Abstract.
Titanium alloys combine outstanding mechanical properties with excellent corrosion resistance, making them desirable for challenging light-weight constructions in the transportation industry. On the other hand, titanium production is energy consuming and expensive since the current method of extraction of titanium from the ore includes smelting, chlorination, and reduction by magnesium (Kroll’s process). Hence, cost reduction is one of the driving factors for titanium research to broaden its field of application. In this overview paper, the basics of titanium metallurgy are first presented. Afterward, the conventional production route from titanium ore to semi-finished products and components is discussed. This includes ore winning and processing, melting and remelting for ingot production, forging, casting, machining of (semi-finished) products, scrap recycling, and additive manufacturing. In the end, a brief overview of titanium application in the transportation industry is given.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords:
- Titanium
- Titanium alloys
- Kroll’s process
- Titanium ore processing
- Melting
- Forging
- Casting
- Machining
- Additive manufacturing
- Powder bed fusion
1 Titanium’s Metallurgy
Titanium belongs to the allotropic metals and can exist in more than one equilibrium lattice modification [1]. Pure titanium crystallizes at 1668 °C in a body-centered cubic (bcc) structure called β-titanium which at 882 °C is transformed to a hexagonal close-packed structure (hcp), the α-titanium, having anisotropic properties. In addition, at very low temperatures and/or high pressures, α-titanium transforms to the ω-phase (hexagonal). A martensitic β-to-α-transformation (called α′- or α″-phase depending on the crystal structure) is possible at significant cooling rates. The transformation temperature named β-transus temperature (Tβ) can be influenced by alloying elements [1].
For titanium alloy production, typical alloying elements are aluminum (Al) and oxygen (O), both α-stabilizers shifting the β-transus temperature to higher temperatures, whereas tin (Sn) and zirconium (Zr) only show a limited influence on the β-transus temperature. The β-stabilizing elements are subdivided into two groups: Niobium (Nb), molybdenum (Mo), tantalum (Ta), and vanadium (V) stabilize the β-phase between the melting point and room temperature and are, therefore, called isomorphous β-stabilizers. Elements like copper (Cu), iron (Fe), or silicon (Si) also stabilize the β-phase to lower temperatures but undergo a eutectoid reaction during cooling. β-titanium then is dissociated to α-titanium and an intermetallic compound. Consequently, these elements are called eutectoid β-stabilizers [2].
Titanium alloy classification and related phase compositions are shown in the schematic quasi-binary phase diagram of titanium with increasing amounts of isomorphous β-stabilizers (see Fig. 1).
According to the phases present at room temperature, titanium alloys are divided into different main groups (see Fig. 1, top line), starting with CP-Titanium (commercially pure titanium, containing low amounts of oxygen, iron, nitrogen, and carbon only), consisting of nearly 100% α-phase. α- and near-α-alloys are composed of α-phase and up to 5% of β-phase at room temperature, respectively. Near-β-alloys contain more than 95% of β-phase and β-alloys are composed of almost 100% β-phase [1]. Finally, the two-phase alloys consist of more than 5% and less than 95% β-phase at room temperature. Typically, one or more α- and β-stabilizers are present in such alloys. They are divided into two subgroups: In (α + β)-alloys, a martensitic transformation during quenching from above β-transus is possible and depending of the exact chemical composition, either the hexagonal α′- or the orthorhombic α′′-martensite can form. The microstructure of (α + β)-alloys can, therefore, be refined and hardened by a (partial) decomposition of martensite. In metastable β-alloys, the martensite start temperature is below room temperature. Therefore, after water quenching, metastable β-alloys consist of supercooled β-phase, and in the case of solute-lean metastable β-alloys, ω-phase might be present [3]. The ω-transus temperature of solute-rich metastable alloys, on the other hand, is below room temperature so that such an alloy consists of a supercooled β-phase only after water quenching from above β-transus. Aging of supercooled β-phase leads to the formation of fine-dispersed α-phase [4] and the metastable ω-phase in some alloys [5]. Thus, in metastable β-alloys, a partial or complete precipitation hardening of the microstructure can be carried out to reach ultimate tensile strengths up to 1400 MPa [6].
