Abstract
There have been a number of studies about the effects and influence of the menstrual cycle on aerobic and anaerobic performance, but most studies that investigated muscle strength responses to resistance exercise over the menstrual cycle have not found any changes. However, since there are many factors that can influence exercise performance, the effect of the menstrual cycle on muscle strength is probably very individual specific, and hence, the effect of menstrual cycle status on the response to acute resistance exercise is not clear. To this end, some studies have demonstrated that the responses of anabolic hormones to acute resistance exercise in women vary with menstrual cycle status. Women with menstrual disorders associated with low serum estradiol and progesterone levels have an attenuated anabolic hormone response to acute resistance exercise, suggesting that menstrual disorders characterized by low ovarian hormone levels may affect exercise-induced changes in anabolic hormones in women. It has also been suggested that estrogen may be protective against exercise-induced muscle damage, and that recovery from exercise-induced muscle damage may differ between the menstrual cycle phases. These differences might critically affect long-term adaptations to resistance training; however, to date there is little data available to examine this point. Further studies are needed to demonstrate the short-term and long-term effects of changes in skeletal muscle response to resistance exercise by menstrual cycle status in women.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
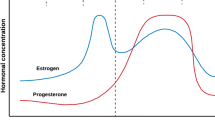
During a normal menstrual cycle, women are exposed to a continuously changing profile of female sex steroid hormones. Estrogen starts to increase halfway through the follicular phase and peaks just prior to ovulation. Estrogen and progesterone are both elevated during the middle of the luteal phase (Table 10.1) and have profound physiological effects (see earlier chapters in this book). To better understand the relationship between the menstrual cycle and resistance exercise in women, it is important to consider the hormonal fluctuations that occur throughout the menstrual cycle. This chapter attempts to address these points.
Estrogen receptors have been localized to skeletal muscle tissue as well as tendons and ligaments. Through these receptors, estrogen is thought to influence the turnover of skeletal muscle and connective tissue proteins at rest in the post-absorptive phase and enhance sensitivity to anabolic stimuli (Hansen and Kjaer 2014). In women, estradiol (the principal estrogen, see Chap. 1) functions as an antioxidant and membrane stabilizer during exercise, particularly exercise that induces high levels of oxidative stress, such as intense aerobic and resistance exercise. The protective role of estradiol appears to be a primary factor in mitigating muscle damage due to exercise stress and is evidenced by the smaller inflammatory response found in women (Fleck and Kraemer 2014; Enns and Tiidus 2010).
Interesting, women experience a rapid decline in muscle mass and strength around menopause when estrogen levels decline dramatically. These changes may at least partly be related to the hormonal changes during aging. The striking decline in muscle strength during perimenopause and after menopause can be reversed with hormone replacement therapy (Jabbour et al. 2006).
In contrast, there is a paucity of data on the effects of progesterone on skeletal muscle, although progesterone is purported to be catabolic (Oosthuyse and Bosch 2010). Another difficulty in interpreting menstrual cycle research stems from the interaction between estrogen and progesterone. During the early follicular phase, estrogen and progesterone levels are both low, but in the late-follicular phase there are high estrogen concentrations and low progesterone concentrations. In the mid- luteal phase, levels of both estrogen and progesterone are high. To compare the increase in estrogen concentration relative to progesterone concentration, some studies have reported the estrogen/progesterone ratio in the luteal phase. This ratio may provide information about the opposing effects of estrogen and progesterone (Janse de Jonge 2003).
To understand the relationship between hormonal fluctuations during the menstrual cycle and exercise in women, researchers, athletes, and coaches should take physical and mental symptoms related to menstruation into account, not only the effects of sex hormones on skeletal muscles. Furthermore, dysmenorrhea (painful menstruation) and premenstrual syndrome (PMS) which can involve mood swings, tender breasts, food cravings, fatigue, irritability, and depression, are commonly seen in eumenorrheic women. These symptoms in turn may affect performance and physical and mental conditions for training and performance during the menstrual cycle phases.
Muscle Strength During the Menstrual Cycle
Several review articles on muscle strength during the menstrual cycle (Constantini et al. 2005; Janse de Jonge 2003; Lebrun 1994) have reported that some studies found greater strength in the follicular phase or in the ovulatory phase than in the luteal phase, whereas other studies have reported that strength was greater in the mid- luteal phase. A majority of studies could not find any changes in muscle strength over the menstrual cycle. In particular, recent studies measuring estrogen and progesterone concentration in order to verify menstrual cycle phase have reported no changes over the menstrual cycle in isokinetic peak torque of knee extensors and flexors and maximum isometric strength of knee extensors (Bambaeichi et al. 2004), maximum voluntary isometric force of the first dorsal interosseous muscle (Elliott et al. 2003), or handgrip strength and isokinetic muscle strength of knee extensors (peak torque), muscle endurance, and one leg hop test (Fridén et al. 2003). Based on these results, although from a very limited number of studies, it can be concluded there is little or no difference in muscle strength at various times during the menstrual cycle. However, since there are many factors that can potentially influence exercise performance such as self-expectations, negative attitudes toward menstruation, and weight gain, the effect of the menstrual cycle on performance is probably very individual specific and much more research is needed.
