Abstract
Phenolic acids are one of the major classes of phenolic compounds occurring as secondary metabolites in plants; among them, hydroxybenzoic and hydroxycinnamic acids are outstanding. These compounds are widespread in the human diet through plant-based foods, where they contribute to sensory and functional properties. Their consumption has also been associated with positive effects in human health, owing to their recognized biological activities (e.g., antioxidant, anti-inflammatory, antimicrobial, antidiabetic, or anticarcinogenic). Technological and functional properties of phenolic acids have made them interesting compounds for food, pharmaceutical, and cosmetic companies, so that suitable preparation processes are required to meet their increasing research and industrial demand. To fulfill these needs, an efficient production of pure compounds is required that cannot be fully satisfied by their isolation from natural sources or chemical synthesis, which suffer limitations such as low yield, time-consuming, or non-environmentally friendly processes. Biotechnological approaches including the construction of heterologous plant or microbial systems can be an alternative for enhancing phenolic acid production or addressing pathways toward the biosynthesis of particular target compounds. This chapter offers an overview on phenolic acids occurrence in food and natural sources, biosynthesis and advances in their biotechnological production.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Phenolic acids are one of the major classes of phenolic compounds (also commonly referred to as polyphenols) that occur as secondary metabolites in plants, where they play essential physiological and metabolic functions for plant life. They have structural roles, regulate processes related with plant development and growth, cell division, seed germination, and plant pigmentation, and are also involved in the mechanisms of natural plant resistance against biotic and abiotic stresses (Lattanzio et al. 2008, 2012). They are also widespread in plant-based foods, in which they contribute to sensory (flavor, astringency, bitterness, or color) and functional properties, and may also serve as food preservatives helping to prevent processes of enzymatic browning. As for other phenolic compounds, the phenolic acids possess a series of biological activities that have been related with positive effects in human health. Among others, different compounds of this family have been reported to act as antioxidant, anti-inflammatory, antimicrobial, antidiabetic, or anticarcinogenic agents (Kiokias and Oreopoulou 2021; Kumar and Goel 2019; Ruwizhi and Aderibigbe 2020; Sova and Saso 2020).
Antioxidant activity is one of their more recognized properties. Indeed, phenolic acids behave as efficient antioxidants helping to counteract cell oxidative damage, owing to their ability to scavenge oxidizing species, e.g., reactive oxygen and nitrogen species, through mechanisms that involve the transfer of an H atom or of a single electron to the radical. They may also act as indirect antioxidants by modulating cell oxidative stress via the induction of endogenous protective pathways, such as the antioxidant response element (ARE) regulatory system (Santos-Buelga et al. 2019). Anti-inflammatory activity of phenolic acids and derivatives has been related to their ability to suppress the activity of the nuclear transcription factor NF-κB, and downregulation of pro-inflammatory cytokines, such as IL-6, IL-8, or TNF-α, as well as to their antioxidant properties (Ali et al. 2020; Nagasaka et al. 2007). Similarly, anticancer effects of different phenolic acids have been associated to their antioxidant and anti-inflammatory activity and the regulation of diverse transcription factors and signaling pathways related to cell proliferation, apoptosis, or angiogenesis, such as NF-κB, cyclin-dependent kinases (CDKs), vascular endothelial growth factor (VEGF), phosphoinositide 3-kinase (PI3K), or protein kinase B (Akt), as mostly demonstrated in in vitro assays and animal studies (De et al. 2011; Abotaleb et al. 2020).
Antimicrobial properties of phenolic acids seem to depend on their lipophilicity. As weak organic acids, they partially exist in undissociated lipophilic form in biological systems, which allow them to diffuse across cell membranes, disturbing the membrane structure and acidifying the cytoplasm causing protein denaturation (Campos et al. 2009). The lipophilicity of hydroxybenzoic acids basically correlates with the type and number of functional groups, decreasing with the number of hydroxyl groups and increasing with that of methoxy substituents; in hydroxycinnamic acids it is much lesser dependent on the substitutions of the aromatic ring, but strongly dependent on the double bond of the side chain (Sanchez-Maldonado et al. 2011). The pH is also determining in the antimicrobial activity of phenolic acids, which increases at lower pH values, making cell membrane more permeable and affecting the sodium-potassium ATPase pump implicated in ATP synthesis (Cueva et al. 2010).
The antidiabetic potential of phenolic acids has been associated to their ability to interfere with glucose metabolism. Several mechanisms have been described, including inhibition of enzymes involved in carbohydrate digestion and intestinal glucose uptake, stimulation of insulin secretion from the pancreatic β-cells, modulation of glucose transporters GLUT2 and GLUT4, or activation of insulin receptor and glucose uptake in insulin-sensitive tissues (Vinayagam et al. 2016). Detailed information on biological activities and health benefits of phenolic acids can be found in recent reviews (Kiokias and Oreopoulou 2021; Kumar and Goel 2019; Ruwizhi and Aderibigbe 2020; Sova and Saso 2020).
Technological and functional properties of phenolic acids make them highly interesting for food, pharmaceutical, and cosmetic industries, so that suitable preparation processes are required to meet their increasing industrial demands. Just as an example, phenolic acids like ferulic, gallic, vanillic, or salicylic acids are widely utilized as ingredients or raw materials for the preparation of preservatives, flavors and fragrances, skin protective agents, or edible films (Valanciene et al. 2020).
This chapter offers an overview on phenolic acids occurrence in food and natural sources, biosynthesis and advances in their biotechnological production.
2 Description
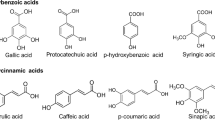
Structure of phenolic acids consists of a phenolic ring with an attached carbon chain bearing a carboxylic group (Fig. 3.1). The most important compounds of this family are hydroxybenzoic (C6-C1) and hydroxycinnamic acids (C6-C3); hydroxyphenylacetic acids (C6-C2) also occur, usually as minor components, in plants and food, being 4-hydroxyphenylacetic acid and 3-methoxy-4-hydroxyphenylacetic (homovanillic) acid and the most representative compounds of this group.
In their natural sources, phenolic acids can be found in free form, but most frequently linked to other compounds such as carbohydrates, organic acids, flavonoids, terpenes, or sterols, as well as bounded to plant matrix components (cellulose, proteins, lignin) through ester, ether, or acetal bonds. Free forms may also appear in processed foods resulting from chemical or enzymatic cleavage during fruit and vegetable processing.
2.1 Hydroxybenzoic Acids
Figure 3.1 shows the structures of the most common hydroxybenzoic acids (HBAs), differing in the number of hydroxyl and methoxyl substituents on their aromatic ring. In general, they are present in plants as conjugates, usually glucosides. Four HBAs, namely p-hydroxybenzoic, vanillic, syringic, and protocatechuic acid, are constituents of lignin; as an approximation, it can be said that plants which do not contain lignin do not contain these acids either (Tomás-Barberán and Clifford 2000). Other common hydroxybenzoates are salicylic and gallic acids.
Gallic acid esterified to glucose and less usually to other polyols, such as hamamelose, quinic acid, shikimic acid, or other cyclitols, giving rise to the so-called hydrolyzable tannins (HTs). Successive substitution of the hydroxyl groups of glucose by gallic acid leads to a series of simpler galloyl esters from monogalloylglucose (1-O-galloyl-β-d-glucose; β-glucogallin) to 1,2,3,4,6-penta-O-galloyl-β-d-glucose (Fig. 3.2). This latter is considered the biosynthetic precursor of both gallotannins (GTs) and ellagitannins (ETs). Complex GTs are formed by substitution of pentagalloylglucose with additional galloyl residues affording polygalloylglucoses. Tergallic and gallagic acids (the gallic acid trimer and tetramer, respectively) may also be found in some HTs. Tannic acid is a generic name given to variable mixtures of GTs that are present in several plant sources. GTs are less distributed in plants than ETs, which also display a much more pronounced structural variability; their strong tendency to form dimeric and oligomeric derivatives contributes significantly to the vast number of described structures (Gross 2008). Gallic acid also makes part of the structure of condensed tannins, in this case esterified to flavan-3-ols (i.e., catechins and proanthocyanidins). ETs derive from the oxidative formation of secondary C-C bonds between adjacent galloyl residues to produce hexahydroxydiphenoyl residues (Gross 2008). Acid or alkaline hydrolysis of ETs releases hexahydroxydiphenic acid that spontaneously rearranges into the dilactone ellagic acid (Fig. 3.2), which explains their denomination.
2.2 Hydroxycinnamic Acids
Hydroxycinnamic acids (HCAs) are more widespread in nature than hydroxybenzoic acids. The main HCAs are p-coumaric, caffeic, ferulic, and sinapic acids (Fig. 3.1). These compounds predominantly occur in plants and food as trans (E)-isomers, although low amounts of cis (Z)-isomers may also be found, mostly originating from isomerization by exposure to UV-irradiation or produced enzymatically. HCAs can be present in plants and food in free form, but more usually they occur in conjugated forms esterified with hydroxyl acids, such as quinic, shikimic, and tartaric acid, sugars or flavonoids. The largest group of HCAs are chlorogenic acids (CGAs), a term that includes a series of compounds resulting from the esterification of HCAs with 1L-(-)-quinic acid. Caffeoylquinic acids (CQAs) are the main group of CGAs. 5-O-Caffeoylquinic acid (5-CQA) is the most abundant CGA in nature and commonly referred to as chlorogenic acid. Nevertheless, in the literature, there is some confusion between 5-CQA and 3-CQA, which are often mistaken, a discrepancy that derives from either using or not the IUPAC numbering for 1L-(-)-quinic acid (Fig. 3.3). Indeed, if the spatial arrangement of the substituents for representing chlorogenic acid is not used, it is not possible to differentiate both enantiomers, as also depicted in Fig. 3.3. Using IUPAC rules, the trivial name “chlorogenic acid” should be retained for 5-CQA, while 3-CQA would correspond to the so-called neochlorogenic acid (Kremr et al. 2016). Unfortunately, as indicated by Clifford et al. (2017), many publications and online sources (including Wikipedia) are unaware of this situation and 3-CQA (non-IUPAC) is treated as the same compound as 3-CQA (IUPAC). Coffee beverage rather than fruits and vegetables is probably the main dietary source of CQAs for many people (Clifford et al. 2017).
HCAs can also occur in polymerized forms, as present in the polyaromatic domain of suberin and other cell wall matrix components like cutin or lignin (Strack 1997), as well as conjugated with amino acids and amines giving rise to phenolamides (Fig. 3.4). These latter can be classified into different subgroups according to the type of amine moiety, namely anthranilic acids (avenanthramides, AVAs), aromatic monoamines (e.g., tryptamine, tyramine, serotonin, dopamine, octopamine), aliphatic di- and polyamines (putrescine, cadaverine, spermine, spermidine), and agmatine. AVAs are unique compounds that are found exclusively in oats, while phenolamides with aromatic monoamines are more widely distributed, being reported in a variety of foods, including tomatoes, paprika, lettuce, garlic, or potatoes. HCAs conjugated to aliphatic di- and polyamines and agmatine have been mainly described in the Poaceae and Solanaceae families, among edible plants (Wang et al. 2020).
3 Biosynthesis
Phenolic acids are synthesized by the shikimate pathway, a route of the primary metabolism for the formation of aromatic amino acids that is present in plants, bacteria, and fungi. Aromatic amino acids, i.e., L-phenylalanine and L-tyrosine, further enter the secondary metabolism through the phenylpropanoid pathway to yield the different polyphenol classes. Main steps of the shikimate and general phenylpropanoid pathways are depicted in Fig. 3.5.
Scheme of main steps of the shikimate and general phenylpropanoid pathways. Enzyme abbreviations: DAHPS, D-arabinoheptulosonate 7-phosphate synthase; DSD, 3-dehydroshikimate dehydratase; QDH, quinate dehydrogenase; QD, quinate dehydratase; CS, chorismate synthase; ICS, PAL, phenylalanine ammonia lyase; TAL, tyrosine ammonia lyase; C4H, cinnamate 4-hydroxylase; 4CL, 4-hydroxycinnamoyl-CoA ligase
The shikimate pathway consists of a sequence of seven enzymatic steps leading to chorismate, which is the common precursor to aromatic amino acids. Firstly, erythrose-4-phosphate (E4P), derived from the non-oxidative branch of the pentose phosphate pathway, and phosphoenolpyruvate (PEP), produced by the glycolytic pathway, are condensed into 3-deoxy-D-arabinoheptulosonate 7-phosphate (DAHP) by DAHP synthase (DAHPS). Following reactions lead successively to the formation of 3-dehydroquinate (DHQ), 3-dehydroshikimate, shikimate, shikimate 3-phosphate (S3P), and 5-enolpyruvylshikimate 3-phosphate (EPSP), to finally produce chorismate catalyzed by chorismate synthase (CS) (Marchiosi et al. 2020).
