Abstract
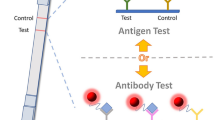
Rapid tests are essential tools for monitoring and containing the COVID-19 pandemic. Lateral flow assays (LFAs) have been introduced for the point-of-care COVID-19 diagnosis, using paper-based devices, and widely used for detecting antigen or antibody related to COVID-19. This book chapter includes a brief overview of the LFAs for rapid test of COVID-19, with focus on nanomaterials for bioconjugation, material selection, human sampling, antibody and antigen tests, viral nucleic acid detection, advantages, limitations, and future perspective.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The damage caused by the COVID-19 pandemic in the last two years (2019–2021) is undeniable. The novel coronavirus (SARS-CoV-2), responsible for the coronavirus disease 2019 (COVID-19) has already more than 5 million deaths and 263 million confirmed cases worldwide [1]. Despite measures taken to decelerate the transmission rate, without global cooperation and mass testing, the virus will remain uncontrolled, as so, its mutants. Traditional laboratory-based analytical methods, such as enzyme-linked immunosorbent assay (ELISA) and real time polymerase chain reaction (RT-PCR) considered gold standard for detection of a large number of disease; however, they are laborious, time-consuming, and require certified molecular testing laboratories [2, 3].
Rapid tests can be a useful tool for monitoring and containing the COVID-19 pandemic. Immunochemical methods provide highly sensitive and specific results, and for that reason, the most utilized screening method for detection is based on immunoassays [2, 4]. Lateral flow assays (LFA) are paper based devices widely used for detecting a target analyte in different matrix [5]. When a LFA is based on the formation of an immunocomplex antigen (Ag)—antibody (Ab), they are called Lateral Flow Immunoassay (LFIA), which the efficacy is related to the efficiency of the Ab-Ag immunocomplex formation and its capacity to detect this phenomenon [6].
Among the assay-based methodologies, the immunochromatographic strip test (ICST), well-known as lateral flow immunoassay (LFIA) or even as rapid diagnostic test (RDT) may be an appropriated alternative solution to mass testing diagnosis of SARS-CoV-2, because it present advances, such as mass-production, rapid response, and good trial tool [7]. First designed in 1960 [8], this device became a breakthrough technology that not only changed the medical practice, but also the medical device market, globally.
The success of LFIA can be seen in their importance in the global market. Only in 2020, the lateral flow market was estimated to be US$7.8 billion, and it is projected to reach a size of US$11.6 billion in 2027, demonstrating that this device has already entered real-life applications [9]. The LFA are versatile in different fields, such as healthcare [10], food safety [11], environmental [12], and in therapeutic drug monitoring [13]. Despite innumerous efforts to improve their sensitivity, depending on the target analyte, in general, the LFIA already have an adequate sensitivity acceptable in diverse government agencies, such as Food and Drug Administration (FDA) [14], with a limit of detection (LOD), varying to 0.1 and 10 ng mL−1[2].
Reviews about LFA already have provided a deep knowledge on specific topics, such as construction [15], Designs [16], formats [15], recognition elements [17], and instrumentalization [18]. Therefore, the aim of this chapter is to give a brief introduction on LFA, general improvements, and trends in lateral flow.
2 LFA Construction
Generally, the construction of LFA includes three principal items: membrane, biomolecules as biorecognition elements, and reporters as signal-transforming element [19]. In addition, the total construction also depends on other components shown in Table 1, which also play a crucial role in the confection of a good device [15, 16].
The detection mechanism is mainly based in capillarity phenomenon. Parameters, such as the fluid used also determine the flow behavior of the membrane, and in some cases, it requires a sample pretreatment (LFA is not an ideal platform for solid samples). Also, there are equations that can be used to predict variables such as the time of fluid transport, optimization of antibody concentration, label concentration and the length of the strip, as Washburn’s equation in wet-out condition, for instance. The appropriated selection of reagents in LFAs also impacts their reproducibility. Steps, such as biomolecules immobilization, drying, washing and the time of the assay must be studied and controlled. Properties like pH, ionic strength, particle size, porosity, flow rate, and viscosity, may also contributes to LFA performance [20].
This device is used innumerous applications, as well as different samples with different characteristics, as we can see in the literature [16]. Further, it can be seen that the design of the LFA depends on two main points: (I) Target analyte and (II) Sample. Taking these points into consideration, it is important to remember that the confection of a reliable LFA is not trivial [21]. Some of the main advantages and disadvantages are present in Fig. 1.
Common advantages and disadvantages of LFIA. Adapted from [21]
The LFIA format is similar to ELISA, although it does not replace them, it can be used as trial tool, as mentioned beforehand. It uses almost the same components as the ELISA. One of the first reports of LFIA construction with immobilized antibodies on a chromatographic paper strip date in the mid of 1980. Combining the advantages of chromatographic principles and immunological recognition elements, lateral flow devices were consolidated as one of the main technologies for screening both in research and in the market [22].
Classified as a challenge, the biomolecule selection for LFA gives a wide possibility of bioreceptors that can be used. Antibodies can be employed as biorecognition elements on the test and control line. They are responsible for binding to the target analyte through the immunocomplex formation in the flux. Although there are commercially available LFIAs for diseases, contaminants, and hormones, they can be synthesized against specific analyte [23]. Aptamers, considered as artificial nucleic acids, are short single-stranded DNA or RNA, having a molecular weight that varies between 10 and 30 kD, and can replace antibodies in biorecognition events in the LFA. Synthesized in SELEX, aptamers can bind to target analytes due to the three-dimensional shape, hydrogen bonding, salt bridges, Van der Waals forces, electrostatic interaction, stacking of aromatic rings, and complementarity shape [24]. Nanobody is an antibody fragment, presenting with a small size (15 kD) and concave shape that can recognize and capture epitopes from antigens, which normally antibodies do not access. Due to the presence of fewer charged groups, there is less problem with cross-reaction phenomenon compared to the whole antibody; however, both only detect immunogenic molecules [25].
