Abstract
The development of 3D physical systems that can realistically reproduce organs and tissues’ morphological and mechanical properties is of fundamental importance in the clinical field for specialist training and planning complex surgical interventions. The rapid advances and expansion of additive 3D printing technologies enabled their introduction to the healthcare area, boosting the development of accurate human anatomical models and simulators for a wide variety of medical applications to provide more efficient and personalised care to patients.
The present chapter reports on recent advancements in the development and realisation of novel 3D haptic organ models and anatomical structures, manufactured using innovative tissue-equivalent polymeric materials and state-of-the-art additive fabrication technologies for advanced surgical training and surgical pre-planning. We present a 3D brain phantom to assist neurosurgery residents in simulating realistic meningioma resection procedures during specialist training and a 3D liver model with biosimilar haptic properties to support hepatobiliary surgeons in preoperative planning of complex procedures during living donor transplantations. For the fabrication of these systems, we selected 3D additive technologies, based on photopolymers, suitable for processing soft and flexible printing materials and identified and developed novel silicone-based polymeric blends for 3D moulding. The synergistic combination of this dual hybrid approach ultimately allowed the creation of biomimetic soft 3D models, which present both anatomical and functional realistic tissue fidelity.
Clinical and functional validation tests demonstrated that the organ models possess realistic haptic and functional responses under surgery-relevant manipulations, with potentially considerable impacts in surgical planning and specialist training.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Additive manufacturing
- 3D organ models
- Haptic feedback
- Tissue-equivalent polymeric materials
- Surgical training
- Preoperative planning
-
Over the years, the emphasis on decreasing patient mortality, surgical complications, operating room time, and surgery-associated costs has driven extraordinary innovations in simulation and the evolution of different techniques to reach these fundamental objectives.
-
We developed and characterised novel soft and transparent materials that resemble biological tissue properties, optimising manufacturing approaches to reproduce tissues and organs with realistic features for the fabrication of innovative morpho-functional 3D.
-
The rapid progress and expansion of 3D printing technologies allowed their introduction to the healthcare area, boosting the development of physical human organ models suitable for a wide range of medical applications, including individual patient care, specialist training, and education.
-
3D printing has been introduced into the surgical arena as a tool to facilitate the diagnostic quality, better understand complex underlying anomalies, and help in planning complex surgical procedures, allowing operators to prepare an approach and rehearse the procedure in advance if needed
-
The medical imaging process typically produces a set of 2D pictures separated with a controlled thickness; the 3D representation is achieved by stacking successive layers of 2D images into a 3D volume.
-
The 3D-printed organ models fabricated provide tactile sensation closer to actual organs, compared to rigid-plastic materials, thereby allowing surgeons to perform different kinds of rehearsal operations.
-
We develop a 3D brain phantom to assist neurosurgery residents in simulating realistic meningioma resection and 3D liver models with biosimilar haptic properties to support hepatobiliary surgeons in preoperative planning of complex procedures during liver surgery and living donor liver transplantations.
1 Role of 3D Printing in Medicine
The use of anatomical models and simulators in medicine traces back to centuries ago when sophisticated wax models were used to replicate complex anatomies and disease conditions [1] [2]. Over the years, the emphasis on decreasing patient mortality, surgical complications, operating room time, and surgery-associated costs has driven extraordinary innovations in simulation and the evolution of different techniques to reach these fundamental objectives [3].
The rapid progress and expansion of 3D printing technologies allowed their introduction to the healthcare area, boosting the development of physical human organ models suitable for a wide range of medical applications, including individual patient care, specialist training, and education [4,5,6,7,8].
3D printing is defined as the process of creating a physical object from 3D digital model data, typically depositing materials layer-upon-layer in succession by means of a series of cross-sectional slices, as opposed to subtractive manufacturing technologies [9, 10]. In this way, objects are fabricated as a succession of layers of controlled thickness, which depends on the accuracy of the method and the resolution of the machine chosen [11].
There is an extensive range of different technologies and materials that can be used in additive manufacturing (e.g. plastics, rubbers, ceramics, glass, metals), each having its own limitations and applications in producing 3D models [12, 13]. The choice of the most suitable 3D printing technology among all the available ones is quite a delicate matter, depending on the combination of the desired final features of the model and the 3D printer and materials characteristics [14]. Multiple factors need to be considered, such as accuracy, speed, machinability, size, appearance (surface quality, transparency), mechanical properties, durability, and biocompatibility [15, 16].
1.1 Surgical Planning
3D printing has been introduced into the surgical arena as a tool to facilitate the diagnostic quality, better understand complex underlying anomalies, and help in planning complex surgical procedures, allowing operators to prepare an approach and rehearse the procedure in advance if needed [17]. Its applications significantly improved diagnosis and treatment due to better 3D appreciation of pathological structures and increased accuracy. In addition, the possibility of simulating complicated surgical steps in advance using prototype models can help identify potential intra-operative complications, especially for complex cases where 2D images or 3D virtual visualisation are insufficient to provide a complete understanding of the pathology. This results in reduced operating time, allow a cost-effective use of operating rooms, and minimised morbidity and even mortality. Some examples in the fields of neurology [18, 19], nephrology [20], and hepatology [21,22,23] can be found in the literature.
1.2 Medical Education and Specialist Training
3D models are also used for the purpose of training surgeons and medical residents for several reasons: (1) 2D or 3D visualisations on a computer screen can be insufficient for obtaining an intuitive understanding of complex anatomical details and structural relationships; (2) reduced working hours, an increase in the number of trainees, and medicolegal issues have reduced the opportunity for trainees to practice on patients under direct supervision; (3) current surgical training often employs the use of cadavers and animal models that either lack pathological similarity or realism and are becoming less accessible and affordable, also having ethical implications [24]. 3D-printed phantoms have anatomical and structural fidelity, allowing students and trainees to learn human anatomy better and simulate a surgical procedure in a risk-free and realistic environment, gaining experience and expertise before operating actual patients. Furthermore, the possibility to practice an entire procedure as many times as needed allowing room for mistakes in a zero-risk environment, repetitive practice, and multiple case studies, not only improves surgeons abilities and provides a unique opportunity to determine the best operating strategy but also makes them feel more confident while entering the operating room for engaging a real surgical operation [25,26,27].
2 Production Workflow
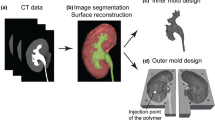
Source data acquired with any imaging modality (i.e. computer tomography (CT) or nuclear magnetic resonance imaging (MRI)) are typically visualised in two dimensions. With the aid of postprocessing tools and algorithms, it is possible to elaborate and produce three-dimensional views of the corresponding anatomy.
