Abstract
Plant pathogenic fungi use the well-conserved MAP kinase (MAPK) pathways to mediate responses to external stimuli and regulate various infection and developmental processes. Most ascomycetous fungal pathogens have three MAPK cascades. In general, the Pmk1/Kss1 invasive growth (IG) pathway is essential for pathogenesis by regulating infection-related morphogenesis, such as formation of appressoria or hyphopodia, penetration, and invasive growth in infected plant tissues. The cell wall integrity (CWI) MAPK pathway is also normally important for plant infection by regulating species-specific infection processes, cell wall integrity, infectious growth, and responses to cell wall stress. Unlike the IG and CWI pathways, the HOG (high osmolarity glycerol) pathway is dispensable for virulence in some fungal pathogens such as Magnaporthe oryzae but plays a critical role in pathogenesis in many others. Besides its conserved role in osmoregulation, the HOG pathway is usually important for responses to oxidative and other environmental stresses. Overall, both conserved and species-specific functions have been identified for individual MAP kinase cascades in plant pathogenic fungi, likely due to variations in upstream signaling and downstream transcriptional regulation. Limited studies in a few fungal pathogens have also shown that there is crosstalk among three MAPK pathways to regulate various infection processes and responses to biotic and abiotic stresses, indicating the complex regulatory networks associated with these MAP kinase pathways.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In fungi and other eukaryotic organisms, mitogen-activated protein (MAP) kinase pathways play critical roles in regulating responses to various extracellular cues. A typical MAP kinase cascade consists of a MAP kinase (MAPK), a MAPK kinase (MEK), and a MEK kinase (MEKK). The sequential activation of these protein kinases results in the dual phosphorylation of MAPKs at the T-X-Y activation motif, which then phosphorylates downstream targets to regulate transcriptional changes and cellular responses. The model organism Saccharomyces cerevisiae has five MAPK genes due to its whole genome duplication event. Fus3 and Kss1 are two paralogous MAPKs that have overlapping functions in pheromone response but only Kss1 is involved in regulating filamentation and invasive growth into agar (Schwartz and Madhani 2004; Chen et al. 2012). From Ste2 and Ste3 pheromone receptors to transcription factors such as Ste12 and Dig1, the pheromone response pathway is the best characterized MAPK pathway in eukaryotic organisms. Slt2 and Hog1 MAPKs mainly regulate cell wall integrity and osmoregulation, respectively, although they are also involved in responses to other stresses. Smk1 is a meiosis-specific MAPK regulating ascospore wall assembly. Unlike other yeast MAPKs, Smk1 lacks upstream MEK or MEKK and it is activated by autophosphorylation and phosphorylation by Cak1 (Schwartz and Madhani 2004; Chen et al. 2012).
Whereas orthologs of Fus3/Kss1, Slt2, and Hog1 are well-conserved in plant pathogenic ascomycetes, Smk1 appears to be unique to S. cerevisiae. In fact, most plant pathogenic ascomycetes have only three MAPKs, three MEKs, and three MEKKs that are orthologous to the key components of the yeast Fus3/Kss1, Slt2, and Hog1 MAP kinase cascades, with a few exceptions such as two MAPKs homologous to yeast Hog1 in Verticillium dahliae and two MEKKs functioning upstream from the cell wall integrity MAPK in Fusarium oxysporum. Various components of these three well-conserved MAPK pathways have been characterized in different plant pathogenic ascomycetes for their functions in pathogenesis, sexual and asexual reproduction, mycotoxin production, and stress responses (Jiang et al. 2018). To date, all three MAPK cascades have been characterized in the rice blast fungus Magnaporthe oryzae, wheat scab fungus Fusarium graminearum, and several other plant pathogenic fungi. Whereas only two MAPKs are important for pathogenesis in M. oryzae, a model for studying fungal–plant interactions, all three MAPKs play critical roles in pathogenesis of F. graminearum, indicating variations in the functions of individual MAPKs among different fungal pathogens.
2 The Pmk1/Kss1 Invasive Growth (IG) Pathway
In general, plant pathogenic ascomycetes have only one MAPK that is orthologous to Fus3 and Kss1, which are activated by upstream MEK Ste7 and MEKK Ste11 in yeast. Studies in a number of fungal pathogens have showed that this MAPK pathway is important for regulating infection-related morphogenesis and invasive growth in plant tissues (Li et al. 2012; Turrà et al. 2014). Although they share similar amino acid sequence identity with yeast Fus3 and Kss1, the IG MAPKs from plant pathogens are considered to be more closely related to the latter because of the role of Kss1 in agar invasion in S. cerevisiae.
2.1 Regulation of Appressorium Formation by the PMK1 Pathway in M. oryzae
Like many other foliar pathogens, M. oryzae forms melanized, dome-shaped appressoria for plant penetration. As the first MAPK gene characterized in plant pathogens, PMK1 (pathogenicity MAP kinase 1) is essential for appressorium formation and pathogenesis in the rice blast fungus. Germ tubes of the pmk1 deletion mutant form subapical swollen bodies instead of appressoria on artificial hydrophobic surfaces and rice leaves. Deletion of PMK1 blocks the formation of appressoria but not surface recognition, which is regulated by the cAMP-PKA pathway in M. oryzae (Xu and Hamer 1996). Pmk1 is activated by the Mst7 MEK, which is in turn activated by Mst11 MEKK (Fig. 8.1). The mst7 and mst11 mutants have the same defects in appressorium formation and plant infection as the pmk1 mutant. Although the Mst11-Mst7-Pmk1 MAPK cascade lacks a scaffold protein, Mst7 directly interacts with Pmk1 via its MAPK docking site and both Mst11 and Mst7 interact with the adaptor protein Mst50 (Zhao and Xu 2007; Park et al. 2006). The Trx2 thioredoxin is involved in the activation of Pmk1 by affecting the folding or intra−/inter-molecular interaction of Mst7 (Zhang et al. 2016). One of the downstream targets of the Pmk1 pathway is Mst12, a Ste12 ortholog that is essential for appressorium penetration and pathogenicity. MST12 is dispensable for appressorium formation but regulates septin-mediated cytoskeleton reorganizations in mature appressoria (Park et al. 2002, 2004; Dagdas et al. 2012). MoMcm1 and MoSfl1 are the other two transcription factors that likely function downstream from the Pmk1 MAPK cascade for appressorium penetration and invasive growth (Li et al. 2011; Zhou et al. 2011). MoSfl1 is identified as one of the proteins phosphorylated by Pmk1 in vitro (Li et al. 2011). Deletion of MoSFL1 rescues the defect of the cpk1 cpk2 mutant in vegetative growth by relieving transcriptional suppression of the Cyc8-Tup1 co-suppressor (Li et al. 2017b), suggesting that it may be functionally related to both Pmk1 and cAMP-PKA pathways.
