Abstract
High lipoprotein(a) [Lp(a)] levels are found in over one billion individuals worldwide. Based on decades of accumulating evidence from mechanistic, epidemiological, and genetic studies, Lp(a) is established as a likely causal risk factor for ischemic cardiovascular and, in particular, coronary artery disease. In recent years, large genetic epidemiologic studies have also provided compelling evidence for high Lp(a) levels as a strong, causal risk factor for calcific aortic valve stenosis. Accumulating evidence also points to high Lp(a) levels as a risk factor for ischemic stroke in adults and children. This chapter will summarize findings for Lp(a) and aortic valve stenosis, stroke, and other noncoronary cardiovascular diseases, focusing on findings from large genetic epidemiologic studies, and provide estimates for Lp(a) level thresholds for increased risk.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
High lipoprotein(a) [Lp(a)] levels are now, based on more than three decades of accumulating evidence from mechanistic, epidemiological, and genetic studies, widely recognized as an important and likely causal risk factor for ischemic cardiovascular and, in particular, coronary artery disease (CAD) (Kamstrup 2021; Reyes-Soffer et al. 2022). High Lp(a) levels in the top 10% of the concentration distribution (Fig. 14.1) associate with a two to threefold increase in risk of myocardial infarction independent of conventional risk factors (Kamstrup 2021). More recently, high Lp(a) levels, found in an estimated >1 billion individuals globally, have also been identified as a risk factor for calcific aortic valve stenosis (AVS) with risk estimates of at least the same magnitude as those found for CAD (Thanassoulis et al. 2013; Kamstrup et al. 2014). Several clinical guidelines on cardiovascular disease (CVD) prevention now recommend once-in-a-lifetime Lp(a) measurements in all to identify individuals at increased risk and optimize management of modifiable risk factors (Mach et al. 2020; Pearson et al. 2021).
Lp(a) concentration distribution. Plasma levels of Lp(a) (total mass and particle number) in the Copenhagen General Population Study (N = 79,718 White individuals of Danish descent, 118 measurements >200 mg/dL not displayed). All measurement values were calibrated to fresh sample measurements by the latex-enhanced Denka Seiken (Denka Seiken, Tokyo, Japan) immunoturbidimetric assay with traceability to an international calibrator (WHO SRM 2B). Conversion to nmol/L was done according to the following equation based on ~13,900 individuals with measurements in both mg/dL and nmol/L (Denka Seiken Roche distributed assay using different calibrations for mg/dL and nmol/L): lipoprotein(a) nmol/L = 2.18*lipoprotein(a) mg/dL–3.83. (Adapted from Clin Chem 2021;67:154–166 with permission)
The mounting evidence that high Lp(a) levels represent an unmet clinical need have spurred the development of potent Lp(a)-lowering drugs opening opportunities for future CVD prevention in individuals with levels above thresholds for increased risk (Reyes-Soffer et al. 2022). In this chapter, we summarize findings for Lp(a) and AVS, stroke, and other noncoronary CVD, focusing on findings from large genetic epidemiologic studies, and provide estimates for thresholds.
Aortic Valve Stenosis
Calcific AVS is a chronic, progressive disease which shares risk factors with atherosclerotic disease and is estimated to affect 3% of adults older than 75 years of age and with a steeply increasing disease burden in high-income countries (Otto and Prendergast 2014; Yadgir et al. 2020). Up to 50% of patients progress to severe disease within 2–4 years, and valve replacement represents the only treatment option for severe disease characterized by obstructive heart failure and increased risk of sudden death (Coisne et al. 2021). While familial aggregation exists for both bi- and tricuspid diseases, up until 2013, no specific genetic risk factors had been identified (Otto and Prendergast 2014). However, in a landmark study from 2013, and using a hypothesis-free genome-wide association study (GWAS) approach, the LPA gene was identified as the only genome-wide significant locus for the presence of aortic valve calcification (AVC) in a White European ancestry cohort (N = 6942) and with replication also in African American and Hispanic American cohorts (Thanassoulis et al. 2013). The LPA rs10455872 single nucleotide polymorphism (SNP), previously found to be strongly associated with Lp(a) levels (Clarke et al. 2009), associated with a twofold increase per allele in risk of AVC considered an early phenotype for AVS (Thanassoulis et al. 2013). Also, based on Lp(a) levels available in a subgroup (N = 3670), an odds ratio of 1.62 (1.27–2.06) for AVC was found per log-unit increase in genetically determined Lp(a) levels. Finally, in two cohort studies, an association with incident AVS and valve replacement was found with hazard ratios per allele of 1.7 (95% confidence interval: 1.3–2.2) and 1.5 (1.1–2.3). The combined findings of the study provided strong genetic evidence of a causal association of Lp(a) with AVC and likely also AVS.
