Abstract
Pollution from potentially toxic metalloids such as arsenic is becoming a major concern for living organisms all over the world. Arsenic (As) is a non-essential metalloid in plants that can build up to toxic levels. As-contaminated soil remediation ought to be sustainable, low-cost, and applicable in the most vulnerable low-to-middle income countries. Phytoremediation is an aesthetically appreciable and successful approach that can be used for As decontamination using the best approach(es) and the most promising plant(s). On the other hand, Phytoremediation lacks the requisite speed, and the stress generated by As often can reduce plants’ ability to remediate. To solve these faults, we need to supplement plants’ potential with appropriate contemporary science means, such as microbial treatments and plant genetic modification, in order to reduce As stress and increase As accumulation in phytoremediator plants. According to the literature, integrated techniques like phytobial, constructed wetlands employing As-resistant microorganisms with vegetation activities have not been substantially researched. For As remediation, integrated phytoremediation techniques with practical application and reliability are seen to be the most promising. Further technology improvements would aid in exploring literature review gaps in various techniques, guiding us toward As phytoremediation sustainability and perfection. This chapter describes how arsenic concentrations, speciation, absorption, bioavailability, uptake, transport, phytotoxicity, and arsenic detoxification in plants may all be linked. This chapter aimed to provide insight into recent breakthroughs in phytoremediation technologies for overcoming arsenic poisoning in ecosystems. Aspects such as the current and future use of assisted phytoremediation approaches are also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
20.1 Introduction
Arsenic (As) is a ubiquitous heavy metalloid ranked as 20th most abundant element of the Earth’s crust. Historically, it appears to be an abundantelement on the original Earth's surface, supplying energy to few early life forms but posing a metabolic challenge to others. As Earth cools, it absorbed heavy elements like iron, nickel, sulfur, and As, leaving barely traces on the surface that allowed life to flourish (Olsen et al. 1990; Roy et al. 2015). Later on, periodic volcanic eruptions and weathering from geogenic rock, brought it to the surface. On the other hand, fungicides, pesticides, and herbicides containing heavy metals like As and careless mining activities all contributed to the abnormally high proportion in soils (Masuda 2018). It’s not unexpected that a vast range of living species, including bacteria and plants, have survived in the presence of this lethal As; rather, it’s the result of millions of years of adaption, selection of nature, and evolution (Oremland et al. 2002). Owing to As toxic nature, it is classified as Class I category carcinogenic heavy metal by the International Agency of Research on Cancer (Cohen et al. 2019). Additionally, As is positioned at the top of the most hazardous substances (ATSDR 2019). According to Akinbile et al. (2012), 150 million people are exposed to As contamination throughout the world. Long-term exposure to sub-acute levels of As poisoning causes arsenicosis, which varies in severity from skin lesions to neurological disorders, cancer, and even mortality (Ozturk et al. 2021; Rahaman et al. 2021). Soil and water both contain very low amounts of arsenic which is responsible for As chronic exposure (Smedley et al. 2002), has evolved As tolerance or detoxifying systems in most, if not all, living species (Rosen 2002), including humans (Apata et al. 2020). Exposure to As has been exacerbated bythe use of contaminated groundwater to irrigate staple food crops like rice and wheat (Smedley et al. 2002, Rahman et al. 2011). Long-term irrigation with As-contaminated water causes As to build up in the soil (Gillispie et al. 2015). Abandoned mines also contaminate nearby agricultural soils with As (Kim et al. 2005; Susaya et al. 2010). Phytoremediation is cost-effective, socially acceptable, and environment friendly compared to conventional methods; that’s way got the attention of researchers to be used as a potential method of As remediation and revegetation of As contaminated land (Ali et al. 2013; Chatterjee et al. 2013; Irfan et al. 2022).
20.2 Origin and Occurrence of Arsenic
Arsenic is considered the 20th most plentiful component in the amount of 5 mg/kg (Garelick et al. 2008) as well an undyed, inodorous and unflavoured toxic substance present in the lithosphere (Katsoyiannis et al. 2006). In nature, it exists in the combined form of minerals such as Realgar (AsS), Orpiment (As2S3), and Arsenopyrite (FeAsS) (Magalhaes 2002). After weathering process, particles of arsenic combine with rain droplets, and through this pathway, arsenic penetrates into aquifers. It is to be noted that aquifers of some Asian and American countries have ahigher amount of arsenic (Melkonian et al. 2011). As it exists naturally, but the elevated use of arsenic in human activities isthe major cause of increasing its concentration in nature (Taylor et al. 2003; Raj, 2019),which badly effecting flora and fauna in multiple ways (Smedley and Kinniburgh 2002; Irshad et al. 2021). Generally, arsenic is present in 4 major forms from which the amount of arsenic (0 oxidation state) and arsenide (-3 oxidation state) is not constant in the soil (Xie and Haung 1998). As they are very poisonous in nature, but when they penetrate the nutrient cycle and convert into low poisonous forms such as MMA (Monomethyl arsine) DMA (Dimethyl arsine) and TMA (Trimethyl arsine) (Edmonds and Francesconi 1988; Lee and Wen 2019). Arsenate is majorly present in areas with higher availability of free oxygen, but the elevated concentration of arsenite exists in an oxygen-deficient (free) environment (Abedin et al. 2002; SignesPastor et al. 2007).
20.3 Historical Usage of Arsenic
In 1250 CE, arsenic was primarily identified. In the past, it was used as a medication for dermatosis, embellishment (Shrivastava et al. 2015) and pest killer chemicals in crops (Smith et al. 2003). Due to the elevated dissolving capacity and arsenic’s fast-poisoning ability, it has been utilized to form chemicals to kill rodents, insects, and herbs. In earlier times, the food of farm animals also contained arsenic as an additional supplement in their food, but after the twentieth century, its use was legally prohibited (Jones 2007). Among the duration of 55 years, from 1900–1955, arsenic was also utilized in tick management that affected cows and buffaloes (Rahman et al. 2019). In the past, arsenic was also considered as a source of causing impurities and pollutants in food stuff materials. The outbreak of Manchester in 1900 occurred by the utilization of beer that was poisoned by arsenic (Phillips and French 1998). Furthermore, the severe Japan epidemic of 1956 happened because of arsenic toxicity in soya sauce (Mizuta et al. 1956).
20.4 Arsenic Phytoremediation
Phytoremediation is one of its kind in green abetment technology. During the process of phytoremediation, soil fertility increases and replenishes soil microbes (Yan et al. 2020a, b). There are various kinds of phytoremediation, such as phytoextraction, phytostabilization, and phytovolatilization; which can be utilized for As removal from soil based on the ground condition, suitability of option, and the objective of the remediation (Guarino et al. 2020, Kowitwiwat and Sampanpanish 2020, Wei et al. 2020). Recent phytoremediation studies to treat As polluted soil are summarized in Table 20.1. These studies revealed that plant suitability for phytoremediation is highly dependent on translocation and bioaccumulation factors. Plants with translocation and bioaccumulation factors greater than one are considered ideal for phytoextraction because they can accumulate high concentrations of As in their above-ground parts (Mateoet al., 2019). Plants with less than one translocation and bioaccumulation factor, on the other hand, cannot uptake and store higher As concentrations in above-ground parts, making them inefficient for phytoextraction but potentially useful for phytostabilization (Shackira and Puthur 2019).
