Abstract
Integrin-mediated interaction of cells with proteins present in the extracellular matrix and basement membranes direct many cellular processes required for both normal and pathological processes. These interactions guide angiogenesis and vessel homeostasis, both in the embryo and the adult. Mouse genetic studies have explored the contribution of individual integrin heterodimers; laminins and their integrin receptors have emerged as important regulators of these processes. This chapter discusses findings from both in vivo studies and organotypic cell culture models that provide insight into the molecular mechanisms by which the laminin-binding integrins α3β1, α6β1, α6β4, and their ligands, laminin-411 and laminin-511, regulate endothelial cell signaling, cell–cell interactions, and gene expression that contribute to the regulation of endothelial cell function in angiogenesis and vessel homeostasis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Angiogenesis is a process that is critical to tissue repair, cancer progression, as well as inflammation, and involves the sprouting of endothelial cells from the preexisting vasculature (Potente et al. 2011). Angiogenic signals, such as vascular endothelial growth factor (VEGF), activate endothelial cells leading to the proteolytic breakdown of the endothelial basement membrane, the loosing of endothelial cell–cell junctions, the dissociation of mural cells, and the sprouting of endothelial cells into surrounding extracellular matrix. Sprouting is guided by endothelial tip cells with proliferating stalk cells in tow (Potente et al. 2011). Tip cells from neighboring sprouts anastomose to create a network of new vessels that form lumens, assemble new basement membranes, and recruit mural cells (Potente et al. 2011) (Fig. 3.1). Sprouting endothelial cells can interact with the plasma proteins, fibronectin, vitronectin, or fibrinogen present in the provisional matrix or with collagens present in the interstitial matrix (Senger and Davis 2011; Eming et al. 2007). Endothelial cells can also interact with endothelial-secreted extracellular matrix (ECM) proteins, including fibronectin, and laminins (Hallmann et al. 2005; Avraamides et al. 2008; Turner et al. 2017). Members of the integrin family of adhesion receptors (Hynes 2002a) mediate these interactions that can contribute to the formation and stabilization of endothelial tubes (Hallmann et al. 2005; Turner et al. 2017; Senger and Davis 2011; Xu et al. 2020; Song et al. 2017; Avraamides et al. 2008).
3.2 Integrins in Angiogenesis
Integrins are heterodimeric transmembrane proteins that contain one α and one β subunit. Mammals have 18 α subunits and 8 β subunits that combine to form 24 α/β heterodimers (Hynes 2002a). Their extracellular domains engage ECM and basement membrane components, including fibronectin, collagens, and laminins. Integrin intracellular domains interact with the cell’s cytoskeleton and signaling networks to regulate many aspects of cell behavior including cell adhesion, migration, proliferation, survival, and invasion, as well as gene expression, which together contribute to complex biological processes, such as tissue morphogenesis and development (Danen and Sonnenberg 2003; Streuli and Akhtar 2009).
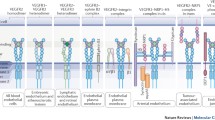
Several endothelial integrin heterodimers, including α1β1, α2β1, α3β1 α5β1, αvβ3, αvβ5, α6β1, and α6β4, are known to regulate angiogenesis, and their individual roles can be context-dependent (Avraamides et al. 2008; Senger and Davis 2011; Van Der Flier et al. 2010; Murphy et al. 2015). Much of what is known about the contribution of integrins has been gleaned from mouse genetic studies. The α5β1, αvβ3, and αvβ5 integrins all bind to the RGD motif in fibronectin (Hynes 2002a). Both the global deletion of fibronectin (Fn1-null) in mice and the global deletion of integrin α5 subunit (Itga5-null) in mice are embryonic lethal, with both mutants exhibiting defects in embryonic vascular development (Avraamides et al. 2008). The specific deletion of fibronectin alleles in endothelial cells demonstrated that the endothelial expression of fibronectin is required for retinal developmental angiogenesis (Turner et al. 2017). However, the endothelial-specific deletion of the integrin α5 subunit alleles was not sufficient to alter developmental angiogenesis; however, endothelial-specific deletion of both integrin α5 (Itga5) and αv (Itgav) subunit alleles inhibited embryonic vascular remodeling, suggesting that α5β1 and αv integrins can compensate for one another’s endothelial function during the development of the embryonic vasculature (Van Der Flier et al. 2010). Significant effort both at the bench and in the clinic has been concerned with the RGD-binding integrins, α5β1, αvβ3, and αvβ5; however, drugs targeting these integrins to suppress tumor angiogenesis have met with discouraging results (Hynes 2002b; Desgrosellier and Cheresh 2010; Paolillo et al. 2016). Additionally, the endothelial-specific deletion of the prominent RGD-binding integrins, as well as fibronectin, failed to inhibit tumor angiogenesis (Murphy et al. 2015). Thus, it seems that other integrins and matrix components contribute to the regulation of angiogenesis.
The α1β1 and α2β1 integrins bind to both interstitial collagens, as well as collagens present in basement membranes (Hynes 2002a). An endothelial-specific deletion of α1 subunit gene (Itga1) has not been generated. However, mice with null mutations in the α1 integrin subunit gene, Itga1-null animals, are viable and fertile (Gardner et al. 1996), indicating that α1β1 is not required for developmental angiogenesis. However, α1β1 can regulate angiogenesis in the adult. Tumor angiogenesis was suppressed in Itga1-null mice, and this was correlated with the increased plasma levels of the metalloproteinases MMP-7 and MMP-9 and defects in endothelial proliferation (Pozzi et al. 2000; Pozzi et al. 2002). However, it is unclear whether endothelial cells are responsible for increased levels of MMPs, as α1β1 is expressed in multiple cell types in addition to microvascular endothelial cells (Belkin et al. 1990; Glukhova et al. 1993; Gullberg et al. 1992; Defilippi et al. 1991; Glukhova et al. 1995; Loeser et al. 1995; Sobel et al. 1998; Senger et al. 1997).
The α2β1 integrin is expressed on microvascular endothelial cells and has been observed on sprouting tips of angiogenic vessels, suggesting a role for α2β1 in angiogenesis (Senger et al. 1997; Enenstein and Kramer 1994). An endothelial-specific deletion of the α2 subunit gene (Itga2) has not been generated. Mice lacking both α2 alleles (Itga2-null mice) have been made. These mice develop normally and are fertile (Chen et al. 2002; Holtkotter et al. 2002); thus, similar to α1β1, α2β1 is not required for developmental angiogenesis. However, adult Itga2-null mice exhibited enhanced wound and tumor angiogenesis, suggesting anti-angiogenic role for α2β1 (Zweers et al. 2007; Grenache et al. 2007; Zhang et al. 2008). Surprisingly, the angiogenic response in Itga2-null mice was found to be tumor-dependent. An enhanced angiogenic response was triggered by B16F10 melanoma cells, but not by Lewis lung carcinoma cells (Zhang et al. 2008). VEGFR1 is the receptor for the VEGF family member, placental growth factor (PlGF) and is upregulated in endothelial cells from Itga2-null mice. B16F10 melanoma cells secrete much higher levels of PlGF compared to Lewis lung carcinoma cells, which explains the differences in the angiogenic response and underlines the importance of crosstalk between tumor and endothelial cells, as well as the tumor microenvironment in the angiogenic response (Zhang et al. 2008). Interestingly, increased expression of metalloproteinases, including MMP-9, was observed in dermal wound tissue from Itga2-null mice compared to control animals. In vitro studies suggested the enhanced protease expression was derived from Itga2-null keratinocytes, pointing to this as a potential mechanism for enhanced wound angiogenesis (Grenache et al. 2007). Others have shown the importance of integrin-regulated secretion of paracrine factors from keratinocytes in the crosstalk between keratinocytes and endothelial cells in the regulation of angiogenesis during dermal wound healing (Mitchell et al. 2009). Nonetheless, it is unclear how increases in MMP expression inhibit angiogenesis in the case of Itga1-null mice and enhance angiogenesis in the case of Itga2-null mice. Possibly the cellular source and localization of increased MMP expression determine the effect on angiogenesis. It is notable that these two collagen receptors have opposing roles in the regulation of angiogenesis, raising the question of whether they balance one another’s function, or whether α1β1 or α2β1 plays a dominant role. Analysis of animals lacking alleles for both the integrin α1 and integrin α2 subunits demonstrated that both wound and tumor angiogenesis were inhibited, indicating that the Itga1-null phenotype is dominant (Ghatak et al. 2016). This was further supported by explant aortic ring angiogenesis assays, which showed that sprouting was inhibited in explants from doubly null mice (Ghatak et al. 2016). Although mostly discussed in terms of their ability to bind to collagen, α1β1 or α2β1 can also bind to a subset of laminin isoforms, which will be discussed in more detail below.