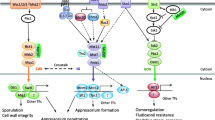
Distinct and overlapping functions of the Pmk1 and Mps1 MAPK pathways in Magnaporthe oryzae. The Mst11-Mst7-Pmk1 MAPK cascade is involved in regulating appressorium formation, penetration, and invasive growth (moving from cell to cell) in infected plant tissues. Both trimeric G-proteins and small GTPase Ras2 have been implicated in activating the Pmk1 pathway via Msb2 and Cbp1 mucins, Sho1, and possibly GPCRs as the receptors for physical and chemical cues such as surface hydrophobicity and hardness, cutin monomers, and primary alcohols. Thioredoxin Trx2 affects the activation of Mst7 but the role of Mst20 and Chm1 PAK kinases in Mst11 activation is not clear. Known downstream transcription factors of Pmk1 include Mst12, Mcm1, and Sfl1 but none of them is essential for appressorium formation, indicating the existence of other Pmk1 targets. The Bck1-Mkk2-Mps1 cascade is involved in regulating appressorium penetration, invasive growth, disease development, and conidiation. M. oryzae has orthologs of Wsc1–3 and Mid1 that may function as the cell wall stress sensors. Based on its conserved functions, this CWI MAPK pathway likely functions downstream from PKC and Rho1. Transcription factors known to function downstream from Mps1 MAPK include Gti1, Swi1, and Mig1. Adaptor protein Mst50 is involved in both Pmk1 MAPK and Mps1 MAPK pathways. Mip11 functions as a RACK protein that interacts with both Mst50 and Mck1
In yeast, the PAK kinase STE20 functions upstream from the pheromone response pathway. In M. oryzae, deletion of the STE20 ortholog does not block appressorium formation or plant infection. Deletion of the only other PAK kinase gene, CHM1, results in pleiotropic defects in growth, conidiation, and plant infection but the chm1 mutant still forms melanized appressoria (Li et al. 2004). Both Mst50 and Mst11 have the Ras-associating domain and Mst50 physically interacts with Ras1 and Ras2 in yeast two-hybrid assays (Park et al. 2006). In M. oryzae, RAS2 is an essential gene and functions upstream of both the cAMP-PKA and MAPK pathways. Expressing the dominant RAS2DA allele in the wild type, but not in the mst50 mutant, results in the formation of melanized appressoria in liquid droplets, indicating the bypass of the requirement of surface attachment and recognition (Zhou et al. 2012; Qi et al. 2015). Besides Ras2, trimeric G-proteins also are involved in regulating appressorium formation and pathogenesis in M. oryzae and Mst50 interacts with Mgb1 Gβ subunit (Nishimura et al. 2003; Park et al. 2006). For upstream receptors, the M. oryzae genome has over 40 putative G protein-coupled receptor (GPCR) genes, including two pheromone receptors. However, deletion of MoSTE2 and/or MoSTE3 has no effect on appressorium formation. Although the CFEM (conserved fungal-specific extracellular membrane-spanning)-domain containing GPCR encoded by PTH11 is important for surface recognition, and plant infection, treatments with cAMP suppress the defects of pth11 mutant in plant infection, indicating that PTH11 mainly functions via cAMP signaling in M. oryzae (DeZwaan et al. 1999; Nishimura et al. 2003). In contrast, the orthologs of yeast Sho1 and Msb2 mucin have overlapping roles in acting as the sensors for plant surface chemicals such as primary alcohols to activate the Pmk1 pathway for regulating appressorium formation (Liu et al. 2011). In addition, the CBP1 gene encoding a putative extracellular chitin-binding protein appears to be involved in sensing hydrophobic surfaces in M. oryzae (Kamakura et al. 2002).
2.2 Regulating the Formation of Various Infection Structures in Fungal Pathogens
The Pmk1/Kss1 MAPK pathway also has been functionally characterized in several other plant pathogenic fungi that form appressoria for plant penetration, including Bipolaris sorokiniana, Cochliobolus heterostrophus, Colletotrichum gloeosporioides, Colletotrichum fructicola, and Colletotrichum lagenarium. In all of them, this MAPK pathway is required for appressorium formation (Leng and Zhong 2015; Li et al. 2012; Liang et al. 2019). Furthermore, transforming the CMK1 gene of C. lagenarium into the pmk1 mutant rescues its defect in appressorium formation. Expression of CPMK1 from Claviceps purpurea, a non-appressorium-forming ascomycete, or PsMAPK1 from the wheat stripe rust Puccinia striiformis f. sp. tritici, a basidiomycete, also complements the pmk1 mutant for appressorium formation and pathogenesis (Mey et al. 2002; Guo et al. 2011a), indicating that this MAPK is well-conserved in sequence and function among different fungal pathogens.
Similar to appressoria formed by foliar pathogens, hyphopodia are formed by root pathogens for plant invasion. Under laboratory conditions, M. oryzae also forms hyphopodia for infection of rice roots. The Pmk1 MAPK cascade, but not the cAMP-PKA pathway, is essential for hyphopodium formation in M. oryzae and likely other root pathogens (Kong et al. 2013; Sesma and Osbourn 2004). On rice leaves or artificial hydrophobic surfaces, hyphal tips of M. oryzae also form melanized, swollen apical structures that are morphologically similar to appressoria formed by germ tubes. PMK1 is also essential for the formation of appressorium-like structures at hyphal tips (Kong et al. 2013). In the gray mold fungus Botrytis cinerea, the formation of infection cushions or compound appressoria by hyphae attached to plant surface is blocked in the msb2 and bmk1 mutants (Leroch et al. 2015). In F. graminearum, the GIV1 GPCR gene that appears to function upstream of Gpmk1 is important for infection cushion formation (Jiang et al. 2019). In Sclerotinia sclerotiorum, SMK1 is characterized for its function in regulating sclerotium formation but has not been examined for its role in infection cushion formation (Chen et al. 2004). Nevertheless, the smk1 mutant is reduced in the expression of the RGB1 type 2A protein phosphatase gene and silencing of RGB1 results in a significant reduction in infection cushion formation (Erental et al. 2007). Although the functions of two PMK1 orthologs in the rice sheath blight fungus Rhizoctonia solani, a basidiomycetous pathogen, have not be directly characterized, expression of the RNA interference (RNAi) construct targeting both RPMK1–1 and RPMK1–2 in transgenic rice plants significantly reduces infection cushion formation and disease severity (Tiwari et al. 2017). Therefore, it is likely that the Pmk1/Kss1 IG MAPK pathway has a conserved role in regulating infection structure formation in plant pathogenic fungi.
2.3 Invasive Growth After Penetration
In M. oryzae, Pmk1 is important for invasive growth after penetration as well, and the pmk1 mutant fails to infect rice leaves through wound sites (Fig. 8.1). As a hemibiotrophic pathogen, invasive hyphae of M. oryzae spread from the initial colonized cell to neighboring compartments before killing plant cells. Pmk1 plays a critical role in cell-to-cell spread of invasive hyphae in infected rice tissues (Sakulkoo et al. 2018). Its orthologs have a conserved role in invasive growth after penetration in other appressorium-forming plant pathogens (Jiang et al. 2018).
PMK1 orthologs also are important for plant infection in various plant pathogenic fungi that do not form appressoria, including the biotrophic pathogen, Claviceps purpurea, vascular wilt pathogens, F. oxysporum and V. dahliae, canker pathogens, Cryphonectria parasítica and Valsa mali, corn stalk and ear rot pathogen, Fusarium verticillioides, wheat pathogens, Zymoseptoria tritici and Parastagonospora nodorum, and the banana pathogen, Mycosphaerella fijiensis (Hamel et al. 2012; Jiang et al. 2018; Li et al. 2012). In Z. tritici and P. nodorum, the Pmk1 ortholog is important for infectious growth in mesophyll tissues after invasion through stomata (Solomon et al. 2005; Cousin et al. 2006). This IG MAPK pathway regulates the expression of various cell wall-degrading enzyme (CWDE) genes in F. oxysporum, F. graminearum, V. mali, and C. parasitica (Jiang et al. 2018). In F. graminearum, the gpmk1 (fmk1) mutant is non-pathogenic and fails to cause disease symptoms on drop-inoculated wheat kernels. Deletion of its upstream MEK and MEKK genes results in the same defects in plant infection and all the mutants disrupted in this MAPK cascade are defective in the production of deoxynivalenol (DON), a potent inhibitor of eukaryotic protein synthesis (Wang et al. 2011). DON is an important virulence factor required for the spread of invasive hyphae from inoculated sites to neighboring spikelets through rachis tissues in F. graminearum. In F. verticillioides, FvMK1 regulates the biosynthesis of fumonisins that are also toxic to plant cells (Zhang et al. 2011).
In summary, the Pmk1/Kss1 IG MAPK pathway is conserved for regulating penetration-related morphogenesis and invasive growth in fungal pathogens. It may regulate the expression of various stage-specific genes during disease development, likely in response to plant signals recognized at different infection stages. In M. oryzae, genes of diverse functions are regulated by the IG MAPK pathway, including PTH11 GPCR, GAS2/GAS2 hypothetical proteins, and MoHOX7 homeobox transcription factor (Jiang et al. 2018; Zhang et al. 2021). For plant signals, ethylene, wheat floral tissue extract, and secreted class III peroxidases are known to activate the IG MAPK cascade in C. gloeosporioides, F. graminearum, and F. oxysporum, respectively (Jiang et al. 2019; Turra et al. 2015; Kim et al. 2000).