Anabolic Hormones and Resistance Exercise in Women
Resistance exercise provides a potent stimulus for muscular adaptation. This process is mediated, at least in part, by acute and chronic hormonal responses to resistance training, including changes in testosterone, growth hormone (GH),dehydroepiandrosterone sulfate (DHEA-S), andinsulin-like growth factor I (IGF-I) (Consitt et al. 2002; Fleck and Kraemer 2014; Kahn et al. 2002; Kraemer and Ratamess 2005). This is true for both sexes. However, the resistance exercise-induced anabolic hormone changes are considerably different in men and women.
Testosterone, a major androgenic-anabolic hormone, exerts a significant influence on anabolic functions in the human body, especially in males. Blood serum testosterone concentration is acutely elevated immediately following heavy resistance exercise in men, although several factors such as muscle mass, exercise intensity and volume, nutrition intake, and training experience play a role in this response (Fleck and Kraemer 2014). At rest, women have a 10- to 40-fold lower blood concentrations of testosterone than men (Kraemer et al. 1991; Vingren et al. 2010). Previous studies have reported that concentrations of testosterone do not change acutely after resistance exercise in women (Kraemer et al. 1991, 1993; Staron et al. 1994; Häkkinen and Pakarinen 1995) (Fig. 10.1), but some other data challenges this construct in relation to other exercise forms (Lane et al. 2015).
Serum testosterone concentrations in men and women after the same resistance training session consisting of three sets of eight exercises at 10RM (ten repetition maximum) with 1 min of rest between sets and exercises (Kraemer et al. 1991). * Significantly different from pre-exercise value in the same sex; + significantly different from females at the same time point
Growth hormone (GH) appears to be involved in the growth of skeletal muscle and many other tissues in the body. It also appears to play a vital role in the body’s adaptation to the stimulus of resistance training (Fleck and Kraemer 2014). The GH response to resistance training is quite similar between the sexes. A high-intensity and high-volume resistance exercise program with short rest periods has been shown to induce a post-exercise increase in GH in both men and women (Kraemer et al. 1991; Häkkinen and Pakarinen 1995) (Fig. 10.2).
Serum growth hormone concentrations in men and women after the same resistance training session consisting of three sets of eight exercises at 10RM with 1 min of rest between sets and exercises (Kraemer et al. 1991). * Significantly different from pre-exercise value in the same sex; + significantly different from females at the same time point
Insulin-like growth factor-I (IGF- I) is a salient biomarker for monitoring health, fitness, and training status. It also reflects nutritional status as well (Fleck and Kraemer 2014). The acute response of IGF-I to resistance exercise remains unclear. Most studies have shown no change in IGF-I during or immediately following an acute bout of resistance exercise (Kraemer et al. 1993; Consitt et al. 2001), whereas a few studies have shown acute elevations during and following resistance exercise (Kraemer et al. 1991; Kraemer and Ratamess 2005). However, long-term studies in women have shown elevations in resting IGF-I, particularly during high-volume training (Marx et al. 2001; Koziris et al. 1999). Interestingly, researchers showed that GH and IGF-I appear to compensate for the attenuated testosterone response to signal muscle tissue growth in women and therefore may play a more central role in muscle hypertrophy than they do in men (Fleck and Kraemer 2014).
In women, about 90 % of circulating testosterone is derived from the metabolism of peripheral precursors, in particular from DHEA- S (Baulieu 1996; Labrie et al. 1997). DHEA-S is actually the predominant adrenal steroid hormone in both sexes. Regrettably, there is little information available on acute responses of DHEA-S to resistance exercise in women (Enea et al. 2011). Riechman et al. (2004) reported that acute resistance exercise increases blood DHEA-S levels in both men and women. Aizawa et al. (2003) have reported a dramatic increase in resting serum DHEA-S concentrations after 8 weeks of resistance training. Furthermore, Aizawa et al. (2006) demonstrated that serum DHEA-S levels are positively correlated with leg extensor power in female athletes, but not in male athletes (Fig. 10.3). These findings suggest that blood DHEA-S levels in female athletes may reflect training-induced adaptation and play an important role in muscular strength development. DHEA- S may also play a major biological role in women through its transformation into active androgens and estrogens (McMurray and Hackney 2000).
Serum DHEA-S levels were positively correlated with leg extensor power (peak torque/body weight) in female athletes but not in males athletes (Aizawa et al. 2006)
Thus, it is clear there are sex (gender) differences in basal anabolic hormone levels and responses to exercise. Nevertheless, women and men display similar relative changes in hypertrophy with resistance exercise. Although not discussed here, other hormones or mechanisms may also be responsive to resistance training and thus affect long-term adaptations to resistance training in women (see McMurray and Hackney 2000).