3 LFA for COVID-19
Normally, LFA for COVID-19 involves different types of detection: (I) Antibody (II) antigen and (III) nucleic acid detection, but commercially, until the date, only antibody and antigen detection are available. Antibody-based test do not confirm of the virus in organism, however, present a larger immunological window, stability of antibodies, and generally used as supplementary tool for monitoring immune response [26]. Although antigen-based LFAs are less sensitive than RT-PCR, is an economic way for mass testing and rapid response [7]. Besides, they can be combined with other techniques, including RT-PCR, Isothermal amplification, and CRISPR [27]. In addition, the use of nanomaterials for bioconjugate fabrication and, collection and handling of the sample is a crucial step for a good sensitivity, being as important as the manufacturing of the device. Here we only will discuss human sampling for COVID-19 detection in LFA, more information of different samples is widely discussed in literature [28].
3.1 Nanomaterials for Bioconjugation
In LFA, the use of labels is indispensable. They can be divided according to the type of readout, such as: (I) Naked-eye detection, (II) fluorescence detection, and (III) non-optical readout detection. The initial labels were the same enzymes applied for enzyme immunoassays [29]. Nowadays, new nanomaterials are classified as alternatives to enzymes, e.g., gold nanoparticles (GNPs), latex beads, carbon nanoparticles, quantum dots, magnetic nanoparticles (MNP) [16], and less often, selenium, carbon, or liposomes can be used as labels [21]. These nanomaterials help in improving their performance, as requested in real sample applications [30]. The limitations of using LFA remain in samples with low concentration of the target analytes, especially in early detection of diseases.
Nanomaterial-based assays still struggle in nonspecific biding in complex matrix. One way to control or minimize this phenomenon relies on manipulating biomolecular recognition events in the nanomaterial [31]. Bioconjugates that are design for naked detection normally involves equipment-free detection once they are based on the visual presence of test line on the LFA. Mainly for gold and silver nanoparticles, the bioconjugate monitoring can be based on localized surface plasmon resonance properties, that allow free electrons to oscillate so it is possible to absorption peaks in the visible regions, as can be seen in Fig. 2 [31]. There are several protocols for conjugation of molecules, biomolecules onto gold surface, as well different mechanism involved it, such as electrostatically, covalent, hydrophobic, ionic interaction, and chemisorption. Characteristics like nanoparticle shape, surface change, size and ligand located on nanoparticle surface show a great influence in the choice of bioconjugation process [32]. Other cautions as time of incubation, temperature, inappropriate tubes used in conjugation process, molecules/biomolecules purity, quantity, concertation of reagents, and buffer used may influence the whole process.

© 2022. b GNP/antibody bioconjugate change color in the presence of different concentration of COVID-19 antigen from 1 pg mL−1 to 1000 pg mL−1. c UV–Vis of GNP/antibody bioconjugate at different concentration of antigen
a Schematic representation for the visual naked-eye detection of SARS-CoV-2. Reprinted from [33] with permission. ACS Copyright
3.2 Human Sampling
For RT-qPCR, the nasopharyngeal, oropharyngeal swab, and saliva are the most specimens used for COVID-19 detection [34]. Also, the combination of nasopharyngeal and saliva is used to confirm viral infection, Once, the infection migrates from the upper to the lower respiratory area (nasopharyngeal, oropharyngeal), to replicate [35]. The sample should be collected promptly, and in an appropriate manner to minimize or avoid false negative results. The collection should considerate which biomarker used, of instance, for acuate phase, virus or genetic material can be detect in 3–7 days onset. In situations that antibodies are the target, de collection of the sample should be 7–15 days after the last symptom [36] (Fig. 3).
Samples normally used in LFA for COVID-19, adapted from [28]
Nasopharyngeal samples (NS) (used in antigen tests) require sterile, flexible, and long rayon swab. The use of calcium alginate swabs, wooden sticks is not allowed, once they may present substances that inactivated some viruses and can influence some molecular assays [37]. According to Centers for Disease Control and Prevention (CDC), the step of collection includes the gently and slowly insert a rayon swab through the nostril parallel to the palate until resistance is encountered. Next, the swab is rubbed and rolled leaving it in place for several seconds to absorb secretions. Then, the swab is slowly removed while rotating it, and finally placed into the transport tube. The storage respiratory specimens should be at 2–8 °C for 72 h after collection. For long store specimens, the temperature should be at −70 °C or below [38]. NS is preferably used for SARS-CoV-2 detection, especially for the reported a high sensitivity than other specimens, although its collection may be uncomfortable [39].
Presenting a similar collection protocol, oropharyngeal sample (OP) differentiate only that the insert of the swab is performed in throat. The swab is also rubbed over both tonsillar pillars and posterior oropharynx. However, studies found that using 19 positive samples for COVID-19, only nine tested positive, even when the samples where recollected [40]. A possible way that can be taken to reduce false negatives is collecting samples from multiple sites.
Blood, plasma, and serum samples are generally used for antigen or antibody detection in LFA. As already described in literature, one or two drops of blood is collected by fingerstick devices in middle or ring finger [38]. Saliva samples normally require 80 µL for the assay [41]. The storage also is taken at −20 to −80 °C [42]. The use of saliva samples is simpler, less invasive way for testing, especially for children, the main disadvantage is that the sensibility is variable [34]. Nowadays, self-test have gained attention and became commercially available, yet, CDC reports that nasopharyngeal and oropharyngeal specimens are not appropriate for self-collection [38]. Furthermore, an important question arises: How do people will interpret the result? How will data be collected and analyzed for case tracking? A study carried with 360 adults, showed that a considerable portion misinterpreted the negative results [43].
