Abstract
Of the over 100 species in the genus Vibrio, approximately twelve are associated with clinical disease, such as cholera and vibriosis. Crucially, eleven of those twelve, including Vibrio cholerae and Vibrio vulnificus, have been isolated from birds. Since 1965, pathogenic Vibrio species have been consistently isolated from aquatic and ground-foraging bird species, which has implications for public health, as well as the One Health paradigm defined as an ecology-inspired, integrative framework for the study of health and disease, inclusive of environmental, human, and animal health. In this meta-analysis, we identified 76 studies from the primary literature which report on or examine birds as hosts for pathogenic Vibrio species. We found that the burden of disease in birds was most commonly associated with V. cholerae, followed by V. metschnikovii and V. parahaemolyticus. Meta-analysis wide prevalence of our Vibrio pathogens varied from 19% for V. parahaemolyticus to 1% for V. mimicus. Wild and domestic birds were both affected, which may have implications for conservation, as well as agriculturally associated avian species. As pathogenic Vibrios become more abundant throughout the world as a result of warming estuaries and oceans, susceptible avian species should be continually monitored as potential reservoirs for these pathogens.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Waterborne pathogens around the globe are experiencing a period of unprecedented global change, with the Vibrionaceae categorized among the most climate-sensitive families of aquatic prokaryotes (Hofstra 2011; Lipp et al. 2002). Evidence continues to mount concerning the uptick in the abundance, distribution, and phenology of the Vibrionaceae, since rising temperatures, humidity, and precipitation have led to their increased survival and rates of replication (Wittman and Flick 1995; Montánchez and Kaberdin 2020; Vezzulli et al. 2020). Within this family resides the genus Vibrio, a genetically diverse group of gram-negative, motile, and facultatively anaerobic bacteria that are endemic to marine and estuarine waters (Pruzzo et al. 2005). With over 100 named species in the Vibrio genus, approximately twelve are known to be pathogenic to human hosts (Huehn et al. 2014). Specifically, eleven of the twelve, i.e., V. alginolyticus, V. cholerae, V. cincinnatiensis, V. hollisae, e.g., Grimontia hollisae, V. furnissii, V. mimicus, V. parahaemolyticus, V. vulnificus, V. harveyi, V. scophthalmi, and V. metschnikovii, are the causative agents of human vibriosis, a term that incorporates a broad range of clinical signs (Ramamurthy et al. 2014; Igbinosa and Okoh 2008; Morris and Acheson 2003). These pathogenic species arguably include some of the greatest public health burdens worldwide, and over the last 40 years, the incidence of Vibrio infections has strikingly increased (Rodrick 1991; Baker-Austin et al. 2010, 2017). The continued rise of the incidence and prevalence of Vibrio pathogens has contributed to an unprecedented worldwide health burden of enteric, diarrheal diseases (Levy et al. 2018; Semenza 2020). Yet, the Vibrionaceae are not only expanding their breadth throughout the human population—over the last one hundred and fifty years, but it also appears that the Vibrio genus is expanding its niche into avian hosts, with ensuing implications for the One Health paradigm, and how we contextualize “human” diseases (Sekyere et al. 2020; Destoumieux-Garzón et al. 2018; Jeamsripong et al. 2020; Sweet et al. 2021).
During the fifth pandemic of cholera (1881–1886), the bacteriologist Gamelaia reported a disease afflicting Rock Pigeons (Columba livia) and domestic chickens (Gallus gallus) in southern Russia. It was described as “a disease of fowls,” of which the etiological agent was indistinguishable by morphological examination from Vibrio cholerae (Gamaleia 1888; Henze 2010). This etiological agent would eventually be classified as Vibrio metschnikovii, and by the early twenty-first century, it would be considered one of the twelve pathogenic Vibrio species that cause disease in human hosts (Huehn et al. 2014; Skerman et al. 1980; Tantillo et al. 2004). The occurrence of another pathogenic Vibrio isolated from birds would not be reported until 1966, when individual species from the Gifu and Higashiyama Zoos (Table 15.2) in Japan tested positive by culture for Biotypes 1 and 2 of Vibrio parahaemolyticus (Ose 1967). Pathogenesis in these zoo birds was not reported (Ose 1967). Based on the literature, it is possible that the bird that had cultured positive for Biotype 2 of Vibrio parahaemolyticus was in fact shedding V. alginolyticus (Sakazaki 1968; Fu et al. 2016).
Pathogenic Vibrio species can be lethal in human hosts. For example, Vibrio vulnificus is a causative agent of primary septicemia with a case fatality rate of up to fifty percent (Bross et al. 2007; Oliver et al. 2012). As one of the world’s leading causes of seafood-related deaths, Vibrio vulnificus is an opportunistic pathogen which causes high morbidity and mortality among the immunocompromised and those with liver disease (Oliver and Sadowsky 2015; López-Pérez et al. 2021). Vibrio cholerae, specifically serotypes O1 and O139, is likely the most well-known member of the Vibrio genus. It is a pathogen which has generated seven pandemics since 1817, and whose ecology and pathogenesis has been covered in depth (Hu et al. 2016; Mutreja et al. 2011; Colwell 1996; Colwell and Spira 1992; Faruque et al. 2003; Almagro-Moreno and Taylor 2014). Vibrio parahaemolyticus is a leading cause of seafood-borne illness, with clinicians reporting gastroenteritis and septicemia as the primary causes of morbidity among patients (Li et al. 2019; Letchumanan et al. 2014). V. alginolyticus and V. fluvialis are considered emerging pathogens and have been linked to gastroenteritis and extraintestinal infections (Ramamurthy et al. 2014; Mustapha et al. 2013). The remaining Vibrio species, V. cincinnatiensis, V. hollisae, e.g., Grimontia hollisae, V. furnissii, V. mimicus, V. harveyi, V. scophthalmi, and V. metschnikovii have been linked to sporadic reports of disease in human hosts (Magalhães et al. 1996; Jean-Jacques et al. 1981; Jäckel et al. 2020; Edouard et al. 2009; Derber et al. 2011; Kay et al. 2012), however, that does not diminish their clinical, veterinary, or ecological importance.
The One Health paradigm is a collaborative endeavor that seeks to incorporate the health of the environment, animals, and humans, given the understanding that the resilience of these individual components is integrated and intertwined (Patz and Hahn 2013; Conrad et al. 2009). Thus, the emergence of pathogenic Vibrio species in birds is not only of public health importance (Islam et al. 2020; Laviad-Shitrit et al. 2017), but also of significance to avian disease ecology, as little is known of the large-scale effects that members of the Vibrio genus may have upon species of conservation concern (Friend 2006; Friend et al. 2001). With few exceptions (Almagro-Moreno and Taylor 2014), little is also known concerning the role that birds may play in the maintenance or potentially cyclical contamination of the brackish, aquatic reservoirs they share with other susceptible vertebrates (Fukushima and Seki 2004; Ogg et al. 1989; West et al. 1983; Vezzulli et al. 2010; Meszaros et al. 2020). Therefore, in this chapter, we build on the work of prior investigators who have identified the presence of pathogenic Vibrio species in avian species to a) identify the avian taxas most likely to excrete the pathogens and b) assess the prevalence of individuals in each community or sample that do so. We further examine whether pathogenic Vibrio species are immunogenic and/or pathogenic to birds and the duration that they shed in experimental infection studies. We focus not just on studies that have identified the presence or absence of pathogenic Vibrio species in wild avian communities, but also include experimental infection and immunity studies. Our objective is to provide a baseline framework by which avian disease ecologists, wildlife management professionals, veterinarians, and One Health personnel can evaluate and/or mitigate the potential risks of emerging pathogenic Vibrio species within our wild birds.
15.2 Methods
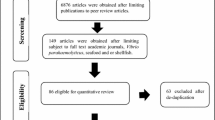
Using Google Scholar and Web of Science (Wiethoelter et al. 2015; Murray et al. 2016), we searched for peer-reviewed studies, pre-prints, abstracts, and graduate theses in which the antibodies against or the antigens of pathogenic Vibrio species were isolated from birds or from the avian environment (e.g., the isolation of Vibrio pathogens from avian fecal matter or their nests) (Ayala et al. 2020). In our search strategy, we used the following search terms and Boolean operators: “Vibrio pathogen of interest” OR “Vibrio pathogen and disease” and “bird*” OR “wild bird*” OR “avian” (n = 14,950). In our search, we systematically searched for studies that examined evidence of infection by the following members of the Vibrio genus: V. alginolyticus, V. cholerae, V. cincinnatiensis, V. furnissii, V. mimicus, V. parahaemolyticus, V. vulnificus, V. harveyi, V. scophthalmi, and V. metschnikovii. Given the relatively recent taxonomic reclassifications of Grimontia hollisae (Thompson et al. 2003) and Photobacterium damselae (Smith et al. 1991) from the genus Vibrio, we also included these pathogens in our analysis. We included experimental infection studies, case reports, and cross-sectional studies published between 1966 and January 1, 2022. In our analysis, we excluded sources that did not serve as primary literature involving investigations of pathogenic Vibrio infections in domestic or wild birds, e.g., retrospective studies and review papers (retrospective and review papers, n = 24) as well as duplicates (duplicates, n = 81). We also excluded any literature without a clear diagnostic and physiological association between domestic or wild birds as hosts of our Vibrio species of interest (exclusion criteria, n = 14,845).
From each study, we extracted the following elements when available: avian species or taxonomic grouping, Vibrio species, country, year the study was conducted or published, the number of birds tested or infected, the number of birds from which Vibrio was isolated, and the method(s) by which pathogenic Vibrio species were identified. Where possible, we identified the prevalence of our Vibrio pathogens of interest, including presence and absence, to determine study-wide prevalence. We also reported serotypes and/or clinically important strains when that information was provided. We further identified whether our Vibrio pathogens of interest were associated with clinical signs or avian mortality events, however, unless specifically stated in the text, we could not determine whether our Vibrio pathogens of interest were the causative agent(s) of reported morbidity or mortality.