Hormonal Responses to Resistance Exercise During Different Menstrual Cycle Phases
It has been suggested that many factors (e.g., sex, age, fitness level, nutritional status, exercise variables) influence hormonal responses to resistance exercise (Consitt et al. 2002; Kraemer and Ratamess 2005). When interpreting a woman’s hormonal response to training, the potential effects of the menstrual cycle must be considered, because the hormonal responses to exercise are modified by the ovarian systems in women. Logically then understanding the potential effects of the menstrual cycle is vital for female athletes and their coaches.
Previous studies have reported that GH concentrations are higher in the peri-ovulatory phase than in the early-follicular phase (Faria et al. 1992; Ovesen et al. 1998). Kraemer et al. (1995) demonstrated that low-volume resistance exercise induces larger increases in estradiol, GH, and androstenedione in the mid- luteal phase than in the early- follicular phase, although they did not compare the responses within the same individuals. Nakamura et al. (2011) have investigated the effect of the menstrual cycle on ovarian and anabolic hormonal responses to acute resistance exercise in young women. Specifically in this work, eight eumenorrheic women and eight women with menstrual disorders including oligomenorrhea and amenorrhea were enrolled in the study. All subjects were recreationally active young women (18–30 years of age) and were not using oral contraceptives. The eumenorrheic women participated in two series of exercise sessions, one in the early-follicular phase (days 4–7 of the menstrual cycle) and one in the mid-luteal phase (7–10 days after ovulation). The women with menstrual disorders participated in a series of exercise session on an arbitrary day. All subjects performed three sets each of resistance exercises (i.e., lat pull-downs, leg curls, bench presses, leg extensions, and squats) at 75–80 % of the one-repetition maximum with 1 min of rest between sets. Blood samples were obtained before exercise and immediately, 30 and 60 min after exercise. The effects of the menstrual cycle phase in eumenorrheic women are described in this section of the chapter, and the effects of menstrual status in women with menstrual disorders are described in the next section.
In the mid- luteal phase, serum estradiol and progesterone increased after exercise, but they did not change after exercise in the early- follicular phase (Fig. 10.4). Serum GH increased after exercise in both the early-follicular and mid-luteal phases. On the other hand, total secretion (area under the curve) of GH was increased significantly after exercise in the mid- luteal phase, but not in the early- follicular phase (Fig.10.5). Hornum et al. (1997) also demonstrated that total secretion of GH after a high-intensity cycling exercise was greater in the peri-ovulatory phase (estradiol levels were high) than in the follicular phase (estradiol levels were low). These findings indicate that menstrual cycle variations in circulating estradiol levels may affect exercise-induced GH secretion. Testosterone and IGF- I concentrations showed no significant increase in response to the resistance exercise protocols during either menstrual cycle phases (Fig. 10.5). These findings are consistent with previous studies (Consitt et al. 2001; Copeland et al. 2002; Häkkinen and Pakarinen 1995; Häkkinen et al. 2000; Kraemer et al. 1993). DHEA-S concentrations showed no significant increase immediately after resistance exercise in either menstrual cycle phases; however, there was a significant increase in DHEA- S 60 min after exercise in the early-follicular phase. Cortisol, a catabolic hormone, also did not increase after resistance exercise in either menstrual cycle phases (Fig. 10.5). Likewise, Häkkinen and Pakarinen (1995) reported no changes in cortisol levels after acute resistance exercise in women. Kraemer et al. (1998), however, found significant elevations in cortisol after acute resistance exercise in men and women. It is possible that these differences in the response of cortisol to exercise are affected by the training status of the subjects (Kraemer et al. 1998), daily hormonal fluctuation, or both factors interacting (Hackney and Viru 2008).
Concentrations of ovarian hormones (estradiol and progesterone) before (Pre) and after resistance exercise: immediately after the end of the resistance exercise: (P0), 30 min after exercise. (P30) and 60 min after exercise (P60). In the mid- luteal phase, these hormones increased after exercise, but they did not change in the early-follicular phase and in women with menstrual disorders. Early-follicular phase:diamond, mid-luteal phase:filled square, women with menstrual disturbance:triangle. Data are expressed as means ± SEM. *P < 0.05, **P < 0.01 versus Pre.aP < 0.001,bP < 0.01 between Pre in the early-follicular phase and Pre in the mid-luteal phase. The hatched box represents resistance exercise (Nakamura et al. 2011)
Concentrations of anabolic hormones (GH, IGF-I, testosterone, and DHEA-S) and cortisol before (Pre) and after resistance exercise (immediately after the end of the resistance exercise (P0), 30 min after exercise (P30), and 60 min after exercise (P60). Early-follicular (EF): diamond, mid-luteal phase (ML): filled square, women with menstrual disturbance (OAM): triangle. Data in the small upper panels represent the area under the curve (AUC). Data are expressed as means ± SEM. *P < 0.05, **P < 0.01 versus Pre.cP < 0.01 between Pre in the early-follicular (EF) phase and Pre in women with menstrual disorders (MD). The hatched box represents resistance exercise.#P < 0.05 compared with zero (Nakamura et al. 2011)
The findings from these studies suggest that anabolic hormone responses to resistance exercise (e.g., levels of ovarian hormones, GH) may be influenced by menstrual cycle phase and the hormonal changes associated with the phases.
