Abstract
Alzheimer’s disease (AD) is a clinically progressive decline in cortical function involving memory and other cognitive domain of executive function and pathologically by two hallmark lesions, amyloid-β plaque cores (APC) and neurofibrillary tangles (NFT). Over 30 years ago, these hallmark lesions were characterized by indirect qualitative analysis, which led to a substantial expansion of molecular studies, as well as optimism about successful therapeutic interventions. Unfortunately, despite copious facts of the biochemical cascades that produce these aggregates, there has been no meaningful progress toward disease modification. The repeated failures in this regard have been attributed to tardiness in intervention, rather than instead an overdue need for a paradigm shift. The ruling theories for AD pathogenesis have their root in pathological lesion removal. It is argued that “pathological” changes instead reflect elaborate neuroprotection mechanisms in response to a decade-long adaptation to an aging-hostile environment. The lesions themselves may similarly be a manifestation of neuroprotection, and likewise the targeting of such lesions as the offending agents is done at the risk of disrupting homeostasis and the body’s attempt at fighting disease. Rather than simply shifting the same ideas and interventions to an earlier age or disease stage, a broadening of the scope of treatment efforts to working with rather than against the biological responses of the brain, and the realities of repeated negative data, should now be embraced with enthusiasm. Sadly, this verdict still holds since the previous version of this book with only marginal benefits if any shown for any lesion removal-based therapy.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
1 Introduction
Alzheimer’s disease (AD) is complex and heterogeneous, differing in many respects from case to case, including age at onset, clinical signs and symptoms, presence or absence of various and numerous risk factors, extent and distribution of neuropathology, and genetic alterations including germline mutation and susceptibility alleles. This heterogeneity is leading to dissection of AD into further diseases, as in prior years it led to Lewy body disease and frontal temporal dementia. Numerous hypotheses with ostensible treatment possibilities are therefore expected, while the treatment avenues themselves have been unrewarding. Whether or not this failure reflects the complexity of the disease, the now numerous failures in the face of almost limitless knowledge of biochemical facts suggest that prevailing paradigms are significantly, if not fatally, flawed (Castellani et al., 2008, 2009).
Presently, standard thought equates protein constituents of insoluble pathological lesions or their assembly intermediates with toxicity (Hardy & Higgins, 1992). For example, AD brains accumulate NFT (the major discovery made by Alois Alzheimer in 1906) that contain phosphorylated tau protein that is toxic to neurons in vitro such that the cascade that produces phosphorylated tau, if left unchecked, causes disease (Spires-Jones et al., 2009). Alternatively, AD brains accumulate amyloid plaque cores containing amyloid-β peptide (Aβ); Aβ is therefore a product of a pathological cascade that is often viewed as inherently deleterious (Selkoe, 2008). Somewhat more recent iterations of this hypothesis have focused on soluble oligomers as the major toxic species, although such species suffer from artificiality, reproducibility, and difficulty with which they are studied directly. If one adds to these issues a putative attack on the synapse, another degree of complexity is added to the concept and therefore further removed from relevance to the human condition (Tanzi, 2005). Such thinking is elegant in its simplicity but presumptuous that it will yield a solution (Benilova et al., 2012).
In this review, the pathophysiology of two proteins implicated in AD pathogenesis, Aβ and tau, is discussed and the concept of pathology as a downstream and possibly beneficial response to the underlying process which is as yet poorly understood, if not completely misunderstood. Alternative hypothesis of AD linked to ApoE, genetics, lifestyle, and alternations in neuronal metabolism is further discussed.
2 Amyloidosis Linked to Neurodegeneration
Amyloid-β peptide (Aβ) was initially identified through purification of insoluble pathological lesions – amyloid plaque cores and cerebral vasculature involved by amyloid angiopathy (Masters et al., 1985). Subsequent identification of Aβ as a metabolic product of amyloid-β protein precursor (AβPP), its localization to the long arm (q) of chromosome 21, the identification of familial AD kindred with pathogenic AβPP mutations, and the increased AD pathology in Down’s syndrome patients who carry an extra copy of chromosome 21 produced a compelling argument in favor of the so-called amyloid cascade as the pathogenic mediator of disease. The cascade was further substantiated by characterization of presenilin proteins, part of the notch signaling complex with γ-secretase activity (see below) and therefore inherently amyloidogenic.