3.3 Antibody Test
When an organism gets in contact with a pathogen, for example SARS-CoV-2, the immune system starts to produce antibodies against the infection, and this specific antibody can be useful to detect the virus using the LFIA platform [44]. This type of test is based on antigen–antibody immunocomplex formation. For example, in SARS-Cov-2, a part of the spike protein, or its immunogenic fragments, such as the Receptor-Binding Domain (RBD) can detect specific immunoglobulins (IgA, IgM and IgG) that are produced as a response to the infection, as described elsewhere [27]. Antibody tests presents some advantages compared to other configurations of LFA, for example, wider detection window, easy and safe for operators to collect blood samples than respiratory samples, higher stability, less susceptibility to degradation, uniform distribution of antibodies in blood and serum [45]. It can detect ongoing (IgM detections) or past infections (IgG detections), in order to understand the transmission dynamics of COVID-19 [46].
It is already reported that many companies provide LFA presenting results within 15–30 min. For example, Roche diagnostic produced Elecsys® Anti-SARS-CoV-2 for IgG antibody detection in serum and plasma with specificity of 99.5% [47]. Abbot developed an 10 min rapid test named Panbio™ COVID-19 IgG/IgM, presenting specificity for serum/plasma of 92.8%, fingerstick whole blood 100.0% and venous whole blood 95.8% [48]. FDA provide a complete open data for serologic test authorized is USA [49]. In academic field, it was reported a colorimetric-fluorescent dual detection of IgM and IgG for SARS-CoV-2 based on quantum dots nanobeads (SiO2@Au@QD nanobeads (NBs) used as label [50]. Completed within 15 min and using 1 µL of serum sample, the LOD was 100 times more sensitive than the use of common GNPs. The proposed device presented 100% of specificity using a total of 16 positive serum human sample and 41 negatives from other viral infections.
The quantification of the SARS-CoV-2 antibodies using a 532 nm laser optical and gold nanoparticles was described [51]. The laser was directed at the LFIA strip, and the readout system provided the quantification of a conventional LFIA. The LOD of 4 × 108 IgG molecules was achieved. A SARS-CoV-2 antigen test immunoassay using latex microspheres to label the antibodies was reported in literature (Fig. 4a, b). This device was tested in 659 samples and showed the sensitivity and specificity of 98.22% and 97.93%, respectively [52]. At present, more than one hundred serology tests have been globally granted since the begin of the pandemic, still it is important to emphasize that antibody test is not recommended by CDC for COVID-19 diagnosis.

Copyright © 2022, Wiley. c homemade water bath used; d the RT-PCR combined with LFA for the simultaneous detection of SARS-CoV-2 and influenza B virus. Reprinted from [63] with authorization. Copyright © 2022, Royal Society of Chemistry. e CRISPR-Cas12a reaction on pre-amplified SARS-CoV-2 target genes, and visualization of the lateral flow strips. f The detection limit of the RT-qPCR assay (left) using PCR machine and RT-LAMP/Cas12a assay (right) using clinical samples. Reprinted from [66] with authorization. Copyright © 2022, MDPI
Schematic representation showing the a Fabrication and detection principle for SARS‐CoV‐2 antigen test using latex microspheres; b Colorimetric signal of LFIA in different positive specimen dilutions. Reprinted from [52] with permission.
3.4 Antigen Test
Governed by the same phenomenon as antibody test, the aim of this format is the detection of the antigens. This device is widely used to identify the presence of spike, membrane or nucleocapsid proteins of SARS-CoV-2 [10]. The development of antigen test for COVID-19 was considered late, and these first-generation devices have been gradually validated independently in literature [10, 53, 54]. The main obstacle remains in the identification of asymptomatic cases, once the diagnosis of COVID-19 is mostly for symptomatic individuals [10]. But successfully can identify symptomatic cases, helping in control the spread of the virus [55].
According to European commission directorate-general for health and food safety, data about commercial LFA antigen test approved for COVID-19 detection and continuous validations updates is required. Therefore, all LFA should present their efficacy to be keep in the market and recognize as a detection tool [56]. The effect of SARS-CoV-2 mutation are the main motivation for these strategies, not only for LFA but for Nucleic Acid Amplification Tests (NAAT) as well. Abbott Rapid Diagnostics created a LFA labelled as Panbio™ COVID-19 Ag Rapid Test, presenting a 98.1% of sensitivity and 99.8% specificity using nasal swab [57]. AAZ-LMB developed a self-test named COVID-VIRO ALL IN® for children. The adapted device has a different configuration for easy sample extraction. Within 15 min, the result can be read and the device presents a correlation of 97.5% with the nasopharyngeal PCR test [58].
Not only sensitivity and specificity, also positive predict value (PPV) and negative predict value (NPV) are essential to measure the risks and consequences of false positive and false negative results in guidance decision protocols in healthcare system. A prospection showed a relationship between test performance, predictive values, and disease prevalence. Here, they concluded that a lower prevalence means lower PPV and a higher number of false positive results. If it is observed an increase of the prevalence, higher is the PPV, consequently, the false positive results decrease. The sensitivity vary in elevated prevalence situations, once leads to lower NPV and a higher number of false negatives. The device should present sensitivity enough to minimize false negative results and, specific enough to avoid/minimize false positives in low prevalence scenarios [59]. The construction and correct use of an antigen test, as well as any type of test is a tiny balance of various factors that is crucial for appropriated results. A study showed an interesting pathway for a complete diagnose of COVID-19 antigen test for patients using LFA. The authors concluded that the device can be effectively adopted with POC-RT-PCR testing during periods of high and low disease prevalence [60].
