Abstract
Lithium ion battery technology is the most promising energy storage system thanks to many advantages such as high capacity, cycle life, rate performance and modularity. Many transportation applications including marine, aerospace and railway have been utilizing lithium ion batteries. Likewise, there is a dramatic transition from conventional vehicles having internal combustion engines to electric vehicles (EVs). In this review, current lithium ion technology and electric vehicles are introduced. Furthermore, expected future technological advancements are discussed from the active materials to scalability. It can be summarized that consumers have three main concerns towards electric vehicles: range, charging time and life time. Possible solutions to overcome the range anxiety, charging time, and lifetime are exhibited from mostly material and cell design perspectives. Although it is possible to enlarge the volume of this review, the key subjects are presented by introducing the most current advancements which can be adapted to current production infrastructures. Those achievements in the battery level will transform the global transportation sector.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Energy storage
- Lithium ion batteries
- Electric vehicles
- Future technologies
- Energy density enhancement
- Fast charging
1 General Battery Types
Energy is generated, transmitted, converted and stored. Importance of energy storage systems is increasing each day as human kind has needs of energy from micro scale to MWhs. There are many types of energy storage systems such as PHES (Pumped hydro energy storage), CAES (Compressed air energy storage), FES (Flywheel energy storage), SMES (Superconducting magnetic energy storage), flow batteries, supercapacitors and so on [1, 2]. In order to evaluate the technical performance of various energy storage systems, there are many parameters to be considered such as the energy density, the working life, the investment cost, the response time, the cycle life, the technology readiness level and so on. Analyzing all parameters; lithium ion batteries, super capacitors and fuel cells are the most appropriate elements to supply energy for mobility applications. A more brilliant idea could be the hybridization of these technologies so that pluses are combined while the disadvantages are compensated by one another. This review focuses on the lithium ion battery technology, current concerns and expected developments in this field.
Energy storage systems for transportation applications are mainly dominated by batteries. In comparison, batteries are effective in size and mass, modular and relatively cheap. When the market is analyzed, there are certain batteries types encountered: Nickel metal hydride (NiMH), nickel cadmium (NiCd), lead acid, (Pb-acid) and lithium ion batteries. Comparison of different battery types in terms of energy and power densities is provided at Fig. 1 along with other critical battery properties.
Comparison of different battery technologies for transportation applications (reproduced from Ref [3] with permission from Wiley)
Lead acid batteries are very commonly used for transportation applications. For vehicles having internal combustion engines, lead acid batteries mostly provide auxiliary energy needs. Historically, this technology is very well studied and commercialized even though the energy density is small compared to others. Lead acid batteries basically consist of the lead oxide and the metallic lead present at the cathode and the anode sides, respectively. Electrodes are immersed in H2S04 solution. Different types of lead acid batteries exist such as the flooded lead acid, the valve regulated lead acid, the absorbent glass mat and so on [4]. Mostly batteries for cars have 12 V nominal voltage, and these batteries can be used for many years (long cycle life) with a relatively low cost. Apart from vehicles; trains and ships are other common applications for lead acid batteries. Another interesting use is submarines [5]. Some types of modern submarines (diesel electric) utilize tons of lead acid batteries where the energy provided by a diesel engine is transferred to batteries. Quite operation of lead acid batteries provides tactical advantage for military operations. Although there is a strong attempt the modernize the submarines by replacing lead acid batteries with lithium ion (Mitsubishi, Thyssenkrupp, Naval Group and so on), it will take some years to develop fully qualified and certified submarines powered by lithium ions.
Ni-Cd battery usually consists of NiO(OH) as the cathode, Cd as the anode, and KOH alkaline solution as the electrolyte. Its operating principle is based on the redox reactions between NiO(OH) and Cd. One major problem of NiCd batteries is their memory effects, where their maximum energy capacity is gradually lost when they are not fully charged for each use. Another limitation of Ni-Cd batteries is their high self-discharge rates. On the other hand, NiCd batteries can be ultra-fast charged with little deformation. Also they offer long shelf life, simple storage and transportation and good temperature performance [6]. Thanks to their advantages (especially high discharge rates), NiCd batteries are mostly used for aerospace and railway applications.
Configuration of Nickel Metal Hydride (Ni-MH) batteries are very similar to that of Ni-Cd battery. They both use the same cathode materials and electrolyte, but instead of Cd, a hydrogen absorbing alloy is used as the anode in Ni-MH battery. The replacement of metal Cd makes Ni-MH batteries less expensive and eco-friendlier when compared to Ni-Cd batteries. Their energy density is more than two times that of lead-acid batteries, about 50% higher than that of Ni-Cd batteries. The largest drawback of Ni-MH batteries is their high self-discharge rate, which is up to three or more times higher than that of Li-ion battery. Considering the energy demand of electric vehicles, Ni-MH are not suitable for PHEV and BEV where high energy densities are required. Rather, HEV are a better application for this technology [7]. It is known that Honda and Toyota have been producing hybrid vehicles using Ni-MH.