AβPP is an integral type I membrane protein on chromosome 21q21 (Wilquet & De Strooper, 2004). Full expression of AβPP results in a cytoplasmic tail, a transmembrane portion, and a large extracellular domain, although only a fraction of newly synthesized AβPP molecules reach the cell surface in cell culture studies. AβPP further exists as multiple alternatively spliced isoforms, three of which predominate: two isoforms, AβPP770/751, contain the Kunitz protease inhibitor domain (exon 7) within the extracellular portion and are the predominant forms in cells other than neurons; AβPP695 is devoid of the Kunitz protease inhibitor domain and is the predominant form in neurons.
Enzyme cleavage results in amyloidogenic and non-amyloidogenic fragments, depending on whether or not the Aβ protein is produced. Inherent in the term “amyloidogenic” is a process that is deleterious, although it is worth noting that Aβ synthesis, including the so-called pathogenic Aβ, Aβ1–42, is a physiological process, with Aβ being synthesized and secreted throughout normal life (Fig. 1). Comparable levels of Aβ in AD and healthy controls are detected in cerebrospinal fluid and plasma.
The amyloidogenic pathway of amyloid-β precursor protein in Alzheimer’s disease. Neuronal AβPP receptor is cleaved by β- and γ-secretases releasing transmembrane Aβ peptide; then after a conformational change, the Aβ peptide aggregates into oligomers, fibrils, and ultimately Aβ plaque cores. This process of Aβ peptide aggregation may be mediated by ApoE
Cleavage of AβPP by either α-secretase or β-secretase produces soluble N-terminal fragments AβPP and C83 and C99 membrane-bound C-terminal fragments, respectively. Further cleavage by γ-secretase leads to the release and secretion of non-pathogenic p3 peptide (previous α-secretase cleavage) and Aβ (previous β-secretase cleavage) (Fig. 1). Moreover, depending on the precise site of γ-secretase cleavage, different lengths of Aβ are produced, varying from 38 to 43 amino acids. The 42 amino acid form, Aβ42, has a greater tendency to form fibrils in vitro compared to other forms, so not surprisingly Aβ42 synthesis and deposition is a hallmark of the amyloid cascade hypothesis.
β-Secretase and γ-secretase cleavage, resulting in the Aβ peptide, was established early on in the study of Aβ metabolism, although the constituents and cell biology of γ-secretase have proven a challenge. The presenilins, initially identified through linkage to early onset familial AD with apparent increased Aβ42 (Citron et al., 1997; Duff et al., 1996; Scheuner et al., 1996), are components of the γ-secretase complex (Maiorini et al., 2002).
Despite the presumption of presenilin as an important component of the multimeric secretase complex, the biochemical mechanism of presenilin action is largely unknown. During development, presenilins appear to cleave a transmembrane protein termed Notch, which in turn is a transcriptional activator of gene involvement in cellular differentiation (De Strooper et al., 1999) . PS1 and PS2 are involved in a range of biological processes, including cell adhesion, G-protein-mediated signal transduction, and the unfolded protein response (Baki et al., 2001; Smine et al., 1998; Niwa et al., 1999). Nicastrin has also been shown to interact strongly with the presenilins and appears to be required for normal Notch signaling in Caenorhabditis elegans (Yu et al., 2000).
AβPP cleavage with generation of Aβ fragments also differs as a function of cellular subcompartment. At the cell surface, AβPP is proteolytically processed, primarily by α-secretases, resulting in shedding of the majority of the extracellular domain. Rapid and efficient internalization is mediated by a “YENPTY” internalization motif (Vetrivel & Thinakaran, 2006). Once endocytosed, AβPP may be recycled to the cell surface, degraded, or further processed. β-Site AβPP cleaving enzyme-1 (BACE1) acts on AβPP in late Golgi/TGN and endosomes, as indicated by the acidic optimal pH of BACE1 activity. γ-Secretase complex activity on the other hand takes place in multiple cellular compartments including endoplasmic reticulum (ER), Golgi, and the plasma membrane; the last is thought to comprise only a small fraction of the γ-secretase activity.
