Abstract
The chapter describes the most common and effective methods of obtaining of II-VI semiconductor compounds (CdS, CdSe, CdTe, ZnS, ZnSe, ZnTe) thin films for modern optoelectronic devices and production of chemical sensors. Special attention is paid to such methods as vacuum thermal deposition and its varieties which differ in ways of sputtering of the precursor material, magnetron sputtering, chemical vapor deposition, epitaxial deposition, ion beam sputtering, chemical bath deposition, aerosol spray pyrolysis, electrochemical deposition, and some other methods that have been developed recently. Physics and chemistry of the methods, process characteristics, necessary equipment, conditions and models of film deposition, common features of II-VI semiconductor film synthesis, and advantages and disadvantages leading to the quality degradation are presented for each method. The focus is made on the evaluation of the influence of the process parameters and conditions on the semiconductive and functional (optic, photoelectric) properties of the films, providing the achievement of sensor characteristics maximum for effective practical use of II-VI compounds. In this aspect, characteristic examples are given for a number of compounds on the effect of the production conditions on the kinetic, structural, semiconductor, and optical characteristics of the deposited films.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- II-VI semiconductors
- Cadmium selenide
- Cadmium sulfide
- Cadmium telluride
- Zinc selenide
- Zinc sulfide
- Zinc telluride
- Chemical sensors
- Methods of deposition
- Aerosol spray pyrolysis
- Atomic layer deposition
- Arc-PVD
- Chemical bath deposition
- Chemical vapor deposition
- Electrochemical deposition
- Electron beam vacuum evaporation
- Ion beam sputtering
- Magnetron sputtering
- Metalorganic vapor phase epitaxy
- Molecular-beam epitaxy
- Physical vapor deposition|
- Pulsed laser deposition technique
- Successive ionic layer adsorption and reaction
- Vacuum thermal sputtering
- Methods of synthesis
- Hydrothermal synthesis
- Sol-gel synthesis
- Photodetectors
- Photonics
- Thin films
1 Introduction
The formation of thin films of II-VI compounds with specified parameters is one of the most critical operations in the manufacturing process of various optoelectronic, photonics, and sensory devices. The experiment showed that films of II-VI compounds on the surface of substrates can be formed using a variety of approaches. The most commonly used methods of film formation used in the manufacture of photodetectors and gas sensors are listed in Table 10.1. It should be noted that the data given in Table 10.1 were obtained without taking into account approaches based on the use of 1D nanostructures and the principles of thick-film technology, when pre-synthesized nanocrystals are deposited on the substrate surface in various ways. In the methods presented in Table 10.1, nanocrystals are synthesized directly on the substrate surface. It can be seen that the most commonly used methods include vacuum thermal evaporation and some of its varieties, deposition from the gas or vapor phase, sputtering, spray pyrolysis, deposition in a chemical bath, electrochemical deposition, and some other methods that have been actively developed in recent years. Given the above, consider these methods in more detail.
2 Vacuum Thermal Evaporation
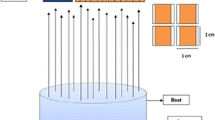
In general, vacuum deposition is a set of processes used to deposit films with a thickness from one monolayer to hundreds of micrometers in negative pressure conditions. The principle of vacuum thermal sputtering (vacuum evaporation) is the transfer of original substances into the vapor phase due to heating by the direct deposition of vapors on a specially prepared substrate (see Fig. 10.1). Historically, this method is one of the first used to produce thin films of II-VI compounds. Any vacuum deposition unit includes an energy source, which provides the evaporation of the deposited material. There are many designs of evaporators implementing resistance, radiation, induction, laser impulse, and other heating techniques.
Film deposition process is carried out at temperatures that provide the necessary pressure of vapors of evaporating substances. The rate of vapor condensation and film growth, in turn, depends on the relative position of the evaporator and the substrate as well as substrate temperature [51]. Thus, the optimal choice of the distance from the evaporator to the substrate is important taking into account the mean free path of molecule in the residual gas atmosphere, which is evaluated experimentally. Three main processes simultaneously occur while applying thin films in vacuum: Reaching a certain concentration of particles of the evaporating substance, their transit in vacuum to the substrate, and vapor condensation on the substrate surface. The purity of the deposited film depends on the vacuum level and the purity of the precursor used. It will be higher as the deposition rate increases since this reduces the inclusion of gaseous impurities in its composition. The film thickness can also vary depending on the geometry of the evaporation chamber. Collisions of particles with molecules and residual gas atoms increase the irregularity of the thickness.
At thermal vacuum deposition of films, the evaporation temperature is nominally taken as its value at which vapor pressure of the substance above its surface is 0.01 mm of mercury. The standard evaporation rate by weight at this pressure for most II-VI semiconductor compounds is about 10−4g·cm−2·s−1. According to kinetic studies to avoid significant contamination of films with atmospheric gases at average growth rate of the layer up to 1 nm·s−1, deposition should be carried out at residual pressure of not more than 10−3 Pa.
Vacuum thermal-furnace evaporated deposition of II-VI compounds has several advantages in that the physical and electrical properties of the films can be easily controlled by the various processing conditions such as deposition temperature, annealing temperature, and deposition pressure. In addition, thermal evaporation technique under certain conditions provides high crystalline quality and uniform films with excellent adherence due to the use of vacuum conditions.
When depositing films by thermal sputtering, various scenarios can be used. For example, when forming ZnTe and CdS films, high-purity zinc and telluride powders or elements Cd and S can be used as a source, which, mixed in a certain weight ratio, are placed on a molybdenum or tungsten boat, heated to temperatures of 400–600°C [60, 99]. Elemental S, Se, and Te are mainly evaporated in the form of polyatomic clusters. CdTe and CdS powders can also be used as a source. During the spraying process, the chamber is usually evacuated to a low-pressure situation (10−3–10−6 Pa). The chamber can also be filled with argon or an Ar/H2 [5] mixture to inhibit oxidation processes.
Evaporation of binary II-VI compounds is usually accomplished by dissociation during their thermal decomposition. Due to both significant difference in the volatility of the compound components and difference in the chemical potential of each of them in vapor and solid states, the vapor composition in the vacuum chamber and the film on the substrate can differ from the starting material. In particular, during CdSe deposition, it was found that the stoichiometry of CdSe films improves with an increase in the substrate temperature up to 550 K. At 550 K, the CdSe films were exactly stoichiometric (atomic ratio Cd/Se = 1) (see Fig. 10.2). Above 550 K substrate temperature, the films were nonstoichiometric and showed deficiency of selenium [99]. This may be due to reevaporation of highly volatile selenium from the substrate. An increase in the Cd/Se ratio, due to depletion of the source, can also be observed with an increase in the thickness of the deposited film. In this case, separate evaporators with different temperatures must be used to provide stoichiometric composition of the deposited layer. For example, to form CdS films, two separate sources of CdS and S can be used to produce Cd and S vapors. This approach allows to grow the films of II-VI compounds with a required composition at appropriate temperature control.
The variation of grain size and elemental atomic ratio Cd/Se of the CdSe thin films deposited at different substrate temperatures keeping constant a thickness of 100 nm (a). Variation of resistivity, carrier mobility, carrier concentration in CdSe thin films with different substrate temperatures at a constant thickness of 100 nm (b). (Adapted with permission from Patel et al. [99]. Copyright 1994: Wiley)
Numerous experiments have shown that the substrate temperature during film deposition is indeed one of the main parameters affecting film parameters. For example, Fig. 10.2 shows the influence of the substrate temperature on the electrophysical properties of CdSe films, and Table 10.2 shows typical results of the effect of substrate temperature on the parameters of ZnS films. These results were reported by Patel et al. [99] and Vishwakarma [121]. Nanocrystalline semiconductor ZnS films 500 nm thick were deposited on a glass substrate at various substrate temperatures. It can be seen that with an increase in the deposition temperature from 300 to 400 K, the grain size increases, while the dislocation density, the electrical resistance of the film, and the band gap decrease. At the same time, when the dislocation density decreases by 1.5 times in this temperature range, the electrical resistance decreases by 2.4 times. As the substrate temperature range increases, the changes in film properties become even greater. As is known, the temperature of the thermal evaporation substrate can vary over a very wide range from RT to 600°С [5, 69, 70, 121]. The argon pressure in the deposition chamber also has a significant effect on the film parameters. For example, Lin et al. [60] found that the relative concentration of carriers in ZnTe films increased with decreasing argon pressure and increasing deposition temperature.
It is important to note that films of ternary compounds can also be obtained using the thermal evaporation method. For example, in [87], 300-nm-thick CdSxSe1-x ternary films with composition, x, varying from 0 to 1 were obtained by this method. High-purity CdS and CdSe materials were used as a source. ZnxCd1-xS films (x = 0, 0.15, 0.30, and 0.45) and CdxZn1-xTe (0.2 ≤ x ≤ 1.0) were obtained by the same method. High-purity CdS and ZnS or CdTe and ZnTe powders were used to deposit these films [6].
Thermal evaporation also makes it possible to deposit doped films of II-VI compounds. In work [5] CdS nanostructures undoped and doped with zinc were grown on Si/SiO2 substrates in the pipe furnace under the Ar/H2 atmosphere. The results of the studies showed the combination of ribbon and rod forms of nanoparticles for undoped layers, whereas the CdS sample doped with Zn had morphology of nanorod with hexagonal crystal structure. Mahdi et al. [70] synthesized nanostructured films doped with aluminum using thermal evaporation in a vacuum. The undoped and Al-doped ZnTe thin films were deposited on glass and Si substrates to produce heterojunction detectors [74]. Doping with aluminum was accompanied by a significant change in the film morphology and crystallite size.
As for the disadvantages of this method, as it is mentioned above, there is a problem with the sputtering of the materials with complex composition due to the difference of vapor pressure of the compound components. In some cases, another problem is the poor crystallinity of the films when deposited on low-temperature substrates and their poor adhesion observed when using relatively low temperatures of the substrate and also due to contamination with atmospheric gases under poor vacuum. For example, Kalita et al. [42] performed a detailed analysis of the structural parameters of ZnSe thin films deposited by thermal evaporation on a substrate heated to a temperature varying in the range of 30–350°C during deposition. It was found that the peak intensities on the X-ray diffraction pattern, indicating the crystallinity of the deposited material, increase noticeably only at a substrate temperature above 300 °C. See [8, 14, 18, 41, 71, 78, 122] for more details on vacuum thermal evaporation of II-VI films, and we strongly recommend to refer to these works.
3 Magnetron Sputtering
Magnetron sputtering is a technique of applying thin films on the substrate using cathode sputtering of the target in crossed electric and magnetic fields [7, 51]. Magnetic field is applied perpendicular to electric field and oriented parallel to the cathode. When negative potential is applied to the cathode, electric lines of force appearing near it are crossed with magnetic lines of force. In the unit with a flat cathode, magnetic field prevents the releasing of electrons from the cathode region directing them along a closed path (see Fig. 10.3). This prevents electron bombardment of the deposited film and increases the ionization efficiency of the working gas atoms due to a series of sequential collisions with them. Thus, most of the electron energy is used for ionization and excitation before it reaches the anode. Positive ions in turn bombarding the cathode surface cause its sputter. Increasing sputtering rate due to plasma localization at the cathode surface and simultaneously decreasing working pressure to 0.1 Pa and lower allow to reduce film contamination with gaseous occlusions significantly. Decreasing the bombardment intensity of the substrate also reduces temperature and radiation effects in deposited materials.