15.3 Results
15.3.1 Literature Review
We identified 76 studies from the primary literature that met our inclusion criteria, resulting in 425 study records of avian species or taxonomic groups from which the presence or absence of pathogenic Vibrio species was recorded (identified species, n = 171, identified families, n = 46). In our meta-analysis, a study record ranges from a single examined bird to 565 examined birds, which reflects the same species or taxonomic group that was tested for a single pathogenic Vibrio species of interest that originated from within the same study (Tables 15.1, 15.2, 15.3, 15.4, and 15.5). In our meta-analysis, 29 countries were represented, constituting all continents except for Antarctica. Of the fifty-five years between 1966 and the start of 2022, studies were either published in or conducted in 41 of them. Sixteen study records did not provide sufficient information from which to identify Vibrio prevalence, as either the number of birds, flocks, nests, or sites were incompletely reported or collected samples were pooled. When the Vibrio pathogens of interest were either not named or not classified into species, it was categorized as “Vibrio spp.”.
15.3.2 Vibrio cholerae
A total of 41 studies in the primary literature examined the role of Vibrio cholerae in wild or domestic birds (Laviad-Shitrit et al. 2017; Ogg et al. 1989; Aberkane et al. 2015; Aguirre et al. 1991, 1992; Akond et al. 2008; Bisgaard and Kristensen 1975; Bisgaard et al. 1978; Bogomolni et al. 2008; Buck 1990; Cardoso et al. 2014, 2018; Contreras-Rodríguez et al. 2019; Cox 1992; Fernández-Delgado et al. 2016; Hirsch et al. 2020; Ismail et al. 2021; Metzner et al. 2004; Laviad-Shitrit et al. 2018; Lee et al. 1982; Huamanchumo 2021; Mehmke et al. 1992; Myatt and Davis 1989; Páll et al. 2021; Rodríguez et al. 2010; Roges et al. 2010; Sack 1973; Salles et al. 1976; Sanyal et al. 1974; Schlater et al. 1981; Siembieda et al. 2011; Singh et al. 1975; Song et al. 1998; Strauch et al. 2020; Szeness et al. 1979; Watanabe et al. 2002; Watts et al. 1993; Sakazaki and Shimada 1977; Wobeser and Rainnie 1987; Zhang et al. 1996; Zheng et al. 2020, 2021). One hundred and fifty-six study records investigated the presence or absence of Vibrio cholerae, with the most common technique utilized being culture alone, followed by culture and PCR, or PCR coupled with sequencing. Twenty-five study records reported multiple serotypes from the same species, in the same study. Five of those 25 study records dealt with individual birds who either excreted or displayed multiple serotypes within the same fecal or blood sample or were sampled longitudinally and subsequently cultured positive for different serotypes at different times (Ogg et al. 1989; Singh et al. 1975). Four study records reported the detection of Vibrio cholerae O1 from within one or a flock of birds, with Inaba and Ogawa each reported at least once (Ogg et al. 1989; Rodríguez et al. 2010; Salles et al. 1976; Sanyal et al. 1974). Serotype distribution across species or taxonomic groups was not analyzed, since the number of birds positive for each serotype was usually not provided in the primary literature. We do, however, report the available data in Table 15.1. The most common “type” of Vibrio cholerae reported from birds was non-O1/O139, however, many study records did not identify or report the serotype of Vibrio cholerae that was isolated. Vibrio cholerae O139 was not reported from any study.
One hundred and seven (n = 107) species were examined for the presence of Vibrio cholerae antigens or antibodies. An additional sixteen records were extracted from the literature, but we were not able to identify those study records to species. The Anatidae (waterfowl) represented 49 study records, Laridae (gulls and terns) represented 20 study records, and the Ardeidae (shorebirds) represented 10 study records. Within our meta-analysis, 5492 reported birds were tested for Vibrio cholerae, and 864 reported birds tested positive, for an overall meta-analysis prevalence of 16%. The prevalence of various studies ranged from 100% in case reports to zero, for example, this often represented rarely captured species that did not yield evidence of exposure to the pathogen. Mallards (Anas platyrhynchos) (n = 381) (Ogg et al. 1989; Cox 1992; Siembieda et al. 2011; Szeness et al. 1979; Zhang et al. 1996) appeared to be the most captured and examined wild species, while domestic chickens (n = 552), both backyard and experimentally inoculated, were the most commonly examined domestic species (Akond et al. 2008; Ismail et al. 2021; Salles et al. 1976; Sanyal et al. 1974; Singh et al. 1975; Sakazaki and Shimada 1977). Wilson’s Plover (Charadrius wilsonia), a species of shorebird that was examined in Venezuela (n = 16/16), had the highest cross-sectional study prevalence for any wild bird captured, with a prevalence of 100% (Huamanchumo 2021). This was followed by Greater Yellowlegs (Tringa melanoleuca), also in Venezuela (n = 6/6), with a prevalence of 100% (Huamanchumo 2021), and Killdeer (Charadrius vociferus) in the western United States (n = 13/15), with a prevalence of 86.7% (Ogg et al. 1989).
Clinical signs were reported from 20 study records and were most often associated with V. cholerae non-O1/O139 (Aguirre et al. 1991; Bisgaard and Kristensen 1975; Hirsch et al. 2020; Metzner et al. 2004; Salles et al. 1976; Schlater et al. 1981; Strauch et al. 2020; Watts et al. 1993; Wobeser and Rainnie 1987; Zheng et al. 2020, 2021). One study reported clinical signs, primarily edema and cellulitis of the gastrointestinal tract, with an experimental inoculation of O1 Ogawa in domestic chickens (Salles et al. 1976). Clinical signs from the literature ranged from respiratory signs to lethargy and sepsis; most infections were associated with other pathogens. However, in a mortality study of American Flamingoes (Phoenicopterus ruber), V. cholerae infection was associated with lead toxicity (Aguirre et al. 1991). The largest cross-sectional study to examine wild birds who had exhibited clinical signs in the wild was performed in China, whereby Ruddy Shelducks (Tadorna ferruginea) (n = 25/55) tested positive for V. cholerae non-O1 (Zheng et al. 2021). This study also examined other taxa of birds, such as waterfowl, gulls, shorebirds, and Great Cormorants (Phalacrocorax carbo) for the presence of Vibrio cholerae, however, study-wide prevalences were generally low when associated with clinical signs (Table 15.1).
15.3.3 Vibrio parahaemolyticus
We identified 20 studies in the literature that examined the role of wild birds as hosts for V. parahaemolyticus (Ose 1967; Bogomolni et al. 2008; Buck 1990; Cardoso et al. 2014, 2018; Contreras-Rodríguez et al. 2019; Cox 1992; Myatt and Davis 1989; Páll et al. 2021; Roges et al. 2010; Watanabe et al. 2002; Zheng et al. 2020; Forrester et al. 1997; Fu et al. 2019; Karunasagar et al. 1986; Kassim et al. 2011; Miyasaka et al. 2006; Reuschel et al. 2020; Wang et al. 2021). We extracted seventy-three study records from these papers that examined the prevalence of the pathogen, however, in an additional two study records, we were unable to determine the number of birds infected and/or the number of birds tested (Roges et al. 2010; Kassim et al. 2011). One paper examined the immunogenicity of V. parahaemolyticus and V. vulnificus in Japanese Quail eggs (Coturnix coturnix) and found that birds elicited a high humoral response to the antigens, as measured by ELISA and Western Blots (Kassim et al. 2011). Most studies utilized culture to determine the presence of V. parahaemolyticus, or suckling mice coupled with culture, however, PCR and sequencing were more commonly utilized in more recent works. The Anatidae were represented by 21 study records, the Phasianidae (turkeys, chickens, and pheasants) represented nine study records, and the Laridae represented six study records. We were able to identify 60 species that had been examined for V. parahaemolyticus, representing 22 families. For eleven study records, we were unable to identify the species or family of the birds involved in the study (Bogomolni et al. 2008; Cardoso et al. 2018; Myatt and Davis 1989; Roges et al. 2010; Watanabe et al. 2002; Zheng et al. 2020; Karunasagar et al. 1986). Common Loons (Gavia immer) were the most common species tested for V. parahaemolyticus, after a multi-year mortality event in Florida (Forrester et al. 1997), however the prevalence was only 0.23% (1/434).
Similar to V. cholerae, prevalences for V. parahaemolyticus ranged from 100% in the cases of individual study records that were examined, or zero when relatively cryptic and/or scarce species were assessed. Out of the seventy-five study records that we extracted, only 44 reported study records contained birds that tested positive for the pathogen. The highest prevalence for wild birds captured in a cross-sectional study was 68%, involving three species of gulls: Herring Gulls (Larus argentatus), Laughing Gulls (Leucophaeus atricilla), and Ring-billed Gulls (Larus delawarensis) captured off the coast of Florida (Buck 1990). This was followed by Herring Gulls and Black-tailed Gulls (Larus crassirostris) captured off the coast of Japan, with a prevalence of 67% (Miyasaka et al. 2006). Across our study records, we found that 3996 birds had been tested for the presence of the pathogen or for antibodies against the pathogen. A total of 761 birds were positive for V. parahaemolyticus, for a meta-analysis prevalence of 19%. Clinical signs were only reported for two studies, in both, co-infection with other organisms was noted (Forrester et al. 1997; Reuschel et al. 2020). Four studies were associated with other Vibrio spp. that were not identified to species (Buck 1990; Cox 1992; Páll et al. 2021; Wang et al. 2021). Few studies overlapped between reporting both V. cholerae and V. parahaemolyticus in birds (Buck 1990; Cox 1992; Roges et al. 2010).