The manufacturing process required for the creation of any 3D-printed patient-specific organ model, shown in Fig. 55.1, consists of four main steps: (1) understanding the clinical need, (2) image acquisition and processing, (3) 3D printing, (4) postprocessing and validation.
Process steps involved from image acquisition to the manufacturing of a patient-specific 3D-printed model of the spine (With permission from [28])
2.1 Understanding the Need
The first step in creating a new model using 3D printing consists of defining the objective [11]. Is the target to teach anatomy, preprocedural planning, or technical skills? Which is the anatomical region of interest, and how much of the organ needs to be included? Which degree of anatomical detail should be rendered? Are the structures surrounding the specific area of interest necessary for the replication? Moreover, the physical characteristics of the constitutive materials of the model will be essential if the possibility to cut, resect, and suture is required.
These considerations will have an impact on the type of imaging used to capture the area of interest, the accuracy and resolution required, the nature of materials employed, and the type of 3D printer to be used.
2.2 Image Acquisition and Processing
The medical imaging process typically produces a set of 2D pictures separated with a controlled thickness; thus, the 3D representation is achieved by stacking successive layers of 2D images into a 3D volume. This explains why the accuracy of the 3D geometry diminishes as the thickness between each slice increases, potentially losing important anatomical details [11]. Therefore, acquiring sufficiently high-resolution imaging data that are also free from artifacts is essential since it directly impacts the quality and accuracy of the printed model.
Several imaging techniques are used to obtain 2D images of the human body, but the most common and non-invasive ones remain CT and MRI [17, 29, 30]. The basic output of medical imaging is essentially a greyscale map, which is then used to discriminate between different anatomical structures.
After the data acquisition in DICOM (Digital Imaging and Communications in Medicine) format, a process known as segmentation is carried out [31]. Segmentation is the delineation of structures by identifying the outline of each anatomical area of interest to define its geometry. It is a threshold-based process performed using colour contrast between tissues of different densities to separate each structure and capture different anatomies. Each 2D slice within the 3D imaging dataset is analysed individually, and then multiple data from all slices are collected to create the surface of the 3D solid objects [11].
At this stage, the anatomical 3D solid data may be further processed using 3D CAD (Computer-Aided Design) software to optimise the surface topologies for 3D printing stampability according to specific manufacturing purposes and intended uses of the model.
A final verification step is essential to verify that the imaging data correctly identify the anatomical structures of interest. This is usually performed by clinicians and radiologists and consists of overlaying the finished manipulated surface file on top of the source imaging data [32].
Once the 3D solid model is finalised, data are finally exported to the Standard Triangle Language (STL) file format, the gold standard for data preparation in the 3D printing field.
2.3 3D Printing the Model
At this point, the STL file is uploaded to a dedicated 3D printing preparation software, commonly referred to as slicer, which slices the model into single 2D layers and generates the list of deposition paths and step-by-step instructions (G-code) to be loaded in the 3D printer [33].
The 3D printer eventually interprets the digitally supplied G-code and executes its instructions to deposit the desired material on the build platform, layer upon layer, until the physical copy of the 3D model is complete.
The central problem in the 3D printing field has been finding materials that are compatible with the printing procedure and can also offer adequate mechanical and functional properties for tissue-mimicking constructs. There is a huge variety of materials that can be used—such as polymers, ceramics, and metals—but the ideal ones should include the following characteristics: (1) printability: the need to facilitate handling and deposition by the printer; (2) mechanical properties: the material should match the mechanical properties of the organs; (3) biomimicry: the desired materials should be based on the knowledge of the tissues to mimic [17].
2.4 Postprocessing and Validation
After printing, models usually require postprocessing operations to smooth rough surfaces and remove any residual material. The degree of postprocessing needed often depends on the selected technology, the type and quality of the 3D printer, and the materials used [32].
Finally, validation is necessary to ensure the printed model’s quality. The most commonly employed methods are (1) the validation of the surgeons by checking the CT and MRI data obtained during the medical imaging step and comparing them with the real prototype or; (2) analysing the prototype into a CT scanner [17].
3 3D-Printed Organ Models for Surgical Applications
3.1 State of the Art
The improved spatial and contrast resolution of radiological images and the rapid advances in additive manufacturing technologies allow printing anatomical and pathological structures with accurate and complex patient-specific details (sub-millimetric resolutions). In combination with rapid transformation times from digital to 3D physical models, these advantages make 3D printing a valuable tool for preoperative planning of surgical procedures and training purposes [34].
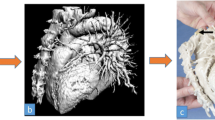
At present, the vast majority of 3D-ghprinted organ models are made of hard materials, such as plastics and tough, rubbery polymers [35, 36]. Although they are adequate to reproduce the anatomical structure of the native organs, they fail in capturing many physical and functional properties of soft living tissues, including tactile sensation, deformability, and physiological features (e.g. blood circulation). This is a severe limitation since these properties ultimately represent an essential add-on to extend the usability of training models and optimise simulation experiences [37]. Nevertheless, these organ models have demonstrated utility in a variety of medical fields, including cardiology, urology, neurology, and hepatology [35, 38].
With the improvement of 3D printing technologies, the palette of materials that can be used in these processes evolved to broaden the application cases. This includes the possibility of 3D printing elastomeric (rubber-like) and flexible materials, in addition to conventional rigid plastics [39]. Some examples of such materials include soft photopolymers or thermoplastic elastomers (TPE) [35]. The 3D-printed organ models fabricated from such materials provide tactile sensation closer to actual organs, compared to rigid-plastic materials, thereby allowing surgeons to perform different kinds of rehearsal operations, as shown in Fig. 55.2.
3D-printed organ models using rubber-like and flexible materials. (a) A 3D-printed cardiac model with a congenital defect for hands-on surgical training (With permission from [40]). (b) A patient-specific 3D-printed hollow hepatic vein model (in blue) affected by an intrahepatic lesion (in red) (With permission from [41]). (c) A 3D-printed “split” kidney model showing the relative position of the renal tumour with respect to the renal artery, vein, and collecting system (With permission from [42]). (d) A 3D-printed tracheobronchial tree model with a fiberoptic view through the bronchus intermedius (inset) (With permission from [43]). (e) Model of a cranium incorporating vascular structures for surgical simulation and planning and (f) the embedded aneurysm model for clipping rehearsal (With permission from [37])
3.2 Design and Fabrication Approaches
Two possible additive-based approaches can be considered to produce 3D organ models for surgical and training applications: the direct approach and the hybrid approach.