2 Lithium Ion Batteries for EVs
Future of electromobility will be dependent on the lithium ion batteries. Although the lead acid, NiMH and NiCd batteries are available for several applications, the lithium ion technology has prevailed in the competition because of many advantages. Cars, buses, trucks, trains, ships, planes, submarines, unmanned aerial vehicles, torpedoes, satellites and many vehicles have been electrified by lithium ion batteries. Any technological development (the material, the system design, cheaper production methods) has the potential to provide huge technical and financial success. Therefore, the rest of this study addresses different aspects of lithium ion batteries.
Briefly, lithium ion batteries offer high energy density, long cycle life, relatively low cost and high discharge rates with respect to the design criteria. These batteries consist of porous anode and cathode which are separated by a polymeric separator. These components are wet by electrolyte; mostly LiPF6 dissolved in carbonates. Overall system is well sealed and preserved from the atmosphere. During charging, Li+ ions are transferred from cathode to anode side due to an applied voltage. During discharge, the lithiated anode material releases the ions to be transferred back to the cathode. Number of lithium ions in cathode chemistry and molecular weight determine the theoretical capacity of the cathode. Many items like the cell chemistry, the electrode quality, design parameters and the electrolyte affect the overall performance of the cell (Fig. 2).
Working mechanism of lithium ion cell (reproduced from Ref. [8] with permission from the Royal Society of Chemistry)
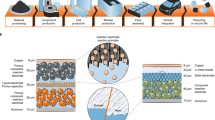
Lithium ion batteries are composed of lithium ion cells, the modules, the pack and the battery management systems (BMS). Cell is the smallest unit of the battery where the electrochemical activity occurs and the energy is stored. What gives this capability is basically the electrodes: the anode and the cathode. First step of cell manufacturing is fabrication of the electrodes [9]. Active materials (NMC, NCA, LFP, graphite and so on) are mixed with polymeric binder (PVDF, CMC, SBR) and the conductive agent (acetylene black, Super P etc.) in a proper solvent to form slurry. In the industry most common solvent is NMP. Apart from NMP, aqueous processing of slurry preparation is also possible. Slurry is then coated on current collectors (aluminum for the cathode and copper for the anode) and simultaneously dried to evaporate the solvent. Resulting electrode is then pressed by heated cylinders, which is called calendaring (Fig. 3).
Common forms of lithium ion cells; pouch (left), cylindrical (middle) and prismatic (right) (reproduced from Ref. [10] with permission from MDPI).
Having prepared the electrodes, next step is manufacturing the cells. There are basically 4 different kinds of lithium ion cells: the button, the cylindrical, the prismatic and the pouch. Button cells are mostly used for scientific purpose and not preferred for industrial applications like EVs. Cylindrical and prismatic cells are similar to each other; i.e. both are produced by rolling the electrodes and the separators. Thanks to continuous rolling, manufacturing speed of these cells is much higher than pouch cells. These cells are placed in aluminum or steel cases which make them stronger for mechanical effects. On the other hand, the pouch cell is placed in a polymeric composite film. For this reason, the weight of pouch cell is automatically lowered, giving higher energy density. Yet, the mechanical strength of pouch cell compared to others is much lower. Therefore, it becomes the preference of the designers to choose the cell type.
Lithium ion batteries can be integrated into many transportation vehicles: buses, ships, trains, planes and so on. Because the problems (the capacity, the weight, the size, the cycle life, the charge rate, the discharge rate) with electrification of vehicles are similar, this report is mainly concentrated on electric vehicles (EVs) for daily use in order to explain the transition from conventional vehicles to battery powered ones. Electric vehicles are powered by batteries instead of combustion engines which need fossil fuels. According to EPA (United States Environmental Protection Agency), 27% of total CO2 emission in USA is coming from transportation sector in 2020 [11]. So both academia and industry have been investing great effort to develop better performing EVs. Sales statistics are a good indication of this development. According to International Energy Agency, more than 16.5 million EVs are driven in by 2021 [12]. Huge increase of EVs, especially in 2021 even though when a global pandemic negatively affected the global economy, shows the motivation of consumers to choose between conventional vehicles and electric vehicles (Fig. 4).