2.4 Sexual Reproduction
Sexual reproduction is important to increase genetic variation in plant pathogenic fungi. In S. cerevisiae that forms naked asci, mating occurs between two regular yeast cells of compatible mating types. Fus3 and Kss1 have overlapping functions in pheromone response and the fus3 kss1 double mutant is sterile. In contrast, most ascomycetous crop pathogens form asci and ascospores inside ascocarps such as perithecia and pseudothecia and often involve the development of female-specific mating structures know as ascogonia. In M. oryzae, a heterothallic fungus, the pmk1 mutant is fertile when mated as the male but sterile when mated as the female. The PMK1 ortholog is also essential for female fertility but dispensable for male fertility in C. heterostrophus, F. verticillioides, and F. graminearum (Jenczmionka et al. 2003; Zhang et al. 2011; Takano et al. 2000).
However, many other genes are known to be essential for female fertility in M. oryzae and other fungal pathogens. In fact, deletion of the CWI MPAK MPS1 also results in the loss of female fertility in M. oryzae. In F. graminearum, a homothallic fungus that can be forced to outcross, mutants deleted of the other two MAPKs also are female sterile in outcrosses. In C. heterostrophus, the mps1 and hog1 MAPK deletion mutants are female fertile although pseudothecia are not developed in the mps1 × mps1 cross (Igbaria et al. 2008). Nevertheless, all these MAPK mutants retain male fertility. Therefore, it appears that none of the MAPKs is essential for male fertility in ascomycetous fungal pathogens but the Pmk1/Kss1 IG pathway has a conserved role in female fertility. The IG MAPK may also play a role in ascus development and ascospore formation because expressing a dominant active FST7 MEK allele rescues the defect of a mutant blocked in ascus/ascospore formation but not perithecium development (Jiang and Xu, unpublished). Overall, in comparison with S. cerevisiae, the regulation of sexual reproduction is much more complex in filamentous ascomycetes that form sexual fruiting bodies. Unlike in yeast, deletion of the individual pheromone or pheromone receptor genes does not block perithecium formation in F. graminearum (Lee et al. 2008).
3 The Cell Wall Integrity (CWI) MAPK Pathway
In the budding yeast, the CWI pathway consisting of the Bck1-Mkk1/Mkk22-Slt2 MAPK cascade is activated by Rho1 and Pkc1 to regulate gene expression changes via transcription factors Rlm1 and Swi6 (Jiménez-Gutiérrez et al. 2020). It is required for remodeling of the fungal cell wall during growth, development, and for responding to environmental stimuli. The key components of this CWI MAPK pathway are conserved in ascomycetous phytopathogens and have been shown to play important roles in regulating various infection and developmental processes besides responses to cell wall stress.
3.1 Penetration and Infectious Growth
In M. oryzae, the MPS1 MAP kinase gene is important for appressorial penetration and infectious growth. The mps1 deletion mutant forms melanized appressoria, but its appressoria are defective in penetration and it fails to infect through wounds (Xu et al. 1998). Deletion of the MoMCK1 MEKK gene results in similar defects with the mps1 mutant in plant infection (Jeon et al. 2008). Interestingly, Mst50 also interacts with MoMck1 and MoMkk2, and both Mst50 and MoMck1 interact with RACK1 protein Mip1 (Li et al. 2017a). Deletion of MST50 or MIP1 reduces the phosphorylation level of Mps1 under stress conditions and results in cell wall integrity defects, indicating the involvement of Mst50 and Mip11 for tethering the CWI MAPK cascade together in M. oryzae (Fig. 8.1). In S. cerevisiae, cell wall stressors or damages are recognized by sensor proteins Mid2, Wsc1-Wsc3, Sho1, and Hkr1. Their orthologs are conserved in M. oryzae and other fungal pathogens, and some of them may function as the sensors for the CWI MAPK pathway (Carbó and Pérez-Martín 2010; Xu et al. 2019). For downstream targets, MIG1 and MoSWI6 encode transcription factors orthologous to yeast Rlm1 and Swi6. Like the mps1 mutant, the mig1 mutant still forms appressoria, but is defective in the differentiation and growth of invasive hyphae, likely due to defects in overcoming plant defense responses (Mehrabi et al. 2008). Whereas the mps1 and mig1 mutants are non-pathogenic, the Moswi6 mutant causes small specks but not typical blast lesions on infected rice leaves (Qi et al. 2012). Appressoria formed by the Moswi6 mutant are defective in appressorium turgor generation. Another likely downstream target of the Mps1 pathway is the MoGti1 transcription factor that is important for penetration peg formation and invasive growth in M. oryzae (Li et al. 2016). Although it forms melanized appressoria with normal turgor pressure, the Mogti1 deletion mutant is non-pathogenic because MoGti1 regulates the expression of many effector genes, including BAS1, BAS2, and PWL1 (Li et al. 2016). Interestingly, expression of the bacterial effector HopAI with the infection-specific MIR1 promoter (Li et al. 2007) significantly reduces the phosphorylation of Mps1 and results in defects in invasive growth and lesion development (Zhang et al. 2017).
The CWI MAPK pathway also is important for plant infection in other fungal pathogens with different tissue specificity or infection mechanisms, such as B. cinerea, C. parasitica, C. purpurea, F. graminearum, Z. tritici, M. fijiensis, and S. sclerotiorum (Sanz et al. 2017; Jiang et al. 2018). However, although it has a conserved role in pathogenesis, this MAPK pathway varies in the actual infection processes under its regulation among different plant pathogenic fungi. Whereas Mps1 is dispensable for appressorium formation in M. oryzae, its ortholog is important for appressorium development in C. lagenarium and C. gloeosporioides (Yong et al. 2013; Kojima et al. 2002). In S. sclerotiorum, SMK3 is important for infection cushion formation and initial infection but it is not essential for lesion expansion (Bashi et al. 2016). In F. graminearum, the Mgv1 and Gpmk1 MAPKs are involved in regulating basal resistance to plant defensin MsDef1 (Ramamoorthy et al. 2007). Similarly, both CWI and HOG pathways are important for responding to cell wall stresses caused by the phytoalexins camalexin and brassinin in Alternaria brassicicola (Joubert et al. 2011). In Z. tritici, the MgSlt2 mutant is normal in stomata penetration but defective in developing invasive hyphae in wheat leaves (Mehrabi et al. 2006). In Aspergillus flavus and F. verticillioides, deletion of the BCK1 MEKK gene results in a significant reduction in virulence. However, the Afbck1 deletion mutant is increased in aflatoxin production but the Fvbck1 mutant is increased in fumonisin production (Zhang et al. 2020). In F. graminearum, mutants deleted of any component of the CWI MAPK cascade are significantly reduced in DON production (Wang et al. 2011). In A. alternata, deletion of AaSLT2 results in failure to produce host-selective toxins and loss of pathogenicity (Yago et al. 2011). In B. sorokiniana, the Bsslt2 mutant is normal in appressorium formation and root infection but has a reduced virulence on leaves (Leng and Zhong 2015). These observations show that the CWI MAPK pathway has species-specific roles in fungal pathogenesis and secondary metabolism.
3.2 Cell Wall Integrity and Hyphal Growth
Like in S. cerevisiae, in all the plant pathogenic fungi that have been studied, mutants disrupted in the CWI MAPK pathway by targeted deletion of its key components are hypersensitive to cell wall lytic enzymes and cell wall stressors such as Congo Red (CR) or Calcofluor White (CFW) (Jiang et al. 2018; Hamel et al. 2012). In M. oryzae, the mps1 mutant is normal in growth rate on oatmeal agar but produces only limited aerial hyphae, conidiophores, and conidia. In cultures older than 1 week, autolysis of aerial hyphae can be observed in the center of mps1 and Mobck1 colonies (Xu et al. 1998; Jeon et al. 2008). Autolysis of aerial hyphae in aging cultures also has been observed in mutants deleted of key components of the CWI MAPK pathway in other fungi, including Sordaria macrospora and Coniothyrium minitans (Zhang et al. 2020).