Hormonal Responses to Resistance Exercise with Different Menstrual Cycle Status
Menstrual disorders such as oligomenorrhea and amenorrhea are functional disorders characterized by altered gonadotropin-releasing hormone pulsatility, loss of pulsatile gonadotropin secretion (FSH and LH), and, in turn, altered ovarian steroidogenesis (Meczekalski et al. 2000). These reproductive hormonal changes have the potential to impact of a variety of other hormones (McMurray and Hackney 2000). For example, Waters et al. (2001) reported that amenorrheic athletes had significantly lower (four to fivefold) GH responses to 50 min of submaximal exercise (70% maximal oxygen consumption) compared with eumenorrheic athletes. Yahiro et al. (1987) reported that serum testosterone levels increased in eumenorrheic runners, but not in amenorrheic runners, after acute treadmill exercise.
As mentioned in the preceding section, Nakamura et al. (2011) investigated changes in ovarian and anabolic hormones after acute resistance exercise in women with menstrual disorders including oligomenorrhea and amenorrhea. Serum estradiol and progesterone concentrations in these women with menstrual disorders did not increase after exercise (Fig. 10.4). Immediately after the end of resistance exercise, GH concentrations in eumenorrheic women increased significantly from pre-exercise levels, but this difference was not observed in women with menstrual disorders. However, a small, but statistically significantly higher increase in IGF- I in response to resistance exercise, was observed immediately after exercise in women with menstrual disorders. Testosterone and DHEA-S concentrations showed no significant increase in response to the resistance exercise protocols either, regardless of menstrual cycle status. However, total secretion (area under the curve) of testosterone was significantly lower in women with menstrual disorders. In addition, DHEA- S increased significantly 60 min after exercise in the early-follicular phase in eumenorrheic women, but it was decreased significantly in women with menstrual disorders. And finally no significant differences in cortisol levels were found within any menstrual cycle status (Fig. 10.5).
The neuroendocrine mechanisms of exercise-induced GH release are not fully understood; however, GH secretion is controlled by hypothalamic hormones. These hormones include GH-releasing hormone, which exerts positive feedback, and somatostatin, which exerts negative feedback, on GH secretion (Giustina and Veldhuis 1998; Jenkins 1999). It is possible that the attenuated GH secretion of these women in response to acute resistance exercise is related to a disturbance in hypothalamic–pituitary function, but the mechanism of these events is not well understood at this time.
Findings from previous studies have suggested that estradiol stimulates GH secretion because estrogen replacement therapy increases GH secretion in postmenopausal women and prepubertal girls with Turner syndrome (Kanaley et al. 2005; Mauras et al. 1990). In addition, Kraemer et al. (1998) and Kanaley et al. (2005) demonstrated that GH responses to endurance exercise are higher in postmenopausal women receiving hormone replacement therapy than those who were not. This effect of estrogen may be due to a combination of withdrawal of somatostatin’s (also known as growth hormone-inhibiting hormone [GHIH]) inhibitory tone, amplification of endogenous GH-releasing hormone release or its pituitary actions, and recruitment of other mechanisms that stimulate GH release (Giustina and Veldhuis 1998).
Specific endocrine differences between eumenorrheic women and women with menstrual disorders include cyclic fluctuations of estrogen and progesterone which are controlled by the hypothalamic–pituitary–gonadal axis (see Chap. 1). Women with menstrual disorders can be estrogen deficient on a long-term basis. Insufficient estrogen and progesterone feedback disturbs hypothalamic–pituitary axis responses (De Crée 1998). Thus, differences in ovarian hormone secretion status, that is, differences in hypothalamic–pituitary function between women with menstrual disorders and eumenorrheic women, may influence the GH response to acute resistance exercise. However, there appears to be no available information on anabolic hormonal responses to resistance exercise in women with menstrual disorders. There is also little information available on acute responses of DHEA-S to resistance exercise in such women. Riechman et al. (2004) reported that acute resistance exercise increases blood DHEA-S levels. Exercise-induced increases in DHEA- S concentrations have been attributed to an increased rate of secretion from the adrenal cortex in response to ACTH stimulation (Johnson et al. 1997). Meczekalski et al. (2000) investigated the hypothalamic–pituitary–adrenal axis in women with hypothalamic amenorrhea and reported that the ACTH response to corticotrophin-releasing hormone was significantly lower in amenorrheic women compared with healthy control women. Nakamura et al. (2011) reported DHEA-S levels were not significantly higher immediately after acute resistance exercise regardless of menstrual cycle status. Enea et al. (2009) reported short-term exercise does not induce increased adrenal steroid production in response to ACTH secretion. It is possible that a higher-volume resistance exercise program for a longer period could induce an increase in DHEA-S.