3.2.1 All-Printed Organ Models
All-printed 3D models rely on the direct 3D printing of complex monolithic structures using multiple materials during a single print job. This approach requires suitable 3D printing facilities and minimises postprocessing operations. On the other hand, it allows for minimal optimisation of manufacturing strategies and processes and implies poor material customisation, therefore, limiting the efficiency in accurately reproducing the high degree of variability of biological organs and tissues.
Implementating of these solutions in daily clinical practice is still limited due to the need for expensive printers, long processing time, and trained technicians not commonly available in hospitals. Moreover, the 3D-printed anatomical models produced with this approach do not adequately reproduce the deformability properties and haptic feedback of native human tissues, hindering the simulated experience of surgical manipulation [44, 45].
3.2.2 Hybrid-Assembling Organ Models
In the hybrid approach, the design and fabrication of anatomical models rely on the production and subsequent 3D assembly of multiple structures manufactured with standard additive printing in combination with other technologies (e.g. 3D moulding and casting). Hybridisation among different techniques makes it possible to fully exploit the advantages of each one in order to optimise each manufacturing strategy and to suitably customise materials properties in terms of softness, tactile and haptic response, echogenicity, radiological response, etc. [21]. This is a multi-process approach that requires high technological skills and excellent care in assembling the models while keeping the correct anatomical constraints and relative spatial features, but it certainly offers many more advantages on tissue-mimicking materials development than the direct approach [46, 47].
4 PRINTMED-3D: The Project
4.1 Integrated Platform for Three-Dimensional Medical Technologies: An Innovative Idea
PRINTMED-3D is a project funded by Regione Lombardia and supported by the European Regional Development Fund (POR FESR) 2014–2020. It brings together complementary skills from both an academic and an industrial perspective, finding their ideal synthesis in a partnership where the University of Milan is the leading institution, involving the Physics Department, the Interdisciplinary Center for Nanostructured Materials and Interfaces (CIMaINa), and the Faculty of Medicine and Surgery.
PRINTMED-3D aims to create a multidisciplinary infrastructure to develop enabling solutions for personalised medicine and specialist training through the combined use of virtual reality (VR) and functional additive manufacturing technologies (AM) [48].
The project is created to address the growing demand for personalised medical services in the clinical, diagnostic, and pre-clinical fields, to improve the quality of the assistance to patients and the economic sustainability of the healthcare system by adopting more efficient and targeted approaches.
The main idea consists of creating a virtual reality environments associated with the corresponding 3D physical models of the target organs or tissues to be used in combination to promote new surgical planning practices, obtain safer therapeutic results, optimise the training of surgery residents, and implement highly effective teaching methodologies.
4.2 A Revolutionary Approach
The development of innovative materials and the use of state-of-the-art printing technologies are essential to reproduce tissues and organs with realistic features and to create anatomical models that are morphologically, mechanically, and functionally similar to their natural counterparts in terms of shape, structure, density, haptic response, and radiological properties.
Thanks to the technological solutions developed by PRINTMED-3D, surgeons can identify efficient patient-specific treatments, optimise operating procedures in advance, and simulate complex surgical interventions in a risk-free environment, in an ethical and repeatable way.
The physical–virtual 3D reproductions of the brain and the liver are initially considered the case studies for the project; these organs have been selected because of their peculiar morpho-structural and functional aspects, but at present also, other anatomies are being considered as well.
Although every biological organ is made up of many tissues and characterised by the complexity of its structures, when it comes to reproducing them using additive and 3D moulding technologies, not all of them need to be faithfully replicated to build a realistic model. Therefore, it is necessary to operate a simplification by identifying structures and materials that summarise the extreme variability encountered in organs and tissues to give particular attention in the digital source model only to the fundamental constitutive structures.
According to the main structures that characterise these target organs, we define three major categories into which they can be divided:
-
parenchymal and bulky tissue structures (e.g. brain and liver parenchyma, tumour lesions)
-
membranes (e.g. cerebral meninges, peritoneum)
-
vascular structures (e.g. arteries, veins, and bile ducts).
The selection of these structures is supported by key-opinion leaders in the reference fields of neurosurgery and hepatobiliary surgery, partners of the PRINTMED-3D project.
4.3 General Manufacturing Method
The manufacturing process of all the 3D organ models presented in this book chapter follows the production workflow idea presented earlier in Sect. 55.2 as a general approach.
Routine CT and MRI images are retrieved from institutional PACS (Picture Archiving and Communication System), anonymised, and loaded into open-source slicing software. Rigid image coregistration and volumetric segmentation of anatomical structures are properly performed with pre-built functions in the software and, when necessary, manually corrected under the supervision of experienced radiologists. Finally, the 3D models are exported to STL files and further processed for 3D printing preparation. The whole process takes about 6–24 h to be completed, substantially depending on the complexity of the target anatomy and availability of automated segmentation tools.
In particular, digital models are processed, keeping a significant detail of the internal anatomical structures to build functional physical correspondents both for training and simulation purposes and for preoperative surgical planning, as shown in Fig. 55.3.
To produce the 3D organ models presented in this study, we adopt the hybrid fabrication approach based on additive manufacturing and casting tissue-mimicking materials into three-dimensional moulds. The core of this methodology is based on the production and subsequent 3D assembly of each structure of interest in the designed models, individually fabricated by choosing the appropriate technique and by developing equivalent synthetic materials to achieve the best possible result in line with the requirements of the model.
The production of models with tissue characteristics similar to human biological ones is still a technological challenge in the 3D printing domain. The advantage of using a hybrid approach for the fabrication of the models is the extremely high degree of customisation of the materials: their properties are finely tuned in terms of tactile feedback (softness, surface finish, compliance), radiological response to diagnostic imaging techniques, and mechanical response to stimuli of interest in surgery, in order to obtain 3D models with anatomical and functional tissue fidelity that allow trainees to learn and develop their clinical and surgical skills and allow surgeons to plan in advance the most appropriate surgical practices.