From chorismate onward the carbon flow is channeled to different branches of secondary metabolism through the phenylpropanoid pathway. Hydroxycinnamic acids (HCAs) result from the deamination of L-phenylalanine involving the enzyme phenylalanine ammonia lyase (PAL) to produce trans-cinnamic acid, as core structure for phenylpropanoids. Further hydroxylation by the action of cinnamate 4-hydroxylase (C4H) yields p-coumaric acid that through a series of sequential hydroxylation and methylation reactions lead to the formation of the rest of common HCAs, caffeic, ferulic, and sinapic acids (Strack 1997). C4H is a cytochrome P450-dependent monooxygenase and composes a multi-enzyme complex with the enzyme p-coumaroyl 3-hydroxylase (C3H), which hydroxylates the p-coumaric acid to caffeic acid. In some plants, tyrosine can also be directly converted into p-coumaric acid by tyrosine ammonia lyase (TAL), thus bypassing C4H. Nevertheless, in most plants phenolic compounds are biosynthesized from phenylalanine, so that PAL reaction represents the usual branching point between primary and secondary metabolism, being an important regulatory step in polyphenol formation (Marchiosi et al. 2020).
HCAs can be then converted to their CoA derivatives catalyzed by 4-hydroxycinnamoyl-CoA ligase (4CL), an enzyme that acts on different HCA substrates, to be subsequently reduced to p-hydroxycinnamoyl alcohols, such as p-coumaryl, sinapyl, and coniferyl alcohols, also termed monolignols, which are precursors of lignin and lignans (Umezawa 2010). Condensation of p-coumaroyl-CoA with three molecules of malonyl-CoA, derived from citrate produced by the tricarboxylic acid (TCA) cycle, catalyzed by chalcone synthase (CHS) leads to the formation of naringenin chalcone, branching the phenylpropanoid pathway toward the formation of flavonoids (Davies and Schwinn 2006).
Hydroxycinnamates are often accumulated in conjugated forms basically with carboxylic acids or carbohydrates, but also with proteins, lipids, amino acids, amines, terpenoids, alkaloids, or flavonoids. Furthermore, insoluble forms of hydroxycinnamates occur in plant cell walls bound to polymers such as cutins and suberins, lignin or polysaccharides (Strack 1997). The widespread chlorogenic acids are formed by esterification of quinic acid with hydroxycinnamoyl-CoA through hydroxycinnamoyl-CoA quinate:hydroxycinnamoyl transferase (HQT). Quinic acid results from the reduction of 3-dehydroquinate (DHQ) or dehydration of shikimate, catalyzed by quinate dehydrogenase (QDH) and quinate dehydratase (QD), respectively (Marchiosi et al. 2020).
The biosynthesis of hydroxybenzoic acids can occur from intermediates of the shikimate or phenylpropanoid pathways. In the first case, 3-dehydroquinate, 3-dehydroshikimate, shikimate, and chorismate act as precursors. Chorismate is a precursor of salicylic, 2,3-dihydroxybenzoic (hypogallic), and 2,5-dihydroxybenzoic (gentisic) acids through isochorismate formed by the action of isochorismate synthase (ICS). Gallic acid can be formed by oxidation of 3-dehydroshikimate, while dehydration of this latter would lead to 3,4-dihydroxybenzoic (protocatechuic) acid that can be further hydroxylated to gallic acid (Marchiosi et al. 2020).
Hydroxybenzoic acids can also derive from the phenylpropanoid pathway from structurally analogous hydroxycinnamic acids by cleavage of an acetate fragment from their side chain. The reaction sequence involves coenzyme A (β-oxidative), but it can also be CoA-independent (non-β-oxidative) or a combination of both pathways (Valanciene et al. 2020). The substitution patterns of hydroxybenzoates may be determined by the hydroxycinnamate precursor, although hydroxylations and methylations may also occur in an analogous way to the hydroxycinnamate pathway (Strack 1997).
Hydrolysable tannins, i.e., gallo- and ellagitannins, are synthesized from gallate and UDP-glucose through UDP-glucose:gallate O-glucosyltransferase yielding 1-O-galloyl-β-d-glucose; sequential incorporation of galloyl residues takes place catalyzed by galloyltransferases up to 1,2,3,4,6-pentagalloylglucose. Further galloylations (depside formation) leading to the gallotannins are also catalyzed by such 1-galloylglucose-dependent acyltransferases. Ellagitannins arise from secondary C-C linkages between adjacent galloyl groups and may further combine to yield oligomeric derivatives coupled through C-O-C linkages (Strack 1997).
The accumulation of phenolic acids, both hydroxybenzoic and hydroxycinnamic acids, varies according to the part of the plant (leaves, flowers, stalks, roots) and is highly dependent on factors like cultivar, seasonality, and agricultural and edafoclimatic conditions, being boosted by biotic (e.g., fungal or bacterial pathogens) or abiotic stresses (e.g., salinity, UV light, water deficit, wounding, metal ions, or treatment with elicitors like ethylene or methyl jasmonate) (Valanciene et al. 2020).
4 Phenolic Acids in Food
4.1 Occurrence
Phenolic acids are widespread in the human diet through plant-based foods, with hydroxycinnamic acids being better represented than hydroxybenzoic acids.
HCAs commonly occur in plants and foods esterified with sugars, organic acids (especially quinic or tartaric acids), or flavonoids, as well as bound to plant structural elements (i.e., cellulose, lignin, and proteins), while they are less usually found in free form. Caffeic and ferulic acids, either free or esterified, are the most abundant HCAs in foodstuffs. Caffeic acid is more usual in fruits and vegetables, being reported that it may account for 75%–100% of the total HCA content in most fruits, while ferulic acid predominates in cereal grains, which represent its major dietary source (Manach et al. 2004).
Chlorogenic acids (CGA), including caffeoyl-, feruloyl-, dicaffeoyl-, and coumaroylquinic acids, are the most ubiquitous phenolic acids, especially the caffeoylquinic acid isomers (CQA), with 5-caffeoylquinic acid as the most common isomer in food products, except in stone fruits and brassicas where the 3-isomer seems to dominate (Clifford 2000). Green coffee beans are among the richest plant sources of CGA, with contents that may range 3.5–7.5% of bean dry matter (Jeszka-Skowron et al. 2016), and 5-CQA representing about 76–84% of total CGAs (Tajik et al. 2017). Nevertheless, the roasting process has a profound effect on CQA contents and isomer distribution. Clifford (2000) estimated a decrease of 8–10% of CGA for every 1% loss of dry matter during roasting, while Crozier et al. (2012) found that in this process 5-CQA was destroyed faster than 3-CQA and 4-CQA, although it still would be the main isomer in coffee with percentages ranging 43–58%. On the other hand, Moeenfard et al. (2014) reported 3-CQA as the major isomer in coffee brews, accounting for about 50% of the total CQAs, followed by 5-CQA and 4-CQA, with around 25–26% each, which was explained not only by their relative rates of destruction of the isomers during roasting but also by the fact that 3-CQA would be more water-soluble. These latter authors determined total CGA concentrations within a large range between 46 and 1662 mg/L in coffee brews, depending on their preparation, with greater levels in boiled and filtered coffees and lowest in instant coffees (Moeenfard et al. 2014). For their part, Crozier et al. (2012) reported an average amount of 145 mg of total CGA (range of 24–422 mg) per espresso serving (43 ml), while Clifford (1999) estimated that a 200 ml-cup of coffee may contain 70–350 mg CGA.
In addition to coffee, tea (Camellia sinensis) and mate (Ilex paraguariensis) are also relevant sources of CGA. Contents of 10–50 g/kg have been reported for green and black tea leaf, while a brew of green mate (approx. 200 ml) may contain 107–133 mg of CGA (Clifford 1999). Other rich dietary sources of CQAs are fruits and vegetables like some berries, apples, stone fruits, potatoes, or aubergines, as well as some species from the Asteraceae family, such as lettuce, endive, and artichoke. Fruits with the highest contents (blueberries, kiwis, plums, cherries, apples) may contain 0.5–2 g/kg fresh weight (Manach et al. 2004), while concentrations of 500–1200 mg/kg dry weight have been described in potato tubers and around 600 mg/kg in aubergines (Clifford 1999). Artichoke (Cynara scolymus) may provide some 450 mg/kg, and levels of total cinnamates of 50–120 mg/kg and 200–500 mg/kg were cited lettuce and endive, respectively (Clifford 1999).
Ferulic acid is particularly abundant in cereal grains, in which it may constitute up to 90% of total phenolic acid content (Manach et al. 2004). It mostly accumulates in the outer parts of the fruit (pericarp and aleurone layers) bound to cell wall polysaccharides. Concentrations as high as 30 g/kg have been reported in maize bran (Clifford 1999), while wheat bran, and especially aleurone, may present more than 8 g/kg (Barron et al. 2007); contents of 0.8–2 g/kg dry weight have been described in whole wheat grain (Manach et al. 2004). Cinnamoyl-tartaric acid esters, namely caftaric, fertaric, and p-coutaric acids, are especially abundant in grapes, being caftaric acid the predominant one; average concentrations about 170 mg/kg have been indicated for Vitis vinifera grapes, although large variations exist depending on the grape variety and geographical and agricultural factors (Waterhouse 2002).
The concentrations of hydroxybenzoic acids in food are usually low, with the exception of certain berries (e.g., blackberry, black and redcurrant, raspberry, or strawberry), green and black tea, or pomegranate. Gallic and ellagic acids are usually the commonest phenolic acids, although they occur in good extent as a part of the hydrolyzable tannins. Contents of gallic acid up to 3.5 g/kg have been reported in black tea leaves, and concentrations of 20–50 mg/l of brew have been estimated for a typically prepared tea infusion (Tomás-Barberán and Clifford 2000). The contents of gallotannins in foods are not well characterized; mango fruits and dried sumac (Rhus coriaria), used as a condiment, could be dietary sources (Clifford and Scalbert 2000). The highest levels of ellagic acid (EA) are found in berries, although a relevant part of it is present as ellagitannins (ETs). Contents of ETs from 1 to 400 mg/100 g fresh weight have been reported in berries, as reviewed by Landete (2011). Greater concentrations of ETs have been found in berries of the genus Rubus, such as raspberries and cloudberries, while they represent the second largest phenolic group in genus Fragaria (strawberry) after anthocyanins (Kähkönen et al. 2001; Koponen et al. 2007). Other relevant sources of EA and ETs are pomegranate and walnut. Contents of ETs from 68 to more than 1800 mg/L have been determined in pomegranate juice, depending on the way of preparation (Gil et al. 2000), while 59 mg of total EA/100 g dry weight have been reported in walnut (Daniel et al. 1989). Berry fruits can also be significant dietary sources of salicylates, with a range from 0.76 mg/100 g for mulberries to 4.4 mg/100 g for raspberries (Swain et al. 1985). Some condiments are particularly rich in salicylates. Contents around 200 mg/100 g were determined in curry powder, thyme, or hot paprika, and between 50 and 100 mg/100 g in dry thyme, dill, oregano, rosemary, or turmeric (Swain et al. 1985). Nevertheless, given the small amounts used in food, they cannot be considered as significant contributors to the dietary intake. Besides plants, some edible mushroom species can also contain significant amounts of phenolic acids, mostly benzoic acids, examples are Ramaria botrytis, which is rich in protocatechuic acid (343 μg/g DW), Agaricus silvicola that mostly contains p-hydroxybenzoic acid (343 μg/g DW) (Barros et al. 2009), or Agaricus brasiliensis (syn. A. blazei), with high amounts of p-hydroxybenzoic (333 μg/g DW), gallic (492 μg/g DW), and ferulic acids (753 μg/g DW) (Bach et al. 2019).
4.2 Dietary Intake
Several studies over different countries have estimated the dietary intake of phenolic acids, obtaining results that vary within a wide range from some tens mg/day to more than 1 g/day depending on the target population and the methodological approach, i.e., the type of dietary assessment and composition databases used. Indeed, the existing databases for phenolic compounds have limitations, lacking data for some compounds or foods, which, on the other hand, can show variable polyphenol contents as influenced by factors like plant origin, cultivation, processing, or storage.
Radtke et al. (1998) in a study on a Bavarian cohort calculated an average intake of total phenolic acids of 222 mg/day, from which 211 mg corresponded to hydroxycinnamic acids and 11 mg to hydroxybenzoic acids. Caffeic acid (206 mg/day) was by far the most important contributor to that intake, while the mean intake of ellagic acid represented up to 5.2 mg/day, p-coumaric acid up to 3.8 mg/day, and ferulic acid up to 1.9 mg/day. Coffee was the major phenolic acid contributor, providing around 92% of the caffeic acid. Very large differences in the individual dietary intakes of phenolic acids were observed, ranging between 6 and 987 mg/day of total cinnamates, mostly depending on coffee consumption. Clifford (1999), in a rough estimation based on composition data collected from the literature and the probable burden of food intake, proposed a similar range of values for total cinnamates consumption by the UK population, comprised between less than 25 mg/day, in people that do not take coffee and have little intake of fresh fruit or vegetables, and 800–1000 mg/day, in great consumers of coffee, bran, and citrus fruits. Again, coffee was the main source of phenolic acids, namely caffeoyl-derived hydroxycinnamates.
