247–55.
Ma L, Waldmann E, Ooi EM, Chan DC, Barrett HP, Watts GF, Parhofer KG. Lipoprotein (a) and low-density lipoprotein apolipoprotein B metabolism following apheresis in patients with elevated lipoprotein (a) and coronary artery disease. Eur J Clin Investig. 2019c;49:e13053.
Mack S, Coassin S, Rueedi R, Yousri NA, Seppälä I, Gieger C, Schönherr S, Forer L, Erhart G, Marques-Vidal P, Ried JS, Waeber G, Bergmann S, Dähnhardt D, Stöckl A, Raitakari OT, Kähönen M, Peters A, Meitinger T, Strauch K, Kedenko L, Paulweber B, Lehtimäki T, Hunt SC, Vollenweider P, Lamina C, Kronenberg F. A genome-wide association meta-analysis on lipoprotein (a) concentrations adjusted for apolipoprotein (a) isoforms. J Lipid Res. 2017;58:1834–44.
Marcoux C, Lussier-Cacan S, Davignon J, Cohn JS. Association of Lp(a) rather than integrally-bound apo(a) with triglyceride-rich lipoproteins of human subjects. Biochim Biophys Acta. 1997;1346:261–74.
Marcovina SM, Koschinsky ML. Lipoprotein(a) concentration and apolipoprotein(a) size—a synergistic role in advanced atherosclerosis? Circulation. 1999;100:1151–3.
Marcovina SM, Hobbs HH, Albers JJ. Relation between number of apolipoprotein(a) kringle 4 repeats and mobility of isoforms in agarose gel: basis for a standardized isoform nomenclature. Clin Chem. 1996;42:436–9.
März W, Beckmann A, Scharnagl H, Siekmeier R, Mondorf U, Held I, Schneider W, Preissner KT, Curtiss LK, Gross W, Huttinger M. Heterogeneous lipoprotein (a) size isoforms differ by their interaction with the low density lipoprotein receptor and the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. FEBS Lett. 1993;325:271–5.
McCormick SPA, Schneider WJ. Lipoprotein(a) catabolism: a case of multiple receptors. Pathology. 2019;51:155–64.
Moriarty PM, Varvel SA, Gordts PL, McConnell JP, Tsimikas S. Lipoprotein(a) mass levels increase significantly according to APOE genotype: an analysis of 431 239 patients. Arterioscler Thromb Vasc Biol. 2017;37:580–8.
Nandakumar R, Matveyenko A, Thomas T, Pavlyha M, Ngai C, Holleran S, Ramakrishnan R, Ginsberg HN, Karmally W, Marcovina SM, Reyes-Soffer G. Effects of mipomersen, an apolipoprotein B100 antisense, on lipoprotein (a) metabolism in healthy subjects. J Lipid Res. 2018;59:2397–402.
Nassir F, Bonen DK, Davidson NO. Apolipoprotein(a) synthesis and secretion from hepatoma cells is coupled to triglyceride synthesis and secretion. J Biol Chem. 1998;273:17793–800.
Nicholls SJ, Tang WW, Scoffone H, Brennan DM, Hartiala J, Allayee H, Hazen SL. Lipoprotein(a) levels and long-term cardiovascular risk in the contemporary era of statin therapy. J Lipid Res. 2010;51:3055–61.
Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57:1953–75.
Ooi EM, Watts GF, Chan DC, Pang J, Tenneti VS, Hamilton SJ, McCormick SP, Marcovina SM, Barrett PH. Effects of extended-release niacin on the postprandial metabolism of Lp(a) and ApoB-100-containing lipoproteins in statin-treated men with type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2015;35:2686–93.
Parham JS, Goldberg AC. Mipomersen and its use in familial hypercholesterolemia. Expert Opin Pharmacother. 2019;20:127–31.
Parhofer KG, Demant T, Ritter MM, Geiss HC, Donner M, Schwandt P. Lipoprotein(a) metabolism estimated by nonsteady-state kinetics. Lipids. 1999;34:325–35.