The customisation of casting materials offers the greatest flexibility in this respect: contrary to printing materials, which are very often limited to proprietary choices, in 3D moulding, we can employ different materials in different combinations of ingredients by varying their concentrations to develop suitable tissue-mimicking soft composites. However, some requirements have to be met in order to design adequate materials that make it possible to develop and manufacture handleable phantom models: (1) the raw materials must have sufficient chemical stability to allow the 3D printing process and manipulation upon casting, and (2) although they have a very low stiffness and soft consistency, they need to be structurally stable so that they maintain their shape and physico-chemical properties at room temperature, avoiding any degree of degradation or swelling. Our choice in terms of casting materials consists of in-house developed polymer-based composites with different physical and chemical properties. We chose to employ silicone-based materials mainly for two reasons:
-
1.
in contrast to hydrogels, agarose gel-based or organic materials often employed in the literature for the production of morpho-functional phantoms [49] that present critical storage and high maintenance conditions, silicone products easily meet the requirements we outlined regarding chemical–physical and structural stability;
-
2.
the Interdisciplinary Center for Nanostructured Materials and Interfaces (CIMaINa) from the University of Milan, partner of the PRINTMED-3D project, has developed numerous studies on functional polymeric materials and nanocomposites over the years, acquiring in-depth knowledge and expertise in the field [50].
Two different uses of 3D printing technologies can be distinguished in our approach: (1) direct production of the parts required for the models, as in the case of hepatic vascular structures; and (2) production of moulds in which we subsequently cast silicone materials to create the parts, as in the case of parenchymal and bulky tissue structures and membranes.
Typically, we design moulds from each 3D component of the digital model and manufacture them, choosing between three options: (1) UV-curable rigid resins (80–90 shoreD); (2) rigid thermoplastics (90–100 shoreD); and (3) soft and fast curing silicone rubbers (25 ShoreA) when the use of softer moulds are required. The choice between these materials is mainly determined by the combination of the moulds’ design and the casting material properties to facilitate the demoulding process of the objects and minimise possible polymerisation inhibition events at the boundary between the silicone materials and the mould surfaces. In addition, the durability of the mould material, its compatibility with postprocessing requirements, and any specific demands for transparency and smoothness in the surface finish of the synthetic artifacts are also considered in the selection.
4.4 Quantitative and Functional Characterisation
The systematic characterisation of materials is of fundamental importance in conceiving and growing a materials library, containing key information on the physical properties of materials and enabling the design, development, and fabrication of novel soft polymeric composites with even more functionally tailored physico-chemical properties. Hence, we are interested in characterising our tissue-mimicking materials both quantitatively and functionally. In particular, we focus our analysis on two fundamental properties:
-
1.
mechanical behaviour under compressive loadings, to assess their compressive stiffness at a rate comparable to surgery conditions [51,52,53];
-
2.
radiological response to CT scans, to evaluate their radiodensity in terms of relative electron density (rED) using CT acquisition protocols similar to those applied in clinical routine [54].
We obtain the values of compressive Young’s modulus and relative electronic density of the materials simulating the brain (E = 2.258 kPa, rED = 1.044) and the liver (E = 21.880 kPa, rED = 1.039) parenchyma that result comparable with those reported in the literature for the corresponding biological soft tissues and testing conditions, assessing these materials as excellent biosimilar candidates in terms of stiffness, compliance, and radiological response to diagnostic imaging techniques [52, 55,56,57,58].
In addition, key-opinion leaders in neurosurgery and hepatobiliary surgery performed further functional validation of the materials and assembled 3D organ models. The evaluation method is based on the experience and expertise of surgeons partners of the PRINTMED-3D project, who empirically assess the functional response of the materials under surgically relevant mechanical stimuli and typical surgical manipulations, procedures, and operations (e.g. laceration in cuts and incisions made with surgical scalpels, elastic springback, compliance, tear strength upon application of surgical stitches) with the aid of custom-developed questionnaires.
The surveys aim to provide a quantitative assessment of the different features of the phantoms, focusing on two main aspects: (1) general evaluation of the realism of the models and their educational potential and (2) specific evaluation of the properties of the developed materials, to serve as a driver in the subsequent materials design and optimisation. A specific evaluation is requested by providing four possible scores: unsatisfactory (score 1), satisfactory (score 2), very good (score 3), and excellent (score 4).
The following sections outline the specific designs and procedures developed to manufacture our 3D physical phantoms and the resulting manufacts.
5 Models for Specialist Training: Neurosurgery
Although the introduction of advanced technological tools in neurosurgery has been an important step to improve safety and achieve optimal clinical outcomes, for brain tumour surgery, total tumour resection while preserving functional integrity is considered a top priority, as it is associated with the longest progression-free and overall patient survival. To achieve these goals, specific case-lectures using 3D-printed disease models have become of great importance as they can be an effective tool for trainees to shorten the learning curve, quickly achieving a high level of experience through repeated practice in a safe environment.
This study aims to develop brain-simulating materials with mechanical properties comparable to those of biological brain tissue and tumours, to develop brain phantoms that can help neurosurgery residents to simulate resection procedures in their medical training programs. In particular, we focus on meningiomas, cerebral tumours originating from the dura mater and typically growing by compressing the brain and adjacent nerve tissues inward [59], as shown in Fig. 55.4.
Axial gadolinium-enhanced MRI showing three different cases of meningiomas with (a) dural tail, (b) brain infiltration, and (c) invasion of the bone (With permission from [60])
The ultimate goal of the project is to develop several simulators that recreate different cases in terms of meningiomas consistency and degree of complexity (e.g. infiltration, bleeding, and recurrence); our first prototype aims to simulate the resection of a WHO (World Health Organisation) grade I meningioma without any parenchyma, bone or vessels infiltration, and with a very dense consistency.
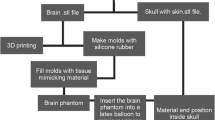
5.1 Mould Design and Manufacturing Approach
Our physical brain simulator combines 3D printing technologies to create the skull and moulds and 3D casting of tissue-mimicking silicone-based materials actually to manufacture the cerebral parenchyma, meningioma, and dura mater. In this first prototype, we assume the simplification of not including the arachnoid mater currently under investigation.
The manufacturing protocol is based on the 3D assembly of parenchyma and lesion models in a dedicated mould reproducing the skull morphology to which the dura mater is fixed, as shown in Fig. 55.5. While assembling the system, it is essential to keep the correct anatomical configuration and relative spatial features (i.e. mutual distances and positions between parenchymal and neoplastic structures and the meningeal membrane), to allow trainees to experience the same conditions they will find in actual clinical scenarios.