However, the main advantages of magnetron sputtering are relatively high sputtering rate and the possibility to obtain the films uniform in thickness on the substrates of a large area, which allows to consider this technique as a very perspective low-temperature technique. The use of magnetron sputtering for II-VI compounds allows to increase the deposition film rate by one order more compared to thermal deposition. If it is necessary to apply films having high ohmic resistance, such as compounds of cadmium and zinc sulfides, high-frequency magnetron sputtering is used for their synthesis. In this case, the target is bombarded with ions only in the negative half-period of the supply voltage.
In work [109] CdS thin films were deposited at room temperature by high-frequency magnetron sputtering at different deposition times. The formation of solid and dense layers without any significant defects such as cracks, pores, and protrusions has been established. Their element analysis showed that films have almost stoichiometric composition. Photodetectors made using the deposited CdS film demonstrated a high value of the photoconductive response to optical radiation. At the same time, some disadvantages of magnetron sputtering technique at the formation of CdS layers were noted in work [49]: relatively small grain size and high surface roughness, which can negatively affect their photovoltaic characteristics. Magnetron deposition was successfully used for the deposition of ZnS films [39, 126] and CdTe and CdS films [57]. Moreover, Li et al. [57] established for deposited CdS and CdTe films the relationships between the physical and optical properties of these films, including how their semiconductor and physical properties are affected by the void fraction, grain size, and developing deformations. Highly conductive polycrystalline ZnSe thin films can also be deposited using reactive d.c. magnetron sputtering of zinc in an Ar + H2Se atmosphere [16]. The composition of the deposited films can be controlled by the zinc deposition power, the substrate temperature, and the partial pressure of H2Se. Shallow donors can be introduced by co-sputtering of Al, Ga, or In or by incorporating In into a zinc target. As a result of such doping, ZnSe films with a resistivity of up to 20 Ohm·cm can be obtained even when ZnSe is deposited on a substrate at a temperature of up to 120 °C.
As a rule, films of II-VI compounds deposited using magnetron sputtering at low temperatures are amorphous or nanocrystalline with a crystallite size from 2–3 nm to 15 nm, increasing with increasing film thickness. Postdeposition heat treatments make it possible to increase the size of nanocrystals [73]. Changing the sputtering conditions and HF power is also accompanied by a change in the degree of orientation of the films and the size of the nanoparticles [126]. An increase in the substrate temperature during deposition also leads to a significant increase in the crystallinity and the size of the crystallites forming the films. As can be seen from Fig. 10.4, clear peaks appear on the X-ray diffraction patterns only at a substrate temperature above 100 °C. With a further increase in the substrate temperature from 100 to 400 °C, the diffraction patterns of films of a nanostructural nature are enhanced [39]. Table 10.3 shows the variation of the structural characteristics in the ZnS film: full width at half maximum (FWHM), the sizes of crystallites according to both X-ray diffraction (XRD) and field emission scanning electron microscopy (FESEM), as well as the Zn/S ratio in the layer when the substrate temperature increases to 400 °C. It can be seen that its growth within the studied limits increases the crystallinity of the films and brings the content of the main elements closer to the stoichiometric ratio.
XRD patterns of ZnS films grown at various substrate temperatures from 100 to 400 °C. (Reprinted from Ref. [39]. Published 2012 by Springer as open access)
4 Pulsed Laser Deposition Technique
Pulsed laser deposition (PLD) is a physical vapor deposition technique that uses a powerful pulsed laser as an evaporator [65]. For these purposes, the CO2 lasers (λ = 10.6 μm) and Nd:YAG lasers (λ = 355 nm) are usually used [55, 65]. But other lasers such as krypton fluoride laser (Kr:F laser) (λ = 248 nm) can also be used [83]. Parameters of these lasers are listed in Table 10.4. Its beam is focused inside the vacuum chamber on a target made of the deposited material. As a target for sputtering, single-crystal plates or polycrystalline films formed by various methods can be used. The pulse lasts about 10–40 μs, depending on the parameters of the laser.
As a rule, the process of PLD takes place in ultrahigh vacuum [34]. A typical installation configuration used for PLD is shown in Fig. 10.5. The particles that have left the target are extremely diverse. These are atoms, molecules, electrons, ions, clusters, and melt particles. The parameters of the working laser, the laser radiation flux density, the time and duration of the pulse, and the degree of ionization of the deposited material will affect the quality of the film during deposition and its stoichiometry.
As a rule, the method of PLD consists of several important parts [65]. Each of them will greatly affect the quality of thin films. According to many researchers, these stages include the interaction of laser radiation with the target, the dynamics of ablative materials, the deposition of ablative materials on a substrate, and the growth of thin films on a substrate. In other words, this deposition method depends on the interaction of photons to create an ejected plume of material from the target. Then the vapor is collected on a substrate located at a small distance from the target. The PLD method during film deposition is characterized by significant supersaturation, which leads to a more intense process of nucleation on the substrate surface compared to thermal vacuum deposition. This increases the uniformity of the film in terms of microrelief and the size of the particles forming the film. According to Zhang et al. [127], PLD makes it possible to control the size and crystalline phase by varying the laser parameters and growth conditions. In addition, this method maintains stoichiometry when materials are transferred from the target to the substrate and exhibits good crystallinity of the films as described by Xin et al. [123]. Thus, the development of laser technologies makes the PLD method very competitive for growing thin homogeneous films of complex stoichiometry.
Experiments have shown that in addition to vacuum, an inert or active gas atmosphere can be used for PLD. Chalana et al. [11] studied the effect of an argon atmosphere (0, 5, 10, 15, and 20 Pa) on the parameters of ZnS films and found that an increase in the argon pressure from 0 to 15 Pa is accompanied by an increase in the crystallinity of the ZnS films. However, the crystallinity decreases as the argon pressure is beyond 15 Pa. Therefore, they concluded that 15 Pa is the best argon pressure for PLD of ZnS films. In addition, these films show the highest photoluminescence intensity for excitation wavelength 325 nm and the best crystallinity. Similar studies with respect to CdS films were carried out by Kumar et al. [55]. They found that at a pressure of 100 mTorr in a vacuum chamber, a cardinal change occurs in the electrophysical, optical, and luminescent properties of the grown films. In this case, the crystallite size decreases from 38.5 nm to 11.5 nm as the argon pressure increases from 0 to 100 mTorr.
The temperature of the substrate during the PLD process can vary over a very wide range. Tong et al. [118] changed the substrate temperature from 100 to 600 °C during the deposition of CdS films and studied the effect of this parameter on the film properties. They found that the crystallinity, optical properties, and surface quality of the films could be improved by increasing the temperature up to 450 °C. However, degradation of the quality of the films could be observed as the temperature was increased to 600 °C. Ming et al. [85] also analyzed the effect of substrate temperature on the properties of ZnS films. However, they were limited to the region of low temperatures of 150–200 °C, which did not allow to determine the truly optimal deposition parameters. They only found that the intensity of (111) peak increased and the surface was more homogenous with the increasing of the substrate.
In principle, given the low deposition rates, PLD technology is most suitable for the formation of thin films (d<100 nm). However, as shown by Zhang et al. [127], thick films can also be formed. Using PLD, Zhang et al. [127] grew uniform stoichiometric ZnS films with a thickness of 3 μm. With an increase in the thickness of the films, a regular process of an increase in the size of the crystallites forming the films is observed. For example, Alias et al. [3] reported that the crystallite size was observed to increase from 12.1 to 51.9 nm as the thickness of CdS film was increased from 130 to 780 nm. In the XRD studies, the grown CdS films were polycrystalline, and the peaks appear sharper when the film thickness increases.
It was shown that in addition to depositing films of binary II-VI compounds, the PLD method can be used to deposit multicomponent II-VI semiconductors such as Cu2ZnSnS4 [88, 89], and others.
Other research results related to film formation of II-VI compounds using the PLD method can be found in other published articles [83, 124]. In work [36], a brief overview of the deposition of thin films applied with a pulsed laser is presented. In [83] a study of manganese-doped luminescent ZnS, thin films obtained using Kr:F-laser demonstrate a good stoichiometric quality of the layers at a high process rate. Moreover, the comparison of the characteristics of the films with the samples obtained by magnetron sputtering technique was made. The results received in [26] at the deposition of CdS thin films by pulsed laser chemical deposition technique in a bath (PLACBD) are of great interest. The results show that irradiation of a chemical bath with a laser during the deposition of films leads to their accelerated growth.
In spite of the mentioned benefits of the pulsed laser deposition method, some shortcomings have been identified by many researchers [51]. A laser beam with sufficient energy density for coating processes has a limited size. Therefore, a laser-applied coating technique is less efficient than other conventional methods like thermal evaporation, spraying, in terms of coverage rate. This means that this method has low productivity. In addition, coverage of large area components by laser-applied coatings requires overlapping of individual tracks. Other disadvantages of PLD include (i) relatively high investment costs; (ii) the composition and thickness depend on many deposition conditions. The processing parameters, such as wavelength, energy, and shape of the laser pulse, focusing geometry, process atmosphere, and substrate temperature, all greatly influence the parameters of the ablative particle fluxes and, hence, the properties of the deposited films and the efficiency of the deposition process; (iii) difficulties in the deposition of thick layers; (iv) difficulties in attaining the necessary stoichiometry of materials containing volatile components, (v) difficulties in scaling up to large wafers; and (vi) as a result of repeated interaction of the laser beam with the target, structural changes occur on the surface with craters forming. Therefore, the composition and properties of the deposited material will depend on the duration of the deposition process.
Given the above, it becomes quite reasonable that this technique has been scarcely employed for the development of sensors and photodetectors aimed for the market.
5 Ion Beam Sputtering (IBS)
The main feature of ion beam sputtering technique (IBS) during film deposition, in contrast to the ion plasma (cathode) methods, is the absence of the necessity to supply an electrical potential to the sputtering target. In ion beam spraying devices the functions of working gas ionization units and material sputtering source are separated. The target is sputtered by beam of ions of certain energy directed from an independent source.
Typically, a beam of inert gas ions, usually argon, is directed to the target as a concentrated flow. Sputtering particles are predominantly neutral atoms, or their conglomerates (clusters), as well as ions. With an increase of the energy of bombarding ions over 100 eV, the sputtering yield increases dramatically, and it comes up to saturation at 5–10 keV. The inert gas ions make the structure of the applied film be more robust and resistant to various effects, ensuring good quality and reproducibility of the applied layers.
One variety of ion beam sputtering is a reactive film application, which involves dosed introduction of so-called reactive gases capable of interacting with the material of the sputtered target. Use of sulfur, selenium, tellurium, or their hydrogen compounds as vapors at varying partial pressure, process parameters, and geometry of sputtering devices allows to obtain and control composition of deposited films of II-VI chalcogenides. The synthesis is usually carried out at moderate deposition rates and substrate temperatures of 300–500 K. In order to obtain ultraclean films, it is necessary, in particular, to use an ultrahigh vacuum. The ion beam technique has a number of significant advantages over the magnetron and other ion plasma methods. These include relatively low process temperatures allowing coatings to be applied to thermally sensitive materials (plastics), possibility to transfer stoichiometric composition of the target material onto the substrate, and possibility of controlling ionic beam energy and target sputtering rate due to its bombardment at an angle to the surface.
In [59], the deposition of a thin film of cadmium sulfide (CdS) demonstrates the absence of impurity elements and good transfer of the target material with an initial Cd/S ratio close to stoichiometric. In work [120] ion beam sputtering was used for nonreactive deposition of adhesive ZnS films of high quality. In [45], using the example of deposition of ZnS thin films, it was concluded that the ion beam sputtering method can be used to adjust the optical band gap of zinc sulfide when used in optoelectronic devices.