A key question that has existed since the elucidation of AβPP is the normal cellular function of this molecule, which is unresolved. One candidate ligand, secreted neuronal protein F-spondin, is implicated in neuronal sprouting and development. F-Spondin binds AβPP as well as APLP-1 and APLP-2, which may interfere with β-secretase cleavage and cell signaling effected by the cytoplasmic domain (Wilquet & De Strooper, 2004). AβPP has been suggested to serve as a receptor for intracellular transport of synaptic vesicles through interaction with kinesin and microtubules (Kamal et al., 2001). Both AβPP and the low density lipoprotein receptor-related protein have been shown to bind the adaptor protein Fe65 via their cytoplasmic domains which increases AβPP proteolytic processing (Pietrzik et al., 2004). Interestingly, both LRP and AβPP are also γ-secretase substrates after cleavage and removal of their extracellular domains. A role of AβPP in heavy metal binding and as an antioxidant may also be an important role with direct implications in the disease process when dysfunctional.
In brief, the fundamentally toxic Aβ42, otherwise a product of normal cellular metabolism, is thought to be overproduced in AD resulting in neurodegeneration or so the amyloid cascade theory postulates. Support for this comes principally if not entirely from Mendelian diseases with pathogenic AβPP mutations linked to extensive Aβ deposits and early onset disease. What is missed in causality is that genetics demonstrate correlation/association; causality instead was not shown because Aß removal in humans did not reverse AD. In vitro toxicity of Aβ42 peptides is considered further evidence for the cascade, although toxicity lies within narrow conditions irrelevant to brain metabolism, at least as toxicity. Despite the commonly held notions, whether Aβ42 is toxic in vivo in human remains to be elucidated, whereas a role of Aβ42 in neuroprotection, which is made intuitively difficult by the prevailing ideas, has been demonstrated (Nunomura et al., 2006). Nevertheless, the collective data not surprisingly led to considerable enthusiasm and a frenzy of activity to not only further characterize, or “prove” the importance of the cascade, but to rush headlong into human immunotherapeutic trials specifically targeting Aβ. The first major and somewhat infamous phase II active immunization approach (AN-1792) may have been the most informative of all trials to date given the now long-term follow-up. The evidence is now clear that removal of Aβ from the brain in mild to moderate AD has no major cognitive benefits. Moreover, the two individuals studied who had almost complete removal of Aβ plaques compressed to dementia at the same rate as placebo and expired with a mini mental status score of 0, effectively answering the question of whether dementia progresses in the face of Aβ removal from the brain whether it be fibers or oligomers (Holmes et al., 2008).
2.1 Does Aβ Correlate with Clinical and Anatomic Indices of Disease?
While the above data seems to have closed the case on whether Aβ causes dementia, the relationship between Aβ pathology and disease has been the subject of a number of studies prior to the AN-1792 trial, and indeed the data indicate unambiguously that the correlation between Aβ and clinical disease is imprecise at best (Castellani et al., 2010). With an early study in the 1960s showing an overall trend toward increased disease severity with plaque burden (Blessed et al., 1968; Giannakopoulos et al., 2003), subsequent studies have relied on this concept. At present, it is accepted that amyloid burden overall correlates poorly with disease severity, and the distribution of Aβ tends to be diffuse throughout the neocortex with no meaningful region specificity. Diffuse deposits of amyloid also occur in the striatum and cerebellar cortex late in disease, with no discernible selectivity in terms of loss of function subserved by these regions. Relative to neocortical Aβ, it is of some interest that medial temporal allocortical tissue involved in episodic declarative memory shows decreased Aβ (Arnold et al., 1991). Given the role of ApoE in facilitating fibrillogenesis of Aβ, it is also interesting that the extent of neocortical Aβ deposits does not correlate with the various Apo E alleles, including ε4 (Berg et al., 1998). Nunomura found that Aβ levels increase with disease progression with ApoE4 but shows less pronounced increase with ApoE3 (Fig. 2).
