Abstract
In order to reduce the use of sodium silicate solutions and hydroxides for the alkaline activation of materials, the combination of alkaline activators has a positive effect to produce alternative cements with environmental advantages in relation to Portland cement-based materials. This research report results of compressive strength and reaction products of blast-furnace slag cements activated with a mixture of MgO-Na2SO4 with a ratio 1:1 added in 4, 6 and 8% relative to the slag weight. Pastes of alkali activated slag were prepared and cured at 20 ℃ and 60 ℃-24h-20 ℃ up to 90 days. Samples at 20 ℃ did not show a strength gain during the first 3 days of curing, but after 7 days the strength increased noticeably of up 53 MPa at 90 days. It also was observed that curing at 60 ℃ favored a gain of compressive strength at early ages between 20-30MPa with a marginal increase at 90 days. The use of the mix MgO-Na2SO4 was favorable for the chemical activation of the slag without detrimental effects on the stability of the formulated samples. The microstructures consisted of C-S-H, ettringite, hydrotalcite and some traces of CaCO3 from the raw material.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Portland cement-based concrete (PC) is the most widely used material for the construction [1], however it generates a great environmental impact due to the CO2 emissions regarded with its manufacture process. For this situation, many worldwide initiatives are currently conducted to investigate alternative cements of lower environmental impact based on raw materials of regional abundance, as silicoaluminate clays or diverse industrial wastes as blast furnace slag (BFS). The latter has demonstrated a great potential as cementitious precursor in traditional PC binders and in alkali activated cements (AAC).
AAC are obtained by the interaction of BFS or other amorphous materials with alkaline solutions of sodium silicates and hydroxides, nonetheless, diverse alternative soft alkalis as Na2CO3, Na2SO4, Ca(OH)2 and CaO can be used and their effectiveness could be enhanced if some of them are used in combined form as proposed in this investigation [2,3,4,5,6].
An interesting additive recently explored, is the MgO which results in the densification of microstructures by the formation of hydrotalcite that fills pores and improves the mechanical properties to a reasonable degree when is combined with different soft and strong alkalis [7].
For the study, diverse cementitious pastes of BFS activated with blends of MgO-Na2SO4 were analyzed. The reaction products were further characterized by thermal analysis and scanning electron microscopy. The results showed that the use of Na2SO4 in combination with MgO was effective to obtain AAC pastes with high compressive strength and dense microstructures.
2 Experimental Procedures
2.1 Materials
BFS was used as a solid precursor with a mean particle size of (d50) = 21.2 µm, the chemical composition obtained by X-ray fluorescence (XRF) is shown in Table 1. The surface area of the BFS was of 5000 cm2/g. The material was mainly amorphous with some traces of crystalline phases of akermanite and gehelenite. To activate the slag, two agents with 1:1 ratio of reactive MgO and Na2SO4 were used and supplied by Jalmek ® with chemical composition shown in Table 1. Purified water was also used to prepare pastes.
2.2 Sample Preparation
Six pastes were elaborated using the combination of MgO:Na2SO4 with a constant ratio 1:1 added at concentrations of 4, 6 and 8% by mass relative to the slag content. The water to binder ratio was 0.33 to ensure good workability and fluidity, Table 2 shows the details of the formulations studied. The pastes where formulated with 100% BFS incorporating the mixture of MgO:Na2SO4 (in 1:1 proportion) at 4, 6 and 8% relative to the mass of the BFS.
The BFS+ alkaline activators where dry mixed and then added to water and mixed for 3 min. The pastes were cast into cubic molds of 2.5 cm per side and vibrated on a shaking table (Controls Model 55-C0159/LZ) for 30 s to eliminate air bubbles. The samples were covered with plastic film to prevent moisture losses. The pastes were cured at 20 and 60 ℃ for 24 h with subsequent curing at 20 ℃ for 90 days.
2.3 Characterization
For compressive strength tests, four cubes of each paste were evaluated at 1, 3, 7, 14, 28 and 90 days using an automatic hydraulic machine (Controls model 50-C7024). Fragments of the fractured samples were immersed in acetone for 2 days and then dried in a vacuum oven (VWR 1430 M) for 48 h at 40 ℃, with the intention of interrupting the advance of the reactions. Some samples were pulverized in a planetary mill (PM 400/2; Restch), to be characterized by thermal and differential analysis using a simultaneous device of instruments TGA/DSC SDT Q-600-TA up to 950 ℃ with a heating rate of 10 ℃/min with Al2O3 crucibles. For scanning electron microscopy (SEM) analysis, dry fragments were mounted on epoxy resin and polished with silicon carbide sand paper and diamond pastes down to 0.25 µm. The samples were covered with gold to make the samples conductive under the microscope (JEOL JSM-66110LV) accessorized with an Oxford instrument X-Max detector.