6 Chemical Vapor Deposition (CVD)
Chemical vapor deposition method (CVD) is a classic method and allows to obtain layers of high quality from different materials [15, 51]. The substrate is usually placed into the vapors of one or several substances which form a required compound on its surface while reacting and/or decomposing. All of them follow the same basic principles depending on the process conditions and the transferring of precursor materials into the gaseous phase (heating, plasma simulation, laser irradiation). Unlike physical deposition methods, vapor phase and film compositions can vary significantly. The film is formed as a result of a chemical reaction both near and on the surface of the substrate. This greatly complicates the deposition but makes it much more universally valid. The main chemical reactions at the vapor deposition of II-VI compounds are reduction and thermal decomposition processes.
The CVD process can be carried out both at atmospheric pressure (atmospheric pressure chemical vapor deposition (APCVD)) and at its reduced value (low-pressure chemical vapor deposition (LPCVD)). Low pressure reduces the probability of undesirable reactions in the system and leads to more homogeneous film deposition on the substrate. In some cases, a vacuum variant of the process is also used, when the pressure in the chamber is 10−6 Pa and lower (ultrahigh vacuum chemical vapor deposition (UHVCVD)) [15]. Chemical deposition, in which precursors are transported to the substrate as an aerosol stream, is called “aerosol-assisted chemical vapor deposition” (AACVD) [97]. The aerosol can be produced in various ways. If plasma is used to decompose precursors, then the method is called “plasma-enhanced chemical vapor deposition” (PECVD). Unlike PECVD, at the “remote plasma-enhanced CVD” method (RPECVD), due to the spatial separation of the decomposition regions of precursor compounds and film deposition, the substrate is not exposed to high temperatures, which excludes radiation damage in it. At flaming decomposition, i.e., at combustion of precursors in an open atmosphere, the method is called “combination chemical vapor deposition” (CCVD), and if organometallic precursors are used, the method is called “metalorganic chemical vapor deposition” (MOCVD). In addition to the abovementioned methods, a number of CVD methods have been developed with their own characteristics. According to literature data, AACVD and MOCVD processes are most widely used methods to form thin films for optoelectronic devices.
In the classic version of the CVD process, two precursors are used as independent sources of elements of II and VI groups for growing II-VI films (Table 10.5). However, as a rule, such precursors are very toxic with different vapor pressures, which significantly complicates the process of film growth [15]. Therefore, in recent decades, great interest has been shown in the search for single-molecule precursors in which the elements of a binary semiconductor are juxtaposed within a single molecule [1, 27, 97]. Typical single-molecule precursors are listed in Table 10.6. The single-molecule precursor has some distinct advantages over such conventional dual precursors. MOCVD with single-molecule precursor is a one-step synthesis featuring improved air/moisture stability, stoichiometric control, limited pre-reactions, and no need for harmful gases such as H2S.
Detailed conditions of CVD synthesis of nanostructured films of II-VI cadmium-containing compounds can be found in monograph [73]. In work [13], ZnS thin films were deposited under vacuum conditions using Zn(S2COCHMe2)2 as a precursor; its decomposition was carried out by a controlled laser during the MOCVD process. Zinc sulfide thin films were deposited by Palve [97] using the aerosol-assisted chemical vapor deposition (AACVD) method. The precursors, Zn(II) thiosemicarbazone complexes, were dissolved in methanol. The deposition was carried out on a substrate with a temperature of 400–500 °C. It was shown that the deposition at 450 °C was reproducible with good adhesion with a glass substrate. At this temperature, ZnS films were stoichiometric with the ratio 1:1 of zinc to sulfur. Below 400 °C the films were very poorly deposited on a glass substrate. The same approach was used by Ketchemen et al. [47] and Buckingham et al. [10]. In [47], cadmium (II) dithiocarbamate and cadmium xanthate complexes in tetrahydrofuran and its mixture with chloroform were used for producing CdS films. In [10], cadmium ethylxanthate complexes Cd(S2COEt)2 decomposing at 225–350 °C were used as precursor materials. Decomposition of various metalorganic compounds was also carried out at low operating pressure in work [125] for obtaining ZnS, ZnSe high-quality films with the thickness of 3 and 1 microns, respectively. In work [107], the influence of the temperature of modified MOCVD synthesis on the photoluminescent properties of CdTe nanocrystallites was studied. As it can be seen from Table 10.7 in the range 130–250 °C, the wavelength photoluminescence (PL) dependence is extreme. The disadvantages of the method of chemical vapor deposition of II-VI films are its comparatively low productivity and the relatively weak adhesion of the films to the substrate.
7 Epitaxial Deposition
In recent years, vapor phase epitaxy methods such as Molecular Beam Epitaxy (MBE) have been widely used to produce functional films of II-VI semiconductors [25, 33, 35]. Figure 10.6 shows a rough schematic representation of MBE system using the low-temperature, low-fux, and UHV conditions typical of most single-crystal epitaxial processes. MBE allows growing films of a given thickness with clear interfacial boundaries and with a given doping profile. In MBE installations, due to the high process control, it is possible to evaluate the thickness and quality of the layers in situ, that is, directly during their growth. The MBE process is based on the interaction of one or more molecular beams of different chemical compositions, produced using effusion cells with a heated substrate. The evaporation and condensation processes take place in an ultrahigh vacuum (10−8 Pa or less). Selecting the temperatures of the substrate and cells, epitaxial films of the desired chemical composition are obtained. The quality of films largely depends on the matching of the parameters of the crystal lattice of the material and the substrate. Thus, the greater the differences in these parameters, the smaller thickness of the grown defect-free film can be.
Schematics of MBE and parameters used in film deposition. (Reprinted from Ref. [17]. Published 2020 by Nature as open access)
The advantage of the MBE method is the relatively low temperature of the process, which reduces the diffusion of impurities from the substrate. The use of inertia-free doping, in its turn, allows to control the process of film growth at the atomic level. The disadvantages of the MBE method include the complexity of the equipment, as well as the low growth rate of the films. A number of fundamental physical mechanisms leads to the slow rates and high costs associated with single-crystal deposition, including the need for [17] (1) sufficient time for atoms to bind at appropriate lattice sites within chemoadsorption, surface transport, and flow rates; (2) single-crystal substrates and careful surface preparation to ensure crystal structure and a clean surface for nucleation; (3) high-purity raw materials to eliminate unintentional impurities that cause improper bonding and structural defects; and (4) ultrahigh vacuum (UHV) and/or ultrahigh-purity (UHP) gases to prevent impurities that cause poor nucleation and/or degrade electro-optical properties.
It should be noted that MBE growth of binary II-VI compounds can be carried out using a single effusion cell containing the compound or, more conveniently, using two cells loaded with the appropriate elements. When using a single effusion cell, it is heated to a congruent sublimation temperature. When using two effusion cells, the ratio of anion and cation fluxes, depending on the vapor pressure of each element (Table 10.8), is adjusted for a given growth temperature in order to obtain the required growth rate with the correct stoichiometry and optimal crystal quality. The smoothness of the surface often depends on this flow ratio, and faceted thick-film surfaces can be obtained if the individual flow values and flow ratios are not properly adjusted [35].
As an example of the implementation of the MBE method in the deposition of epitaxial layers of II-VI compounds, we describe the process used in the formation of CdTe films by Colegrove et al. [17]. For deposition of quality layers, state-of-the-art vacuum equipment is required to maintain the background ambient pressure to 10−9–10−10 torr; this provides excellent impurity control to avoid both nucleation defects and carrier compensation. It also makes it possible to extremely reduce the flow of atoms from the primary source, usually from the CdTe effusion cell. Too much flux can result in Cd and Te atoms on the surface not having enough time to bond at their appropriate locations, resulting in a higher defect density and possibly a polycrystalline material. If the substrate temperature rises too high, then the atoms on the crystal surface sublimate at a dangerous rate, resulting in film loss instead of crystal growth. The dopant atoms are introduced from a separate source and the dopant flow rate is varied according to target concentration levels. Because of these physical limitations, CdTe MBE is usually carried out at a substrate temperature of 180–250 °C. The temperature of the effusion cells, depending on the source used, can reach several hundred degrees. The pressure generated by the flow of Cd and Te molecules, represented by the red and purple spheres in Fig. 10.5, known as the beam equivalent pressure, is about 10–6 Torr, resulting in a deposition rate of about 15 nm/min. Similar modes are also used in the deposition of epitaxial films of other II-VI compounds [33, 35].
One of the variants of MBE method is a method of vapor phase epitaxy using metalorganic compounds (metalorganic vapor phase epitaxy (MOVPE)) [106]. Another abbreviation for this method is metalorganic chemical vapor deposition (MOCVD) [25]. MOVPE is a method of epitaxial films chemical deposition, when the initial gaseous reagents pyrolytically decompose at the surface of the substrate, releasing film-forming components. The process is carried out in a flow reactor at vapor pressure of 10−3–10−1 Pa. Pressure reduction allows to obtain more homogeneous films with thickness control up to several angstroms. MOVPE method, successfully competing in its simplicity and productivity with MBE, has a relatively worse reproductivity of film composition and properties. This is due to the use of both higher temperatures and longer deposition time. Optimization of the process even when obtaining films of binary II-VI compounds: CdS, CdSe, ZnS, and ZnSe, is a rather laborious and costly process [53, 54, 56]. Precursors are usually methyl, ethyl, and isobutyl metal compounds, as well as their hydrides. For example, for the deposition of II-VI binary compounds, such as CdS and CdSe, diethyl sulfide (C2H5)2S and dimethyl selenide (CH3)2Se, as well as metal methylates and ethylates, are used as initial components. The results of the study [82] demonstrated good photoluminescent properties in ZnS polycrystalline films grown by the MBE method by congruent sublimation from one Knudsen cell. Figure 10.7 shows the variation of the growth time of zinc sulfide films with a thickness of 200 nm doped with manganese and the intensity of their photoluminescence at 580 nm depending on the temperature of the Knudsen cell. As found in the work, at the given wavelength, the intensity of irradiation is maximum and differs in a pronounced dependence on the cell temperature.
Variation of the growth time of ZnS:Mn films with a thickness of 200 nm and the intensity of their photoluminescent radiation at 580 nm depending on the temperature of Knudsen cell. (Data extracted from Ref. [82])
Atomic layer deposition (ALD) or atomic layer epitaxy (ALE) is an epitaxial film deposition technology that is based on a cycle of consequent chemical reactions on the gas-solid interfacial boundary [35]. This technique is based on a self-regulated layer-by-layer growth mode. Precursors from the gaseous phase alternately react with the surface layer of the film and gradually grow it. The growth of layers consists of specific four stages, which are cyclically repeated:
-
1.
The action of the first precursor. At this stage the substrate is exposed alternately to the vapor beam of effusion cells loaded with the elements (anion and cation) that constitute the compound to be deposited: One cell shutter is open while the other is closed.
-
2.
Exposure and removal of unreacted residues from the reactor. The exposure time is chosen so that the exposed surface is saturated, and the chemisorption process leads to the deposition of a complete or partial monolayer (ML) of one of the elements. Excess atoms adsorbed on atoms of the same type are physisorbed and easily reevaporate after closing the appropriate shutter.
-
3.
The action of the second precursor.
-
4.
Exposure and removal of reagent residues from the reactor.