Using commercially viable plants in phytoremediation also makes it practical for farmers (Ali et al. 2013; Irfan et al. 2022). Plants having high biomass, fast growth rate, and high shoot As accumulation are suitable for phytoremediation (Ye-Tao et al. 2012). However, it has proven a challenge to discover all three traits in one plant for the scientists. Some plants with high As accumulation capacity in shoots areshort-lived and have poor biomass, whereas others have high biomass but low As accumulation efficiency (Chatterjee et al. 2013). Further, several economically beneficial plants having high biomass suffer from As toxicity and cannot develop to their full potential. To overcome such obstacles optimal combination of physicochemical and biological technologies for successful sustained rehabilitation of polluted regions. To address such issues, integrated approaches like microbe-assisted phytoremediation have been applied to boost plants’ development and biomass and enhance plant As accumulation efficiency (Mesa et al. 2017). Nanoparticles have become an accepted strategy forthe reclamationof degraded ecosystems (Zuverza-Mena et al. 2017; Ranjan et al. 2021). The idea of nano-phytoremediation technology has been developed to remove toxins from soil/water, integrating nanotechnology and phytoremediation (Srivastav et al. 2018; Liu et al. 2020; Usman et al. 2020). As phytoremediation, there are numerous ways that may be applied strategically to cleanse polluted environments.
20.4.1 Phytoextraction
It is the simplest and most appropriate method in which plants are used to eliminate the pollutants from soil and water samples. In this process, the roots of plants consume the pollutants and transfer them to the leaves, but a little proportion of the pollutants can eliminate from soil after reaping the crops. This process occurred in “hyperaccumulators” plants (Nedjimi 2021). The best features of hyperaccumulators include higher biomass content on the ground surface, a great production ratio, effective transportation of materials, and they are very simple to grow and reap. Generally, these plants assemble metals in tissues and donot generate poisonous circumstances, and the plants having deep roots become suitable for greater consumption of pollutants (Bhargava et al. 2012). Usually, arsenic is restricted to certain locations in plants such as epidermis, mesophyll, and vascular tissues (especially xylem) (Vithanage et al. 2012). The shoots of naturally occurring plants have a great influence on the rate of photosynthesis and also on the capability of producing flowers. Therefore, these natural plants accumulate metals in their root system and inhibit the movement of metals towards the shoot system. It is noticed that this feature is not present in hyperaccumulator plants. Therefore, they are considered suitable plants for phytoextraction. Some hyperaccumulators are Hydrilla verticilata, Vallisnerlaneotropicals (Chen et al. 2015; Li et al. 2018). Another difference is hyperaccumulator plants have no elevated biomass.
On the other hand, naturally occurring plants have elevated biomass. Yet they donot possess the higher accumulation of selected metal, but they are able to produce assuring consequences such as Brassica juncea (Niazi et al. 2017). When the pollutants are diminished in a definite range from the soil, harvesting starts. After harvesting, the plants are carefully discarded in the form of contaminated pollutants or get smelted to rehabilitate metals. By determining some components such as translocation factor (TF), enrichment co-efficient of the shoot (ECS), and bioaccumulation factor (BF) the transportation ability of the plant can evaluate. Furthermore, by determining the quantity of arsenic in shoots transferred from roots, the phytoremediation capability of plants can be determined (Rahman et al. 2011).
20.4.1.1 Translocation Factor (TF)
It evaluates the plant’s capability to transfer the metals in shoots from roots. It is the proportion of the amount of component in the shoot (mg.g−1) to the amount of identical component in the root (mg.g−1). Hyperaccumulator plants have anelevated rate of translocation factor, but generally, the rate of translocation factor in normal plants is not greater than 1 (Francesconi et al. 2002).
20.4.1.2 Enrichment Co-efficient of Shoot (ECS)
It can utilize to evaluate the consumption-ability of metals in plants Bienert. It is the proportion of the amount of metal in the shoot to the amount of the same metal in the soil. Once the value of ECS in the plant is higher than one, this exhibits the shifting capability of the plant to shift metals towards shoots, mainly in vacuoles (Elshamy et al. 2019).
20.4.1.3 Bioaccumulation Factor (BF)
It can utilize to assess the consumption ability of heavy metals in roots from the soil. It is the amount of components accumulated in roots (mg.g-1) to the amount of identical components in the soil (mg.g−1). Grasses such as barnyard grass (Sultana and Kobayashi 2011) and rice cutgrass (Klaber et al. 2014) are suggested for trees because they possess greater biomass and production ratio and are much more suitable for unfavorable conditions (Ali et al. 2013). It is necessary to adopt protective measures to inhibit the attack of consumers as they become the cause of the entrance of pollutants into the nutrient cycle.
20.4.2 Phytostabilization
It is an effective controlling method in regions near mines. It maintains the pollutants also diminish the accessibility and motility of the pollutants. Therefore, it helps in diminishing ex-situ pollution (Shrivastava et al. 2015). Arsenate reductase is an enzyme excreted by plants that maintain the pollutant (arsenic) via sorption, complexation/metal valence reduction or precipitation it to low poisonous shape (Thakur et al. 2020). Therefore, that procedure is not indicated to produce insignificant debris, as it elevates soil productivity. There should be extended roots in plants that become suited for phytostabilization and give adequate vegetation to the soil, have resistance to the pollutants, and restricted the pollutant in roots and soil, diminishing accessibility of arsenic and erosion (Gonzaga et al. 2006).
Furthermore, there should be less contaminant assemblage capacity in shoots of plants thatare selected for phytostabilization, such as Eucalyptus and Arundo donax L. because if the pollutant assembles in the shoots of other plants then they penetrate nutrient cycle (Bolan et al. 2011; Mirza et al. 2011). The plants of the Eucalyptus family have wood;when they face the pollutant, these plants accumulate less concentration of metals than the other plants. Due to the presence of terpenes and phenolics in shoots of these plants, they are infrequently occupied by organisms and inhibit the entrance of pollutants in the nutrient cycle (King et al. 2008). It is also noticed that the motility of heavy metals is constricted in phytostabilization and is not considered an enduring solution tothat issue (Ali et al. 2013). Therefore, the location should be regularly observedto make sure that all the circumstances are controlled.
