Abstract
In the process of hot metal pretreatment desulfurization, it is necessary to add desulfurizer to promote the desulfurization reaction. At the same time, as the key to measure the sulfur content in molten iron, the study of sulfur distribution ratio is of great significance to achieve the goal of desulfurization. Based on the theoretical sulfur ratio of desulfurization molecules and the theoretical sulfur ratio of ions, this paper analyzes the factors affecting the sulfur content in molten iron, and concludes that the suitable desulfurization conditions are high temperature, low oxygen level and high oxygen anion concentration. Thermodynamic analysis and related introduction of common desulfurization are carried out, and it is pointed out that CaO–Mg composite desulfurizer has the highest removal efficiency and the lowest consumption, which is conducive to desulfurization.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
In the desulfurization process of hot metal pretreatment, a certain amount of desulfurizer is often added to promote the desulfurization reaction. The desulfurization reaction is the interface reaction between desulfurizer and hot metal. When the two phases are in contact, the desulfurization reaction occurs [1–5]. In the process of using desulfurizer, it is necessary to overcome the surface tension, resistance and buoyancy of molten iron. In order to meet the requirements of steel-making for sulfur content, desulfurizer suitable for steel smelting and desulfurization objectives should be selected. This can only improve the desulfurization efficiency, and the use of cost-effective desulfurizer can also reduce the consumption of desulfurization powder and save production costs. There are many kinds of desulfurizers, four of which are widely used, namely CaC2, CaO Na2CO3 and Mg.
Thermodynamic Analysis of Desulfurization
Sulfur partition ratio is one of the key factors to determine the sulfur content in molten iron. The molecular reaction process of desulfurization is as follows [6]:
The equilibrium constant of the reaction is:
The sulfur distribution ratio is:
According to the molecular reaction process of desulfurization, the low FeO reaction proceeds to the right. At the same time, increasing the alkalinity in the slag and reducing the oxygen potential in the slag are conducive to the desulfurization reaction, resulting in the increase of sulfur distribution ratio ls.
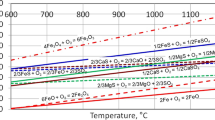
According to the ionic structure theory of molten slag, the desulfurization reaction between desulfurized slag and molten iron is carried out by diffusion on the slag iron interface. O2− in the desulfurized slag combines with s in molten iron to form S2− and enters the slag to maintain balance with cations such as Ca+, Mg+ in molten iron [7]. The schematic diagram and process of desulfurization ion reaction are as follows (Fig. 1).
The reaction process is:
Its equilibrium constant is:
The sulfur distribution ratio is:
KS, γS are the equilibrium constant and sulfur partition coefficient of the reaction in turn;
w(S), w[S], w[O] are sulfur content in slag, sulfur content in molten iron, and oxygen content in molten iron;
acao, a[FeO] are the activity of CaO in slag and the activity of ferrous oxide in molten iron respectively;
\(a_{{O^{2 - } }} ,a_{{S^{2 - } }}\) are the activity of O2− and S2− in the slag in turn;
fS is the activity coefficient of sulfur in molten iron;
LS is the ratio of sulfur content in slag to that in molten iron, i.e. sulfur distribution ratio.
According to the viewpoint of chemical equilibrium, when sulfur in slag is quantitative, its oxygen potential is inversely proportional to the concentration of oxygen anion, that is, the lower the oxygen potential, the higher the concentration of oxygen anion in slag. Therefore, the lower activity of sulfur ions in the slag, so as to promote the chemical reaction, resulting in the easier s in molten iron to enter the slag, and the increase of S. Because FeO appears in the numerator denominator, FeO decreases and the numerator denominator decreases, which has a greater impact on the denominator, so the desulfurization effect can be improved. At the same time, the ion reaction is an endothermic reaction. The increase of temperature and the addition of a certain amount of FeO are conducive to the dissolution of CaO, improve the fluidity of slag, and promote the desulfurization reaction. Therefore, high temperature, low oxygen level, high oxygen anion concentration and low FeO content are required in hot metal pretreatment desulfurization.