3.5 Viral Nucleic Acid Detection
For viral detection, RT-PCR is the gold standard for most viral detection. Based on the reverse transcription of RNA and amplification of specific complementary DNA (cDNA) fragments, RT-PCR provides a quantitative information of viral loads in organism. The main advantage of this technique is that it is not limited by the analyte concentration; however, their accessibility may be difficult in remote regions, which requires trained staffs, and it is time-consuming with high cost of equipment and materials [27, 61]. When based on nucleic detection, LFA is named as Nucleic acid lateral flow assay (NALFA), and it is designed for testing the presence of an amplified double-stranded nucleic acid sequence using primers with two different tags [62]. Despite the limitations in early detection (since normally target concentrations can be low in the infected individual), the combination of different techniques (e.g. RT-PCR, LAMP, Clustered Regularly Interspaced Short Palindromic Repeats—CRISPR, and Loop-mediated Isothermal Amplification—LAMP) and LFA are a promising tool for a simple way to increase the accessibility of those molecular techniques [27, 61]. They are present in this chapter as improvement of the LFA.
4 General Improvements Using LFA
Nucleic acid rapid tests are extremely desired, and integration with different methods and techniques is an interesting way for popularization of this technology. In this way, a water bath PCR combined with fluorescent lateral flow assay for SARS-CoV-2 and influenza B detection was proposed (Fig. 4c, d). After the amplification process, the PCR targeted products were detected in an LFA with two test lines and streptavidin-conjugated quantum dot. The LOD were 8.44 copies per µL for SARS-CoV-2 and 14.23 copies per µL for influenza B virus [63]. Variations of RT-PCR have been proposed. Here a multiplex for three different regions of SARS-CoV-2 was developed. The device was able to detect the three genomic regions (ORF3a RdRp, and N genes). The LoD was 10 copies/test, in a total time for the detection about 2 h (100 min PCR, 12 min LFA assay) [64].
A portable RT-LAMP machine combined with LFA and gold nanoparticles was described [65]. The device was designed for simultaneous detection of SARS-CoV-2 and the flu in a single reaction. The evaluation was proceeded after pre-amplified steps of viral loads and control RNA targets assays followed by the LFA readout. The LOD of 35 copies per liter was obtained in 35 min of test (Fig. 4e, 4f) [66]. The use of a near-infrared (NIR) and nanoparticle as the fluorescent labelled is reported elsewhere [65]. The aggregation-induced emission nanoparticle was designed for early detection of IgM and IgG for SARS-CoV-2 in serum sample. The LOD of IgM was 0.236 and IgG 0.125 μg mL–1. The sensitivity was 78 and 95% for IgM and IgG respectively. The main difference was observed in the time of detecting IgM or IgG, that using the proposed label, clinical samples were analysed in 1–7 days after symptom onset, earlier than the common use of GNPs-based test strip (8–15 days).
The LAMP also can be optimized, a multiplex LAMP for SARS-CoV-2 was developed. The target genes N and ORF1ab were simultaneously amplified and detected using a LFA strip [67]. Completed within 1 h, the LOD obtained was 12 copies for both targets. Parameters such as sensitivity and the specificity were both 100%.
Electrophoresis, dialysis, and magnetic enrichment can be used to improve the performance of LFA [16, 68]. Extractions and pre-concentration based on two-phase system to obtain biomarkers before loading them on LFA can also be used. Other approaches can be focused on the components of the LFA, for example. NC membrane size change, inclusion of new components of LFA, such as wax barrier to delay the flux, different pre-treatments in sample and conjugate pad, blocking agents etc. [16, 68]. Figure 5 is a schematic representation of the general points for LFA enhancement.
Schematic representation of the main points for the enhancement of LFA detection performance. Adapted from [69]
LFA technology holds a great importance as an useful tool for screening and mass-testing for COVID-19 and different diseases. Despite to be easy handle, the construction itself is not trivial, and in the most of cases requires improvement of their performance. Obviously, the upgrade that have been applied in LFA assays were absurd in these almost 3 years. Validation steps are crucial, though, the access to positive and negative samples is not easy task in some cases, also, the poor sample collection, and the use of non-fresh specimen also impact in the device performance.
LFA also is vastly explored in multi target detection as already mentioned. This trend is clearly observed in the number of publications focusing in multi-target detection and in commercial tests available [70]. A great number of multiplexing technology in the LFA has been developed for a few years [71]. Additionally, different configurations of LFA is already described, such as the addition of two conjugate pads for the simultaneous detection of two proteins [5] and different conformations of LFA strips for the simultaneous detection of 10 different antibodies by using a 10-channel disc configuration for 10 different LFIA [72]. Lateral flow platform for COVID-19 might not be restricted to only to detect a single type of target, once the simultaneous detection of antibodies and antigens against SARS-CoV-2 and its mutants by a single LFA can be a great instrument for vaccine and pathology studies. Understanding how each LFA test strip performs when different targets are utilized as test objects is critical to achieving this goal [27].
5 Future Perspectives
Lateral flow technology has date over 50 years ago and between all these years, innumerous applications such as medical, veterinary field, agricultural etc. have been developed. LFA shows great importance in the global market because their facilities as simplicity, since only few drops of the sample are required, with relativity low-cost production, compared to other technologies. However, the detection and diagnosis of many diseases, especially SARS-CoV-2 infection remains as a challenge for LFA to be accepted as a gold standard like RT-PCR or ELISA.