Whereas MPS1 orthologs also are dispensable for normal growth rate in Colletotrichum species, mutants disrupted in the CWI MAPK pathway have severe growth defects in other fungal pathogens, including A. flavus, B. cinerea, F. graminearum, and F. verticillioides (So et al. 2017; Hou et al. 2002; Rui and Hahn 2007; Zhang et al. 2020). In F. graminearum, C. parasitica, and B. cinerea, mutants deleted of the CWI MAPK form compact colonies with limited whitish aerial hyphae. In S. sclerotiorum, the smk3 mutant is reduced in growth rate, blocked in sclerotium formation, but increased in aerial hyphal growth (Bashi et al. 2016). In fungal pathogens, reduced growth rate and increased sensitivity to cell wall stresses may directly contribute the defects of CWI mutants in plant infection. Nevertheless, a functional CWI MAPK pathway may be necessary for masking cell wall components to avoid being degraded or recognized by the host to trigger immunity response.
In C. parasitica, the Cpslt2 and Cpbck1 deletion mutants often produce spontaneous suppressors with faster growth rate although these suppressor strains still grow slower than the wild type and are similar to the original mutants in virulence (So et al. 2017). Therefore, only the defects of the CWI mutants in growth, but not their defects in plant infection, are partially rescued by spontaneous mutations that remain to be identified in these suppressor strains. In F. graminearum, the mgv1 mutant also is unstable and produces spontaneous suppressors with faster growth rate that have nonsense or frameshift mutations in FgHOG1, an ortholog of yeast HOG1 MAPK (Ren et al. 2019). Deletion of FgHOG1 is confirmed to partially rescue the growth defect of the mgv1 mutant but not its defect in pathogenesis. One possible explanation is that deletion of MGV1 results in the overstimulation of the HOG pathway, which is detrimental to hyphal growth (see below) but can be suppressed by nonsense or frameshift mutations in the FgHOG1 ortholog (Ren et al. 2019). Similar suppressor mutations may occur in the suppressor strains of Cpslt2 and Cpbck1 mutants in C. parasitica and other fungi.
3.3 Hyphal Fusion and Parasexual Reproduction
Hyphal fusion between hyphae of different strains can lead to heterokaryon formation and parasexual reproduction that are unique to fungi and contribute to genetic variations in many asexual fungal pathogens (Clutterbuck 1996; Daskalov et al. 2017). The first fungal MAPK gene found to be essential for hyphal fusion and heterokaryon formation is MGV1 of F. graminearum (Hou et al. 2002). Anastomosis is not observed in the mgv1 mutant and the mgv1 nit1 mutant fails to form heterokaryons with a nitM mutant (Hou et al. 2002). In the model filamentous fungus Neurospora crassa, Mak-1 (Slt2) and Mak-2 (Kss1) MAPKs interact with Cot-1 to regulate hyphal fusion. Further studies showed that the Mak-1 and Mak-2 MAPK pathways crosstalk to regulate hyphal fusion together with the striatin-interacting protein phosphatase and kinase (STRIPAK) complex (Dettmann et al. 2014; Fischer and Glass 2019). In N. crassa, the So protein functions as a scaffold for the upstream components of the CWI MAPK pathway. Interestingly, So is one of the proteins phosphorylated by Mak-2. However, unlike the N. crassa so and mak-2 mutants, hyphal fusion still occurs in the Fgso (Fgsoft) and Gpmk1 deletion mutants in F. graminearum (Zheng et al. 2013). Therefore, the functions of So and other components of STRIPAK in hyphal fusion may be not conserved in all the phytopathogenic ascomycetous species. Furthermore, the roles of MAPKs in steps of parasexual reproduction after hyphal fusion and heterokaryon formation, such as fate and stability of heterokaryons, diploidization, and somatic recombination, remain to be characterized.
4 The High-Osmolarity Glycerol (HOG) Pathway
Whereas the other two fungal MAPKs have the TEY dual phosphorylation site, Hog1 and its orthologs have the TGY motif, which is similar to p38 stress activated MAP kinases (SAPKs) in animals. In yeast, the Ssk2/Ssk22-Pbs2-Hog1 MAPK cascade mainly regulates responses to hyperosmotic stress. In plant pathogenic fungi, besides its conserved role in osmoregulation, the HOG pathway in general is important for regulating responses to other environmental stresses, including antifungal chemicals, reactive oxygen species (ROS), and plant defense compounds (Dunayevich et al. 2018; Lee et al. 2017; Yang et al. 2020a, b).
4.1 Species-Specific Roles in Pathogenesis
In M. oryzae, the osm1 deletion mutant is normal in appressorium formation and plant infection (Dixon et al. 1999). Although deletion of OSM1 affects glycerol accumulation in vegetative hyphae under hyperosmotic conditions, the osm1 mutant has no defects in appressorium turgor generation (Fig. 8.2), indicating that glycerol accumulation in appressoria is not regulated by OSM1. Its upstream sensor histidine kinases MoSln1 and MoHik1, phosphotransfer protein MoYpd1p, and MoSsk1 MEKK also are important for osmoregulation during vegetative growth but dispensable for pathogenesis (Jacob et al. 2016).
The HOG MAPK pathway in Magnaporthe oryzae and Fusarium graminearum. All of the key components of HOG pathway, including the three-tiered protein kinase cascade and upstream phosphorelay and sensor proteins, are conserved in M. oryzae (Mo) and F. Graminearum (Fg). Besides its conserved function in osmoadaptation, the Hog1 MAPK pathway also has species-specific roles in these two important plant pathogens. Although this pathway is dispensable for pathogenesis in M. oryzae, it is important for plant infection in F. graminearum. In F. graminearum, the FgHog1 MAPK is also important for sexual development and secondary metabolism
Like in M. oryzae, the Hog1 ortholog is dispensable for plant infection in some fungal pathogens, such as Cochliobolus orbiculare and Bipolaris oryzae (Jiang et al. 2018; Moriwaki et al. 2006). However, the HOG MAPK pathway is important for plant infection in many other plant pathogenic fungi. For example, Sak1 is important for appressorium development and penetration of epidermal cells in B. cinerea (Liu et al. 2008). Silencing of Hog1 and Pbs2 reduces infectious growth and virulence in F. oxysporum (Pareek and Rajam 2017). In F. graminearum (Fig. 8.2), the Fghog1 mutant is defective in DON production and fails to spread through rachis tissues in wheat heads after the initial infection (Zheng et al. 2012). In Ustilaginoidea virens, UvHog1 regulates the production of secondary metabolites that are toxic to plant cells (Zheng et al. 2016). Therefore, the HOG pathway likely has species-specific roles during plant infection in fungal pathogens.
The best characterized downstream target of the HOG MAPK in plant pathogenic fungi is the Atf1 bZIP transcription factor. In F. graminearum, Atf1 interacts with FgOs2 (FgHog1) in the nucleus under osmotic stress and constitutive expression of FgATF1 suppresses the defects of Fgos2 mutant in osmoregulation and pathogenesis (Van Nguyen et al. 2013). Atf1 orthologs also are important for virulence in M. oryzae, F. verticillioides, V. dahliae, and other fungal pathogens (Jiang et al. 2018; Szabó et al. 2020; Tang et al. 2020). However, the ATF1 ortholog mainly regulates responses to oxidative stress instead of osmoregulation in these plant pathogenic fungi. For orthologs of yeast Skn7, a response regulator of the HOG pathway, they are important for plant infection in a number of fungi, but MoSKN7 is dispensable for virulence in M. oryzae (Motoyama et al. 2008), further indicating the differences among various plant pathogenic fungi in the roles of HOG pathway during plant infection.
4.2 Osmoregulation and Survival
Although the importance of the HOG pathway for plant infection varies, its function in regulating adaptive responses to hyperosmotic stress is well-conserved in fungal pathogens. Deletion of the HOG1 ortholog results in increased sensitivity to hyperosmotic stress in all the plant pathogenic fungi studied, including Z. tritici and F. graminearum. Like in yeast, Hog1 orthologs are rapidly phosphorylated in response to hyperosmotic stress in fungal pathogens such as C. heterostrophus (Yoshimi et al. 2005). In M. oryzae, the osm1 deletion mutant is hypersensitive to osmotic stress and desiccation (Dixon et al. 1999). Although it is normal in plant infection under laboratory conditions, the osm1 mutant will face problems to survive in desiccated plant tissues in the field. In fact, the HOG pathway may be important for survival in nature in many other plant pathogens because of its essential role in adaptive responses to hyperosmotic stress associated with desiccation.