In summary, women with certain forms of menstrual disorders, associated with low estradiol and progesterone levels, appear to have an attenuated anabolic hormonal response to acute resistance exercise. However, there is extremely limited evidence on this topic and more research is needed.
Resistance Exercise-Induced Muscle Damage and Recovery During Different Menstrual Cycle Phases
As estrogen receptors have been localized to skeletal muscle tissue, variations in hormonal concentrations due to the menstrual cycle may influence resistance training outcomes. Haines et al. (2018) compared estrogen receptor activation and subsequent effects on myogenic-related genes in response to eccentric exercise in the mid-follicular phase (MF) and mid-luteal phase (ML). Skeletal muscle estradiol levels were not significantly impacted by either menstrual phase despite greater levels of serum estradiol during ML. This is consistent with data from rats showing no significant change in muscle estradiol levels following treadmill exercise at 30 m/min for 30 min (Aizawa et al. 2008). However, both skeletal muscle estrogen receptor-α (ER-α) mRNA and protein were significantly increased during MF. ER activation and subsequent expression of Myo-D mRNA occurred independent of the circulating estradiol levels. Furthermore, skeletal muscle cyclin D1 mRNA expression was increased by eccentric exercise to a much greater extent during MF and may play a role in ER activation during periods of lower circulating estradiol. These results suggests that greater potential for recovery and repair in MF following eccentric exercise.
It has been suggested that endogenous estrogen may be protective against exercise-induced muscle damage, and if so, it could be speculated that endogenous estrogen may play a role in enhancing recovery from exercise-induced muscle damage (Thompson et al. 2020). Despite the problem of combining oral contraceptives and menstrual cycle participants into one group, the results of Markofski et al. (2014) examining the effect of menstrual cycle phase on markers of exercise-induced muscle damage following acute high-volume eccentric exercise are very interesting. After high-volume eccentric exercise, creatine kinase (CK) was significantly lower and strength recovery was better at 96 hours post-session in the follicular phase than in the luteal phase (Fig. 10.6). Blood estrogen levels were significantly higher in the luteal phase than in the follicular phase; however, the effects of estrogen in skeletal muscle tissue are very complex. To understand the effects of estrogen on muscle damage, it will be necessary to consider the interactions between estrogen and ER and estrogen and progesterone.
Strength recovery over the period of 1 week after eccentric-biased extension exercises performed during the follicular and luteal phases (Markofski et al. 2014). A significant (p < 0.0001) main effect was present for time. * Strength was significantly higher than baseline at all other time points. † Follicular phase significantly (p = 0.009) higher strength than luteal phase. ‡ Follicular phase significantly (p = 0.001) higher strength than luteal phase
Menstrual Cycle and Resistance Training Program
As discussed in this chapter, the responses of sex hormones and anabolic hormones to acute resistance exercise, as well as muscle damage and recovery are affected by hormonal fluctuations during the menstrual cycle, suggesting that menstrual cycle may influence exercise training-induced skeletal muscular adaptation. Thus, it would be possible that training programs for eumenorrheic women could be timed in accordance with the menstrual cycle for enhancing resistance training outcomes.
Recent studies have begun investigating the trainability of muscle strength with menstrual cycle-triggered training. Sung et al. (2014) compared the effects of two different menstrual cycle-based leg strength training programs (follicular phase-based training versus luteal phase-based training) on muscle volume and microscopic morphological parameters. The increase in maximum isometric force with follicular phase-based training was higher than with luteal phase-based training (Fig. 10.7), which was consistent with earlier findings of Reis et al. (1995). Follicular phase-based training was also associated with a higher increase in muscle diameter than luteal phase-based training. Moreover, they found significant increases in fiber type II diameter and cell nuclei-to-fiber ratio after follicular phase-based training but not luteal phase-based training.
In contrast, Sakamaki-Sunaga et al. (2015) reported no major differences among different training frequencies for arm curls during menstrual cycle phases with regard to muscle hypertrophy and strength, but this study did not use hormone testing to confirm menstrual cycle phase. Another study investigated the effects of menstrual and oral contraceptive cycle on a high-frequency periodized leg resistance training in trained women (Wikström-Frisén et al. 2017). Significant increase in squat and countermovement jump, and peak torque values in hamstrings for follicular/early oral contraceptive cycle phase-based training group were observed, and significant increase in lean body mass of the legs. These results suggest that follicular phase-based training may be superior to both regular training and luteal phase-based training for developing strength and muscle mass in eumenorrheic women (Thompson et al. 2020).