Global EV stock according to years [12]
Basically there are three types of electric vehicles: BEV (Battery Electric Vehicle), PHEV (Plug-in Hybrid Electric Vehicle), HEV (Hybrid Electric Vehicle). Since HEVs cannot be charged by an outer plug, lithium ion batteries are more important for PHEV and especially for BEVs. General classification of electric vehicles and schematic showing the power flow for PHEV and BEVs are shown in Fig. 6 (Fig. 5).
Schematic showing different types of electric vehicles: a) Hybrid electric vehicle (HEV), b) Plug-in Hybrid Electric Vehicle (PHEV), c) Battery Electric Vehicle (BEV) (Reproduced from Ref. [13] with permission from MDPI)
A fully electric vehicle is consisted of items like the battery, the electric motor, the DC/DC converter, the onboard charger, the power electronic and the transmission. Most important components are the electric motor and the batteries. In order to compete with conventional vehicles, EVs have to offer higher driving range (higher KWh capacity). Meanwhile other important criteria that consumers might be concerned about is the life of the battery. Drivers may also intend to charge their cars in a very short time. Therefore, EVs have to respond many question marks from the range capability to the environmental compatibility. In this regards, manufacturers offer novel design, different cell chemistries, the BMS systems, the packaging engineering, charging methods and novel ideas like regenerative braking [14, 15].
3 Future of Batteries for Transportation Technologies: Practical and Quickyl Adaptable Solutions
Lithium ion battery prices are going down each year [17]. Technological developments related to improvement in manufacturing and installation of huge capacity factories (Giga factories) make prices affordable for users. Although the transition from conventional vehicles to electric vehicles is very obvious, there are still realistic concerns at the consumer side. Technology and infrastructure regarding conventional vehicles are well established. In comparison, drivers rightfully may have fear of purchasing electric cars mainly due to the battery related problems. Three of the main concerns are the driving range, the charging and the battery life time that could concern individuals who plan to purchase electric vehicles. This chapter tries to evaluate the reasons behind the worries and present the technological trends (Fig. 7).
Lithium ion technology prices at cell and pack level. (Reproduced from Ref. [16] with permission from MPDI)
Schematic representation of energy decrease from electrode to system level by addition of inactive components (reproduced from Ref. [18] with permission from European commission)
3.1 Overcoming Range Anxiety
Electrochemical properties of lithium ion cells are mostly determined by the active material. Available capacity that a battery package can offer is limited by the type and the amount of the active material. In order to form a functional battery pack, many inactive materials are integrated from the electrode level to the pack level. In this regards, an increase in the energy density can be achieved by either eliminating the inactive parts as much as possible or increasing the unit energy of active material (Fig. 8).
Future projection of cathode chemistries and market share forecast by IEA Stated Policies Scenario (reproduced from Ref. [19] with permission from the Springer Nature)
At the cell level, the specific capacity of lithium ion cells is in the range of 200–300 Wh/kg. At the pack level, overall capacity lowers than 200 Wh/kg, making the batteries “heavy”. For this reason, there are great efforts in material development studies. Currently, lithium ion batteries are commonly composed of layered oxides such as NMC111, NMC532 and NMC811 chemistries. In order to increase the energy efficiency, higher Ni content (NMC811, NMC955) and Li-rich chemistries are on the way for commercialization. Other motivations behind replacing cobalt with Ni are high cost and the availability of cobalt. Other very common cathode chemistry is lithium iron phosphate (LFP) which is considered as safer and cheaper compared to layered oxides. Besides, high discharging rates are possible with LFP chemistry. Market share of LFP is expected to decrease in the following decades because of lower energy density according to International Energy Agency Stated Policies (STEP) NCX scenario [19]. Another strategy to increase the energy density is increasing the cell voltage. There are many high voltage cathodes (>4.5 V) (spinel LiNi0.5Mn1.5O4 (LNMO), inverse spinel LiNiVO4, olivine LiCoPO4 (LCPO), post-5 V ultra-high voltage cathodes such as olivine LiNiPO4 (LNPO), spinel LiCoMnO4 (LCMO), orthorhombic Li2CoPO4F (LCPOF) which are under investigation [20]. Technical problems such as low cycle life and degradation of electrolyte must be handled for these types of cathodes.
Anode side of the batteries are mostly dominated by the graphite. Theoretical capacity of graphite (372 mAH/g) is almost achieved (around 330 mAh/g), and researchers are looking for new anode chemistries such as alloys and silicon. Great capacity of silicon compared to the graphite makes it a perfect candidate for future batteries. Thanks to higher energy anode materials such as silicon and the lithium metal, thinner and lighter electrode production will make it possible to develop higher energy cells in terms of weight and volume [21] (Fig. 9).