Raal FJ, Kallend D, Ray KK, Turner T, Koenig W, Wright RS, Wijngaard PLJ, Curcio D, Jaros MJ, Leiter LA, Kastelein JJP. Inclisiran for the treatment of heterozygous familial hypercholesterolemia. N Engl J Med. 2020;382:1520–30.
Rader DJ, Mann WA, Cain W, Kraft HG, Usher D, Zech LA, Hoeg JM, Davignon J, Lupien P, Grossman M. The low density lipoprotein receptor is not required for normal catabolism of Lp(a) in humans. J Clin Invest. 1995;95:1403–8.
Ramos-Cáceres M, Lamiquiz-Moneo I, Cenarro A, Calmarza P, Marco-Benedí V, Bea AM, Mateo-Gallego R, Puzo J J, Ordovas JM, Civeira F, Laclaustra M. Triglyceride metabolism modifies lipoprotein(a) plasma concentration. J Clin Endocrinol Metab. 2022;107:3594–602.
Ray KK, on Behalf of the ROSE Investigators. Obicetrapib lowers LDL-C in patients on high-intensity statin: results from the ROSE trial. Presented at EAS Congress 2022, 23 May 2022.
Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PLJ, Kastelein JJP. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382:1507–19.
Reblin T, Niemeier A, Meyer N, Willnow TE, Kronenberg F, Dieplinger H, Greten H, Beisiegel U. Cellular uptake of lipoprotein[a] by mouse embryonic fibroblasts via the LDL receptor and the LDL receptor-related protein. J Lipid Res. 1997;38:2103–10.
Reyes-Soffer G, Moon B, Hernandez-Ono A, Dionizovik-Dimanovski M, Jimenez J, Obunike J, Thomas T, Ngai C, Fontanez N, Donovan DS, Karmally W, Holleran S, Ramakrishnan R, Mittleman RS, Ginsberg HN. Complex effects of inhibiting hepatic apolipoprotein B100 synthesis in humans. Sci Transl Med. 2016;8:323ra12.
Reyes-Soffer G, Pavlyha M, Ngai C, Thomas T, Holleran S, Ramakrishnan R, Karmally W, Nandakumar R, Fontanez N, Obunike J, Marcovina SM, Lichtenstein AH, Matthan NR, Matta J, Maroccia M, Becue F, Poitiers F, Swanson B, Cowan L, Sasiela WJ, Surks HK, Ginsberg HN. Effects of PCSK9 inhibition with alirocumab on lipoprotein metabolism in healthy humans. Circulation. 2017;135:352–62.
Reyes-Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, Lloyd-Jones DM, Marcovina SM, Yeang C, Koschinsky ML. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement From the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42:e48–60.
Romagnuolo R, Scipione CA, Boffa MB, Marcovina SM, Seidah NG, Koschinsky ML. Lipoprotein(a) catabolism is regulated by proprotein convertase subtilisin/kexin type 9 through the low density lipoprotein receptor. J Biol Chem. 2015;290:11649–62.
Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–22.
Saleheen D, Haycock PC, Zhao W, Rasheed A, Taleb A, Imran A, Abbas S, Majeed F, Akhtar S, Qamar N, Zaman KS, Yaqoob Z, Saghir T, Rizvi SNH, Memon A, Mallick NH, Ishaq M, Rasheed SZ, Memon FU, Mahmood K, Ahmed N, Frossard P, Tsimikas S, Witztum JL, Marcovina S, Sandhu M, Rader DJ, Danesh J. Apolipoprotein(a) isoform size, lipoprotein(a) concentration, and coronary artery disease: a mendelian randomisation analysis. Lancet Diabetes Endocrinol. 2017;5:524–33.
Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a). J Lipid Res. 2016;57:1339–59.
