Abstract
Even after a careful and complete diagnostic process, which also includes thoracoscopic pleural biopsy, a certain number of pleural effusions remain without a definite diagnosis. The histological diagnoses we receive in these situations are generic and not indicative: usually non-specific pleuritis, fibrinous pleuritis or pachypleuritis; non-specific pleuritis is common to many benign pathologies. The incidence of NSP varies in the different experiences from 5% to 30%.
During the follow-up, a diagnosis of benign effusion (of differing causes) is made or the diagnosis is not reached, and therefore, the effusion is defined as ‘idiopathic’. But in a variable percentage, from 5% to 12.3%, the follow-up leads to the diagnosis of malignancy (mainly mesothelioma). We believe that the term ‘idiopathic pleural effusion’ should be used only in cases in which, in addition to the negative pleural biopsies performed by medical thoracoscopy or VATS, an adequate follow-up has been undertaken and a definite diagnosis has not been reached.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Idiopathic pleural effusion is a topic for which, because of the different interpretations and different approaches in managing it, there is no clarity in the scientific literature.
Without claiming to solve the problem, let’s try to tackle this topic with the hope of shedding some light and, if possible, even giving some advice!
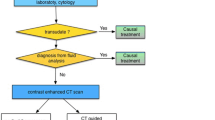
The diagnostic path of the pleural effusion includes a complete clinical evaluation of the patient with radiological tests, all the indicated laboratory blood tests, all possible investigations on the pleural fluid, and also the use of pleural biopsies, indispensable in many clinical pictures, as in about 25% of cases, the precise diagnosis can only be reached with pleural histology.
Medical Thoracoscopy is certainly the best method of obtaining biopsies on account of its high diagnostic yield and because it usually (in approximately 90%–95% of cases) allows us to successfully complete the diagnostic path. But, even after a careful and complete diagnostic process, a certain number of pleural effusions remain without a definite diagnosis. All of us have happened to be disappointed when a non-orientative histological diagnosis arrived from Pathological Anatomy – from samples on which we had placed all our hopes for a definite diagnosis!
The histological diagnoses we receive in these situations are generic and not indicative: usually non-specific pleuritis, fibrinous pleuritis or pachypleuritis. It is like calling it ‘pleuritis X’.
A histological diagnosis of ‘non-specific pleuritis/fibrosis’ (NSP) is defined if the histology report of the pleural tissue revealed any of the following: reactive fibrous pleural thickening, fibrinous pleurisy, fibrosis, florid reactive change, fibrous connective tissue, chronic inflammation, benign change or dense fibrous tissue, in the absence of malignant pleural infiltration, granulomata, pleural vasculitis or evidence of bacterial infection [1].
Therefore, although NSP is a detailed and precise definition, it only helps us to exclude, at least temporarily – as in some cases, the patient’s follow-up yields some surprises – neoplasms and TB, and is not indicative of other pathologies. In fact, non-specific pleuritis is common to many benign pathologies (also in transudative effusions) [2], the diagnosis of which hinges not only on the histological data but also on a correct diagnostic approach taking into account the clinical context: in effusions associated with drugs or in those associated with connective tissue diseases, or in benign asbestosic effusions and in many other situations.
The incidence of NSP varies in the different experiences from 5% to 30% [2].
It is therefore essential, after completing the diagnostic process, to plan a careful follow-up of the patient, of varying duration in the different clinical experiences, but certainly not less than 1 year [3].
◘ Table 14.1 shows some clinical experiences relating to patients for whom the histological diagnosis of NSP was made and who were followed up over time with follow-ups varying in duration [1, 3,4,5,6,7,8].
The most important datum that emerges from this table, and from other similar experiences published in the literature, is that in most of these patients during the follow-up (of differing duration) a diagnosis of benign effusion (of different causes) is made or the diagnosis is not reached, and therefore, the effusion is defined as ‘idiopathic’. But in a variable percentage, from 5% to 12.3%, the follow-up leads to the diagnosis of malignancy (mainly mesothelioma). The final diagnosis of idiopathic pleuritis was in a percentage of between 25% and 80%.
1 When Can We Talk About Idiopathic Pleural Effusion?
Are NSP and idiopathic pleuritis the same? There is discrepancy in the literature; for example, for Reuter [9] the two diagnoses are considered the same. For some authors, the term ‘idiopathic’ is arbitrarily attributed to a pleural effusion in which repeated cytological examinations of the pleural fluid are negative, for others when the definitive diagnosis is not reached even with pleural biopsies. We believe that the term ‘idiopathic pleural effusion’ should be used only in cases in which, in addition to the negative pleural biopsies performed by medical thoracoscopy or VATS, an adequate follow-up has been undertaken and a definite diagnosis has not been reached; in reality, therefore, ‘idiopathic pleural effusion’ does not express a diagnosis, but only our diagnostic ‘limits’!