In a large study across ten European countries in the frame of the European Prospective Investigation into Cancer and Nutrition (EPIC), Zamora-Ros et al. (2013) calculated a mean phenolic acid intake of 512 mg/day, with hydroxycinnamic acids as the predominant class (around 85% of total phenolic acids), followed by hydroxybenzoic acids (some 14%) and hydroxyphenylacetic acids (below 1%). Significant differences were observed among individuals and regions. Total phenolic acid intake was greater in northern than in southern European countries, although coffee was the principal source in all cases. Caffeic acid was again the most consumed phenolic acid, followed by ferulic acid, with cereals, bran, and whole-grain products as main dietary sources, while intake of p-coumaric acid was low and primarily provided by fruits, nuts, and spices. Tea, wine, and rosaceous fruits were the principal sources of hydroxybenzoic acids, whose intake was higher in southern countries associated to their greater consumption of nuts, seeds, and fruit and vegetables. Among hydroxyphenylacetic acids, homovanillic acid was the most important compound (77.8%), being primarily found in olives and olive oils more frequently consumed in southern European diets.
In a highly cited paper, Scalbert and Williamson (2000) proposed that flavonoids contributed around two-thirds of the dietary intake of total polyphenols, while phenolic acids accounted for one-third, an assertion that was assumed and spread by many other authors. However, several prospective studies over different populations seem not support it as a general rule, but similar consumption of both classes of polyphenols, or even higher of phenolic acids, are usually found in most studies, although notable variations obviously exist, reflecting the distinct dietary behaviors, e.g., coffee, fruits, or cereal consumption.
In a survey over a random sample of Finnish people using a 48-h dietary recall and data on phenolic composition collected in the Finnish National Food Composition database, Ovaskainen et al. (2008) calculated a daily polyphenol intake of 863 mg, from which 75% corresponded to phenolic acids. Coffee was the primary food item contributing to phenolic acid intake and also to the total intake of polyphenols; other relevant contributors were cereals (especially rye bread), tea, and fruits. Average intakes of 639 ± 273 mg/day and 506 ± 219 were calculated for phenolic acids and flavonoids, respectively, in a French cohort, being hydroxycinnamates the most largely consumed polyphenols, mostly originating from coffee (83%), followed by potatoes (4%), apples (2%), and green chicory (2%) (Pérez-Jiménez et al. 2011). Similar intakes of flavonoids (897 ± 423 mg/day) and phenolic acids (800 ± 345 mg/day) were found by Grosso et al. (2014) in a prospective study over a Polish cohort. Hydroxycinnamic acids were far more abundant (around 88% of total phenolic acids) than hydroxybenzoic acids. Newly, coffee was the primary food item contributing to phenolic acid intake (66%) and caffeoylquinic acids the most consumed polyphenols.
In a study within the frame of the EPIC study, Zamora-Ros et al. (2016) calculated mean total polyphenol intakes in the range 584–744 mg/day in Greece (the lowest consumption) to 1626–1786 mg/day in Denmark (the highest). In general, phenolic acids were the best represented phenolic class (52.5–56.9%). Higher or similar intakes of phenolic acids and flavonoids were also calculated in other cohorts from Italy (Godos et al. 2017; Vitale et al. 2018), Spain (Tresserra-Rimbau et al. 2014), or Brazil (Miranda et al. 2016; Nascimento-Souza et al. 2018). By contrast, in a large survey on the UK population, Ziauddeen et al. (2019) determined significant higher intakes of flavonoids than phenolic acids in all age subgroups from children of 1.5–3 years to people aged more than 65 years. The group with greater consumption of polyphenols was that of 50–64 years with an average intake of flavonoids of 714.5 ± 415.2 mg/day for 336.7 ± 292.0 mg/day of phenolic acids, from which 231.8 ± 289.9 mg/day hydroxycinnamic acids and 104.6 ± 82.5 mg/day hydroxybenzoic acids.
All in all, the overall intake of polyphenols is highly variable. Phenolic acid derivatives seem to represent the more prevalent class of phenolic compounds in most individuals, although flavonoids can predominate over phenolic acids in some populations. Hydroxycinnamoyl derivatives, and especially caffeoylquinic acids, are the main phenolic compounds in usual diets, with coffee as the most important contributor to their intake, followed by tea and cereals, with fruits and vegetables in the next level. Nevertheless, it is necessary to consider that the observations from prospective studies are determined by their estimative nature, owing to the limitations of the dietary assessments and composition databases, incomplete and lacking data on bound phenolics and complex tannins, including gallo- and ellagitannins, which are difficult to quantify and can represent a relevant part of food polyphenols that are, thus, overlooked in most studies. Actually, the non-extractable polyphenols fraction was estimated by Saura-Calixto et al. (2007) that may contribute up to double amount than extractable phenolic compounds to the dietary phenolic intake.
4.3 Influence on Sensory Properties
Phenolic acids are considered important contributors to the sensory properties of food due to their inherent taste but also because they can prevent rancid flavors by acting as antioxidants influencing lipid oxidation (Duizer and Langfried 2016). Classically they have been classified as possessing sensory properties described as being sour, astringent, and bitter. Nevertheless, studies carried out with isolated phenolic acids have shown the ability of these compounds to elicit a complex mixture of taste and oral sensations (Maga 1978; Peleg and Noble 1995).
Physiologically, to detect taste, compounds must first be dissolved in saliva and transported to taste cells, located at the base of the tongue, where they can either interact with taste receptors on the surfaces of these cells, leading to sweet and bitterness detection, or with ion channels, leading to salty and sour detection (Rawson and Li 2004). Taste thresholds of individual phenolic acids range from 5 ppm for the m-anisic acid to 240 ppm for syringic acid (Maga 1978), although combinations of them, as occurring in foods, may decrease the taste threshold due to synergistic effects (Ferrer-Gallego et al. 2014). For example, a recent study has shown that phenolic acids and quercetin rutinoside interact synergistically in tea infusion enhancing bitterness and astringency of tea infusion (Chen et al. 2022). On the other hand, it is known that sensory properties of phenolic acids can be influenced by their structure. Thus, it has been reported that functional groups in the meta position increase sensitivity, whereas the same functional groups in the ortho position decrease sensitivity. For benzoic acid derivatives, a methoxy group results in a more tasting compound than a hydroxy group (Maga 1978).
Astringency is not a taste, but a tactile sensation described as drying, roughening, and constricting within the oral cavity. In general, astringency has been thought to be the result of the interactions of astringent compounds, such as phenolic compounds, with salivary proteins. Interestingly, the precipitation of salivary proteins was not found to be required for the development of astringency of ferulic, vanillic, or gallic acid, suggesting that salivary protein binding activity may not be an accurate measurement of the astringency of all phenolic compounds. Indeed, it looks like that the perceived astringency of organic acids is a function of pH, suggesting that the inverse relationship between pH and astringency may be explained by reduced salivary lubricity due to denaturation of salivary proteins under conditions of reduced pH (Lawless et al. 1996).
Studies carried out with individual phenolic acids have established that among benzoic acid derivatives, gentisic acid had the highest sourness, astringency, and bitterness intensity, while salicylic had similar astringency than gentisic acid and m-hydroxybenzoic acid was the sweetest sample, and the prickling feeling was especially contributed by benzoic acid (Peleg and Noble 1995). However, based on the amounts found in food and their respective taste threshold, vanillic, p- and o-coumaric and ferulic acids are the principal phenolic acids to be organoleptically detectable (Maga 1978). p-Coumaric acid is perceived as bitter, astringent, and unpleasant at 48 ppm while ferulic acid is perceived as a sour at 90 ppm. However, the combination of p-coumaric and ferulic acids is felt as bitter and sour at 20 ppm (Huang and Zayas 1991). Vanillic acid taste is significantly sourer than ferulic acid, while ferulic acid is considered to be significantly more bitter than vanillic acid.
Chlorogenic acid isomers are precursors of caffeic acid and quinic acid that contribute to the formation of sensory attributes and are related to the sensory characteristics of coffee beverage, mainly to astringent, acid, and bitter ones (Aree 2019; Moon and Shibamoto 2010). In the same way, it has been described that the sensory properties of virgin olive oil may be differently affected by its phenolic acid content depending on the type of cultivar (Rivas et al. 2013).
In general, the native phenolic compounds in plant-based foods have been considered to negatively influence food selection due to imparting negative bitter flavor attributes to foods (Drewnowski et al. 1997; Heiniö et al. 2008). On the other hand, it is also well known that some hydroxycinnamic acids undergo decarboxylation and oxidative reactions during thermal processing leading to volatile products that can impact on both aroma and taste attributes of foods as well as food palatability (Jiang and Peterson 2010). For example, under thermal processing, ferulic acid generates aroma compounds such as 4-vinylguaiacol, guaiacol, and vanillin (Fiddler et al. 1967), while catechol and 4-ethylcatechol can derive from thermal degradation of caffeic acid and 5-caffeoylquinic acid (Frank et al. 2006). Decarboxylation processes can also occur during fermentation by lactic acid bacteria (LAB) (Couto et al. 2006) or yeast (Shinohara et al. 2000), resulting in the production of 4-vinylplenols, or their reduced form 4-ethylphenols, with great impact on the sensorial characteristics of foods. In fermentation by LAB, p-coumaric acid had the highest conversion efficiency, followed by caffeic acid and lastly ferulic acid (Miyagusuku-Cruzado et al. 2020), while yeast strains are very unselective and decarboxylate cinnamic, p-coumaric, and ferulic acids with similar conversion rates (Goodey and Tubb 1982). In fermented products such as wine, HCA degradation by yeast results in the formation of 4-vinylplenols that are responsible for undesirable phenolic off-flavors in the final product (Shinohara et al. 2000). On the other hand, phenolic acids remarkably inhibit terpene glycosides hydrolysis and free terpene volatilization and affect the profile and amounts of free terpenes, thus influencing the tropical, sweet, small berry, and floral aromas of wine (Wang et al. 2021). However, these compounds may be desirable, for example, in wheat beer, where the reduction of HCA in 4-vinylphenol and 4-vinylguaiacol results in a less pronounced wheat beer aroma (Kalb et al. 2020; Langos and Granvogl 2016), or in bread where decarboxylation of ferulic acid generates 4-vinylguaiacol contributing to its flavor profile (Wang et al. 2012).
4.4 Effect of Processing and Storage on Phenolic Acids
Most food processing techniques involve a sequence of operations bringing about changes in the raw material, with each operation having impact on the food constituents. In general, food processing operations, such as thermal processing, fermentation, or freezing lead to a release of matrix-bound phenolic acids increasing the contents of free forms (Dewanto et al. 2002). The changes in free and bound forms of phenolic acids induced by processing depend on the type of matrix and the processing technique employed (Nayak et al. 2015). Detrimental effects can also be produced, being temperature, oxygen, and enzymes activity major factors affecting the stability of phenolic acids (Dewanto et al. 2002).
A majority fraction of phenolic compounds present in cereal and cereal-based products are bound to cellulose and hemicellulose structures, which are partially broken down during thermal processing. Thus, after thermal processing of wheat bran, increases in the content of ferulic, vanillic, and p-coumaric acids of 70% have been reported, while in oat bran dihydroxybenzoic acids increased almost 40% (Călinoiu and Vodnar 2019). Likewise, extrusion processes (120–200 °C) enhanced by two to three times the levels of free forms of vanillic, syringic, and ferulic acids (Zielinski et al. 2001). Bryngelsson et al. (2002) found that autoclaving of oats increased the content of p-coumaric, vanillin, and ferulic acids, while it had a negative impact on caffeic acid. Szwajgier et al. (2014) reported detrimental effects after heating of different fruits, with a significant reduction of total phenolic acids in compotes and jams compared with the corresponding fresh-frozen (thawed) fruits; the most dramatic loss of phenolic acids was predominantly observed in the case of jams. In the same way, a significant decrease in the levels of p-hydroxybenzoic, p-coumaric, caffeic, chlorogenic, and syringic acids was observed in dried fruit homogenates in comparison with fresh fruits. Thermal processing of orange juice resulted in the hydrolysis of ferulic acid esters with subsequent release of the free acids, which later undergo decarboxylation, leading to the formation of 4-vinyl guaiacol, a potent off-flavor compound imparting an unpleasant odor to the final product (Lee and Nagy 1990). Changes in free and bound phenolic acids were found in artichokes during cooking, being the decrease in total caffeoylquinic acids higher in boiling than in frying and griddling, although the formation of new isomers due to transesterification phenomenon partially compensated the loss of total mono- and di- caffeoylquinic acids (Domínguez-Fernández et al. 2021). As for coffee roasting, the higher the roasting degree the lower the content of chlorogenic acids, however, as they are decomposed a rise in caffeic and quinic acids levels is produced (Awwad et al. 2021; Fuller and Rao 2017).