The remainder of this chapter focuses on the contribution of endothelial laminins and their integrin receptors in the regulation of normal and pathological angiogenesis, as well as in vessel homeostasis. We discuss results from both mouse genetic models as well as in vitro angiogenesis assays.
3.3 Endothelial Laminins
Laminins are heterotrimeric proteins, each containing an α, β, and γ chain, are assembled intracellularly and are secreted as heterotrimers (Yurchenco et al. 1997). Laminins are important contributors to the formation and structure of basement membranes, that are present in epithelial, endothelial, muscle, and neural tissues (Colognato and Yurchenco 2000; Yurchenco 2011). In mammals, there are five distinct α chains, four β chains, and three γ chains, that can associate to form at least 15 laminin isoforms, with distinct tissue distribution (Yurchenco 2011). Endothelial cells express two laminin isoforms: laminin-411, which contains the α4, β1, and γ1 chains, and laminin-511, which contains the α5, β1, and γ1 chains. It is important to note that laminin-411 and laminin-511 were previously named laminin-8 and laminin-10 respectively (Aumailley et al. 2005).
Since laminin-411 and laminin-511 contain the same β and γ chains, their endothelial expression in the vasculature can be monitored by immunostaining with antibodies that recognize the laminin α4 chain or the laminin α5 chain, respectively. In mice, the laminin α4 chain is expressed ubiquitously by endothelial cells and is observed in the vasculature beginning at embryonic day 8.8 (Frieser et al. 1997; Iivanainen et al. 1997; Miner et al. 1997). The endothelial expression of the α5 chain of laminin is first observed a few weeks after birth and is detected primarily on capillaries and venules (Patton et al. 1997; Sorokin et al. 1997). Because laminin-411 and laminin-511 share the same β and γ chains, the effects of deletion of laminin-411 and laminin-511 on endothelial function can be examined by deletion of the α4 (Lama4) or α5 (Lama5) chain alleles, respectively. To date, an endothelial-specific deletion of Lama4 gene has not been generated. Analysis of Lama4-null mice revealed significant hemorrhaging both during embryonic and neonatal development. This was accompanied by defects in the assembly and structure of endothelial basement membranes. Collagen IV and nidogen are also key components of basement membranes; their expression was lacking in endothelial basement membranes of developing Lama4-null mice. Electron microscopy confirmed the presence of defective basement membranes associated with capillary endothelial cells. Together these data suggest that laminin-411 is required for the proper assembly of the endothelial basement membrane that in turn functions to stabilize the embryonic vasculature (Thyboll et al. 2002). Interesting, Lama4-null mice recover and develop into adulthood due to the endothelial expression of laminin-511, which becomes more widely expressed by vascular endothelial cells in mutant mice; thus, laminin-511 can compensate for the loss of laminin-411 (Thyboll et al. 2002; Zhou et al. 2004). Mice null for the laminin α5 chain, die during embryogenesis, before laminin-511 is expressed by endothelial cells (Miner et al. 1998). However, an endothelial-specific deletion of the laminin α5 chain alleles (Lama5) has been generated. The vasculature in these mice appears to develop normally with no significant defects in vasculature at homeostasis (Song et al. 2013).
3.4 Endothelial Laminin-Binding Integrins
Multiple integrin heterodimers bind laminin isoforms (Hynes 2002a). Endothelial cells express the α3β1, α6β1, and α6β4 laminin-binding integrins, as do many epithelial cells (Avraamides et al. 2008; Hynes 2002a). The α chains of laminin heterotrimers contain the binding sites for integrins, and thus, the identity of the α chain present in a particular laminin isoform determines its integrin binding partners (Yurchenco 2011). Several approaches have been employed to determine whether α3β1, α6β1, and α6β4 can bind to laminin-411 and/or laminin-511. Purified laminin-411 or laminin-511 has been used in cell adhesion assays together with antibodies to inhibit the function of specific integrin subunits. Endothelial cell adhesion to laminin-411 was inhibited by function-blocking antibodies to either the α6 or β1 integrin subunit, demonstrating that laminin-411 is a ligand for α6β1 (Kortesmaa et al. 2000). K562 cells do not express any laminin receptors. The ability of K562 cells expressing individual recombinant laminin-binding integrins demonstrated that the α3β1, α6β1, and α6β4 integrins can each function as adhesion receptors for laminin-411 (Kortesmaa et al. 2000; Fujiwara et al. 2001). Endothelial cell adhesion to laminin-511 was inhibited using function-blocking antibodies to integrin α3β1 (Doi et al. 2002). The ability of α6β4 to bind laminin-511 was demonstrated using the murine β1 Itgb1-null GD25 cells that express only one laminin-binding integrin, α6β4 (Kikkawa et al. 2004). The ability of α6β1 to bind laminin-511 was demonstrated using in vitro binding assays (Nishiuchi et al. 2006), as well as in cell adhesion assay with antibodies to block the function of α6 integrins. Although α1β1 and α2β1 are usually considered collagen-binding integrins, they have also been shown to bind to a subset of laminin isoforms (Humphries et al. 2006, Yurchenco 2011 41). The α2β1 was shown to function in cell adhesion to laminin-411, but not laminin-511 (Stenzel et al. 2011). The ability of α1β1 to bind to these laminin isoforms has not been tested. It is also important to note that the αvβ3 integrin was shown to bind a recombinant fragment of the laminin α4 chain (Gonzalez et al. 2002). The significance of the interaction between αvβ3 and the α4 chain of laminin-411 to endothelial cell function is not yet fully appreciated. The laminin α5 chain contains an RGD sequence and the α5β1, αvβ3, αvβ1 and αvβ5 were shown to bind recombinant fragments of the α5 chain containing the motif (Hallmann et al. 2005; Sasaki and Timpl 2001). Whether these interactions contribute to angiogenesis or vessel homeostasis is yet to be appreciated; however, adhesion to laminin-511 mediated by β1 and β3 integrins was implicated in maintaining the surface expression of the adherens junction protein, VE-cadherin (Song et al. 2017), as discussed below.