The process is repeated as many times as necessary in order to reach the expected thickness. For example, the formation and growth of the zinc sulfide film are due to cyclic reactions between sulfur and zinc vapors in the adsorbed layer. Thus, it is important to have a monomolecular adsorption of the particles that is provided by the substrate temperature. During ALD chemical reactions usually take place in the temperature range of 200–400 °C and at an operating pressure range of 0.1–10 kPa. The film is doped by adding an impurity into the gaseous phase. The final thickness of the layer is determined only by the number of treatment cycles of the substrate with precursors.
A description of several processes of epitaxial growth of several II-VI semiconductors can be found in [43, 50]. For example, Konagai et al. [50] using ALD method deposited epitaxial layer of ZnSe and ZnTe. Nitrogen gas was introduced into the chamber. The shutters of zinc and chalcogen were alternately opened and closed for 7 s with an interval of 1 s. In total, 1000 cycles were used for the ALD of ZnSe and ZnTe during the entire experiment. Figure 10.8 shows the change in the growth rate of ZnSe and ZnTe films depending on the growth temperature. This clearly shows that ideal ALD growth was achieved in the substrate temperature range of 250–350 °C for ZnSe and around 220–250 °C for ZnTe.
Growth rates of ZnSe and ZnTe grown at various substrate temperatures by ALD. (Reprinted with permission from Konagai et al. [50]. Copyright 1993: Elsevier)
The ALD method offers several advantages over MBE growth modes for the deposition of high-quality epitaxial films of II-VI compounds. In particular, the ALD method is very promising for obtaining homogeneous films necessary for further miniaturization of optoelectronic elements. ALD is also of great interest for the development of quantum structures, in particular for quantum wells, where a small number of layers are used and precise control of thickness and composition is required. It is important to note that the method is a simplified version of MBE, since it does not require ultrahigh vacuum. However, ALD growth of thick films takes much longer and requires intensive use of shutters.
8 Successive Ionic Layer Adsorption and Reaction (SILAR)
Successive ionic layer adsorption and reaction (SILAR) is a widely used method for the layer-by-layer synthesis of thin films, including films of II-VI compounds [92, 93]. The method has several names: in addition to SILAR, this method is referred to in various articles as layer-by-layer (LbL) synthesis or SILD (successive ionic layer deposition) [117]. The SILAR method consists of successive, multiple processes of adsorption of cations and anions with the formation of a poorly soluble compound on the substrate (see Fig. 10.9) [20, 117].
In the pioneering works of Nicolau – one of the founders of the method [92, 93] – it was shown that alternate dipping of the substrate into the solution containing cadmium and zinc soluble salts and then into the solution containing sulfur-anions followed by washing-up in deionized water allows to form polycrystalline epitaxial ZnS and CdS layers. Precursors used during these processes are listed in Table 10.9. According to Nicolau [92], the growth mechanism involves three most important steps: (i) specific adsorption of the most strongly adsorbed ions of the compound to be grown by substrate immersion in a solution of one of its salts, (ii) water rinsing of the excess solution still adhering to the substrate, and (iii) chemical reaction between the most strongly specifically adsorbed ions and the less strongly adsorbed ones by the subsequent substrate immersion in a solution containing the latter, entailing the growth. The application of this method for fabricating thin films for solar converters, photodetectors, LEDs, etc. is described in [117].
Numerous reactions at ionic layer deposition can be divided into different subgroups according to the basic types of interaction of cations and anions in the solutions [116, 117]. The main group involves the reactions that proceed without changing of the oxidation state of the ions. Such reactions include, for example, the reaction between adsorbed Cd2+, Zn2+ cations and HS− anions. The result of this interaction is the formation of sulfide layer on the substrate. The essential condition of the targeted SILAR synthesis is the washing-up of the grown layer from the remaining reagents of the previous stage. It is obvious that the compounds for which the best synthesis conditions of ionic layer deposition are satisfied involve those, which have the lowest solubility product values in the range of used pH values. They are cadmium and zinc sulfides and selenides: CdS, CdSe, ZnS, and ZnSe. When the substrate is washed in distilled water, some of the zinc ions are removed from the surface, but the pH at the surface is changed to 7 by the water, causing hydrolysis of the Zn and Cd ions which opposes the desorption.
If the substrate is c of Na2S, the S2− and HS− ions react on the surface, giving a layer of CdS or ZnS:
Despite the simplicity of the process, the synthesis due to its multistage nature is preferably carried out using special installations that operate in the automated mode according to the pre-defined program. As noted in review [100], the SILAR method has proven to be an inexpensive and convenient process for applying metal chalcogenides films on large regions. It is possible to use the substrates made of organic materials because the deposition takes place at or near room temperature. The main condition for obtaining the high-quality films is optimization of process parameters, selection of reagent concentrations, pH, and improvement of washing-up quality.
The concentration of the salt solutions should be high enough in order to promote the specific adsorption, but at the same, time the rinsing time must be sufficient to entail ion diffusion from the diffusion layer. Optimum magnitudes for the solution concentration-rinsing time parameters should be experimentally determined. The pH of solutions plays an important role. OH- may interfere with the specific anion adsorption entailing the contamination of the sulfide with hydroxide, may change the substrate surface state, especially for oxides, and may change the sulfide and substrate solubility. For example, the optimal parameters of ZnS films (homogeneous, adherent, compact, specularly reflecting) when deposited from ZnSO4 and Na2S reagents were obtained using ZnSO4 (0.005M, pH = 3.9) and Na2S (0.005M, pH = 11.6) solutions [92]. The growth rate in one cycle is usually in the range of 0.1–0.8 nm (Table 10.10). This means that more than 100 deposition cycles are needed to obtain a film with a thickness of 100–200 nm. One cycle usually takes 1–3 min. This means that the SILAR method is characterized by a very low deposition rate. However, in some studies, higher growth rates were also achieved due to the use of highly concentrated solutions. For example, in work [12] the average growth rate of the CdSe film was about 6–8 nm (see Fig. 10.10).
Variation of CdSe film thickness and cadmium and selenium ratio depending on number of immersion cycles under SILAR synthesis method. (Data extracted from Chaudhari et al. [12])
The experiment showed that the deposited ZnS and CdS films had good adhesion. It was also found that the synthesized films can be doped during the synthesis. There are many publications where the SILAR method is used to form cadmium and zinc sulfide films from aqueous and nonaqueous solutions with various dopants (Mn, Ni, Ti, Zn, et al.) [63, 64, 67, 90]. It should be noted that the complete coating of the substrate in the case of CdS occurs after about 50 synthesis cycles and the crystallinity of ZnS layers appears for the films with a thickness of about 250 nm. It is also shown that the composition of the deposited films, as well as microstrains and dislocation density, depends on the number of deposition cycles. As can be seen from Fig. 10.10, as the number of cycles increases, the composition of the CdSe film gradually approaches the stoichiometric ratio. Using ZnSe as an example, it was shown in [28] that, with an increase in the number of cycles, the number of defects and microstrains in the film decreases, while the band gap increases.
9 Chemical Bath Deposition (CBD)
Chemical bath deposition method (CBD) is an analogue of chemical vapor deposition method but carried out in a liquid medium. The method has proved to be one of the most available for obtaining II-VI films with high photovoltaic and sensor properties. It is most effectively used for deposition of CdS, CdSe, CdTe, ZnS, and ZnSe layers. In CBD, deposition of thin films takes place from aqueous solutions at low temperatures by a chemical reaction between dissolved precursors, with the help of a complexing agent. Among the advantages of the CDB method are the simplicity of its technological process (see Fig. 10.11), the use of temperatures up to 100oC, the ease of film doping, and high efficiency. CBD is suitable for growing nanofilms over large areas at low temperature. This method can also be used to synthesize multicomponent nanofilms. In addition, the CBD method allows the use of a wide range of substrates (in terms of material and geometry). The deposition of layers on the substrate is usually carried out without the risk of the substrate dissolving in the chemical bath.
A great number of reviews on CBD method are published nowadays [14, 37, 51, 62, 66, 91, 110]. In reviews [62, 66] the results of the CBD synthesis of metal sulfide, selenide, and telluride films with the analysis of their nucleation and growth processes are presented. The data of chemical synthesis of II-VI compound films in nanocrystalline form for photonics as well as cadmium and zinc sulfides are given in [37, 52, 91, 110].
It is believed that chemical bath deposition is based on the following steps [94]:
-
1.
Equilibrium between the complexing agent and water
-
2.
Formation/dissociation of ionic metal-ligand complexes (M(L)i)2−ik, where Lk− denotes one or more ligands
-
3.
Hydrolysis of the source of chalcogenides
-
4.
Formation of a solid body
In this process, the hydrolysis of the chalcogenide source (step 3) is critical because it provides the desired nonmetal particles that draw metal cations out of solution to form a solid film. This step is very sensitive to the pH and temperature of the solution.
At the CDB method of II-VI compounds, the reaction mixture is typically a mixture of an aqueous solution of Мn+ metal salt, a chalcogenizer (a source of chalcogenide-ions S2−, Se2−, Te2−), and complexing agents. Thiourea CS(NH2)2, hydrogen sulfide H2S, thiosulfate S2O32−, or thioacetamide CH3CSNH2 are used as a chalcogenizers in the production of sulfides: selenium hydrogen H2Se, selenourea C(NH2)2Se, and selenosulfate Na2SeSO3 – in the production of selenides, metal tellurides – in the production of H2Te telluride films. As a rule, aqueous ammonia or KOH is used as complexing agents. Some deposition modes for Cd-based compounds are shown in Table 10.11.
The ways of prediction of boundary conditions of solid phase deposition by analysis of ion equilibrium in the reaction system are suggested in [76]. The necessary condition of its formation is the supersaturation in the deposited compound. The use of such ligands for cadmium and zinc as chloride ions, tartrate ions, citrate ions, ammonia, ethylenediamine, triethanolamine, hydrazine, etc. in the reaction bath provides the control of phase formation. The strength of obtaining metal complex forms and the nature of ligands influence both the nucleation rate and its growth and due to the spatial structure the interaction between initial particles defining microstructure and morphology of the synthesized films [68, 79].
The ways of prediction of boundary conditions of solid phase deposition by analysis of ion equilibrium in the reaction system are suggested in [76]. The necessary condition of its formation is the supersaturation in the deposited compound. The use of such ligands for cadmium and zinc as chloride ions, tartrate ions, citrate ions, ammonia, ethylenediamine, triethanolamine, hydrazine, etc. in the reaction bath provides the control of phase formation. The strength of obtaining metal complex forms and the nature of ligands influence both the nucleation rate and its growth and due to the spatial structure the interaction between initial particles defining microstructure and morphology of the synthesized films [68, 79].
Work [79] provides comparative data on the influence of three different reaction baths varying in complexing agent composition: citrate, ammonia, and citrate-ammonia on activation energy of the cadmium sulfide synthesis process, thickness, average particle size, roughness, and elemental composition of deposited CdS films (see Table 10.12).
The concentration of complexing agents and the type of chalcogenizer allow to regulate the crystallographic orientation of the particles, which can vary from several nanometers to several microns. In addition, ligands are necessary for prevention of the formation of impurity phases (hydroxides, hydroxide salts, cyanamides) in the system. At the same time, as it is noted in [14, 80], the main role of nucleation and adhesive contact of the film with the substrate belongs to the hydroxide phase which activates the substrate surface. Thiourea C(NH2)2S, which is sulfocarbonate diamide, is most frequently used in the synthesis of CdS and ZnS films for photodetectors and other optoelectronic devices. A detailed review of its chemical properties and decomposition was made by the authors in [80, 84]. According to Markov and Maskaeva [76, 80], thiourea is susceptible to reversible hydrolytic decomposition in an alkaline solution with the formation of hydrogen sulfide and cyanamide. But there are fewer studies of the cadmium and zinc telluride deposition by the CBD method. The film deposition in this case is carried out using sodium telluride or TeO2 in the presence of strong reducing agents capable of converting tellurium (IV) into Te2− [21].