20.4.3 Phytofiltration
It is utilized to purify the water (the surface, below the surface, waste, etc.) having less pollutants (Garg et al. 2011; Shrivastava et al. 2015). This procedure shows that the pollutants are consumed by plants, lowering the quantity of contaminants in water, i.e., purifying heavy metals from water to roots (Mykolenko et al. 2013). That is why the plants with great absorbing capacity are selected. Micranthemumumbrosum is considered a powerful assembler of metals because it accumulates 1000 mg As g-1 in its shoot parts and lowers the quantity of arsenic in a solution of 10 folds (Islam et al. 2015). It has 3 kinds that depend on the components of the plant utilized for that method. Rhizofiltration (roots), blastofiltration (seedling) and caulofiltration (shoots) (Ali et al. 2013). The more productive plants, ineffective metal carriers (carry metal towards shoot, causing rhizofiltrationto become inefficient) and extended roots should be selected, such as Eucalyptus globules, Faidherbia albida etc. (Anawar et al. 2008). When the procedure reaches its end point, roots harvesting occurs and then desiccates. Metals can remove by acid analysis or ignite at unhealthy debris locations (Dushenkov et al. 1995). Therefore, this method is considered as a productive, environmentally sound procedure to decrease the pollution in naturally occurring marshlands and waterway zones. Because of the great metal assimilation capability in Lemnagibba is used to remove the contaminated metals from the water coming from mining areas (Anawar et al. 2008).
20.4.4 Phytovoltalization
This procedure exhibits the consumption of pollutants from the soil and its discharge in fewer amounts into the atmosphere in a gas form via transpiration (Ranjan et al. 2020). It is noticed that poisonous contaminants got weakened or probably transformed into a rarely poisonous type in the environment (Guarino et al. 2020). Direct and indirect are two kinds of this procedure. Direct includes vapourization from shoots or roots, but indirect has underground vapourization because of the actions of roots (Pandey et al. 2018). It is considered as acontentious type of phytoremediation. It exhibits the shifting movement from one form to another and can return back into its actual form. Therefore, it shows low or no command of the mobility of pollutants (Bolan et al. 2011). Another benefit of this procedure is that no physical work or stress is required to shift or eliminate plants’ polluted components, andit needs low controlling effort (Heaton et al. 1998). Generally, arsenic is used in the shape of trimethylarsine in phytovolatilization, the concluding outcome of the methylation route where arsenic is passed from the methylation process and converted into dimethylarsenic acid then to trimethylarsenine oxide, which faces a reduction process and producesthe final product called trimethylarsine (Mirza et al. 2011). In phytovolatilization, P. vittatacan be utilized to discharge heavy metals into the atmosphere (Sakakibara et al. 2007).
20.5 Consumption and Transportation of Arsenic in Plants
Generally, the roots of plants consume As. Mostly the accessibility of As in four major states in plants such as As (III), As (V), MMA and DMA. In the soil, these states are developed, and at the same time, their consumption occurs particularly by the roots through various routes and carriers. The consumption process shows involvement in the use of phosphate carriers required in the route of phosphate transportation. Due to the structural similarity of As (V) with phosphate, the entrance of As (V) becomes possible in roots.
20.5.1 Transportation of Arsenic in Plants by Phosphate Carriers
Different scientists explained their work that phosphate carriers have great importance in promotingthe transportation and bearing capacity of arsenic in plants (Fig. 20.2). Cao et al. (2019) examined the role of the phosphate carrier named Pteris vittata phosphate transporter (PvPht1;3) in increasing the adaptation and transportation of arsenic in shoots of Nicotiana tobaccum (grow in both terrestrial and aquatic mediums). Research shows the proof of arsenic consumption and addition in Oryza sativa through phosphate carriers. Generally, OsPT1, OsPT4, and OsPT8 (genes) are used to increase response to the stimulus in rice plants’ root and shoot system and showed strong attraction for As (V). Sun et al. (2019) examined the upregulation of PvPht1;4 decreased transportation and poisonous effects of arsenic in tobacco plants. The tobacco plants using that gene assimilated less arsenic concentration of arsenic upto 37–55% in shoots than other plants.
Schematic representation of transporter-assisted arsenic acquisition and transport in plant tissues. a Upregulation of phosphate (Pi) transporters (Pht1; 1, Pht1; 2, Pht1; 3) mediate As(V) uptake inside the roots cell under aerobic conditions. a1 Vacuolar phosphate transporter (VPT1) contributes towards vacuolar phosphate sequestration and is associated with As(V) quenching inside the vacuole, hence confer plant tolerance towards arsenic toxicity. a2 The cytoplasmic enzyme arsenate reductase reduces As(V) to As(III) and provides resistance to the plants against As(V) toxicity. b Overexpression of aquaporines (AQPs) like nodulin 26-like intrinsic proteins (NIPs) (Nip1; 1, Nip3; 1, Nip5; 1, Nip6; 1) induce As(III) uptake inside the root cell under anaerobic conditions. b1 Two ATP binding cassette transporters (ABCC1/ABCC2) are involved in the transport of As(III)-PC complex inside the vacuole and a member of the same sub-family transporter ABCC7 mediate its transport to shoots via xylem. b2 As(III) from root cell is exported to the xylem by the silicon transporter (Lsi2), resulting in root to shoot transport of As(III). c Organic species monometylarsonic acid (MMA) and dimethylarsinic acid (DMA) are taken up by plant roots via silicon transporter (Lsi1) and is transported to aerial parts via xylem. d Arsenic as MMA/DMA or As(III) is transported to shoots by expression of PTR7 and NIPs. e To phloem, arsenic is transported by PTR7 as DMA or As(III). f Arsenic transport as DMA or As(III) from phloem to the grain is mediated by (MATEs) MATE1/2 and a long-distance transporter PTR7.
20.5.2 Transport of Arsenic by Aquaporins
Aquaporins give definite functions in As acquirement of plants. The groups of aquaporin proteins determined the consumption of As (III) by plants. Kamiya et al. (2009) observed that nodulin 26-like intrinsic proteins (NIPs) belonged to aquaporin proteins and participated in the consumption of arsenic (III). NIPs are classified into three types due to their porous configuration. Ma et al. (2008) revealed the accessibility of NIP 1 protein to water, lactic acid, and glycerol. Mitani et al. (2008) showed the participation of NIP II proteins in the transportation of greater solutes such as formamide, boric acid, and urea due to their big porous structure. Still, NIP III proteins are accessible to silicic acid. Protein carriers related to NIP family showed involvement in assimilation and transportation of arsenic in Oryza sativa plants. Sun et al. (2018) recognized the overexpression of two NIP group carriers OsNIP1:1 and OsNIP3:3 decreased the amount and transportation of As (III) in shoots of rice plants. The upregulation of NIP genes caused the effluence of As (III) from the stele, constricted As’ storage in vascular tissues (xylem) and its adaptation in rice plants. Xu et al. (2015) reported the analytical part given by 9 NIP group carriers in the accession and transportation of As in shoots from the roots of Arabidopsis thaliana. The overexpression of NIP3:1 in the roots of variants increased the consumption of As (III) in roots, and transfer to aerial components of plants showed powerful bearing capacity against the poisonous effect of As (III). Kamiya et al. (2009) observed the involvement of NIP1; 1 in the susceptibility of As (III) in plant tissues. He et al. (2016) carried out a study and developed a new protein PvT1P4; 1 from Pteris vittata showed significant As (III) consumption. It is a protein carrier restricted toplasma membrane.
