Abstract
The charoite and denisovite are rare minerals. They are found as thin needle asbestos-like fibres. The structures of these minerals are composed the edge-sharing (Ca,Na)- octahedra and some types of dreier silicate chains. The chains are running along [001] and are located between the ribbons and walls of octahedra. In polytype charoite-90 three adjacent fragments, produced by silicate chains, are followed each other without shifts. In polytype charoite-96 the same fragments are displaced by a translation of ½c. In the structure of denisovite the layers parallel to (100) formed by silicate chains and the octahedra walls can be distinquished. The chains are located between neighboring octahedra walls and can be displaced either Δz = + c/4 or –c/4 between neighboring layers. Such an ambiguity with respect to their z-position along octahedra walls can lead to the formation of polytypic sequences, to twinning, to new ordered structure, or to disorder when the directions of the reverse randomly.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In silicate minerals such phenomenon as polytypism occurs quite often (e.g., Ferraris et al. 2004). In dreier chain silicates (nomenclature according to Liebau 1985) this phenomenon occurs most often due to stacking faults or shifts of crystal structure fragments (Jefferson and Brown 1973; Wenk et al. 1976). In these silicates, the octahedral columns of walls and separate bands and tetrahedral silicate chains are streched along the z axis. The parameter along the z axis c ≈ 7.3 Å spans one repeat period of the tetrahedral silicate chains and two octahedra edges, respectively. So, the metrical relationships between these fragments of the structure allow the tetrahedral silicate chain to be attached to the octahedral column in two different positions, ≈ 3.65 Å apart, along the chain axis. The regular alternation of the displacements could result to a new ordered structure (polytype), or to twinning. When the directions of the displacements reverse randomly, it leads to disorder. Charoite and denisovite relates to Ca-bearing alkaline silicates with various dreier silicate chains and lattice parameter c ≈ 7.3 Å (Table 1).
2 Order-Disorder in Charoite Structure
Charoite, is a beautiful semi-precious, violet stone. It was found in the alkaline intrusion of the Murun massif in Yakutiya, Siberia, Russia (Konev et al. 1996) and recently in the Patynskiy massif, Gornaya Shoriya, Southern Siberia, Russia (Kasatkin et al. 2019). The charoite was confirmed as a new mineral species in 1978 (Rogova et al. 1978). The chemical composition of charoite from both localities is close. Charoite from Patynskiy massif differ from the Murun charoite by the total absence of admixed Sr and F, lower Mn and substantially higher Ca content. The main difference between the charoite varieties is the color. Charoite from Murun is famous for its purple to violet coloration caused by traces of Mn3+, whereas charoite from the Patynskiy massif is whitish-grey to brown. Charoite is usually found in close association with other alkaline calcium silicates such as frankamenite, canasite, miserite, tokkoite and tinaksite (Konev et al. 1996). In the structures of all of these minerals various types of dreier silicate chains are located between bands of edge-sharing [(Ca,Na)O6] octahedra. The silicate chains and octahedra bands are parallel to the z-axis with the lattice parameter c ≈ 7.3 Å (Rozhdestvenskaya and Nikishova 2002).
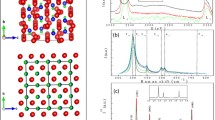
The crystal chemical formula of charoite is \( \left( {{\text{K}}_{{{\text{13}}.{\text{88}}}} {\text{Sr}}_{{{\text{1}}.0}} {\text{Ba}}_{{0.{\text{32}}}} {\text{Mn}}_{{0.{\text{36}}}} } \right)_{{\Sigma {\text{15}}.{\text{56}}}} \) \( \left( {{\text{Ca}}_{{{\text{25}}.{\text{64}}}} {\text{Na}}_{{{\text{6}}.{\text{36}}}} } \right)_{{\Sigma {\text{32}}}} [({\text{Si}}_{{\text{6}}} \left( {{\text{O}}_{{{\text{11}}}} \left( {{\text{O}},{\text{OH}}} \right)_{{\text{6}}} } \right)_{{\text{2}}} ({\text{Si}}_{{{\text{12}}}} \left( {{\text{O}}_{{{\text{18}}}} \left( {{\text{O}},{\text{OH}}} \right)_{{{\text{12}}}} } \right)_{{\text{2}}} \) (Si17(O25(O,OH)18)2](OH,F)4.0·3.18H2O, or in the general case (K,Sr,Ba,Mn)15–16(Ca,Na)32 [Si70(O,OH)180](OH,F)4.0·nH2O. Three different silicate chains can be distinquished in the structure: a dreier double chain, [Si6O17]10− (DC), a tubular dreier triple chain, [Si12O30]12− (TC) and a tubular hybrid dreier quadruple chain, [Si17O43]18− (QC) (Fig. 1a) (Rozhdestvenskaya et al. 2009, 2010, 2011).