One of the principal limitations is the quantitative or semi-quantitative detections, of which some diseases require quantification of their biomarker. Ideally, rapid test must provide a reliable early detection, help to personalized treatment, or be used for mass-testing in healthcare. Various formats of LFA platform have sprung up in last 10 years, and the advance in nanomaterials field allows the achievement of lower LOD. In this way, the lateral flow assay is considered a helpful complement to RT-PCR and ELISA, once it is widely available in low-resource settings.
If large-scale testing of is already a reality, in the next few years, we might expect an efficient implementation of different technologies for performance, processing and collection of the data generated by lateral flow devices. Nevertheless, those technological integration of LFAs in the future can increase the cost of analysis, thereby making them unsuitable for self-test at home or in low-resource areas.
References
World Health Organization (WHO), WHO Coronavirus (COVID-19) Dashboard, (2021). https://covid19.who.int/info/
F. Di Nardo, M. Chiarello, S. Cavalera, C. Baggiani, L. Anfossi, Ten years of lateral flow immunoassay technique applications: trends, challenges and future perspectives. Sensors 21, 5185 (2021). https://doi.org/10.3390/s21155185
I.A. Mattioli, A. Hassan, O.N. Oliveira Jr., F.N. Crespilho, O.N. Oliveira, F.N. Crespilho, On the challenges for the diagnosis of SARS-CoV-2 based on a review of current methodologies. ACS Sensors 5, 3655–3677 (2020). https://doi.org/10.1021/acssensors.0c01382
J.M. Van Emon, Immunoassay and Other Bioanalytical Techniques (CRC Press, Boca Raton, 2016)
K.M. Koczula, A. Gallotta, Lateral flow assays. Essays Biochem. 60, 111–120 (2016). https://doi.org/10.1042/EBC20150012
H. Chen, A.E.V. Hagström, J. Kim, G. Garvey, A. Paterson, F. Ruiz-Ruiz, B. Raja, U. Strych, M. Rito-Palomares, K. Kourentzi, J.C. Conrad, R.L. Atmar, R.C. Willson, Flotation immunoassay: masking the signal from free reporters in sandwich immunoassays. Sci. Rep. 6, 24297 (2016). https://doi.org/10.1038/srep24297
B.D. Grant, C.E. Anderson, J.R. Williford, L.F. Alonzo, V.A. Glukhova, D.S. Boyle, B.H. Weigl, K.P. Nichols, SARS-CoV-2 coronavirus nucleocapsid antigen-detecting half-strip lateral flow assay toward the development of point of care tests using commercially available reagents. Anal. Chem. 92, 11305–11309 (2020). https://doi.org/10.1021/acs.analchem.0c01975
R.S. Yalow, S.A. Berson, Immunoassay of endogenous plasma insulin in man. J. Clin. Invest. 39, 1157–1175 (1960). https://doi.org/10.1172/JCI104130
R. and Markets, Lateral Flow Assays - Global Market Trajectory & Analytics, (2021). https://www.researchandmarkets.com/reports/4805627/lateral-flow-assays-global-market-trajectory (toegang verkry 12 Februarie 2022)
T. Peto, D. Affron, B. Afrough, A. Agasu, COVID-19: rapid antigen detection for SARS-CoV-2 by lateral flow assay: a national systematic evaluation of sensitivity and specificity for mass-testing. EClinicalMedicine. 36, 0–6 (2021). https://doi.org/10.1016/j.eclinm.2021.100924
J. Liu, S. Gao, L. Kang, B. Ji, W. Xin, J. Kang, P. Li, J. Gao, H. Wang, J. Wang, H. Yang, An ultrasensitive gold nanoparticle-based lateral flow test for the detection of active botulinum neurotoxin type A. Nanoscale Res. Lett. 12, 227 (2017). https://doi.org/10.1186/s11671-017-1944-9
C. Liu, Q. Jia, C. Yang, R. Qiao, L. Jing, L. Wang, C. Xu, M. Gao, Lateral flow immunochromatographic assay for sensitive pesticide detection by using Fe3O4 nanoparticle aggregates as color reagents. Anal. Chem. 83, 6778–6784 (2011). https://doi.org/10.1021/ac201462d
L. Bian, J. Liang, H. Zhao, K. Ye, Z. Li, T. Liu, J. Peng, Y. Wu, G. Lin, Rapid monitoring of vancomycin concentration in serum using europium (III) Chelate nanoparticle-based lateral flow immunoassay. Front. Chem. 9 (2021). https://doi.org/10.3389/fchem.2021.763686
FDA, EUA authorized serology test performance (2021). https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance (toegang verkry 12 Februarie 2022)
M. Sajid, M. Daud, Designs, formats and applications of lateral flow assay: a literature review. J. Saudi Chem. Soc. 19, 689–705 (2015). https://doi.org/10.1016/j.jscs.2014.09.001
C. Parolo, A. Sena-Torralba, J.F. Bergua, E. Calucho, C. Fuentes-Chust, L. Hu, L. Rivas, R. Álvarez-Diduk, E.P. Nguyen, S. Cinti, D. Quesada-González, A. Merkoçi, Tutorial: design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 15, 3788–3816 (2020). https://doi.org/10.1038/s41596-020-0357-x
V. Perumal, U. Hashim, Advances in biosensors: principle, architecture and applications. J. Appl. Biomed. 12, 1–15 (2014). https://doi.org/10.1016/j.jab.2013.02.001