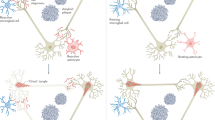
Aβ attack on the synapse. A relatively new paradigm that is more functional than structural has emerged, in part in response to the growing realization of the imprecise relationship between Aβ and clinical disease, and the clinical trials that have effectively removed Aβ and have not altered the neurodegenerative process. It is now suggested that soluble, low-n Aβ oligomers, identified by high-speed centrifugation and immunoblotting of the supernatant, cause synaptic damage and functional neurologic deficits (Selkoe, 2008). Experimental studies involving injection of conditioned medium, derived from oligomer-secreting AβPP V717F Chinese hamster ovary cells into rat lateral ventricle, demonstrated alterations in long-term potentiation (LTP) that was to be related to low-n oligomers per se and not monomers (Walsh et al., 2002). LTP alteration was also shown in vitro in hippocampal mouse slices, along with concomitant changes in cell cycle signaling cascades and behavioral abnormalities. Here again, however, there is a premature juxtaposition of experimental data and human brain separate from each other by several degrees of relevance, in addition to lack of insight into those soluble species that are the most toxic and the overall lack of reproducibility of many of the studies.
3 Phosphorylated Tau
Alzheimer’s neurofibrillary tangle. While plaques were a known accompaniment of senile dementia in the late 1800s, the first description of the neurofibrillary tangles (NFT) in 1907 can be attributed to Alzheimer (1907; Castellani et al., 2010). It is also interesting to note that Alzheimer devoted ten sentences and two paragraphs to his initial description of the NFT, compared to only two sentences to the senile plaque. This, and the fact that plaques were a known component of senile dementia at that time, suggests that the NFT was the more intriguing lesion. Nevertheless, in spite of copious literature written by Alzheimer and his contemporaries, it is difficult to find firm allusions to the cause of the basic disease process. Rather, the importance and controversy rested for the most part on whether this condition affecting a relatively young patient represented a new disease or, instead, was a form of senile dementia with early onset.
Not long after the purification of amyloid deposits and the identification of the Aβ protein, NFTs were purified and the microtubule-associated protein tau determined as a protein component. The enthusiasm for tau phosphorylation as a primary process in AD was however blunted by the absence of genetic linkage, which has forever relegated pathological tau events to a downstream position on the popular algorithms. Instead, germline tau mutations are more closely related to the frontotemporal dementia phenotype.
Tau as the major protein component of neurofibrillary pathology. Similar to the situation with Aβ, knowledge of tau has expanded considerably since it was purified and molecular species elucidated from the insoluble lesions. It is now known that the phosphorylated tau is a major protein component of neurofibrillary pathology, which in turn promoted the study of tau in copious detail (Fig. 3).
The tau gene is comprised of over 100 kb and contains 16 exons. Upstream of the first exon are consensus binding sites for transcription factors, including AP2 and SP1. Alternative splicing of tau nuclear RNA in the adult brain involving exons two, three, and ten results in six tau isoforms. These 6 isoforms in turn differ in the presence of either 3 of 4 repeats of 31 or 32 peptide residues in the C-terminal region (exon 10). This peptide repeat region also comprises the microtubule binding domain and therefore has direct implications in tau pathophysiology. Moreover, tau isoforms differ in the expression of zero, one, or two inserts encoded on exons two and three. The relative amounts of these tau isoforms as well as their phosphorylation status changes during development; 3 repeat tau with no inserts is expressed in the fetus and early post natal infant, while heterogeneous isoforms are expressed in the adult brain. This switch in RNA splicing also corresponds to an overall reduction in tau phosphorylation. Tau is relatively abundant in neurons but is present in all nucleated cells. Its major physiologic function appears to be in binding microtubules and in stabilizing microtubule assembly for polymerization.
In disease, tau is abnormally hyperphosphorylated at proline directed serine/threonine phosphorylation sites, including Ser-202/Thr-205 (AT8 site), Ser-214 and/or Ser-212 (AT100 site), Thr-231 and/or Ser-235 (TG3 site), and Ser-396/Ser-404 (PHF-1 site). In addition, alternative tau splicing differs according to pathological phenotype, such that tau accumulation in AD is a mixture of 3R and 4R tau, Pick disease tends to be 3R tau, corticobasal degeneration and progressive supranuclear palsy tends to be 4R tau, and so-called argyrophilic grain disease accumulates small inclusions comprised of 3R tau.