Interestingly, phenylpyrrole fungicides, fludioxonil and fenpiclonil, overstimulate the HOG pathway and result in the accumulation of intracellular glycerol and cell burst. Mutants deleted for key components of the HOG MAPK pathway are resistant to these fungicides in N. crassa, C. lagenarium, and other fungi (Brandhorst et al. 2019; Jiang et al. 2018). Remarkably, fludioxonil and fenpiclonil have been applied to control foliar pathogens for over 30 years, but field isolates with complete resistance against these phenylpyrrole fungicides have not emerged and spread widely in crop fields (Kilani and Fillinger 2016), which may be related to the defects of HOG mutants in stress response and survival in nature. Resistance against dicarboximide fungicides also has been observed in HOG pathway mutants in N. crassa and fungal pathogens (Zhang et al. 2002; Fujimura et al. 2003). For example, the hog1 mutant has increased tolerance to vinclozolin in A. alternata (Yu et al. 2016). However, the direct targets of these fungicides are not key components of the HOG pathway and remain to be identified in plant pathogenic fungi.
4.3 Oxidative Stress
In fungal pathogens, the HOG pathway plays a critical role in regulating responses to oxidative stress caused by oxidants produced by plant cells or present in the environment. Mutants deleted of the Hog1 MAPK or other key components of this pathway have increased sensitivity to oxidative stress, which may be related to defects in plant infection observed in some fungal HOG mutants as described above. In pathogens where the HOG MAPK is dispensable for virulence, they may use effector proteins to effectively suppress the oxidative burst in infected plant tissues. In general, the Atf1 ortholog is one major transcription factor functioning downstream from the HOG MAPK to regulate the expression of genes important for oxidative responses in fungal pathogens (Guo et al. 2011b; Tang et al. 2020). In contrast, the role of the Skn7 ortholog in oxidative stress response differs significantly among different pathogens, such as being dispensable in M. oryzae but critical in A. alternata (Motoyama et al. 2008; Chen et al. 2012). AP1 is another transcription factor known to be involved in regulating oxidative stress-related genes in fungi but its relationship with the HOG MAPK pathway is not clear. In yeast, YAP1 is not known to be related to the HOG pathway. In M. oryzae, whereas the osm1 mutant is normal in pathogenesis, the MoAP1 deletion mutant is defective in plant infection (Guo et al. 2011b).
In some fungal pathogens, the Hog1 MAPK pathway also has been implicated in regulating responses to other environmental stresses, such as UV irradiation, hypoxia-inducing NaNO2-treatment, and heavy metals (for reviews, see Zhang et al. 2021). However, although Hog1 MAPK plays a major role, the other two MAPK pathways often are involved in stress responses by directly regulating downstream targets or crosstalk with the HOG pathway. For example, mutants deleted of key components of the CWI MAPK pathway have increased sensitivities to oxidative stress in B. cinerea (Yin et al. 2018) and F. verticillioides (Zhang et al. 2015). In F. graminearum, the Gpmk1 mgv1 Fghog1 mutant, the only triple MAPK mutant that has been reported in plant pathogenic fungi, is viable but hypersensitive to various environmental stresses (Ren et al. 2022).
5 Concluding Remarks
The well-conserved MAPK pathways regulate various plant infection and developmental processes in ascomycetous plant pathogens. Most of them have three linear MAPK cascades without redundancy at the MAPK, MEK, or MEKK level. In different plant pathogenic ascomycetes, individual MAPK pathways have both conserved and species-specific functions, such as the regulation of invasive growth and DON biosynthesis by the IG MAPK pathway in F. graminearum (Hamel et al. 2012; Jiang et al. 2018). MAPK signaling also has been characterized in basidiomycetous plant pathogens but mainly limited to U. maydis, in which two Kss1-like MAPKs, Kpp2 and Kpp6, have overlapping functions in plant infection (Brachmann et al. 2003; Di Stasio et al. 2009). For the diverse roles of MAPKs that have been observed, fungal pathogens must be able to recognize various plant and environmental signals with upstream sensors or receptors. Among the predicted sensor or receptor genes, only GPCRs are significantly expanded in fungal pathogens in comparison with the budding yeast. For example, M. oryzae and F. graminearum have over 40 and 100 putative GPCRs, respectively, which is more than 10 times the three GPCRs found in yeast. Some of these GPCRs may be responsible for sensing host and environmental signals to regulate plant infection processes, such as PTH11 in M. oryzae and GIV1 in F. graminearum (Jiang et al. 2019; Kulkarni et al. 2005).
Unlike their roles in pathogenesis and stress response, the functions of fungal MAPK pathways in defense against mycoviruses, bacteria, and other fungi have not been well characterized although limited studies indicate their involvement in fungal–fungal/bacterial/viral interactions. Ascomycetous fungal pathogens lack receptor kinases or receptor-like kinases but have putative nucleotide-binding and leucine-rich repeat domain-containing (NLR) immune receptors (Uehling et al. 2017). Like in plants and animals, these NLRs may recognize certain microbe-associated molecular patterns (MAMPs) and function upstream from MAPK cascades in fungal pathogens to regulate the expression of genes related to defense or antagonistic interactions with bacteria or other fungi. Plant pathogenic fungi are known to produce various anti-microbial/fungal compounds and secrete various hydrolytic enzymes such as chitinases and glucanases. Therefore, it is not only interesting to characterize the possible functional relationships between MAMP recognition by NLRs and MAPK signaling to regulate anti-microbial/fungal activities but also helpful to improve biocontrol agents.
In yeast, MAPKs are hubs of protein–protein interaction networks and they influence each other as part of the interconnected signaling networks to ensure appropriate cellular responses to external cues (Saito 2010; Van Drogen et al. 2020). Plant pathogenic fungi have much more complex developmental and infection processes and they likely use these three MAPK pathways to coordinately regulate responses to host and environmental signals. To date, most of the MAPK studies in plant pathogenic fungi deal with individual MAPKs or MAPK pathways. There are only a few reports on mutants disrupted in two MAPK pathways, such as the mps1 hog1 mutant of C. heterostrophus and mgv1 Fghog1 mutant of F. graminearum (Ren et al. 2019; Igbaria et al. 2008). To better understand the crosstalk among these MAPKs, systematic transcript profiling with mutants disrupted in multiple MAPK pathways and different components of MAPK pathways is needed to establish the regulatory networks involving these MAPKs in fungal pathogens. Similarly, systematic proteomics analysis is needed to establish protein–protein interaction networks and determine the positions and links of individual MAPKs in M. oryzae or other plant pathogenic fungi.