W.C. Mak, V. Beni, A.P.F. Turner, Lateral-flow technology: from visual to instrumental. TrAC Trends Anal. Chem. 79, 297–305 (2016). https://doi.org/10.1016/j.trac.2015.10.017
F. Li, M. You, S. Li, J. Hu, C. Liu, Y. Gong, H. Yang, F. Xu, Paper-based point-of-care immunoassays: recent advances and emerging trends. Biotechnol. Adv. 39, 107442 (2020). https://doi.org/10.1016/j.biotechadv.2019.107442
S. Kasetsirikul, M.J.A. Shiddiky, N.-T. Nguyen, Challenges and perspectives in the development of paper-based lateral flow assays. Microfluid. Nanofluidics. 24, 17 (2020). https://doi.org/10.1007/s10404-020-2321-z
G.A. Posthuma-Trumpie, J. Korf, A. van Amerongen, Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 393, 569–582 (2009). https://doi.org/10.1007/s00216-008-2287-2
D.J. Litman, T.M. Hanlon, E.F. Ullman, Enzyme channeling immunoassay: a new homogeneous enzyme immunoassay technique. Anal. Biochem. 106, 223–229 (1980). https://doi.org/10.1016/0003-2697(80)90141-4
R.-M. Lu, Y.-C. Hwang, I.-J. Liu, C.-C. Lee, H.-Z. Tsai, H.-J. Li, H.-C. Wu, Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 27, 1 (2020). https://doi.org/10.1186/s12929-019-0592-z
M. Majdinasab, M. Badea, J.L. Marty, Aptamer-based lateral flow assays: current trends in clinical diagnostic rapid tests. Pharmaceuticals 15 (2022). https://doi.org/10.3390/ph15010090
Y. Liu, L. Zhan, Z. Qin, J. Sackrison, J.C. Bischof, Ultrasensitive and highly specific lateral flow assays for point-of-care diagnosis. ACS Nano 15, 3593–3611 (2021). https://doi.org/10.1021/acsnano.0c10035
G. Liu, J.F. Rusling, COVID-19 antibody tests and their limitations. ACS Sens. 6, 593–612 (2021). https://doi.org/10.1021/acssensors.0c02621
Y. Zhou, Y. Wu, L. Ding, X. Huang, Y. Xiong, Point-of-care COVID-19 diagnostics powered by lateral flow assay. Trends Analyt. Chem. 145, 116452 (2021). https://doi.org/10.1016/j.trac.2021.116452
S. Purohit, P.K. Rao, D. Rawtani, in Sampling and Analytical Techniques for COVID-19 (Chap. 4), ed. by D. Rawtani, C.M. Hussain, N.B.T.-C.-19 in the E. Khatri (Reds) (Elsevier, 2022), bll 75–94. https://doi.org/10.1016/B978-0-323-90272-4.00008-7
M.G. Pappas, R. Hajkowski, W.T. Hockmeyer, Dot enzyme-linked immunosorbent assay (Dot-ELISA): a micro technique for the rapid diagnosis of visceral leishmaniasis. J. Immunol. Methods. 64, 205–214 (1983). https://doi.org/10.1016/0022-1759(83)90399-X
D. Quesada-González, A. Merkoçi, Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 73, 47–63 (2015). https://doi.org/10.1016/j.bios.2015.05.050
A. Pramanik, Y. Gao, S. Patibandla, K. Gates, P.C. Ray, Bioconjugated nanomaterial for targeted diagnosis of SARS-CoV-2. Accounts Mater. Res. 3, 134–148 (2022). https://doi.org/10.1021/accountsmr.1c00177
M.H. Jazayeri, H. Amani, A.A. Pourfatollah, H. Pazoki-Toroudi, B. Sedighimoghaddam, M. Hadi, H. Amani, A. Akbar, H. Pazoki-Toroudi, B. Sedighimoghaddam, M.H. Jazayeri, H. Amani, A.A. Pourfatollah, H. Pazoki-Toroudi, B. Sedighimoghaddam, Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens. Bio-Sensing Res. 9, 17–22 (2016). https://doi.org/10.1016/j.sbsr.2016.04.002
P. Moitra, M. Alafeef, K. Dighe, M.B. Frieman, D. Pan, Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 14, 7617–7627 (2020). https://doi.org/10.1021/acsnano.0c03822
M. Ahmadzadeh, H. Vahidi, A. Mahboubi, F. Hajifathaliha, L. Nematollahi, E. Mohit, Different respiratory samples for COVID-19 detection by standard and direct quantitative RT-PCR: a literature review, Iran. J. Pharm. Res. 20, 285–299 (2021). https://doi.org/10.22037/ijpr.2021.115458.15383
D. Wang, B. Hu, C. Hu, F. Zhu, X. Liu, J. Zhang, B. Wang, H. Xiang, Z. Cheng, Y. Xiong, Y. Zhao, Y. Li, X. Wang, Z. Peng, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. China. JAMA 323, 1061–1069 (2020). https://doi.org/10.1001/jama.2020.1585
J.A. Al-Mughales, T.J. Al-Mughales, O.I. Saadah, Monitoring specific IgM and IgG production among severe COVID-19 patients using qualitative and quantitative immunodiagnostic assays: a retrospective cohort study. Front. Immunol. 12 (2021). https://doi.org/10.3389/fimmu.2021.705441
Pan American Health Organization, Sample collection (2020). https://www3.paho.org/hq/index.php?option=com_content&view=article&id=7918:2012-videos-sample-collection&Itemid=40295&lang=pt (toegang verkry 27 April 2022)
Centers for Disease Control and Prevention, Interim guidelines for collecting and handling of clinical specimens for COVID-19 testing (2021). https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html (toegang verkry 27 April 2022)