3.1 Does Phospho-Tau Correlate with Clinical and Anatomic Indices of Disease?
In spite of the fact that tau tends to appear in the cortex subsequent to Aβ and is generally considered a secondary or downstream phenomenon, it is interesting that neurofibrillary pathology correlates closely with clinical signs and much more closely than Aβ deposits. Phosphorylated tau deposition, for example, has a striking tendency to involve memory circuitry early in disease as well as in the aging process. It is also remarkable that abundant neocortical neurofibrillary pathology is virtually always associated with clinical signs of AD, whereas extensive neocortical Aβ deposits are often seen in aged individuals in the absence of significant cognitive impairment or evidence of neuronal loss. In other words, heavy tau “burden” is generally incompatible with preserved cerebral function, while heavy amyloid burden often is not.
Tau attack on the synapse. The role of phosphorylated tau in functional disease is progressing in a manner very much similar to Aβ. Recent studies, for example, indicate that insoluble tau accumulation is somewhat benign, while oligomeric phospho-tau intermediates may be more toxic and may be toxic at the specific level of the synapse (Santacruz et al., 2005). Also similar to Aβ studies, support for this concept is limited to highly experimental models. In another AD-like model, axonal pathology with accumulation of tau preceded plaque deposition, while studies of a P301S tauopathy model demonstrated microglia activation and synapse loss prior to NFT formation. The results highlight the growing theme that insoluble pathological lesions, and in this case NFT formation, are late-stage non-toxic events and that attention might be better directed toward upstream soluble tau intermediates. That the entire process, from changes in synthesis of soluble species, to putative soluble assembly intermediates, to insoluble pathological lesions, has yet to be embraced as a response to an underlying pathogenic process. With recent failure of the cascades, testing of biological alternatives is long overdue.
4 Apolipoprotein E
Polymorphism in the apolipoprotein E (APOE) gene is a major genetic risk of late-onset AD. The APOE gene exists as three polymorphic alleles – ε2, ε3, and ε4 – with a frequency of 8.4%, 77.9%, and 13.7%, respectively, although the frequency of the ε4 allele is significantly higher among patients with AD, being around 40%. Also, there are racial and ethnic differences associated with APOE alleles. A study with 4917 patients in the USA indicated that Black/African Americans have a higher proportion of APOE-ε2 allele than White/European Americans (ε2ε2/ε2ε3/ε2ε4; 22% vs 13%) and APOE-ε4 allele (ε3ε4/ε4ε4; 33% vs 24%) (Rajan et al., 2017). Clinical and basic research support the correlation between APOE-ε4 with an increased risk of AD and more abundant amyloid pathology in the brain. Human ApoE is a 34 kDa glycoprotein composed of 299 amino acids, with key functions in lipid transport throughout the body, playing a critical role in atherosclerosis, metabolic diseases, and neurodegeneration. ApoE isoforms only differ by one amino acid at the structural level; ApoE2 has C112/C158, ApoE3 has C112/R158, and ApoE4 has R112/R158, providing isoform-specific properties and binding specificities (Fig. 4).
ApoE4 is a strong risk factor for late-onset AD by altering Aβ aggregation and clearance, NFT aggregation, microglia and astrocyte responses, alteration of the blood-brain barrier, and alteration in synapse formation contributing to AD pathogenesis. In particular, ApoE4 disrupts lipid homeostasis in human glia, causing increased unsaturation of fatty acids and accumulation of lipid droplets (Sienski et al., 2021). In contrast, ApoE3 did not affect intracellular lipid metabolism. APOE4+ carriers present a higher susceptibility to developing AD, including Aβ and tau accumulation, brain atrophy in the medial temporal lobe, and overall greater memory impairment compared to APOE4- subjects (Emrani et al., 2020). ApoE3 is the most common variant and not related to AD risk. Individuals with two copies of APOE3 (Christchurch R136S mutation) showed resistance to autosomal dominant AD, even though accompanied with unusually high brain Aβ levels, limited tau, and no development of mild cognitive impairment (Arboleda-Velasquez et al., 2019). In contrast ApoE2 is rare and has showed some neuroprotective effects against AD. A clinical study of AD with 5000 subjects indicated that APOE2/2 homozygotes are associated with an exceptionally low likelihood of AD compared to APOE2/3, APOE3/3, and obviously the APOE4/4 (Reiman et al., 2020). Subjects with the APOE2/2 genotype have a 66% lower odds ratio (OR) to develop AD than those with the APOE2/3 genotype, an 87% lower OR than subjects with the APOE3/3 genotype, and an overall 99.6% lower OR than those with APOE4/4 genotype.