20.5.3 Involvement of Silicon Carriers in Transportation of Arsenic
Two silicon carriers Lsi1 and Lsi2 of NIP III group, took part in the consumption and transportation of As III in plants. Lsi1 is present in the plasma membrane (roots) and participated in consuming As III in Oryza sativa. The overexpression of Lsi1 in Xenopus laevis oocytes increased the accession of As III (Yamaji et al. 2015). Ma et al. (2008) observed that Lsi2 variants work more efficiently than Lsi1 variants in lowering accession and transportation of As III in rice plants.
20.5.4 Consumption and Transportation of Methylated Arsenic Species in Plants
The use of arsenic in chemicals for killing pests, herbs, and methylation arsenic by microorganisms has been mixed in minute amounts of As group such as MMA, DMA inthe soil (Chen et al. 2020; Rahman et al. 2019). In plants, anelevated amount of methylated arsenic has been identified. The plants consumed methylated arsenic slowly as compared to inorganic arsenic. However, DMA is wholly transferred to the sexual and above-ground components of the plants (Tang et al. 2016; Zhu et al. 2017). The carriers of organic compounds caused the transportation of DMA to sexual parts of the plants. It is observed that rice granules have an elevated amount of DMA than inorganic arsenic (Yan et al. 2020a, b). OsPTR7 is a putative peptide transporter in Oryza sativa. Successive variants of OsPTR7 considerably reduced the transportation of DMA in shoots from roots than the brown rice plants.
20.5.5 Consumption and Transportation of Thioarsenate Species in Plants
The structure of thioarsenates is related to As (V) and derived from As (III) in sulfate-reducing circumstances. In the case of monothioarsenates, μM arsenic is more poisonous than As (V) and becomes less poisonous than As (III). Planer-Friedrich et al. (2017) elevated the toxic effect and bearing capacity of arsenic is influenced by monothioarsenate in Arabidopsis thalliana. After studies, the writers discovered that adaptation of arsenic in the roots was less on adding monothioarsenate, possibly because of increased levels of As in roots. Inspite the As transportation in shoots from roots was higher for monothioarsenate than arsenate. Introduction to monothioarsenates brought comparatively elevated adaptation to phytochelatins in the ferocious variants (Col-o), thus presenting Arabidopsis thaliana to fight arsenic stimulated toxic effects (Planer-Friedrich et al. (2017). The latest research performed by Wang et al. (2020) shows that oxygen-deficient soil growth at various pHs caused thiolation of arsenic polluted soils. Soils with neutral sulphur (pH greater than 6.5) demonstrated the supremacy of thioarsenates. In addition, soils having methylated oxyarsenates (pH lower than 7.0) showed the existence of methyl thioarsenates. It is highlighted that arsenic thiolation and surplus sulphate in the soil exhibited the same results, but increased amounts of soluble Fe in the soil reduce arsenic thiolation (Wang et al. 2020). On the other hand, the translocation of inorganic and methylated thioarsenates interceded by carriers was not recognized until now. Future endeavors in this area are definitely approaching the scientists regarding the probable part of protein carriers in the attainment and uptake of thioarsenates in the plant tissues.
20.5.6 Process of Arsenic Decontamination in Plants
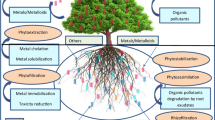
The secure method forthe production of reactive oxygen species (ROS) because of the existence of arsenic is the generation of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), gluthathione reductase (GR) and ascorbate peroxidase (APX) to maintain radicals without charge. In plants the generation of some osmolites such as proline (Sayantan 2017), glyanebetaine and mannitol are noted below oxidation conditions because of conservation and durability (Abbas et al. 2018). The main route of antioxidant protection to clean H2O2 is the Ascorbate–Glutathione route. Four enzymes such as ascorbate peroxidise, monodehydroascorbate reductase, dehydroascorbate reductase, and gluththione reductase have a major part in cleaning ROS in that route and conserving the plant from numerous abiotic conditions (Hasanuzzaman et al. 2019). The other method involves the arsenic complexity with ligands. When arsenic penetrates the plant after the reduction process, As (V) converts into As (III) by using the arsenate reductase enzyme (Zhao et al. 2003). Arsenite is considered the causative agent affecting metabolism in the cytoplasm, so decontamination takes place. All of this is noted in plants such as H. Verticillata, Brassica juncea, tomato, and rice (Chen et al. 2015).
20.6 Integrated Approaches for Enhanced Phytoremediation
20.6.1 Phytobial Remediation
Bioremediation and phytoremediation are combined in phytobial remediation techniques to abate pollution. The micro soil biota helps plants in numerous ways to improve health and productivity by regulating nutrients (Mehmood et al. 2021c; Glick 2012), enhancing the status of growth limiting factors such as nitrogen and phosphorus, and to improve soil enzyme activities (van der Heijden et al. 2008, Ullah et al. 2015). The microbiota in the rhizosphere helps increase the plant biomass and raise bioavailability of As to plant (Khan 2005; Alka et al. 2020; Srivastava et al. 2021). Therefore, it is essential to select such species of microbes that could enhance plant productivity andAs phytoremediation to achieve good results. The functions of the plant-bacteria symbiotic association are phytoimmobilization, rhizofiltration, phytostabilization, chelation, As solubilization, and phytoextraction. Several studies have shown that plants and their root communities work better together than plants or bacteria alone in soil and water systems with high levels (Mehmood et al. 2021a, 2022a; Irshad et al. 2021).
20.6.2 Transgenic Phyto and Phytobial Remediation
Genetically modified organisms (GMO) based technology has the potential to augment the innate bioremediation capability of plants and microorganisms in such as a way to enhance symbiosis betweenplant and soil microbiota for better As bioremediation (Guarino et al. 2020). Transgenic plants are developed either with increased capacity to extract As from soil or stable food crops with increased ability to restrict As absorption from soil providing new hope for As phytoremediation. Genes encoding As absorption channels and transporters have been identified in As accumulator and hyperaccumulator plants (Roy et al. 2015). As a result, preventing the uptake of As(V)/As(III) via roots is possible by inactivating or deleting the genes encoding several phosphate transporter variants, NIP aquaporins, and Lsi2-like carrier proteins (Roy et al. 2015).