H. Wang, Q. Liu, J. Hu, M. Zhou, M. Yu, K. Li, D. Xu, Y. Xiao, J. Yang, Y. Lu, F. Wang, P. Yin, S. Xu, Nasopharyngeal swabs are more sensitive than oropharyngeal swabs for COVID-19 diagnosis and monitoring the SARS-CoV-2 load. Front. Med. 7 (2020). https://doi.org/10.3389/fmed.2020.00334
C. Xie, L. Jiang, G. Huang, H. Pu, B. Gong, H. Lin, S. Ma, X. Chen, B. Long, G. Si, H. Yu, L. Jiang, X. Yang, Y. Shi, Z. Yang, Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 93, 264–267 (2020). https://doi.org/10.1016/j.ijid.2020.02.050
A. Roda, S. Cavalera, F. Di Nardo, D. Calabria, S. Rosati, P. Simoni, B. Colitti, C. Baggiani, M. Roda, L. Anfossi, Dual lateral flow optical/chemiluminescence immunosensors for the rapid detection of salivary and serum IgA in patients with COVID-19 disease. Biosens. Bioelectron. 172, 112765 (2021). https://doi.org/10.1016/j.bios.2020.112765
B.S. Henson, D.T. Wong, in Collection, Storage, and Processing of Saliva Samples for Downstream Molecular Applications BT—Oral Biology: Molecular Techniques and Applications, ed. by G.J. Seymour, M.P. Cullinan, N.C.K. Heng (Reds) (Humana Press, Totowa, 2010), bll 21–30. https://doi.org/10.1007/978-1-60761-820-1_2
S. Woloshin, B. Dewitt, T. Krishnamurti, B. Fischhoff, Assessing how consumers interpret and act on results from at-home COVID-19 self-test kits a randomized clinical trial. JAMA Intern. Med. 182, 332–341 (2022). https://doi.org/10.1001/jamainternmed.2021.8075
C. Wang, W. Li, D. Drabek, N.M.A. Okba, R. van Haperen, A.D.M.E. Osterhaus, F.J.M. van Kuppeveld, B.L. Haagmans, F. Grosveld, B.-J. Bosch, A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 11, 2251 (2020). https://doi.org/10.1038/s41467-020-16256-y
R. Jalandra, A.K. Yadav, D. Verma, N. Dalal, M. Sharma, R. Singh, A. Kumar, P.R. Solanki, Strategies and perspectives to develop SARS-CoV-2 detection methods and diagnostics. Biomed. Pharmacother. 129, 110446 (2020). https://doi.org/10.1016/j.biopha.2020.110446
A. Petherick, Developing antibody tests for SARS-CoV-2. Lancet 395, 1101–1102 (2020). https://doi.org/10.1016/S0140-6736(20)30788-1
Roche Diagnostics, Elecsys® Anti-SARS-CoV-2 (2022). https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2.html (toegang verkry 27 April 2022)
Abbott, PANBIOTM COVID-19 IgG/IgM rapid test device (2020). https://www.globalpointofcare.abbott/en/product-details/panbio-covid-19-igg-igm-antibody-test.html (toegang verkry 07 April 2022)
FDA, Independent evaluations of COVID-19 serological tests (2020). https://open.fda.gov/apis/device/covid19serology/
C. Wang, X. Yang, B. Gu, H. Liu, Z. Zhou, L. Shi, X. Cheng, S. Wang, Sensitive and simultaneous detection of SARS-CoV-2-specific IgM/IgG using lateral flow immunoassay based on dual-mode quantum dot nanobeads. Anal. Chem. 92, 15542–15549 (2020). https://doi.org/10.1021/acs.analchem.0c03484
T. Peng, X. Liu, L.G. Adams, G. Agarwal, B. Akey, J. Cirillo, V. Deckert, S. Delfan, E. Fry, Z. Han, P. Hemmer, G. Kattawar, M. Kim, M.-C. Lee, C. Lu, J. Mogford, R. Nessler, B. Neuman, X. Nie, J. Pan, J. Pryor, N. Rajil, Y. Shih, A. Sokolov, A. Svidzinsky, D. Wang, Z. Yi, A. Zheltikov, M. Scully, Enhancing sensitivity of lateral flow assay with application to SARS-CoV-2. Appl. Phys. Lett. 117, 120601 (2020). https://doi.org/10.1063/5.0021842
L. Shen, Q. Zhang, X. Luo, H. Xiao, M. Gu, L. Cao, F. Zhao, Z. Chen, A rapid lateral flow immunoassay strip for detection of SARS-CoV-2 antigen using latex microspheres. J. Clin. Lab. Anal. 35, e24091 (2021). https://doi.org/10.1002/jcla.24091
A.K. Lindner, O. Nikolai, C. Rohardt, F. Kausch, M. Wintel, M. Gertler, S. Burock, M. Hörig, J. Bernhard, F. Tobian, M. Gaeddert, F. Lainati, V.M. Corman, T.C. Jones, J.A. Sacks, J. Seybold, C.M. Denkinger, F.P. Mockenhaupt, Diagnostic accuracy and feasibility of patient self-testing with a SARS-CoV-2 antigen-detecting rapid test. J. Clin. Virol. 141, 104874 (2021). https://doi.org/10.1016/j.jcv.2021.104874
P. Merino et al., Multicenter evaluation of the PanbioTM COVID-19 rapid antigen-detection test for the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 27, 758–761 (2021). https://doi.org/10.1016/j.cmi.2021.02.001
W.-Y. Hsieh, C.-H. Lin, T.-C. Lin, C.-H. Lin, H.-F. Chang, C.-H. Tsai, H.-T. Wu, C.-S. Lin, Development and efficacy of lateral flow point-of-care testing devices for rapid and mass COVID-19 diagnosis by the detections of SARS-CoV-2 antigen and anti-SARS-CoV-2 antibodies. Diagnostics (Basel, Switzerland). 11 (2021). https://doi.org/10.3390/diagnostics11101760