References
Bashi ZD, Gyawali S, Bekkaoui D, Coutu C, Lee L, Poon J, Rimmer SR, Khachatourians GG, Hegedus DD (2016) The Sclerotinia sclerotiorum SLT2 mitogen-activated protein kinase ortholog, SMK3, is required for infection initiation but not lesion expansion. Can J Microbiol 62:836–850
Brachmann A, Schirawski J, Müller P, Kahmann R (2003) An unusual MAP kinase is required for efficient penetration of the plant surface by Ustilago maydis. EMBO J 22:2199–2210
Brandhorst TT, Kean IRL, Lawry SM, Wiesner DL, Klein BS (2019) Phenylpyrrole fungicides act on triosephosphate isomerase to induce methylglyoxal stress and alter hybrid histidine kinase activity. Sci Rep 9:1–17
Carbó N, Pérez-Martín J (2010) Activation of the cell wall integrity pathway promotes escape from G2 in the fungus Ustilago maydis. PLoS Genet 6:e1001009
Chen C, Harel A, Gorovoits R, Yarden O, Dickman MB (2004) MAPK regulation of sclerotial development in Sclerotinia sclerotiorum is linked with pH and cAMP sensing. Mol Plant-Microbe Interact 17:404–413
Chen LH, Lin CH, Chung KR (2012) Roles for SKN7 response regulator in stress resistance, conidiation and virulence in the citrus pathogen Alternaria alternata. Fungal Genet Biol 49:802–813
Clutterbuck AJ (1996) Parasexual recombination in fungi. J Genet 75:281–286
Cousin A, Mehrabi R, Guilleroux M, Dufresne M, Van der Lee T, Waalwijk C, Langin T, Kema GH (2006) The MAP kinase-encoding gene MgFus3 of the non-appressorium phytopathogen Mycosphaerella graminicola is required for penetration and in vitro pycnidia formation. Mol Plant Pathol 7:269–278
Dagdas YF, Yoshino K, Dagdas G, Ryder LS, Bielska E, Steinberg G, Talbot NJ (2012) Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science 336:1590–1595
Daskalov A, Heller J, Herzog S, Fleißner A, Glass NL (2017) Molecular mechanisms regulating cell fusion and heterokaryon formation in filamentous fungi. Microbiol Spectr 5(2)
Dettmann A, Heilig Y, Valerius O, Ludwig S, Seiler S (2014) Fungal communication requires the MAK-2 pathway elements STE-20 and RAS-2, the NRC-1 adapter STE-50 and the MAP kinase scaffold HAM-5. PLoS Genet 10:e1004762
DeZwaan TM, Carroll AM, Valent B, Sweigard JA (1999) Magnaporthe grisea pth11p is a novel plasma membrane protein that mediates appressorium differentiation in response to inductive substrate cues. Plant Cell 11:2013–2030
Di Stasio M, Brefort T, Mendoza-Mendoza A, Münch K, Kahmann R (2009) The dual specificity phosphatase Rok1 negatively regulates mating and pathogenicity in Ustilago maydis. Mol Microbiol 73:73–88
Dixon KP, Xu JR, Smirnoff N, Talbot NJ (1999) Independent signaling pathways regulate cellular turgor during hyperosmotic stress and appressorium-mediated plant infection by Magnaporthe grisea. Plant Cell 11:2045–2058
Dunayevich P, Baltanás R, Clemente JA, Couto A, Sapochnik D, Vasen G, Colman-Lerner A (2018) Heat-stress triggers MAPK crosstalk to turn on the hyperosmotic response pathway. Sci Rep 8:1–15
Erental A, Harel A, Yarden O (2007) Type 2A phosphoprotein phosphatase is required for asexual development and pathogenesis of Sclerotinia sclerotiorum. Mol Plant-Microbe Interact 20:944–954
Fischer MS, Glass NL (2019) Communicate and fuse: how filamentous fungi establish and maintain an interconnected mycelial network. Front Microbiol 10:619. https://doi.org/10.3389/fmicb.2019.00619
Fujimura M, Ochiai N, Oshima M, Motoyama T, Ichiishi A, Usami R, Horikoshi K, Yamaguchi I (2003) Putative homologs of SSK22 MAPKK kinase and PBS2 MAPK kinase of Saccharomyces cerevisiae encoded by os-4 and os-5 genes for osmotic sensitivity and fungicide resistance in Neurospora crassa. Biosci Biotechnol Biochem 67:186–191
Guo J, Dai X, Xu JR, Wang Y, Bai P, Liu F, Duan Y, Zhang H, Huang L, Kang Z (2011a) Molecular characterization of a Fus3/Kss1 type MAPK from Puccinia striiformis f. sp. tritici, PsMAPK1. PLoS One 6:e21895
Guo M, Chen Y, Du Y, Dong Y, Guo W, Zhai S, Zhang H, Dong S, Zhang Z, Wang Y (2011b) The bZIP transcription factor MoAP1 mediates the oxidative stress response and is critical for pathogenicity of the rice blast fungus Magnaporthe oryzae. PLoS Pathog 7:e1001302
Hamel LP, Nicole MC, Duplessis S, Ellis BE (2012) Mitogen-activated protein kinase signaling in plant-interacting fungi: distinct messages from conserved messengers. Plant Cell 24:1327–1351
Hou Z, Xue C, Peng Y, Katan T, Kistler HC, Xu JR (2002) A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol Plant-Microbe Interact 15:1119–1127
Igbaria A, Lev S, Rose MS, Lee BN, Hadar R, Degani O, Horwitz BA (2008) Distinct and combined roles of the MAP kinases of Cochliobolus heterostrophus in virulence and stress responses. Mol Plant-Microbe Interact 21:769–780
Jacob S, Schüffler A, Thines E (2016) Hog1p activation by marasmic acid through inhibition of the histidine kinase Sln1p. Pest Manag Sci 72:1268–1274
Jenczmionka NJ, Maier FJ, Lösch AP, Schäfer W (2003) Mating, conidiation and pathogenicity of Fusarium graminearum, the main causal agent of the head-blight disease of wheat, are regulated by the MAP kinase Gpmk1. Curr Genet 43:87–95
Jeon J, Goh J, Yoo S, Chi MH, Choi J, Rho HS, Park J, Han SS, Kim BR, Park SY (2008) A putative MAP kinase kinase kinase, MCK1, is required for cell wall integrity and pathogenicity of the rice blast fungus, Magnaporthe oryzae. Mol Plant-Microbe Interact 21:525–534
Jiang C, Zhang X, Liu H, Xu JR (2018) Mitogen-activated protein kinase signaling in plant pathogenic fungi. PLoS Pathog 14:e1006875
Jiang C, Cao S, Wang Z, Xu H, Liang J, Liu H, Wang G, Ding M, Wang Q, Gong C (2019) An expanded subfamily of G-protein-coupled receptor genes in Fusarium graminearum required for wheat infection. Nat Microbiol 4:1582–1591
Jiménez-Gutiérrez E, Alegría-Carrasco E, Sellers-Moya Á, Molina M, Martín H (2020) Not just the wall: the other ways to turn the yeast CWI pathway on. Int Microbiol 23:107–119
Joubert A, Bataille-Simoneau N, Campion C, Guillemette T, Hudhomme P, Iacomi-Vasilescu B, Leroy T, Pochon S, Poupard P, Simoneau P (2011) Cell wall integrity and high osmolarity glycerol pathways are required for adaptation of Alternaria brassicicola to cell wall stress caused by brassicaceous indolic phytoalexins. Cell Microbiol 13:62–80
Kamakura T, Yamaguchi S, Saitoh KI, Teraoka T, Yamaguchi I (2002) A novel gene, CBP1, encoding a putative extracellular chitin-binding protein, may play an important role in the hydrophobic surface sensing of Magnaporthe grisea during appressorium differentiation. Mol Plant-Microbe Interact 15:437–444
Kilani J, Fillinger S (2016) Phenylpyrroles: 30 years, two molecules and (nearly) no resistance. Front Microbiol 7:2014. https://doi.org/10.3389/fmicb.2016.02014
Kim YK, Kawano T, Li D, Kolattukudy PE (2000) A mitogen-activated protein kinase kinase required for induction of cytokinesis and appressorium formation by host signals in the conidia of Colletotrichum gloeosporioides. Plant Cell 12:1331–1343
Kojima K, Kikuchi T, Takano Y, Oshiro E, Okuno T (2002) The mitogen-activated protein kinase gene MAF1 is essential for the early differentiation phase of appressorium formation in Colletotrichum lagenarium. Mol Plant-Microbe Interact 15:1268–1276