3.5 Laminins in Pathological Angiogenesis
As mentioned above, embryonic vessels that lack the expression of laminin-411 are leaky, suggesting that laminin-411 functions to promote vessel stability, at least in the embryo (Thyboll et al. 2002). To determine the role of laminin-411 in new vessel formation in adult animals, the effects of inhibiting the expression of laminin-411 were assessed in cornea angiogenesis assays. Compared with wild-type littermates, Lama4-null mice exhibited enhanced endothelial sprouting in response to FGF2 (Thyboll et al. 2002), suggesting that laminin-411 may function as a negative regulator of angiogenesis. Interestingly, in wild-type corneas, a well-organized vasculature developed in response to FGF2, but most of these vessels had regressed by 42 days (Zhou et al. 2004). Vessels that formed in Lama4-null corneas were disorganized and dilated, but surprisingly at day 42, these vessels had undergone dramatic remodeling into what appeared as a normal vasculature (Zhou et al. 2004). This correlated with the upregulation of laminin-511 expression, suggesting that laminin-511 promotes vessel maturation. To examine the contribution of laminin-411 to tumor angiogenesis, control mice and mice deficient in the α4 chain of laminin-411 were challenged with subcutaneously implanted Lewis lung carcinoma cells or B16-F10 melanoma cells (Zhou et al. 2004). Tumor growth and angiogenesis were enhanced in mutant compared to control in response to both tumor types. Additionally, greater B16-F10 lung metastases were observed in Lama4-null compared to control mice; however, the mechanisms involved have yet to be identified. Although the tumor vasculature in control mice mostly expressed laminin-411, in Lama4-null mice the tumor vasculature exhibited strong expression of laminin-511, which presumably compensated for the loss of laminin-411 (Zhou et al. 2004).
In post-natal retinal developmental angiogenesis, the expression of laminin α4 chain RNA localized to extending endothelial sprouts, suggesting a positive role for laminin-411 in regulating endothelial sprouting. Lama4-null mice, however, showed enhanced endothelial sprouting, suggesting that the expression of laminin-411 puts the breaks on sprouting (Stenzel et al. 2011). This is consistent with the studies discussed above, which reported enhanced tumor angiogenesis in Lama4-null mice.
As mentioned in the Introduction, sprouting is guided by endothelial tip cells, which send out filopodia that direct the growing sprout. VEGF-VEGFR2 signaling in tip cells promotes the secretion of the Notch ligand Dll4 to limit the generation of additional tip cells nearby (Potente et al. 2011). Dll4-Notch signaling is critical to the regulation of angiogenesis; the loss of Dll4/Notch signaling is known to lead to hyper-sprouting (Eilken and Adams 2010; Phng and Gerhardt 2009). Thus, the loss of laminin-α4 expression phenocopied the defects observed when Dll4/Notch signaling is inhibited. Indeed, Notch signaling was inhibited in Lama4-null retinas (Stenzel et al. 2011). Additionally, in vitro adhesion of endothelial cells to laminin-411, but not laminin-511, significantly increased the expression of Dll4 RNA (Stenzel et al. 2011). Antibody inhibition studies and siRNA knockdown experiments indicated that the α2β1 and α6β1 integrins contributed to this regulation (Stenzel et al. 2011). Taken together, these results suggest that α2β1- and α6β1-dependent endothelial cell adhesion to laminin-411 regulates the expression of Dll4 to promote properly regulated endothelial sprouting (Stenzel et al. 2011).
An endothelial-specific deletion of the laminin α5 chain is available (Song et al. 2013). No developmental angiogenesis defects were observed, as expected, since the endothelial expression of laminin α5 chain occurs postnatally. The contribution of laminin-511 to adult or pathological angiogenesis has yet to be explored. However, loss of endothelial laminin-511 decreases endothelial barrier function (Song et al. 2017). Such a phenotype might be permissive to angiogenesis. The contribution of laminin-511 to the regulation of endothelial barrier function is discussed below.
3.6 Laminin-Binding Integrins in Pathological Angiogenesis
Mouse genetic models were established to characterize the function of the laminin-binding integrins α3β1, α6β1, and α6β4 during development. Mice null for the α3 (Itga3), α6 (Itga6), or β4 subunit gene (Itgb4) died soon after birth with no defects in developmental angiogenesis reported (Kreidberg et al. 1996; Georges-Labouesse et al. 1996; Dowling et al. 1996; da Silva et al. 2010; Germain et al. 2010; Bouvard et al. 2012). To study the role of α3β1 and α6 integrins during angiogenesis in the adult, several labs used conditional endothelial deletion of either the Itga3, Itga6, or Itgb4 gene (Germain et al. 2010; da Silva et al. 2010; Bouvard et al. 2012; Bouvard et al. 2014; Seano et al. 2014; Welser-Alves et al. 2013). To conditionally delete integrin α3 subunit alleles in endothelial cells, Cre recombinase was expressed from the Tie-1 promoter (da Silva et al. 2010). This mutant will be referred as ec-Itga3-null. The α3β1 integrin was not expressed by angiogenic vessels in mutant mice; however, α3β1 expression was maintained in quiescent endothelial cells in dermal vasculature of ec-Itga3-null mice at levels similar to littermate controls (da Silva et al. 2010). Ec-Itga3-null mice exhibited increased angiogenesis in three distinct models. Tumor angiogenesis was analyzed in response to the subcutaneous injection of either B16F0 melanoma cells or CMT19T lung carcinoma cells (da Silva et al. 2010). Both tumor cell types showed enhanced angiogenesis in mutant compared to control mice. Hypoxia-induced retinal angiogenesis was also greater in ec-Iga3-null mice compared to control, as was endothelial sprouting in aortic ring explant angiogenesis assays. These results supported a role for α3β1 as a general negative regulator of angiogenesis in the adult. The mechanism reported for the ec-Itga3-null phenotype was surprisingly elaborate. The α3β1 integrin was shown to be a positive regulator of the endothelial expression of VEGF, which led to the inhibition of the VEGFR2 RNA expression (da Silva et al. 2010). Thus, endothelial cells from mutant mice express higher levels of VEGFR2, explaining their increased angiogenic activity (da Silva et al. 2010).
There are conflicting reports as to whether and how α6 integrins (α6β1 and α6β4) regulate adult angiogenesis. The phenotype of targeting the endothelial expression of α6 integrins is dependent upon the promoter used to express Cre recombinase in endothelial cells (Germain et al. 2010; Bouvard et al. 2014). Like the Tie-1-dependent deletion of the integrin α3 subunit genes, the Tie1-Cre-dependent deletion of the integrin α6 subunit alleles (Itga6), referred to as ec1-Itga6-null, resulted in enhanced tumor angiogenesis (Germain et al. 2010). Subcutaneous injection of either B16F0 melanoma cells or Lewis lung carcinoma cells resulted in larger more vascularized tumors in ec1-Itga6-null, compared to those in littermate controls, suggesting that α6 integrins, like α3β1, are negative regulators of angiogenesis. The authors note that this is consistent with the decreased expression of α6 integrins in angiogenic vessels associated with invasive ductal carcinoma as compared to normal breast tissue (Germain et al. 2010). The observed enhanced angiogenesis was not due to the upregulation of other integrins reported to promote angiogenesis, such as the α1β1, α3β1, α5β1, and αvβ3 integrins (Germain et al. 2010). Endothelial cells from ec1-Itga6-null mice displayed increased surface expression of VEGFR-2 and downstream signaling, which explain the enhanced angiogenesis observed ec1-Itgα6-null mice (Germain et al. 2010).
Tie-2 (Tek) driven Cre recombinase was also used to examine the effect of the endothelial deletion of α6 integrins (Bouvard et al. 2012, 2014). This mutant is referred to as ec2-Itgα6-null. The angiogenic phenotype of this mutant was first examined following ischemic injury to mouse hind limb (Bouvard et al. 2012). A significant reduction in angiogenesis was observed in mutant animals. It is important to note that in addition to endothelial cells, the Tie-2-lineage includes pericytes and subsets of endothelial progenitors and macrophages (Kisanuki et al. 2001). The decrease in angiogenesis observed in ec2-Itgα6-null was accompanied by a reduction in the recruitment of bone marrow-derived endothelial progenitor cells and Tie-2 expressing macrophages to the site of injury. Thus, the phenotype of ec2-Itgα6-null could be due to the loss of α6 integrins in multiple cell types (Bouvard et al. 2012). Notably, the loss of expression of α6 integrins did not affect the surface expression of other integrin heterodimers, or surprisingly, the expression of VEGFR2.