Schematic showing the development of energy density by changing anode with respect same cathode material [22] (Reproduced from MIT News website)
At the electrolyte level, higher voltage electrolytes and solid-state electrolytes are at the study level. Any improvement at the cell voltage automatically increases the energy density of a cell (Wh = Ah * V). Also, solid state electrolytes offer improved safety which is another critical aspect of the technology. Solid state electrolytes will also make the use of metallic anode which will even increase the energy density further. All these achievements could lead to development of cells of 300–500 Wh/kg [23]. It will be a great relief for drivers to overcome the “range anxiety” (Fig. 10).
Technological road map for lithium ion batteries (Reproduced from Ref. [24] with permission from Elsevier)
Apart from material level developments, new progresses have been also utilized at the electrode level for better electrode properties (higher energy density); and cheaper and faster production route. In this regard, several strategies can be implemented at electrodes level such as foil engineering. Thickness of the aluminum foil and the copper foil are 15–20 micron and 8–12 micron, respectively. In this regard, thinner current collectors will provide less amount of inactive material leading to higher energy density. 3-D current collectors will be another great achievement for lithium ion batteries. Nickel foam, 3D porous nickel, 3D copper foils are some types of high- tech current collectors. Basically, those current collectors provide better electronic conductivity due to increased surface area, improved mechanical strength and the flexibility, which is hard to achieve for safety issues [25].
Electrode manufacturing is one of the most crucial steps of the lithium ion cell production. Considering the cost, even coating and drying consists of 15% of the total production cost (formation and aging: %32, enclosing: %12,5) [26]. Therefore, developments for electrode manufacturing are crucial for the electrode quality together with time-cost perspectives. During fabrication of the electrodes, NMP has been conventionally used as organic solvent to mix active material with other ingredients. Instead, cheaper electrode manufacturing can be achieved by aqueous processing of both anode and cathode electrodes. Industry has greatly adapted to water based processing of graphitic materials. However, there are some problems associated with water based processing of cathode such as the electrode cracking, the electrochemical incompatibility and the foil corrosion. Progress in aqueous cathode processing could bring great manufacturing advantages as well as positive environmental outcomes [27]. One step further for NMP-free processing is fabrication of electrodes by dry techniques. Solvent free techniques remove the need for toxic NMP, and may provide faster and conformal coating. Spray deposition, the temperature pressing and 3D printing are commonly observed in the research studies [28]. Production rate of electrodes are mostly limited by slurry preparation. In the conventional planetary mixers, it may take hours to prepare slurry. At this point, Bühler group has come with a great idea for slurry preparation. Similar to plastic extrusion, their novel system continuous form slurry to be fed to coater [29]. It is believed that this technique is able to raise production speed and lower cost (Fig. 11).
Top) Ni foam 3D current collector (reproduced from Ref. [30] with permission from Elsevier), middle) dry electrode processing (reproduced from Ref. [31], Ludwig, B., Zheng, Z., Shou, W. et al. Solvent-Free Manufacturing of Electrodes for Lithium-ion Batteries. Sci Rep 6, 23150 (2016)) and bottom) continuous slurry preparation presented by Bühler
3.2 Fast Charging
Another concern of EV drivers is the charging time. For the safe and long use of lithium ion batteries, the charging rate should be minimized to smallest possible currents such as C/10. Most of the current battery systems are not suitable with fast charging (%80 SoC in 10–15 min). Therefore, both material and system level advancements are needed.
Firstly, the chemistry and the electrode design should allow the faster diffusion of Li ions and the intercalation. High rates of lithium transfer mostly causes the lithium plating (as negative electrode potential close to the lithium metal) over anode surface. Additionally temperature of the cell increases which degrades the cell components like the electrolyte over time [32]. Diffusion of lithium ions through cathode active material, the electrolyte and the charge transfer kinetics are other limiting factors for fast charging. At the electrode level, the active particle distribution, the porosity and the thickness are other rate limiting factors.
Considering the graphite anode, the charge transfer barrier, Ea, is about 0.6 eV and while it’s 0.33 eV for LTO. Level of charge transfer barrier for the lithium diffusion greatly affects the rate capability. Anode surface is covered by SEI (solid electrolyte interface). It is the protective layer covering anode surface from further degradation with electrolyte interactions. Over fast charging, SEI layer is damaged causing to capacity and cycle life performance loss. Many techniques are available to form artificial SEI layer suitable for fast charging applications [33]. Also, degradation of the anode by time (Li plating, heating, particle cracking) can be prevented by extra lithium metals coating on the graphite electrode. Also, aligning the graphite particles by magnets is another great technique for fast charging [34]. At the electrode level thinner and porous electrode provide batter fast charging performance [17]. Coating the cathode active material, novel chemistries and the electrolyte offering lower charge transfer resistance at the boundaries must be developed for fast charging cells. To sum up, novel designs are required in order to charge batteries in shorter durations as well as widespread charging points for EVs to penetrate daily life (Fig. 12).