Kong L, Li G, Liu Y, Liu M, Zhang S, Yang J, Zhou X, Peng Y, Xu JR (2013) Differences between appressoria formed by germ tubes and appressorium-like structures developed by hyphal tips in Magnaporthe oryzae. Fungal Genet Biol 56:33–41
Kulkarni RD, Thon MR, Pan H, Dean RA (2005) Novel G-protein-coupled receptor-like proteins in the plant pathogenic fungus Magnaporthe grisea. Genome Biol 6:1–14
Lee J, Leslie JF, Bowden RL (2008) Expression and function of sex pheromones and receptors in the homothallic ascomycete Gibberella zeae. Eukaryot Cell 7:1211–1221
Lee YM, Kim E, An J, Lee Y, Choi E, Choi W, Moon E, Kim W (2017) Dissection of the HOG pathway activated by hydrogen peroxide in Saccharomyces cerevisiae. Environ Microbiol 19:584–597
Leng Y, Zhong S (2015) The role of mitogen-activated protein (MAP) kinase signaling components in the fungal development, stress response and virulence of the fungal cereal pathogen Bipolaris sorokiniana. PLoS One 10:e0128291
Leroch M, Mueller N, Hinsenkamp I, Hahn M (2015) The signalling mucin Msb2 regulates surface sensing and host penetration via BMP1 MAP kinase signalling in Botrytis cinerea. Mol Plant Pathol 16:787–798
Li L, Xue C, Bruno K, Nishimura M, Xu JR (2004) Two PAK kinase genes, CHM1 and MST20, have distinct functions in Magnaporthe grisea. Mol Plant-Microbe Interact 17:547–556
Li L, Ding S, Sharon A, Orbach M, Xu JR (2007) MIR1 is highly upregulated and localized to nuclei during infectious hyphal growth in the rice blast fungus. Mol Plant-Microbe Interact 20:448–458
Li G, Zhou X, Kong L, Wang Y, Zhang H, Zhu H, Mitchell TK, Dean RA, Xu JR (2011) MoSfl1 is important for virulence and heat tolerance in Magnaporthe oryzae. PLoS One 6:e19951
Li G, Zhou X, Xu JR (2012) Genetic control of infection-related development in Magnaporthe oryzae. Curr Opin Microbiol 15:678–684
Li Y, Wang G, Xu JR, Jiang C (2016) Penetration peg formation and invasive hyphae development require stage-specific activation of MoGTI1 in Magnaporthe oryzae. Mol Plant-Microbe Interact 29:36–45
Li GT, Zhang X, Tian H, Choi YE, Tao WA, Xu JR (2017a) MST50 is involved in multiple MAP kinase signaling pathways in Magnaporthe oryzae. Environ Microbiol 19:1959–1974
Li Y, Zhang X, Hu S, Liu H, Xu JR (2017b) PKA activity is essential for relieving the suppression of hyphal growth and appressorium formation by MoSfl1 in Magnaporthe oryzae. PLoS Genet 13:e1006954
Liang X, Wei T, Cao M, Zhang X, Liu W, Kong Y, Zhang R, Sun G (2019) The MAP kinase CfPMK1 is a key regulator of pathogenesis, development, and stress tolerance of Colletotrichum fructicola. Front Microbiol 10:1070
Liu W, Leroux P, Fillinger S (2008) The HOG1-like MAP kinase SAK1 of Botrytis cinerea is negatively regulated by the upstream histidine kinase Bos1 and is not involved in dicarboximide-and phenylpyrrole-resistance. Fungal Genet Biol 45:1062–1074
Liu W, Zhou X, Li G, Li L, Kong L, Wang C, Zhang H, Xu JR (2011) Multiple plant surface signals are sensed by different mechanisms in the rice blast fungus for appressorium formation. PLoS Pathog 7:e1001261
Mehrabi R, Zwiers LH, de Waard MA, Kema GH (2006) MgHog1 regulates dimorphism and pathogenicity in the fungal wheat pathogen Mycosphaerella graminicola. Mol Plant-Microbe Interact 19:1262–1269
Mehrabi R, Ding SL, Xu JR (2008) MADS-box transcription factor Mig1 is required for infectious growth in Magnaporthe grisea. Eukaryot Cell 7:791–799
Mey G, Oeser B, Lebrun MH, Tudzynski P (2002) The biotrophic, non-appressorium-forming grass pathogen Claviceps purpurea needs a Fus3/Pmk1 homologous mitogen-activated protein kinase for colonization of rye ovarian tissue. Mol Plant-Microbe Interact 15:303–312
Moriwaki A, Kubo E, Arase S, Kihara J (2006) Disruption of SRM1, a mitogen-activated protein kinase gene, affects sensitivity to osmotic and ultraviolet stressors in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol Lett 257:253–261
Motoyama T, Ochiai N, Morita M, Iida Y, Usami R, Kudo T (2008) Involvement of putative response regulator genes of the rice blast fungus Magnaporthe oryzae in osmotic stress response, fungicide action, and pathogenicity. Curr Genet 54:185–195
Nishimura M, Park G, Xu JR (2003) The G-beta subunit MGB1 is involved in regulating multiple steps of infection-related morphogenesis in Magnaporthe grisea. Mol Microbiol 50:231–243
Pareek M, Rajam MV (2017) RNAi-mediated silencing of MAP kinase signalling genes (FMK1, HOG1, and PBS2) in Fusarium oxysporum reduces pathogenesis on tomato plants. Fungal Biol 121:775–784
Park G, Xue C, Zheng L, Lam S, Xu JR (2002) MST12 regulates infectious growth but not appressorium formation in the rice blast fungus Magnaporthe grisea. Mol Plant-Microbe Interact 15:183–192
Park G, Bruno KS, Staiger CJ, Talbot NJ, Xu JR (2004) Independent genetic mechanisms mediate turgor generation and penetration peg formation during plant infection in the rice blast fungus. Mol Microbiol 53:1695–1707
Park G, Xue C, Zhao X, Kim Y, Orbach M, Xu JR (2006) Multiple upstream signals converge on the adaptor protein Mst50 in Magnaporthe grisea. Plant Cell 18:2822–2835
Qi Z, Wang QI, Dou X, Wang W, Zhao Q, Lv R, Zhang H, Zheng X, Wang P, Zhang Z (2012) MoSwi6, an APSES family transcription factor, interacts with MoMps1 and is required for hyphal and conidial morphogenesis, appressorial function and pathogenicity of Magnaporthe oryzae. Mol Plant Pathol 13:677–689
Qi L, Kim Y, Jiang C, Li Y, Peng Y, Xu JR (2015) Activation of Mst11 and feedback inhibition of germ tube growth in Magnaporthe oryzae. Mol Plant-Microbe Interact 28:881–891
Ramamoorthy V, Cahoon EB, Li J, Thokala M, Minto RE, Shah DM (2007) Glucosylceramide synthase is essential for alfalfa defensin-mediated growth inhibition but not for pathogenicity of Fusarium graminearum. Mol Microbiol 66:771–786
Ren J, Li C, Gao C, Xu JR, Jiang C, Wang G (2019) Deletion of FgHOG1 is suppressive to the mgv1 mutant by stimulating Gpmk1 activation and avoiding intracellular turgor elevation in Fusarium graminearum. Front Microbiol 1073
Ren J, Zhang Y, Wang Y, Li C, Bian Z, Zhang X, Liu H, Xu JR, Jiang C (2022) Deletion of all three MAP kinase genes results in severe defects in stress responses and pathogenesis in Fusarium graminearum. Stress Biol 2:1–13
Rui O, Hahn M (2007) The Slt2-type MAP kinase Bmp3 of Botrytis cinerea is required for normal saprotrophic growth, conidiation, plant surface sensing and host tissue colonization. Mol Plant Pathol 8:173–184
Saito H (2010) Regulation of cross-talk in yeast MAPK signaling pathways. Curr Opin Microbiol 13:677–683
Sakulkoo W, Osés-Ruiz M, Oliveira Garcia E, Soanes DM, Littlejohn GR, Hacker C, Correia A, Valent B, Talbot NJ (2018) A single fungal MAP kinase controls plant cell-to-cell invasion by the rice blast fungus. Science 359:1399–1403
Sanz AB, Garcia R, Rodriguez-Pena JM, Arroyo J (2017) The CWI pathway: regulation of the transcriptional adaptive response to cell wall stress in yeast. J Fungi 4:1. https://doi.org/10.3390/jof4010001
Schwartz MA, Madhani HD (2004) Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae. Annu Rev Genet 38:725–748
Sesma A, Osbourn AE (2004) The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 431:582–586
So KY, Kim SH, Jung KT, Lee HY, Oh SH (2017) MAPK/JNK1 activation protects cells against cadmium-induced autophagic cell death via differential regulation of catalase and heme oxygenase-1 in oral cancer cells. Toxicol Appl Pharmacol 332:81–91