Tumor angiogenesis was also examined in ec2-Itgα6-null mice. Angiogenesis was inhibited following the subcutaneous injection of B16F10 melanoma cells (Bouvard et al. 2014). This was associated with a decreased recruitment of Tie-2 expressing macrophages to the site of tumor growth. Macrophages significantly contribute to the tumor microenvironment; thus, their loss could affect the angiogenic response (Coussens and Werb 2002). Notably, pericyte coverage of tumor vessels was similar in wild-type and mutant mice (Bouvard et al. 2014). Although the reduction in macrophage recruitment to the site of tumor formation in ec2-Itga6-null mice, may explain the difference in the angiogenic responses in ec2-Itga6-null versus ec1-Itga6-null mice, it is difficult to understand how the loss of α6 integrins affects VEGFR2 expression in one case and not the other.
A pro-angiogenic role for α6β1 is supported by the finding that the α6β1 integrin localizes invasive structures referred to as podosomes (Seano et al. 2014) These structures are integrin-extracellular matrix contact sites that aid in targeting proteases to sites of invasion (Linder and Aepfelbacher 2003; Gimona et al. 2008). Antibodies or RNAi approaches to inhibit the activity or expression of α6 integrins suppressed VEGF-induced formation of podosomes and the recruitment of the metalloproteinase MT1-MMP (Seano et al. 2014). The importance of α6β1 in the formation of these structures was further supported by the finding that podosome formation was impaired in aortic explants from ec2-Itga6- null mice, compared to littermate controls (Seano et al. 2014). A tight balance in integrin-laminin interactions appears critical to the formation of these structures in endothelial cells, as more VEGF-induced podosomes were observed in explants from Lama4-null mice compared to control (Seano et al. 2014).
As noted above, the integrin α6 subunit heterodimerizes with either the β1 or β4 subunit. Thus, the effects of deletion of the α6 subunit could be due to the loss of either α6β1 or α6β4. Since the β1 subunit dimerizes with multiple α subunits, distinct roles for endothelial α6β1 and α6β4 have been inferred from the effects of targeted endothelial deletion of the β4 integrin subunit gene (Itgb4) together with information on the endothelial expression of α6β4 in different vascular beds.
The β4 integrin subunit has a very large intracellular domain compared with other β subunit intracellular domains. The membrane-proximal region is known to connect the α6β4 integrin to the intermediate filament cytoskeletal system (te Molder et al. 2021; Mercurio et al. 2001): keratin intermediate filaments in the case of epithelial cells and vimentin intermediate filaments in endothelial cells (te Molder et al. 2021; Homan et al. 1998, 2002). Mice null for the β4 integrin subunit gene (Itgb4) die after birth due to extensive defects in epithelial tissues thought to be mostly due to the loss of a transmembrane connection between the basement membrane and the keratin intermediate filament system (Dowling et al. 1996; Van Der Neut et al. 1996). The membrane distal region is involved in the ability of α6β4 to initiate intracellular signaling events to control cell behavior (te Molder et al. 2021; Mercurio et al. 2001). The global deletion of only the signaling portion of the β4 subunit cytoplasmic domain was reported to inhibit angiogenesis in several types of assays (Nikolopoulos et al. 2004). The loss of the signaling portion of the β4 cytoplasmic domain inhibited hypoxia-induced retinal angiogenesis. The subcutaneous injection of a number of different tumor cell lines, including B16F0 melanoma cells and Lewis lung carcinoma cells resulted in significantly decreased tumor angiogenesis in mutant mice compared to wild-type littermate controls (Nikolopoulos et al. 2004). The loss of the signaling portion of the β4 cytoplasmic domain correlated with reduced nuclear localization of ERK and NFκB, which are known to become activated downstream of α6β4 and to promote endothelial sprouting and invasion (Nikolopoulos et al. 2004).
As indicated above, a conditional endothelial deletion mutant has been generated using Tie-2-dependent expression of Cre recombinase (Welser-Alves et al. 2013). This mutant is referred to as ec-Itgb4-null. No defects in vascular development were observed and analysis of endothelial expression of α6β4 was reported to be restricted to arterioles (Welser-Alves et al. 2013). However, it is important to note that others reported the expression of α6β4 in angiogenic vessels associated with tumors and dermal wound repair, although the α6β4 expression appeared dynamic in angiogenic endothelial cells in some contexts (Nikolopoulos et al. 2004; Desai et al. 2013). Additionally, single-cell RNA sequencing of vascular cells from the murine lung, found β4 mRNA widely expressed in endothelial cells in veins, venules and with higher expression in capillary endothelial cells (He et al. 2018), consistent with the ability of α6β4 to contribute to angiogenesis. Also of note, the endothelial expression pattern of α6β4 is similar to the endothelial expression of the α5 chain of laminin (Patton et al. 1997; Sorokin et al. 1997). The same single-cell RNA sequencing study indicated that arterial endothelial cells of the brain vasculature had relatively higher levels of β4 mRNA expression (He et al. 2018). The function of α6β4 in brain endothelial cells was analyzed in a chronic hypoxia model (Welser-Alves et al. 2013). Although wild-type and ec-Itgb4-null mice did not exhibit a significant difference in the early angiogenic response to hypoxia, the number of arteriolar size vessels was significantly lower in ec-Itgb4-null mice, implicating α6β4 in regulating arteriolar remodeling. Previous work indicated a role for TGF-β signaling in this process (Seki et al. 2003). Consistent with this previous work, the expression of the type-I TGF-β receptor, activin-like kinase 1 (ALK1) and the activation of downstream signaling protein was inhibited in ec-Itgb4-null mice, providing a mechanism for defective arterial remodeling in mutant mice (Welser-Alves et al. 2013). Nonetheless, with reports of α6β4 expression in venules, capillaries, and angiogenic vessels, it will be interesting to examine the effect of an endothelial-specific deletion of Itgb4 employing other models of adult angiogenesis.
3.7 Laminin-511 and α6β4 in the Regulation of Endothelial Barrier Function
Previous studies demonstrated that the endothelial expression of α6β4 integrin was upregulated during neuroinflammation (Milner and Campbell 2006). To understand the implication of the upregulation, the effect of the endothelial deletion of β4 was examined using ec-Itgb4-null mice in experimental autoimmune encephalomyelitis (EAE), which is a mouse model for multiple sclerosis (Welser et al. 2017). Although the timing of the onset of disease was similar in mutant and wild-type mice, the clinical outcome was worse in ec-Itgb4-null mice, which exhibited increased leukocyte infiltration and was accompanied by the loss of the tight junction proteins claudin-5 and ZO-1 from cell–cell junctions (Welser et al. 2017). These studies implicate the endothelial expression of α6β4 in the protection of the blood–brain barrier by regulating the integrity of cell–cell junctions (Welser et al. 2017).
Leucocyte extravasation from the vasculature occurs predominately at postcapillary venule sites with both laminin-411 and laminin-511 expression (Hallmann et al. 2005). In the EAE model of neuroinflammation, T lymphocytes were found to transmigrate at areas of the endothelial basement membrane that contained laminin-411, but low levels of laminin-511 (Sixt et al. 2001; Wu et al. 2009). In Lama4-null mice, the expression of laminin-511 is ubiquitously present in endothelial basement membranes. In the EAE model, Lama4-null mice exhibited reduced T-cell extravasation across the blood–brain barrier and decreased disease severity, suggesting that laminin-511 promotes endothelial barrier function (Wu et al. 2009 38). Later studies demonstrated that laminin-511 regulates endothelial barrier function by stabilizing the localization of VE-cadherin at endothelial cell–cell junctions, known as adherens junctions, to limit leukocyte extravasation (Song et al. 2017). Endothelial adhesion to laminin-511 by β1 and β3 integrins regulates the localization of VE-cadherin by promoting the activation of the small GTPase protein RhoA (Song et al. 2017). Taken together these results suggest that multiple laminin-511-binding integrins may cooperate to promote the blood–brain barrier in the brain, with β1 and β3 integrins functioning to stabilize adherens junctions and α6β4 stabilizing tight junctions. It will be interesting to know whether similar interactions function to limit inflammation in other vascular beds.