The detoxification process is indicated when ligands (glutathione (GSH), phytochelatins (PCs) or metallothioneins (MTs)) are integrated into complexes, they are sequestered or compartmentalized in trichomes or vacuoles which are dedicated tissues such for this task. Consequently, genetic engineering has increased the capacity of As accumulating plant to either sequester As in its roots via rhizofiltration or improved hyperaccumulation in shoots/fronds (phytoextraction) phytovolatilization pathways. Enhanced root surface area and plant biomass may be obtained by developing such phenotypes having more hairy roots (Eapen et al. 2003).
Metal tolerance could be promoted by activating oxidative stress-related genes. Targeting C synthesis and transporter genes may promote enhanced translocations into shoots and higher vacuole storage (Cherian et al. 2005). Genes involved in As methylation might possibly be a target. In transgenic rice plants, as has been methylated and volatilized. Novel biotechnological approaches, such as the development of transgenic plants, not only have the ability to phytoextract and accumulate large amounts of As but also possess toxin or conditional lethality genes which could be resisting Asa transfer to the food chain by distracting herbivores and resisting pest attack, can alleviate concerns about food chain contamination (Eapen et al. 2003; Zhao et al. 2009).
20.6.3 Phytoaugmentation (Addition of Abiotic Factors)
Nature’s attenuation process may be accelerated by introducing various biotic (microbes) and abiotic (addition of various chemicals; bioaugmentation). The As-immobilizing microbes and abiotic chemicals may be put into the soil to achieve phytoimmobilization. To achieve long-term As immobilization by solid-phase sorption, acidic and oxidizing conditions must be maintained (Adriano et al. 2004). The pH buffering agents should be used to improve and stabilize As sorption and inhibit As remobilization. If Fe salts, such as FeCl2 and FeSO4, are combined with H2O2, they may precipitate As from groundwater. The presence of H2O2 maintains as an oxidizing environment for As(V) sorption bypromoting the oxidation of As(III) species to As(V) species (Wang et al. 2006). It is feasible to use such oxidant types, ensuring that subterranean soil and water are adequately oxygenated. It is possible to increase Fe bioavailability by using naturally abundant soil organic compounds such as humic acids. (Adriano et al. 2004).
Researchers studied the impact of several soil amendments on the plant P. vittata (Cao et al. 2003; Paz-Ferreiro et al. 2014). Calcium (Ca2 + ), selenium (S), and nitrogen (N) have been demonstrated to increase P. vittataAs accumulation (Liao et al. 2007; Paz-Ferreiro et al. 2014). According to Huang et al. (2012) adding organic matter to the soil of a rice field significantly boosted the methylation and volatilization of As from the soil. It has been shown that using various kinds of nutrients and microbial growth-enhancing agents, such as compost and biochar, lowers As stress in plants (Mehmood et al. 2017, 2021b; Irshad et al. 2021).
20.6.4 Nano Phytoremediation
Nano-phytoremediation has recently emerged as a possible approach for improving plants’ capacity to grow in a polluted and stressed environment while accumulating arsenic in plants (Fig. 20.3). The development of efficient and environmentally friendly nanoparticles for the successful treatment of widespread contamination by hazardous metalloids has gotten a lot of attention (Ganie et al. 2021). Nanoparticles (NPs) have the potential to improve plant stress tolerance to promote phytoremediation and minimize toxicity (Srivastava et al. 2021; Mehmood et al. 2022b). Nanophytoremediation, which treats contaminated soils and water using plants with high NPs/metal absorption efficiency, has the potential to be an efficient alternative to As phytoremediation (Gul et al. 2022).

Copyright 2022 MDPI. Reproduced from (Srivastava et al. 2021)
The use of nanoparticles through foliar spray and via roots can effectively enhance the tolerance of plants to arsenic.
Nanoparticles, according to studies, may be utilized to manage polluted agricultural areas and stimulate plant growth and development. Nanostructured silicon dioxide has been shown to be a feasible agent for enhancing the phytoremediation process and achieving the necessary results (Bao-Shan et al. 2004). Nanoparticles of aluminium oxide (nAl2O3) were shown to have no deleterious effects on Arabidopsis thaliana when tested at doses of up to 4000 mg/L (Lee et al. 2010). The inclusion of nanoscale zero-valent iron aided the phytoremediation process (Song et al. 2019). It was discovered that nano-TiO2 reduced As accumulation in rice by 40–90% when administered at a concentration of 1000 mg/L (Wu et al. 2021). Adding zinc oxide to rice seedlings improved rice seedling development, decreased As buildup in roots and shoots, and increased phytochelatin levels (Yan et al. 2021). Nano-phytoremediation advances have the potential to pave the way for the development of safe, economical, and environmentally sustainable As phytoremediation technologies for awide range of environmental settings (Zhou et al. 2020).
20.6.5 Phytosuctionpartition
Phytosuction partitioning is a newly designed and improved phytoremediation technology (Katoh et al. 2016a). According to several research investigations phytoremediation of hazardous metal and/or metalloid polluted soils is both cost-effective and environmentally acceptable (Ali et al. 2013; Chatterjee et al. 2013; Jha et al. 2022). However, the PE technique requires longer time periods, making it unworkable even with hyperaccumulators. This approach employs heavy metal(oid)-contaminated bottom soil and heavy metal-free planting soil. A layer of immobilisation material separates these two kinds of soils. Plants are cultivated by growing them in soil (devoid of heavy metals) (Kabiraj et al. 2022). Following this method involves spraying a chemical like ferrihydrite over contaminated soil and then growing plants on top of it, potentially immobilizing the target hazardous metal and/or metalloid. Roots sucking up water attract metals and metalloids, causing them to get immobilized. Arsenic may be removed from ferrihydrite-polluted soil using a novel technology that employs ferrihydrite’s immobilizing agent. When the phytosuction partitioning approach was compared to classic PE, it was discovered that the phytosuction partitioning method produced superior ratios.
Furthermore, as compared to the PE approach, the removal ratios were greater at shallow soil levels of up to 0.25 cm (Arita and Katoh 2019). This approach has been shown to remove 8–25 times more lead (Pb) and 69–533 times more antimony (Sb) from the environment than PE (Katoh et al. 2016b). It takes less time than the PE technique since it does not need root systems to absorb metals or metalloids. According to our findings, one of the most important aspects influencing phytosuction efficiency is the mobility of the metal/metalloid under consideration.
20.6.6 Electrokinesis Assisted Phytoremediation
Electrokinetic remediation has sparked considerable interest as a method of boosting plant absorption of pollutants (inorganic and organic), whereas the majority of research which is focused on combining electrokinetics assisted phytoremediation has concentrated on improving phytoextraction of heavy metals (Gomes et al. 2012). The electric field makes contaminants mobile, thus increasing bioavailability, which is evident by improved plant growth (Cameselle et al. 2013). Low-intensity direct current is transferred between two electrodes implanted vertically into the soil without causing structural damage to the soil. Organic and inorganic molecules are separated using an electric current. Water and electromigration are two routes for heavy metal cations to reach the cathode. Electromigration transports anions and other small-charged particles towards the anode. Applied electric field to the soil regulates the movement of pore fluid, ions, and colloids via electroosmosis, electromigration and electrophoresis, respectively, allowing for higher metal buildup in the rhizosphere interstitial fluid and absorption by the plant (Chirakkara et al. 2016).