15.3.4 Vibrio vulnificus
Eight studies reported examining wild or domestic birds for the presence of V. vulnificus or V. vulnificus antibodies in the literature, from which we were able to extract 21 study records (Cardoso et al. 2018; Páll et al. 2021; Roges et al. 2010; Kassim et al. 2011; Miyasaka et al. 2006; Wang et al. 2021; Adebowale and Adeyemo 2018; Zhao et al. 2020). At least 17 species were represented in this dataset, categorized into 10 families. We were unable to determine within-study prevalences for five of those 21 study records, however, due to the pooling of samples (Roges et al. 2010; Kassim et al. 2011; Zhao et al. 2020). The most commonly utilized method of determining exposure to V. vulnificus in birds was the use of a biochemical panel coupled with culture (Páll et al. 2021; Adebowale and Adeyemo 2018); similar to V. parahaemolyticus, PCR and sequencing were more commonly used in later papers (Wang et al. 2021; Zhao et al. 2020). The prevalence of relevant studies ranged from zero for rarely captured and/or examined species to 50%, which was attributed to one of the two Black-tailed Godwits (Limosa limosa) captured in China that was positive by PCR and sequencing (Wang et al. 2021). This was followed by a prevalence of 26% for Herring Gulls and Black-tailed Gulls sampled off the coast of Japan (Miyasaka et al. 2006). The largest cross-sectional study was performed in Ogun State, Nigeria, from which multiple farms, representing 565 domestic chickens, were sampled for the presence of exposure to V. vulnificus (Adebowale and Adeyemo 2018). The study-wide prevalence was 0.7%.
No clinical signs or mortality events were reported from any study. In a cross-sectional sampling of urban birds in Houston, Texas, Zhao et al. (2020) reported that Muscovy Ducks (Cairina moschata) and Laughing Gulls excreted more V. vulnificus (vvh) than American Crows in the winter as compared to the summer. The greatest diversity of pathogenic Vibrio species was reported from a study of stranded seabirds (n = 17/69, Vibrio spp., prevalence of 25%) in Brazil, from which V. vulnificus was isolated along with V. cholerae, V. parahaemolyticus, V. cincinnatiensis, V. fluvialis, V. harveyi, and V. mimicus (Roges et al. 2010). The individual prevalence of V. vulnificus, or the avian species afflicted in this study was not reported, however a follow-up study reported a V. vulnificus prevalence of 1.7% in Brazilian seabirds (Cardoso et al. 2018). Overall, 1231 birds were examined for evidence of exposure to V. vulnificus, and 94 were positive, for a meta-analysis wide prevalence of 8%.
15.3.5 Vibrio alginolyticus
In the literature, we recovered 15 studies in which the role of domestic and wild birds as hosts for V. alginolyticus was examined, providing us with 49 study records (Bogomolni et al. 2008; Buck 1990; Cardoso et al. 2014, 2018; Contreras-Rodríguez et al. 2019; Cox 1992; Páll et al. 2021; Siembieda et al. 2011; Forrester et al. 1997; Kassim et al. 2011; Adebowale and Adeyemo 2018; Byrum and Slemons 1995; Cooper et al. 1986; de Moura et al. 2012; Work and Rameyer 1999). Two study records, involving seabirds off the coast of Brazil, did not identify the sampled birds to species (Cardoso et al. 2018; Roges et al. 2010). Forty-seven species were represented in this data subset, categorized into 18 families. Nineteen study records were attributed to the Anatidae, five study records to the Laridae, and three study records were represented by the Falconidae family, known for its small falcons and hawks. Culture, followed by biochemical panels, were the most commonly utilized methods to identify the pathogen. PCR was rarely utilized. The highest prevalence of V. alginolyticus recovered from a cross-sectional study of wild birds involved Herring Gulls, Laughing Gulls, and Ring-billed Gulls captured off the coast of Florida, with a prevalence of 68% (Buck 1990). The next highest prevalence of the pathogen was 55%, originating from Herring Gulls and Great Black-backed Gulls captured along coastal Connecticut (Buck 1990). Across the board, prevalences ranged from zero to 68%, no study record reached a prevalence of 100%. In a study performed in Ogun State, Nigeria, V. alginolyticus was isolated from 2% of domestic chickens (Adebowale and Adeyemo 2018).
Clinical signs and mortality were recorded by two studies, one involving a multi-year mortality event of Common Loons in Florida, and the second involved a mortality event off the coast of Oahu, Hawaii, of Wedge-tailed Shearwaters (Ardenna pacifica), which demonstrated a prevalence of 10% (Forrester et al. 1997; Work and Rameyer 1999). Clinical signs ranged from emaciation and lethargy to toxemia and sepsis; bacteremia in the case of the Wedge-tailed Shearwaters was strongly suspected (Forrester et al. 1997; Work and Rameyer 1999). In a diagnostic examination of critically endangered Mauritius Kestrels (Falco punctatus), a captive individual (1/6) was positive by culture for V. alginolyticus, yet no clinical signs were noted (Cooper et al. 1986). In general, waterfowl demonstrated the lowest prevalences for any group of birds, besides passerines, for the pathogen (Cox 1992; Páll et al. 2021). Throughout our dataset, 258 birds of 2967 sampled birds tested positive for V. alginolyticus, for a meta-analysis prevalence of 9%.
15.3.6 Vibrio fluvialis
From the literature, we found 15 studies that reported examining wild or domestic birds for the presence of V. fluvialis or V. fluvialis antibodies, from which we were able to extract 26 study records (Bogomolni et al. 2008; Buck 1990; Cardoso et al. 2014, 2018; Huamanchumo 2021; Myatt and Davis 1989; Páll et al. 2021; Roges et al. 2010; Kassim et al. 2011; de Moura et al. 2012; Bönner et al. 2004; Jubirt 2012; Moreki et al. 2011; Shimada and Sakazaki 1983; Shnawa et al. 2014; Wobeser and Kost 1992). At least 20 species were reported in this dataset, representing 15 families. Four records did not provide sufficient data from which to identify birds to species or family. Culture was the most commonly utilized method to identify V. fluvialis, followed by a biochemistry panel. The largest cross-sectional study examining the prevalence of V. fluvialis in wild birds was performed on Canada Geese in Germany, however, only one of 289 birds cultured positive for the pathogen (Bönner et al. 2004). V. fluvialis was associated with one mortality event—a die-off of overwintering Mallards in Canada which was attributed to a Vitamin A deficiency (Wobeser and Kost 1992). The reported prevalence of the pathogen for these birds was 8%. In a study of captive study of Great Egrets (Ardea alba) captured from the Mississippi Delta, control birds shed V. fluvialis for four of seven days in captivity (Jubirt 2012). The highest prevalence was associated with a study performed in Connecticut involving Herring Gulls and Great Black-backed Gulls, with 55% culturing positive for V. fluvialis. Studies with a prevalence of 100% involved two experiments, one involving of avian-sourced strains, and a mitogenicity study on domestic chickens (Shimada and Sakazaki 1983; Shnawa et al. 2014). Overall, the meta-analysis prevalence, including experimental infection studies (88/834) was 11% percent (85/834).
Other Pathogenic Vibrio spp.: V. cincinnatiensis, V. hollisae, e.g., Grimontia hollisae, V. furnissii, V. mimicus, V. harveyi, V. scophthalmi, V. metschnikovii, and Photobacterium damselae.
The abundance of studies that reported on other pathogenic Vibrio species that were isolated from wild or domestic birds varied (Table 15.6). V. cincinnatiensis was examined by five studies and provided five study records (Jäckel et al. 2020; Cardoso et al. 2014, 2018; Roges et al. 2010; de Moura et al. 2012). Unspecified seabirds were the taxa that were examined most frequently (Cardoso et al. 2018; Roges et al. 2010), however overall prevalences were low across all studies for a mean prevalence of 3%. The presence or absence of Photobacterium damselae was examined by three studies (Buck 1990; Forrester et al. 1997; Colvile et al. 2012), and yielded three study records from the United Kingdom and the United States. Two studies involved mortality events, one of Common Loons in Florida, and the second of British passerines (Forrester et al. 1997; Colvile et al. 2012). Across these cross-sectional studies, the overall meta-analysis prevalence was approximately 5%. Our literature search of V. furnissii yielded four study records from three cross-sectional studies (Cardoso et al. 2018; Huamanchumo 2021; de Moura et al. 2012), two of those study records did not identify the number of birds positive or the number of individuals examined. The Laridae were the most prevalent species identified in association with V. furnissii, specifically Kelp Gulls (Larus dominicanus), Laughing Gulls, and Brown Boobys (Sula leucogaster). The overall meta-analysis prevalence for this Vibrio pathogen was approximately 2%.
The search for studies involving V. harveyi and avian species yielded four cross-sectional studies and four study records (Cardoso et al. 2014, 2018; Roges et al. 2010; Wang et al. 2021), involving seabirds and Manx Shearwaters (Puffinus puffinus). A single study involving a coastal sandpiper, the Common Greenshank (Tringa nebularia), had a prevalence of 0% out of five birds that were tested by PCR (Wang et al. 2021). From the two studies that provided individual birds positive in contrast to individual birds examined, we were able to calculate a V. harveyi prevalence of approximately 13%. No study that tested for this pathogen reported clinical signs or a mortality event. Grimontia hollisae was rarely detected in birds, as we found only two studies, resulting in three study records, that searched for the pathogen in avian hosts (Fu et al. 2020; Albuixech-Martí et al. 2021). One study examined the shared microbiota between wild Hooded Cranes (Grus monacha) and domestic geese (Anser anser) using MiSeq—Grimontia hollisae was identified as a potential pathogen, but the total number of birds colonized was not reported (Fu et al. 2020). A longitudinal microbiome study involving shorebirds off the coast of Cork, Ireland discovered Grimontia hollisae in fecal samples, however, the number of samples positive/examined was not enumerated (Albuixech-Martí et al. 2021). Clinical signs or mortality were not reported from either study.