Three intermetallic titanium aluminides are known: Ti3Al, TiAl, and TiAl3. In particular, the phases α2-Ti3Al (hexagonal) and γ-TiAl (tetragonal) exhibit low-density and good high-temperature properties, but both phases show limited ductility at room temperature. Nevertheless, in the last decades, different γ-TiAl alloys have been developed [3].
2 Ore Winning, Semi-finished Part Production and Recycling
The most crucial titanium minerals are anatase, rutile (both TiO2), and ilmenite (FeTiO3), which contains up to 53% TiO2. Primary deposits occur in Australia, Canada, South Africa, Brazil, India, and Norway. The most useful mineral for the extraction of titanium is rutile. Although it is rarer than ilmenite, its TiO2 content is higher. Titanium metal is mainly produced by reducing titanium tetrachloride (TiCl4) which is manufactured from natural rutile or from ilmenite, called Kroll’s process. Iron is separated from the ilmenite during a smelting operation, and a TiO2-rich slag is produced which is then transformed to TiCl4 by chlorination. In a final step, TiCl4 is reduced to pure titanium by liquid magnesium reacting to magnesium chloride (mainly MgCl2). Finally, a titanium sponge is obtained by vacuum distillation to remove the remaining MgCl2 and other byproducts [6].
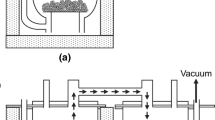
During the industrial titanium alloy production, the titanium sponge coming from Kroll’s process is mixed with alloying elements and compressed into parts. The resulting compacts are welded together to form an electrode [1]. The electrodes are often molten to the first ingot in a vacuum arc furnace (VAR). To ensure sufficient homogeneity of the alloy, the first ingot has to be remolten once for standard applications or twice for safety-critical applications, e.g., compressor disks of aircraft engines. For first ingot production or in the case of non-safety-critical applications, nowadays, electron-beam cold hearth (re)melting (EBCHR) is used [7]. The two most essential melting processes (VAR and EBCHR) in industrial titanium production are shown in Fig. 2.
Schematic sketches of the VAR process (left) and the EBCHR process (right). In vacuum arc remelting, an electric arc heats the electrode, drops of the molten material fall down and crystallize in the mold to form the ingot. In electron-beam melting, the material coming from a feedstock is molten by electron guns (EB guns), flows along a water-cooled hearth and crystallizes in a mold.
In the case of vacuum arc remelting of alloys containing refractory elements with high melting temperatures (e.g., Nb or Mo), the resulting ingots might contain small particles of these elements, the so-called high-density inclusions (HDI). To some extent, this is unavoidable, as during vacuum arc remelting, superheating of the melt is not possible, and crystallization occurs naturally in the mold (see, Fig. 2, left). Increasing electric currents would only lead to increased melting velocity. If refractory elements are required in titanium alloys, master alloys like Al-Mo or Al-Cr-V are advisable to reduce their melting temperatures [1]. In electron-beam melting, the process-related risk of HDI formation is drastically reduced as unmolten refractory element particles normally sink to the ground of the water-cooled hearth and are trapped in the skull before they can reach the ingot (see Fig. 2, right).
Independent of the melting procedure, the final ingots are typically deformed by (hot) forging, rotary swaging, rod extrusion, or rolling to form bars, rods, plates, sheets, or semi-finished products [1]. As these operations are normally performed in air, oxygen can diffuse into the surface of processed parts as oxygen is a strong α-stabilizer. The so-called α-case formation can occur at the subsurface, a partial transformation to a-phase. Along with the phase transformation, increased hardness, reduced toughness, and notch sensitivity are observed in the α-case due to the interstitially dissolved oxygen. Therefore, the α-case is generally removed by stripping or grinding before applying semi-finished products [3]. Usually, a recrystallization treatment and subsequent aging produce the final microstructure (see section microstructure).
Ingots coming from VAR or EBCHR can be remolten and used for precision casting of semi-finished parts or components. Also, skull melting of titanium sponge and the addition of alloying elements is possible, followed by casting to reduce the number of necessary production steps. After casting, a hipping treatment or further heat treatments might be applied to improve the part’s quality and mechanical properties and produce the final microstructure [8].