3.8 Dissecting the Contribution of Laminin-Binding Integrins to Processes Involved in Angiogenesis Using In vitro Angiogenesis Assays
There are several in vitro angiogenesis assays that have been used to identify molecular mechanisms involved in regulating the morphogenesis of endothelial cells into endothelial cords and tubes (Simons et al. 2015). Two organotypic co-culture angiogenesis assays have been particularly useful. These are the “bead sprout” and the “planar co-culture” assays (Nakatsu et al. 2003; Nakatsu and Hughes 2008; Bishop et al. 1999; Donovan et al. 2001; Bajaj et al. 2012; Li et al. 2018). In the bead sprout assay, endothelial cells, usually human umbilical vein endothelial cells, are adhered to gelatin-coated beads and embedded in a fibrin gel (Nakatsu et al. 2003; Nakatsu and Hughes 2008). Endothelial sprouting from beads can easily be observed microscopically by phase contrast or by confocal following immunostaining (Fig. 3.2). Fibrin is present in the provisional matrix of tumors and wounds and provides an adhesive ligand for migration through interactions with RGD-binding integrins (Eming et al. 2007). In the planar co-culture assay, endothelial cells are plated at low density onto dermal fibroblasts, which have grown to confluence (Bishop et al. 1999; Donovan et al. 2001; Bajaj et al. 2012). Endothelial morphogenesis then occurs in the dense fibroblast-secreted ECM, containing fibronectin and fibrillar collagen, like what is observed in wound and tumor ECM (Eming et al. 2007) (Fig. 3.3).
Endothelial cells secrete collagen IV (Col IV) and laminin-411 and laminin-511 on their basal surfaces, as they form tubes in both co-culture assays (Fig. 3.4 and (Xu et al. 2020)). Nonetheless, without the demonstration that other components of basement membrane are present and without ultrastructure analysis, it is unclear whether a basement membrane is fully assembled in these assays. However, the expression of these laminins in co-culture allowed for the examination of their contribution to endothelial morphogenesis. RNAi-dependent depletion of either the α4 or α5 chains of laminin-411 or laminin-511 inhibited endothelial sprouting and the formation of endothelial tubes in both the bead-sprout and planar co-culture assays, respectively (Xu et al. 2020).
Laminin-411 and laminin-511 are deposited by growing sprouts in organotypic cultures. (a) Confocal images of sprouting endothelial cells stained in green for collagen IV (COLIV), laminin β1 and γ1 chains (LM 111), laminin α4 chain (LM-α4) and laminin α5 chain (LM-α5). CD31 staining is shown in red. Scale bar, 100 μm. (b) High magnification confocal images of lumenized sprouts and basement membrane components expressed on the endothelial basal surface. Main images show xy sections, with xz and yz shown below. Nuclei are stained with DAPI. Scale bar, 6 μm (Xu et al. 2020)
As discussed above, α6β1 and α6β4 bind to both laminin-411 and laminin-511. The same organotypic angiogenesis assays were used to explore the contribution of α6 integrins to endothelial morphogenesis. RNAi-dependent depletion of α6 integrins inhibited both endothelial sprouting and tube formation (Xu et al. 2020). The surface expression of the α2β1, α5β1 or αvβ3 integrins, previously shown to regulate angiogenesis, was not altered by depletion of α6 integrins. Notably, the expression of α3β1 was significantly increased (Xu et al. 2020), which is interesting as the endothelial-specific depletion of the α3 subunit gene promoted angiogenesis (da Silva et al. 2010). However, depletion of α3β1 did not enhance endothelial sprouting in our co-culture assays, as discussed further below (Xu and Laflamme 2022).
The bead-sprout assay allows the easy isolation of endothelial cells for the analysis of gene expression, as the layer of fibroblasts is easily removed (Li et al. 2018). Several genes have been associated with endothelial sprouting and angiogenesis, including VEGFR2, CXCR4, ANGPT2, DLL4, PDGFB, NRP1, JAG1, and MMP14 (MT1-MMP) (de Smet et al. 2009; del Toro et al. 2010; Strasser et al. 2010). To determine whether endothelial α6 integrins or their laminin substrates regulate the expression of any of these genes in the bead-sprout assay, RNAi technology was employed to deplete endothelial cells of either α6 integrins, laminin-411 or laminin-511 and changes in gene expression were analyzed by qPCR. Defects in endothelial morphogenesis correlated with significant decreases in the expression of the chemokine receptor (CXCR4) and angiopoietin-2 (ANGPT2), as well as α5 chain of laminin-511, LAMA5 (Xu et al. 2020). A similar decrease in the expression of chemokine CXCR4 receptor and ANGPT2 was observed when endothelial cells were depleted of the α5 chain of laminin-511, suggesting that the interaction of α6 integrins with laminin-511 significantly contributes to the regulation of these pro-angiogenic genes (Xu et al. 2020). Importantly, the expression of recombinant CXCR4 in α6-depleted endothelial cells partially rescued endothelial tube formation (Xu et al. 2020). It will be interesting to test whether the expression of recombinant ANGPT2 alone or in combination with recombinant CXCR4 is sufficient to restore endothelial morphogenesis in the absence of α6 integrins and whether α6 integrins regulate the expression of these pro-angiogenic genes in vivo. Notably, no overlap was observed in the genes regulated by α6 integrins and laminin-411. Of note, Dll4 was upregulated in endothelial cells depleted of the α4 laminin chain, but this increase did not reach statistical significance (Xu et al. 2020).
To distinguish the contribution of α6β4 in organotypic co-culture assays, RNAi was employed to inhibit the expression of the β4 subunit (Xu and Laflamme 2022). The depletion of the α6β4 integrin also inhibited endothelial sprouting. Interestingly, this was accompanied by a decrease in the expression of ANGPT2 mRNA. It is unclear whether α6β4 acts alone or collaborates with α6β1 in this regulation. The depletion of α6β4, however, did not alter the expression of CXCR4, implicating α6β1 in this regulation (Xu and Laflamme 2022).
As discussed above, in vivo studies implicated the α3β1 integrin as a negative regulator of angiogenesis (da Silva et al. 2010). Also, the expression of the α3β1 integrin was enhanced in endothelial cells depleted of α6 integrins in bead sprout assays (Xu et al. 2020), suggesting a potential inhibitory role in organotypic assays, as well. Surprisingly, RNAi-dependent depletion of α3β1 also inhibited endothelial sprouting in co-culture angiogenesis assays. However, this inhibition was not accompanied by the downregulation of CXCR4, ANGPT2, or LAMA5 expression, suggesting that α3β1 regulates sprouting by distinct mechanisms. This is consistent with the inability of α6 integrins to compensate for the loss of α3β1 and vice versa. Notably, the expression of neuropilin-1 (NRP1) mRNA was most significantly decreased in α3-depleted cells (Xu and Laflamme 2022). NRP1 is enriched in endothelial tip cells and functions as a co-receptor for VEGFR2 receptor signaling (Kofler and Simons 2015). Thus, the downregulation of NRP1 expression may be responsible for the inhibition of sprouting observed when endothelial cells are depleted of α3β1. A comparison of gene expression regulated by the α3β1, α6β1, and α6β4 integrins suggested that these laminin-binding integrins regulate distinct but overlapping sets of genes previously identified to be important in angiogenesis (Xu and Laflamme 2022).