3.3 Life of Batteries: Electrolyte View
Lastly, life time of batteries is another great concern for drivers. Life time of the lithium ion batteries are dependent on many internal and external factors. Parameters like the usage profile, the cycle number, the environmental conditions (temperature, humidity etc.), the storage conditions, the cell chemistry, the pack design, the battery management system are all important factors to determine how long the battery will last. End of life capacity (EoL capacity) is one of the critical indications of the quality of the batteries. Generally, the number of cycles to reach 70 or 80% of the initial capacity is considered as the overall life time of batteries. This chapter of the review presents the technical reasons causing the battery degradation from the electrolyte perspective. Authors are aware of the fact that electrolyte is not the only component determining the life time of the batteries. Yet, electrolyte remains as one of the most critical component as it is directly related to cell functioning and reactions occurring within anode and cathode interfaces.
Lithium ion cells are the electrochemical energy storage systems. Therefore, putting aside any external effect, regular use of the lithium ion batteries causes aging and internal degradations. Failure mechanisms can be very diverse such that all active and inactive components of batteries contribute to degradation over use. Some of the common failure reasons are SEI growth, Li plating, the electrolyte decomposition, active material contact loss, structural changes within active material, the active material dissolution, the oxidation of electrolyte, separator failures and so on [36]. For this reason, studies on the cycle life developments should consider all components of the cells and compatibility between those. In this report, two main perspectives to achieve longer life time for batteries are introduced: Good SEI formation and high voltage electrolyte stability. It is very important to keep in mind that many other parameters also exist waiting for improvement such as the electrode adhesion to the foil, the type of separator, good assembly of cell and so on (Fig. 13).
Lithium ion cell failure mechanisms (Reproduced from Ref. [37] with permission from the Elsevier)
Solid electrolyte interface (SEI) is basically a passivation layer formed on negative electrode material, dominantly formed during formation process. Because of the reactions between electrolyte and negative electrode material, a thin layer of SEI is formed which acts also as protective layer for anode from further degradation. During continuous use of lithium ion batteries, SEI growth occurs causing to increase of internal resistance and capacity fading due to consumption of active lithium and the electrolyte decomposition [38]. At this point formation protocol is vital for SEI stability. There are many formation protocols applied in academia and industry. Constant current (CC), the constant voltage (CV), the constant power (CP), CCCV, multistage constant current (MSCC), the pulse charging (PC), the boost charging (BC) and many other protocols are applied for the lithium ion batteries mainly due to better SEI formation and shorter times if possible [39 40]. It is important to consider that each cell has its own characteristics because of the chemistry, the electrode and the cell design. Therefore, unique protocols should be developed for different lithium ion cells. Charging current, upper and lower cut off voltages, the wetting time, the temperature and many other parameters should be considered for an optimum formation procedure. Formation duration of conventional lithium ion cells having graphitic materials can take couple of days, however it does not necessarily mean that longer formation time would yield better electrochemical properties [41]. Apart from graphitic anode materials, future lithium ion cells are likely to utilize the lithium metal thanks to increased energy density. Formation of SEI on lithium metal is challenging because of continuous reactions between the electrolyte and the anode material. Upon charging and discharging, inhomogeneous lithium plating occurs causing the dendrite formation which is detrimental. Therefore, alternative methods are needed for the lithium metal anode. A novel idea is artificial solid electrolyte interface (ASEI) which is formed mostly by the atomic layer deposition, the aeration and coating [42]. Design of ASEI requires three main issues to engineer: the mechanical stability, uniform ion transport and the chemical passivation [43]. Artificial SEI formation can eliminate the difficulty to control native SEI during formation and provide higher energy densities with prolonged cycle life.