Machining is a standard operation in titanium component manufacturing, especially if complex geometries have to be produced. Here, up to 50% of the semi-finished parts are removed. However, the machining of titanium alloys involves relatively high production costs because of their poor machinability. This difficulty arises from titanium’s physical, chemical, and mechanical properties [2]. Due to titanium’s relatively poor thermal conductivity, heat generated by the cutting action cannot diffuse quickly into the chip’s material, so heat is concentrated in front of the tool’s rake face [9]. Titanium’s relatively low modulus of elasticity results in the spring back of the workpiece during the cutting action, causing tool rubbing at the flank face, chatter marks, and tolerance problems. The high strength of most of the titanium alloys only allows relatively low cutting speeds compared to the machining of aluminum and steel. Furthermore, titanium’s high chemical reactivity limits the number of possible materials for the cutting tools. Finally, titanium’s strong alloying tendency causes galling, welding, and smearing, leading to the rapid destruction of the tool and decreasing the finished workpiece’s quality. In addition, titanium machining operations like turning or drilling cannot be automated due to the formation of long chips. These chips can wrap around the turning chisel during turning or might get stuck between the drill and the hole, causing poor surface quality and tool failure [10]. In recent years, severe progress in titanium machining has been made by the introduction of new tool coatings [11], the application of enhanced machining processes [12], minimum quantity lubrication [13], and high-pressure cooling [14].
With increasing titanium production, titanium scrap as a raw material instead of titanium sponge has become increasingly important. Titanium scrap is produced during the production of semi-finished (e.g., during titanium ingot production in which potentially contaminated surfaces are often removed by machining between the different remelting steps) and finished (mainly chips coming from machining operations) products. Before reuse, titanium scrap must be pretreated. Adhering particles and other residues must be removed from the surface by abrasive blasting or pickling. Depending on the melting process, the scrap may be mixed with a new sponge for EBCHR melting or can be welded together for addition to a consumable electrode to be used in titanium recycling, EBCHR melting is often preferred to avoid HDI formation as scrap might be contaminated with cemented-carbide particles coming from machining operations [6].
3 Microstructures
α- and β-alloys consist of a single-phase microstructure of typically equiaxed α- or β-grains. Due to the relatively large self-diffusion rate in the β-phase, grain growth and, thus, coarsening of the microstructure might become a problem if a related alloy is annealed above β-transus.
(α + β)-alloys are normally applied with one of three different basic microstructures, namely, (i) a globular, (ii) a lamellar, or (iii) a duplex structure, a combination of a globular and a lamellar structure (see Fig. 3). Vacuum arc or electron-beam melting typically leads to lamellar microstructures with relatively large grains. After deformation, the microstructure typically consists of strongly deformed lamellae which could be broken up completely to allow subsequent recrystallization [3].
Three typical microstructures of (α + β)-alloys. Left: Globular microstructure consisting of equiaxed αp-grains (bright) surrounded by β-phase (dark), Center: Lamellar microstructure of altering α- and β-lamellae, colonies of parallel laths are clearly visible. Right: Duplex microstructure consisting of αp-phase in a lamellar matrix.
Globular microstructures (see Fig. 3, left) in (α + β)-alloys are typically achieved by recrystallization at comparably low temperatures. According to the binary phase diagram, as shown in Fig. 2, the amount of α-phase at annealing temperature (the so-called primary α-phase, αp) is comparably large (typically >60%). After recrystallization, the alloy is air cooled. Strongly deformed materials heat treated at short annealing times typically obtain fine-grained microstructures with fully equiaxed α-grains surrounded by small amounts of β-phase. Structures with αp grain sizes <5 μm show excellent mechanical properties.