Vibrio metschnikovii was reported from three studies, resulting in 10 study records (Páll et al. 2021; Zheng et al. 2021; Lee et al. 1978). Approximately half the study records examined passerines of Romania as hosts (Páll et al. 2021), including members of the Laniidae, Sylviidae, and Paridae families, all of which were negative for the pathogen by biochemical panels. The highest prevalence was reported from sites in Inner Mongolia, China, by Black-headed Gulls (Chroicocephalus ridibundus), from which an overall study prevalence of 38% was reported (Zheng et al. 2021). Clinical signs and a mortality event that spanned multiple waterfowl and waterbird species were documented in the study by Zheng et al. (2021). The number of total tested birds was not available for analysis in the latter study; thus, we could not report a meta-analysis prevalence of V. metschnikovii with confidence. Vibrio mimicus was examined by six studies, yielding 18 study records (Cardoso et al. 2018; Páll et al. 2021; Roges et al. 2010; Fu et al. 2019; Adebowale and Adeyemo 2018; Foti et al. 2020). Biochemical panels were the most common diagnostic tool used to identify the pathogen, however, overall prevalences were very low across studies. In a study of wading birds and songbirds performed along the Danube Delta of Romania, all sampled birds (n = 38) were negative for V. mimicus (Páll et al. 2021). On the other hand, wading birds and seabirds sampled in China, Brazil, and Italy demonstrated evidence of shedding the pathogen (Cardoso et al. 2018; Fu et al. 2019; Foti et al. 2020). The only study to examine the role of domestic birds as hosts was performed in Ogun State, Nigeria—this study yielded a prevalence of approximately 1% (Adebowale and Adeyemo 2018). Across studies, 806 birds were examined for the presence of the pathogen, with 21 testing positive, resulting in a meta-analysis prevalence of 2%. No clinical signs or mortality events were reported from any study that examined the role of birds as hosts for V. mimicus.
Vibrio scophthalmi was only reported from one study, resulting in a single study record (Fu et al. 2019). A Common Greenshank (1/26) that was sampled using whole genome sequencing was positive for the pathogen (Fu et al. 2019). This study was not associated with clinical signs or a mortality event. Uncategorized Vibrio spp. were reported from 14 studies (Bogomolni et al. 2008; Buck 1990; Cardoso et al. 2018; Cox 1992; Fernández-Delgado et al. 2016; Huamanchumo 2021; Páll et al. 2021; Watanabe et al. 2002; Zheng et al. 2020; Wang et al. 2021; Albuixech-Martí et al. 2021; White et al. 1973; Negruțiu et al. 2017; Saiful Islam et al. 2021). Two studies were associated with clinical signs and/or mortality events, however, these outbreaks were attributed to other causal pathogens (Zheng et al. 2020; White et al. 1973). Culture followed by biochemical panels were the most commonly utilized methods of identifying Vibrio spp. Given that many studies did not identify these Vibrio spp. to species or identify the number of birds excreting them, we were unable to calculate a meta-analysis wide prevalence.
15.4 Discussion
The question of pathogenic Vibrio spp. as the etiological agents of disease in birds remains only partially answered. Of the 76 studies that surveyed birds for pathogenic Vibrio species, 19 reported disease or death from individuals, scaling up to community-level events (Aguirre et al. 1991; Bisgaard and Kristensen 1975; Hirsch et al. 2020; Metzner et al. 2004; Salles et al. 1976; Schlater et al. 1981; Strauch et al. 2020; Watts et al. 1993; Wobeser and Rainnie 1987; Zheng et al. 2020, 2021; Forrester et al. 1997; Reuschel et al. 2020; Work and Rameyer 1999; Moreki et al. 2011; Wobeser and Kost 1992; Colvile et al. 2012; Lee et al. 1978; White et al. 1973). Yet, it remains uncertain whether these pathogenic Vibrio species are opportunistic pathogens that contribute to morbidity and/or mortality in already stressed individuals, or whether they can be the primary arbiters of disease (Zhao et al. 2020). Experimental inoculation studies reported contrasting results, if they reported clinical signs at all (Laviad-Shitrit et al. 2017; Salles et al. 1976; Zhang et al. 1996; Shnawa et al. 2014). In addition, avian susceptibility to pathogenic Vibrio species may also be conflated by host species, natural history, and prior exposure, resulting in an as yet-understood degree of immunity (Roche et al. 2009; Gamble et al. 2019). In our meta-analysis, disease was most commonly associated with V. cholerae, followed by V. metschnikovii and V. parahaemolyticus—notably, 11 of 39 study records were associated with domestic ducks (Anas platyrhynchos or Anser anser) or domestic chickens (Bisgaard and Kristensen 1975; Bisgaard et al. 1978; Hirsch et al. 2020; Metzner et al. 2004; Salles et al. 1976; Watts et al. 1993). This may have implications for agriculturally associated species in areas of the world where backyard birds are the primary protein source for pastoral families (Conan et al. 2012; Hamilton-West et al. 2012; Kariithi et al. 2021).
Of the 425 study records we extracted from the literature, interestingly, the Anatidae represented 105 of them, including wild and domesticated Mallards, which represented 16 study records. The Laridae represented 39 study records, prominently represented by Laughing Gulls, Herring Gulls, and Ring-billed Gulls. Shorebirds and waders, categorized into the Ardeidae family, represented 16 study records, primarily of egrets and herons. These bird species are often highly associated with coastal estuarine and marine environments (Barnes and Thomas 1987; Waldenström et al. 2002; Chatterjee et al. 2020), which are also inhabited by autochthonous and halophilic Vibrio species. These results are congruent with what is known of avian foraging ecology and Vibrio habitat specificity (Pruzzo et al. 2005; Almagro-Moreno and Taylor 2014; Vezzulli et al. 2010; Grimes et al. 2009; Johnson et al. 2012; Grimes 2020). What was unexpected were the number of ground-foraging birds that tested positive for pathogenic Vibrio species that are often not strictly associated with aquatic environments, such as Great Tits (Parus major), Garden Warblers (Sylvia borin), and Hooded Crows (Corvus cornix) (Mehmke et al. 1992; Páll et al. 2021).
For example, in a study of Egyptian backyard poultry (chickens, turkeys, and waterfowl), 36% of examined birds were positive for V. cholerae, including chickens and turkeys (Ismail et al. 2021). Domestic chickens accounted for 13 total study records, across geographical areas as varied as the United States, Bangladesh, Egypt, Ghana, Nigeria, Iraq, and India, and reported as early as 1972 (Akond et al. 2008; Ismail et al. 2021; Salles et al. 1976; Sanyal et al. 1974; Singh et al. 1975; Sakazaki and Shimada 1977; Adebowale and Adeyemo 2018; Byrum and Slemons 1995; Shnawa et al. 2014; Lee et al. 1978). On the other hand, another surprising result was the low prevalence of pathogenic Vibrio species cultured from seabirds that were sampled from the New England region of the United States, with only one of 192 birds testing positive for Vibrio cholerae, non-O1 (Bogomolni et al. 2008). This result may be due to several reasons, many of which are not mutually exclusive (Chatterjee et al. 2020). For one, as seabirds tend to spend more time in marine versus coastal habitats, they may be less susceptible to exposure from pathogenic Vibrio species that tend to congregate in lower salinity, brackish habitats (Hsieh et al. 2008). In addition, the northern Atlantic may harbor a lower abundance of pathogenic Vibrios during the cooler months as a result of low sea surface temperatures (Baker-Austin et al. 2010, 2012). Lastly, it may be possible that although pathogenic Vibrio spp. may cause disease in seabirds, that the recovery of carcasses or diseased individuals may be reduced due to minimal mortality, low carcass persistence, and increased distances from urbanized centers (Piatt and Ford 1996; Ford 2006; Ward et al. 2006).
Meta-analysis prevalence varied across pathogenic Vibrio species, but all were below 20% (e.g., 19% for V. parahaemolyticus, 16% for V. cholerae, 13% for V. harveyi, 11% for V. fluvialis, 9% for V. alginolyticus, 8% for V. vulnificus, 5% for P. damselae, 2% for V. furnissii, and 1% for V. mimicus). Given that we utilized experimental inoculation studies coupled with cross-sectional studies, there is likely a degree of reporting bias in our meta-analysis prevalences (Lachish and Murray 2018), however, we speculate that this reporting bias is likely offset by the reportedly few studies that have targeted these pathogens for investigation in wild and domestic birds. To determine true “prevalence,” and avian susceptibility under ecological conditions, longitudinal studies that sought to recover these pathogens from a community of birds would be more informative (Wobeser 2007; Brown et al. 2013). In addition, these studies would need to utilize large sample sizes, as well as represent various ecological foraging guilds, in geographic locations with both low and high recovery rates of these pathogens from their aquatic, environmental reservoir (Stallknecht 2007; Cardoso et al. 2021; Watsa and Wildlife Disease Surveillance Focus Group 2020; Sleeman et al. 2012).
With a meta-analysis Vibrio prevalence of 16% coupled with the reports of clinical signs, there is a possibility that pathogenic Vibrio species—specifically V. parahaemolyticus, V. cholerae, and V. metschnikovii—may be emerging pathogens of wild and domestic aquatic or wetland birds (Daszak et al. 2000; Robinson et al. 2010). Gire et al. (2012) defined emerging pathogens as falling into two categories: introduced microbes and existing microbes that rapidly increase in prevalence and/or incidence in a population. Given that so little is known of non-cholera Vibrio species in human hosts, however, it is difficult to distinguish between the two categories in our avian hosts given the currently available data. Speculation suggests that these Vibrio pathogens may have a long-standing relationship with aquatic birds. However, as climate change alters and influences the abundance and distribution of pathogenic Vibrio species in marine and estuarine environments, so too may the incidence of these pathogens in wild and domestic birds (Fuller et al. 2012).
In summary, we have offered a rigorous meta-analysis that examines the prevalence of Vibrio spp. across bird species. In doing so, we also reveal a plethora of data that fortifies the notion that birds are both an underappreciated object of study and potential reservoirs for pathogenic bacterial species. In the context of a dynamic ecology defined by climate change and human-associated activities, we suggest that avian reservoirs should be the focus of more rigorous study, as they may be an actor in Vibrio emergence events. Transcending the case of birds, our study proposes that more attention should be paid to animal species that may harbor pathogens of interest to human health.