Increase in the strength of maximum isometric knee extension (Fmax) after two different menstrual cycle-based leg strength training programs were compared to each other (follicular phase-based- [FT] versus luteal phase-based training [LT]) (Sung et al. 2014). Pre: before training, control: control cycle, training: training cycle, day 11: analysis around day 11; day 25: analysis around day 25; *P < 0.05 compared to pre-training, †P < 0.05 FT versus LT
On the other hand, many reproductive-aged women, including athletes, experience menstrual irregularities and amenorrhea. In addition, the use of oral contraceptives (OC) is becoming more common among both the general population and athletes. It is notable that women with menstrual disorders characterized by low estradiol and progesterone serum concentrations show an attenuated GH response to acute resistance exercise (Nakamura et al. 2011). Women who take OC also have significantly lower levels of circulating estrogen compared to eumenorrheic women throughout the menstrual cycle. Several studies reported a higher level of post-exercise CK in OC group than eumenorrheic group (Hicks et al. 2017; Minahan et al. 2015; Roth et al. 2001). These appear that endogenous estrogen may be protective against exercise-induced muscle damage, suggesting that recovery from exercise-induced muscle damage may be different between OC users and eumenorrheic women (Thompson et al. 2020). This may have implications for long-term adaptations to resistance training, but at this time there is no data available to clearly demonstrate these. Further studies are needed to demonstrate the short-term and long-term effects of changes in hormonal responses to resistance exercise on skeletal muscle by menstrual cycle status and OC use.
Conclusion
The fluctuations of sex steroid hormones have the potential influence muscle strength performance and response to resistance exercise in women. However, based on a number of studies investigating the effects of the menstrual cycle on muscle strength performance, it can be concluded there is little or perhaps no difference in muscle strength at various times during the menstrual cycle. On the other hand, though, there is very limited data about responses to resistance exercise in women as related to their sex hormone levels. A few studies do indicate that menstrual cycle phase and status variations affect exercise-induced secretion of some hormones and muscle damage and recovery. Therefore, there is a possibility of an effect on trainability of muscle strength to resistance training programs. But much more research work is needed on these topics. In addition, exercise performance and physiological response to exercise, resistance and otherwise, are probably very individual specific and influenced by many factors, and more research is also needed taking into account these individual specific factors.
References
Aizawa K, Akimoto T, Inoue H, et al. Resting serum DHEAS level increases after 8-week resistance training among young. Eur J Appl Physiol. 2003;90:575–80.
Aizawa K, Hayashi K, Mesaki N. Relationship of muscle strength with dehydroepiandrosterone sulfate (DHEAS), testosterone and insulin-like growth factor-I in male and female athletes. Adv Exerc Sports Physiol. 2006;12:29–34.
Aizawa K, Iemitsu M, Otsuki T, Maeda S, Miyauchi T, Mesaki N. Sex differences in steroidogenesis in skeletal muscle following a single bout of exercise in rats. J Appl Physiol. 2008;104(1):67–74.https://doi.org/10.1152/japplphysiol.00558.2007.
Bambaeichi E, Reilly T, Cable NT, et al. The isolated and combined effects of menstrual cycle phase and time-of-day on muscle strength of eumenorrheic females. Chronobiol Int. 2004;21:645–60.
Baulieu EE. Dehydroepiandrosterone (DHEA): a fountain of youth? J Clin Endocrinol Metab. 1996;81:3147–51.
Consitt LA, Copeland JL, Tremblay MS. Hormone responses to resistance vs. endurance exercise in premenopausal females. Can J Appl Physiol. 2001;26:574–87.
Consitt LA, Copeland JL, Tremblay MS. Endogenous anabolic hormone responses to endurance versus resistance exercise and training in women. Sports Med. 2002;32:1–22.
Constantini NW, Dubnov G, Lebrun CM. The menstrual cycle and sport performance. Clin Sports Med. 2005;24:e51–82.
Copeland JL, Consitt LA, Tremblay MS. Hormonal responses to endurance and resistance exercise in females aged 19–69 years. J Gerontol A Biol Sci Med Sci. 2002;57:B158–65.
De Crée C. Sex steroid metabolism and menstrual irregularities in the exercising female. A review. Sports Med. 1998;25:369–406.
Elliott KJ, Cable NT, Reilly T, et al. Effect of menstrual cycle phase on the concentration of bioavailable 17-beta oestradiol and testosterone and muscle strength. Clin Sci (Lond). 2003;105:663–9.
Enea C, Boisseau N, Ottavy M, et al. Effects of menstrual cycle, oral contraception, and training on exercise-induced changes in circulating DHEA-sulphate and testosterone in young women. Eur J Appl Physiol. 2009;106:365–73.https://doi.org/10.1007/s00421-009-1017-6.