Schmidt AF, Hunt NB, Gordillo-Marañón M, Charoen P, Drenos F, Kivimaki M, Lawlor DA, Giambartolomei C, Papacosta O, Chaturvedi N, Bis JC, O’Donnell CJ, Wannamethee G, Wong A, Price JF, Hughes AD, Gaunt TR, Franceschini N, Mook-Kanamori DO, Zwierzyna M, Sofat R, Hingorani AD, Finan C. Cholesteryl ester transfer protein (CETP) as a drug target for cardiovascular disease. Nat Commun. 2021;12:5640.
Schwartz GG, Ballantyne CM. Existing and emerging strategies to lower lipoprotein(a). Atherosclerosis. 2022;349:110–22.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, Leitersdorf E, McMurray JJ, Mundl H, Nicholls SJ, Shah PK, Tardif JC, Wright RS, dal OI. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99.
Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–107.
Schwartz GG, Szarek M, Bittner VA, Diaz R, Goodman SG, Jukema JW, Landmesser U, López-Jaramillo P, Manvelian G, Pordy R, Scemama M, Sinnaeve PR, White HD, Gabriel SP. Lipoprotein(a) and benefit of PCSK9 inhibition in patients with nominally controlled LDL cholesterol. J Am Coll Cardiol. 2021;78:421–33.
Smith KW, White CM. Inclisiran: a novel small interfering RNA drug for low-density lipoprotein reduction. J Clin Pharmacol. 2022; https://doi.org/10.1002/jcph.2045. Epub ahead of print.
Takagi H, Umemoto T. Atorvastatin decreases lipoprotein (a): a meta-analysis of randomized trials. Int J Cardiol. 2012;154:183–6.
Tall AR. Plasma cholesteryl ester transfer protein. J Lipid Res. 1993;34:1255–74.
Thomas T, Zhou H, Karmally W, Ramakrishnan R, Holleran S, Liu Y, Jumes P, Wagner JA, Hubbard B, Previs SF, Roddy T, Johnson-Levonas AO, Gutstein DE, Marcovina SM, Rader DJ, Ginsberg HN, Millar JS, Reyes-Soffer G. CETP (Cholesteryl ester transfer protein) inhibition with anacetrapib decreases production of lipoprotein(a) in mildly hypercholesterolemic subjects. Arterioscler Thromb Vasc Biol. 2017;37:1770–5.
Tsimikas S. Lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69:692–711.
Tsimikas S, Fazio S, Ferdinand KC, Ginsberg HN, Koschinsky ML, Marcovina SM, Moriarty PM, Rader DJ, Remaley AT, Reyes-Soffer G, Santos RD, Thanassoulis G, Witztum JL, Danthi S, Olive M, Liu L. NHLBI working group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71:177–92.
Tsimikas S, Gordts PLSM, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein (a) levels. Eur Heart J. 2020;41:2275–84.
Villard EF, Thedrez A, Blankenstein J, Croyal M, Tran TT, Poirier B, Le Bail JC, Illiano S, Nobécourt E, Krempf M, Blom DJ, Marais AD, Janiak P, Muslin AJ, Guillot E, Lambert G. PCSK9 modulates the secretion but not the cellular uptake of lipoprotein(a) ex vivo: an effect blunted by alirocumab. JACC Basic Transl Sci. 2016;1:419–27.
Wade DP, Knight BL, Harders-Spengel K, Soutar AK. Detection and quantitation of apolipoprotein(a) mRNA in human liver and its relationship with plasma lipoprotein(a) concentration. Atherosclerosis. 1991;91:63–72.
Wang X, Li J, Ju J, Fan Y, Xu H. Effect of different types and dosages of statins on plasma lipoprotein(a) levels: a network meta-analysis. Pharmacol Res. 2021;163:105275.
Ward NC, Chan DC, Watts GF. A tale of two new targets for hypertriglyceridaemia: which choice of therapy? BioDrugs. 2022;36:121–35.