We manufactured the moulds for the cerebral parenchyma and meningioma using an 80–90 ShoreD and 65 MPa ultimate tensile strength hard photopolymer [61] with 0.1 mm layer resolution to create accurate and robust smooth surface finished parts.
Following initial tests, the rigid 3D-printed mould of the parenchyma was eventually replicated for process optimisation, producing a mask made of a soft, high viscosity, and fast-curing silicone (25 ShoreA), shown in Fig. 55.6. This choice improved both the ease of demoulding and the polymerisation quality at the surface boundaries.
We used two different silicone blends formulated in-house to manufacture these specific models as casting materials.
We moulded the meningioma using a poorly cross-linked tacky dielectric gel (10 Shore000 hardness), mixed with a 20% v/v 30 ShoreA platinum catalysed hardener with brittle properties, and coloured with white pigments. Additional liquid additives were employed to reduce surface tension (1.5% w/w) and lower the blend’s viscosity (10% v/v). We chose this formulation because we identified it as the most accurate to reproduce the dense texture of the meningioma, as shown in Fig. 55.7.
For the cerebral parenchyma, we chose a composite material made from 60% silicon oil and 40% silicone blend created using a poorly cross-linked tacky dielectric gel (10 Shore000 hardness), mixed with a 7.5% v/v room-temperature vulcanising (RTV) silicone rubber hardener (20 ShoreA) with brittle properties, and coloured with a mixture of white and light flesh-tone pigments. Additional liquid additives were employed both to reduce surface tension (1.5% w/w) and to lower the viscosity of the blend (10% v/v). We chose this formulation because it was suitable to reproduce the soft consistency of biological cerebral parenchyma, allowing realistic manipulation during meningioma resection simulations.
The curing of the silicone blends took approximately 48 hours at room temperature after which demoulding took place to obtain the models.
The manufacturing approach to produce the dura mater only involved 3D moulding techniques. To obtain a thin membrane, we used two slabs of glass separated by a frame made from a silicone sheet to set the thickness of the layer. Since the known thickness range for human dura mater is 0.4–1.4 mm [62, 63], we decided to use a thickness of about 0.8 mm to be compatible with the corresponding biological tissue.
After pouring the casting material, which is platinum catalysed 18 ShoreA silicone rubber compound coloured with white pigments, the mould was closed with clips to allow the mixture to expand correctly over the entire glass, thus obtaining a membrane of uniform height, as shown in Fig. 55.8. The curing time was approximately 24 h at room temperature, after which demoulding and assembling took place to obtain the model.
A first prototype of the skull case model was manufactured employing an 80–90 ShoreD and 65 MPa ultimate tensile strength hard photopolymer [61].
5.2 Meningioma Resection Simulator
We obtained a compact and optimised 3D brain simulator with high-quality overall haptic and functional perception, which preserves the spatial configuration of the anatomy and morphology when compared to the original MRI acquisitions. Figure 55.9 presents images of the final 3D physical phantom.
The evaluation method was developed as part of our ongoing clinical trial to assess the educational potential of the 3D brain phantom in different simulation settings. The model was validated by Professor A. Perin, the key-opinion leader in the field of neurosurgery and scientific coordinator of the BestaNeuroSim Center (Department of Neurosurgery, IRCCS ‘Carlo Besta’ Neurological Institute Foundation).
The 3D anatomical details and the relative size and spatial relationships of the structures constituting the haptic phantom were rated as effective both in providing accessible manipulation of surgical tools during training simulations and in mimicking realistic meningioma resection procedures. Furthermore, the educational potential of this model was positively evaluated, considering its usefulness in acquiring basic surgical and manipulation skills, orienting trainees during resection procedures, and improving their confidence and clinical competence, as also reported in the literature [56, 64]. This general evaluation provided an overall score of 3.0 (very good quality).
In addition, the brain model was rated to realistically simulate the overall haptic feedback of the native organs and tissues (i.e. dura mater, meningioma, and brain parenchyma) with different degrees of fidelity, considering stiffness, compliance, and response to typical surgical tools and procedures (e.g. surgical scalpels and tweezers, digitoclasia, application of surgical stitches) as evaluation parameters, as shown in Fig. 55.10. Due to the different physical properties of the tissue-mimetic silicone blends employed for each structure, textures, densities, and functional responses to surgery-relevant stimuli were extensively diversified, even in terms of optimisation of production processes. The results of this specific evaluation provided a score of 2.0 (satisfactory quality) for the dura mater, a score of 3.0 (very good quality) for the meningioma, and an intermediate score ranging from 2.0 (satisfactory quality) to 3.0 (very good quality) for the brain parenchyma.
6 Models for Surgical Planning: Liver Surgery
The understanding of liver anatomy has evolved greatly in recent years [65, 66]. A greater comprehension of vascular anatomy, along with advances in technologies for intra-operative mapping and parenchymal dissection, has made hepatobiliary surgery safer and more effective. However, the complexities and nuances of liver anatomy require continuous practice and rehearsal of surgical procedures. To this aim, 3D-printed models allow pre-planning of complex procedures and identifying of potential intra-operative complications and complex underlying anomalies in advance, ensuring maximum patient safety during operations and optimising surgical outcomes.
This study aims to develop liver-simulating materials with mechanical properties comparable to those of biological tissues to develop phantoms that can help hepatobiliary surgeons identify anatomical variabilities, and plan liver transplantation procedures during preliminary sessions and surgery.
Living donor liver transplantation (LDLT) has steadily increased in recent years due to the growing demand for liver transplantation and the concomitant shortage and inadequacy of cadaveric grafts [44]. Living donors are healthy individuals, so ensuring their safety and that of the recipients is of utmost importance. Therefore, preoperative identification of vascular and biliary structures and their relationship to the surrounding parenchyma through a careful study of a 3D printing model may allow better surgical planning, avoid unnecessary surgery in patients with a complex anatomy potentially unsuitable for a safe liver resection, minimizing the risk of life-threatening living donor complications (e.g. extensive bleeding and large areas of hepatic ischaemia or infarction) [67].
Our attempt to produce a highly accurate 3D-printed model of a patient’s liver aims to overcome these obstacles and improve the safety of LDLT. The aim of this study is to establish anatomical precision, volumetric accuracy, and realistic tactile response in the 3D-printed model of the donor undergoing LDLT.