The apical oxygens of dreier silicate chains link them to (Ca,Na)-octahedra bands.
The blocks four octahedra wide are linked by apical oxygens and form continuous zigzag walls parallel to (100) (Fig. 1a). Furthermore, a double column and two single columns bonded by common apical oxygens present isolated block four octahedra wide.
The dreier double chain (DC), [Si6O17]10− and the tubular dreier triple chain (TC), [Si12O30]12− were found in the xonotlite structure (Kudoh and Takeuchi 1979) and in synthetic Li2Mg2[Si4O11] (Czank and Bissert 1993), respectively. The tubular hybrid dreier quadruple chain, [Si17O43]18−, is more complicated. It consists of two loop-branched dreier single chains, a dreier single chain and a dreier double chain. The dreier single chain is the same as found in pectolite and the dreier double chain is of the type found in okenite (Merlino 1983).
All dreier chains (DC), (TC) and (QC) have horizontal Si2O7 groups. The groups Si2O7 of neighboring chains are connected to different vertices of octahedron edge (Fig. 1a). Therefore, adjacent chains are displaced relative to each other by the length of one octahedron edge, i.e. c/2.
So, in charoite structure three silicate chains (DC), (TC) and (QC) are located one after another along the x-axis and form a fixed block (Fig. 2) (Rozhdestvenskaya et al. 2011). In the polytype charoite-90 neighboring fixed blocks follow each other without shifts. In the polytype charoite-96 neighboring fixed blocks are shifted by octahedron edge, i.e. a translation of c/2. The shifts take place between a hybrid dreier quadruple chain (QC) and a double dreier chain (DC). The disorder along [100] (charoite-d) have been observed actually. This is due to the random distributions + or - shifts.
The monoclinic lattice parameters of charoite from the Patynskiy massif were calculated from the XRPD pattern. They were very close to the parameters of charoite-90 polytype from Murun (Kasatkin et al. 2019). However, the polytypes of charoite are very difficult to determine from the XRPD because these patterns of various polytypes are very close because of the specific features of the charoite structure (Rozhdestvenskaya, Drits 2013).
The dashed line indicates the shift or “stacking fault” plane in charoite structure (Rozhdestvenskaya et al. 2011)
3 Order-Disorder in Denisovite Structure
Rare mineral denisovite occurring as aggregates of fibres typically 200–500 nm diameter. It was discovered uniquely in the Eveslogchorr and Yukspor Mountain in Khibini massif, Kola Peninsula and in the Murun massif, Yakutia, Russia (Konev et al. 1987). It was confirmed as new mineral in 1984 year by CNMNM of IMA (Menshikov 1984). Denisovite occurs in close association with nepheline, potassic feldspar, aegirine, fluorite, apatite, biotite and yuksporite in Khibini massif (Menshikov 1984) and with aegirine, kalsilite, feldspar in Murun massif (Konev et al. 1987, 1996). The crystal chemical formula of denisovite is K14.44(Ca41.96Na6.04)Σ=48[(Si6O17)6(Si12O30)2]F16(O0.4OH3.6)•2H2O, Z = 1 or in general K14Ca42Na6[Si60(O,OH)162]F16(O,OH)8•2H2O. The denisovite structure is formed by building modules the same as those found in charoite (Rozhdestvenskaya et al. 2010, 2011) and other Ca-bearing alkaline silicates (Rozhdestvenskaya, Nikishova 2002).
In the structure the edge-sharing octahedra are arranged in columns along the z-axis with a repeat unit cell of two octahedra, corresponding to the c-lattice parameter. The individual columns are linked in wall with either one or two neighboring columns. Neighboring columns are mutually displaced along [001] by c/4 with respect to each other or half of the length of a vertical octahedron edge. Because of symmetry, it makes no difference if the displacement is + c/4 or –c/4.
In the denisovite structure the octahedra walls form a rigid framework with channels along [001] direction. Channels are situated between zig-zag sequences of walls and form strips along [010] (Fig. 1b).