Electrokinetics and phytoremediation have shownpromising results in laboratory experiments for heavy metals such as Zn, Pb, Cd, and As (Cameselle et al. 2013). It is reported that the efficiency of phytoremediation may be increased if the soil is kept from becoming too acidic or basic by modulating the electric field. Soil with acidic or alkaline conditions hasa detrimental impact on the metabolism of plants, growth, and biomass yield. Keeping the electric current intensity low will limit the extent of the electrolysis of water and, as a result, the fast changes in pH in the region surrounding the electrodes. Two possible methods for reducing pH variations in soil include periodic polarity inversion in the case of DC or the application of AC current (Aboughalma et al. 2008). The other main issue of the application of electric field in phytoremediation is elevated exposure of heavy metals to the plants, which may exacerbate plant stress. To solve this issue, researchers have suggested to use plants that can withstand elevated metal concentrations (i.e., hyperaccumulator plants with short growth cycles) in electrokinetic assisted phytoremediation (Cameselle et al. 2013). Even yet, further large-scale testing is required to establish if this technology can be employed in the future as a low-cost remediation option. Combining many methods proved to be more successful than using just one.
20.6.7 Co-cultivation and Intercropping
In agriculture, intercropping is a typical approach for improving soil conditions for plant development and soil enzyme activity and nutrient availability by cultivating two different crops together to improve soil conditions (Srivastava et al. 2021). The reduction of As contamination in field and to reduce the stress of As on sensitive and non-accumulator plants Intercropping is utilized. The P.vittata (As hyperaccumulator) is cultivated with either As sensitive or a non-As accumulator plants. It is investigated that in the intercropping of two commercially important plants; P.vittata and Panax notoginseng the rhizospheric concentrations of As for Panax plants were lowered compared to Pteris (Lin et al. 2015). When P.vittata was intercropped with Morus alba; Pteris has accumulated As, leaving behind lower As levels for Morus alba plants (Wan et al. 2017).
The P.vittata intercropping with maize (Zea mays) plant is studied in coordinate and malposed intercropping settings. When the rate of As accumulation in P.vittata was compared in both settings, the As phytoextraction was more in malposed intercropping than in coordinate intercropping. Malposed intercropping had a 2.4-fold higher rate of As removal than coordinated intercropping. (Ma et al. 2018). Ma et al. (2018) also found that following malposed intercropping, maize grains indicated decreased As levels in grains, below the threshold maximum contamination limit. Therefore, intercropping Pteris could achieve promising results with other cash crops/economically essential crops. Intercropping has long been recognized as the ideal technique for remediating soil and using the land for economic advantage (Lin et al. 2015; Srivastava et al. 2021). The P. vittatacocultivation with rice has significantly reduced rice’s As and DMA concentration (Ye et al. 2011).
20.7 Disposal of Plants After Remediation
The aim of phytoremediation cannot be encountered if plants utilized in this process are not accurately discarded or controlled after discharging metals in the atmosphere because of the deposition of metals in the biomass of plant (Ghosh et al. 2005). Phytoremediation becomes a much more environmentally safe method due to the recycling ability of final materials of this process. Composting is known as the secure discharging method of heavy metals. It helps in diminishing the amount of biomass and simple transportation (Mohanty 2016; Newete and Byrne 2016). The main disadvantage is the transportation of poisonous materials from one location to another (Ghosh et al. 2005).
The procedures lime can use to lower the leachability of heavy metals (Vocciante et al. 2019) are known as stabilization procedures. Plants that use such procedures are not able to discard at any place, but they discard in specific locations such as areas near mines. Generally, incineration and generated charcoal are other procedures thatare the causative agents of producing energy for cooking fires (Ghosh et al. 2005). It confirms that biomass is not utilized for producing chemicals forplant growth and food foranimals. As it can pollute the air, thus incineration is not suggested forexecution in uncovered areas. Pyrolysis is another substitute in which biomass is heated in the absence of oxygen between 350 and 650 ℃ (Vocciante et al. 2019).The final materials are pyrolytic fluid oil and coke (Newete and Byrne 2016). In biogasification, methanol (gas) and some liquids are generated, and they are used as an origin of fuel (Monanty 2016). The sorption of methylene blue (dye) can occur by producing biochar (Gong et al. 2018).
20.8 Conclusion and Future Perspective
The conventional As remediation technologies have evident limits, whereas phytoremediation is now a realistic, cost-effective, and trustworthy solution for abating As toxicity. Along with technological and scientific research development, phytoremediation will be made more sustainable and utilized in an excellent manner. To aid comprehension, the following options are stated:
-
(1)
One of the most important criteria for the successful restoration of contaminated areas and polluted fields is proper plant selection. Plant variety and variability increased throughout time, providing a wide range of plant alternatives. Economic plant species that are not edible may be used for safe phytoremediation and revenue production.
-
(2)
The use of plant growth-promoting microbes, nanoparticles, and other integrated approaches have tremendous potential to significantly reduce As pollution in plants and the environment. However, extensive study is needed to fully understand the potential of microbe/nano-assisted phytoremediation to purify As-contaminated soils.
-
(3)
Although the number of microbes associated with plants is growing all the time, more research into the methods and roles of individual genomes and the enzymatic activities involved in Photobiol As cleaningis still needed. In addition, further focus on functional tests is necessary to determine if microbiota boosted with As stress improves the plant.
-
(4)
For practical ramifications, a better knowledge of the processes involved in bacterial As oxidation is required. In addition, the use of genetic engineering in the utilization of As in bioenergy generation and microbial fuel cell applications might bring fresh insights.
-
(5)
Phytoextraction of As by hyperaccumulators is one of the promising techniques, as shown by a number of practical implications. However, the fate of acquired biomass must be considered; composting, pyrolysis, or biogasification may all be viable options.
-
(6)
Currently, no specific advice is available on the design criteria for establishing a big wetland for arsenic removal. Extensive lab, pilot, and field-scale research and geological modeling studies are necessary to develop a constructed wetland.
-
(7)
The crucial need for a successful phytoremediation application is a cost–benefit analysis and computation of landowner economic advantages in the clean-up process.
More research is needed to improve phytoremediation technology, and new technology may be developed to separate heavy metalloids. For example, waste biomass and the reuse of safe biomass with a high quantity of stored carbon for biofuel or feed. Finally, we might claim that phytoremediation cleaning, either alone or in cooperation with others, represents a potential low-cost option. As a result of restoration throughout a large portion of As polluted soil and visually pleasing to the community.