References
Aberkane S, Compain F, Barraud O, Ouédraogo A-S, Bouzinbi N, Vittecoq M et al (2015) Non-O1/Non-O139 Vibrio cholerae Avian isolate from France cocarrying the bla(VIM-1) and bla(VIM-4) genes. Antimicrob Agents Chemother 59(10):6594–6596. https://doi.org/10.1128/AAC.00400-15
Adebowale O, Adeyemo O (2018) Characterization of bacterium types isolated from commercial laying hen farms in Ogun State Nigeria. Rev Elev Med Vet Pays Trop 71(3):137–141. https://doi.org/10.19182/remvt.31642
Aguirre AA, Cook RS, McLean RG, Quan TJ, Spraker TR (1991) Occurrence of potential pathogens in Wild Caribbean Flamingos (Phoenicopterus ruber ruber) during a lead poisoning die off in Yucatán, Mexico. J Zoo Wildl Med 22(4):470–475
Aguirre AA, Quan TJ, Cook RS, McLean RG (1992) Cloacal flora isolated from wild black-bellied whistling ducks (Dendrocygna autumnalis) in Laguna La Nacha, Mexico. Avian Dis 36(2):459–462. https://doi.org/10.2307/1591530
Akond MA, Alam S, Hasan S, Uddin SN, Shirin M (2008) Antibiotic resistance of Vibrio cholerae from poultry sources of Dhaka, Bangladesh. Adv Biol Res 2:60–67
Albuixech-Martí S, Lynch SA, Culloty SC (2021) Connectivity dynamics in Irish mudflats between microorganisms including Vibrio spp., common cockles Cerastoderma edule, and shorebirds. Sci Rep 11(1):22159. https://doi.org/10.1038/s41598-021-01610-x
Almagro-Moreno S, Taylor RK (2014) Cholera: environmental reservoirs and impact on disease transmission. In: Atlas RM, Maloy S (eds) One health. ASM Press, Washington, DC, pp 149–165
Ayala AJ, Yabsley MJ, Hernandez SM (2020) A review of pathogen transmission at the Backyard Chicken–Wild bird interface. Front Vet Sci 7(662). https://doi.org/10.3389/fvets.2020.539925
Baker-Austin C, Stockley L, Rangdale R, Martinez-Urtaza J (2010) Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: a European perspective. Environ Microbiol Rep 2(1):7–18. https://doi.org/10.1111/j.1758-2229.2009.00096.x
Baker-Austin C, Trinanes JA, Taylor NGH, Hartnell R, Siitonen A, Martinez-Urtaza J (2012) Emerging Vibrio risk at high latitudes in response to ocean warming. Nat Clim Chang 3(1):73–77. https://doi.org/10.1038/nclimate1628
Baker-Austin C, Trinanes J, Gonzalez-Escalona N, Martinez-Urtaza J (2017) Non-cholera vibrios: the microbial barometer of climate change. Trends Microbiol 25(1):76–84. https://doi.org/10.1016/j.tim.2016.09.008
Barnes GG, Thomas VG (1987) Digestive organ morphology, diet, and guild structure of North American Anatidae. Can J Zool 65(7):1812–1817. https://doi.org/10.1139/z87-274
Bisgaard M, Kristensen KK (1975) Isolation, characterization and public health aspects of Vibrio cholerae nag isolated from a Danish Duck Farm. Avian Pathol 4(4):271–276. https://doi.org/10.1080/03079457509353875
Bisgaard M, Sakazaki R, Shimada T (1978) Prevalence of non-cholera vibrios in cavum nasi and pharynx of ducks. Acta Pathol Microbiol Scand Sect B Microbiol 86(1–6):261–266. https://doi.org/10.1111/j.1699-0463.1978.tb00042.x
Bogomolni AL, Gast RJ, Ellis JC, Dennett M, Pugliares KR, Lentell BJ et al (2008) Victims or vectors: a survey of marine vertebrate zoonoses from coastal waters of the Northwest Atlantic. Dis Aquat Org 81(1):13–38. https://doi.org/10.3354/dao01936
Bönner BM, Lutz W, Jäger S, Redmann T, Reinhardt B, Reichel U et al (2004) Do Canada geese (Branta canadensis Linnaeus, 1758) carry infectious agents for birds and man? Eur J Wildl Res 50(2):78–84. https://doi.org/10.1007/s10344-004-0044-1
Bross MH, Soch K, Morales R, Mitchell RB (2007) Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician 76(4):539–544
Brown VL, Drake JM, Stallknecht DE, Brown JD, Pedersen K, Rohani P (2013) Dissecting a wildlife disease hotspot: the impact of multiple host species, environmental transmission and seasonality in migration, breeding and mortality. J R Soc Interface 10(79):20120804. https://doi.org/10.1098/rsif.2012.0804
Buck JD (1990) Isolation of Candida albicans and halophilic Vibrio spp. from aquatic birds in Connecticut and Florida. Appl Environ Microbiol 56(3):826–828. https://doi.org/10.1128/aem.56.3.826-828.1990
Byrum BR, Slemons RD (1995) Detection of proteolytic bacteria in the upper respiratory tract flora of poultry. Avian Dis 39(3):622–626. https://doi.org/10.2307/1591817
Cardoso MD, de Moura JF, Tavares DC, Gonçalves RA, Colabuono FI, Roges EM et al (2014) The Manx shearwater (Puffinus puffinus) as a candidate sentinel of Atlantic Ocean health. Aquat Biosyst 10(1):6. https://doi.org/10.1186/2046-9063-10-6
Cardoso MD, Lemos LS, Roges EM, de Moura JF, Tavares DC, Matias CAR et al (2018) A comprehensive survey of Aeromonas sp. and Vibrio sp. in seabirds from southeastern Brazil: outcomes for public health. J Appl Microbiol 124(5):1283–1293. https://doi.org/10.1111/jam.13705
Cardoso B, García-Bocanegra I, Acevedo P, Cáceres G, Alves PC, Gortázar C (2021) Stepping up from wildlife disease surveillance to integrated wildlife monitoring in Europe. Res Vet Sci. https://doi.org/10.1016/j.rvsc.2021.11.003
Chatterjee A, Adhikari S, Pal S, Mukhopadhyay SK (2020) Foraging guild structure and niche characteristics of waterbirds wintering in selected sub-Himalayan wetlands of India. Ecol Indic 108:105693. https://doi.org/10.1016/j.ecolind.2019.105693
Colvile KM, Lawson B, Pocknell AM, Dagleish MP, John SK, Cunningham AA (2012) Chlamydiosis in British songbirds. Vet Rec 171(7):177. https://doi.org/10.1136/vr.100506
Colwell RR (1996) Global climate and infectious disease: the cholera paradigm. Science 274(5295):2025–2031. https://doi.org/10.1126/science.274.5295.2025
Colwell RR, Spira WM (1992) The ecology of Vibrio cholerae. In: Barua D, Greenough WB (eds) Cholera. Springer, Boston, MA, pp 107–127
Conan A, Goutard FL, Sorn S, Vong S (2012) Biosecurity measures for backyard poultry in developing countries: a systematic review. BMC Vet Res 8(1):240. https://doi.org/10.1186/1746-6148-8-240
Conrad PA, Mazet JA, Clifford D, Scott C, Wilkes M (2009) Evolution of a transdisciplinary “One Medicine–One Health” approach to global health education at the University of California, Davis. Prev Vet Med 92(4):268–274. https://doi.org/10.1016/j.prevetmed.2009.09.002
Contreras-Rodríguez A, Aguilera-Arreola MG, Osorio AR, Martin MD, Guzmán RL, Velarde E et al (2019) Detection of potential human pathogenic bacteria isolated from feces of two colonial seabirds nesting on Isla Rasa, Gulf of California: Heermann’s gull (larus heermanni) and Elegant Tern (thalasseus elegans). Trop Conserv Sci 12:1940082919855673. https://doi.org/10.1177/1940082919855673
Cooper JE, Needham JR, Fox NC (1986) Bacteriological, haematological and clinical chemical studies on the Mauritius kestrel (Falco puncta TUS). Avian Pathol 15(3):349–356. https://doi.org/10.1080/03079458608436298
Cox MB (1992) Prevalence of Vibrio species in waterfowl of Jefferson County, Texas. Lamar University, Ann Arbor, p 46
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife--threats to biodiversity and human health. Science 287(5452):443–449. https://doi.org/10.1126/science.287.5452.443
de Moura JF, Roges EM, de Souza RL, Siciliano S, dos Prazeres RD (2012) Marine environment and public health. In: Biodiversity conservation and utilization in a diverse world. IntechOpen, London, p 263. https://doi.org/10.5772/48412
Derber C, Coudron P, Tarr C, Gladney L, Turnsek M, Shankaran S et al (2011) Vibrio furnissii: an unusual cause of bacteremia and skin lesions after ingestion of seafood. J Clin Microbiol 49(6):2348–2349. https://doi.org/10.1128/JCM.00092-11
Destoumieux-Garzón D, Mavingui P, Boetsch G, Boissier J, Darriet F, Duboz P et al (2018) The one health concept: 10 years old and a long road ahead. Front Vet Sci 5(14). https://doi.org/10.3389/fvets.2018.00014
Edouard S, Daumas A, Branger S, Durand JM, Raoult D, Fournier PE (2009) Grimontia hollisae, a potential agent of gastroenteritis and bacteraemia in the mediterranean area. Eur J Clin Microbiol Infect Dis 28(6):705–707. https://doi.org/10.1007/s10096-008-0678-0
Faruque SM, Sack DA, Sack RB, Colwell RR, Takeda Y, Nair GB (2003) Emergence and evolution of Vibrio cholerae O139. Proc Natl Acad Sci 100(3):1304–1309. https://doi.org/10.1073/pnas.0337468100
Fernández-Delgado M, Sanz V, Giner S, Suárez P, Contreras M, Michelangeli F et al (2016) Prevalence and distribution of Vibrio spp. in wild aquatic birds of the Southern Caribbean Sea, Venezuela, 2011–12. J Wildl Dis 52(3):621–626. https://doi.org/10.7589/2015-06-154