5 Genetic Aspects Associated with Alzheimer’s Disease Risk
Pathophysiology of AD is linked to several variants in key genes linked to neurodegeneration, especially in the case of early-onset familial AD (eFAD) which is inherited in an autosomal dominant pattern. Early development of AD is associated with the presence of mutations or duplication in AβPP, PSEN1 (presenilin 1), or PSEN2 (presenilin 2) genes. Back in 1963, several cases of early-onset AD indicated the presence of autosomal-dominant mutations. This was later confirmed in 1987, indicating that the genetic defect causing eFAD maps on loci 21q11.2 to 21q22.2 in chromosome 21, causing a single-point mutation Val-Ile in codon 717 of APP. There are at least 68 variants of the APP gene that, in most of the cases, occur close to the sites of cleavage by γ-secretase. Several of these APP mutations have not shown clear pathogenicity, but others such as the Swedish (KM670/671NL), English (H677R), Taiwanese (D678H), Osaka (E693del), Italian (E693K), Dutch (E693Q), Iowa (D694N), or Artic (E693G) have shown an increased expression and deposition of Aβ into senile plaques. Remarkably, the Icelandic APP mutation A673T seems to have a neuroprotective activity with reduced deposition of Aβ and age-related cognitive decline. PSEN1 and PSEN2 encode for presenilin 1 and 2, respectively, which are subunits of γ-secretase complex that cleave AβPP to produce Aβ40/42 peptide. Databases indicate that there are more than 300 variants of the PSEN1 gene and 64 different mutations in the PSEN2 gene. The presence of rare variants in APP, PSEN1, and PSEN2 may increase the risk for AD (Cruchaga et al., 2012). Although eFAD constitutes around 1–5% of all AD cases, understanding the molecular and biochemical features of these genetic variants is critical to understand the pathological deposition of Aβ, alterations in neuronal metabolism, and overall clinical progression of AD (Fig. 5).
MAPT gene encodes for microtubule-associated protein tau, which is the second pathological hallmark of AD forming neurofibrillary tangles (NFT). Mutations in MAPT are mainly related to tauopathies and frontotemporal dementia, altering the structure of neuronal microtubules and promoting tau aggregation. More than 100 different mutations in MAPT have been reported with variable degrees of pathogenicity, propensity to alter microtubules, and overexpression of tau isoforms and to promote aggregation of NFT within affected neurons. Mutations in the MAPT gene in chromosome 17 have been linked to a higher risk of developing neurodegeneration, promoting tauopathies and abnormal aggregation of NFT within affected neurons. The gene TREM2 encodes for Triggering Receptor Expressed on Myeloid cells 2, and this cellular receptor is expressed in microglia in the brain. Several mutations in TREM2 have now been shown to increase the risk of developing late-onset AD. The relevance of TREM2 is its participation in microglial metabolism, stress responses, and microglial responses to Aβ deposition. Around 70 mutations in TREM2 have been observed; these variants are linked to a high risk of AD, frontotemporal dementia, Parkinson’s disease, amyotrophic lateral sclerosis, and Nasu-Hakola disease (NHD), an autosomal recessive early-onset dementia.