Increases in the contents of some phenolic acids in thermally processed fruits have been reported by several authors. Thus, the concentration of ellagic acid was found to raise in preserves of strawberry in relation to fresh fruit due to partial degradation of ellagitannins, while it was reduced during processing of blueberries into jams (Häkkinen et al. 2000), highlighting the importance of the matrix effect. Similarly, an increase in the level of caffeic acid was observed in pear juice in relation to fresh fruit, which was attributed to the thermal hydrolysis of chlorogenic acids (Spanos and Wrolstad 1990). Pressure cooking of legumes also resulted in improved bioaccessibility of phenolic acids, released from plant matrix (Chen et al. 2015). Significant enhancement in phenolic acids contents was also found in black currant juices submitted to pasteurization, and further increase by a factor of two to four was produced in the levels of hydroxycinnamic acids during one-year storage, which could be possibly explained by acid hydrolysis of conjugated forms (Mäkilä et al. 2017). However, no changes were observed in the concentration of total phenolic acids (the sum of p-hydroxybenzoic, vanillic, chlorogenic, caffeic, syringic, ferulic, and o-coumaric acids) in blueberry juices, either pressed, clarified, pasteurized, or concentrate, submitted to heat and SO2 treatments (Lee et al. 2002).
Other technological processes, such as pulsed electric field (Agcam et al. 2014; Morales-De la Pena et al. 2011), gamma radiation (Breitfellner et al. 2002), or high pressure processing (Marszałek et al. 2017; Pérez-Lamela et al. 2021) have been reported to increase the content of individual phenolic acids when applied on juices or fruits, also attributable to the release of bound compounds through hydrolysis reactions.
Fermentation processes on matrices rich in phenolic acids lead to similar events as thermal processing, due to the activity of hydrolytic enzymes. Thus, an increase in the levels of free phenolic acids initially bound, mainly but not exclusively ferulic acid, is produced during fermentation of cereal doughs (Amaya Villalva et al. 2018; Bhanja et al. 2009; Katina et al. 2007). Different lactic acid bacteria (LAB) have shown capacity to release caffeic acid from chlorogenic acids from fruit and vegetable substrates as a result of their cinnamoyl esterases activity (Filannino et al. 2015; Fritsch et al., 2016). Biotransformation of phenolic acids by LAB and yeast decarboxylases or reductases has also been documented in numerous investigations. Yeasts present variable hydroxycinnamate decarboxylase activity according to the strain, which is responsible for the transformation of hydroxycinnamic acids into vinylphenols during wine fermentation, a process with significant influence on red wine color, while their further conversion to ethylphenols have a negative impact on wine flavor (Morata et al. 2016). Ripari et al. (2019) observed that co-fermentation of wheat and rye malt sourdoughs by L. plantarum and L. hammesii released bound ferulic acid, which was further converted to dihydroferulic acid and volatile compounds (e.g., vinylguaiacol and ethylguaiacol) with an impact on bread flavor; however, no conversion of other phenolic acids, including coumaric, sinapic, and vanillic acids was noticed. Svensson et al. (2010) found that ferulic acid was reduced into dihydroferulic acid by L. plantarum and L. fermentum but not decarboxylated during fermentation of sorghum doughs. However, while L. plantarum decarboxylated caffeic acid to vinylcatechol and ethylcatechol, L. fermentum reduced and decarboxylated it to dihydrocaffeic acid and vinylcatechol, respectively, without production of ethylcatechol. Filannino et al. (2015) showed that degradation of caffeic acid would depend on the L. plantarum strain, whereas some strains mostly degraded it into vinylcatechol, others released dihydrocaffeic acid or ethylcatechol as end products. Leonard et al. (2021) also reported that p-coumaric acid was generally degraded into p-vinylphenol and phloretic acid by L. plantarum, but the proportion of these two metabolites and the percentage of degraded p-coumaric acid substantially differed among strains. Hydroxybenzoic acids can also be transformed by LAB. Catechol was identified as main end product from protocatechuic acid conversion by L. plantarum in cherry juice, while no formation of catechol was observed when inoculated with L. reuteri (Filannino et al. 2015). The ability of different strains of L. plantarum, Enterococcus mundtii, and Pediococcus pentosaceus to decarboxylate caffeic, ferulic, and p-coumaric acids into 4-vinylcatechol, 4-vinylguaiacol, and 4-vinylphenol, respectively, in model in vitro systems was demonstrated by Miyagusuku-Cruzado et al. (2020). All these studies reveal that different LAB strains can show distinct metabolic pathways and substrate specificities leading to different products when degrading phenolic acids.
Changes in phenolic acids content and composition have also been observed during storage. Ellagic acid was found to be released from the hydrolysis of ellagitannins during refrigerated storage of fruits such as strawberry and juices (Häkkinen et al. 2000), as well as during their storage under modified atmospheres (Gil et al., 1997). By contrast, Klaiber et al. (2005) observed that the level of phenolic acids strongly diminished in fresh vegetables during storage in the absence of oxygen or at carbon dioxide levels >30%, which was explained by inhibition of PAL activity; however, high accumulation was seen when they were stored in air. An increase in the content of hydroxycinnamic acids was found in cherries during postharvest storage at 15 ± 5 °C for 6 to 30 days, although a tendency to decline was observed at 1–2 °C (Goncalves et al. 2004). Several phenolic acids (protocatechuic, p-hydroxybenzoic, syringic, salicylic, p-coumaric, chlorogenic, ferulic, and β-resorcylic acids) were analyzed in soybean flour stored under different conditions of time and temperature by Prabakaran et al. (2019), concluding that whenever temperature and time increased an enhancement was also produced in total contents of phenolic acids.
Light is another important factor that influences phenolic acids evolution during storage of fruits and vegetables. Continuous light irradiation (30 μmol m2 s−1) was shown to produce positive effects in the accumulation of phenolic acids in minimally processed spinachs, which was explained by the activation of PAL activity (Zhan et al. 2020). Friedman (1997) and Griffiths et al. (1995) also reported significant greater increases in chlorogenic acid levels in light-stored potatoes than in those stored in darkness. By contrast, Slimestad and Verheul (2005) found that the content of chlorogenic acid felt in cherry tomatoes from 0.51 to 0.06 mg/100 g after 3 weeks of postharvest storage at 20 °C in the absence of light.
Overall, processing can have both positive and detrimental effects on phenolic acid content and composition depending on the plant matrix and the processing method. Therefore, it is critical to determine not only optimal processing conditions to extend the shelf life of products, but also to reduce the degradation of bioactive compounds (Ifie and Marshall 2018).
5 Biotechnological Production
5.1 Preparation from Natural Sources
In view to their use by food, cosmetic, or pharmaceutical industries, large production of phenolic acids is required. Although plant extracts or compounds mixtures can be appropriate for some purposes (e.g., dietary supplements, cosmeceuticals), the preparation of pure compounds is necessary for their use as food ingredients or additives, as well as for the adequate assessment of their technological, healthy, therapeutic, or safety characteristics.
Concentrations of phenolic acids in plants are usually low and strongly variable depending on plant species and tissues, as well as geographical, seasonal, and edafoclimatic factors. Despite some plant materials are very rich in some particular compounds, such as coffee beans (chlorogenic acids) or cereal brans (ferulic acid derivatives), their extraction on a large-scale is normally unaffordable. Extraction is usually carried out with organic solvents and only allows obtaining free extractable phenolic acids; furthermore, the process may involve the use of non-environmentally friendly solvents and, in general, extraction yields are low and lead to complex mixtures of compounds, so that further purification is required, making the process tedious and expensive. Although chemical synthesis might be an alternative for the preparation of some compounds, the existing approaches are often inefficient, especially in the case of complex products, and suffer from similar limitations of low yields, formation of side-products, challenging reactions, use of non-green or toxic solvents and reagents, laborious purification, waste production, and heavy pollution.
Hydrolysis or fermentation processes allow releasing phenolic acids from complex structures (e.g., hydrolyzable tannins) or bound to matrix components, thus increasing the extraction yields. Acid and alkaline hydrolyses can provide an effective cleavage of bound phenolics, although these treatments are environmentally unsuitable; while enzymatic hydrolysis is more friendly it can be expensive. In this respect, processes taking advantage of the enzymatical machinery of microorganisms would be more appropriate, such as extracellular enzymes produced during microbial growth like pectinases, cellulases, amylases, xylanases, or esterases able to degrade cell walls. Residues from agro-food industries can be particularly suitable sources, as they are rich in lignin, from which phenolic acids like ferulic, p-coumaric, syringic, vanillic, or p-hydroxybenzoic acids can be released. In this respect, biotechnological processes using different microorganisms and substrates have been explored, either in liquid media containing essential nutrients for the growth of the microorganisms (submerged fermentation processes) or in the absence of free-flowing water on a moist solid substrate that acts both as physical support and source of nutrients for microorganisms (i.e., solid-state fermentation) (Šelo et al. 2021). Solid-state fermentation (SSF) offers particular advantages such as low energy requirements and not requiring complex machinery and control systems, thus providing a low-cost process. Nevertheless, no high yields are usually obtained, and process scaling-up and compound purification are challenging. Yeasts and fungi are the microorganisms most commonly used due to their lower water activity requirements compared to bacteria (Thomas et al. 2013). Some processes developed for phenolic acids production are below given as examples.
Saccharomyces cerevisiae was employed by Santos da Silveira et al. (2019) in an SSF process to obtain chlorogenic acid from coffee pulp; optimization of the process at a pilot scale allowed the preparation of extracts 400% richer in chlorogenic acids (600 mg/kg of coffee pulp) and with lower sugar amounts, which facilitated further purification. Solid-state fermentation with the fungus Rhizopus oryzae was found to strongly enhance the content of free phenolic acids in rice bran, especially gallic and ferulic acids, reaching levels 170 and 765 mg/g in the fermented bran, respectively (Schmidt et al. 2014). Significant increases in the contents of ferulic, sinapic, vanillic, caffeic, syringic, and 4-hydroxybenzoic acids were also found in rice bran by Abd Razak et al. (2015), following SSF with single and mixed cultures of two fungi, Rhizopus oligosporus and Monascus purpureus.
A range of bacteria, including strains of genera such as Streptomyces, Rhodococcus, Pseudomonas, Bacillus, Alcaligenes, Arthrobacter, or Nocardia, have also been reported to be capable of degrading lignin to low molecular weight compounds (Bugg et al. 2011; Lee et al. 2019). An anaerobic strain from the Acetoanaerobium genus, isolated from the sludge of a pulp and paper mill, was used by Duan et al. (2015) for the production of ferulic and syringic acids as final metabolites from the biodegradation of kraft lignin derived from pulp and paper industries, using it as the sole carbon source.
Mushrooms have also been used to recover phenolic acids from different substrates. The food grade fungus Lentinus edodes (shiitake mushroom) has been shown to be able to produce high amounts of extracellular β-glucosidase, an enzyme capable of hydrolyzing phenolic glycosides to release free phenolic acids, during solid-state growth. SSF processing of cranberry pomace with L. edodes was employed for effective production of free gallic, p-hydroxybenzoic and p-coumaric acids (Zheng and Shetty 2000), and ellagic acid (Vattem and Shetty 2003). The ability of the white-rot fungus Trametes versicolor to produce lignolytic enzymes was explored by Bucic-Kojic et al. (2017) to release phenolic acids from corn silage, reaching 10.4, 3.4, 3.0, and 1.8-fold increases in the yield of free syringic, vanillic, p-hydroxybenzoic, and caffeic acids, respectively, after 20 days of treatment with the fungus.
Nevertheless, these and other studies were intended to optimize fermentation conditions in order to increase the extraction yields of phenolics. However, to obtain a ready-to-market product, downstream concentration and purification of the compounds using sustainable techniques is still required and the processes have to be scaled-up, so that their cost-effectiveness and environmental impact can be evaluated, which is yet a long way to go. To overcome some of the constraints posed by the extraction of phenolics acids from natural sources, genetic and molecular biology techniques have been explored for engineering plants or microorganisms, some of them aimed at channeling the synthesis toward target compounds, thus requiring minimum purification.
5.2 Plant Genome Engineering
As above discussed, polyphenols are produced through the shikimate-phenylpropanoid pathway, a route that is involved in the biosynthesis of both phenolic acids and flavonoids, as well as other phenolic compounds, like stilbenes or lignans.
Plants present several advantages over microorganisms for the construction of heterologous systems in view to produce phenolic compounds. Firstly, plants already contain genes of the phenylpropanoid pathway, so that there is no need to reconstitute the entire pathway. Actually, producing the array of enzymes needed to reproduce all the phenylpropanoid diversity seems unaffordable in microbial hosts; besides, plant enzymes may not behave as efficiently in microbes as in plants, especially in prokaryotes lacking post-translational modifications and organelles for enzyme compartmentalization. While phenolic compounds can be accumulated in large amounts in plant vacuoles, their accumulation in microorganisms would be more limited; further, some phenylpropanoids may also have antimicrobial activity, thus killing hosts. On the other hand, plants can be readily edible or require minimal processing, which is advantageous for their delivery, reducing costs associated with the production chain (Ferreira and Antunes 2021). By contrast, plant growth is slow and strongly influenced by external factors (soil, climate, biotic, and abiotic stresses) (Rainha et al. 2020). Another difficulty for phenolic acid production in plants is that specialized enzymes involved in the biosynthesis of phenylpropanoids are able to utilize multiple substrates and, therefore, pathways are not straightforward, but rather structured as complex grids, with coexistence of multiple and sometimes competitive pathways, so that it is difficult to foresee the metabolic consequences of gene engineering. Furthermore, plants are more difficult to manipulate genetically than microorganisms.