Good formation of SEI is directly related to electrolyte composition as well. Mostly for lithium ion cells having graphitic anode material, EC and LiPF6 are utilized as solvent and salt, respectively. In order to increase the lifetime of lithium ion cells, the cathode also must be protected from further failure by means of the electrolyte reactions. Combinations of carbonate based solvents and additives can be used for conventional lithium ion battery systems. As indicated earlier, high voltage cathode materials are good candidates for increasing overall cell energy. Therefore, more work is needed to engineer electrolyte formulations which are suitable with high voltage cathode materials. Literature suggests the following reasons for degradation of high voltage cells: the oxidation of the electrolytes on the high voltage cathodes, transition metal dissolution at the CEI, cracks due to large volume change during lithiation/delithiation processes [20]. Although EC is very compatible with anode graphite forming stable SEI, the protective layer formed on cathode (CEI) is much worse in comparison [44]. Delithiated cathode surface and electrolyte have parasitic interaction which leads to reconstitution of cathode reducing metal valence and inducing metal dissolution. This process causes increase in the charge transfer resistance in the cathode. Upon continuous cycling, more cracks are formed on the cathode surface creating new fresh regions where the electrolyte can capture oxygen from delithiated cathode. This loop mechanism continues at high voltages, at the end, leading to overall cell degradation. Therefore, it is very critical to maintain the oxidative stability of electrolyte at high voltages. Many methods are proposed in literature for stabilization of the cathode-electrolyte interface. One of them is use of additives in the electrolyte system. Tris(pentafluorophenyl) phosphine (TPFPP), ethylenedioxythiophene (EDT), o-terphenyl (OTP), furan, tris(trimethylsilyl) borate (TMSB), methylenemethanedisulfonate(MMDS), trimethylphosphite (TMP), 3-hexylthiophene, LiBF2(C2O4) and tetramethoxytitanium (TMTi) are the other additives which can be encountered in literature for stable CEI formation [47]. One very attractive additive is Tris(trimethylsilyl) Phosphite (TMSPi) which has been reported as a promising additive increasing the discharge capacity and slowing down the surface impedance. Effect of 2 wt% TMSPi additive in the baseline electrolyte (1 m LiPF6 in EC:DEC) for NMC811/Si-Gr (%12,5–77,5) full cell has been studied [45]. Authors found that the additive helps forming very robust CEI and SEI stabilizing anode and cathode. TMSPi breaks down Li2CO3 on cathode side and believed to be acting as HF “scavenger”. One of the primary functions of this additive is that TMSPi is oxidized before solvent and form stable interface preventing further metal dissolution [46]. Another study suggests [48] that a combination of 1 vol. % of TMSPi and 1 vol. % of VC additives with a standard electrolyte (1M LiPF6 in EC:DMC) develops NMC811-graphite cell performance. They suggest TMSPi is able to lower the active lithium trapped on SEI and slows down the metal dissolution from cathode. This work presents that TMSPi also acts as HF scavenger and reduce Al dissolution from cathode current collector. Apart from additives, there are many other methods for improvement of high voltage electrolytes such as surface modification of cathode materials [49], sulfone based electrolytes [50] and ionic liquids [46]. All the progress mentioned could bring out the possibility of using voltage cathode materials with increased energy density and prolonged stability for lithium ion cells upon cycling (Fig. 14).
Working schematic of TMSPi and effect on electrochemical performance (reproduced from Ref. [45] with permission from the Wiley)
4 Conclusion
Electric vehicles will be in the center of our life in the future. Current technology is still at a level below the expected performance level. Drivers may hesitate to buy electric vehicles because of the range, charging (both time and lack of charging stations) and the life time issues. For this reason, new generations of lithium ion batteries must evolve for common use of electric vehicles. In this report, some aspects of future lithium ion batteries are discussed. Higher energy material developments, fast charging requirements and solid electrolyte interface enhancement to prolong the life time are explained. The basic intention of this report is to combine the consumer concerns and show that the technological trend is very promising. There are many other academic work in the literature as most of them are in the R&D stage being away from producibility. This report presents the technological achievements which are believed to be easily adapted to mass scale production facilities and provide economic benefits as well. It is believed that further battery developments will make electric vehicles more preferable for consumers. This report intends to show the pathway for electric powered transportation sector.