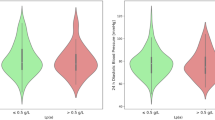
Prior to 2013, associations of high Lp(a) levels with increased risk of AVC or AVS had only been sporadically reported in smaller epidemiological studies (Gotoh et al. 1995; Stewart et al. 1997; Glader et al. 2003; Bozbas et al. 2007). However, the 2013 GWAS study generated considerable interest in high Lp(a) levels as a possible causal risk factor for calcific AVS. Thus, in 2014, risk estimates for incident AVS at different levels of Lp(a) were reported from large general population studies. The first was a combined analysis of the historic Copenhagen City Heart Study and the contemporary Copenhagen General Population Study (Kamstrup et al. 2014). Risk of AVS was increased for Lp(a) levels in the top third of the concentration distribution (≥20 mg/dL, ≥40 nmol/L), and individuals with levels ≥90th percentile (≥65 mg/dL, ≥138 nmol/L) had a two to threefold increased risk of AVS as compared to individuals with levels <5 mg/dL (N = 29,016, Fig. 14.2). Notably, the risk estimates appeared independent of the presence or absence of CAD. On a continuous scale, a tenfold increase in plasma Lp(a) levels is associated with a hazard ratio of 1.4 (1.2–1.7) comparable to the 1.6 (1.2–2.1)-fold increase in risk found for a similar increase in genetically determined levels based on three LPA genotypes (rs10455872, rs3798220, KIV-2) explaining 41% of the total variation in plasma levels. The second was from the European Prospective Investigation into Cancer (EPIC)-Norfolk study (N = 17,553), where individuals in the top third of the Lp(a) concentration distribution had a 1.6 (1.0–2.4)-fold increased risk of AVS with consistent genetic findings as hetero- and homozygous minor allele rs10455872 carriers had a 1.8 (1.1–2.9)- and 4.8 (1.8–13.2)-fold increased risk, as compared to noncarriers (Arsenault et al. 2014).
Risk of aortic valve stenosis by Lp(a) levels. Analyses were adjusted for age, sex, total cholesterol, HDL (high-density lipoprotein) cholesterol, systolic blood pressure, smoking, and diabetes. Lipoprotein(a) in mg/dL is shown as median (interquartile range). (Adapted from J Am Coll Cardiol 2014;63:470–7 with permission)
In subsequent years, additional large genetic studies provided evidence that high Lp(a) levels were at least as strong a risk factor for AVS as for CAD (Emdin et al. 2016; Gudbjartsson et al. 2019). Thus, in a study including 112,338 UK Biobank participants and using a genetic risk score based on four LPA SNPs associated with decreased Lp(a) plasma levels, a one standard deviation decrease in genetically determined Lp(a) levels is associated with a 37% reduced risk of AVS, compared to effect sizes of 31% for peripheral vascular disease, 29% for CAD, 17% for heart failure, and 17% for stroke (Emdin et al. 2016). Similarly, in a large case-control study of 143,087 Icelanders from 2019, genetically imputed Lp(a) levels were associated with similar risk estimates for CAD and AVS of 16–17% risk increase per 50 nM (~25 mg/dL) increase in genetically determined Lp(a) levels. The association appeared entirely mediated by increased Lp(a) levels and not by the concomitant small apolipoprotein(a) isoform size, a previous point of contention (Gudbjartsson et al. 2019).