Ford RG (2006) Using beached bird monitoring data for seabird damage assessment: the importance of search interval. Mar Ornithol 34:91–98
Forrester DJ, Davidson WR, Lange RE Jr, Stroud RK, Alexander LL, Christian Franson J et al (1997) Winter mortality of common loons in florida coastal waters. J Wildl Dis 33(4):833–847. https://doi.org/10.7589/0090-3558-33.4.833
Foti M, Grasso R, Fisichella V, Mascetti A, Zafarana MA, Colnaghi M et al (2020) Analysis of Eurasian stone curlew (Burhinus oedicnemus) microbial flora reveals the presence of multi-drug resistant pathogens in agro-pastoral areas of Sicily (Italy). Heliyon 6(10):e05401. https://doi.org/10.1016/j.heliyon.2020.e05401
Friend M (2006) Disease emergence and resurgence: the wildlife-human connection. Circular 1285. Other Publications in Wildlife Management. USGS, Reston, VA
Friend M, McLean RG, Joshua DF (2001) Disease emergence in birds: challenges for the twenty-first century. Auk 118(2):290–303. https://doi.org/10.1093/auk/118.2.290
Fu K, Li J, Wang Y, Liu J, Yan H, Shi L et al (2016) An innovative method for rapid identification and detection of Vibrio alginolyticus in different infection models. Front Microbiol 7(651):651. https://doi.org/10.3389/fmicb.2016.00651
Fu S, Hao J, Yang Q, Lan R, Wang Y, Ye S et al (2019) Long-distance transmission of pathogenic Vibrio species by migratory waterbirds: a potential threat to the public health. Sci Rep 9(1):16303. https://doi.org/10.1038/s41598-019-52791-5
Fu R, Xiang X, Dong Y, Cheng L, Zhou L (2020) Comparing the intestinal bacterial communies of sympatric wintering Hooded Crane (Grus monacha) and domestic goose (Anser anser domesticus). Avian Res 11(1):13. https://doi.org/10.1186/s40657-020-00195-9
Fukushima H, Seki R (2004) Ecology of Vibrio vulnificus and Vibrio parahaemolyticus in brackish environments of the Sada River in Shimane Prefecture, Japan. FEMS Microbiol Ecol 48(2):221–229. https://doi.org/10.1016/j.femsec.2004.01.009
Fuller T, Bensch S, Müller I, Novembre J, Pérez-Tris J, Ricklefs RE et al (2012) The ecology of emerging infectious diseases in migratory birds: an assessment of the role of climate change and priorities for future research. EcoHealth 9(1):80–88. https://doi.org/10.1007/s10393-012-0750-1
Gamaleia MN (1888) Vibrio metschnikovi (n. sp.) et ses rapports avec le microbe du choléra asiatique. Ann Inst Pasteur 2:482–488
Gamble A, Garnier R, Jaeger A, Gantelet H, Thibault E, Tortosa P et al (2019) Exposure of breeding albatrosses to the agent of avian cholera: dynamics of antibody levels and ecological implications. Oecologia 189(4):939–949. https://doi.org/10.1007/s00442-019-04369-1
Gire SK, Stremlau M, Andersen KG, Schaffner SF, Bjornson Z, Rubins K et al (2012) Emerging disease or diagnosis? Science 338(6108):750–752. https://doi.org/10.1126/science.1225893
Grimes DJ (2020) The Vibrios: scavengers, symbionts, and pathogens from the sea. Microb Ecol 80(3):501–506. https://doi.org/10.1007/s00248-020-01524-7
Grimes DJ, Johnson CN, Dillon KS, Flowers AR, Noriea NF, Berutti T (2009) What genomic sequence information has revealed about Vibrio ecology in the ocean—a review. Microb Ecol 58(3):447–460. https://doi.org/10.1007/s00248-009-9578-9
Hamilton-West C, Rojas H, Pinto J, Orozco J, Hervé-Claude LP, Urcelay S (2012) Characterization of backyard poultry production systems and disease risk in the central zone of Chile. Res Vet Sci 93(1):121–124. https://doi.org/10.1016/j.rvsc.2011.06.015
Henze CE (2010) Disease, health care and government in late imperial Russia: life and death on the Volga, 1823-1914. Routledge, New York
Hirsch N, Kappe E, Gangl A, Schwartz K, Mayer-Scholl A, Hammerl JA et al (2020) Phenotypic and genotypic properties of Vibrio cholerae non-O1, non-O139 isolates recovered from domestic ducks in Germany. Microorganisms. 8(8):1104. https://doi.org/10.3390/microorganisms8081104
Hofstra N (2011) Quantifying the impact of climate change on enteric waterborne pathogen concentrations in surface water. Curr Opin Environ Sustain 3(6):471–479. https://doi.org/10.1016/j.cosust.2011.10.006
Hsieh JL, Fries JS, Noble RT (2008) Dynamics and predictive modelling of Vibrio spp. in the Neuse River Estuary, North Carolina, USA. Environ Microbiol 10(1):57–64. https://doi.org/10.1111/j.1462-2920.2007.01429.x
Hu D, Liu B, Feng L, Ding P, Guo X, Wang M et al (2016) Origins of the current seventh cholera pandemic. Proc Natl Acad Sci 113(48):E7730–E77E9. https://doi.org/10.1073/pnas.1608732113
Huamanchumo LF (2021) Aislamiento e identificación de Vibrio cholerae y otras especies de vibrios halofílicos patógenos a partir de varios reservorios acuáticos naturales en la zona de la bocana del río Lurín. Universidad Ricardo Palma, Santiago de Surco, Peru
Huehn S, Eichhorn C, Urmersbach S, Breidenbach J, Bechlars S, Bier N et al (2014) Pathogenic vibrios in environmental, seafood and clinical sources in Germany. Int J Med Microbiol 304(7):843–850. https://doi.org/10.1016/j.ijmm.2014.07.010
Igbinosa EO, Okoh AI (2008) Emerging Vibrio species: an unending threat to public health in developing countries. Res Microbiol 159(7):495–506. https://doi.org/10.1016/j.resmic.2008.07.001
Islam MS, Hassan-uz-Zaman M, Islam MS, Clemens JD, Ahmed N (2020) Chapter 1 - Emerging waterborne pathogens in the context of climate change: Vibrio cholerae as a case study. In: Vara Prasad MN, Grobelak A (eds) Waterborne pathogens. Butterworth-Heinemann, Oxford, pp 1–14
Ismail EM, Kadry M, Elshafiee EA, Ragab E, Morsy EA, Rizk O et al (2021) Ecoepidemiology and potential transmission of Vibrio cholerae among different environmental niches: an upcoming threat in Egypt. Pathogens 10(2):190. https://doi.org/10.3390/pathogens10020190
Jäckel C, Hammerl JA, Arslan H-H-T, Göllner C, vom Ort N, Taureck K et al (2020) Phenotypic and genotypic characterization of veterinary Vibrio cincinnatiensis isolates. Microorganisms 8(5):739. https://doi.org/10.3390/microorganisms8050739
Jeamsripong S, Khant W, Chuanchuen R (2020) Distribution of phenotypic and genotypic antimicrobial resistance and virulence genes in Vibrio parahaemolyticus isolated from cultivated oysters and estuarine water. FEMS Microbiol Ecol 96(8). https://doi.org/10.1093/femsec/fiaa081
Jean-Jacques W, Rajashekaraiah KR, Farmer JJ, Hickman FW, Morris JG, Kallick CA (1981) Vibrio metschnikovii bacteremia in a patient with cholecystitis. J Clin Microbiol 14(6):711–712. https://doi.org/10.1128/jcm.14.6.711-712.1981
Johnson CN, Bowers JC, Griffitt KJ, Molina V, Clostio RW, Pei S et al (2012) Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the Coastal and Estuarine Waters of Louisiana, Maryland, Mississippi, and Washington (United States). Appl Environ Microbiol 78(20):7249–7257. https://doi.org/10.1128/AEM.01296-12
Jubirt MM (2012) Potential of great egrets to be vectors for the transmission of virulent strain of aeromonas hydrophila between channel catfish culture ponds. College of Veterinary Medicine. Mississippi State University, Starkville, Mississippi
Kariithi HM, Ferreira HL, Welch CN, Ateya LO, Apopo AA, Zoller R et al (2021) Surveillance and genetic characterization of virulent newcastle disease virus subgenotype V.3 in indigenous chickens from backyard poultry farms and live bird markets in Kenya. Viruses 13(1):103. https://doi.org/10.3390/v13010103
Karunasagar I, Venugopal MN, Karunasagar I, Segar K (1986) Role of chitin in the survival of Vibrio parahaemolyticus at different temperatures. Can J Microbiol 32(11):889–891. https://doi.org/10.1139/m86-162
Kassim N, Mtenga AB, Lee W-G, Kim J-S, Shim W-B, Chung D-H (2011) Production of Coturnix quail immunoglobulins Y (IgYs) against Vibrio parahaemolyticus and Vibrio vulnificus. Food Sci Biotechnol 20(6):1577–1583. https://doi.org/10.1007/s10068-011-0218-z
Kay MK, Cartwright EJ, MacEachern D, McCullough J, Barzily E, Mintz E et al (2012) Vibrio mimicus infection associated with Crayfish Consumption, Spokane, Washington, 2010. J Food Prot 75(4):762–764. https://doi.org/10.4315/0362-028x.Jfp-11-410
Lachish S, Murray KA (2018) The certainty of uncertainty: potential sources of bias and imprecision in disease ecology studies. Front Vet Sci 5(90). https://doi.org/10.3389/fvets.2018.00090
Laviad-Shitrit S, Lev-Ari T, Katzir G, Sharaby Y, Izhaki I, Halpern M (2017) Great cormorants (Phalacrocorax carbo) as potential vectors for the dispersal of Vibrio cholerae. Sci Rep 7(1):7973. https://doi.org/10.1038/s41598-017-08434-8
Laviad-Shitrit S, Izhaki I, Arakawa E, Halpern M (2018) Wild waterfowl as potential vectors of Vibrio cholerae and Aeromonas species. Trop Med Int Health 23(7):758–764. https://doi.org/10.1111/tmi.13069