References
Om Krishan, S.S.: An updated review of energy storage systems: classification and applications in distributed generation power systems incorporating renewable energy resources. Int. J. Energy Res. 43(12), 6171–6210 (2018)
Behabtu, H.A., et al.: A Review of energy storage technologies’ application potentials in renewable energy sources application potentials in renewable energy sources. Sustainability 12 (2020)
Liang, Y., et al.: A review of rechargeable batteries for portable electronic devices. InfoMat, 1, 6–32 (2019)
Aksoy, H., Soytaş, S.H.: Enerji ve Ulastırma Sektörleri Dönüsümünde Batarya Teknolojilerinin Rolü: Eğilimler, Fırsatlar ve Yenilikçi Uygulamalar, Sabancı Üniversitesi, İstanbul (2019)
Lus, T.: Waiting for breakthrough in conventional submarine’s prime movers. Trans. Maritime Sci. 8(1), 37–45 (2019)
Rajender, B., Inamuddin, I., Pothu, R., Asiri, A.M.: Rechargeable Batteries: History, Progress, and Applications. Scrivener Publishing LLC (2020)
Fetcenko, M., Koch, J., Zelinsky , M.: Nickel–metal hydride and nickel–zinc batteries for hybrid electric vehicles and battery electric vehicles. In: Advances in Battery Technologies for Electric Vehicles, pp. 103–126. Woodhead Publishing Series in Energy (2015)
Qi, W., Shapter, J.G., Wu, Q., Yin, T., G ao, G., Cui, D.: Nanostructured anode materials for lithium-ion batteries: principle, recent progress and future perspectives. Royal Soc. Chem. 5, 19521–19540 (2017)
Gonçalves, R., Lanceros-Méndez , S., Costa, C.M.: Electrode fabrication process and its influence in lithium-ion battery performance: State of the art and future trends. Electrochem. Commun. 135, 107210 (2022)
Löbberding, H., et al.: From cell to battery system in BEVs: analysis of system packing efficiency and cell types. World Electric Vehicle J. 11(4) (2020)
EPA: Fast Facts on Transportation Greenhouse Gas Emissions, US Environmental Protection Agency (2022)
I. E. AGENCY: Global EV Outlook 2022 Securing supplies for an electric future, IEA (2022)
Nour, M., Chaves-Ávila , J.P., Magdy, G., Sánchez-Miralles, A.: Review of positive and negative impacts of electric vehicles charging on electric power systems. Energies 13(18) (2020)
Yonga, J.Y., Ramachandaramurthy, V.K., Tan, K.M., Mithulananthan, N.: A review on the state-of-the-art technologies of electric vehicle, its impacts and prospects. Renew. Sustain. Energy Rev. 49, 365–38549 (2015)
Saw, L.H., Ye, Y., Tay, A.A.O.: Integration issues of lithium-ion battery into electric vehicles battery pack. J. Clean. Product. 113, 1032–1045 (2016)
Kim, S.S., et al.: Return of interest planning for photovoltaics connected with energy storage system by considering maximum power demand. Appl. Sci. 10(3) (2020)
Masias, A., Marcicki, J., Paxton, W.A.: Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett. 6, 621–630 (2021)
S. F. E. Policy: Towards the battery of the future. European Commission. Bristol (2018)
Xu, C., Dai, Q., Gaines, L., Hu, M., Tukker, A., Steubing, B.: Future material demand for automotive lithium-based batteries. Commun. Mater. 1(1), 1 (2020). https://doi.org/10.1038/s43246-020-00095-x
Fan, X., Wang, C.: High-voltage liquid electrolytes for Li batteries: progress and perspectives. Chem. Soc. Rev. 50, 10486 (2021)
Placke, T., Kloepsch, R., Dühnen, S., Winter, M.: Lithium ion, lithium metal, and alternative rechargeable battery technologies: the odyssey for high energy density. J. Solid State Electrochem. 21(7), 1939–1964 (2017). https://doi.org/10.1007/s10008-017-3610-7
Matheson, R.: Doubling Battery Power of Consumer Electronics. MIT News (2016). https://news.mit.edu/2016/lithium-metal-batteries-double-power-consumer-electronics-0817. Accessed 27 June 2022
Lua, Y., Rong, X., Hu, Y.-S., Chen, L., Li, H.: Research and development of advanced battery materials in China. Energy Storage Mater. 23, 144–153 (2019)
Yoo, H.D., Markevich , E., Salitra, G., Sharon, D., Aurbach , D.: On the challenge of developing advanced technologies for electrochemical energy storage and conversion. Mater. Today 17(3), 110–121 (2014)
Jin, S., Jiang, Y., Ji, H.: Advanced 3D current collectors for lithium-based batteries. Adv. Mater. 30(48) (2018)
Liu, Y., Zhang, R., Wang, J., Wang, Y.: Current and future lithium-ion battery manufacturing. iScience 24(4) (2021)
Demiryürek, R., et al.: Roll-to-roll manufacturing method of aqueous-processed thick LiNi0.5Mn0.3Co0.2O2 electrodes for lithium-ion batteries. Int. J. Energy Res. 45(15), 21182–21194 (2021)
Verdier, N., et al.: Challenges in solvent-free methods for manufacturing electrodes and electrolytes for lithium-based batteries. Polymers 13(3) (2021)