As shown in Fig. 3, center, lamellar microstructures are produced by annealing the alloy above β-transus followed by air or furnace cooling. After crossing β-transus, α-seeds develop at grain boundaries or other areas of increased energy and grow into the former β-grains following the burgers relationship [3]. Due to element partitioning, β-stabilizers are concentrated in the remaining β-phase (as well as the α-stabilizers are diffused into the newly formed α-phase) so that parallel, altering laths of α- and β-phase develop the so-called colonies. Depending on the number of active α-seeds and the cooling conditions, the colony size and the length and width of individual lamellae can be varied. In general, lamellar microstructures are relatively damage tolerant and show the best impact properties of all three microstructures [1]. Water quenching from above β-transus leads to a fully (metastable, as it is supersaturated with β-stabilizing elements) martensitic structure. Due to its notch sensitivity, relatively low fatigue limit, and the difficulty to obtain homogeneous microstructures after quenching, primarily if more significant parts are concerned, fully martensitic structures are not applied [2].
Duplex structures (see Fig. 3, right) consist of globular αp-grains surrounded by lamellar structures, i.e., they are composed of a mixture of globular and lamellar microstructures. Annealing temperatures are typically chosen close to (between 30 and 50 K below) β-transus, followed by air cooling. Optimized mechanical properties are achieved once a volume fraction between 10% and 20% of αp-phase is reached. Subsequent aging is possible [3].
Besides these basic microstructures, multi-step heat treatments might be applied to (α + β)-alloys, including precipitation hardening of α- (e.g., by Ti3Al) or β-phase (e.g., by α-phase) and martensite decomposition once the duplex heat treatment is followed by water quenching instead of air cooling. During subsequent aging at relatively low temperatures, the martensite platelets are transformed to α- and β-phase and, thus, further refine the structure [15].
Metastable β-alloys are typically used with a certain amount of αp-phase (approx. 15%), which is achieved by heat-treating related alloys below β-transus followed by fast cooling or water quenching [1]. As a martensitic transformation is not possible, besides αp-phase, retained β-phase is present. Subsequent aging leads to the precipitation of fine-dispersed α-particles and, thus, to excellent mechanical properties, i.e., the highest strengths among all titanium alloys [2].
4 Additive Manufacturing
Additive manufacturing (AM) is a modern technology in titanium manufacturing in which a component is built-up layer-wise from a powder according to a digital model [16]. In general, spherical powders (made of CP-Titanium as well as of titanium alloys), mainly being produced by gas atomization like electrode induction melting gas atomization (EIGA) or plasma melting induction guiding gas atomization (PIGA) with particle sizes between approx. 10 μm and 100 μm are used in titanium additive manufacturing. Both powder production processes are shown in Fig. 4.
Processes used for the production of spherical titanium (alloy) powders applied in additive manufacturing. Left: Electrode induction melting gas atomization (EIGA). Right: Plasma melting induction guiding gas atomization (PIGA). The powder production process is shielded from oxygen containing atmospheres.
In titanium additive manufacturing, besides sintering processes like metal injection molding (MIM), in which titanium powder is processed together with an organic binder [8], a direct energy source, such as a laser or electron beam, is applied to melt the alloy powders locally [17]. Related processes are laser- or electron-beam powder bed fusion (PBF-L or PBF-EB, respectively). Consequently, crystallization occurs at high solidification rates in these processes, and the titanium alloys undergo a unique thermal history so that specific microstructures develop, see below [16].
Mainly two different titanium alloys, i.e., CP-Titanium and Ti-6Al-4V, have been successfully used to produce dense and reliable components [18]. From the materials’ point of view, one of the most significant advantages of powder bed fusion is the near-net-shape forming ability. Parts with complex geometries can be produced in one step without intensive and expensive machining procedures. On the other hand, post-processing is required, e.g., thermo-mechanical treatments like hipping to close existing pores and improve the microstructure or surface finishing of functional surfaces by grinding or machining. Nevertheless, besides reducing raw materials’ scrap, powder bed fusion is particularly effective and allows designs containing undercuts and, hence, to realize high-level functional integration in light-weight constructions.