While genetic studies have provided strong evidence for high Lp(a) levels as a cause of AVS, the pathophysiological mechanism is not fully understood. In vitro studies have, however, demonstrated osteogenic differentiation of valvular interstitial cells exposed to Lp(a) and associated pro-inflammatory oxidized phospholipids (Zheng et al. 2019), and both measurements have in AVS patient cohort studies been associated with the progression of both mild to moderate and more advanced valvular diseases (Zheng et al. 2019; Capoulade et al. 2018), and most recently also with aortic valve microcalcification in individuals without macroscopically detectable valve pathology (Despres et al. 2019), thus also pointing to a role in earlier-stage disease. The totality of evidence pointing to high Lp(a) levels as a potentially modifiable cause of calcific AVS thus holds great promise for improved prevention of symptomatic AVS upon future availability of effective Lp(a)-lowering drugs.
Stroke
Ischemic Stroke
Lp(a) is a firmly established risk factor for myocardial infarction and AVS by proposed pathophysiological mechanisms such as atherosclerosis and thrombosis. The association of high Lp(a) levels with risk of ischemic stroke (IS) is not as firmly established as results from several studies are unclear and somewhat conflicting. Most studies did find increased risk of IS with high Lp(a) levels such as in the large prospective Atherosclerosis Risk in Communities (ARIC) study from the USA, which included both White and Black individuals with a 79% higher risk ratio for high versus low levels (Ohira et al. 2006). Another large prospective contemporary study from Denmark, the Copenhagen General Population Study (N = 46,699), found increased risk of IS per 50 mg/dL higher Lp(a) levels with a hazard ratio of 1.2 (95% CI: 1.1–1.3) in observational analyses and with genetic estimates of 1.2 (1.0–1.4) via KIV2 and 1.3 (1.1–1.5) via rs10455872, indicating a causal role (Fig. 14.3) (Langsted et al. 2019a). In support of a genetic association, the previously mentioned large UK Biobank study found an odds ratio of 0.87 (0.79–0.96) for risk of IS for one standard deviation genetically lower Lp(a) levels (Emdin et al. 2016). Also, meta-analyses of observational studies, for example, from the Emerging Risk Factors Collaboration including data from 24 studies (Emerging Risk Factors Collaboration et al. 2009) and from India including data from 41 studies (Kumar et al. 2021) on IS, find an association of high Lp(a) levels with increased risk of IS. In the Prospective Epidemiological Study of Myocardial Infarction (PRIME) study, a large prospective study from Northern Ireland and France, the association was not significant, but the point estimate indicated an increased risk of IS with high Lp(a) levels (Canoui-Poitrine et al. 2010). On the contrary, data from the Physicians’ Health Study including White middle-aged males from the USA found no association between high Lp(a) levels and risk of IS (Ridker et al. 1995).
Risk of ischemic stroke in the Copenhagen General Population Study. Age- and sex-adjusted observational odds ratio and causal genetic risk ratios by LPA KIV2 and LPA rs10455872 with 95% confidence intervals for ischemic stroke for 50 mg/dL (105 nmol/L) higher Lp(a) levels. (Adapted from J Am Coll Cardiol. 2019;74(1):54–66 with permission from Elsevier)
Notably, the risk estimates for IS for high Lp(a) levels or corresponding genetic variants are lower than those reported for myocardial infarction and AVS, perhaps indicating that the pathophysiology for IS might be different.
Hemorrhagic Stroke
In most cases, the pathophysiology of hemorrhagic stroke differs greatly from the causes of IS. High Lp(a) levels have previously been associated with a low risk of bleeding (Langsted et al. 2017) perhaps due to the proposed antifibrinolytic effects of Lp(a) because of its homology with plasminogen. Most studies examining the role of Lp(a) in stroke have focused on ischemic or overall stroke as described above, and results on hemorrhagic stroke are even more mixed with both protective and pathological effects of high Lp(a) levels reported. In a study from Japan, it was found that high Lp(a) levels were associated with low risk of hemorrhagic stroke, most significantly in men with a hazard ratio of 0.44 (0.21–0.96) for highest versus lowest tertile of Lp(a) levels (Ishikawa et al. 2013). The meta-analysis from the Emerging Risk Factors Collaboration et al. (2009) including nine studies on hemorrhagic stroke found no association of Lp(a) with hemorrhagic stroke, in contrast to findings from two Chinese studies (Sun et al. 2003; Fu et al. 2020) which found high Lp(a) levels to be associated with increased risk of hemorrhagic stroke.
The complex nature and fundamentally different causes of hemorrhagic stroke compared to ischemic stroke might be one of the reasons for these highly conflicting results. Studies including subtypes of hemorrhagic stroke based on the underlying pathophysiology are needed to find meaningful associations.