"Efficient continuous electrode slurry production. https://www.buhlergroup.com/content/buhlergroup/global/en/industries/batteries/Continuous-electrode-slurry-production.html. Accessed 16 June 2022
Issatayev, N., Nuspeissova, A., Bakenov , Z.: Three-dimensional foam-type current collectors for rechargeable batteries: a short review. J. Power Sources Adv. 10 (2021)
Ludwig, B. Zheng, Z., Shou, W., Wang, Y., Pan, H.: Solvent-free manufacturing of electrodes for lithium-ion batteries. Sci. Rep. 6 (2016)
Deng, J., Bae, C., Denlinger, A., Miller, T.: Electric vehicles batteries: requirements and challenges. Joule 4, 509–515 (2020)
Weiss, M., et al.: Fast charging of lithium-ion batteries: a review of materials aspects. Adv. Energy Mater. 11(33) (2021)
Bayındır, O.: Controlling the crystallographic orientation of graphite electrodes for fast-charging li-ion batteries. ACS 14(1), 891–899 (2021)
Tomaszewska, A., et al.: Lithium-ion battery fast charging: a review. eTransportation 1 (2019)
Gandoman, F.H., et al: Concept of reliability and safety assessment of lithium-ion batteries in electric vehicles: basics, progress, and challenges. Appl. Energy 251 (2019)
Birkl, C.R., Roberts , M.R., McTurk, E., Bruce , P.G., Howey , D.A.: Degradation diagnostics for lithium ion cells. J. Power Sources 341, 373–386 (2017)
Wang, A., Kadam, S., Li, H., Shi, S., Qi, Y.: Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. Comput. Mater. 15 (2018)
Keil, P., Jossen, A.: Charging protocols for lithium-ion batteries and their impact on cycle life—an experimental study with different 18650 high-power cells. J. Energy Storage 6, 125–141 (2016)
Guo, Z., Liaw, B.Y., Qiu , X., Gao, L., Zhang , C.: Optimal charging method for lithium ion batteries using a universal voltage protocol accommodating aging. J. Power Sources 274, 957–964 (2015)
Mao, C.: Balancing formation time and electrochemical performance of high energy lithium-ion batteries. J. Power Sources 402(31), 107–115 (2018)
Thanner, K., Varzi, A., Buchholz, D., Sedlmaier, S.J., Passerini, S.: Artificial solid electrolyte interphases for lithium metal electrodes by wet processing: the role of metal salt concentration and solvent choice. ACS 12(29), 32851–32862 (2020)
Yu, Z., Yi, C., Zhenan, B.: Design principles of artificial solid electrolyte interphases for lithium-metal anodes. Cell Reports Phys. Sci. 1(7) (2020)
Choi, N.S., Han, J.-G., Ha, S.-Y., Parka, I., Backb , C.-K.: Recent advances in the electrolytes for interfacial stability of high-voltage cathodes in lithium-ion batteries. RSC Adv. 5, 2732–2748 (2015)
Liu, H., Naylor, A.J., Menon, A.S., Brant, W.R., Edström, K., Younesi, R.: Understanding the Roles of Tris(trimethylsilyl)Phosphite (TMSPi) in LiNi0.8Mn0.1Co0.1O2 (NMC811)/Silicon–Graphite (Si–Gr) Lithium-Ion Batteries. Adv. Mater. Interf. 7(15) (2020)
Haregewoin, A.M.: Electrolyte additives for lithium ion battery electrodes: progress and perspectives. Energy Environ. Sci. 9, 1955–1988 (2016)
Tan, S., Ji, Y.J., Zhang, Z.R., Yang, Y.: Recent progress in research on high-voltage electrolytes for lithium-ion batteries. ChemPhysChem Rev. 15(10), 1956–1969 (2014)
Laveda, J.V.: Stabilizing capacity retention in NMC811/Graphite Full Cells via TMSPi electrolyte additives. ACS 2(10) (2019)
Liu, J., Manthiram, A.: Improved electrochemical performance of the 5 V Spinel Cathode LiMn1.5Ni0.42Zn0.08O4 by surface modification. J. Electrochem. Soc. 156(1) (2008)
Flamme, B., Światowska, J., Haddad, M., Phansavath, P.: Sulfone based-electrolytes for lithium-ion batteries: cycling performances and passivation layer quality of graphite and LiNi 1/3 Mn 1/3 Co 1/3 O 2 electrodes. J. Electrochem. Soc. 167(7), 070508 (2020)
Acknowlegments
We gratefully thank the authors of the all journals which are referred in this article. Also, authors are grateful to The Scientific and Technological Research Council of Turkey (TUBITAK) RTTI Energy Storage Division and Marmara University Metallurgical and Materials Engineering Department.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Demiryürek, R., Ateş, M.N., Tunaboylu, B. (2023). Future of Lithium Ion Batteries for Electric Vehicles: Problems and Expected Developments. In: Durakbasa, N.M., Gençyılmaz, M.G. (eds) Towards Industry 5.0. ISPR 2022. Lecture Notes in Mechanical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-24457-5_42
Download citation
DOI: https://doi.org/10.1007/978-3-031-24457-5_42
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24456-8
Online ISBN: 978-3-031-24457-5
eBook Packages: EngineeringEngineering (R0)