Change history
17 March 2023
Correction to: N. K. Niazi et al. (eds.), Global Arsenic Hazard, Environmental Science and Engineering, https://doi.org/10.1007/978-3-031-16360-9
Abbreviations
- AC:
-
Alternating current
- Ag:
-
Silver
- As:
-
Arsenic
- As (III):
-
Arsenite
- As (V):
-
Arsenate
- AsS:
-
Realgar
- As2S3:
-
Orpiment
- APX:
-
Ascorbate peroxidise
- BF:
-
Bioaccunulation factor
- CAT:
-
Catalase
- Ca:
-
Calcium
- Cd:
-
Cadmium
- DMA:
-
Dimethyl arsine
- DC:
-
Direct current
- ECS:
-
Enrichment co-efficient of shoot
- FeAsS:
-
Arsenopyrite
- Fe:
-
Iron
- GR:
-
Gluthathione reductase
- GMO:
-
Genetically modified organisms
- GSH:
-
Glutathione
- MMA:
-
Monomethyl arsine
- MTs:
-
Metallothioneins
- N:
-
Nitrogen
- NPs:
-
Nanoparticles
- NIPs:
-
Nodulin intrinsic proteins
- PvPht:
-
Pteris vittata
- PCs:
-
Phytochelatins
- Pb:
-
Lead
- ROS:
-
Reactive oxygen species
- S:
-
Selenium
- SOD:
-
Superoxide dismutase
- TMA:
-
Trimethyl arsine
- TF:
-
Translocation factor
- Zn:
-
Zinc
References
Abbas G et al (2018) Int J Environ Res and Public Health 15:59
Abedin MJ et al (2002) Plant Physiol 128:1120–1128
Aboughalma H et al (2008) J Environ Sci Health—Toxic/h 43:926–933
Adriano DC et al (2004) Geoderma 122:121–142
Akinbile CO et al (2012) Trends Appl Sci Res 7:331
Ali H et al (2013) Chemosphere 91:869–881
Alka S et al (2020) Environ Technol Innov 17:100602
Anawar HM et al (2008) Int J Environ Pollut 33:292–312
Ancheta MH et al (2020) J Degraded Mining Lands Manage
Apata M et al (2020) Heredity 124:253–262
Bao-Shan L et al (2004) J for Res 15:138–140
Bhargava A et al (2012) J Environ Manage 105:103–120
Bolan NS et al (2011) Advan Agron 112:145–204
Budzyńska S et al (2017) Microchem J 132:333–340
Cameselle C et al (2013) Chemosphere 93:626–636
Canatto RA et al (2021) Int J Phytorem 23:102–110
Cao X et al (2003) Environ Pollut 126:157–167
Cao Y et al (2019) Environ Sci Technol 53:10636–10644
Chatterjee S et al (2013) Plant-based remediation processes, pp 1–18
Chen G et al (2015) Int J Phytorem 17:249–255
Chen J et al (2020) Front. Environ Sci 8:43
Cherian S et al (2005) Environ Sci Technol 39:9377–9390
Chirakkara RA et al (2016) Rev Environ Sci Bio/Technol 15(2):299–326
Cohen SM et al (2019) Curr Opin Toxicol 14:8–13
de Souza TD et al (2019) Chemosphere 234:402–408
Debiec-Andrzejewska K et al (2020) Environ Pollut 264:114692
DiTusa SF et al (2016) New Phytol 209:762–772
Duan X et al (2017) Resour-Efficient Technol 3:29–36
Dushenkov V et al (1995) Environ Sci Technol 29:1239–1245
Eapen S et al (2003) Environ Res 91:127–133
Edmonds JS et al (1988) Appl Organomet Chem 2:297–302
Elshamy MM et al (2019) Chemosphere 225:678–687
Francesconi K et al (2002) Sci Total Environ 284:27–35
Fresno T et al (2016) Environ Pollut 216:215–222
Gabarrón M et al (2018) J Environ Manage 212:292–300
Ganie AS et al (2021) Chemosphere 275:130065
Garelick H et al (2009) Rev Environ Contam Toxicol 197:17–60
Garg N et al (2011) Environ Chem Lett 9:303–321
Ghosh M et al (2005) Asian J Energy Environ 6:18
Gillispie EC et al (2015) Curr Pollut Rep 1:1–12
Glick BR (2012) Scientifica 2012:963401
Gomes HI et al (2012) Chemosphere 87:1077–1090
Gong X et al (2018) Bioresou Technol 253:64–71
Gonzaga MIS et al (2006) Scientia Agricola 63:90–101
Guarino F et al (2020) Chemosphere 251:126310
Gul et al (2022) Phytoremediation 115–138
Ha NTH et al (2019) Appl Geochem 108:104368
Han Y-H et al (2016) Chemosphere 149:366–372
Hasanuzzaman M et al (2019) Antioxidants 8:384
He Z et al (2016) New Phytol 209:746–761
Heaton ACP et al (1998) J Soil Contamin 7:497–509
Huang H et al (2012) Environ Sci Technol 46:2163–2168
Irfan S et al (2022) Adv Bioremediat Phytorem Sustain Soil Manage, pp 1–16
Irshad S et al (2021) Environ Sci Pollut Res 28:18870–18892
Islam MS et al (2015) Ecotoxicol Environ Saf 112:193–200
Jones FT (2007) Poult Sci 86:2–14
Kabiraj A et al (2022) Curr Microbiol 79:1–15
Kamiya T et al (2009) J Biol Chem 284:2114–2120
Katoh M et al (2016) Clean—Soil Air Water 44:1717–1724
Katsoyiannis IA et al (2006) Rev Environ Health 21:25–42
Khan AG (2005) J Trace Elem Med Biol 18:355–364
Kim J-Y et al (2005) Environ Geochem Health 27:193–203
King DJ et al (2008) Sci Total Environ 406:35–42
Klaber NS et al (2014) Commun Soil Sci Plant Anal 45:810–818
Kofroňová M et al (2019) Ecotoxicol Environ Saf 174:295–304
Kowitwiwat A et al (2020) Heliyon 6:e04552
Lebrun M et al (2020) Chemosphere 244:125397
Lee C-P et al (2019) Mar Chem 209:128–138
Lee CW et al (2010) Environ Toxicol Chem 29:669–675
Lei M et al (2018) Environ Sci Pollut Res 25:124–131
Li B et al (2018) Ecotoxicol Environ Saf 165:224–231
Liao X-Y et al (2007) Int J Phytorem 9:269–280
Lin LY et al (2015) Water Air Soil Pollut 226:113
Liu W et al (2020) Sci Rep 10:1–10
Ma J et al (2018) Chemosphere 194:737–744
Ma JF et al (2008) Proc Natl Acad Sci 105:9931–9935
Ma LQ et al (2001) Nature 409:579–579
Magalhaes MCF (2003) Pure Appl Chem 75:139–139
Masuda H (2018) Prog Earth Planet Sci 5:1–11
Mateo C et al (2019) Environmental chemistry and recent pollution control approaches. p 189
Mehmood M et al (2017) J Geochem Explor 178:83–91
Mehmood M et al (2021a) J Environ Manage 294:113108
Mehmood M et al (2021b) Int J Phytoremediation 23:899–910
Mehmood M et al (2021c) J Environ Manage 292:112654
Mehmood M et al (2022a) Assisted Phytorem, pp 345–370.