Lee JV, Donovan TJ, Furniss AL (1978) Characterization, taxonomy, and emended description of Vibrio metschnikovii. Int J Syst Evol Microbiol 28(1):99–111. https://doi.org/10.1099/00207713-28-1-99
Lee JV, Bashford DJ, Donovan TJ, Furniss AL, West PA (1982) The incidence of Vibrio cholerae in water, animals and birds in Kent, England. J Appl Bacteriol 52(2):281–291. https://doi.org/10.1111/j.1365-2672.1982.tb04852.x
Letchumanan V, Chan K-G, Lee L-H (2014) Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques. Front Microbiol 5(705). https://doi.org/10.3389/fmicb.2014.00705
Levy K, Smith SM, Carlton EJ (2018) Climate change impacts on waterborne diseases: moving toward designing interventions. Curr Environ Health Rep 5(2):272–282. https://doi.org/10.1007/s40572-018-0199-7
Li L, Meng H, Gu D, Li Y, Jia M (2019) Molecular mechanisms of Vibrio parahaemolyticus pathogenesis. Microbiol Res 222:43–51. https://doi.org/10.1016/j.micres.2019.03.003
Lipp EK, Huq A, Colwell RR (2002) Effects of global climate on infectious disease: the cholera model. Clin Microbiol Rev 15(4):757–770. https://doi.org/10.1128/CMR.15.4.757-770.2002
López-Pérez M, Jayakumar JM, Grant T-A, Zaragoza-Solas A, Cabello-Yeves PJ, Almagro-Moreno S (2021) Ecological diversification reveals routes of pathogen emergence in endemic Vibrio vulnificus populations. Proc Natl Acad Sci USA. 118(40). https://doi.org/10.1073/pnas.2103470118
Magalhães V, Branco A, Lima RA, Magalhães M (1996) Vibrio metschnikovii among diarrheal patients during cholera epidemic in Recife Brazil. Rev Inst Med Trop Sao Paulo 38:1–3. https://doi.org/10.1590/S0036-46651996000100001
Mehmke U, Gerlach H, Kösters J, Hausmann S (1992) The aerobic bacterial flora of songbird nests. Dtsch Tierarztl Wochenschr 99(12):478–482
Meszaros VA, Miller-Dickson MD, Francis Baffour-Awuah J, Almagro-Moreno S, Ogbunugafor CB (2020) Direct transmission via households informs models of disease and intervention dynamics in cholera. PLoS One 15(3):e0229837. https://doi.org/10.1371/journal.pone.0229837
Metzner M, Köhler-Repp D, Erhard M, Köhler B (2004) New bacterial pathogens of Turkey? Bordetella hinzii, Enterococcus cecorum and others. In: 9th International Symposium on Turkey Diseases 2004. Deutsche Veterinärmedizinische Gesellschaft, Berlin, Germany)
Miyasaka J, Yahiro S, Arahira Y, Tokunaga H, Katsuki K, Hara-Kudo Y (2006) Isolation of Vibrio parahaemolyticus and Vibrio vulnificus from wild aquatic birds in Japan. Epidemiol Infect 134(4):780–785. https://doi.org/10.1017/S0950268805005674
Montánchez I, Kaberdin VR (2020) Vibrio harveyi: a brief survey of general characteristics and recent epidemiological traits associated with climate change. Mar Environ Res 154:104850. https://doi.org/10.1016/j.marenvres.2019.104850
Moreki J, Chiripasi S, Montsho T, Chibua R, Gabanakgosi K (2011) Prevalence of poultry diseases and parasites in Botswana. J Anim Feed Res 1(5):214–217
Morris JG Jr, Acheson D (2003) Cholera and other types of vibriosis: a story of human pandemics and oysters on the half shell. Clin Infect Dis 37(2):272–280. https://doi.org/10.1086/375600
Murray MH, Becker DJ, Hall RJ, Hernandez SM (2016) Wildlife health and supplemental feeding: A review and management recommendations. Biol Conserv 204:163–174. https://doi.org/10.1016/j.biocon.2016.10.034
Mustapha S, Mustapha EM, Nozha C (2013) Vibrio alginolyticus: an emerging pathogen of food borne diseases. Int J Sci Technol 2(4):302–309
Mutreja A, Kim DW, Thomson NR, Connor TR, Lee JH, Kariuki S et al (2011) Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 477(7365):462–465. https://doi.org/10.1038/nature10392
Myatt DC, Davis GH (1989) Isolation of medically significant Vibrio species from riverine sources in south east Queensland. Microbios 60(243):111–123
Negruțiu V, Niculae M, Páll E, Șandru C, Brudașcă F, Vasiu A et al (2017) Microbial diversity influenced by migratory and feeding behavior in birds from the Danube Delta. Lucrari Stiintifice-Universitatea de Stiinte Agricole a Banatului Timisoara, Medicina Veterinara 50(3):93–97
Ogg JE, Ryder RA, Smith HL (1989) Isolation of Vibrio cholerae from aquatic birds in Colorado and Utah. Appl Environ Microbiol 55(1):95–99. https://doi.org/10.1128/aem.55.1.95-99.1989
Oliver JD, Sadowsky M (2015) The biology of Vibrio vulnificus. Microbiol Spectr 3(3):3. https://doi.org/10.1128/microbiolspec.VE-0001-2014
Oliver JD, Pruzzo C, Vezzulli L, Kaper JB (2012) Vibrio species. Food Microbiol 2012:401–439
Ose Y (1967) Studies on the Vibrio parahaemolyticus isolated from excrements: III isolation of Vibrio parahaemolyticus from bird excrement. Annu Proc Gifu Coll Pharm 17:132–136
Páll E, Niculae M, Brudașcă GF, Ravilov RK, Șandru CD, Cerbu C et al (2021) Assessment and antibiotic resistance profiling in Vibrio species isolated from wild birds captured in Danube Delta Biosphere Reserve, Romania. Antibiotics. 10(3):333. https://doi.org/10.3390/antibiotics10030333
Patz JA, Hahn MB (2013) Climate change and human health: a one health approach. In: Mackenzie JS, Jeggo M, Daszak P, Richt JA (eds) One health: the human-animal-environment interfaces in emerging infectious diseases: Food Safety and Security, and International and National Plans for Implementation of One Health Activities. Berlin, Springer, pp 141–171
Piatt JF, Ford RG (1996) How many seabirds were killed by the Exxon Valdez oil spill? American Fisheries Society Symposium, pp 712–719
Pruzzo C, Huq A, Colwell RR, Donelli G (2005) Pathogenic Vibrio species in the marine and estuarine environment. In: Belkin S, Colwell RR (eds) Oceans and health: pathogens in the marine environment. Springer, Boston, MA, pp 217–252
Ramamurthy T, Chowdhury G, Pazhani G, Shinoda S (2014) Vibrio fluvialis: an emerging human pathogen. Front Microbiol 5(91). https://doi.org/10.3389/fmicb.2014.00091
Reuschel M, Pantchev N, Vrhovec MG, Jung A, Gerhauser I, Sannella AR et al (2020) Occurrence and molecular typing of Giardia psittaci in Parakeets in Germany—a case study. Avian Dis 64(2):228–233. https://doi.org/10.1637/0005-2086-64.2.228
Robinson RA, Lawson B, Toms MP, Peck KM, Kirkwood JK, Chantrey J et al (2010) Emerging infectious disease leads to rapid population declines of common British birds. PLoS One 5(8):e12215. https://doi.org/10.1371/journal.pone.0012215
Roche B, Lebarbenchon C, Gauthier-Clerc M, Chang C-M, Thomas F, Renaud F et al (2009) Water-borne transmission drives avian influenza dynamics in wild birds: the case of the 2005–2006 epidemics in the Camargue area. Infect Genet Evol 9(5):800–805. https://doi.org/10.1016/j.meegid.2009.04.009
Rodrick GE (1991) Indigenous pathogens: vibrionaceae. In: Ward DR, Hackney C (eds) Microbiology of marine food products. Springer, Boston, MA, pp 285–300
Rodríguez J, López P, Muñoz J, Rodríguez N (2010) Detección de Vibrio cholerae no toxigénico en aves migratorias y residentes (Charadriiformes) en una laguna costera del nororiente de Venezuela. SABER Revista Multidisciplinaria del Consejo de Investigación de la Universidad de Oriente 22(2):122–126. https://www.redalyc.org/articulo.oa?id=427739444003
Roges E, Souza R, Santos A, Siciliano S, Ott P, Moreno I et al (2010) Distribution of Vibrio sp. in marine mammals, seabirds and turtles beached or accidentaly captured in fishing nets in coastal regions of Brazil. Vibrios Environ
Sack RB (1973) A search for canine carriers of Vibrio. J Infect Dis 127(6):709–712. https://doi.org/10.1093/infdis/127.6.709
Saiful Islam M, Paul A, Talukder M, Roy K, Abdus Sobur M, Ievy S et al (2021) Migratory birds travelling to Bangladesh are potential carriers of multi-drug resistant Enterococcus spp., Salmonella spp., and Vibrio spp. Saudi J Biol Sci 28(10):5963–5970. https://doi.org/10.1016/j.sjbs.2021.06.053
Sakazaki R (1968) Proposal of Vibrio alginolyticus for the biotype 2 of Vibrio parahaemolyticus. Jpn J Med Sci Biol 21(5):359–362
Sakazaki R, Shimada T (1977) Serovars of Vibrio cholerae identified during 1970-1975. Jpn J Med Sci Biol 30(5):279–282
Salles CA, Voros S, Marbell EC, Amenuvour L (1976) Multiplication, survival and excretion of Vibrio cholerae in chicken. Ghana Med J 199-201
Sanyal SC, Singh SJ, Tiwari IC, Sen PC, Marwah SM, Hazarika UR et al (1974) Role of household animals in maintenance of cholera infection in a community. J Infect Dis 130(6):575–579. https://doi.org/10.1093/infdis/130.6.575
Schlater LK, Blackburn BO, Harrington R, Draper DJ, Van Wagner J, Davis BR (1981) A non-O1 Vibrio Cholerae isolated from a goose. Avian Dis 25(1):199–201. https://doi.org/10.2307/1589842