Solomon PS, Waters OD, Simmonds J, Cooper RM, Oliver RP (2005) The Mak2 MAP kinase signal transduction pathway is required for pathogenicity in Stagonospora nodorum. Curr Genet 48:60–68
Szabó Z, Pákozdi K, Murvai K, Pusztahelyi T, Kecskeméti Á, Gáspár A, Logrieco AF, Emri T, Ádám AL, Leiter É (2020) FvATFA regulates growth, stress tolerance as well as mycotoxin and pigment productions in Fusarium verticillioides. Appl Microbiol Biotechnol 104:7879–7899
Takano Y, Kikuchi T, Kubo Y, Hamer JE, Mise K, Furusawa I (2000) The Colletotrichum lagenarium MAP kinase gene CMK1 regulates diverse aspects of fungal pathogenesis. Mol Plant-Microbe Interact 13:374–383
Tang C, Li T, Klosterman SJ, Tian C, Wang Y (2020) The bZIP transcription factor VdAtf1 regulates virulence by mediating nitrogen metabolism in Verticillium dahliae. New Phytol 226:1461–1479
Tiwari IM, Jesuraj A, Kamboj R, Devanna BN, Botella JR, Sharma TR (2017) Host delivered RNAi, an efficient approach to increase rice resistance to sheath blight pathogen Rhizoctonia solani. Sci Rep 7:1–14
Turrà D, Segorbe D, Di Pietro A (2014) Protein kinases in plant-pathogenic fungi: conserved regulators of infection. Annu Rev Phytopathol 52:267–288
Turra D, El Ghalid M, Rossi F, Di Pietro A (2015) Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature 527:521–524
Uehling J, Deveau A, Paoletti M (2017) Do fungi have an innate immune response? An NLR-based comparison to plant and animal immune systems. PLoS Pathog 13:e1006578
Van Drogen F, Dard N, Pelet S, Lee SS, Mishra R, Srejić N, Peter M (2020) Crosstalk and spatiotemporal regulation between stress-induced MAP kinase pathways and pheromone signaling in budding yeast. Cell Cycle 19:1707–1715
Van Nguyen T, Kröger C, Bönnighausen J, Schäfer W, Bormann J (2013) The ATF/CREB transcription factor Atf1 is essential for full virulence, deoxynivalenol production, and stress tolerance in the cereal pathogen Fusarium graminearum. Mol Plant-Microbe Interact 26:1378–1394
Wang C, Zhang S, Hou R, Zhao Z, Zheng Q, Xu Q, Zheng D, Wang G, Liu H, Gao X (2011) Functional analysis of the kinome of the wheat scab fungus Fusarium graminearum. PLoS Pathog 7:e1002460
Xu JR, Hamer JE (1996) MAP kinase and cAMP signaling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev 10:2696–2706
Xu JR, Staiger CJ, Hamer JE (1998) Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc Natl Acad Sci U S A 95:12713–12718
Xu L, Wang M, Tang G, Ma Z, Shao W (2019) The endocytic cargo adaptor complex is required for cell-wall integrity via interacting with the sensor FgWsc2B in Fusarium graminearum. Curr Genet 65:1071–1080
Yago JI, Lin CH, Chung KR (2011) The SLT2 mitogen-activated protein kinase-mediated signalling pathway governs conidiation, morphogenesis, fungal virulence and production of toxin and melanin in the tangerine pathotype of Alternaria alternata. Mol Plant Pathol 12:653–655
Yang G, Cao X, Ma G, Qin L, Wu Y, Lin J, Ye P, Yuan J, Wang S (2020a) MAPK pathway-related tyrosine phosphatases regulate development, secondary metabolism and pathogenicity in fungus Aspergillus flavus. Environ Microbiol 22:5232–5247
Yang Q, Song L, Miao Z, Su M, Liang W, He Y (2020b) Acetylation of BcHpt lysine 161 regulates Botrytis cinerea sensitivity to fungicides, multistress adaptation and virulence. Front Microbiol 10:2965
Yin WX, Adnan M, Shang Y, Lin Y, Luo CX (2018) Sensitivity of Botrytis cinerea from nectarine/cherry in China to six fungicides and characterization of resistant isolates. Plant Dis 102:2578–2585
Yong HY, Bakar FD, Illias RM, Mahadi NM, Murad AM (2013) Cgl-SLT2 is required for appressorium formation, sporulation and pathogenicity in Colletotrichum gloeosporioide. Braz J Microbiol 44:1241–1250
Yoshimi A, Kojima K, Takano Y, Tanaka C (2005) Group III histidine kinase is a positive regulator of Hog1-type mitogen-activated protein kinase in filamentous fungi. Eukaryot Cell 4:1820–1828
Yu PL, Chen LH, Chung KR (2016) How the pathogenic fungus Alternaria alternata copes with stress via the response regulators SSK1 and SHO1. PLoS One 11:e0149153
Zhang Y, Lamm R, Pillonel C, Lam S, Xu JR (2002) Osmoregulation and fungicide resistance: the Neurospora crassa OS-2 gene encodes a HOG1 mitogen-activated protein kinase homologue. Appl Environ Microbiol 68:532–538
Zhang Y, Choi YE, Zou X, Xu JR (2011) The FvMK1 mitogen-activated protein kinase gene regulates conidiation, pathogenesis, and fumonisin production in Fusarium verticillioides. Fungal Genet Biol 48:71–79
Zhang C, Wang J, Tao H, Dang X, Wang Y, Chen M, Zhai Z, Yu W, Xu L, Shim WB (2015) FvBck1, a component of cell wall integrity MAP kinase pathway, is required for virulence and oxidative stress response in sugarcane Pokkah Boeng pathogen. Front Microbiol 6:01096
Zhang S, Jiang C, Zhang Q, Qi L, Li C, Xu JR (2016) Thioredoxins are involved in the activation of the PMK1 MAP kinase pathway during appressorium penetration and invasive growth in Magnaporthe oryzae. Environ Microbiol 18:3768–3784
Zhang X, Liu WD, Li Y, Li GT, Xu JR (2017) Expression of HopAI interferes with MAP kinase signalling in Magnaporthe oryzae. Environ Microbiol 19:4190–4204
Zhang F, Huang L, Deng J, Tan C, Geng L, Liao Y, Yuan J, Wang S (2020) A cell wall integrity-related MAP kinase kinase kinase AflBck1 is required for growth and virulence in fungus Aspergillus flavus. Mol Plant-Microbe Interact 33:680–692
Zhang X, Wang Z, Jiang C, Xu JR (2021) Regulation of biotic interactions and responses to abiotic stresses by MAP kinase pathways in plant pathogenic fungi. Stress Biol 1:1–19
Zhao X, Xu JR (2007) A highly conserved MAPK-docking site in Mst7 is essential for Pmk1 activation in Magnaporthe grisea. Mol Microbiol 63:881–894
Zheng D, Zhang S, Zhou X, Wang C, Xiang P, Zheng Q, Xu JR (2012) The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum. PLoS One 7:e49495
Zheng Q, Hou R, Ma J, Wu Z, Wang G, Wang C, Xu JR (2013) The MAT locus genes play different roles in sexual reproduction and pathogenesis in Fusarium graminearum. PLoS One 8:e66980
Zheng D, Wang Y, Han Y, Xu JR, Wang C (2016) UvHOG1 is important for hyphal growth and stress responses in the rice false smut fungus Ustilaginoidea virens. Sci Rep 6:1–12
Zhou X, Liu W, Wang C, Xu Q, Wang Y, Ding S, Xu JR (2011) A MADS-box transcription factor MoMcm1 is required for male fertility, microconidium production and virulence in Magnaporthe oryzae. Mol Microbiol 80:33–53
Zhou X, Zhang H, Li G, Shaw B, Xu JR (2012) The cyclase-associated protein Cap1 is important for proper regulation of infection-related morphogenesis in Magnaporthe oryzae. PLoS Pathog 8:e1002911
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wang, Z., Zhang, X., Jiang, C., Xu, JR. (2023). Regulation of Plant Infection Processes by MAP Kinase Pathways in Ascomycetous Pathogens. In: Scott, B., Mesarich, C. (eds) Plant Relationships. The Mycota, vol 5. Springer, Cham. https://doi.org/10.1007/978-3-031-16503-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-16503-0_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-16502-3
Online ISBN: 978-3-031-16503-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)