Arterial Ischemic Stroke in the Young
In 2011, pediatric guidelines for cardiovascular disease risk reduction introduced screening for high Lp(a) levels in children and adolescents with a previous ischemic or hemorrhagic stroke (Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute 2011). Several studies have examined the relationship of high Lp(a) levels and risk of arterial IS in the young. A meta-analysis including 4 studies and a total of 90 events found that children with high Lp(a) levels have an odds ratio of 4.2 (2.9–6.1) for risk of arterial IS (Sultan et al. 2014). Another meta-analysis including five studies (with two studies also included in the former meta-analysis) found an odds ratio for high Lp(a) levels of 6.3 (4.5–8.7) in children with arterial IS (Kenet et al. 2010).
Of note, as arterial IS is much less prevalent in children than in adults and is often associated with underlying medical conditions, most of the studies on Lp(a) have excluded children with other risk factors which may, therefore, limit the generalizability of the study findings; however, the risk estimates are substantial and should be investigated further.
Other Noncoronary Cardiovascular Diseases
Heart Failure
The two to threefold increased risk of myocardial infarction and calcific AVS found in individuals with Lp(a) levels in the top decile is consistent with Lp(a) also being a possible risk factor for heart failure (HF), representing a major and increasing health-economic burden in aging populations (Kamstrup 2021; Heidenreich et al. 2013). In 2015, a clear stepwise association of Lp(a) levels with risk of incident HF was reported from the combined Copenhagen general population studies including >98,000 adult individuals of Danish descent (Kamstrup and Nordestgaard 2016). Lp(a) levels >90th percentile (>67 mg/dL) are associated with a 1.6–1.8-fold increased risk as compared to individuals with levels in the lower third of the concentration distribution and with comparable genetic risk estimates in support of a causal association. Notably, the association appeared largely driven by the likely causal associations of Lp(a) with myocardial infarction and/or AVS, with 63% of the Lp(a)-driven HF risk mediated by these two conditions (Kamstrup and Nordestgaard 2016). Further, a 9% population attributable risk of HF was estimated for high Lp(a) levels indicating that, given the development of future effective Lp(a)-lowering treatments, a notable decrease in HF incidence may also be achieved.
The observational association of Lp(a) levels with HF was since replicated in both the Atherosclerosis Risk in Communities (ARIC) study and in the Multi-Ethnic Study of Atherosclerosis (MESA) (Agarwala et al. 2017; Steffen et al. 2018). Findings from the large 2019 Icelandic case-control study with information on measured and genetically imputed Lp(a) plasma levels also provided additional evidence of a causal association of Lp(a) with HF (Gudbjartsson et al. 2019). Thus, a 50 nM (~25 mg/dL) increase in genetically determined Lp(a) levels is associated with a 5% increase in risk of HF. This is in addition to the more pronounced risk increases reported for CAD, AVS, and peripheral arterial disease (PAD).
Peripheral Arterial Disease
The proposed pathophysiological pathway of lipoprotein(a) through interference with fibrinolysis and thereby promoting thrombosis and the causal association with myocardial infarction could also result in arteriosclerotic or thrombotic PAD. In a prespecified analysis of the ODYSSEY OUTCOMES trial evaluating a PCSK9 inhibitor, it was found that the highest versus lowest quartile of lipoprotein(a) was associated with a hazard ratio of 2.2 (1.4–3.6) for risk of PAD and further that lowering of high lipoprotein(a) levels reduced the risk (Schwartz et al. 2020). In a general population study from Denmark, highest versus lowest tertile of lipoprotein(a) was associated with an odds ratio of 1.6 (1.3–2.0) for risk of peripheral arterial stenosis (Kamstrup et al. 2012). Further, large genetic studies examining genetically determined high lipoprotein(a) levels have also found a solid likely causal association with risk of PAD (Emdin et al. 2016; Laschkolnig et al. 2014).
Summary
Lp(a) has since its discovery been a lipoprotein particle of high interest in cardiovascular research due to a composition consistent with both proatherosclerotic and prothrombotic effects. It is now well established that high levels are associated both observationally and genetically, and therefore likely causally, with increased risk of CAD, calcific AVS, HF, IS, PAD, and mortality (Langsted et al. 2019b). The exact pathophysiology of high Lp(a) levels has not yet been elucidated and may involve, in addition to proatherosclerotic and prothrombotic effects, also proinflammatory and procalcific effects, and the exact mechanisms behind different CVD manifestations may differ.