The Tie-1-dependent deletion of α3 resulted in increased mRNA expression of VEGFR2 in α3β1-deficient endothelial cells (da Silva et al. 2010). However, depletion of α3β1 in bead sprout assays did not result in increased VEGFR2 expression. In fact, RNAi-dependent depletion of the α3 subunit resulted in a small but significant decrease in VEGFR2 with two of the three siRNA targeting sequences (Xu and Laflamme 2022). The differences in the effects of depleting the endothelial expression of α3β1 in vivo versus in cell culture experiments may be due to the timing when α3β1 expression is lost. As mentioned above, expression of α3β1 in ec-Itga3-null mice appears to occur at some point after the initial response to angiogenic stimuli (da Silva et al. 2010); in contrast, in bead sprout assays, the expression of α3β1 was already inhibited before the onset of the assay. Thus, this in vivo model may not be ideal to examine the role of α3β1 during the initial steps of angiogenesis. Perhaps the use of a promoter that is active in quiescent endothelial cells to drive the inducible expression of Cre recombinase, such as the VE-Cadherin promoter may be better for this purpose.
The stable interaction of endothelial cells with their underlying basement membrane promotes vessel stability. As mentioned above, the endothelial expression of laminin-511 promotes endothelial barrier function (Song et al. 2017). Since α6 integrins, but not α3β1, were shown to regulate the endothelial expression of laminin-511, the effect of deleting α6 integrins or α3β1 was examined in the planar co-culture assay ((Xu et al. 2020) and Xu and LaFlamme, unpublished data). The inducible expression of either an α6, α3, or non-targeting shRNA was used to inhibit integrin expression after the formation of endothelial tubes and to analyze the effect on the maintenance of endothelial tube stability. Indeed, depletion of α6 integrins after the establishment of endothelial tubes resulted in the loss of detectable laminin-511 expression, as well as normal tube morphology (Fig. 3.5). Notably, the expression of laminin-411 was maintained as expected (Fig. 3.5 and (Xu et al. 2020)). The depletion of α3β1 integrins did not affect laminin-511 expression or tube morphology (Fig. 3.5). This suggests an important role for the association of α6 integrins and laminin-511 in stabilizing endothelial structures. Unfortunately, the role of α6β4 in the process was not examined in a similar assay, as the endothelial expression of both α6β4 and laminin-511 have been associated with promoting endothelial barrier function in vivo (Welser et al. 2017; Wu et al. 2009).
Laminin-511 expression and tube morphology are regulated by α6 integrins and not by the α3β1 integrin. Planar co-cultures were set up with endothelial cells transduced with lentiviral vectors to direct the doxycycline-inducible expression of α6 (a), α3 (b) or non-targeting (NT) shRNA, together with fluorescent protein as a readout of shRNA expression. (a) After the formation of endothelial tubes, cultures were treated with doxycycline for up to 8 days and imaged. Induction of α6-targeting led to the loss of laminin-511 expression and changes in endothelial tube morphology (Xu et al. 2020). (b) This did not occur following the induction of non-targeting or α3-targeting shRNAs. (c) Changes in tube morphology are even more pronounced 12 days after induction of α6 shRNA (Xu et al. 2020)
Laminin-binding integrins likely regulate angiogenesis by multiple mechanisms, as they are known to activate pathways to promote migration and invasion not only in endothelial cells, but in other cellular contexts. Additionally, the laminin-binding integrins, α3β1, α6β1, and α6β4 form complexes with the tetraspanin CD151 (Sterk et al. 2002). Cd151-null mice exhibit decreased angiogenesis in multiple in vivo and ex vivo models, including tumor angiogenesis and aortic ring explant assays (Takeda et al. 2007). Additionally, integrin-laminin-dependent activation of multiple signaling pathways was decreased in Cd151-null endothelial cells that adhered to laminin (Takeda et al. 2007). In the case of α3β1, CD151 forms a complex with the membrane-anchored matrix metalloproteinase MT1-MMP (MMP14) and α3β1 to promote appropriate proteolysis, and the loss of CD151 results in a dramatic loss of α3β1/MT1-MMP association (Yanez-MO et al. 2008), suggesting an additional mechanism by which α3β1 can contribute to angiogenesis.
3.9 Concluding Remarks
Mouse genetic models have provided important insights into mechanisms that both positively and negatively regulate angiogenesis and vessel stability. These studies have highlighted the importance of individual laminins and laminin-binding integrins in limiting angiogenesis and inflammation. Organotypic cell culture models have provided additional insight into the underlying molecular mechanisms. Cross-fertilization of ideas regenerated from in vivo and cell culture experimental approaches are likely to facilitate the identification of new therapeutic targets to limit pathological angiogenesis and inflammation.
References
Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD (2005) A simplified laminin nomenclature. Matrix Biol 24:326–332
Avraamides CJ, Garmy-Susini B, Varner JA (2008) Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer 8:604–617
Bajaj A, Li QF, Zheng Q, Pumiglia K (2012) Loss of NF1 expression in human endothelial cells promotes autonomous proliferation and altered vascular morphogenesis. PLoS One 7:e49222
Belkin VM, Belkin AM, Koteliansky VE (1990) Human smooth muscle VLA-1 integrin: purification, substrate specificity, localization in aorta, and expression during development. J Cell Biol 111:2159–2170
Bishop ET, Bell GT, Bloor S, Broom IJ, Hendry NF, Wheatley DN (1999) An in vitro model of angiogenesis: basic features. Angiogenesis 3:335–344
Bouvard C, de Arcangelis A, Dizier B, Galy-Fauroux I, Fischer AM, Georges-Labouesse E, Helley D (2012) Tie2-dependent knockout of alpha6 integrin subunit in mice reduces post-ischaemic angiogenesis. Cardiovasc Res 95:39–47
Bouvard C, Segaoula Z, de Arcangelis A, Galy-Fauroux I, Mauge L, Fischer AM, Georges-Labouesse E, Helley D (2014) Tie2-dependent deletion of alpha6 integrin subunit in mice reduces tumor growth and angiogenesis. Int J Oncol 45:2058–2064
Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM (2002) The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol 161:337–344
Colognato H, Yurchenco PD (2000) Form and function: the laminin family of heterotrimers. Dev Dyn 218:213–234
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
da Silva RG, Tavora B, Robinson SD, Reynolds LE, Szekeres C, Lamar J, Batista S, Kostourou V, Germain MA, Reynolds AR, Jones DT, Watson AR, Jones JL, Harris A, Hart IR, Iruela-Arispe ML, Dipersio CM, Kreidberg JA, Hodivala-Dilke KM (2010) Endothelial alpha3beta1-integrin represses pathological angiogenesis and sustains endothelial-VEGF. Am J Pathol 177:1534–1548
Danen EH, Sonnenberg A (2003) Integrins in regulation of tissue development and function. J Pathol 201:632–641
de Smet F, Segura I, de Bock K, Hohensinner PJ, Carmeliet P (2009) Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol 29:639–649
Defilippi P, van Hinsbergh V, Bertolotto A, Rossino P, Silengo L, Tarone G (1991) Differential distribution and modulation of expression of alpha 1/beta 1 integrin on human endothelial cells. J Cell Biol 114:855–863
del Toro R, Prahst C, Mathivet T, Siegfried G, Kaminker JS, Larrivee B, Breant C, Duarte A, Takakura N, Fukamizu A, Penninger J, Eichmann A (2010) Identification and functional analysis of endothelial tip cell-enriched genes. Blood 116:4025–4033