3 Results and Discussion
3.1 Compressive Strength (CS)
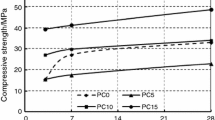
Figure 2 shows the CS development of MgO:Na2SO4 activated pastes at 90 days of curing. Samples cured at 20 ℃ (Fig. 1(a)) did not show CS at 1 and 3 days of curing. After 7 days the formulations showed a slight increase of strength up to 5 MPa for samples with 4 and 8% MgO:Na2SO4. After 28 days, all pastes developed between 30–40 MPa. The lower dosage of activator, resulted in the highest compressive strength of 55 MPa at 90 days of curing while those with 6 and 8% MgO:Na2SO4 showed 45 MPa. The values of strength were higher than those previously reported for BFS activated individually with MgO that reached ̴32 MPa suggesting that the combined use of MgO and Na2SO4 is more beneficial for the formation of cementitious phases [8,9,10,11].
On the other hand, a clear effect of the curing temperature was observed in Fig. 2(b) where the temperature at 60 ℃ per 24 h, favored a rapid gain of CS for all the pastes at early ages of curing. In this case, the temperature accelerated the kinetics of the dissolution of the BFS leading to the precipitation of reaction products with high strength. At 1 day, the binders with 8% MgO:Na2SO4 showed mechanical resistance of ̴ 25 MPa. Over time, the samples showed a marginal increase of CS that stabilized in 30 MPa at 90 days regardless the dosage of activator used. This behavior can be related with the loss of moisture that occurs at high temperature at early ages limiting the further advance of the reactions [12]. From the results, the curing at 20 ℃ was even more feasible because under these conditions a slower dissolution of the reactive species occurred, leading to a more homogeneous condensation of mechanically strong reaction products in the matrix, due to the water retention within the microstructures as the reported elsewhere [12, 13].
3.2 Thermogravimetric Analysis
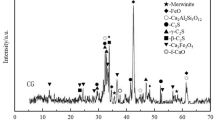
Results of thermal analysis for BFS pastes with 4 and 8% MgO:Na2SO4 cured at 20 ℃ and 60 ℃-24 h-20 ℃ at 90 days are respectively shown in Fig. 3. Table 3 includes the weight losses corresponding to the decomposition of the different reaction products observed.
The first endothermic peak in the derivative weight loss curves at 200 ℃ is representative of the dehydration of C-S-H gel and ettringite phases according to [6, 9, 11]; this is also consistent with the evaporation of free water absorbed into the porous structure of the materials [14]. For samples containing 4% MgO:Na2SO4, in Figure (a) the endothermic signal intensity of C-S-H decreases as the curing temperature increased. This suggests a higher formation of C-S-H in samples at 20 ℃, which agrees with the CS results previously discussed and with the higher retention of water in the samples necessary for the advance of the chemical reactions and precipitation of products.
For both dosages of activator (4 and 8% MgO:Na2SO4) an additional weight loss was observed between 250 and 400 ℃ by decomposition of hydrotalcite-like phases, which is a reaction product frequently reported in alkali activated BFS cements due to the presence of MgO [14, 15]. The progressive weight loss after 600 ℃ was essentially stimulated by the decarbonation of CaCO3 present in raw materials or in products formed during the hydration reactions. For both concentrations of MgO:Na2SO4 the intensity of the endothermic signal was similar, indicating that the formation of this phase did not progress by increasing the % of alkaline activating agent [10, 14].
3.3 Scanning Electron Microscopy
Figure 4 shows the microstructures of pastes activated with 4% MgO:Na2SO4 at 20 ℃ and 60 ℃-24h-20 ℃ after 90 days of curing. According to Figure (a) the curing at 20 ℃ favored the consolidation of a dense and compact microstructure with fully and partially reacted BFS particles of light grey tone and irregular morphology homogeneously distributed in the matrix of reaction products of darker grey tone. According to the energy dispersive spectroscopy (EDS) results, the formed products showed high concentration of Si, Ca, Mg, Al and Na, proposing the formation of the C-(A)-S-H type gel intermixed with hydrotalcite phases, some traces of S where also present and possibly incorporated in ettringite, although such phase cannot be seen in the microstructure [11, 16].
As the curing temperature increased to 60 ℃-24h (Fig. 4(b)) the microstructure seemed to be less dense with a higher presence of anhydrous BFS grains dispersed in the matrix of reaction products justifying the lower values of compressive strength developed. The EDS results did not show significant differences in the chemical composition of the microstructure as the matrix also consisted of Mg, Si, Ca and Al, indicating the formation of C-(A)-S-H and hydrotalcite. Finally, evidence about the presence of unreacted MgO or Na2SO4 was not observed, suggesting their complete integration in the reaction products.
4 Conclusions
-
The mixture of MgO-Na2SO4 is efficient as alkaline activator of blast furnace slag.
-
Under the conditions assessed, the addition of 4% MgO-Na2SO4 resulted in higher compressive strength than samples with 6 and 8% of activator
-
The pastes cured at 20 ℃ developed of up to 53 MPa at 90 days of curing, while those exposed at 60 ℃–24h-20 ℃ had a rapid strength gain at early ages, with a limited gain over time.