Guidelines today are transitioning from recommending measurement of Lp(a) only in individuals at increased risk of cardiovascular disease to once-in-a-lifetime measurement in all. Currently, promising lipoprotein(a)-lowering agents are being tested, and studies will hopefully show that lowering of lipoprotein(a) will lower the risk of CVD.
References
Agarwala A, Pokharel Y, Saeed A, Sun W, Virani SS, Nambi V, et al. The association of lipoprotein(a) with incident heart failure hospitalization: atherosclerosis risk in communities study. Atherosclerosis. 2017;262:131–7.
Arsenault BJ, Boekholdt SM, Dube MP, Rheaume E, Wareham NJ, Khaw KT, et al. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet. 2014;7(3):304–10.
Bozbas H, Yildirir A, Atar I, Pirat B, Eroglu S, Aydinalp A, et al. Effects of serum levels of novel atherosclerotic risk factors on aortic valve calcification. J Heart Valve Dis. 2007;16(4):387–93.
Canoui-Poitrine F, Luc G, Bard JM, Ferrieres J, Yarnell J, Arveiler D, et al. Relative contribution of lipids and apolipoproteins to incident coronary heart disease and ischemic stroke: the PRIME study. Cerebrovasc Dis. 2010;30(3):252–9.
Capoulade R, Yeang C, Chan KL, Pibarot P, Tsimikas S. Association of mild to moderate aortic valve stenosis progression with higher lipoprotein(a) and oxidized phospholipid levels: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2018;3(12):1212–7.
Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–28.
Coisne A, Montaigne D, Aghezzaf S, Ridon H, Mouton S, Richardson M, et al. Association of mortality with aortic stenosis severity in outpatients: results from the VALVENOR study. JAMA Cardiol. 2021;6(12):1424–31.
Despres AA, Perrot N, Poulin A, Tastet L, Shen M, Chen HY, et al. Lipoprotein(a), oxidized phospholipids, and aortic valve microcalcification assessed by 18F-sodium fluoride positron emission tomography and computed tomography. CJC Open. 2019;1(3):131–40.
Emdin CA, Khera AV, Natarajan P, Klarin D, Won HH, Peloso GM, et al. Phenotypic characterization of genetically lowered human lipoprotein(a) levels. J Am Coll Cardiol. 2016;68(25):2761–72.
Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–23.
Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–56.
Fu H, Zhang D, Zhu R, Cui L, Qiu L, Lin S, et al. Association between lipoprotein(a) concentration and the risk of stroke in the Chinese Han population: a retrospective case-control study. Ann Transl Med. 2020;8(5):212.
Glader CA, Birgander LS, Soderberg S, Ildgruben HP, Saikku P, Waldenstrom A, et al. Lipoprotein(a), Chlamydia pneumoniae, leptin and tissue plasminogen activator as risk markers for valvular aortic stenosis. Eur Heart J. 2003;24(2):198–208.
Gotoh T, Kuroda T, Yamasawa M, Nishinaga M, Mitsuhashi T, Seino Y, et al. Correlation between lipoprotein(a) and aortic valve sclerosis assessed by echocardiography (the JMS Cardiac Echo and Cohort Study). Am J Cardiol. 1995;76(12):928–32.
Gudbjartsson DF, Thorgeirsson G, Sulem P, Helgadottir A, Gylfason A, Saemundsdottir J, et al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol. 2019;74(24):2982–94.
Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–19.
Ishikawa S, Kotani K, Kario K, Kayaba K, Gotoh T, Nakamura Y, et al. Inverse association between serum lipoprotein(a) and cerebral hemorrhage in the Japanese population. Thromb Res. 2013;131(2):e54–8.
Kamstrup PR. Lipoprotein(a) and cardiovascular disease. Clin Chem. 2021;67(1):154–66.
Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased risk of heart failure in the general population. JACC Heart Fail. 2016;4(1):78–87.
Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32(7):1732–41.
Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol. 2014;63(5):470–7.