The CDB method usually produces stable, uniform in thickness, and almost stoichiometric in composition II-VI semiconductor films, which results in high dark resistivity and high photosensitivity of these films. The thickness of the deposited films is usually in the range from 0.02 μm to 1 μm for various materials. However, the quality of the films is higher in the lower thickness range of 0.02–0.05 μm. It is important that complete coverage of the substrate surface is observed even at a very small film thickness.
The main control over the CBD process is the selection and regulation of metal salt concentration, chalcogenizer, complexing agents, temperature, and pH of the reaction mixture [40, 77, 80]. In work [40] the complex study of the influence of deposition conditions (thiourea concentration, additives of ammonium sulfate and time of process) on thickness, element composition, and electrical resistance of chemically deposited CdS films was carried out (see Table 10.13).
Analysis of Table 10.13 shows that the thickness of the CdS films naturally increases with increasing thiourea concentration and the time of deposition and rather dramatically decreases at the introduction of ammonium sulfate into the reaction mixture. The used additive also decreases the Cd/S ratio, which is probably a consequence of the formation of impurity phases.
An important factor of the increasing film thickness is also the optimization of conditions and the correct choice of chalcogenizer. It is shown in work [80], providing data on deposition ZnSe films with a thickness of more than 1000 nanometers when using the sodium selenosulfate Na2SeSO3. Figure 10.12 shows the influence of temperature and synthesis time on the thickness of zinc selenide films.
Dependence of ZnSe film thicknesses on temperature and time of the CDB process with a sodium selenosulfate chalcogenizer. (Data extracted from Ref. [80])
Subsequent annealing of deposited films of II-VI compounds also has a significant effect on the electrical and structural properties of the films. As a rule, after annealing, the crystallinity of the films improves, the size of the crystallites increases, and the dislocation density decreases. As for the concentration of charge carriers, its value largely depends on the atmosphere used in the annealing process. It was also found that doping and microwave radiation are effective ways of film property modification in obtaining high-functioning II-VI films for photonics [38, 81].
10 Aerosol Spray Pyrolysis (ASP)
Aerosol spray pyrolysis (ASP) is a film production method based on thermal decomposition of aerosol obtained by sputtering the reaction mixture. The method is attractive in terms of its simplicity. Aerosol pyrolysis is a low-cost method in its use, and it is a preferable method for production of II-IV compounds nowadays. It allows to synthesize highly functional films with a large surface area, a high package density, and homogeneousness of particles in their size.
The method is characterized by a relatively high rate of film growth up to 100 nm/s and an ease of doping process [51]. The technology of this method consists of sputtering an aqueous solution containing metal salt, a chalcogenizer, and various functional additives on the substrate heated up to 100–500 °C in order to obtain metal chalcogenide films [31, 103].
The basic elements of technological installation for spray pyrolysis are the chamber where a substrate with a heating system is installed and the sputtering system. Pneumatic, electrostatic, and ultrasonic sputtering methods are commonly used for aerosol production. The wide review on influence of sputtering parameters on film quality to demonstrate the importance of optimization process is given in [103].
At pneumatic sputtering method, the flow rate of the gas carrier, typically air or nitrogen [51], is about 50–300 m/s, and the average size of droplets is in the range of 100–200 μm. The method is characterized by low-energy efficiency and relatively large droplet size. In the electrostatic sputtering method, the energy is supplied to a thin layer of disperse liquid by electrostatic charge. Coulomb forces applied to a film of liquid make it break down into droplets of 0.1–10 μm. The electrostatic sputtering method is influenced by the value of surface tension, viscosity, liquid conductivity, and electric field intensity. The main disadvantages of the method are the high energy costs and complexity of the equipment. The use of ultrasonic sputtering method converts liquid into aerosol state due to application of mechanical oscillations of ultrasonic frequency. The method is quite economical but has a relatively low sputtering rate (less than 2 cm3/min). The aerosol particles, depending on the specified conditions, have a diameter from 0.1 to 10 μm and are characterized by high monodispersity.
The properties of the synthesized films primarily depend on the choice of aerosol precursors (molecular solutions, sols, colloid dispersions), the bulk sputtering rate, the size of the aerosol droplets, and the substrate temperature. The use of aqueous solutions is associated with a large range of water-soluble metal salts, as well as safety conditions. For the deposition of cadmium and zinc sulfide films, solutions containing chlorides or organometallic metal compounds and thiourea are most commonly used. Precursors usually used for deposition of Cd-based compounds are listed in Table 10.14.
The mechanism of film formation in the application of spray pyrolysis is very complex and has been poorly studied so far [51]. In order to obtain a homogeneous solid layer, it is important that the droplet appears at the substrate immediately after evaporation of the solvent from it. However, during movement, the aerosol droplets gradually dry out and a solid crust is formed on their surface. When completely dried, hollow solid particles are formed, which deposition on the substrate leads to a deterioration of the film properties. To prevent this phenomenon, optimization of process parameters such as solution concentration, aerosol flow rate, substrate temperature, particle size, and distance to the substrate is necessary. It has been found that at low temperatures of the substrate, the deposited film is often cracked, and a loose low-adhesion coating is formed at considerable heating due to the rapid removal of the solvent. It should also be considered that the different volatility of the solution components can cause deviation of the film composition from the stoichiometric one while forming. A large number of works on synthesis of II-VI films for photodetectors and chemical sensors by spray pyrolysis method have been published [4, 22,23,24, 44, 114, 115, 119]. In addition to the evaluation of the influence of substrate temperature on their morphology, semiconductor and photoelectric properties [22], spray gas pressure [44], solution concentration [23], and optimization of synthesis conditions [114], a significant number of publications relate to film doping with various chemical elements: Al, La, Sm, Pb, F, Br, Fe, etc. ([4, 24, 115]; 89–91). In most cases, the basic functional properties of the deposited films are improved with the introduction of the dopаnt of 5–6% by weight into the film.
The crystallinity and the size of crystallites in deposited II-VI films depend on the time of deposition and the temperature of substrate. For example, it was established for CdS films, an increase in crystal quality was observed when substrate temperature was raised to 300 °C. At this temperature the grown CdS films were stoichiometric, polycrystalline in nature, having a hexagonal structure and containing uniformly distributed, and uneven spherical grains of size about 120 nm. The films deposited at 300 °C were n-type semiconducting with direct band gap of 2.44 eV and have minimum resistivity [73]. The effect of deposition time on film parameters is shown in Fig. 10.13 and Table 10.15. They refer to ZnS films obtained by spray pyrolysis at 450 °C [22]. As it can be seen from these results, after 10 minutes, the thickness of the layer reaches 1750 nm with an increase in crystallite sizes to 102 nm. The average film growth rate also increases to 205 nm/min by the 8th minute of the process. It also should be noted that as the deposition time increases, the crystallinity of the ZnS films increases with a decrease in dislocation density and microstrains in them.
Dependence of the thickness and average grain size of the ZnS films on the time of the spray pyrolysis process at a substrate temperature of 450 °C. (Data extracted from Ref. [22])
Thermal annealing of as-deposited films also strongly affects the properties of II-VI films. The lattice constant and grain size increased consistently with increase in annealing temperature of the films. Another factor affecting the crystallite size is film doping during deposition. It has been observed that, as a rule, crystallite size consistently decreases with an increase in metal doping concentration in the spray solution.
Since SPD is a solution-based chemical deposition method, the nature of the solution also plays an important role in determining the quality of the grown materials. Recently, Kerimova et al. [46] deposited nanocrystalline CdS films at different solution pH values using the SPD method. The films grown at pH 6.7 and 9.5 had a wurtzite crystal structure, while the film grown at pH = 10.2 was almost amorphous. Based on the structural and optical characteristics, it was concluded that the crystallinity and grain size of films deposited with SP decrease with increasing pH of the solution used.
11 Electrochemical Deposition
Electrodeposition is the process of depositing metals or semiconductors onto a conductive substrate by passing an electric current through an ionic electrolyte containing metal or semiconductor ions. It should be noted that the electroplating technique in the deposition of II-VI semiconductor materials dates back to the 1970s [30, 98]. A typical two-electrode configuration electrodeposition setup consists of a deposition vessel with an electrolyte, a magnetic stirrer, a hotplate, a power supply, a working electrode (conductive substrate), a counter electrode, and an additional reference electrode in the case of a three-electrode configuration. The use of a potentiostatic power supply is necessary due to the influence of the deposition voltage on the atomic percentage composition of the elements in the electroplated layer, which is one of the factors that determine the type of conduction of II-VI compounds [96, 111].
Both cathodic and anodic methods can be used to precipitate II-VI compounds in an aqueous medium, the first of which includes the joint precipitation of higher-valence metal ions and chalcogenide ions, and the second is based on metal corrosion in a chalcogenide medium. For example, for the deposition of CdTe layers, the component processes can be represented by Eqs. (10.3, 10.4, 10.5, 10.6 and 10.7) [108, 111]:
-
Cathodic
-
Anodic
It must be emphasized, however, that the actual electrodeposition chemistry that is involved is much more complex than that indicated by Eqs. (10.3, 10.4, 10.5, 10.6, and 10.7). It should be noted that in indicated above reactions the solution pH plays an important role. Cathodic electrodeposition is routinely carried out in acidic media; anodization on the other hand is facilitated at alkaline pHs by the stability of low-oxidation state chalcogen species in this range. The main disadvantage of anodic chemical synthesis is that thin film growth is self-limiting. Thus, films thicker than a few tens of nanometers are difficult to achieve. At the same time, much thicker films (several μm) can be realized through the cathodic strategy [108]. As a rule, cathodic deposition is mainly utilized due to its ability to produce stoichiometric thin films with good adherence to the substrate as compared to anodic deposition [96].
The precipitation of Cd-based compounds (CdSe, CdTe, CdS) can be carried out in both acidic and basic solutions [111]. The particle size depends on the anions used in the bath (chloride or perchlorate). For zinc compounds (ZnTe, ZnSe, ZnS), electrodeposition from standard acidic aqueous solutions is more difficult [61]. The films often contain an excess of Se or Te, which indicates that the self-regulation mechanism predicted by the Kroger theory is less effective. This may be due to the fact that the zinc potential is more negative than the cadmium potential by 0.36 V, and it is known that deposition close to the metal potential is favorable. Increasing the negative precipitation potential leads to new problems due to hydrogen evolution and further reduction of chalcogenide elements by parallel reactions. These parasitic reactions disrupt film growth. Examples of precursors that can be used for electrodeposition of II-VI semiconductors are listed in Table 10.16.
The deposition temperature depends on the electrolyte used. For electroplated semiconductor materials from aqueous solution, there is a limitation on the growth temperature due to the boiling temperature of water at 100 °C under standard atmospheric pressure, while the electroplating from other electrolytic baths can go as high as 160 °C. The electrolytic bath temperature increases the transport of species participated in reactions, which results in an increase in the deposition current density and rate of deposition of constituent elements or compounds. It was also shown that an increase in the crystallinity of as-deposited semiconductor material is achievable at higher growth temperature. Deposition of materials at a higher temperature provides the energy needed for the ions/atoms to move and deposit in a regular crystal structure. Current dc density during deposition process can vary over a very wide range from 0.8 to 200 mA/cm2. Platinum is usually used as the cathode.