We therefore agree with Wrightson [10]: ‘the term “idiopathic pleuritis” is, by definition, a diagnosis of exclusion following exhaustive investigations and judicious follow-up, usually over a period of at least 2 years’.
A 1970 NEJM editorial [11] stated that there seemed to be an agreement to abandon this term, quoting the 1966 issue of Harrison’s textbook of internal medicine [12], which said that ‘the term ‘idiopathic pleural effusion’ is idiotic from the standpoint of the physician and pathetic from the standpoint of the patient’. After more than 50 years, however, we are still here talking about idiopathic pleural effusion!
Other definitions – such as cryptogenic effusion, which expresses the lack of knowledge of the aetiology of pleural effusion – have been completely abandoned in the recent scientific literature on pleural pathology; in fact, there is no trace of cryptogenic effusion either in the BTS guidelines [13] or in the latest version of Light’s book on Pleural Pathology [14]. The term currently used for a pleural effusion whose cause is unknown is ‘idiopathic pleural effusion’.
Therefore, faced with the diagnosis of NSP obtained from histological samples, the most important question is whether it really is a benign effusion or a ‘false negative’ [2, 15,16,17]. A false negative can derive from errors in the execution of the biopsy, not performed in sufficient depth (it is necessary to perform a ‘biopsy within the biopsy’), or from too limited a number of samples, or from the presence of widespread adhesions that do not allow for the complete exploration of the pleural cavity. In his important book on Practical Thoracoscopy, Boutin recommends taking up to 15 to 20 biopsies of parietal pleura, from widely separate regions of the pleura, including the diaphragm and the costovertebral gutter, even in the case of normal-looking pleura [18].
Some might also think that Medical Thoracoscopy has limits in diagnostics and that VATS instead improves diagnostic yield; however, there is an interesting and recent work by Froudarakis, who compared the diagnostic yield of Medical Thoracoscopy and VATS [17] and concluded that the results were the same.
A careful and personalised follow-up of the patient is important, as suggested by much authoritative literature, especially if there are reasons for suspicion: history of suspected exposure to asbestos, recurrent effusion, doubtful endoscopic picture on thoracoscopy, pleural thickening on CT, fever, weight loss or chest pain. The thoracoscopy must always be repeated in case of suspicion! If the medical thoracoscopy did not allow for the complete exploration of the pleural cavity because of the presence of adhesions, the patient must be sent to the thoracic surgeon for an exploratory thoracotomy!
2 Conclusions
-
The patient’s diagnostic workup must be complete and include pleural biopsies performed with thoracoscopy.
-
If a histological diagnosis of NSP or similar is reached, it is important to perform a careful follow-up of the patient (there is no uniformity of behaviour in the literature in the various clinical experiences regarding duration); this follow-up should therefore be personalised for each patient based on age, clinical onset, occupational exposure, possible recurrence of the pleural effusion, possible radiological doubts, etc. [8]. Clinical guidelines recommend close observation of patients with undiagnosed exudative effusions, although the follow-up duration and regime is not defined [19]. In the case of recurrent effusion, the follow-up should include, in addition to clinical checks, the repetition of imaging investigations, laboratory tests and certainly all laboratory investigations on the pleural fluid and, if the diagnosis is not reached, the repetition of the thoracoscopy too.
-
There is no justification for the unfortunately common practice of performing ex adiuvantibus therapies, e.g., anti-tuberculous therapy or cortisone therapy, without knowing what is being treated.
References
Davies HE, Nicholson JE, Rahman NM, Wilkinson EM, Davies RJ, Lee YC. Outcome of patients with nonspecific pleuritis/fibrosis on thoracoscopic pleural biopsies. Eur J Cardiothorac Surg. 2010;38(4):472–7. https://doi.org/10.1016/j.ejcts.2010.01.057. Epub 2010 Mar 12. PMID: 20219385.
Janssen J, Maldonado F, Metintas M. What is the significance of non-specific pleuritis? A trick question. Clin Respir J. 2018;12(9):2407–10. https://doi.org/10.1111/crj.12940. PMID: 30004629.
DePew ZS, Verma A, Wigle D, Mullon JJ, Nichols FC, Maldonado F. Nonspecific pleuritis: optimal duration of follow-up. Ann Thorac Surg. 2014;97(6):1867–71. https://doi.org/10.1016/j.athoracsur.2014.01.057. Epub 2014 Mar 28. PMID: 24681036.
Ferrer JS, Muñoz XG, Orriols RM, Light RW, Morell FB. Evolution of idiopathic pleural effusion: a prospective, long-term follow-up study. Chest. 1996;109(6):1508–13. https://doi.org/10.1378/chest.109.6.1508. PMID: 8769502.