Enea C, Boisseau N, Fargeas-Gluck MA. Circulating androgens in women: exercise-induced changes. Sports Med. 2011;41:1–15.https://doi.org/10.2165/11536920-000000000-00000.
Enns DL, Tiidus PM. The influence of estrogen on skeletal muscle: sex matters. Sports Med. 2010;40:41–58.https://doi.org/10.2165/11319760-000000000-00000.
Faria AC, Bekenstein LW, Booth Jr RA, et al. Pulsatile growth hormone release in normal women during the menstrual cycle. Clin Endocrinol (Oxf). 1992;36:591–6.
Fleck SJ, Kraemer WJ. Designing resistance training programs. 4th ed. Champaign, IL: Human Kinetics; 2014.
Fridén C, Hirschberg AL, Saartok T. Muscle strength and endurance do not significantly vary across 3 phases of the menstrual cycle in moderately active premenopausal women. Clin J Sport Med. 2003;13:238–41.
Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–97.
Hackney AC, Viru A. Research methodology: endocrinologic measurements in exercise science and sports medicine. J Athl Train. 2008;43:631–9.
Häkkinen K, Pakarinen A. Acute hormonal responses to heavy resistance exercise in men and women at different ages. Int J Sports Med. 1995;16:507–13.
Häkkinen K, Pakarinen A, Kraemer WJ, et al. Basal concentrations and acute responses of serum hormones and strength development during heavy resistance training in middle-aged and elderly men and women. J Gerontol A Biol Sci Med Sci. 2000;55:B95–105.
Hansen M, Kjaer M. Influence of sex and estrogen on musculotendinous protein turnover at rest and after exercise. Exerc Sport Sci Rev. 2014;42:183–92.https://doi.org/10.1249/JES.0000000000000026.
Haines M, McKinley-Barnard SK, Andre TL, et al. Skeletal muscle estrogen receptor activation in response to eccentric exercise up-regulates myogenic-related gene expression independent of differing serum estradiol levels occurring during the human menstrual cycle. J Sports Sci Med. 2018;17:31-39.
Hicks KM, Onambélé-Pearson G, Winwood K, Morse CI. Oral contraceptive pill use and the susceptibility to markers of exercise-induced muscle damage. Eur J Appl Phys 2017;117(7):1393-1402.https://doi.org/10.1007/s00421-017-3629-6.
Hornum M, Cooper DM, Brasel JA, et al. Exercise-induced changes in circulating growth factors with cyclic variation in plasma estradiol in women. J Appl Physiol. 1997;82:1946–51.
Jabbour HN, Kelly RW, Fraser HM, et al. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46.
Janse de Jonge XA. Effects of the menstrual cycle on exercise performance. Sports Med. 2003;33:833–51.
Jenkins PJ. Growth hormone and exercise. Clin Endocrinol (Oxf). 1999;50:683–9.
Johnson LG, Kraemer RR, Haltom R, et al. Effects of estrogen replacement therapy on dehydroepiandrosterone, dehydroepiandrosterone sulfate, and cortisol responses to exercise in postmenopausal women. Fertil Steril. 1997;68:836–43.
Kahn SM, Hryb DJ, Nakhla AM, et al. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002;175:113–20.
Kanaley JA, Giannopoulou I, Collier S, et al. Hormone-replacement therapy use, but not race, impacts the resting and exercise-induced GH response in postmenopausal women. Eur J Endocrinol. 2005;153:527–33.
Koziris LP, Hickson RC, Chatterton Jr RT, et al. Serum levels of total and free IGF-I and IGFBP-3 are increased and maintained in long-term training. J Appl Physiol. 1999;86:1436–42.
Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35:339–61.
Kraemer WJ, Gordon SE, Fleck SJ, et al. Endogenous anabolic hormonal and growth factor responses to heavy resistance exercise in males and females. Int J Sports Med. 1991;12:228–35.
Kraemer WJ, Fleck SJ, Dziados JE, et al. Changes in hormonal concentrations after different heavy-resistance exercise protocols in women. J Appl Physiol. 1993;75:594–604.
Kraemer RR, Heleniak RJ, Tryniecki JL, et al. Follicular and luteal phase hormonal responses to low-volume resistive exercise. Med Sci Sports Exerc. 1995;27:809–17.
Kraemer RR, Johnson LG, Haltom R, et al. Effects of hormone replacement on growth hormone and prolactin exercise responses in postmenopausal women. J Appl Physiol. 1998;84:703–8.
Labrie F, Belanger A, Cusan L, et al. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–402.
Lane AR, O'Leary CB, Hackney AC. Menstrual cycle phase effects free testosterone responses to prolonged aerobic exercise. Acta Physiol Hung. 2015;102(3):336–41.
Lebrun CM. The effect of the phase of the menstrual cycle and the birth control pill on athletic performance. Clin Sports Med. 1994;13:419–41.