Genome-wide association studies (GWAs) have discovered common risk variants for late-onset AD. These large GWAs of patients diagnosed with AD have found a strong genetic correlation in 29 risk loci, with more than 215 potential causative genes. The genes with significantly associated regions identified in AD include ADAMTS4 and CR1 in chromosome 1; BIN1 and INPPD5 in chromosome 2; HESX1 in chromosome 3; CLNK and HS3ST1 in chromosome 4; HLA-DRB1, TREM2, and CD2AP in chromosome 6; ZCWPW1, EPHA1, and CNTNAP2 in chromosome 7; CLU/PTK2B in chromosome 8; ECHDC3 in chromosome 10; MS4A6A, PICALM, and SORL1 in chromosome 11; SLC24A4 in chromosome 14; ADAM10 and APH1B in chromosome 15; KAT8 in chromosome 16; SCIMP, ADI3, and BZRAP1-AS1 in chromosome 17; SUZ12P1 and ALPK2 in chromosome 18; ABCA7, APOE, AC074212.3, and CD33 in chromosome 19; and CASS4 in chromosome 20 (Jansen et al., 2019). These genes are associated with immune responses, lipid-related processes, and processing or degradation of AβPP. Genetic meta-analysis of AD confirmed risk loci and additional GWAs loci (IQCK, ACE, ADAM10, ADAMTS1, and WWOX) implicated with Aβ processing, tau, immune responses, and lipid processing (Kunkle et al., 2019). An integrative approach that combines proteomics with GWAs found new proteins implicated in AD pathogenesis. Eleven genes showed significance via cis-regulated brain protein abundance (CTSH, DOC2A, ACA1L, LACTB, SNX32, ACE, RTFDC1, CARHSP1, STX6, STX4, and PLEKHA1), which are independent of APOE e4 phenotype (Wingo et al., 2021). Whole-genome sequencing (WGS) of 3347 subjects from 605 multiplex AD families and 1669 unrelated individuals discovered rare genomic variants associated with AD. These 13 new AD candidate loci implicated genes FNBP1L, SEL1L, LINC00298, PRKCH, C15ORF41, C2CD3, KIF2A, APC, LHX9, NALCN, CTNNA2, SYTL3, and CLSTN2, as rare variants contributing to AD risk mainly affecting synaptic function, in contrast to other common AD-associated genes previously described. The discovery of all these loci and genes implicated directly or indirectly in AD pathogenesis opens new insights to understand the complex molecular mechanisms and brain responses during neurodegeneration.
6 Influence of Lifestyle Factors in Alzheimer’s Disease
Different lifestyle factors may contribute to the development of AD. Physical activity or exercise, the nutritional content of the diet, and even sleep patterns are risk factors that directly impact brain health and influence the advancement of AD (Fig. 6). Subjects with long-term exercise interventions show improved blood flow, increased hippocampal volume, and improved neurogenesis. Physical inactivity is, in general, the most common preventable risk factor for developing AD; evidence confirms that subjects with a higher physical activity present a reduced risk of AD. Around half of the AD risks factors are avertible, including diabetes, midlife hypertension, midlife obesity, physical inactivity, depression, smoking, and low educational attainment; in particular, lack of physical activity is the highest attributable lifestyle factor with 21–21.8% of population-attributable risk of AD (Norton et al., 2014). A meta-analysis that included 29 studies concluded that aerobic exercise training is associated with modest improvements in attention, memory, processing speed, and executive function (Smith et al., 2010). In particular, physical activity has an impact in hippocampal volume in elderly subjects; those patients that exercised showed better health, larger hippocampus, and better spatial memory. Diet is an important lifestyle factor influencing etiology of AD. Subjects that follow a Mediterranean diet show lower blood pressure and cognitive benefits. The Mediterranean diet is based on natural fruits, vegetables, whole grains, a variety of legumes, fish, seafood, unsaturated fats from olive oil, and moderated amounts of red meat, eggs, and sweets. The individuals under this diet have shown a lower risk for AD and reduced oxidative stress and inflammation. In contrast, the Western diet has been linked to a higher risk of cognitive decline and AD, possibly linked by the high amounts of processed and refined foods, such as red meat, processed meat, processed carbohydrates, saturated fat, and fried foods. This type of diet has demonstrated a high correlation with development of neurodegenerative processes in the brain, such as elevated Aβ levels, formation of senile plaques, and small vessel disease. This is linked a high-salt diet that may promote AD-like changes, including cognitive impairment and abnormal protein aggregation. Sleep changes have been linked with deteriorating brain health and promoting AD pathology. Alterations in quality sleep measured as non-rapid eye movement (NREM) showed an inverse relationship with cognitive impairment and negatively correlated with Aβ/tau deposition in several areas of the brain (Lucey et al., 2019). Remarkably, alteration of sleep-wake cycle in chronic sleep deprivation has a direct impact in brain damage and cognitive decline, linked with an acute chronical accumulation of Aβ and tau that drive pathology spreading in the affected brain (Holth et al., 2019).
7 Alternative Hypothesis for Pathogenesis of Alzheimer’s Disease
The exact mechanisms of Alzheimer’s disease neuropathology remain unclear; besides the mainstream hypothesis of amyloid-β and tau, alternative ideas have been explored. These alternative hypotheses for explaining the pathogenesis of AD include oxidative stress, impaired glucose-insulin, infection by microorganisms, metal accumulation, arrest in the cell cycle, prion-like transmission, and inflammation.