Sekyere JO, Reta MA, Summers ZM (2020) Genomic and resistance epidemiology of gram-negative bacteria in Africa: a systematic review and phylogenomic analyses from a one health perspective. mSystems 5(6):e00897–e00820. https://doi.org/10.1128/mSystems.00897-20
Semenza JC (2020) Cascading risks of waterborne diseases from climate change. Nat Immunol 21(5):484–487. https://doi.org/10.1038/s41590-020-0631-7
Shimada T, Sakazaki R (1983) Serological studies on Vibrio fluvialis. Jpn J Med Sci Biol 36(6):315–323. https://doi.org/10.7883/yoken1952.36.315
Shnawa IM, Alzamily KY, Omran R (2014) Separation, partial purification, and mitogenicity of enteric Vibrio fluvialis and Aeromonas hydrophila lipopolysaccharides in chicken and rat. World J Pharm Res 3(6):124–136
Siembieda JL, Miller WA, Byrne BA, Ziccardi MH, Anderson N, Chouicha N et al (2011) Zoonotic pathogens isolated from wild animals and environmental samples at two California wildlife hospitals. J Am Vet Med Assoc 238(6):773–783. https://doi.org/10.2460/javma.238.6.773
Singh SJ, Sanyal SC, Sen PC, Tewari IC, Marwah SM, Singh H et al (1975) Studies on bacteriology of cholera infection in Varanasi. Indian J Med Res 63(8):1089–1097
Skerman VBD, McGowan V, Sneath PHA (1980) Approved lists of bacterial names. Int J Syst Evol Microbiol 30(1):225–420. https://doi.org/10.1099/00207713-30-1-225
Sleeman JM, Brand CJ, Wright SD (2012) Strategies for wildlife disease surveillance. In: Aguirre AA, Daszak P (eds) New directions in conservation medicine: applied cases in ecological health. Oxford University Press, New York
Smith SK, Sutton DC, Fuerst JA, Reichelt JL (1991) Evaluation of the Genus Listonella and reassignment of Listonella damsela (Love et al.) MacDonell and Colwell to the genus photobacterium as photobacterium damsela comb. nov. with an emended description. Int J Syst Evol Microbiol 41(4):529–534. https://doi.org/10.1099/00207713-41-4-529
Song H, Baoxi M, Luqiu C (1998) Distribution of serum type of non-O1 Vibrio cholerae separated from migratory water birds in Xinjiang in 1994. J Shenyang Med Coll 1998:01
Stallknecht DE (2007) Impediments to wildlife disease surveillance, research, and diagnostics. In: Childs JE, Mackenzie JS, Richt JA (eds) Wildlife and emerging zoonotic diseases: the biology, circumstances and consequences of cross-species transmission. Springer, Berlin, pp 445–461
Strauch E, Jäckel C, Hammerl JA, Hennig V, Roschanski N, Dammann I et al (2020) Draft genome sequences of Vibrio cholerae non-O1, non-O139 isolates from common tern chicks (Sterna hirundo) following a mass mortality event. Microbiol Resour Announc 9(46):e01053–e01020. https://doi.org/10.1128/MRA.01053-20
Sweet M, Burian A, Bulling M (2021) Corals as canaries in the coalmine: towards the incorporation of marine ecosystems into the ‘One Health’ concept. J Invertebr Pathol 186:107538. https://doi.org/10.1016/j.jip.2021.107538
Szeness L, Sey L, Szeness A (1979) Bacteriological studies of the intestinal content of aquatic birds, fishes, and frogs with special reference to the presence of non-cholera vibrios (NCV) (author’s transl). Zentralbl Bakteriol Orig A 245(1–2):89–95
Tantillo GM, Fontanarosa M, Di Pinto A, Musti M (2004) Updated perspectives on emerging vibrios associated with human infections. Lett Appl Microbiol 39(2):117–126. https://doi.org/10.1111/j.1472-765X.2004.01568.x
Thompson FL, Hoste B, Vandemeulebroecke K, Swings J (2003) Reclassification of Vibrio hollisae as Grimontia hollisae gen. nov., comb. nov. Int J Syst Evol Microbiol 53(5):1615–1617. https://doi.org/10.1099/ijs.0.02660-0
Vezzulli L, Pruzzo C, Huq A, Colwell RR (2010) Environmental reservoirs of Vibrio cholerae and their role in cholera. Environ Microbiol Rep 2(1):27–33. https://doi.org/10.1111/j.1758-2229.2009.00128.x
Vezzulli L, Baker-Austin C, Kirschner A, Pruzzo C, Martinez-Urtaza J (2020) Global emergence of environmental non-O1/O139 Vibrio cholerae infections linked with climate change: a neglected research field? Environ Microbiol 22(10):4342–4355. https://doi.org/10.1111/1462-2920.15040
Waldenström J, Broman T, Carlsson I, Hasselquist D, Achterberg RP, Wagenaar JA et al (2002) Prevalence of Campylobacter jejuni, Campylobacter lari, and Campylobacter coli in different ecological guilds and taxa of migrating birds. Appl Environ Microbiol 68(12):5911–5917. https://doi.org/10.1128/AEM.68.12.5911-5917.2002
Wang Q, Zhang Y, Yang Q, Fu S, Qu B, Defoirdt T (2021) One health pathogen surveillance demonstrated the dissemination of gut pathogens within the two coastal regions associated with intensive farming. Gut Pathogens 13(1):47. https://doi.org/10.1186/s13099-021-00442-4
Ward MR, Stallknecht DE, Willis J, Conroy MJ, Davidson WR (2006) Wild bird mortality and West Nile virus surveillance: biases associated with detection, reporting, and carcass persistence. J Wildl Dis 42(1):92–106. https://doi.org/10.7589/0090-3558-42.1.92
Watanabe M, Okatani AT, Yoshimitsu S, Iwata T, Horisaka T, Horikita T et al (2002) Status of possession of Vibrio spp. in waterfowl flying to Japan. Vet Epidemiol Mag 6(2):77–83. https://doi.org/10.2743/jve.6.77
Watsa M, Wildlife Disease Surveillance Focus Group (2020) Rigorous wildlife disease surveillance. Science 369(6500):145–147. https://doi.org/10.1126/science.abc0017
Watts JL, Salmon SA, Yancey RJ Jr, Nersessian B, Kounev ZV (1993) Minimum inhibitory concentrations of bacteria isolated from septicemia and airsacculitis in ducks. J Vet Diagn Investig 5(4):625–628. https://doi.org/10.1177/104063879300500423
West PA, Lee JV, Bryant TN (1983) A numerical taxonomic study of species of Vibrio isolated from the aquatic environment and birds in Kent, England. J Appl Bacteriol 55(2):263–282. https://doi.org/10.1111/j.1365-2672.1983.tb01324.x
White FH, Simpson CF, Williams LE Jr (1973) Isolation of Edwardsiella tarda from aquatic animal species and surface waters in Florida. J Wildl Dis 9(3):204–208. https://doi.org/10.7589/0090-3558-9.3.204
Wiethoelter AK, Beltrán-Alcrudo D, Kock R, Mor SM (2015) Global trends in infectious diseases at the wildlife–livestock interface. Proc Natl Acad Sci 112(31):9662–9667. https://doi.org/10.1073/pnas.1422741112
Wittman RJ, Flick GJ (1995) Microbial contamination of shellfish: prevalence, risk to human health, and control strategies. Annu Rev Public Health 16(1):123–140. https://doi.org/10.1146/annurev.pu.16.050195.001011
Wobeser GA (2007) Disease in wild animals. Springer
Wobeser G, Kost W (1992) Starvation, Staphylococcosis, and Vitamin A deficiency among Mallards overwintering in Saskatchewan. J Wildl Dis 28(2):215–222. https://doi.org/10.7589/0090-3558-28.2.215
Wobeser G, Rainnie DJ (1987) Epizootic necrotic enteritis in wild geese. J Wildl Dis 23(3):376–385. https://doi.org/10.7589/0090-3558-23.3.376
Work TM, Rameyer RA (1999) Mass stranding of wedge-tailed shearwater chicks in Hawaii. J Wildl Dis 35(3):487–495. https://doi.org/10.7589/0090-3558-35.3.487
Zhang X, Ma B, Chen L, Sun Y, Zhang Z, Wang L et al (1996) Vibrio cholerae separating from water birds infecting Anas poecilorhyncha and Anas platyrhynchos. Zhongguo Gonggong Weisheng Xuebao 15(3):149–150
Zhao H, Sun R, Yu P, Alvarez PJJ (2020) High levels of antibiotic resistance genes and opportunistic pathogenic bacteria indicators in urban wild bird feces. Environ Pollut 266(Pt 2):115200. https://doi.org/10.1016/j.envpol.2020.115200
Zheng L, Zhu L-W, Guan J-y, Wang Y, Jing J, Liang B et al (2020) Bacterial community research of migratory bird in Chifeng, Neimeng via high-throughput sequencing. Res Sq. https://doi.org/10.21203/rs.3.rs-30522/v1
Zheng L, Zhu L-W, Jing J, Guan J-y, Lu G-J, Xie L-H et al (2021) Pan-genome analysis of Vibrio cholerae and Vibrio metschnikovii strains isolated from migratory birds at Dali Nouer Lake in Chifeng, China. Front Vet Sci 8(557):638820. https://doi.org/10.3389/fvets.2021.638820
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ayala, A.J., Ogbunugafor, C.B. (2023). When Vibrios Take Flight: A Meta-Analysis of Pathogenic Vibrio Species in Wild and Domestic Birds. In: Almagro-Moreno, S., Pukatzki, S. (eds) Vibrio spp. Infections. Advances in Experimental Medicine and Biology, vol 1404. Springer, Cham. https://doi.org/10.1007/978-3-031-22997-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-22997-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22996-1
Online ISBN: 978-3-031-22997-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)