Strategies to engineer plants in order to increase the biosynthesis of secondary metabolites can target enzymes or transcription factors. A key enzyme in phenylpropanoid biosynthesis is phenylalanine ammonia lyase (PAL), whose activity represents the branching point between primary and secondary metabolism, so that PAL gene expression is a relevant target for the activation of the phenylpropanoid pathway and increasing phenolic production (Marchiosi et al. 2020). Other relevant enzymes are cinnamate 4-hydroxylase (C4H), 4-coumaroyl CoA ligase (4CL), responsible for the formation p-coumaroyl CoA, the activated intermediate for the various branches of phenylpropanoid metabolism, and cinnamoyl-CoA reductase (CCR) that catalyzes the reduction of cinnamoyl-CoA esters to their corresponding cinnamaldehydes, a key step in the biosynthesis of lignin (Umezawa 2010). Pathway perturbations modifying the activity of these enzymes alter the synthesis of phenylpropanoids. Accumulation of phenolic acids, such as p-coumaric, caffeic, ferulic, 5-hydroxyferulic, and sinapic acids and derivatives has been demonstrated to occur in low producing lignin mutants, e.g., c4h, 4cl1, or ccr1 mutants (Vanholme et al. 2012). Production of high levels of ferulic acid was shown by Xue et al. (2015) in Arabidopsis ccr-1 mutants, which also presented multiple developmental defects including increased cell proliferation, explained by the cell proliferative effect of ferulic acid.
Compound accumulation resulting from enzyme activation may trigger a feedback inhibition in the activities of pathway enzymes with subsequent arrest in metabolite production. A strategy to overcome this shortcoming is the introduction into plants of genes encoding feedback-insensitive enzymes from microbes or plants with unique phenotypes (Yuan and Grotewold 2015). The introduction in Arabidopsis of a bacterial AroG gene encoding a feedback-insensitive 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase (DAHPS), a key enzyme of the primary metabolism regulating the carbon flow through the shikimate pathway, resulted in the enhancement in the plant of the levels of aromatic amino acids, i.e., phenylalanine and tryptophan, but also of derived secondary metabolites, including phenylpropanoids (Tzin et al. 2012).
Transcription factors (TFs) usually control genes of multiple enzymes within a metabolic pathway; thus, their use can be an effective strategy to engineer plants in order to increase the biosynthesis of secondary metabolites. Metabolic engineering can be focused on early TF genes so as to push the flux downstream, or on late pathway genes to address substrates toward a particular final product (Yuan and Grotewold 2015). Regulatory genes encoding the MYB family of transcription factors are particular good candidates, as they are present in all eukaryotes and regulate a variety of plant-specific processes, including phenylpropanoid biosynthesis (Ambawat et al. 2013). Several MYB transcription factors involved in phenylpropanoid biosynthesis have been characterized and proposed to engineer plants in order to promote phenolic acid production. The expression of the R2R3-MYB transcription factor ZmMyb-IF35, with high identity with the P regulator of 3-deoxy flavonoid biosynthesis in maize, does not induce the accumulation of flavonoids, but that of ferulic and chlorogenic acids not found in Black Mexican Sweet maize cells (Dias and Grotewold 2003). Tang et al. (2021) observed that overexpression of another R2R3 MYB transcription factor, LmMYB15 from Lonicera macranthoides, led to increased accumulation of chlorogenic acid (CGA) in tobacco leaves, underlying new breeding strategies to enhance CGA content in L. macranthoides, the main biologically active compound in this plant used in traditional Chinese medicine.
Methyl jasmonate is an elicitor that has been used to activate the transcripts of PAL and C4H enzymes from the phenylpropanoid pathway, as well as tyrosine aminotransferase (TAT) and 4-hydroxyphenylpyruvate reductase (HPPR) from the tyrosine-derived pathway (Xiao et al. 2009, 2011). Overexpression of the methyl jasmonate-responsive R2R3-MYB transcription factors SmMYB1 (Zhou et al. 2021) and SmMYB2 (Deng et al. 2020) was shown to upregulate the expression of genes encoding key enzymes in the phenolic acid biosynthesis leading to increased accumulation of phenolic acids and anthocyanins in Salvia miltiorrhiza, especially salvianolic acids such as rosmarinic acid and salvianolic acid B, which are important bioactive components in this medicinal herb. By contrast, the content of p-coumaric acid, rosmarinic acid, salvianolic acid B, salvianolic acid A, and total phenolics was dramatically decreased in S. miltiorrhiza following overexpression of SmMYB39, suggesting that this TF acts as a repressor through suppressing transcripts of key enzyme genes (Zhang et al. 2013).
Attention has also been paid to the basic helix loop-helix (bHLH) MYC family of transcription factors, such as MYC2, the up-regulation of which enhances the production of phenolic acids in S. miltiorrhiza, namely rosmarinic acid and lithospermic acid B, whose concentrations increased by 2.46-fold and 1.88-fold, respectively (Yang et al. 2017). Previously, Bovy et al. (2002) also showed that ectopical expression in tomatoes of genes of two transcription factors from maize, i.e., MYB-type C1 and MYC-type LC, upregulated the phenylpropanoid pathway resulting in a strong accumulation of phenolic compounds, and especially flavonols, in tomato flesh, a tissue that normally does not produce flavonoids. However, the own authors indicated that the results are difficult to predict, and the type and content of metabolites can vary between plant species and varieties, owing to the induced expression of structural genes and the substrate specificities of the enzymes involved may differ from plant to plant. Similarly, expression in transgenic tomato of AtMYB12, a TF regulating flavonol biosynthesis in Arabidopsis thaliana, led to the accumulation of high levels of chlorogenic acids and flavonols in the fruit, which might represent a way for the effective production of these phenylpropanoids in tomato (Zhang et al. 2015).
In order to minimize possible adverse effects derived from pathway modification, the use of autoregulatory synthetic genetic circuits controlled by biosensors may help the transgenic organism address the metabolic output and trigger an on or off response as needed. Several phenylpropanoid-related TF sensors have been characterized in prokaryotes, although at present their use has been mostly limited to microbial systems, such as bacterial or yeast strains engineered for flavonoid production (Ferreira and Antunes 2021). A promising phenylpropanoid-related biosensor is the Q-system from the fungus Neurospora crassa. This is a gene cluster involved in the catabolism of quinic acid for its use as a carbon source. The cluster contains a transcriptional activator (QF) and its repressor (QS); the binding of QF to a minimal promoter presenting the QUAS regulatory sequence triggers downstream gene expression, while binding of QS to QF inhibits the activity of QF. It has been shown that quinic acid can regulate this system by restoring QF activity through inhibition of QS. The functionality of the Q-system in plants has been demonstrated through expression in Glycine max protoplasts and Nicotiana benthamiana leaves (Persad et al. 2020), envisaging its use as a tool for precise control of gene expression in plant metabolic engineering. As quinic acid is an essential metabolite for redirecting phenylpropanoids toward chlorogenic acids, the Q-system could be a suitable biosensor to regulate this pathway. Nevertheless, de-repression of the system by quinic acid has not been yet tested in plants, although the fact that it functions in other heterologous systems, such as Caenorhabditis elegans (Wei et al. 2012) or drosophila (Riabinina et al. 2015), allows thinking that quinic acid-dependent de-repression might also be possible in plants (Ferreira and Antunes 2021).
Biosensors are only able to detect intracellular ligand concentration, which represents a limitation when cells secrete the desired product (Ferreira and Antunes 2021). A biosensor allowing detection of extracellular p-coumaric acid in yeasts was developed by Siedler et al. (2017). The system was based on a Bacillus subtilis transcriptional repressor (PadR), which was introduced in Escherichia coli to generate E. coli-biosensing cells that were further encapsulated into p-coumaric acid-producing yeasts, which could, thus, be sorted using the fluorescent E. coli biosensor signal. Other phenylpropanoid-related TF sensor proteins have been characterized in prokaryotes, as reviewed by Ferreira and Antunes (2021), a few of which have been engineered for high-throughput screening of metabolites in bacterial or yeast strains, especially for flavonoids. As biosensors become available, they are expected to find applications for detecting metabolites not only in microbial systems but also in plants.
Despite relevant progresses have been made, up to now, plant engineering developments have mostly been carried out at lab scale and not upgraded to industrial production, where outcomes are subject to cultivation factors and difficult to predict and standardize.
5.3 Engineered Microorganisms for Phenolic Acid Production
As above indicated, microorganisms do not produce polyphenols naturally, so that the phenylpropanoid pathway has to be reconstructed. However, primary metabolism is similar to plants and, therefore, they are able to provide the aromatic amino acid precursors for the phenolic biosynthesis, representing an attractive platform when the plant pathways can be functionally introduced (Milke et al. 2018). Indeed, microorganisms may have advantages over plants for the production of secondary metabolites. They can grow in inexpensive substrates and have a rapid growth rate, so that the process is faster, more economical, and easier to upgrade to a large-scale production. Furthermore, the diversion of intermediate products to competing pathways often present in the natural host can be reduced, and the process addressed to the compounds of interest, avoiding simultaneous formation of related molecules, which facilitates further purification (Krivoruchko and Nielsen 2015). Saccharomyces cerevisiae and Escherichia coli have been most commonly used microorganisms for metabolic engineering purposes, having the advantage of being well-characterized and genetically tractable, with multiple tools available for their genetic manipulation; also, other bacteria such as Lactococcus lactis, Corynebacterium glutamicum, or Pseudomonas spp. have been explored (Dudnik et al. 2018; Valanciene et al. 2020).
Although successful developments have been achieved using E. coli as a platform, S. cerevisiae presents advantages, due to its robustness and tolerance toward harsh fermentation conditions, as well as its superior capability of expressing membrane-bound cytochrome P450 oxidases (e.g., C4H), which are key catalysts in most relevant plant-based biosynthetic pathways (Liu et al. 2019b). As a eukaryote, S. cerevisiae is able to perform post-translational modifications such as glycosylation and it possesses intracellular organelles similar to plant cells, which is crucial to functionally express membrane-bound cytochrome P450 enzymes. Besides, unlike E. coli, it has a GRAS status, that is important to produce both nutraceutical and pharmaceutical compounds (Gomes et al. 2022). Nevertheless, it is to say that most attempts of using S. cerevisiae as a chassis have been addressed to the production of flavonoids, prenylflavonoids, or stilbenes, while less developments have been focused on phenolic acids (Gomes et al. 2022; Rainha et al. 2020).
Aromatic amino acids (AAA) are the primary substrates for the biosynthesis of phenolic compounds and their availability represents a major bottleneck and a metabolic engineering challenge for microbial polyphenol production. AAA can be directly added into the media as precursors, although this makes the process more expensive for industrial applications. An alternative is the use of microbial strains capable of producing phenolic compounds directly from glucose (de novo production), which can be achieved by engineering the pentose phosphate and the shikimate pathways to provide sufficient amounts of L-phen or L-tyr.
Aromatic amino acid biosynthesis is subject to feedback inhibition by the product (i.e., L-phen or L-tyr). To overcome it, the most successful strategies have dealt with overexpression of the feedback-insensitive mutant enzymes 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase (DAHPS) and chorismate mutase (CM), which catalyze the first steps in the AAA biosynthesis and are the branching point toward the production of tyrosine and phenylalanine, respectively (Liu et al. 2019b). A tyrosine-insensitive ARO4 allele in conjunction with deletion of the ARO3 allele of the DAHP synthase was introduced by Koopman et al. (2012) in S. cerevisiae engineered with Arabidopsis thaliana genes for phenylpropanoid synthesis. This allowed accumulation of aromatic amino acids that, together with elimination of phenylpyruvate decarboxylase to reduce pathway diversion to phenylethanol formation, led to a notable increase in the extracellular accumulation of the target polyphenols (in this case, the flavanone naringenin), using glucose as sole carbon source. Liu et al. (2019b) constructed a S. cerevisiae platform able to produce high levels of p-coumaric acid. The AAA biosynthesis pathway was modified by introducing a feedback-insensitive DAHPS and CM, and a heterologous phosphoketalose-based pathway to divert glycolytic flux toward formation of the shikimate precursor erythrose 4-phosphate; distribution of the carbon flux from glycolysis toward the AAA biosynthesis was optimized by replacing the promoters of key node genes between these two pathways. To increase production of p-coumaric acid two different strategies were explored consisting of introducing (i) PAL, C4H and a cytochrome P450 reductase from A. thaliana, together with the native cytochrome (CYB5) from S. cerevisiae, or (ii) a highly specific TAL from Flavobacterium johnsoniae. The authors concluded that the PAL branch was far more efficient than the TAL branch.
Efficient production of caffeic acid in S. cerevisiae from L-tyrosine was achieved by Liu et al. (2019a) by recruiting a heterologous tyrosine ammonia lyase (TAL) from Rhodosporidium toruloides and two enzymes, HpaB from P. aeruginosa and HpaC from E. coli, to take on the role of the plant-specific cytochrome P450-dependent monooxygenase, p-coumarate 3-hydroxylase (C3H) to hydroxylate p-coumaric acid to caffeic acid.