Poor adhesion and nonuniform films are common problems when depositing films of II-VI compounds onto a smooth surface, as the contaminated surface provides nucleation sites facilitating the growth, which results in nonuniform film growth. Therefore, during the deposition of thin film, substrate cleaning is very important.
Ternary II-VI compounds can also be deposited using electrodeposition techniques. Thus, thin films of CdxSe1-x, CdxTe1-x, and Hg1-xCdxTe were formed using these methods [108, 111]. However, due to the presence of a greater number of active species in the solution, the choice of operational parameters for the electrosynthesis of semiconductor alloys in this case becomes more laborious.
Electroplating is a simple and cheap method for depositing II-VI semiconductor compounds. However, this method also has disadvantages. According to Ojo and Dharmadasa [96], the disadvantages of this method include:
-
Instability of current density during deposition
-
Difficulties with control and regulation of ions within the electrolytic bath
-
Extrinsic doping of electrolytic bath by the electrodes
-
Nonuniformity of electrodeposited semiconductor layers
-
Need for post-growth treatment
Despite intensive development, electrochemical methods have not yet found a wide practical application for the production of photonics materials and chemical sensors. There is a high degree of dependence of the properties of the deposited films on the deposition mode and electrolyte composition [29]. Using ZnS as an example, Table 10.17 and Fig. 10.13 show how strong the influence of deposition modes on the parameters of films deposited by this method can be. It can be seen that a change in the potential applied to the electrode from -1.0 to -1.3 V is accompanied by a doubling of the growth rate and film thickness, a threefold decrease in the dislocation density, a significant increase in the roughness and microstress in the layer, and a decrease in the size of crystallites (see Fig. 10.14).
Dependence of the average size of crystallites and microstrains in electrodeposited ZnS films on the magnitude of the potential at the electrode. (Adapted with permission from Ref. [29]. Copyright 2017: Elsevier)
Recently, much attention has been paid to the development of pulsed electrodeposition of II-VI compounds, which allows to increase the deposition rate significantly and obtain films with improved morphological and structural properties due to the effect of short high-voltage pulses (10–4–10–5 s) of polarizing voltage on the electrode-solution phase boundary [75].
There is also an electrophoretic deposition of II-VI semiconductors, which is based on the phenomenon of electrophoresis in colloid systems [30]. An overview of electrophoretic film deposition is published in [9]. The deposited layers are loose and weakly bonded to the substrate. Thus, their heat treatment is required.
As regards the structure of the deposited films of the II-VI compounds, in most cases the deposited samples consist of an amorphous phase. Therefore, to transfer them to the crystalline state, additional heat treatment after deposition is required. For example, Ohta and Ohgai [95] used annealing at 653–683 K for 5 h to crystallize electrodeposited ZnTe films. They found that the electrodeposited thin films of zinc telluride crystallized at an annealing temperature above 653 K. The electrodeposited thin films of zinc telluride, post-annealed at 653–673 K, consisted of binary phases (ZnTe and Te). On the contrary, the electrodeposited thin film after annealing at 683 K consisted of single-phase zinc telluride. Crystallization was also accompanied by an increase in the optical band gap and a decrease in resistivity.
12 Close-Space Sublimation Method
Close-space sublimation (CSS) method is inherently a form of thermal evaporation. The CSS technique is one of the most economical and cheap. The CSS process offers the advantages of a simple deposition apparatus and high transport efficiency under low vacuum conditions at moderate temperatures. This simplifies the scaling of the process, which is necessary for the organization of large-scale production. Films and crystals of II-VI semiconductors such as CdS [104], CdTe [48, 113], CdSe [58], CdTe/CdS [104], ZnTe [105], ZnSe [119], and others were obtained by this method.
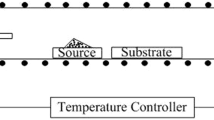
The CSS method is based on the thermal-heating-induced sublimation of a material followed by vapor condensation onto a closely spaced substrate [112]. Figure 10.15 shows the schematic diagram of the CSS system and substrate sandwiched in the growth chamber. The source and substrate are separated by spacers enclosed in a reaction chamber and held between two graphite susceptors inside a quartz reactor. The cross dimensions of the source and the substrate greatly exceed the distance between them. The distance between the source material and the substrate usually does not exceed 4 mm. The source of heating is usually from visible or infrared lamps outside the reactor facing the blocks of graphite, used to reduce the temperature gradient in the chamber. Other heaters may also be used. In particular, Plaza et al. [104] used the SiC electrical heating elements for these purposes. A set of thermocouples monitored the temperature of the graphite blocks for both the source and the substrate temperatures. A diffusion-controlled transport mechanism takes place during the temperature difference between the source and substrate. The material to be deposited is placed on a boat heated to temperatures above the substrate temperature. Polycrystals of II-VI compounds, synthesized by various methods, are usually used as a source. You can also use single-crystal or polycrystalline wafers, powders, pellets or pieces, and a thick II-VI film deposited onto glass or molybdenum substrate. Due to the temperature difference, the material sublimating from the source transfers to the substrate. The deposition rate depends upon the source, spacing, substrate temperature, and ambient gas nature.
In “classic” CSS, the substrate to be coated and the source material are both placed in a vacuum chamber, which is pumped out [104, 113]. In particular, in [113] the chamber for CSS was evacuated down to 10−3 mbar. Plaza et al. [104] deposited films at pressures below 1 mbar. However, it has been shown that the precipitation of II-VI compounds can be carried out without the use of vacuum [48]. It has been found that a hydrogen or inert gas atmosphere is also suitable for this process [86]. For example, Cruz et al. [19] for deposition of CdS/CdTe films used the atmosphere of argon, nitrogen, or a mixture of oxygen/argon with a pressure between 5 and 15 Torr.
The temperature of the source material and the substrate depends on the deposited material. For example, during the deposition of CdS films, the source temperature was 680 °C, and substrate was kept at 520 °C [32]. The film growth rate was ~7–8 nm/min. Cruz et al. [19] deposited CdS films, using for the source and substrate the temperatures 660 °C and 540 °C, respectively. The rate of deposition varied in the range from 50 to 150 nm/min. The size of crystallites in CdS films increased with increasing temperature and film thickness (Fig. 10.16). Typically, film thickness increases linearly with deposition time. According to Plaza et al. [104], the best source temperature for the homogeneous deposition of CdS films was 800 °C. Deposited films were quite compact, with microcrystals close to 30 μm in size. For higher-source temperatures, the structure of the crystals was distorted. Shah et al. [113] for deposition of CdTe films used source and substrate heated to 450 °C and 300 °C, respectively. Similar parameters for deposition of CdTe films were used by Potlog et al. [105]. CdTe thin films were deposited at a temperature of the source and substrate of 340 °C and 590 °C, respectively. At the same time, Plaza et al. [104] believe that the source and the substrate during CdTe deposition should have temperatures 700 °C and 590 °C, respectively. It was established that the grain size in CdTe films was much smaller (typically in the range between 2 and 3 μm) than in the case of the CdS films. For the deposition of ZnTe films, Potlog et al. [105] used source and substrate heated to temperatures of 660 °C and 430 °C. Undoped ZnTe layers had a resistivity of (106–108) Ω·cm. The resistivity of ZnTe thin films grown from ZnTe crystals doped with As reached a value of (102–103) Ω·cm.
(a) Average grain sizes of CSS CdS layers deposited at different temperature. (b, c) Plan-view SEM images of CSS CdS films grown at (b) 500 °C (d = 25 nm) and (c) 560 °C (d = 500 nm). (Reprinted with permission from Paudel et al. [101]. Copyright 2014: Springer)
Depending on the conditions, the film growth process can be described using the following mechanisms [2]: (a) Diffusion-limited transport: The deposited materials migrate to the substrate through the ambient gas and collide several times with gas molecules before condensing on the substrate. (b) Langmuir’s theory: The deposited materials are moved directly to the substrate without any gas phase collision.
If we compare CSS deposition technology with sputtering and chemical bath deposition (CBD) methods, often used in the formation of II-VI compound films, then due to the high substrate temperatures, grain size and roughness in CSS films are larger than in CBD and sputtered films [19]. CBD and sputtering have also much lower deposition rate than CSS method. In addition, CBD is a wet process; therefore, in terms of manufacture compatibility, CSS technology is the most preferred growth method. However, as CSS is a high-rate deposition technique, it is difficult to get very thin films (d<100 nm) with nanosize crystallites using this process. In this case, the control of some parameters, such as pressure and source/substrate temperatures, is essential for obtaining thin continuous films. In addition, Paudel et al. [101] believe that a significant disadvantage of CSS films is also the formation of voids (holes) in the films, caused by the large grain size due to growth at high temperature. It is these shortcomings of CSS deposition technology that significantly limit its use in the development of gas sensors and photodetectors.