Markofski MM, Braun William A. Influence of menstrual cycle on indices of contraction-induced muscle damage. J Strength Conditioning Res. 2014;28(9):2649–2656.https://doi.org/10.1519/JSC.0000000000000429.
Marx JO, Ratamess NA, Nindl BC, et al. Low-volume circuit versus high-volume periodized resistance training in women. Med Sci Sports Exerc. 2001;33:635–43.
Mauras N, Rogol AD, Veldhuis JD. Increased hGH production rate after low-dose estrogen therapy in prepubertal girls with Turner’s syndrome. Pediatr Res. 1990;28:626–30.
McMurray RG, Hackney AC. Endocrine responses to exercise and training. In: Garrett W, Kirkendall DT, editors. Exercise and sport science. Philadelphia, PA: Lippincott, Williams & Wilkins; 2000. p. 135–61.
Meczekalski B, Tonetti A, Monteleone P, et al. Hypothalamic amenorrhea with normal body weight: ACTH, allopregnanolone and cortisol responses to corticotropin-releasing hormone test. Eur J Endocrinol. 2000;142:280–5.
Minahan C, Joyce S, Bulmer AC, Cronin N, Sabapathy S. The influence of estradiol on muscle damage and leg strength after intense eccentric exercise. Eur J Appl Phys. 2015;115(7):1493–1500.https://doi.org/10.1007/s00421-015-3133-9.
Nakamura Y, Aizawa K, Imai T, et al. Hormonal responses to resistance exercise during different menstrual cycle states. Med Sci Sports Exerc. 2011;43:967–73.https://doi.org/10.1249/MSS.0b013e3182019774.
Oosthuyse T, Bosch AN. The effect of the menstrual cycle on exercise metabolism: implications for exercise performance in eumenorrhoeic women. Sports Med. 2010;40:207–27.https://doi.org/10.2165/11317090-000000000-00000.
Ovesen P, Vahl N, Fisker S, et al. Increased pulsatile, but not basal, growth hormone secretion rates and plasma insulin-like growth factor I levels during the periovulatory interval in normal women. J Clin Endocrinol Metab. 1998;83:1662–7.
Reis E, Frick U, Schmidtbleicher D. Frequency variations of strength training sessions triggered by the phases of the menstrual cycle. Int J Sports Med. 1995;16:545–50.
Riechman SE, Fabian TJ, Kroboth PD, et al. Steroid sulfatase gene variation and DHEA responsiveness to resistance exercise in MERET. Physiol Genomics. 2004;17:300–6.
Roth SM, Gajdosik R, Ruby BC, Effects of circulating estradiol on exercise-induced creatine kinase activity. J Exerc Physiol 2001;4:10–7.
Sakamaki-Sunaga M, Min S, Kamemoto K, et al. Effects of menstrual phase-dependent resistance training frequency on muscular hypertrophy and strength. J Strength Cond Res. 2015;30(6):1727–34.
Staron RS, Karapondo DL, Kraemer WJ, et al. Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. J Appl Physiol. 1994;76:1247–55.
Sung E, Han A, Hinrichs T, et al. Effects of follicular versus luteal phase-based strength training in young women. SpringerPlus. 2014;3:668.https://doi.org/10.1186/2193-1801-3-668.
Thompson B, Almarjawi A, Sculley D, Janse de Jonge X. The effect of the menstrual cycle and oral contraceptives on acute responses and chronic adaptations to resistance training: a systematic review of the literature. Sports Med. 2020;50(1):171–85.https://doi.org/10.1007/s40279-019-01219-1.
Vingren JL, Kraemer WJ, Ratamess NA, et al. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. 2010;40:1037–53.https://doi.org/10.2165/11536910000000000-00000.
Waters DL, Qualls CR, Dorin R, et al. Increased pulsatility, process irregularity, and nocturnal trough concentrations of growth hormone in amenorrheic compared to eumenorrheic athletes. J Clin Endocrinol Metab. 2001;86:1013–9.
Wikström-Frisén L, Boraxbekk CJ, Henriksson-Larsén K. Effects on power strength and lean body mass of menstrual/oral contraceptive cycle based resistance training. J Sports Med Phys Fitness. 2017;57(1–2).https://doi.org/10.23736/S0022-4707.16.05848-5.
Yahiro J, Glass AR, Fears WB, et al. Exaggerated gonadotropin response to luteinizing hormone-releasing hormone in amenorrheic runners. Am J Obstet Gynecol. 1987;156:586–91.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nakamura, Y., Aizawa, K. (2023). Sex Hormones, Menstrual Cycle, and Resistance Exercise. In: Hackney, A.C. (eds) Sex Hormones, Exercise and Women. Springer, Cham. https://doi.org/10.1007/978-3-031-21881-1_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-21881-1_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-21880-4
Online ISBN: 978-3-031-21881-1
eBook Packages: MedicineMedicine (R0)