The expression of heterologous genes in S. cerevisiae can lead to translation errors, resulting in the expression of non-functional enzymes; thus, codon optimization can be required for an efficient production of phenolic compounds (Rainha et al. 2020). For instance, expression of the TAL gene from Rhodobacter sphaeroides into S. cerevisiae failed to produce p-coumaric acid when feeding with tyrosine substrate, despite bacterial TAL activity had been confirmed in vitro and the transcripts of TAL were present at high levels in the transgenic yeast. This was attributed to differences in codon usage, with many of the Rhodobacter codons having very low usage frequency in the yeast. Using multi rounds of PCR-based mutagenesis, Wang et al. (2011) succeeded to replace inefficient bacterial codons by yeast favorable codons, which strongly increased p-coumaric acid and resveratrol biosynthesis.
Escherichia coli has also been extensively explored for the heterologous production of phenolic compounds. Comparing with S. cerevisiae, it has the advantage of having higher growth rates and shorter time of doubling, which is relevant to develop competitive processes of production. Besides, the expression levels of heterologous genes in E. coli are usually higher than those in S. cerevisiae. However, it does not perform post-translational modifications and does not possess intracellular organelles, which can be unfavorable for the functional expression of plant-derived enzymes (Gomes et al. 2022).
De novo biosynthesis of p-coumaric acid from glucose as the sole carbon source in E. coli was achieved by Li et al. (2018), using a plant-specific cytochrome P450 enzyme (C4H) encoding the gene LauC4H gene isolated from Lycoris aurea fused with the CYP450 redox partner from A. thaliana, as well as PAL from A. thaliana into the recombinant E. coli cells. Synthesis of four cinnamates (cinnamic, p-coumaric, caffeic, and ferulic acids) was obtained by An et al. (2016) in engineered E. coli harboring different combination of four genes: PAL from A. thaliana, TAL and a monooxygenase (Sam5) from Saccharothrix espanaensis, and an O-methyltransferase (ROMT9) from Oryza sativa. Rodrigues et al. (2015) engineered E. coli for production of caffeic acid adding tyrosine as a precursor in a two-step conversion using TAL from Rhodotorula glutinis (RgTAL) and 4-coumarate 3-hydroxylase from S. espanaensis or cytochrome P450 CYP199A2 from R. palustris. A limitation of the system was the accumulation of p-coumaric acid, owing to its cytotoxicity and feedback inhibition of TAL activity. Improved caffeic acid production could be obtained by changing genes arrangement through codon optimization and different plasmid constructions, together with recurrent addition of the substrate (L-tyr). The same authors further demonstrated that this biosynthetic pathway could be triggered using heat shock promoters, suggesting its potential for the future production of these compounds at industrial scales without chemical induction (Rodrigues et al. 2017).
Jendresen et al. (2015) screened a series of PAL and TAL enzymes from different origins, finding that TAL from Herpetosiphon aurantiacus and Flavobacterium johnsoniae showed higher substrate specificity than other characterized enzymes, resulting in enhanced production of p-coumaric acid in bacteria like E. coli and Lactococcus lactis. A system for caffeic acid production was developed by Haslinger and Prather (2020) by expressing TAL from F. johnsoniae, which performed better at low L-tyr concentrations, and CYP199A2 from R. palustris altering the redox partners to increase the efficiency of the cytochrome P450. Using this strategy, the pathway performance was enhanced under low L-tyr conditions, which allowed de novo production of caffeic acid with glucose as the only carbon source in an otherwise wild type E. coli without supplementing L-tyr. For their part, Lin and Yan (2012) used a dual pathway to convert tyrosine to caffeic acid consisting of the enzymes TAL from Rhodobacter capsulatus and an E. coli native hydroxylase complex (i.e., 4-hydroxyphenylacetate 3-hydroxylase, 4HPA3H). Through this heterologous pathway, they avoided the use of the cytochrome P450-dependent monooxygenases C4H and C3H difficult to be functionally expressed in prokaryotic systems.
Bacterial platforms have also been used for the biosynthesis of hydroxybenzoic acids. A Corynebacterium glutamicum strain was constructed by Kallscheuer and Marienhagen (2018) for the production of hydroxybenzoates derived from chorismate. In this microorganism, the existence of a catabolic network for aromatic molecules prevents the microbial production of aromatic compounds other than aromatic amino acids. To allow formation of hydroxybenzoates, the genes involved in the catabolism of aromatic compounds were deleted. Further, the carbon flux into the shikimate pathway was increased by manipulation of the glucose transport and key enzymatic activities of the central carbon metabolism. Allosteric inhibition of DAHP synthase by L-tyr was avoided by introduction of tyrosine-insensitive DAHP synthases from E. coli (aroH or aroF*) in combination with a second gene (ubiC) coding for an enzyme capable of converting shikimate pathway intermediates to the desired hydroxybenzoate. The obtained strain (C. glutamicum DelAro5) allowed efficient production of 2-hydroxybenzoic (salicylic), 3-hydroxybenzoic, 4-hydroxybenzoic, and protocatechuic acids. Microbial production of protocatechuic acid was also achieved by Okai et al. (2017) from ferulic acid by expressing the vanillate O-demethylase gene (vanAB) from Corynebacterium efficiens in C. glutamicum.
Further detailed information about phenolic acids production by heterologous microorganisms can be found in several recent reviews (Hernández-Chávez et al. 2019; Gomes et al. 2022; Rainha et al. 2020; Valanciene et al. 2020; Vargas-Tah and Gosset 2015).
6 Concluding Remarks and Prospects
Plant phenolic acids are recognized to possess a range of noteworthy biological activities that are thought to contribute to the health-promoting effects associated to the consumption of plant-derived foods. Their antioxidant, anti-inflammatory, or antimicrobial properties have had them interesting for food, cosmetic, and pharmaceutical companies, so that there is an increasing research and industrial demand for these compounds. In order to fulfill these needs, an efficient production of pure compounds is required, so that they can be available for adequately assessing their biological activity and effects, and sufficient amounts can be delivered to the industry for their applications as nutraceutical, cosmeceutical, or functional food ingredients.
Over the last decades, relevant advances have been made in the knowledge of the biosynthetic pathways involved in the formation of the distinct phenolic classes including phenolic acids, and many approaches have been developed to enhance their production or address it toward particular compounds, such as processes of plant selection, tissue culture, fermentation, or construction of heterologous plant or microbial platforms. However, despite improvements in plant optimization and breeding, plants are subject to cultivation factors and production outcomes are difficult to predict and standardize. Although plant tissue cultures allow more control and may overcome some of these shortcomings, they also show problems associated with low and variable product yields, largely influenced by the type of culture, e.g., differentiated (shoots or roots) or non-differentiated cells (callus or suspensions), their instability over time, susceptibility to several stresses, or formation of aggregates limiting oxygen and nutrient diffusion (Wilson and Roberts 2012). Critical aspects for obtaining high yields in bioreactors are the application of adequate selection of process parameters, medium optimization, precursor feeding, or elicitation (Marchev et al. 2020).
Plant engineering constitutes an attractive alternative to increase phenolic acids production. However, plants are difficult to manipulate genetically, and challenges are even higher when the interest is focused on particular compounds and not in the production of a phenolic pool. In this respect, heterologous microorganisms incorporating specialized genes from plants or other microorganisms represent a promising approach. Genetic manipulation is much easier in microorganisms than in plants and, as the polyphenol pathway has to be reconstructed, more straightforward routes to target compounds can be designed avoiding diversion of substrates through competing pathways of secondary metabolism. Microbes also possess the advantages of a rapid growth and the possibility of growing in inexpensive carbon sources. Nonetheless, pathways for phenolic biosynthesis are not always easy to reproduce in microorganisms. Plant enzymes may not work efficiently in microorganisms and gene introduction may entail errors and sequence mismatch at DNA assembly, such as long repeat sequence and hairpin loop structure, which hinder the precise construction of biosynthetic pathways (Chen et al. 2020). Novel technologies have become available as powerful tools for site-specific genome modification, such as the Clustered Regularly Interspaced Short Palindromic Repeats associated caspase 9 endonuclease system (CRISPR/Cas9), which can be expected to break ground for large-scale genome editing and thus provide more rapid developments in plants and microbes genome engineering in the near future (Yuan and Grotewold 2015). Actually, CRISPR/Cas9 approaches for genome engineering of plant secondary metabolism have already been applied in plant in vitro systems (Marchev et al. 2020). The use of these innovative genomic tools together with the advances in computational omics also enables the construction of microbial chassis increasingly adapted to the synthesis of phenolic compounds and other groups of secondary metabolites. Nevertheless, there are still many challenges to overcome before profitable industrial processes for phenolic acid production using microbial cell factories become a reality (Rainha et al. 2020).
References
Abd Razak DL, Abd Rashid NY, Jamaluddin A et al (2015) Enhancement of phenolic acid content and antioxidant activity of rice bran fermented with Rhizopus oligosporus and Monascus purpureus. Biocatal Agric Biotechnol 4:33–38
Abotaleb M, Liskova A, Kubatka P, Büsselberg D (2020) Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomol Ther 10:221
Agcam E, Akyıldız A, Evrendilek GA (2014) Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurization. Food Chem 143:354–361
Ali SS, Ahmad WANW, Budin SB, Zainalabidin S (2020) Implication of dietary phenolic acids on inflammation in cardiovascular disease. Rev Cardiovasc Med 21:225–240
Amaya Villalva MF, González-Aguilar G, Sánchez OR et al (2018) Bioprocessing of wheat (Triticum aestivum cv. Kronstad) bran from Northwest Mexico: effects on ferulic acid bioaccessibility in breads. CyTA J Food 16:570–579
Ambawat S, Sharma P, Yadav NR, Yadav RC (2013) MYB transcription factor genes as regulators for plant responses: an overview. Physiol Mol Biol Plants 19:307–321
An DG, Cha MN, Nadarajan SP et al (2016) Bacterial synthesis of four hydroxycinnamic acids. Appl Biol Chem 59:173–179
Aree T (2019) Understanding structures and thermodynamics of β-cyclodextrin encapsulation of chlorogenic, caffeic and quinic acids: implications for enriching antioxidant capacity and masking bitterness in coffee. Food Chem 293:550–560
Awwad S, Issa R, Alnsour L et al (2021) Quantification of caffeine and chlorogenic acid in green and roasted coffee samples using HPLC-DAD and evaluation of the effect of degree of roasting on their levels. Molecules 26:7502
Bach F, Zielinski AAF, Helm CV et al (2019) Bio compounds of edible mushrooms: in vitro antioxidant and antimicrobial activities. LWT 107:214–220
Barron C, Surget A, Rouau X (2007) Relative amounts of tissues in mature wheat (Triticum aestivum L.) grain and their carbohydrate and phenolic acid composition. J Cereal Sci 45:88–96
Barros L, Dueñas M, Ferreira ICFR et al (2009) Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem Toxicol 47:1076–1079
Bhanja T, Kumari A, Banerjee R (2009) Enrichment of phenolics and free radical scavenging property of wheat koji prepared with two filamentous fungi. Bioresour Technol 100:2861–2866
Bovy A, De Vos R, Kemper N et al (2002) High-flavonol tomatoes through heterologous expression of the maize transcription factor genes LC and C1. Plant Cell 14:2509–2526
Breitfellner F, Solar S, Sontag G (2002) Effect of gamma-irradiation on phenolic acids in strawberries. J Food Sci 67:517–521
Bryngelsson S, Dimberg LH, Kamal-Eldin A (2002) Effects of commercial processing on levels of antioxidants in oats (Avena sativa L.). J Agric Food Chem 50:1890–1896