Desai D, Singh P, Van de Water L, Laflamme SE (2013) Dynamic regulation of integrin alpha6beta4 during angiogenesis: potential implications for pathogenic wound healing. Adv Wound Care (New Rochelle) 2:401–409
Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10:9–22
Doi M, Thyboll J, Kortesmaa J, Jansson K, Iivanainen A, Parvardeh M, Timpl R, Hedin U, Swedenborg J, Tryggvason K (2002) Recombinant human laminin-10 (alpha5beta1gamma1). Production, purification, and migration-promoting activity on vascular endothelial cells. J Biol Chem 277:12741–12748
Donovan D, Brown NJ, Bishop ET, Lewis CE (2001) Comparison of three in vitro human ‘angiogenesis’ assays with capillaries formed in vivo. Angiogenesis 4:113–121
Dowling J, Yu QC, Fuchs E (1996) Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol 134:559–572
Eilken HM, Adams RH (2010) Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol 22:617–625
Eming SA, Brachvogel B, Odorisio T, Koch M (2007) Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem 42:115–170
Enenstein J, Kramer RH (1994) Confocal microscopic analysis of integrin expression on the microvasculature and its sprouts in the neonatal foreskin. J Invest Dermatol 103:381–386
Frieser M, Nockel H, Pausch F, Roder C, Hahn A, Deutzmann R, Sorokin LM (1997) Cloning of the mouse laminin alpha 4 cDNA. Expression in a subset of endothelium. Eur J Biochem 246:727–735
Fujiwara H, Kikkawa Y, Sanzen N, Sekiguchi K (2001) Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins. J Biol Chem 276:17550–17558
Gardner H, Kreidberg J, Koteliansky V, Jaenisch R (1996) Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol 175:301–313
Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, le Meur M (1996) Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet 13:370–373
Germain M, de Arcangelis A, Robinson SD, Baker M, Tavora B, D'amico G, Silva R, Kostourou V, Reynolds LE, Watson A, Jones JL, Georges-Labouesse E, Hodivala-Dilke K (2010) Genetic ablation of the alpha 6-integrin subunit in Tie1Cre mice enhances tumour angiogenesis. J Pathol 220:370–381
Ghatak S, Niland S, Schulz JN, Wang F, Eble JA, Leitges M, Mauch C, Krieg T, Zigrino P, Eckes B (2016) Role of integrins alpha1beta1 and alpha2beta1 in wound and tumor angiogenesis in mice. Am J Pathol 186:3011–3027
Gimona M, Buccione R, Courtneidge SA, Linder S (2008) Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol 20:235–241
Glukhova M, Koteliansky V, Fondacci C, Marotte F, Rappaport L (1993) Laminin variants and integrin laminin receptors in developing and adult human smooth muscle. Dev Biol 157:437–447
Glukhova M, Koteliansky V, Sastre X, Thiery JP (1995) Adhesion systems in normal breast and in invasive breast carcinoma. Am J Pathol 146:706–716
Gonzalez AM, Gonzales M, Herron GS, Nagavarapu U, Hopkinson SB, Tsuruta D, Jones JC (2002) Complex interactions between the laminin alpha 4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo. Proc Natl Acad Sci U S A 99:16075–16080
Grenache DG, Zhang Z, Wells LE, Santoro SA, Davidson JM, Zutter MM (2007) Wound healing in the alpha2beta1 integrin-deficient mouse: altered keratinocyte biology and dysregulated matrix metalloproteinase expression. J Invest Dermatol 127:455–466
Gullberg D, Gehlsen KR, Turner DC, Ahlen K, Zijenah LS, Barnes MJ, Rubin K (1992) Analysis of alpha 1 beta 1, alpha 2 beta 1 and alpha 3 beta 1 integrins in cell--collagen interactions: identification of conformation dependent alpha 1 beta 1 binding sites in collagen type I. EMBO J 11:3865–3873
Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM (2005) Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev 85:979–1000
He L, Vanlandewijck M, Mae MA, Andrae J, Ando K, del Gaudio F, Nahar K, Lebouvier T, Lavina B, Gouveia L, Sun Y, Raschperger E, Segerstolpe A, Liu J, Gustafsson S, Rasanen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C (2018) Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types. Sci Data 5:180160
Holtkotter O, Nieswandt B, Smyth N, Muller W, Hafner M, Schulte V, Krieg T, Eckes B (2002) Integrin alpha 2-deficient mice develop normally, are fertile, but display partially defective platelet interaction with collagen. J Biol Chem 277:10789–10794
Homan SM, Mercurio AM, Laflamme SE (1998) Endothelial cells assemble two distinct alpha6beta4-containing vimentin-associated structures: roles for ligand binding and the beta4 cytoplasmic tail. J Cell Sci 111(Pt 18):2717–2728
Homan SM, Martinez R, Benware A, Laflamme SE (2002) Regulation of the association of alpha 6 beta 4 with vimentin intermediate filaments in endothelial cells. Exp Cell Res 281:107–114
Humphries JD, Byron A, Humphries MJ (2006) Integrin ligands at a glance. J Cell Sci 119:3901–3903
Hynes RO (2002a) Integrins: bidirectional, allosteric signaling machines. Cell 110:673–687
Hynes RO (2002b) A reevaluation of integrins as regulators of angiogenesis. Nat Med 8:918–921
Iivanainen A, Kortesmaa J, Sahlberg C, Morita T, Bergmann U, Thesleff I, Tryggvason K (1997) Primary structure, developmental expression, and immunolocalization of the murine laminin alpha4 chain. J Biol Chem 272:27862–27868
Kikkawa Y, Yu H, Genersch E, Sanzen N, Sekiguchi K, Fassler R, Campbell KP, Talts JF, Ekblom P (2004) Laminin isoforms differentially regulate adhesion, spreading, proliferation, and ERK activation of beta1 integrin-null cells. Exp Cell Res 300:94–108
Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M (2001) Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol 230:230–242
Kofler NM, Simons M (2015) Angiogenesis versus arteriogenesis: neuropilin 1 modulation of VEGF signaling. F1000Prime Rep 7:26
Kortesmaa J, Yurchenco P, Tryggvason K (2000) Recombinant laminin-8 (alpha(4)beta(1)gamma(1)). Production, purification, and interactions with integrins. J Biol Chem 275:14853–14859
Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R (1996) Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122:3537–3547
Li QF, Decker-Rockefeller B, Bajaj A, Pumiglia K (2018) Activation of Ras in the vascular endothelium induces brain vascular malformations and hemorrhagic stroke. Cell Rep 24:2869–2882
Linder S, Aepfelbacher M (2003) Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol 13:376–385
Loeser RF, Carlson CS, Mcgee MP (1995) Expression of beta 1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. Exp Cell Res 217:248–257
Mercurio AM, Rabinovitz I, Shaw LM (2001) The alpha 6 beta 4 integrin and epithelial cell migration. Curr Opin Cell Biol 13:541–545
Milner R, Campbell IL (2006) Increased expression of the beta4 and alpha5 integrin subunits in cerebral blood vessels of transgenic mice chronically producing the pro-inflammatory cytokines IL-6 or IFN-alpha in the central nervous system. Mol Cell Neurosci 33:429–440
Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR (1997) The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alpha3 isoform. J Cell Biol 137:685–701
Miner JH, Cunningham J, Sanes JR (1998) Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol 143:1713–1723
Mitchell K, Szekeres C, Milano V, Svenson KB, Nilsen-Hamilton M, Kreidberg JA, Dipersio CM (2009) Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J Cell Sci 122:1778–1787
Murphy PA, Begum S, Hynes RO (2015) Tumor angiogenesis in the absence of fibronectin or its cognate integrin receptors. PLoS One 10:e0120872
Nakatsu MN, Hughes CC (2008) An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods Enzymol 443:65–82
Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez-Del-Pulgar S, Carpenter PM, Hughes CC (2003) Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc Res 66:102–112
Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG (2004) Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell 6:471–483
Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, Tsuji T, Yamada M, Sekiguchi K (2006) Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol 25:189–197