Mehmood M et al (2022b) Hazard Waste Manage, pp 185–215
Melkonian S et al (2011) American J Epidemiol 173:183–191
Meng XY et al (2011) New Phytol 191:49–56
Mesa V et al (2017) Appl Environ Microbiol 83:e03411-e3416
Mirza N et al (2011) Ecol Eng 37:1949–1956
Mirza N et al (2014) Sci Vision 20:39–48
Mishra S et al (2017) Sci Rep 7:1–13
Mitani N et al (2008) Pflügers Archiv-Eur J Physiol 456:679–686
Mizuta N et al (1956) Bull Yamaguchi Med Sch 4:131–149
Mohanty M (2016) J Environ Anal Toxicol 6:398
Mykolenko S et al (2013) Global J Environ Res 70:135–151
Nedjimi B (2021) SN Appl Sci 3:1–19
Negi S (2018) J Pharmacogn Phytochem 7:2978–2982
Newete SW, Byrne MJ (2016) Environ Sci Pollut Res 23:10630–10643
Niazi NK et al (2017) Int J Phytorem 19:670–678
Olsen EJ et al (1990) Meteorit Planet Sci 34:285–300
Oremland RS et al (2002) Appl Environ Microbiol 68:4795–4802
Ozturk M et al (2021) Biol Trace Elem Res, pp 1–14
Pandey D et al (2018) Int J Life Sci Res 6:146–162
Paz-Ferreiro J et al (2014) Solid Earth 5:65–75
Phillips J et al (1998) Rural Hist 9:195–209
Picco P et al (2019) Int J Phytorem 21:693–698
Pickering IJ et al (2000) Plant Physiol 122:1171–1178
Planer-Friedrich B et al (2017) Environ Sci Technol 51:7187–7196
Rahaman MS et al (2021) Environ Pollut 289:117940
Rahman MA et al (2011a) Chemosphere 83:633–646
Rahman MA et al (2011b) Sci Total Environ 409:4645–4655
Rahman MS et al (2019) Appl Geochem 111:104444
Raj D (2019) Hum Ecol Risk Assess 25:659–671
Ranjan A et al (2020) Biotica Res Today 2:680–683
Ranjan A et al (2021) Environ Nanotechnol Monit Manag 15:100457
Rosen BP (2002) Comp Biochem Physiol A Mol Integr Physiol 133:689–693
Roy M et al (2015) Environ Int 75:180–198
Sahito ZA et al (2021) J Clean Prod 291:125226
Sakakibara M et al (2007) Proc annu int conf soils sediments. Water Energy 12:258–263
Salido AL et al (2003) Int J Phytorem 5:89–103
Sampanpanish P et al (2019) Bull Environ Contam Toxicol 02:140–145
Sayantan D (2017) Proc National Acad Sci India Section b: Biol Sci 87:1343–1353
Shackira AM, Puthur JT (2019) Plant-metal interactions, pp 263–282
Shrivastava A et al (2015) Curr Pollut Rep 1:35–46
Signes-Pastor A et al (2007) Geoderma 137:504–510
Singh S et al (2021) Int J Phytorem 23:1310–1318
Smedley PL et al (2002) Appl Geochem 17:517–568
Smith E et al (2003) J Environ Sci Health Part A 38:223–239
Song B et al (2019) Crit Rev Environ Sci Technol 49:791–824
Souri Z et al (2020) Ecotoxicol Environ Saf 206:111336
Srivastava S et al (2021) Minerals 11:936
Sultana R, Katsuichiro K (2011) Weed Biol Manag 12–17
Sun D et al (2019) Environ Sci Technol 54:1045–1053
Sun SK et al (2018) New Phytol 219:641–653
Susaya J et al (2010) J Hazard Mater 182:427–438
Tang Z et al (2016) J Agric Food Chem 64:2674–2681
Thakur S et al (2020) J Plant Growth Regul 39:532–543
Tripti K et al (2017) Evaluation of arsenic removal potential of arsenic resistant bacteria with the role of physiological and genomic factors
Ullah A et al (2015) Environ Exp Bot 117:28–40
Usman M et al (2020) Sci Total Environ 721:137778
Van-Der-Heijden MGA et al The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11(3):296–310
Vithanage M et al (2012) Environ Chem Let 10:217–224
Vocciante M et al (2019) J Environ Manage 237:560–568
Wan X et al (2017) Sci Total Environ 579:1467–1475
Wang J et al (2020) Nat Geosci 13:282–287
Wang S et al (2006) J Hazard Mater 138:459–470
Wei X et al (2020) J Hazard Mater 388:121756
Wu X et al (2021) J Hazard Mater 405:124047
Xie ZM et al (1998) Commun Soil Sci Plant Anal 29:2471–2477
Xu W et al (2015) Mol Plant 8:722–733
Yamaji N et al (2015) Proc Natl Acad Sci USA 112:11401–11406
Yan A et al (2020a) Front Plant Sci 11:359
Yan M et al (2020b) J Hazard Mater 388:121795
Yan S et al (2021) BMC Plant Biol 21:1–11
Yang G-M et al (2017) Environ Technol Innov 8:366–372
Yang J et al (2018) Int J Phytoremediation 20:1300–1306
Ye W-L et al (2011) Environ Pollut 159:3739–3743
Ye-Tao T et al (2012) Pedosphere 22:470–488
Zhao FJ et al (2003) New Phytol 159:403–410
Zhao FJ et al (2009) New Phytol 181:777–794
Zhao Y et al (2020) J Environ Sci 90:244–252
Zhou P et al (2020) Nanomaterials 11:26
Zhu M et al (2017) Chemosphere 168:1677–1683
Zuverza-Mena N et al (2017) Plant Physiol Biochem 110:236–264
Acknowledgements
Dr. Tariq Mehmood acknowledges the Postdoctoral ResearchFellowship awarded by the Chinese government at Hainan University, Haikou, Hainan, China.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mehmood, T. et al. (2023). Modern Aspects of Phytoremediation of Arsenic-Contaminated Soils. In: Niazi, N.K., Bibi, I., Aftab, T. (eds) Global Arsenic Hazard. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-16360-9_20
Download citation
DOI: https://doi.org/10.1007/978-3-031-16360-9_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-16359-3
Online ISBN: 978-3-031-16360-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)