Watts GF, Chan DC, Dent R, Somaratne R, Wasserman SM, Scott R, Burrows S, Barrett PHR. Factorial effects of evolocumab and atorvastatin on lipoprotein metabolism. Circulation. 2017;135:338–51.
Watts GF, Chan DC, Somaratne R, Wasserman SM, Scott R, Marcovina SM, Barrett PHR. Controlled study of the effect of proprotein convertase subtilisin-kexin type 9 inhibition with evolocumab on lipoprotein(a) particle kinetics. Eur Heart J. 2018;39:2577–85.
Watts GF, Chan DC, Pang J, Ma L, Ying Q, Aggarwal S, Marcovina SM, Barrett PHR. PCSK9 inhibition with alirocumab increases the catabolism of lipoprotein(a) particles in statin-treated patients with elevated lipoprotein(a). Metabolism. 2020;107:154221.
White AL, Hixson JE, Rainwater DL, Lanford RE. Molecular basis for “null” lipoprotein(a) phenotypes and the influence of apolipoprotein(a) size on plasma lipoprotein(a) level in the baboon. J Biol Chem. 1994;269:9060–6.
Wu H, Luan J, Forgetta V, Engert JC, Thanassoulis G, Mooser V, Wareham NJ, Langenberg C, Richards JB. Utility of genetically predicted lp(a) (lipoprotein [a]) and apoB levels for cardiovascular risk assessment. Circ Genom Precis Med. 2021;14:e003312.
Yahya R, Berk K, Verhoeven A, Bos S, van der Zee L, Touw J, Erhart G, Kronenberg F, Timman R, Sijbrands E, Roeters van Lennep J, Mulder M. Statin treatment increases lipoprotein(a) levels in subjects with low molecular weight apolipoprotein(a) phenotype. Atherosclerosis. 2019;289:201–5.
Ying Q, Chan DC, Watts GF. New insights into the regulation of lipoprotein metabolism by PCSK9: lessons from stable isotope tracer studies in human subjects. Front Physiol. 2021;12:603910.
Ying Q, Croyal M, Chan DC, Blanchard V, Pang J, Krempf M, Watts GF. Effect of omega-3 fatty acid supplementation on the postprandial metabolism of apolipoprotein(a) in familial hypercholesterolemia. J Atheroscler Thromb. 2022; https://doi.org/10.5551/jat.63587. Epub ahead of print.
Youssef A, Clark JR, Marcovina SM, Boffa MB, Koschinsky ML. Apo(a) and apoB interact noncovalently within hepatocytes: implications for regulation of lp(a) levels by modulation of apoB secretion. Arterioscler Thromb Vasc Biol. 2022;42:289–304.
Zekavat SM, Ruotsalainen S, Handsaker RE, Alver M, Bloom J, Poterba T, Seed C, Ernst J, Chaffin M, Engreitz J, Peloso GM, Manichaikul A, Yang C, Ryan KA, Fu M, Johnson WC, Tsai M, Budoff M, Vasan RS, Cupples LA, Rotter JI, Rich SS, Post W, Mitchell BD, Correa A, Metspalu A, Wilson JG, Salomaa V, Kellis M, Daly MJ, Neale BM, McCarroll S, Surakka I, Esko T, Ganna A, Ripatti S, Kathiresan S, Natarajan P. Deep coverage whole genome sequences and plasma lipoprotein(a) in individuals of European and African ancestries. Nat Commun. 2018;9:2606.
Acknowledgement
JP was supported by the National Health and Medical Research Council (HMRC) Investigator Grant.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chan, D.C., Pang, J., Watts, G.F. (2023). Contemporary Aspects of Lp(a) Metabolism and Therapies Based on Tracer Kinetic Studies in Humans. In: Kostner, K., Kostner, G.M., Toth, P.P. (eds) Lipoprotein(a). Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-031-24575-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-24575-6_5
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-031-24574-9
Online ISBN: 978-3-031-24575-6
eBook Packages: MedicineMedicine (R0)