Bucic-Kojic A, Šelo G, Zelic B et al (2017) Recovery of phenolic acid and enzyme production from corn silage biologically treated by Trametes versicolor. Appl Biochem Biotechnol 181:948–960
Bugg TD, Ahmad M, Hardiman EM, Singh R (2011) The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol 22:394–400
Călinoiu LF, Vodnar DC (2019) Thermal processing for the release of phenolic compounds from wheat and oat bran. Biomol Ther 22(10):21
Campos FM, Couto JA, Figuereido AR et al (2009) Cell membrane damage induced by phenolic acids on wine lactic acids bacteria. Int J Food Microbiol 135:144–151
Chen PX, Dupuis JH, Marcone MF et al (2015) Physicochemical properties and in vitro digestibility of cooked regular and nondarkening cranberry beans (Phaseolus vulgaris L.) and their effects on bioaccessibility, phenolic composition, and antioxidant activity. J Agric Food Chem 63:10448–10458
Chen R, Yang S, Zhang L, Zhou YJ (2020) Advanced strategies for production of natural products in yeast. iScience 23(3):100879. https://doi.org/10.1016/j.isci.2020.100879
Chen YH, Zhang YH, Chen GS et al (2022) Effects of phenolic acids and quercetin-3-O-rutinoside on the bitterness and astringency of green tea infusion. NPJ Sci Food 27(6):8
Clifford MN (1999) Chlorogenic acids and other cinnamates—nature, occurrence and dietary burden. J Sci Food Agric 79:362–372
Clifford MN (2000) Chlorogenic acids and other cinnamates—nature, occurrence and dietary burden. J Sci Food Agric 80:1033–1043
Clifford MN, Scalbert A (2000) Ellagitannins – nature, occurrence and dietary burden. J Sci Food Agric 80:1118–1125
Clifford MN, Jaganath IB, Ludwig IA, Crozier A (2017) Chlorogenic acids and the acyl-quinic acids: discovery, biosynthesis, bioavailability and bioactivity. Nat Prod Rep 34:1391–1421
Couto JA, Campos FM, Figueiredo AR, Hogg TA (2006) Ability of lactic acid bacteria to produce volatile phenols. Am J Enol Vitic 57:166–171
Crozier TWM, Stalmach A, Lean MEJ, Crozier A (2012) Espresso coffees, caffeine and chlorogenic acid intake: potential health implications. Food Funct 3:30–33
Cueva C, Moreno-Arribas MV, Martín-Alvarez PJ et al (2010) Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res Microbiol 161:372–382
Davies KM, Schwinn KE (2006) Molecular biology and biotechnology of flavonoid biosynthesis. In: Andersen ØM, Markham KR (eds) Flavonoids. Chemistry, biochemistry and applications. CRC Press, Boca Raton, pp 143–218
De P, Baltas M, Bedos-Belval F (2011) Cinnamic acid derivatives as anticancer agents – a review. Curr Med Chem 18:1672–1703
Deng C, Wang Y, Huang F et al (2020) SmMYB2 promotes salvianolic acid biosynthesis in the medicinal herb Salvia miltiorrhiza. J Integr Plant Biol 62:1688–1702
Dewanto V, Wu XZ, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014
Dias AP, Grotewold E (2003) Manipulating the accumulation of phenolics in maize cultured cells using transcription factors. Biochem Eng J 14:207–216
Domínguez-Fernández M, Irigoyen A, Vargas-Alvarez MA et al (2021) Influence of culinary process on free and bound (poly)phenolic compounds and antioxidant capacity of artichokes. Int J Gastron Food Sci 25:100389
Drewnowski A, Henderson SA, Shore AB (1997) Taste responses to naringin a flavonoid and the acceptance of grapefruit juice are related to genetic sensitivity to 6-npropylthiouracil. American J Clin Nutr 66(2):391–397. https://doi.org/10.1093/ajcn/66.2.391
Duan J, Huo H, Du WJ et al (2015) Biodegradation of Kraft lignin by a newly isolated anaerobic bacterial strain, Acetoanaerobium sp. WJDL-Y2. Lett Appl Microbiol 62:55–62
Dudnik A, Almeida AF, Andrade R et al (2018) BacHBerry: BACterial hosts for production of bioactive phenolics from bERRY fruits. Phytochem Rev 17:291–326
Duizer LM, Langfried A (2016) Sensory characterization during repeated ingestion of small-molecular-weight phenolic acids. J Sci Food Agric 96:513–521
Ferreira SS, Antunes MS (2021) Re-engineering plant phenylpropanoid metabolism with the aid of synthetic biosensors. Front Plant Sci 12:701385
Ferrer-Gallego R, Hernández-Hierro JM, Rivas-Gonzalo JC, Escribano-Bailón MT (2014) Sensory evaluation of bitterness and astringency sub-qualities of wine phenolic compounds: synergistic effect and modulation by aromas. Food Res Int 62:1100–1107
Fiddler W, Parker W, Wasserman A, Doerr R (1967) Thermal decomposition of ferulic acid. J Agric Food Chem 15:757–761
Filannino P, Bai Y, Di Cagno R et al (2015) Metabolism of phenolic compounds by lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol 46:272–279
Frank O, Zehentbauer G, Hofmann T (2006) Bioresponse-guided decomposition of roast coffee beverage and identification of key bitter taste compounds. Eur Food Res Technol 222:492–508
Friedman M (1997) Chemistry biochemistry and dietary role of potato polyphenols. A review. Rev. J Agric Food Chem 45(5):1523–1540. https://doi.org/10.1021/jf960900s
Fritsch C, Heinrich V, Vogel RF, Toelstede S (2016) Phenolic acid degradation potential and growth behavior of lactic acid bacteria in sunflower substrates. Food Microbiol 57:178–186
Fuller M, Rao NZ (2017) The effect of time, roasting temperature, and grind size on caffeine and chlorogenic acid concentrations in cold brew coffee. Sci Rep 7:17979
Gil MI, Holcroft DM, Kader AA (1997) Changes in strawberry anthocyanins and other polyphenols in response to carbon dioxide treatments. J Agric Food Chem 45:1662–1667
Gil MI, Tomás-Barberán FA, Hess-Pierce B et al (2000) Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem 48:4581–4589
Godos J, Marventano S, Mistretta A et al (2017) Dietary sources of polyphenols in the mediterranean healthy eating, aging and lifestyle (MEAL) study cohort. Int J Food Sci Nutr 68:750–756
Gomes D, Rodrigues LR, Rodrigues JL (2022) Perspectives on the design of microbial cell factories to produce prenylflavonoids. Int J Food Microbiol 367:109588
Goncalves B, Landbo AK, Knudsen D et al (2004) Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). J Agric Food Chem 52:523–530
Goodey AR, Tubb RS (1982) Genetic and biochemical analysis of the ability of Saccharomyces cerevisiae to decarboxylate cinnamic acids. J Gen Microbiol 128:2615–2610
Griffiths DW, Bain H, Dale MFB (1995) Photo-induced changes in the total chlorogenic acid content of potato (Solanum tuberosum) Tubers. J Sci Food Agric 68(1):105–110. https://doi.org/10.1002/jsfa.2740680117
Gross GG (2008) From lignins to tannins: forty years of enzyme studies on the biosynthesis of phenolic compounds. Phytochemistry 69:3018–3031
Grosso G, Stepaniak U, Topor-Mądry R et al (2014) Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 30:1398–1403
Häkkinen SH, Kärenlampi SO, Mykkänen HM et al (2000) Ellagic acid content in berries: influence of domestic processing and storage. Eur Food Res Technol 212:75–80
Haslinger K, Prather KLJ (2020) Heterologous caffeic acid biosynthesis in Escherichia coli is affected by choice of tyrosine ammonia lyase and redox partners for bacterial cytochrome P450. Microb Cell Factories 19:26
Heiniö R-L, Liukkonen K-H, Myllymäki O, Pihlava J-M, Adlercreutz H, Heinonen S-M, Poutanen K (2008) Quantities of phenolic compounds and their impacts on the perceived flavour attributes of rye grain. J Cereal Sci 47(3):566–575. https://doi.org/10.1016/j.jcs.2007.06.018
Hernández-Chávez G, Martinez A, Gosset G (2019) Metabolic engineering strategies for caffeic acid production in Escherichia coli. Electron J Biotechnol 38:19–26
Huang CJ, Zayas JF (1991) Phenolic acid contributions to taste characteristics of corn germ protein flour products. J Food Sci 56:1308–1310
Ifie I, Marshall LJ (2018) Food processing and its impact on phenolic constituents in food. Cogent Food Agric 4(1):1507782
Jendresen CB, Stahlhut SG, Li M et al (2015) Highly active and specific tyrosine ammonia-lyases from diverse origins enable enhanced production of aromatic compounds in bacteria and Saccharomyces cerevisiae. Appl Environ Microbiol 81:4458–4476
Jeszka-Skowron M, Sentkowska A, Pyrzynska K, De Peña MP (2016) Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: influence of green coffee bean preparation. Eur Food Res Technol 242:1403–1409
Jiang D, Peterson DG (2010) Role of hydroxycinnamic acids in food flavor: a brief overview. Phytochem Rev 9:187–193
Kähkönen MP, Hopia AI, Heinonen M (2001) Berry phenolics and their antioxidant activity. J Agric Food Chem 9:4076–4082
Kalb V, Seewald T, Hofmann T, Granvogl M (2020) Studies on the impact of malting and mashing on the free, soluble ester-bound, and insoluble ester-bound forms of desired and undesired phenolic acids aiming at styrene mitigation during wheat beer brewing. J Agric Food Chem 68:12421–12432
Kallscheuer N, Marienhagen J (2018) Corynebacterium glutamicum as platform for the production of hydroxybenzoic acids. Microb Cell Factories 17:70
Katina K, Laitila A, Juvonen R et al (2007) Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol 24:175–186
Kiokias S, Oreopoulou V (2021) A review of the health protective effects of phenolic acids against a range of severe pathologic conditions (including coronavirus-based infections). Molecules 26:5405
Klaiber RG, Baur S, Koblo A, Carle R (2005) Influence of washing treatment and storage atmosphere on phenylalanine ammonia-lyase activity and phenolic acid content of minimally processed carrot sticks. J Agric Food Chem 53:1065–1072
Koopman F, Beekwilder J, Crimi B et al (2012) De novo production of the flavonoid naringenin in engineered Saccharomyces cerevisiae. Microb Cell Factories 11:1–15
Koponen J, Happonen AM, Mattila PH, Törrönen AR (2007) Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J Agric Food 55:1612–1619
Kremr D, Bajert T, Bajerová P et al (2016) Unremitting problems with chlorogenic acid nomenclature: a review. Quim Nova 39:530–533
Krivoruchko A, Nielsen J (2015) Production of natural products through metabolic engineering of Saccharomyces cerevisiae. Curr Opin Biotechnol 35:7–15
Daniel EM, Krupnick AS, Heur YH et al (1989) Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J Food Comp Anal 2:338–349
Kumar N, Goel N (2019) Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep 24:e00370
Landete JM (2011) Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Res Int 44:1150–1160
Langos D, Granvogl M (2016) Studies on the simultaneous formation of aroma-active and toxicologically relevant vinyl aromatics from free phenolic acids during wheat beer brewing. J Agric Food Chem 64:2325–2332
Lattanzio V, Kroon PA, Quideau S, Treutter D (2008) Plant phenolics: secondary metabolites with diverse functions. In: Daayf F, Lattanzio V (eds) Recent advances in polyphenol research, vol 1. Wiley-Blackwell, Chichester, UK, pp 1–35
Lattanzio V, Cardinali A, Linsalata V (2012) Plant phenolics: a biochemical and physiological perspective. In: Cheynier V, Sarni-Manchado P, Quideau S (eds) Recent advances in polyphenol research, vol 3. Wiley-Blackwell, Chichester, UK, pp 1–39
Lawless HT, Horne J, Giasi P (1996) Astringency of organic acids is related to pH. Chem Senses 21:397–403
Lee H, Nagy S (1990) Formation of 4-vinyl guaiacol in adversely stored orange juice as measured by an improved HPLC method. J Food Sci 55:162–163
Lee J, Durst RW, Wrolstad RE (2002) Impact of juice processing on blueberry anthocyanins and polyphenolics: comparison of two pretreatments. J Food Sci 67:1660–1667
Lee S, Kang M, Bae J-H, Sohn J-H, Sung BH (2019) bacterial valorization of lignin: strains enzymes conversion pathways biosensors and perspectives. Front Bioeng Biotechnol 7209. https://doi.org/10.3389/fbioe.2019.00209
Leonard W, Zhang P, Ying D et al (2021) Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol Adv 49:107763
Li Y, Li J, Qian B et al (2018) De novo biosynthesis of p-coumaric acid in E. coli with a trans-cinnamic acid 4-hydroxylase from the Amaryllidaceae plant Lycoris aurea. Molecules:23:31
Lin Y, Yan Y (2012) Biosynthesis of caffeic acid in Escherichia coli using its endogenous hydroxylase complex. Microb Cell Factories 11:42–51
Liu L, Liu H, Zhang W et al (2019a) Engineering the biosynthesis of caffeic acid in Saccharomyces cerevisiae with heterologous enzyme combinations. Engineering 5:287–295
Liu Q, Yu T, Li X et al (2019b) Rewiring carbon metabolism in yeast for high level production of aromatic chemicals. Nat Commun 10:1–13
Maga JA (1978) Simple phenol and phenolic compounds in food flavor. Crit Rev Food Sci Nutr 10:323–372
Mäkilä L, Laaksonen O, Kallio H, Yang B (2017) Effect of processing technologies and storage conditions on stability of black currant juices with special focus on phenolic compounds and sensory properties. Food Chem 221:422–430
Manach C, Scalbert A, Morand C et al (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Marchev AS, Yordanova ZP, Georgiev MI (2020) Green (cell) factories for advanced production of plant secondary metabolites. Crit Rev Biotechnol 40(4):443–458. https://doi.org/10.1080/07388551.2020.1731414