Paolillo M, Serra M, Schinelli S (2016) Integrins in glioblastoma: still an attractive target? Pharmacol Res 113:55–61
Patton BL, Miner JH, Chiu AY, Sanes JR (1997) Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol 139:1507–1521
Phng LK, Gerhardt H (2009) Angiogenesis: a team effort coordinated by notch. Dev Cell 16:196–208
Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146:873–887
Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA (2000) Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A 97:2202–2207
Pozzi A, Levine WF, Gardner HA (2002) Low plasma levels of matrix metalloproteinase 9 permit increased tumor angiogenesis. Oncogene 21:272–281
Sasaki T, Timpl R (2001) Domain IVa of laminin alpha5 chain is cell-adhesive and binds beta1 and alphaVbeta3 integrins through Arg-Gly-Asp. FEBS Lett 509:181–185
Seano G, Chiaverina G, Gagliardi PA, di Blasio L, Puliafito A, Bouvard C, Sessa R, Tarone G, Sorokin L, Helley D, Jain RK, Serini G, Bussolino F, Primo L (2014) Endothelial podosome rosettes regulate vascular branching in tumour angiogenesis. Nat Cell Biol 16(931–41):1–8
Seki T, Yun J, Oh SP (2003) Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res 93:682–689
Senger DR, Davis GE (2011) Angiogenesis. Cold Spring Harb Perspect Biol 3:a005090
Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M (1997) Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci U S A 94:13612–13617
Simons M, Alitalo K, Annex BH, Augustin HG, Beam C, Berk BC, Byzova T, Carmeliet P, Chilian W, Cooke JP, Davis GE, Eichmann A, Iruela-Arispe ML, Keshet E, Sinusas AJ, Ruhrberg C, Woo YJ, Dimmeler S, American Heart Association Council On Basic Cardiovascular S, Council On Cardiovascular, S. Anesthesia (2015) State-of-the-art methods for evaluation of angiogenesis and tissue vascularization: a scientific statement from the American Heart Association. Circ Res 116:e99–e132
Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM (2001) Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol 153:933–946
Sobel RA, Hinojoza JR, Maeda A, Chen M (1998) Endothelial cell integrin laminin receptor expression in multiple sclerosis lesions. Am J Pathol 153:405–415
Song J, Lokmic Z, Lammermann T, Rolf J, Wu C, Zhang X, Hallmann R, Hannocks MJ, Horn N, Ruegg MA, Sonnenberg A, Georges-Labouesse E, Winkler TH, Kearney JF, Cardell S, Sorokin L (2013) Extracellular matrix of secondary lymphoid organs impacts on B-cell fate and survival. Proc Natl Acad Sci U S A 110:E2915–E2924
Song J, Zhang X, Buscher K, Wang Y, Wang H, di Russo J, Li L, Lutke-Enking S, Zarbock A, Stadtmann A, Striewski P, Wirth B, Kuzmanov I, Wiendl H, Schulte D, Vestweber D, Sorokin L (2017) Endothelial basement membrane laminin 511 contributes to endothelial junctional tightness and thereby inhibits leukocyte transmigration. Cell Rep 18:1256–1269
Sorokin LM, Pausch F, Frieser M, Kroger S, Ohage E, Deutzmann R (1997) Developmental regulation of the laminin alpha5 chain suggests a role in epithelial and endothelial cell maturation. Dev Biol 189:285–300
Stenzel D, Franco CA, Estrach S, Mettouchi A, Sauvaget D, Rosewell I, Schertel A, Armer H, Domogatskaya A, Rodin S, Tryggvason K, Collinson L, Sorokin L, Gerhardt H (2011) Endothelial basement membrane limits tip cell formation by inducing Dll4/notch signalling in vivo. EMBO Rep 12:1135–1143
Sterk LM, Geuijen CA, Van Den Berg JG, Claessen N, Weening JJ, Sonnenberg A (2002) Association of the tetraspanin CD151 with the laminin-binding integrins alpha3beta1, alpha6beta1, alpha6beta4 and alpha7beta1 in cells in culture and in vivo. J Cell Sci 115:1161–1173
Strasser GA, Kaminker JS, Tessier-Lavigne M (2010) Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood 115:5102–5110
Streuli CH, Akhtar N (2009) Signal co-operation between integrins and other receptor systems. Biochem J 418:491–506
Takeda Y, Kazarov AR, Butterfield CE, Hopkins BD, Benjamin LE, Kaipainen A, Hemler ME (2007) Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood 109:1524–1532
te Molder L, de Pereda JM, Sonnenberg A (2021) Regulation of hemidesmosome dynamics and cell signaling by integrin alpha6beta4. J Cell Sci 134
Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K (2002) Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol Cell Biol 22:1194–1202
Turner CJ, Badu-Nkansah K, Hynes RO (2017) Endothelium-derived fibronectin regulates neonatal vascular morphogenesis in an autocrine fashion. Angiogenesis 20:519–531
Van Der Flier A, Badu-Nkansah K, Whittaker CA, Crowley D, Bronson RT, Lacy-Hulbert A, Hynes RO (2010) Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development 137:2439–2449
Van Der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A (1996) Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat Genet 13:366–369
Welser JV, Halder SK, Kant R, Boroujerdi A, Milner R (2017) Endothelial alpha6beta4 integrin protects during experimental autoimmune encephalomyelitis-induced neuroinflammation by maintaining vascular integrity and tight junction protein expression. J Neuroinflammation 14:217
Welser-Alves JV, Boroujerdi A, Tigges U, Wrabetz L, Feltri ML, Milner R (2013) Endothelial beta4 integrin is predominantly expressed in arterioles, where it promotes vascular remodeling in the hypoxic brain. Arterioscler Thromb Vasc Biol 33:943–953
Wu C, Ivars F, Anderson P, Hallmann R, Vestweber D, Nilsson P, Robenek H, Tryggvason K, Song J, Korpos E, Loser K, Beissert S, Georges-Labouesse E, Sorokin LM (2009) Endothelial basement membrane laminin alpha5 selectively inhibits T lymphocyte extravasation into the brain. Nat Med 15:519–527
Xu H, Laflamme SE (2022) Contribution of endothelial laminin-binding Integrins to cellular processes associated with angiogenesis. Cell 11
Xu H, Pumiglia K, Laflamme SE (2020) Laminin-511 and alpha6 integrins regulate the expression of CXCR4 to promote endothelial morphogenesis. J Cell Sci 133
Yanez-MO M, Barreiro O, Gonzalo P, Batista A, Megias D, Genis L, Sachs N, Sala-Valdes M, Alonso MA, Montoya MC, Sonnenberg A, Arroyo AG, Sanchez-Madrid F (2008) MT1-MMP collagenolytic activity is regulated through association with tetraspanin CD151 in primary endothelial cells. Blood 112:3217–3226
Yurchenco PD (2011) Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 3
Yurchenco PD, Quan Y, Colognato H, Mathus T, Harrison D, Yamada Y, O’rear JJ (1997) The alpha chain of laminin-1 is independently secreted and drives secretion of its beta- and gamma-chain partners. Proc Natl Acad Sci U S A 94:10189–10194
Zhang Z, Ramirez NE, Yankeelov TE, Li Z, Ford LE, Qi Y, Pozzi A, Zutter MM (2008) alpha2beta1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor cell-specific manner. Blood 111:1980–1988
Zhou Z, Doi M, Wang J, Cao R, Liu B, Chan KM, Kortesmaa J, Sorokin L, Cao Y, Tryggvason K (2004) Deletion of laminin-8 results in increased tumor neovascularization and metastasis in mice. Cancer Res 64:4059–4063
Zweers MC, Davidson JM, Pozzi A, Hallinger R, Janz K, Quondamatteo F, Leutgeb B, Krieg T, Eckes B (2007) Integrin alpha2beta1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J Invest Dermatol 127:467–478
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
LaFlamme, S.E., Xu, H. (2023). Endothelial Cell–Matrix Interactions in Angiogenesis and Vessel Homeostasis: A Focus on Laminins and Their Integrin Receptors. In: Papadimitriou, E., Mikelis, C.M. (eds) Matrix Pathobiology and Angiogenesis. Biology of Extracellular Matrix, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-031-19616-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-19616-4_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-19615-7
Online ISBN: 978-3-031-19616-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)