The structure of the mineral contains topologically distinct dreier silicate chains: two symmetry-related tubular dreier triple chain, [Si12O30]12− (TC) and six dreier double chain, [Si6O17]10− (DC) (Fig. 1b). The chains (DC) can be subdivided into two distinct classes. Four of the xonotlite-like chains (DC2) are related by the twofold axis and the glide plane. The remaining two xonotlite-like chains (DC1) are mapped onto themselves by the twofold axis. The chains (DC1) and (DC2) are differently surrounded by octahedra walls. Thus they are symmetrically and topologically distinct.
The silicate chains are located within tubes, tunnels or half-tubes formed by octahedra walls. Thereby both, their conformation and their relative positions with respect to the octahedra framework are affected.
To vertical octahedron edges of walls the horizontal Si2O7 groups of a given chain (TC) and of three adjacent chains (DC1) and (DC2) are bonded. Therefore they are displaced with respect to each other by the length of one octahedron edge, i.e. c/2. The position of chain (TC) and of the neighbouring (DC1) and (DC2) is thus invariant with respect to the z-direction. Furthermore, since chains (DC1) and (DC2) on opposite sides of a v-shaped octahedra walls are bonded to octahedra which have only equatorial corners available for bonds with Si, the vertical Si2O7 groups of both (DC1) and (DC2) are at the same height. Thus, all silicate chains are fixed with respect to the z- and to the y-direction within one strip along [010]. Furthermore, because the relative shifts of all chains (DC1) and (DC2) with respect to chain (TC) are c/2, they are highly correlated and the whole strip is invariant with respect to the y- and z-direction. This explains why these directions are not affected by disorder.
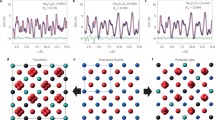
The situation changes significantly in the x-direction. Two neighbouring strips in the x-direction are symmetrically equivalent. Each one is invariant according to what has been considered above. However, the silicate chains in both strips are displaced with respect to each other along the z-direction. Two chains (DC2) on opposite sides of the horizontal octahedra wall belong to two neighbouring strips. The horizontal octahedra wall has inversion centre. The neighbouring columns of horizontal octahedra wall are displaced by c/4 with respect to each other (Fig. 3). Each vertical Si2O7 group of chain (DC2) on opposite sides of the horizontal octahedra wall is connected with a vertical edge of one of the symmetrically equivalent octahedra. Consequently, two chains (DC2) on opposite sides of the horizontal octahedra wall are displaced by c/4 with respect to each other too, and thus the displacement is for the whole strip. In contrast to the octahedra columns, a silicate chain (DC2) does not have inversion symmetry and, hence, displacements by + c/4 or –c/4 are not equivalent. In the real structure shifts in the opposite direction could result in twinning, in a new ordered structure, where shifts + c/4 and –c/4 alternate regularly, or to disorder when the directions of the shifts reverse randomly. The stacking faults ½[001] could also lead to lost correlation. Indeed, HRTEM images (Fig. 4) show that twinning and stacking faults occur already on the length scale of a few nm. The reversal of c/4 displacements of silicate chains with respect to octahedra walls in combination ½[001] stacking faults explain the observed strongly diffuse l odd diffractions.