European Commission Directorate-General for Health and Food Safety, EU health preparedness: a common list of COVID-19 rapid antigen tests; a common standardised set of data to be included in COVID-19 test result certificates; and a common list of COVID-19 laboratory based antigenic assays (2021). https://ec.europa.eu/health/sites/health/files/preparedness_response/docs/common_testingapproach_covid-19_en.pdf
Abbott, PanbioTM COVID-19 Ag rapid test device (2022). https://www.abbott.co.uk/panbio.html
AAZ, Autotest covid suitable for children—autotest COVID-VIRO ALL IN® (2022). https://www.covid19aaz.com/en/autotest-covid-viro-all-in/
R.W. Peeling, P.L. Olliaro, D.I. Boeras, N. Fongwen, Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect. Dis. 21, e290–e295 (2021). https://doi.org/10.1016/S1473-3099(21)00048-7
B. Merrick, M. Noronha, R. Batra, S. Douthwaite, G. Nebbia, L.B. Snell, S. Pickering, R.P. Galao, J. Whitfield, A. Jahangeer, R. Gunawardena, T. Godfrey, R. Laifa, K. Webber, P.R. Cliff, E. Cunningham, S.J.D. Neil, H. Gettings, J.D. Edgeworth, H.L. Harrison, Real-world deployment of lateral flow SARS-CoV-2 antigen detection in the emergency department to provide rapid, accurate and safe diagnosis of COVID-19. Infect. Prev. Pract. 3, 100186 (2021). https://doi.org/10.1016/j.infpip.2021.100186
S. Agarwal, C. Warmt, J. Henkel, L. Schrick, A. Nitsche, F.F. Bier, Lateral flow–based nucleic acid detection of SARS-CoV-2 using enzymatic incorporation of biotin-labeled dUTP for POCT use. Anal. Bioanal. Chem. (2022). https://doi.org/10.1007/s00216-022-03880-4
M. Blažková, M. Koets, P. Rauch, A. van Amerongen, Development of a nucleic acid lateral flow immunoassay for simultaneous detection of Listeria spp. and Listeriamonocytogenes in food. Eur. Food Res. Technol. 229, 867 (2009). https://doi.org/10.1007/s00217-009-1115-z
H. Chen, Y. Wang, H. Wei, Z. Rong, S. Wang, A rapid water bath PCR combined with lateral flow assay for the simultaneous detection of SARS-CoV-2 and influenza B virus. RSC Adv. 12, 3437–3444 (2022). https://doi.org/10.1039/d1ra07756b
S. Yu, S.B. Nimse, J. Kim, K.-S. Song, T. Kim, Development of a lateral flow strip membrane assay for rapid and sensitive detection of the SARS-CoV-2. Anal. Chem. 92, 14139–14144 (2020). https://doi.org/10.1021/acs.analchem.0c03202
R. Chen, C. Ren, M. Liu, X. Ge, M. Qu, X. Zhou, M. Liang, Y. Liu, F. Li, Early detection of SARS-CoV-2 seroconversion in humans with aggregation-induced near-infrared emission nanoparticle-labeled lateral flow immunoassay. ACS Nano 15, 8996–9004 (2021). https://doi.org/10.1021/acsnano.1c01932
M. Rezaei, S. Razavi Bazaz, D. Morshedi Rad, O. Shimoni, D. Jin, W. Rawlinson, M. Ebrahimi Warkiani, A portable RT-LAMP/CRISPR machine for rapid COVID-19 screening. Biosensors 11 (2021). https://doi.org/10.3390/bios11100369
X. Zhu, X. Wang, L. Han, T. Chen, L. Wang, H. Li, S. Li, L. He, X. Fu, S. Chen, M. Xing, H. Chen, Y. Wang, Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens. Bioelectron. 166, 112437 (2020). https://doi.org/10.1016/j.bios.2020.112437
Y. Deng, H. Jiang, X. Li, X. Lv, Recent advances in sensitivity enhancement for lateral flow assay. Mikrochim. Acta. 188, 379 (2021). https://doi.org/10.1007/s00604-021-05037-z
L. Napione, Integrated nanomaterials and nanotechnologies in lateral flow tests for personalized medicine applications. Nanomaterials 11 (2021). https://doi.org/10.3390/nano11092362
F. Mahmoudinobar, D. Britton, J.K. Montclare, Protein-based lateral flow assays for COVID-19 detection. Protein Eng. Des. Sel. 34 (2021). https://doi.org/10.1093/protein/gzab010
L. Anfossi, F. Di Nardo, S. Cavalera, C. Giovannoli, C. Baggiani, Multiplex lateral flow immunoassay: an overview of strategies towards high-throughput point-of-need testing. Biosensors 9, 2 (2018). https://doi.org/10.3390/bios9010002
Y. Zhao, H. Wang, P. Zhang, C. Sun, X. Wang, X. Wang, R. Yang, C. Wang, L. Zhou, Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci. Rep. 6, 21342 (2016). https://doi.org/10.1038/srep21342
Acknowledgements
The authors gratefully acknowledge the Coordinating Agency for Advanced Training of Graduate Personnel (CAPES), MeDiCo Network CAPES, Brazil grant number 88881.504532/2020-01 and 88887.511448/2020-00. Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP, grant numbers: 2019/12053-8, 2019/15333-1, 2018/22214-6 and 2021/10911-7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Castro, K.R., Silva, B.G.R., Crespilho, F.N. (2023). Lateral Flow Assays for COVID-19. In: Crespilho, F.N. (eds) COVID-19 Metabolomics and Diagnosis. Springer, Cham. https://doi.org/10.1007/978-3-031-15889-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-15889-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15888-9
Online ISBN: 978-3-031-15889-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)