6.1 Mould Design and Manufacturing Approach
The physical model of the liver is created using 3D printing technologies to produce the vasculo-biliary branching and 3D moulding to reproduce the parenchyma, using the same approach reported in our previous work [21]. We exploit an in-house developed manufacturing protocol based on the 3D assembly of vasculo-biliary branch models inside a dedicated mould that reproduces the parenchymal morphology, as shown in Fig. 55.11. After assembling the vascular system while keeping the correct anatomical configuration and relative spatial features (i.e. distances between vascular branches, mutual position between vascular and biliary trees), a tissue-equivalent silicone-based blend is poured into a custom-designed mould, giving shape to the parenchymal structure and embedding the intrahepatic arterial–venous branch and the biliary duct.
We manufactured the portal and hepatic veins, and the hepatic arteries as hollow vessels to reproduce the haptic response of biological vessel walls, while for the biliary tree, characterised by small-diameter branches, we preferred a solid structure allowing the model to be self-standing and more robust, as shown in Fig. 55.12. They were directly printed, choosing a commercial 80 ShoreA and 8.9 MPa ultimate tensile strength low-hardness photopolymer [61] with 0.1 mm layer resolution to create accurate and robust smooth surface finished parts.
We also printed a second replica of the vasculature branching, in this case without the surrounding parenchyma, using the same UV-curable resin and equipped with detachable connecting pins to allow surgeons to handle the phantom, even with the possibility of separating and subsequently reassembling the components for improved visualisation purposes.
We chose ABS thermoplastic to produce the 3D mould required to assemble the structures and cast the parenchyma, employing a 0.25 mm layer resolution to create robust parts, as shown in Fig. 55.13.
We used an in-house formulated silicone blend as casting material to mould the haptic liver parenchyma. This consisted of a poorly cross-linked tacky dielectric gel (10 Shore000 hardness), mixed with a 10% v/v platinum catalysed 30 ShoreA hardener with brittle properties. We chose this formulation because we identified it as the most suitable in our library to reproduce the haptic response of the liver parenchyma of an adult individual, allowing realistic manipulation of the model during LDLT pre-planning sessions.
The curing time of the silicone blend was approximately 48 h at room temperature, after which demoulding took place to unfold the liver model.
6.2 Liver Transplantation Phantom
We obtained an optimised transparent 3D liver phantom with high-quality haptic perception and a vasculo-biliary branching model with deeply detailed intra-parenchymal structures. Figure 55.14 presents images of the final 3D physical phantoms. Each part of the vascular anatomy (venous outflow, portal venous system, arterial branching, and intrahepatic biliary tree) was independently positioned, preserving the spatial configuration of the anatomy and morphology in fully assembled models. Internal vasculo-biliary structures can be clearly identified by different colour codes, providing a unique understanding of their complex relationship with the surrounding parenchyma.
The evaluation method was developed as part of our ongoing clinical trial to assess the potential benefit of the 3D liver model in different clinical settings. The models were validated by P. Aseni, the key-opinion leader in the field of liver surgery and transplantation (Emergency Department, ASST Grande OspedaleMetropolitano Niguarda, Milan).
The 3D anatomical details of the haptic phantoms were rated as effective in both providing a more comprehensive and clear visualisation of the spatial relationships between internal structures when compared to the standard imaging techniques and the 3D digital model, as shown in Fig. 55.15, and in highlighting underlying patient-specific anomalies, allowing surgeons to optimise preoperative assessments and identify the safest surgical strategy in advance, as also reported in the literature [44, 67]. The result of this general evaluation provided an overall score ranging from 3.0 (very good quality) to 4.0 (excellent quality).
In addition, the liver model was rated to realistically simulate with high-fidelity overall haptic feedback of the native organ in terms of stiffness, consistency, and compliance due to the combined use of hollow vascular structures produced with soft photopolymers and the developed transparent tissue-mimetic silicone blend representing the parenchyma, engineered with optimised texture, density, and functional response under surgery-relevant mechanical stimuli. The result of this specific evaluation provided an overall score ranging from 2.0 (satisfactory quality) to 3.0 (very good quality).
Its huge potential in preoperative surgical planning had also been demonstrated by the great resonance obtained following a living donor liver transplantation that was successfully planned and performed at ASST Grande OspedaleMetropolitano Niguarda (Milan, June 2021) with the aid of our 3D liver and vascular-branching models [68, 69].
7 Conclusion
In this study, we developed and characterised novel soft materials that resemble biological tissue properties and optimised manufacturing approaches to reproduce tissues and organs with realistic features for the fabrication of innovative morpho-functional 3D models in view of applications in surgical planning and specialist training, as part of the PRINTMED-3D project.
To manufacture the phantoms, we adopted a hybrid manufacturing approach. The design and fabrication of anatomical models rely on multi-process production and subsequent 3D assembly of multiple structures manufactured by state-of-the-art additive printing in combination with other technologies (e.g. 3D moulding and casting).
We selected 3D additive technologies based on liquid resins, suitable for processing soft and flexible printing materials (50 to 80 ShoreA hardness) and identified and developed novel customised silicone-based polymeric blends and composites for 3D moulding. Hybridisation among different techniques ultimately allowed a synergistic combination of both processes to fully exploit the advantages of each one in order to optimise each manufacturing strategy and to suitably design materials properties to create custom biomimetic soft 3D models with both realistic anatomical and functional tissue fidelity.
The results are particularly promising for further development and refinement of the materials and 3D physical models presented and constitute an important innovation in the realisation of morpho-functional 3D systems for an increasingly personalised approach to medicine and surgical training.
Further systematic materials characterisations are of fundamental importance in developing our preliminary materials library to enable the design, development, and fabrication of novel soft polymeric composites with even more functionally tailored physicochemical properties. This aim will be pursued in future project developments by extensively characterising our collection of materials by considering additional physical and physiological properties, such as magnetic response to NMR scans, mechano-acoustic behaviour in terms of speed of sound, acoustic impedance and attenuation, fluidic compliance in response to physiological flows (e.g. blood circulation), and mechanical properties under tensile or shear loads.
References
Lemire M. Representation of the human body: the colored wax anatomic models of the 18th and 19th centuries in the revival of medical instruction. Surg Radiol Anat. 1992;14:283–91.
Owen H. Early use of simulation in medical education. Simul Healthc. 2012;7:102–16.
Badash I, Burtt K, Solorzano CA, Carey JN. Innovation in surgery simulation: a review of past, current and future techniques. Ann Transl Med. 2016;4:453.
Witowski J, Budzyński A, Grochowska A, et al. Decision-making based on 3D printed models in laparoscopic liver resections with intraoperative ultrasound: a prospective observational study. Eur Radiol. 2020;30:1306–12.