Marchiosi R, dos Santos W, Constantin RP et al (2020) Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem Rev 19:865–906
Marszałek K, Woźniak Ł, Skąpska S, Mitek M (2017) High pressure processing and thermal pasteurization of strawberry purée: quality parameters and shelf-life evaluation during cold storage. J Food Sci Technol 54:832–841
Milke L, Aschenbrenner J, Marienhagen J, Kallscheuer N (2018) Production of plant-derived polyphenols in microorganisms: current state and perspectives. Appl Microbiol Biotechnol 102:1575–1585
Miranda AM, Steluti J, Fisberg RM, Marchioni DM (2016) Dietary intake and food contributors of polyphenols in adults and elderly adults of Sao Paulo: a population-based study. Br J Nutr 115:1061–1070
Miyagusuku-Cruzado G, García-Cano I, Rocha-Mendoza D et al (2020) Monitoring hydroxycinnamic acid decarboxylation by lactic acid bacteria using high-throughput UV-Vis spectroscopy. Molecules 25:3142
Moeenfard M, Rocha L, Alves A (2014) Quantification of caffeoylquinic acids in coffee brews by HPLC-DAD. J Anal Methods Chem 2014:965353
Moon JK, Shibamoto T (2010) Formation of volatile chemicals from thermal degradation of less volatile coffee components: quinic acid, caffeic acid, and chlorogenic acid. J Agric Food Chem 58:5465–5470
Morales-De La Pena M, Salvia-Trujillo L, Rojas-Graü MA, Martín-Belloso O (2011) Changes on phenolic and carotenoid composition of high intensity pulsed electric field and thermally treated fruit juice–soymilk beverages during refrigerated storage. Food Chem 129:982–990
Morata A, Loira I, Suárez-Lepe JA (2016) Influence of yeasts in wine colour. In: Morata A, Loira I (eds) Grape and wine biotechnology. IntechOpen, London
Nagasaka R, Chotimarkorn C, Shafiqul IM et al (2007) Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem Biophys Res Commun 358:615–619
Nascimento-Souza MA, de Paiva PG, Pérez-Jiménez J et al (2018) Estimated dietary intake and major food sources of polyphenols in elderly of Viçosa, Brazil: a population-based study. Eur J Nutr 57:617–627
Nayak B, Liu RH, Tang JM (2015) Effect of processing on phenolic antioxidants of fruits, vegetables, and grains: a review. Crit Rev Food Sci Nutr 55:887–918
Okai N, Masuda T, Takeshima Y et al (2017) Biotransformation of ferulic acid to protocatechuic acid by Corynebacterium glutamicum ATCC21420 engineered to express vanillate o-demethylase. AMB Express 7:130
Ovaskainen ML, Törrönen R, Koponen JM et al (2008) Dietary intake and major food sources of polyphenols in Finnish adults. J Nutr 138:562–566
Peleg H, Noble AC (1995) Perceptual properties of benzoic acid derivatives. Chem Senses 20:393–400
Pérez-Jiménez J, Fezeu L, Touvier M et al (2011) Dietary intake of 337 polyphenols in French adults. Am J Clin Nutr 93:1220–1228
Pérez-Lamela C, Franco I, Falqué E (2021) Impact of high-pressure processing on antioxidant activity during storage of fruits and fruit products: a review. Molecules 26:5265
Persad R, Reuter DN, Dice LT et al (2020) The Q-system as a synthetic transcriptional regulator in plants. Front Plant Sci 11:245
Prabakaran M, Chung I-M, Son N-Y, Chi H-Y, Kim S-Y, Yang Y-J, Kwon C, An Y-J, Ahmad A, Kim S-H (2019) Analysis of selected phenolic compounds in organic pesticide-free conventional rice (Oryza sativa L.) using LC-ESI-MS/MS. Molecules 24(1):67. https://doi.org/10.3390/molecules24010067
Radtke J, Linseisen J, Wolfram G (1998) Phenolsäurezufuhr Erwachsener in einem bayerischen Teilkollektiv der Nationalen Verzehrsstudie. Z Ernahrungswiss 37:190–197
Rainha J, Gomes D, Rodrigues LR, Rodrigues JL (2020) Synthetic biology approaches to engineer Saccharomyces cerevisiae towards the industrial production of valuable polyphenolic compounds. Life 10:56
Rawson NE, Li X (2004) The cellular basis of flavour perception: taste and aroma. In: Taylor AJ, Roberts DD (eds) Flavour perception. Blackwell Publishing, Iowa, pp 57–85
Riabinina O, Luginbuhl D, Marr E et al (2015) Improved and expanded Q-system reagents for genetic manipulations. Nat Methods 12:219–222
Ripari V, Bai Y, Gänzle MG (2019) Metabolism of phenolic acids in whole wheat and rye malt sourdoughs. Food Microbiol 77:43–51
Rivas A, Sanchez-Ortiz A, Jimenez B et al (2013) Phenolic acid content and sensory properties of two Spanish monovarietal virgin olive oils. Eur J Lipid Sci Technol 115:621–630
Rodrigues JL, Araújo RG, Prather KLJ et al (2015) Heterologous production of caffeic acid from tyrosine in Escherichia coli. Enzyme Microbial Technol 71:36–44
Rodrigues JL, Couto MR, Araújo RG et al (2017) Hydroxycinnamic acids and curcumin production in engineered Escherichia coli using heat shock promoters. Biochem Eng J 125:41–49
Ruwizhi N, Aderibigbe BA (2020) Cinnamic acid derivatives and their biological efficacy. Int J Mol Sci 21:5712
Sanchez-Maldonado AF, Schieber A, Ganzle MG (2011) Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J Appl Microb 111:1176–1184
Santos da Silveira J, Durand N, Lacour S et al (2019) Solid-state fermentation as a sustainable method for coffee pulp treatment and production of an extract rich in chlorogenic acids. Food Bioprod Process 115:175–184
Santos-Buelga C, González-Paramás AM et al (2019) Plant phenolics as functional food ingredients. Adv Food Nutr Res 90:183–257
Saura-Calixto F, Serrano J, Goñi I (2007) Intake and bioaccessibility of total polyphenols in whole diet. Food Chem 101:492–501
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073S–2085S
Schmidt CG, Gonçalve LM, Prietto L et al (2014) Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem 146:371–377
Šelo G, Planinic M, Tišma M et al (2021) Comprehensive review on valorization of agro-food industrial residues by solid-state fermentation. Foods 10:927
Shinohara T, Kubodera S, Yanagida F (2000) Distribution of phenolic yeasts and production of phenolic off-flavors in wine fermentation. J Biosci Bioeng 90:90–97
Siedler S, Khatri NK, Zsohár A et al (2017) Development of a bacterial biosensor for rapid screening of yeast p-coumaric acid production. ACS Synth Biol 6:1860–1869
Slimestad R, Verheul MJ (2005) Content of chalconaringenin and chlorogenic acid in cherry tomatoes is strongly reduced during postharvest ripening. J Agric Food Chem 53:7251–7256
Sova M, Saso L (2020) Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients 12:2190
Spanos GA, Wrolstad RE (1990) Influence of variety, maturity, processing, and storage on the phenolic composition of pear juice. J Agric Food Chem 38:817–824
Strack D (1997) Phenolic metabolism. In: Dey PM, Harborne JB (eds) Plant biochemistry. Academic, London, pp 387–416
Svensson L, Sekwati-Monang B, Lutz DL et al (2010) Phenolic acids and flavonoids in nonfermented and fermented red sorghum (Sorghum bicolor (L.) Moench). J Agric Food Chem 58:9214–9220
Swain AR, Dutton SP, Truswell AS (1985) Salicylates in foods. J Am Diet Assoc 85:950–960
Szwajgier D, Halinowski T, Helman E et al (2014) Influence of different heat treatments on the content of phenolic acids and their derivatives in selected fruits. Fruits 69:167–178
Tajik N, Tajik M, Mack I, Enck P (2017) The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur J Nutr 56:2215–2244
Tang N, Cao Z, Yang C et al (2021) A R2R3-MYB transcriptional activator LmMYB15 regulates chlorogenic acid biosynthesis and phenylpropanoid metabolism in Lonicera macranthoides. Plant Sci 308:110924
Thomas L, Larroche C, Pandey A (2013) Current developments in solid-state fermentation. Biochem Eng J 81:146–161
Tomás-Barberán TA, Clifford MN (2000) Dietary hydroxybenzoic acid derivatives – nature, occurrence and dietary burden. J Sci Food Agric 80:1024–1032
Tresserra-Rimbau A, Rimm EB, Medina-Remón A et al (2014) Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr Metab Cardiovasc Dis 24:639–647
Tzin V, Malitsky S, Zvi MMB et al (2012) Expression of a bacterial feedback-insensitive 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase of the shikimate pathway in Arabidopsis elucidates potential metabolic bottlenecks between primary and secondary metabolism. New Phytol 194:430–439
Umezawa T (2010) The cinnamate/monolignol pathway. Phytochem Rev 9:1–17
Valanciene E, Jonuskiene I, Syrpas M et al (2020) Advances and prospects of phenolic acids production, biorefinery and analysis. Biomol Ther 10:874
Vanholme R, Storme V, Vanholme B et al (2012) A systems biology view of responses to lignin biosynthesis perturbations in Arabidopsis. Plant Cell 24(9):3506–3529
Vargas-Tah A, Gosset G (2015) Production of cinnamic and p-hydroxycinnamic acids in engineered microbes. Front Bioeng Biotechnol 3:116
Vattem DA, Shetty K (2003) Ellagic acid production and phenolic antioxidant activity in cranberry pomace (Vaccinium macrocarpon) mediated by lentinus edodes using a solid-state system. Process Biochem 39:367–379
Vinayagam R, Jayachandran M, Xu B (2016) Antidiabetic effects of simple phenolic acids: a comprehensive review. Phytother Res 30:184–199
Vitale M, Masulli M, Rivellese AA et al (2018) Dietary intake and major food sources of polyphenols in people with type 2 diabetes: the TOSCA.IT study. Eur J Nutr 57:679–688
Wang Y, Halls C, Zhang J et al (2011) Stepwise increase of resveratrol biosynthesis in yeast Saccharomyces cerevisiae by metabolic engineering. Metab Eng 13:455–463
Wang HE, Hwang CF, Tzeng YM et al (2012) Quality of white bread made from lactic acid bacteria-enriched dough. J Food Process Preserv 36:553–559
Wang W, Snooks HD, Sang S (2020) The chemistry and health benefits of dietary phenolamides. J Agric Food Chem 68:6248–6267
Wang XJ, Li YK, Song HC et al (2021) Phenolic matrix effect on aroma formation of terpenes during simulated wine fermentation - Part I: phenolic acids. Food Chem 341:128288
Waterhouse AL (2002) Wine phenolics. Ann N Y Acad Sci 957:21–36
Wei X, Potter CJ, Luo L, Shen K (2012) Controlling gene expression with the Q repressible binary expression system in Caenorhabditis elegans. Nat Methods 9:391–395
Wilson SA, Roberts SC (2012) Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol J 10:249–268
Xiao Y, Gao S, Di P, Chen J et al (2009) Methyl jasmonate dramatically enhances the accumulation of phenolic acids in Salvia miltiorrhiza hairy root cultures. Physiol Plant 137:1–9
Xiao Y, Zhang L, Gao S et al (2011) The c4h, tat, hppr and hppd genes prompted engineering of rosmarinic acid biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. PLoS One 6:e29713
Xue J, Luo D, Xu D et al (2015) CCR1, an enzyme required for lignin biosynthesis in Arabidopsis, mediates cell proliferation exit for leaf development. Plant J 83:375–387
Yang N, Zhou W, Su J et al (2017) Overexpression of SmMYC2 increases the production of phenolic acids in Salvia miltiorrhiza. Front Plant Sci 8:1804
Yuan L, Grotewold E (2015) Metabolic engineering to enhance the value of plants as green factories. Metab Eng 27:83–91
Zamora-Ros R, Rothwell JA, Scalbert A et al (2013) Dietary intakes and food sources of phenolic acids in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr 110:1500–1511
Zamora-Ros R, Knaze V, Rothwell JA et al (2016) Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr 55:1359–1375
Zhan L, Li J, Huang W et al (2020) Light irradiation affects the total antioxidant capacity, total phenolic compounds, phenolic acids, and related enzyme activities of minimally processed spinach (Spinacia oleracea L.). J Food Process Preserv 244:e14825
Zhang S, Ma P, Yang D et al (2013) Cloning and characterization of a putative R2R3 MYB transcriptional repressor of the rosmarinic acid biosynthetic pathway from Salvia miltiorrhiza. PLoS One 8:e73259
Zhang Y, Butelli E, Alseekh S et al (2015) Multi-level engineering facilitates the production of phenylpropanoid compounds in tomato. Nat Commun 6:8635
Zheng Z, Shetty K (2000) Solid-state bioconversion of phenolics from cranberry pomace and role of Lentinus edodes beta-glucosidase. J Agric Food Chem 48:895–900
Zhou W, Shi M, Deng C et al (2021) The methyl jasmonate-responsive transcription factor SmMYB1 promotes phenolic acid biosynthesis in Salvia miltiorrhiza. Hortic Res 8:10
Ziauddeen N, Rosi A, Del Rio D et al (2019) Dietary intake of (poly)phenols in children and adults: cross-sectional analysis of UK National Diet and Nutrition Survey Rolling Programme (2008–2014). Eur J Nutr 58:3183–3198
Zielinski H, Kozlowska H, Lewczuk B (2001) Bioactive compounds in the cereal grains before and after hydrothermal processing. Innov Food Sci Emerg Technol 2:159–169
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Santos-Buelga, C., González-Paramás, A.M., González-Manzano, S. (2023). Phenolic Acids and Derivatives: Description, Sources, Properties, and Applications. In: Carocho, M., Heleno, S.A., Barros, L. (eds) Natural Secondary Metabolites. Springer, Cham. https://doi.org/10.1007/978-3-031-18587-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-18587-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-18586-1
Online ISBN: 978-3-031-18587-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)