Desulfurization Mechanism and Development of Desulfurizer
CaO
CaO is a desulfurizer commonly used in hot metal pretreatment. It is rich in resources, cheap, safe and pollution-free [8]. When using CaO for desulfurization, in order to ensure the desulfurization efficiency, it needs to be ground into powder, but the ground lime powder is prone to moisture deterioration, so it needs moisture-proof storage. Its mechanism is to use CaO and sulfur in molten iron to form CaS. The relevant chemical reaction formula is:
Formula 10 shows that the increase of temperature leads to the decrease of \(\Delta G^{{\uptheta }}\), and high temperature is conducive to lime desulfurization. The oxygen content in molten iron plays a vital role in the desulfurization reaction. Low oxygen concentration makes the desulfurization reaction more thorough.
When molten iron contains Al, the reaction formula of Al participating in desulfurization reaction is:
There is free oxygen in the product after the Cao desulfurization reaction, which reacts with Si in molten iron to produce CaO·SiO2 with higher melting point, forming a dense film on the surface of CaO to prevent the desulfurization reaction from continuing. The relevant chemical reaction formula is:
At 1350 °C, the equilibrium constant of molten iron treated with CaO is 6.489, and the end-point sulfur content can reach 0.00037%. Moreover, the desulfurization efficiency of CaO is lower than that of Mg, and the melting point of CaO is high. When it is used as desulfurizer in molten iron, it is solid. Flux CaF2 is often added to reduce the melting point and become liquid. After adding flux, the sulfur content in molten iron will decrease significantly. Because CaO is easily hygroscopic and converted into Ca(OH)2, Ca(OH)2 will cause hot metal splashing during hot metal pretreatment and desulfurization, resulting in the reduction of hot metal temperature.
Na2CO3
As the first desulfurizer used outside the furnace, Na2CO3 has strong desulfurization ability and low melting point. At 1350 °C, soda is used in high-carbon molten iron for desulfurization outside the furnace, and the equilibrium constant of desulfurization reaction is 5 × 104, the sulfur content in molten iron is 4.7 × 10–7, the desulfurization capacity is stronger than CaO. Only a part of sodium vapor participates in the desulfurization reaction, and most of the remaining sodium vapor will be oxidized in the air, releasing a large amount of smoke, causing serious environmental pollution, which is inconsistent with the current carbon neutral carbon reduction emissions, and is rarely used alone now. The chemical equation is:
Due to high temperature, Na2CO3 is rapidly decomposed into Na2O and CO2 after entering the molten iron, and then Na2O will continue to react with carbon and silicon in the molten iron to generate CO and Na2SiO3 respectively.
The sodium oxide generated by the decomposition of soda at high temperature is liquid, and its content in the slag is high. The fluidity of the slag is good, it is difficult to scrape the slag mechanically, and it erodes the refractory materials in the hot metal tank seriously. Above 1250 °C, Na2S will be oxidized by air, and the generated Na2O may continue to be reduced to gas sodium. Sodium vapor and carbon monoxide burn in the air, producing a large amount of smoke, causing great damage to the environment, which is inconsistent with the current carbon neutralization, so its use should be reduced.
Mg
The melting point of magnesium is low, only 650 °C, and the boiling point is 1107 °C. The desulfurization reaction is mainly the homogeneous reaction of molten iron. For low-temperature molten iron, magnesium is one of the strongest desulfurizers. After reacting with molten iron, the amount of desulfurization slag is small and the iron loss is small [9]. Most of magnesium exists as magnesium vapor in molten iron, and the other part of magnesium dissolves in molten iron and reacts with sulfur. The final products of both are magnesium sulfide.
At 1350 °C and PMg = 0.1 MPa, apply \(\Delta G^{{\uptheta }} = - RT\ln K\) to calculate the sulfur activity when the reaction reaches equilibrium as:
According to the calculation of sulfur activity, it is very low, indicating that magnesium has strong desulfurization ability, and the end-point sulfur content after desulfurization with magnesium is 1.6 × 10–5, its desulfurization capacity is far greater than CaO. Magnesium metal has high activity and is easily oxidized. It is flammable and explosive. Magnesium particles can be safely transported and used only after surface passivation treatment. After passivation treatment, a protective film is formed on the surface of magnesium particles, which limits the activity of magnesium and enables magnesium to smoothly participate in desulfurization reaction in molten iron. At the same time, the price of magnesium is expensive, and the desulfurized slag after desulfurization is thin, which brings the problem of slag removal and causes the pressure of sulfur recovery to the converter. Therefore, when using magnesium desulfurizer, some non-metallic magnesium mixed desulfurizer is often added. Table 1 shows the desulfurization effect ratio of composite magnesium desulfurizer [10, 11].