HRTEM of denisovite, showing ordered P2/a areas, a stacking fault (SF), and a twin boundary TB. A unit cell is indicated in white, yellow rhomboids are a guide to the eyes (Rozhdestvenskaya et al. 2017)
References
Czank, M., Bissert, G.: The crystal structure of Li2Mg2[Si4O11], a loop-branched dreier single chain silicate. Z. für Kristallographie. 204, 129–142 (1993)
Dornberger-Schiff, K.: On order-disorder structures OD-structures Acta Crystallogr. 9, 593–601 (1956)
Dornberger-Schiff, K.: Grundzüge einer Theorie der OD-Strukturen aus Schichten Abhandlungen der Deutschen Akademie der Wissenschaften zu Berlin, Klasse fur Chemie, Geologie und Biologie. 3, 1–107 (1964)
Dornberger-Schiff, K.: Lehrgang über OD Strukturen, p. 135. Akademie Verlag, Berlin (1996)
Ferraris, G., Makovicky, E., Merlino, S.: Crystallography Of Modular Materials, p. 370. Oxford University Press, New York (2004)
Jefferson, D.A., Bown, M.G.: Polytypism and stacking disorder in wollastonite. Nat. Phys. Sci. 245, 43–44 (1973)
Liebau, F.: Structural Chemistry of Silicates, p. 412. Springer, Heidelberg (1985)
Kasatkin, A.V., et al.: Patynit, NaKCa4[Si9O23], a New Mineral from the Patynskiy Massif, Southern Siberia Russia. Minerals 9, 611–630 (2019)
Konev, A.A., Vorob'ev, E.I., Lazebnik, K.A.: Mineralogy of Murunskii Alkaline Massif. Nauchno-Izdatelsky Tsentr Ob'edinennogo Instituta Geologii, Geofiziki, Mineralogii. Siberian Branch of Russian Academy of Science. Novosibirsk. Russia, p. 221 (1996). (in Russian)
Konev, A.A., Vorob'ev, Y.I., Paradina, L.F., Sapozhnikov, A.N.: Denisovite from the Murun pluton, its second find in the world. Doklady Acad. Nauk SSSR. 293, 196–198 (1987). (in Russian)
Kudoh, Y., Takeuchi, Y.: Polytypism in xonotlite: (I) Structure of an A-1 polytype. Mineral. J. 9, 349–373 (1979)
Menshikov, Yu.P.: Denisovite Ca4(K1.4Na0.6)2Si6O16(F,OH)2 – a new mineral from Khibini massif. Zapiski Vsesoyuznogo mineralogicheskogo obshchestva. 113, 718–723 (1984). (in Russian)
Merlino, S.: Okenite, Ca10Si18O46*18H2O: the first example of a chain and sheet silicate. Am. Mineral. 68, 614–622 (1983)
Rogova, V.P., Rogov, Yu.P., Drits, V.A., et al.: Charoite – a new mineral and new semi-precious stone. Zapiski Vsesojuznogo Mineralogicheskogo Obshchestva. 1, 94–97 (1978). (in Russian)
Rozhdestvenskaya, I.V., Drits, V.A.: Peculiarities of the charoite powder diffraction pattern. Zapiski Rossiiskogo Mineralogicheskogo Obshchestva.V. CXLII. 4, 101–112 (2013)
Rozhdestvenskaya, I.V., Nikishova, L.V.: Characteristics of Alkali Calcium Silicates from Charoitites. Crystallography Rep. 47(4), 545–554 (2002)
Rozhdestvenskaya, I.V., Kogure, T., Abe, E., Drits, V.A.: Structural model of charoite. Mineralogical Magazin. 73(5), 883–890 (2009)
Rozhdestvenskaya, I., et al.: The structure of charoite, (K,Sr,Ba,Mn)15–16(Ca,Na)32[(Si70(O,OH)180)](OH,F)4.0 * nH2O, solved by conventional and automated electron diffraction. Mineral. Mag. 74(1), 159–177 (2010)
Rozhdestvenskaya, I., Mugnaioli, E., Czank, M., Depmeier, W., Kolb, U., Merlino, S.: Essential features of the polytypic charoite-96 structure compared to charoite-90. Mineral. Mag. 75(6), 2833–2846 (2011)
Rozhdestvenskaya, I.V., et al.: The structure of denisovite, a fibrous nanocrystalline, polytypic disordered very complex silicate, studied by a synergistic multi-disciplinary approach employing methods of electron crystallography and X-ray powder diffraction. IUCrJ. 4(3), 223–242 (2017)
Wenk, H.R., Muller, W.F., Liddell, N.A., Phakey, P.P.: Polytypism in wollastonite Wenk H.R., et al (Eds.). Electron Microscopy in Mineralogy. Berlin, Heidelberg, New York: Springer-Verlag. 564, pp. 324–331 (1976) https://doi.org/10.1007/978-3-642-66196-9_24
Acknowledgements
A description of the OD character (Dornberger-Schiff, 1956, 1964, 1966; Ferraris et al., 2004) of charoite and denisovite has been presented in the papers by Rozhedestvenskaya et al. (2010, 2011, 2017).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Rozhdestvenskaya, I.V., Depmeier, W. (2023). Order-Disorder in Charoite and Denisovite Structures. In: Marin, Y. (eds) XIII General Meeting of the Russian Mineralogical Society and the Fedorov Session. GMRMS 2021. Springer Proceedings in Earth and Environmental Sciences. Springer, Cham. https://doi.org/10.1007/978-3-031-23390-6_85
Download citation
DOI: https://doi.org/10.1007/978-3-031-23390-6_85
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23389-0
Online ISBN: 978-3-031-23390-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)