In titanium powder bed fusion, two significant problems occur: First of all, even if the process is well shielded from the air by the use of inert gases (PBF-L) or vacuum (PBF-EB), during manufacturing, the amount of oxygen in the alloys processed is slightly increased (as the powder particles typically contain a thin oxide layer). Therefore, high-purity (in particular with low-oxygen levels) and, hence, more expensive powders are needed so that the final component fulfills the requirements of the related materials’ standards regarding the chemical composition. Second, due to the rapid cooling, the sharp thermal gradient, and the resulting directional solidification, columnar, textured β-grains develop having their <100> directions oriented approximately parallel to the building direction [19]. The β-grains either nucleate at the building platform or grow at the interface between the melt pool and the previously deposited layer, extending the β-grains to a length far larger than the deposition layer thickness [16]. During further cooling, most of this β phase is transformed into α′-martensite with a limited number of orientations [20]. Even if post-processing can transform the martensite into lamellar structures, then the remaining texture and, thus, anisotropic properties of the part are still present [21]. Globular or duplex structures cannot be produced by powder bed fusion. These two problems are the subject of ongoing research activities, which is aimed at process design, i.e., choosing adequate process parameters or a dedicated post-processing procedure or developing new titanium alloys dedicated to additive manufacturing.
5 Application of Titanium in the Transportation Industry
The largest consumer of CP-Titanium and titanium alloys in the transportation sector is the aircraft industry, in which the used amounts continuously increase [1]. In modern aircrafts, the amount of titanium (mainly applied in airframe structures and aircraft engines) can reach 15% of the airplanes’ weight, e.g., 14% of the aerostructure of the Airbus A350-900 XWB and 15% of the Boeing 787 are made of titanium. In addition, 25% of the GE CF6 aero-engine consists of titanium alloys [22]. A typical buy-to-fly ratio for the aerospace industry is 6:1 and can be as high as 20:1 for some complex aero-engine parts [22]. Typical alloys used for airframe structures are Ti-6Al-4V, a medium-strength (α + β) alloy applied, e.g., in fuselage frames or fasteners, Ti-10V-2Fe-3Al and Ti-5Al-5V-5Mo-3Cr, both high-strength metastable β-alloys mainly used in landing gear components. In aircraft engines, fan components (e.g., blades and disks) are made of Ti-6Al-4V, Ti-6Al-2Sn-4Zr-6Mo, or Ti-5Al-2Sn-2Zr-4Mo-4Cr (Ti-17). Ti-6Al-2Sn-4Zr-6Mo and Ti-17 are primarily used as fan disks at higher strength levels in some newer, larger aircraft engines [23], whereas Ti-6Al-4V can be found in several fans of small and medium-sized commercial aircraft engines.
The current temperature application limit for conventional titanium alloys is approx. 530 °C. Hence, titanium alloys can only be used in the low-pressure compressor. This can be explained by the relatively poor oxidation resistance of titanium alloys. At room temperature, titanium spontaneously forms a TiO2 layer once a fresh metallic surface is exposed to air [3]. However, at temperatures above 550 °C, this oxidation resistance is dramatically lost. This can be explained by (i) a partial transformation of Ti4+ ions present in TiO2 to a mixture of Ti4+ and Ti3+ ions producing vacancies in the oxygen sub-lattice and (ii) a partial transformation of TiO2 to TiO, TiO2, and Ti2O3, producing additional phase boundaries and lattice mismatches. Both effects support accelerated oxygen diffusion through the oxide layer [1]. Consequently, at the metal-oxide interface, additional oxidation occurs, leading to a volume increase so that the oxide layer finally peels off, and the process starts again. The poor oxidation resistance hinders the application of titanium alloys in high-temperature environments, e.g., in aircraft engines’ high-pressure compressor or turbine applications, for which they are otherwise very well suited [1]. Considerable efforts have been made since the late 1980ies to improve the oxidation and creep resistance at elevated temperatures of both conventional titanium alloys and titanium aluminides [24]. The two most compelling elements are Si and Nb [25]. In the case of Si, this can be explained by (1) the formation of a thin SiO2 layer at the metal-oxide interface forming a diffusion barrier for oxygen penetration and, thus, reducing further oxidation, and (2) the precipitation of Ti3Si or Ti5Si3 intermetallic phase at the grain boundaries decelerating grain boundary oxidation [25]. On the other hand, the mechanisms of Nb additions leading to improved oxidation resistance of titanium alloys have been intensively discussed for several years [26]. Still, a satisfying, final explanation has not been found so far.