It can be seen from Table 1 that the desulfurization effect of using metal magnesium desulfurizer alone is not the best, and the desulfurization rate of No. 4 and No. 5 metal magnesium mixed desulfurization slag is the highest. However, No. 4 contains CaF2, which seriously erodes the ladle lining, so the use of CaO–Mg composite desulfurizer is a more reasonable desulfurizer.
CaC2
The main component of calcium carbide powder is CaC2. Industrial CaC2 is actually used (containing about 80% of CaC2, 16% of CaO, and the rest is carbon), and the price is relatively expensive. Its desulfurization rate can reach 90%, and the reaction speed is fast. The reaction formula is:
The equilibrium constant of calcium carbide desulfurization reaction is usually 6.90 × 105. When the reaction reaches equilibrium, the sulfur content in molten iron can reach 4.9 × 10–7. The desulfurization reaction with calcium carbide is exothermic, which is conducive to reducing the temperature loss of molten iron. The melting point of desulfurization product CaS is 2450 °C, so after desulfurization, loose solid slag will be formed on the surface of molten iron to reduce the sulfur recovery of molten iron. And the corrosion to the lining of molten iron tank is light, which is convenient for slag removal. However, it is very easy to deliquesce and deteriorate, and the following reactions are produced rapidly when it contacts with water in the atmosphere:
Acetylene (C2H2) gas produced by this reaction is very explosive, so it needs to be sealed during transportation and storage to prevent the occurrence of the above reaction. In addition, when calcium carbide is mixed with other desulfurizers, it will also absorb the above water and react, so calcium carbide should be mixed before injection.
Conclusions
-
(1)
Through thermodynamic analysis of desulfurization, it is found that hot metal pretreatment desulfurization requires high temperature, low oxygen level, high oxygen anion concentration and low FeO content.
-
(2)
By analyzing and comparing the desulfurization efficiency of the three desulfurizers, it is concluded that mg has the highest desulfurization efficiency, followed by CaC2, and CaO has the lowest efficiency. At the same time, it is pointed out that CaO–Mg composite desulfurizer is an ideal desulfurizer.
References
Vuolio T, Visuri V, Sorsa A, et al. (2019) Genetic algorithm‐based variable selection in prediction of hot metal desulfurization kinetics. Steel Res Int 90(8)
Fei X (2013) Metallurgical physicochemical study on magnesite based desulfurizer. Liaoning University of science and technology
Dong C (2021) Analysis and reduction measures of steel material consumption in converter. Metall Manag (11):1–2
Wei W, Li H (2019) Production practice of ultra-low sulphur pipeline steel L245NCS without pretreatment of hot metal
Yunzong G, Benliang Z, Hui W (2015) Research on hot metal desulfurization technology by Kr method. Wide Thick Plate (03):36–40
Jialong Q (2016) CaO-Al2O3-SiO2-MgO-TiO2-Na2O Study on Desulfurization Kinetics of Six Element Slag System and Thermodynamic Properties of CaO in Slag. Jiangxi University of science and technology
Zhiming Y (2019) Basic research on the Structure and Properties of Aluminosilicate Based Blast Furnace Slag. Chongqing University
Xiulan P, Yanhong W, Huizhi L, et al. (2010) Development status and prospect of hot metal pretreatment technology. World Steel (06):29–36
Hanjie G (2007) Dynamics of hot metal desulfurization process with magnesium particles. Steel 42(005): 7–41
Xianhui W, Siming Z, Xiaofen M (2013) Analysis of factors affecting desulfurization efficiency of Cao based composite desulfurizer. Steelmaking 029(002):30–33
Wu W, Han Z, Hu Y (2008) Desulfurizer desulphurization kinetics by the injection method. J Beijing Univ Sci Technol (Engl Ed)
Acknowledgements
National Natural Science Foundation of China (No. 51274084), Hebei Natural Science Foundation (E2018209323), and Project of North China University of Science and Technology GP201507. National Natural Science Foundation of China (No. 51274084), Hebei Natural Science Foundation (E2018209323), and Project of North China University of Science and Technology GP201507.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Tian, L., Jiang, W., Hao, S., Zhang, Y. (2023). Effect of Different Desulfurizers on Hot Metal Pretreatment. In: TMS 2023 152nd Annual Meeting & Exhibition Supplemental Proceedings. TMS 2023. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-031-22524-6_75
Download citation
DOI: https://doi.org/10.1007/978-3-031-22524-6_75
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22523-9
Online ISBN: 978-3-031-22524-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)