Venekamp LN, Velkeniers B, Noppen M. Does ‘idiopathic pleuritis’ exist? Natural history of non-specific pleuritis diagnosed after thoracoscopy. Respiration. 2005;72(1):74–8. https://doi.org/10.1159/000083404. PMID: 15753638.
Gunluoglu G, Olcmen A, Gunluoglu MZ, Dincer I, Sayar A, Camsari G, Yilmaz V, Altin S. Long-term outcome of patients with undiagnosed pleural effusion. Arch Bronconeumol. 2015;51(12):632–6. https://doi.org/10.1016/j.arbres.2014.09.016. English, Spanish. Epub 2015 Jul 26. PMID: 26216715.
Yang Y, Wu YB, Wang Z, Wang XJ, Xu LL, Tong ZH, Shi HZ. Long-term outcome of patients with nonspecific pleurisy at medical thoracoscopy. Respir Med. 2017;124:1–5. https://doi.org/10.1016/j.rmed.2017.01.005. Epub 2017 Jan 23. PMID: 28284315.
Yu YX, Yang Y, Wu YB, Wang XJ, Xu LL, Wang Z, Wang F, Tong ZH, Shi HZ. An update of the long-term outcome of patients with nonspecific pleurisy at medical thoracoscopy. BMC Pulm Med. 2021;21(1):226. https://doi.org/10.1186/s12890-021-01596-2. PMID: 34253218; PMCID: PMC8276511.
Reuter SB, Clementsen PF, Bodtger U. Incidence of malignancy and survival in patients with idiopathic pleuritis. J Thorac Dis. 2019;11(2):386–92. https://doi.org/10.21037/jtd.2018.12.136. PMID: 30962981; PMCID: PMC6409269.
Wrightson JM, Davies HE. Outcome of patients with nonspecific pleuritis at thoracoscopy. Curr Opin Pulm Med. 2011;17(4):242–6. https://doi.org/10.1097/MCP.0b013e3283470293. PMID: 21519267.
Gaensler EA. “Idiopathic” pleural effusion. N Engl J Med. 1970;283(15):816–7. https://doi.org/10.1056/NEJM197010082831514. PMID: 5456242.
Branscomb BV, Harrison TR. Diseases of the pleura, mediastinum, and diaphragm. In: Harrison TR, et al., editors. Principles of internal medicine. 5th ed. New York, NY: McGraw-Hill Book Company; 1966. p. 946–51.
Hooper C, Lee YC, Maskell N, BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(Suppl. 2):ii4–17. https://doi.org/10.1136/thx.2010.136978. PMID: 20696692.
Light RW, Gary Lee YC, editors. Textbook of pleural diseases. 3rd ed. Boca Raton, FL: CRC Press; 2016.
Vakil E, Ost D, Vial MR, Stewart J, Sarkiss MG, Morice RC, Casal RF, Eapen GA, Grosu HB. Non-specific pleuritis in patients with active malignancy. Respirology. 2018;23(2):213–9. https://doi.org/10.1111/resp.13187. Epub 2017 Oct 12. PMID: 29024191.
Nugent K, Berdine G, Test V. What is the next step in a patient with an undiagnosed exudative pleural effusion? Am J Med Sci. 2020;360(3):211–2. https://doi.org/10.1016/j.amjms.2020.05.009. Epub 2020 May 13. PMID: 32482349.
Karpathiou G, Anevlavis S, Tiffet O, Casteillo F, Mobarki M, Mismetti V, Ntolios P, Koulelidis A, Trouillon T, Zadel N, Hathroubi S, Peoćh M, Froudarakis ME. Clinical long-term outcome of non-specific pleuritis (NSP) after surgical or medical thoracoscopy. J Thorac Dis. 2020;12(5):2096–104. https://doi.org/10.21037/jtd-19-3496. PMID: 32642113; PMCID: PMC7330408.
Boutin C, Viallat J, Aelony Y. Practical thoracoscopy. 1st ed. Berlin: Springer; 1991.
Maskell NA, Butland RJ, Pleural Diseases Group, Standards of Care Committee, British Thoracic Society. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax. 2003;58(Suppl. 2):ii8–17. https://doi.org/10.1136/thorax.58.suppl_2.ii8. PMID: 12728146; PMCID: PMC1766019.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Casalini, A.G. (2023). Idiopathic Pleural Effusion. In: Casalini, A.G. (eds) Practical Manual of Pleural Pathology. Springer, Cham. https://doi.org/10.1007/978-3-031-20312-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-20312-1_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-20311-4
Online ISBN: 978-3-031-20312-1
eBook Packages: MedicineMedicine (R0)