-
The curing of samples at 20 ℃ benefits the dissolution of reactive species and condensation of dense microstructures with higher strength, while the curing at 60 ℃–24h-20 ℃, accelerated the reactions at early ages with a limited further gain of strength.
-
The reaction products consisted of C-S-H type-gels, ettringite due to the sodium sulfate, and hydrotalcite-like phases.
-
The handling of the blended MgO:Na2SO4 activators is more friendly than those containing strong alkalis as NaOH or sodium silicates. The use of powdered activators is suitable to obtain pastes just by adding water to the precursors (BFS+activator).
References
Provis, J.L., Bernal, S.A.: Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 44, 3.1–3.29 (2014)
Kovtun, M., Kearsley, E.P., Shekhovtsova, J.: Chemical acceleration of a neutral granulated blast-furnace slag activated by sodium carbonate. Cem Concr. Res. 72, 1–9 (2015)
Y. Jeong, H. Park, Y. Jun, J.H. Jeong, J. Eun Oh. Influence of slag characteristics on strength development and reaction products in CaO-activated slag system. Cem. Concr. Compos. 72 (2016), 155–167
Burciaga-Díaz, O., Magallanes-Rivera, R.X., Escalante-García, J.I.: Alkali-activated slag-metakaolin pastes: strenght, structural and microstructural characterization. J. Sustain. Cement-Based Mater. 2, 111–127 (2013)
Shi, C., Krivenko, P.V., Roy, D.: Alkali-Activated Cements and Concretes: Taylor & Francis group (2006)
Kim, M.S., Jun, Y., Lee, C., Oh, J.E.: Use of CaO as an activator for producing Price competitive non-cement structural binder using ground granulated blast furnace slag. Cem. Concr. Res. 54, 208–214 (2013)
He, J., Zheng, W., Bai, W., Hu, T., He, J., Song, X.: Effect of reactive MgO on hydration and properties of alkali-activated slag pastes with different activators. Constr. Build. Mater. 271, 121608 (2021)
Yi, Y., Liska, M., Al-Tabbaa, A.: Properties and microstructure of GGBS-magnesia pastes. Adv. Cem. Res. 26(2), 114–122 (2014)
Abdalqader, A.F., Jin, F., Al-Tabbaa, A.: Characterization of reactive magnesia and sodium carbonate-activated fly ash/slag paste blends. Constr. Build. Mater. 93, 506–513 (2015)
Burciaga-Díaz, O.: Irma Betancourt-Castillo, Characterization of novel blast-furnace slag cement pastes and mortars activated with a reactive mixture of MgO-NaOH. Cem. Concr. Res. 105, 54–63 (2018)
Tan, H., et al.: Compressive strength and hydration process of wet-grinded granulated blast-furnace slag activated by sodium sulfate and sodium carbonate. Cem. Concr. Compos. 97, 387–398 (2019)
Burciaga-Díaz, O., Gómez-Zamorano, L.Y., Escalante-García, J.I.: Influence of the long term curing temperature on the hydration of alkaline binders of blast furnace slag-metakaolin. Constr. Build. Mater. 113, 917–926 (2016)
Barnett, S.J., Soutsos, M.N., Millard, S.G., Bungey, J.H.: Strength development of mortars containing ground granulated blast-furnace slag: Effect of curing temperature and determination of apparent activation energies. Cem. Concr. Res. 36, 434–440 (2006)
Lee, N.K., Koh, K.T., Kim, M.O., An, G.H., Ryu, G.S.: Physicochemical changes caused by reactive MgO in alkali-activated fly ash/slag blends under accelerated carbonation. Ceram. Int. 43, 12490–12496 (2017)
Gu, K., Jin, F., Al-Tabbaa, A.: Bin Shi, Activation of ground granulated blast furnace slag by using calcined dolomite. Constr. Build. Mater. 68, 252–258 (2014)
Gu, K., Jin, F., Al-Tabbaa, A., Shi, B., Liu, J.: Mechanical and hydration properties of ground granulated blast-furnace slag pastes activated with MgO-CaO mixtures. Constr. Build. Mater. 69, 101–108 (2014)
Acknowledgements
The Technological National of Mexico (TecNM) and the National Council of Science and Technology (Conacyt) are acknowledged for the financial support of the project 5609.15-P and for the scholarship (711629) to I. Betancourt-Castillo, respectively.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Betancourt-Castillo, I.E., Díaz-Guillén, J.A., Escalante-García, J.I., Burciaga-Díaz, O. (2023). One Part Blast-Furnace Slag Cements Activated with a Blend of MgO-Na2SO4. In: Escalante-Garcia, J.I., Castro Borges, P., Duran-Herrera, A. (eds) Proceedings of the 75th RILEM Annual Week 2021. RW 2021. RILEM Bookseries, vol 40. Springer, Cham. https://doi.org/10.1007/978-3-031-21735-7_69
Download citation
DOI: https://doi.org/10.1007/978-3-031-21735-7_69
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-21734-0
Online ISBN: 978-3-031-21735-7
eBook Packages: EngineeringEngineering (R0)