References
Afzaal M, Crouch D, O’Brien P, Park J-H (2002) Metal-organic chemical vapour deposition of II-VI semiconductor thin films using single-source approach. MRS Proc 730:V3.5.1–V3.5.6
Alamri SN (2003) The growth of CdTe thin film by close space sublimation system. Phys Status Solidi 200(2):352–360
Alias MFA, Salman M, Al-Douri AAJ (2014) The role of thickness on structural properties of thin CdS films prepared by pulse laser deposition. Int J Adv Sci Tech Res 4:620–626
Attaf A, Derbali A, Saidi H, Benamra H, Aida MS, Attaf N et al (2020) Physical properties of Pb doped ZnS thin films prepared by ultrasonic spray technique. Phys Lett A 384:126199
Baghchesara MA, Yousefi R, Cheraghizade M, Jamali-Sheini F, Sáaedi A (2016) Photocurrent application of Zn-doped CdS nanostructures grown by thermal evaporation method. Ceram Int 42(1):1891–1896
Barman B, Bangera KV, Shivakumar GK (2019) ZnxCd1-xS thin films: A study towards its application as a reliable photodetector. Superlattice Microst 137:106349
Berlin EV, Seidman LA (2014) Obtaining Thin films by Reactive Magnetron Sputtering. Technosphere, Moscow. (in Russian)
Berlin EV, Dvinin SA, Seidman LA (2007) Vacuum technology and equipment for the applying and etching thin films. Technosphere, Moscow, p 176. (in Russian)
Besra L, Liu M (2007) A review on fundamentals and applications of electrophoretic deposition (EPD). Progr Mater Sci 52:1–61
Buckingham MA, Catherall AL, Hill MS, Johnson AL, Parish JD (2017) Aerosol-assisted chemical vapor deposition of CdS from xanthate single source precursors. Cryst Growth Des 17(2):907–912
Chalana SR, Vinodkumar R, Navas I, Ganesan V, Pillai VPM (2012) Influence of argon ambience on the structural, morphological and optical properties of pulsed laser ablated zinc sulfide thin films. J Lumin 132:944–952
Chaudhari KB, Gosavi NM, Deshpande NG, Gosavi SR (2016) Chemical synthesis and characterization of CdSe thin films deposited by SILAR technique for optoelectronic applications. J Sci Adv Mater Dev 1(4):476–481
Cheon JW, Talaga DS, Zink JI (1997) Photochemical deposition of ZnS from the gas phase and simultaneous luminescence detection of photofragments from a single-source precursor, Zn (S2COCHMe2)2. J Am Chem Soc 119(1):163–168
Chopra KL, Das SR (1983) Thin Film solar cells. Plenum Press, New York–London
Choy KL (2003) Chemical vapour deposition. Progr Mater Sci 48:57–170
Chu TL, Chu SS (1995) Thin film II-VI photovoltaics. Solid State Electron 38(3):533–549
Colegrove E, Albin DS, Moutinho HR, Amarasinghe M, Burst JM, Metzger WK (2020) Scalable ultrafast epitaxy of large-grain and single-crystal II-VI semiconductors. Sci Rep 10:2426
Coutts TJ (ed) (1978) Active and Passive Thin Film Devices. Academic Press, London, p 858
Cruz LR, Sousa Fernandes JA, Ferreira CL, Pinheir WA (2014) Microstructural and optical properties of CSS and CBD-CdS thin films for photovoltaic solar cells. Revista Mater 19(3):228–234
Decher G, Schlenoff JB (eds) (2003) Multilayer thin films: Sequential assembly of nanocomposite materials. Wiley-VCH, New York, p 511
Deivanayaki S, Jayamurugan P, Mariappan R, Ponnuswamy V (2010) Optical and structural characterization of CdTe thin films by chemical bath deposition technique. Chalcogenide Lett 7:159–163
Derbali A, Attaf A, Saidi H, Benamra H, Nouadji M, Aida MS et al (2018a) Investigation of structural, optical and electrical properties of ZnS thin films prepared by ultrasonic spray technique for photovoltaic applications. Optik 154:286–293
Derbali A, Saidi H, Attaf A, Benamra H, Bouhdjer A, Attaf N et al (2018b) Solution flow rate influence on ZnS thin films properties grown by ultrasonic spray for optoelectronic application. J Semicond 39:093001
Derbali A, Attaf A, Saidi H, Aida MS, Benamra H, Attaf R et al (2021) Br doping effect on structural, optical and electrical properties of ZnS thin films deposited by ultrasonic spray. Mater Sci Eng A 268:115135
Doering R, Nishi Y (eds) (2008) Handbook of Semiconductor Manufacturing Technology. CRC press, New York
Garcia LV, Loredo SL, Shaji S, Aguilar Martinez JA, Krishnan B (2016) Structure and properties of CdS thin films prepared by pulsed laser assisted chemical bath deposition. Mater Res Bull 83:495–467
Garje SS, Ritch JS, Eisler DJ, Afzaal M, O’Brien P, Chivers T (2006) Chemical vapour deposition of II-VI semiconductor thin films using M[(TePiPr2)2N]2 (M = Cd, Hg) as single-source precursors. J Mater Chem 16:966–969
Geethanjali PM, Deepa K, Remadevi TL (2021) Effect of number of cycles on SILAR deposited ZnSe thin films. AIP Conf Proc 2352:020011
Ghezali K, Mentar L, Boudine B, Azizi A (2017) Electrochemical deposition of ZnS thin films and their structural, morphological and optical properties. J Electroanal Chem 794:212
Glasstone S (1960) The fundamentals of electrochemistry and electrodeposition. American Electroplaters Society/ Franklin Pub Co, p 90
Gurav A, Kodas T, Pluym T, Yun X (1993) Aerosol processing of materials. Aerosol Sci Technol 19:411–452
Han J, Liao C, Jiang T, Spanheimer C, Haindl G, Fu G et al (2011) An optimized multilayer structure of CdS layer for CdTe solar cells. J Alloys Compounds 509(17):5285–5289
Hartmann H. (1975) Vapour phase epitaxy of II-VI compounds: a review, In: Vapour growth and epitaxy, proceedings of the third international conference on vapour growth and epitaxy, Amsterdam, The Netherlands, 18–21 August 1975, pp 323–332
Hendi AA (2014) Optical characterizations of CdS thin films grown by pulsed laser deposition technique. J Nano Electron Optoelectron 9:596–600
Hernandez-Calderon I (2013) Epitaxial growth of thin films and quantum structures of II-VI visible-bandgap semiconductors. In: Henini M (ed) Molecular Beam Epitaxy. Elsevier, pp 311–346
Ho S (2016) A brief review of the growth of pulsed laser deposited thin films. J Appl Sci Technol 14(6):1–6
Hodes G (2002) Chemical Solution Deposition of Semiconductor Films. Marcel Dekker Inc., New York, p 388
Husham M, Hassan Z, Selman A (2016) Synthesis and characterization of nanocrystalline CdS thin films for highly photosensitive self-powered photodetector. Eur Phys J Appl Phys 74:10101
Hwang DH, Ahn JH, Hui KN, Hui KS, Son YG (2012) Structural and optical properties of ZnS thin films deposited by RF magnetron sputtering. Nanoscale Res Lett 7:26
Islam S, Hossain T, Sarwar H, Junaebur RM (2020) A systematic study on chemically deposited cadmium sulfide (CdS) thin film. J Theor Appl Phys 14(3):265–274
Jaeger RC (2002) Film Deposition: Introduction to Microelectronic Fabrication. Prentice Hall Upper Saddle River, New Jersey, p 303
Kalita PKR, Sarma BK, Das HL (2000) Structural characterization of vacuum evaporated ZnSe thin films. Bull Mater Sci 23:313–317
Karam NH, Wolfson RG, Bhat IB, Ehsani H, Ghandhi SK (1993) Growth and characterization of CdTe, HgTe and HgCdTe by atomic layer epitaxy. Thin Solid Films 225:261–264
Kathalingam A, Valanarasu S, Ahamad T, Alshehri SM, Kim H (2020) Spray pressure variation effect on the properties of CdS thin films for photodetector applications. Ceram Int 47:7608–7616
Kennedy J, Murmu PP, Gupta PS, Carder DA, Chong SV, Leveneur J, Rubanov S (2014) Effects of annealing on the structural and optical properties of zinc sulfide thin films deposited by ion beam sputtering. Mater Sci Semicond Process 26:567–566
Kerimova A, Bagiyev E, Aliyeva E, Bayramov A (2017) Nanostructured CdS thin films deposited by spray pyrolysis method. Phys Status Solidi C 14(6):1600144
Ketchemen KIY, Mlowe S, Nyamen LD, Ndifon PT, Revaprasadu N, O’Brien P (2018) CdS thin films deposition by AACVD: effect of precursor type, decomposition temperature and solvent. J Mater Sci Mater Electron 29(17):14462–14470
Khomchenko VS, Sopinskyy MV, Dan’ko VA, Olkhovik GP (2020) Doping the thin films by using the original close space sublimation method. Semicond Phys Quant Electron Optoelectron (SPQEO) 23(1):5–28
Kim D, Park Y, Kim M, Choi Y, Park YS, Lee J (2015) Optical and structural properties of sputtered CdS films for thin film solar cell applications. Mater Res Bull 69:78–83
Konagai M, Takemura Y, Yamasaki K, Takahashi K (1993) Self-limiting growth of zinc chalcogenides and their superlattices. Thin Solid Films 225:256–260
Korotcenkov G, Cho BK (2010) Synthesis and deposition of sensor materials. In: Korotcenkov G (ed) Chemical Sensors: Fundamentals of Sensing Materials, General Approaches, vol 1. Momentum Press, New York, USA, pp 215–304
Kozhevnikova NS, Vorokh AS, Uritskaya AA (2015) Cadmium sulfide nanoparticles obtained by chemical bath deposition. Russ Chem Rev 84:225–250
Kozlovsky VI, Martovitsky VP, Sannikov DA, Kuznetsov PI, Yakushcheva GG, Jitov VA (2003) MOVPE growth and characterization of hexagonal CdS epilayers and CdS-based QW structures on CdS and ZnCdS substrates. J Cryst Growth 248:62–66
Kumar P, Saxena N, Gupta V (2018) Ion beam assisted fortification of photoconduction and photosensitivity. Sens Actuators A 279:343–350
Kumar P, Saxena N, Gupta V (2020) Vital role of Ar ambient pressure in controlled properties of nanocrystalline CdS thin films. J Mater Sci - Mater Electron 31:6755–6763
Kuznetsov PI, Yakushcheva GG, Jitov VA, Zakharov LY, Shchamkhalova BS, Kozlovsky VI et al (2006) MOVPE growth and study of ZnCdSe/ZnSSe MQW structures for green VCSELs. Phys Status Solidi 3:771–775
Li J, Chen J, Sestak MN, Thornberry C, Collins RW (2009) Spectroscopic ellipsometry studies of thin film CdTe and CdS: From dielectric functions to solar cell structures. In: Proceeding of 34th IEEE photovoltaic specialists conference (PVSC). 11152208
Li K, Lin X, Song B, Kondrotas R, Wang C, Lu Y et al (2021) Rapid thermal evaporation for cadmium selenide thin-film solar cells. Fron Optoelectron 14(4):482–490
Liang G-X, Fan P, Zheng Z-H, Luo J-T, Zhang D-P, Chen C-M, Cao P-J (2013) Room-temperature preparation and properties of cadmium sulfide thin films by ion-beam sputtering deposition. Appl Surf Sci 273:491–495
Lin J-S, Wei S-S, Yu Y-T, Hsu C-H, Kao W-H, Chen W-S et al (2013) Synthesis and characterization of ZnTe thin films on silicon by thermal-furnace evaporation. Proc SPIE 8913:89130K
Lincot D (2005) Electrodeposition of semiconductors. Thin Solid Films 487:40–48
Lincot D, Froment M, Cachet H (1999) Advances in Electrochemical Science and Engineering. Wiley-VCH, Weinheim, p 165
Lindroos S, Kanniainen T, Leskela M, Rauhala E (1995) Deposition of manganese-doped zinc sulfide thin films by the successive ionic layer adsorption and reaction (SILAR) method. Thin Solid Films 263:79–84
Lindroos S, Kanniainen T, Leskelä M (1997) Growth of zinc sulfide thin films by the successive ionic layer adsorption and reaction (Silar) method on polyester substrates. Mater Res Bull 32(12):1631–1636
Liu Z (2010) Laser applied coatings. In: Materials Science and Materials Engineering Materials Science and Materials Engineering, Shreir’s Corrosion, vol 4. Elsevier, pp 2622–2635
Lokhande CD (1991) Chemical deposition of metal chalcogenide thin films. Mater Chem Phys 27:1–43