Kenet G, Lutkhoff LK, Albisetti M, Bernard T, Bonduel M, Brandao L, et al. Impact of thrombophilia on risk of arterial ischemic stroke or cerebral sinovenous thrombosis in neonates and children: a systematic review and meta-analysis of observational studies. Circulation. 2010;121(16):1838–47.
Kumar P, Swarnkar P, Misra S, Nath M. Lipoprotein(a) level as a risk factor for stroke and its subtype: a systematic review and meta-analysis. Sci Rep. 2021;11(1):15660.
Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and low risk of major bleeding in brain and airways in the general population: a Mendelian randomization study. Clin Chem. 2017;63(11):1714–23.
Langsted A, Nordestgaard BG, Kamstrup PR. Elevated lipoprotein(a) and risk of ischemic stroke. J Am Coll Cardiol. 2019a;74(1):54–66.
Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and high risk of mortality. Eur Heart J. 2019b;40(33):2760–70.
Laschkolnig A, Kollerits B, Lamina C, Meisinger C, Rantner B, Stadler M, et al. Lipoprotein(a) concentrations, apolipoprotein(a) phenotypes, and peripheral arterial disease in three independent cohorts. Cardiovasc Res. 2014;103(1):28–36.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Ohira T, Schreiner PJ, Morrisett JD, Chambless LE, Rosamond WD, Folsom AR. Lipoprotein(a) and incident ischemic stroke: the atherosclerosis risk in communities (ARIC) study. Stroke. 2006;37(6):1407–12.
Otto CM, Prendergast B. Aortic-valve stenosis—from patients at risk to severe valve obstruction. N Engl J Med. 2014;371(8):744–56.
Pearson GJ, Thanassoulis G, Anderson TJ, Barry AR, Couture P, Dayan N, et al. 2021 Canadian Cardiovascular Society Guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37(8):1129–50.
Reyes-Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42(1):e48–60.
Ridker PM, Stampfer MJ, Hennekens CH. Plasma concentration of lipoprotein(a) and the risk of future stroke. JAMA. 1995;273(16):1269–73.
Schwartz GG, Steg PG, Szarek M, Bittner VA, Diaz R, Goodman SG, et al. Peripheral artery disease and venous thromboembolic events after acute coronary syndrome: role of lipoprotein(a) and modification by alirocumab: prespecified analysis of the ODYSSEY OUTCOMES randomized clinical trial. Circulation. 2020;141(20):1608–17.
Steffen BT, Duprez D, Bertoni AG, Guan W, Tsai MY. Lp(a) [Lipoprotein(a)]-related risk of heart failure is evident in whites but not in other racial/ethnic groups. Arterioscler Thromb Vasc Biol. 2018;38(10):2498–504.
Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29(3):630–4.
Sultan SM, Schupf N, Dowling MM, Deveber GA, Kirton A, Elkind MS. Review of lipid and lipoprotein(a) abnormalities in childhood arterial ischemic stroke. Int J Stroke. 2014;9(1):79–87.
Sun L, Li Z, Zhang H, Ma A, Liao Y, Wang D, et al. Pentanucleotide TTTTA repeat polymorphism of apolipoprotein(a) gene and plasma lipoprotein(a) are associated with ischemic and hemorrhagic stroke in Chinese: a multicenter case-control study in China. Stroke. 2003;34(7):1617–22.
Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503–12.
Yadgir S, Johnson CO, Aboyans V, Adebayo OM, Adedoyin RA, Afarideh M, et al. Global, regional, and National Burden of calcific aortic valve and degenerative mitral valve diseases, 1990–2017. Circulation. 2020;141(21):1670–80.
Zheng KH, Tsimikas S, Pawade T, Kroon J, Jenkins WSA, Doris MK, et al. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol. 2019;73(17):2150–62.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Ethics declarations
PRK reports talks and consultancies sponsored by Physicians Academy of Cardiovascular Education (PACE), Silence Therapeutics and Novartis. AL has nothing to disclose.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Langsted, A., Kamstrup, P.R. (2023). Lp(a) and Aortic Valve Stenosis, Stroke, and Other Noncoronary Cardiovascular Diseases. In: Kostner, K., Kostner, G.M., Toth, P.P. (eds) Lipoprotein(a). Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-031-24575-6_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-24575-6_14
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-031-24574-9
Online ISBN: 978-3-031-24575-6
eBook Packages: MedicineMedicine (R0)