Typical alloys used in low-pressure compressor applications are Ti-6Al-2Sn-4Zr-2Mo-0.1Si and Ti-5.8Al-4Sn-3.5Zr-0.7Nb-0.5Mo-0.35Si-0.06C both Si-containing near-α-alloys and in case of the latter Nb containing as well. In low-pressure turbines of modern aircraft engines, titanium aluminides (γ-TiAl) such as Ti-48Al-2Cr-2Nb, a cast alloy, and Ti-43.5Al-4Nb-1Mo-0.1B (TNM-B7), a wrought alloy, are applied, e.g., in the PW 1000 engine series of Pratt & Whitney [27].
Besides the application in the (aero)space industry, in racing cars and high-quality bikes, titanium in the transportation sector is rather limited due to the relatively high cost of titanium and its alloys. Nevertheless, parts of Diesel engines like compressor impeller wheels of turbochargers used in trucks, locomotives, and large ships are manufactured of titanium alloys or γ-TiAl-based intermetallics, and thus, this shows that serial applications of titanium exist; whenever, a high specific fatigue limit in combination with corrosion resistance is needed.
6 Summary
This review paper presents a brief overview of titanium’s metallurgy, and the state-of-the-art titanium production from ore extraction and processing, melting and remelting, and semi-finished-product manufacturing, including casting, forging, and machining, powder production, and additive manufacturing to scrap recycling has been given. Finally, the application of titanium in the transportation industry has been discussed. Even if titanium and its alloys are expensive materials, the unique combination of a high specific (fatigue) strength with corrosion resistance makes them desirable for challenging light-weight constructions, especially in the transportation industry.
References
Peters M, Leyens C, editors. Titanium and titanium alloys. Weinheim: Wiley-VCH; 2002.
Boyer R, Welsh G, Collings EW, editors. Materials properties handbook: titanium alloys. Ohio: ASM International; 1994.
Lütjering G, Williams JC. Titanium. Berlin: Springer; 2007.
Bowen AW. Strength enhancement in a metastable β-titanium alloy: Ti-15Mo. J Mater Sci. 1977;12:1355–60.
Ng HP, Douguet E, Bettles CJ, Muddle BC. Age-hardening behaviour of two metastable beta-titanium alloys. Mater Sci Eng A. 2010;527:7017–26.
Sibum H, Güther V, Roidl O, Habashi F, Wolf HU, Siemers C. Titanium, titanium alloys and titanium compounds. In: Ullmann’s encyclopedia of industrial chemistry. Weinheim: Wiley-VCH; 2017. https://doi.org/10.1002/14356007.a27_095.pub2.
Okano H, Hatta Y, Tada O, Tanaka H. Titanium ingot production at Toho Titanium Co., Ltd. In: Niinomi M, Akiyama S, Hagiwara M, Ikeda M, Maruyama K, editors. Eleventh world conference on titanium. Kyoto; 2007. p. 155–8.
Siemers C, Stöcker C. Developments in titanium research and applications in Germany. MATEC Web Conf. 2020;321:01003. https://doi.org/10.1051/matecconf/202032101003.
Siemers C, Bäker M, Mukherji D, Rösler J. Microstructure evolution in shear bands during the chip formation of Ti 6Al 4V. In: Lütjering G, Albrecht J, editors. Tenth world conference on titanium (Ti-2003). Hamburg: Wiley VCH; 2003. p. 839–46.
Siemers C, Laukart J, Zahra B, Rösler J, Spotz Z, Saksl K. Development of advanced and free-machining titanium alloys. In: Gallienne D, Bilodeau M, editors. Fortynineth conference of metallurgists, section light metals 2010 – advances in materials and processes. Vancouver; 2010. p. 311–22.
Li G, Lü W, Liu S, Li C, Zhou Y, Wang Q. Multilayer-growth of TiAlN/WS self-lubricating composite coatings with high adhesion and their cutting performance on titanium alloy. Compos Part B. 2021;211:108620. https://doi.org/10.1016/j.compositesb.2021.108620.
Muhammad R, Hussain MS, Maurotto A, Siemers C, Roy A, Silberschmidt VV. Analysis of a free machining α+β titanium alloy using conventional and ultrasonically assisted turning. J Mater Process Technol. 2014;214:906–15. https://doi.org/10.1016/j.jmatprotec.2013.12.002.