Youssef A, Hollister SJ, Dalton PD. Additive manufacturing of polymer melts for implantable medical devices and scaffolds. Biofabrication. 2017;9:012002.
Cogswell PM, Rischall MA, Alexander AE, et al. Intracranial vasculature 3D printing: review of techniques and manufacturing processes to inform clinical practice. 3D Print Med. 2020;6:18.
Grillo FW, Souza VH, Matsuda RH, et al. Patient-specific neurosurgical phantom: assessment of visual quality, accuracy, and scaling effects. 3D Print Med. 2018;4:3.
Wake N, Rosenkrantz AB, Huang R, et al. Patient-specific 3D printed and augmented reality kidney and prostate cancer models: impact on patient education. 3D Print Med. 2019;5:4.
Alexander AE, Wake N, Chepelev L, et al. A guideline for 3D printing terminology in biomedical research utilizing ISO/ASTM standards. 3D Print Med. 2021;7:8.
ASTM International. ISO/ASTM52900 15 standard terminology for additive manifacturing—general principles—terminology. West Conshohocken; 2015.
Garcia J, Yang Z, Mongrain R, et al. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4:27–40.
Gibson I, Rosen D, Stucker B. Additive manufacturing technologies. Springer; 2015.
Mele M, Campana G. Tecnologie Additive. Introduzione ai processi e alle strategie produttive. Esculapio; 2021.
Ligon SC, Liska R, Stampfl J, et al. Polymers for 3D printing and customized additive manufacturing. Chem Rev. 2017;117:10212–90.
Diment LE, Thompson MS, Bergmann JHM. Clinical efficacy and effectiveness of 3D printing: a systematic review. BMJ Open. 2017;7:e016891.
Martelli N, Serrano C, van den Brink H, et al. Advantages and disadvantages of 3-dimensional printing in surgery: a systematic review. Surgery. 2016;159:1485.
Tejo-Otero A, Buj-Corral I, Fenollosa-Artés F. 3D printing in medicine for preoperative surgical planning: a review. Ann Biomed Eng. 2020;48:536–55.
Błaszczyk M, Jabbar R, Szmyd B, et al. 3D printing of rapid, low-cost and patient-specific models of brain vasculature for use in preoperative planning in clipping of intracranial aneurysms. J Clin Med. 2021;10:1201.
Dho YS, Lee D, Ha T, et al. Clinical application of patient-specific 3D printing brain tumor model production system for neurosurgery. Sci Rep. 2021;11:7005.
Adams F, Qiu T, Mark A, et al. Soft 3D-printed phantom of the human kidney with collecting system. Ann Biomed Eng. 2017;45:963–72.
Aseni P, Santaniello T, Rizzetto F, et al. Hybrid additive fabrication of a transparent liver with biosimilar haptic response for pre-operative planning. Diagnostics. 2021;11:1734. https://doi.org/10.3390/diagnostics11091734.
Blanco AM, Krauel L, FenollosaArtés F. Development of a patients-specific 3D-printed preoperative planning and training tool, with functionalized internal surfaces, for complex oncologic cases. Rapid Prototyp J. 2019;25:363–77.
Tejo-Otero A, Lustig-Gainza P, Fenollosa-Artés F, et al. 3D printed soft surgical planning prototype for a biliary tract rhabdomyosarcoma. J Mech Behav Biomed Mater. 2020;109:103844.
Bohl MA, McBryan S, Spear C, et al. Evaluation of a novel surgical skills training course: are cadavers still the gold standard for surgical skills training? World Neurosurg. 2019;127:63–71.
Liu Y, Gao Q, Du S, et al. Fabrication of cerebral aneurysm simulator with a desktop 3D printer. Sci Rep. 2017;7:44301.
Nagassa RG, McMenamin PG, Adams JW, Quayle MR, Rosenfeld JV. Advanced 3D printed model of middle cerebral artery aneurysms for neurosurgery simulation. 3D Print Med. 2019;5:11.
Vitagliano G, Mey L, Rico L, et al. Construction of a 3D surgical model for minimally invasive partial nephrectomy: the urotrainerVK-1. Curr Urol Rep. 2021;22:48.
Provaggi E, Leong JJH, Kalaskar DM. Applications of 3D printing in the management of severe spinal conditions. Proc Inst Mech Eng H. 2017;231:471–86.
Kondo K, Harada N, Masuda H, et al. A neurosurgical simulation of skull base tumors using a 3D printed rapid prototyping model containing mesh structures. Acta Neurochir. 2016;158:1213–9.
Waran V, Narayanan V, Karuppiah R, et al. Utility of multimaterial 3D printers in creating models with pathological entities to enhance the training experience of neurosurgeons. J Neurosurg. 2014;120:489–92.
Kamio T, Suzuki M, et al. DICOM segmentation and STL creation for 3D printing: a process and software package comparison for osseous anatomy. 3D Print Med. 2020;6:17.
Bastawrous S, Wu L, Strzelecki B, et al. Establishing quality and safety in hospital-based 3D printing programs: patient-first approach. Radiographics. 2021;41:1208–29.
Gross BC, Erkal JL, Lockwood SY, et al. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. 2014;86:3240–53.
Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: review of medical applications. Int J CARS. 2010;5:335–41.
Qiu K, Haghiashtiani G, McAlpine MC. 3D printed organ models for surgical applications. Annu Rev Anal Chem. 2018;11:287–306.
Young RJ, Lovell PA. Introduction to polymers. Boca Raton: CRC Press; 2011.
Patel A, et al. Three-dimensional printing technology in Surgery. Surg Curr Res. 2015;6(1):1000255. https://doi.org/10.4172/2161-1076.1000255.
Jin Z, Li Y, Yu K, et al. 3D printing of physical organ models: recent developments and challenges. Adv Sci. 2021;8:2101394.
Loadman MJ. Analysis of rubber and rubber-like polymers. Springer; 2012.
Yoo SJ, Spray T, Austin EH III, et al. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J Thorac Cardiovasc Surg. 2017;153:1530–40.
Kiraly L, Tofeig M, Jha NK, et al. Three-dimensional printed prototypes refine the anatomy of post-modified Norwood-1 complex aortic arch obstruction and allow presurgical simulation of the repair. Interact CardiovascThorac Surg. 2016;22:238–40.
Wake N, Chandarana H, Huang WC, et al. Application of anatomically accurate, patient-specific 3D printed models from MRI data in urological oncology. Clin Radiol. 2016;71:610–4.