Lokhande CD, Sankapal BR, Pathan HM, Muller M, Giersig M, Tributsch H (2001) Structural studies on successive ionic layer adsorption (SILAR)-deposited CdS thin films. Appl Surf Sci 181:277–282
Londhe PU, Rohom AB, Bhand GR, Jadhav S, Lakhe MG, Chaure NB (2017) Effect of complexing agent on the chemically deposited ZnS thin film. J Mater Sci Mater Electron 28(7):5207–5214
Mahdi MA, Hassan JJ, Ng SS, Hassan Z, Ahmed NM (2012) Synthesis and characterization of single-crystal CdS nanosheet for high-speed photodetection. Physica E Low Dimens Syst Nanostruct 44(7-8):1716–1721
Mahdi MA, Hassan JJ, Ahmed NM, Ng SS, Hassan Z (2013) Growth and characterization of CdS single-crystalline micro-rod photodetector. Superlattice Microst 54:137–145
Maissel LI, Glang R (eds) (1970) Handbook of Thin Film Technology. McGraw-Hill Book Company, New York
Majid A, Bibi M (2018a) Wet chemical synthesis methods. In: Cadmium based II-VI Semiconducting Nanomaterials, Topics in Mining, Metallurgy and Materials Engineering. Springer, Cham, Switzerland, pp 43–101
Majid A, Bibi M (2018b) Vapor deposition synthesis. In: Cadmium based II-VI Semiconducting Nanomaterials, Topics in Mining. Metallurgy and Materials Engineering. Springer, Cham, Switzerland, pp 103–144
Maki SA, Hassun HK (2018) Effect of Aluminum on characterization of ZnTe/n-Si heterojunction photo detector. J Phys Conf Ser 1003:012085
Maliy L, Mamaev A, Mamaeva V (2017) Electrochemical high-energy deposition of CdSe nanostructures: modelling, synthesis and characterization. J Appl Electrochem 47(9):1073–1082
Markov VF, Maskaeva LN (2010) Calculating the boundary conditions of the formation of solid-phase metal sulfides and selenides by deposition with thio- and selenourea. Russ J Phys Chem A 84:1288–1293
Markov VF, Maskaeva LN, Ivanov PN (2006) Chemical bath deposition of metal sulfide films: modeling and experiment. Ural Branch of RAS, Ekaterinburg, p 218
Martin PM (2009) Handbook of Deposition Technologies for Films and Coatings: Science, Applications and Technology. William Andrew, p 936
Maskaeva LN, Markov VF, Forostyanaya NA, Kutyavina AD, Mokrousova OA (2016) Kinetic aspects of hydrochemical deposition of cadmium sulfide from solutions with diverse ligand backgrounds. Russ J Gen Chem 86(10):2273–2281
Maskaeva LN, Markov VF, Fedorova EA, Kuznetsov MV (2018) Influence of the conditions of the chemical bath deposition of thin ZnSe films on their morphology and internal mechanical stresses. Russ J Appl Chem 91(9):1528–1537
Maskaeva LN, Markov VF, Lipina OA, Pozdin AV, Anokhina LA (2020) Morphology, structure and optical properties of nanocrystalline CdSe films doped with copper. Russ J Phys Chem A 94(12):2441–2449
McClean IP, Thomas CB (1992) Photoluminescence study of MBE-grown films on ZnS. Semicond Sci Technol 7(11):1394
McLaughlin M, Sakeek H, Maguire PD, Graham WG, Molloy J, Morrow T, Laverty S (1993) Properties of ZnS thin films prepared by 248-nm pulsed laser deposition. Appl Phys Lett 63(14):1865–1867
Mertschenk B, Beck F, Bauer W (2005) Thiourea and Thiourea Derivatives. Wiley-VCH Verlag Gmb H and Co, KGaA, Weinheim, p 15
Ming Z, Ding C, Li B, Zhang J, Li W, Wu L, Feng L, Wu J (2013) Properties study of ZnS thin films fabricated at different substrate temperatures by pulsed laser deposition. Adv Mater Res 821-822:835–840
Mirsagatov SA, Uteniyazov AK (2012) Injection photodiode based on p-CdTe film. Tech Phys Lett 38(1):34–37
Moger SN, Mahesha MG (2020) Colour tunable co-evaporated CdSxSe1-x (0 < x < 1) ternary chalcogenide thin films for photodetector applications. Mater Sci Semicond Proc 120:105288
Moholkar AV, Shinde SS, Babar AR, Sim K, Lee HK, Rajpure KY, Patil PS (2011) Synthesis and characterization of Cu2ZnSnS4 thin films grown by PLD: Solar cells. J Alloys Compd 509:7439–7446
Moholkar AV, Shinde SS, Agawane GL, Jo SH, Rajpure KY, Patil PS, Bhosale CH, Kim JH (2012) Studies of compositional dependent CZTS thin film solar cells by pulsed laser deposition technique: An attempt to improve the efficiency. J Alloys Compd 544:145–151
Munusamy G, Peruma S (2020) Structural and optical properties of Silar deposited CdS and Ni, Ti & Zn doped CdS thin films. Int J Thin Film Sci Tec 9(3):169–174
Nair PK, Nair MTS, Garcia VM, Arenas OL, Pena Y, Castillo A et al (1998) Semiconductor thin films by chemical bath deposition for solar energy related applications. Sol Energy Mater Sol Cells 52:313–344
Nicolau YF (1985) Solution deposition of thin solid compound films by a successive ionic-layer adsorption and reaction process. Appl Surf Sci 22:1061–1074
Nicolau YF, Dupuy M, Brunel M (1990) ZnS, CdS, and Zn1−xCdxS thin films deposited by the successive ionic layer adsorption and reaction process. J Electrochem Soc 137:2915
Niesen TP, De Guire MR (2001) Review: Deposition of ceramic thin films at low temperatures from aqueous solutions. J Electroceramics 6:169–207
Ohta J, Ohgai T (2021) Post-annealing effects on the structure and semiconductor performance of nanocrystalline ZnTe thin films electrodeposited from an aqueous solution containing citric acid. Appl Sci 11:10632
Ojo AA, Dharmadasa IM (2018) Electroplating of semiconductor materials for applications in large area electronics: A review. CoatingsTech 8:262
Palve AM (2019) Deposition of Zinc Sulfide thin films from Zinc(II) Thiosemicarbazones as single molecular precursors using aerosol assisted chemical vapor deposition technique. Front Mater 6:46
Pandey RK, Sahu SN, Chandra S (1996) Handbook of Semiconductor Electrodeposition. Marcel Dekker, New York, NY
Patel NG, Panchal CJ, Makhija KK (1994) Use of Cadmium Selenide thin films as a Carbon dioxide gas sensor. Cryst Res Technol 29:1013–1020
Pathan HM, Lokhande CD (2004) Deposition of metal chalcogenide thin films by successive ionic layer adsorption and reaction (SILAR) method. Bull Mater Sci 27(2):85–111
Paudel JL, Xiao C, Yan Y (2014) Close-space sublimation grown CdS window layers for CdS/CdTe thin-film solar cells. J Mater Sci Mater Electron 25:1991–1998
Pawar SM, Pawar BS, Kim JH, Joo O-S, Lokhande CD (2011) Recent status of chemical bath deposited metal chalcogenide and metal oxide films. Current Appl Phys 11:117–161
Perednis D, Gauckler LJ (2005) Thin film deposition using spray pyrolysis. J Electroceramics 14:103–111
Plaza JL, Martinez O, Rubio S, Hortelano V, Diegueza E (2013) СdS and CdTe films by close space vapour sublimation by using SiC resistive elements. Cryst Eng Comm 15:2314–2318
Potlog T, Spalatu N, Fedorov V, Maticiuc N, Antoniuc C, Botnariuc V, et al (2011) The performance of thin film solar cells employing photovoltaic ZnSe/CdTe, CdS/CdTe and ZnTe/CdTe heterojunctions, In: Proceedings of 37th IEEE Photovoltaic Specialists Conference. 19–24 June 2011, Seattle, WA, US, 12710867
Putkonen M, Niinistö L (2005) Organometallic precursors for atomic layer deposition. Top Organomet Com 9:125–145
Qi X-Y, Pu K-Y, Fang C, Fan Q-L, Tang D-F, Wen G-A et al (2008) Cadmium telluride nanocrystals: synthesis, growth mode and effect of reaction temperature on crystal structures. NANO Brief Reports and Reviews 3(2):109–115
Rajeshwar K (1992) Electrosynthesized thin films of group II-VI compound semiconductors, alloys and superstructures. Adv Mater 4(1):23–29
Rondiya S, Rokade A, Funde A, Karthab M, Pathanb H, Jadkar S (2017) Synthesis of CdS thin films at room temperature by RF-magnetron sputtering and study of its structural, electrical, optical and morphology properties. Thin Solid Films 631:41–49
Sadovnikov I (2019) Synthesis, properties and applications of semiconductor nanostructured zinc sulfide. Russ Chem Rev 88:571–593
Saha S, Johnson M, Altayaran F, Wang Y, Wang D, Zhang Q (2020) Electrodeposition fabrication of chalcogenide thin films for photovoltaic applications. Electrochemistry 1:286–321
Seth A, Lush GB, McChue JC, Singh VP, Flood D (1999) Growth and characterisation of CdTe by CSS on metal substrates. Sol Energy Mater Sol Cells 59:35–41
Shah NA, Ali A, Aqili AKS, Maqsood A (2006) Physical properties of Ag-doped cadmium telluride thin films fabricated by closed–space sublimation technique. J Cryst Growth 290(2):452–458
Shelke NT, Karle SC, Karche BR (2020) Photoresponse properties of CdSe thin film photodetector. J Mater Sci Mater Electron 31:15061–15069
Shkir M, Khan ZR, Alshammari AS, Gandouzi M, Ashraf IM, AlFaify S (2021) A comprehensive experimental investigation of La@CdS nanostructured thin films: Structural, opto-nonlinear and photodetection properties. Surf Interfaces 24:101063
Tolstoi VP (1993) Synthesis of thin-layer structures by the ionic layer deposition method. Russ Chem Rev 62(3):237–242
Tolstoy VP (2006) Successive ionic layer deposition. The use in nanotechnology. Russ Chem Rev 75(2):161–175
Tong XL, Jiang DS, Li Y, Liu ZM, Luo MZ (2006) Femtosecond pulsed laser deposition of CdS thin films onto quartz substrates. Phys Status Solidi A 203:1992–1998
Triboulet R, N’tep JM, Barbe M, Lemasson P, Mora-Sero I, Munoz V (1999) Some fundamentals of the vapor and solution growth of ZnSe and ZnO. J Cryst Growth 198(199):968–974
Varitimos TE, Tustison RW (1987) Ion beam sputtering of ZnS thin films. Thin Solid Films 151(1):27–33
Vishwakarma R (2015) Еffect of substrate temperature on ZnS films prepared by thermal evaporation technique. J Theor Appl Phys 9:185–192
Vossen JL, Kern W (eds) (1978) Thin Film Processes. Academic, New York, p 564
Xin ZJ, Peaty RJ, Rutt HN, Eason RW (1999) Epitaxial growth of high quality ZnS films on sapphire and silicon by pulsed laser deposition. Semicond Sci Technol 14:695–698
Yano S, Schroeder R, Ullrich B, Sakai H (2003) Absorption and photocurrent properties of thin ZnS films formed by pulsed laser deposition on quartz. Thin Solid Films 423:273–276
Yoshikawa A, Yamaga S, Tanaka K, Kasai H (1985) Growth of low-resistivity high-quality ZnSe, ZnS films by low-pressure metalorganic chemical vapour deposition. J Cryst Growth 72(1–2):13–16
Zhang R, Wang B, Wei L (2008) Influence of RF power on the structure of ZnS thin films grown by sulfurizing RF sputter deposited ZnO. Mater Chem Phys 112:557–561
Zhang W, Zeng X, Lu J, Chen L (2013) Phase controlled synthesis and optical properties of ZnS thin films by pulsed laser deposition. Mater Res Bull 48:3843–3846
Acknowledgments
G. Korotcenkov is grateful to the State Program of the Republic of Moldova, project 20.80009.5007.02, for supporting his research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Markov, V.F., Korotcenkov, G., Maskaeva, L.N. (2023). Thin Films of Wide Band Gap II-VI Semiconductor Compounds: Features of Preparation. In: Korotcenkov, G. (eds) Handbook of II-VI Semiconductor-Based Sensors and Radiation Detectors. Springer, Cham. https://doi.org/10.1007/978-3-031-19531-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-19531-0_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-19530-3
Online ISBN: 978-3-031-19531-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)