Roushan A, Rao US, Patra K, Sahoo P. Performance evaluation of tool coatings and nanofluid MQL on the micro-machinability of Ti-6Al-4V. J Manuf Process. 2020;73:595–610. https://doi.org/10.1016/j.jmapro.2021.11.030.
Lu Z, Zhang D, Zhang X, Peng Z. Effects of high-pressure coolant on cutting performance of high-speed ultrasonic vibration cutting titanium alloy. J Mater Process Technol. 2020;279:116584. https://doi.org/10.1016/j.jmatprotec.2019.116584.
Morita T, Hatsuoka K, Iizuka T, Kawasaki K. Strengthening of Ti-6Al-4V alloy by short-time duplex heat treatment. Mater Trans. 2005;46(7):1681–6.
Zhang T, Liu C-T. Design of titanium alloys by additive manufacturing: a critical review. Adv Powder Mater (in print). 2021. https://doi.org/10.1016/j.apmate.2021.11.001.
Martin J, Yahata B, Hundley J, Mayer JA, Schaedler TA, Pollock TM. 3D printing of high-strength aluminium alloys. Nature. 2017;549:365–9. https://doi.org/10.1038/nature23894.
Lewandowski JJ, Seifi M. Metal additive manufacturing: a review of mechanical properties. Annu Rev Mater Res. 2016;46:151–86. https://doi.org/10.1146/annurev-matsci-070115-032024.
DebRoy T, Wei HL, Zuback JS, Mukherjee T, Elmer JW, Milewski JO, Beese AM, Wilson-Heid A, De A, Zhang W. Additive manufacturing of metallic components – process, structure and properties. Prog Mater Sci. 2018;92:112–224. https://doi.org/10.1016/j.pmatsci.2017.10.001.
Liu S, Shin YC. Additive manufacturing of Ti6Al4V alloy: a review. Mater Des. 2019;164:107552. https://doi.org/10.1016/j.matdes.2018.107552.
Barriobero-Vila P, Gussone J, Stark A, Schell N, Haubrich J, Requena G. Peritectic titanium alloys for 3D printing. Nat Commun. 2018;9(1):3426. https://doi.org/10.1038/s41467-018-05819-9.
Shokrani A, Al-Samarrai I, Newman ST. Hybrid cryogenic MQL for improving tool life in machining of Ti-6Al-4V titanium alloy. J Manuf Process A. 2019;43:229–43. https://doi.org/10.1016/j.jmapro.2019.05.006.
Williams JB, Boyer RR. Review – opportunities and issues in the application of titanium alloys for aerospace components. Metals. 2020;10(6):705. https://doi.org/10.3390/met10060705.
Tsuyama S, Mitao S, Minakawa K. Alloy modification of γ-base titanium aluminide for improved oxidation resistance, creep strength and fracture toughness. Mater Sci Eng A. 1992;153:451–6.
Maki K, Shioda M, Sayashi M. Effect of silicon and niobium on oxidation resistance of TiAl intermetallics. Mater Sci Eng A. 1992;153:591–6.
Tegner BE, Zhu L, Siemers C, Saksl K, Ackland JG. High temperature oxidation resistance in titanium-niobium alloys. J Alloys Compd. 2015;643:100–5. https://doi.org/10.1016/J.JALLCOM.2015.04.115.
Siemers C, Kiese J. Developments in titanium research and applications in Germany. In: Venkatesh V, Pilchak AL, Allison JE, editors. Thirteenth world conference on titanium (Ti-2015). San Diego; 2015. p. 41–55. https://doi.org/10.1002/9781119296126.ch5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Siemers, C., Haase, F. (2023). Titanium: From Ore Extraction and Processing to Its Applications in the Transportation Industry. In: Proceedings of the 61st Conference of Metallurgists, COM 2022. COM 2022. Springer, Cham. https://doi.org/10.1007/978-3-031-17425-4_41
Download citation
DOI: https://doi.org/10.1007/978-3-031-17425-4_41
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17424-7
Online ISBN: 978-3-031-17425-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)