Bustamante S, Bose S, Bishop P, et al. Novel application of rapid prototyping for simulation of bronchoscopic anatomy. J Cardiothorac Vasc Anesth. 2014;28:1122–5.
Nalbant B, Egeli T, Ünek T, et al. Utilizing 3 dimensional print of the liver in living donor liver transplantation for preoperative evaluation. J Basic Clin Health Sci. 2020;1:85–7.
Witowski JS, Pedziwiatr M, Major P, et al. Cost-effective, personalized, 3D-printed liver model for preoperative planning before laparoscopic liver hemihepatectomy for colorectal cancer metastases. Int J CARS. 2017;12:2047–54.
Igami T, Nakamura Y, Hirose T, et al. Application of a three-dimensional print of a liver in hepatectomy for small tumors invisible by intraoperative ultrasonography: preliminary experience. World J Surg. 2014;38:3163–6.
Perica E, Sun Z. Patient-specific three-dimensional printing for pre- surgical planning in hepatocellular carcinoma treatment. Quant Imaging Med Surg. 2017;7:668–77.
PRINTMED-3D. Integrated platform for three-dimensional medical technologies. 2021. https://printmed-3d.com/. cited 18/01/2022.
Culjat MO, Goldenberg D, Tewari P, et al. A review of tissue substitutes for ultrasound imaging. Ultrasound Med Biol. 2010;36:861–73.
CIMaINa. The interdisciplinary center for nanostructured materials and interfaces. http://cimaina.unimi.it/. cited 18/01/2022.
Karimi A, Shojaei A. An experimental study to measure the mechanical properties of the human liver. Dig Dis. 2018;36(2):150–5.
Lozoya MN, Kennedy MS, Dean D, et al. Development of phantom material that resembles compression properties of human brain tissue for training models. Materialia (Oxf). 2019;8:10.1016/j.mtla.2019.100438.
Miller K, Chinzei K. Constitutive modelling of brain tissue: experiment and theory. J Biomechanics. 1997;30:1115–21.
Legnani E, Gallo P, Pezzotta F, et al. Additive fabrication of a vascular 3D phantom for stereotactic radiosurgery of arteriovenous malformations. 3D Print Addit Manuf. 2021;8:217–26.
Lozoya MN. Development of a tissue-mimicking brain phantom for neurosurgical pre-operative planning and training. All theses; 2016.
Ploch CC, Mansi CSSA, Jayamohan J, Kuhl E. Using 3D printing to create personalized brain models for neurosurgical training and preoperative planning. World Neurosurg. 2016;90:668–74.
Mattei G, Ahluwalia A. Sample, testing and analysis variables affecting liver mechanical properties: a review. Acta Biomater. 2016;45:60–71.
Umale S, Deck C, Bourdet N, et al. Experimental mechanical characterization of abdominal organs: liver, kidney spleen. J Mech Behav Biomed Mater. 2013;17:22–33.
Andersen MS, Pedersen CB, Mathiesen T, et al. Meningioma. UgeskrLaeger. 2019:181.
Fathi AR, Roelcke U. Meningioma. Curr Neurol Neurosci Rep. 2013;13:337.
Formlabs Inc. Materials data sheet, 2019. https://archive-media.formlabs.com/DataSheet.pdf. cited 18/01/2022.
Singh V. Textbook of clinical neuroanatomy. Elsevier India; 2009.
Zwirner J, Scholze M, Waddell JN, et al. Mechanical properties of human dura mater in tension—an analysis at an age range of 2 to 94 years. Sci Rep. 2019;9:16655.
Vakharia VN, Vakharia NN, Hill CS. Review of 3-dimensional printing on cranial neurosurgery simulation training. World Neurosurg. 2016;88:188–98.
Abdel-Misih SRZ, Bloomston M. Liver anatomy. Surg Clin North Am. 2010;90:643–53.
Majno P, Mentha G, Toso C, et al. Anatomy of the liver: an outline with three levels of complexity—a further step towards tailored territorial liver resections. J Hepatol. 2014;60:654–62.
Zein NN, Hanouneh IA, Bishop PD, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19:1304–10.
La Repubblica Milano. Milano, figlio dona al padre metà del suo fegato e il trapianto si prepara con la stampa in 3D dell’organo—L’avveniristico trapianto da vivente avvenuto all’ospedale Niguarda, 29 giugno 2021. https://milano.repubblica.it/cronaca/2021/06/29/news/. cited 09/11/2021.
Corriere della Sera. Ospedale Niguarda, il trapianto preparato con un fegato stampato in 3D: così il figlio ha salvato il padre—Un’operazione unica nel suo genere. Il modello tridimensionale realizzato con un gel biosimilare che mima la consistenza dei tessuti biologici. Bettoni S. 29 giugno 2021. https://milano.corriere.it/notizie/cronaca/21_giugno_29/. cited 18/01/2022.
Acknowledgement
This work was supported by RegioneLombardia under the Program “Call Hub Ricerca e Innovazione” (POR-FESR 2014–2020, project ID 1170989—PRINTMED-3D). Prof. Alessandro Perin from the “Fondazione I.R.C.C.S. IstitutoNeurologico Carlo Besta” (Milan, Italy) and Prof. Paolo Aseni from the “Department of Emergency, ASST Grande OspedaleMetropolitano Niguarda” (Milan, Italy) are greatly acknowledged for their support and contribution to the organ models design and clinical validation. Prof. Maurizio Vertemati from the “Department of Biomedical and Clinical Sciences “L. Sacco”—University of Milan” (Milan, Italy) and Dr. Francesco Rizzetto from the “Department of Radiology, ASST Grande OspedaleMetropolitano Niguarda” (Milan, Italy) are thankfully acknowledged for their work on 3D reconstructions of medical images and organ models design. Dr. Lorenzo Gentili (CIMAINA) is also greatly acknowledged for his contribution to additive fabrication and 3D systems modelling.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mazzoleni, G. et al. (2023). 3D Printing Technology in Medicine: A Personalised Approach Towards a Safer Surgical Practice. In: Aseni, P., Grande, A.M., Leppäniemi, A., Chiara, O. (eds) The High-risk Surgical Patient. Springer, Cham. https://doi.org/10.1007/978-3-031-17273-1_55
Download citation
DOI: https://doi.org/10.1007/978-3-031-17273-1_55
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17272-4
Online ISBN: 978-3-031-17273-1
eBook Packages: MedicineMedicine (R0)