Oxidative stress or alteration of brain redox and the antioxidant system is frequently found in AD, pointing to a probable hypothesis for understanding the pathogenesis and progression of dementia. This is characterized by altered mitochondrial activity, accumulation of reactive oxygen species (ROS), and formation of free radicals. Oxidative stress leads to protein oxidation, protein nitration, glycoxidation, lipid peroxidation, and ultimately synaptic dysfunction and neuronal death. Dysfunctional glucose metabolism contributed to cognitive decline and progression of neurodegeneration. The hypothesis of metal accumulation in AD suggests that alterations in transport-storage of copper, iron, zinc, aluminum, and calcium present a potential factor in AD pathogenesis. These biometals alter mitochondrial functions and redox state and may trigger protein aggregation forming Aβ plaques and NFT. Amyloid-β aggregates isolated from AD brain have shown to have copper, iron, zinc, and calcium, but not only in the ionic form but also aggregated into nanometer-scale deposits (Plascencia-Villa et al., 2016; Everett et al., 2018). Alterations of biometals in the brain could be related to mitochondrial dysfunction, high oxidative stress, synaptic dysfunction, and cell death (Plascencia-Villa & Perry, 2021). Alterations in glucose and insulin in the brain may cause extensive oxidative damage, protein oxidation and nitrosylation, mitochondrial dysfunction, and oxidative damage to mitochondrial DNA. Decreased ATP production in the brain cells is linked to synaptic dysfunction, neuronal death, and cognitive impairment. The changes in energy metabolism are also associated with mitochondrial dysfunction, microglia, and astrocyte activation in AD. Infectious microorganisms have been proposed to explain late-onset AD etiology, even though it is a controversial topic. Several bacteria, viruses, and fungi in the brain are linked to the onset of dementia, triggering overproduction and aggregation of Aβ senile plaques (Fig. 7). The infectious hypothesis of AD is based on analysis of postmortem brain of AD patients, showing the presence of herpes simplex virus 1 (HSV1) (Jamieson et al., 1991; Readhead et al., 2018), but also several bacteria such as Chlamydophila pneumoniae, Borrelia burgdorferi, and Porphyromonas gingivalis. Fungi and gut microbes are associated with a higher risk of mild cognitive impairment (MCI) and AD. Fungal material was detected in AD subjects in different brain regions, including the frontal cortex, entorhinal cortex/hippocampus, and choroid plexus (Pisa et al., 2015). Alterations in gut microbiota are linked to neuroinflammation and AD in the proposed microbiota-gut-brain axis. This hypothesis proposed that gut microbiota-related products can cause systemic inflammation and brain amyloidosis via endothelial dysfunction.
8 Conclusion
Current hypotheses of AD pathogenesis encompass copious and sophisticated data, but nevertheless have their origin in hallmark pathological lesions described more than a century ago. The literature is now contradictory on whether hallmark lesions are best considered manifestations of neurotoxicity or instead insoluble epiphenomena to the more important events involving soluble, toxic intermediates and the synapse: events that, ironically, are difficult to observe. Based on considerable evidence, however, it is now believed that pathological lesions as well as their constituent proteins of whatever species, be they monomeric, oligomeric, or insoluble fibrils, can be tied to molecular pathogenesis on the basis of disease expression, or a host response, that is fundamentally adaptive over a long period of time. Targeting of such lesions for therapeutic intervention should be entertained only with considerable care, if not the sober realization that it will more likely do more harm than good, as has been demonstrated in abundance.
9 Cross-References
-
Experimental Approach to Alzheimer’s Disease with Emphasis on Insulin Resistance in the Brain
-
Mechanisms Underlying Long-Latency Neurodegenerative Diseases of Environmental Origin
-
Microglial Cell Dysregulation in the Aged Brain and Neurodegeneration
-
Neurotoxicity: A Complex Multistage Process Involving Different Mechanisms
Abbreviations
- AD:
-
Alzheimer’s disease
- APC:
-
Amyloid-β plaque cores
- ApoE:
-
Apolipoprotein E
- Aβ:
-
Amyloid-β peptide
- AβPP:
-
Amyloid-β protein precursor
- BACE1:
-
β-Site AβPP cleaving enzyme-1
- eFAD:
-
Early-onset familial Alzheimer’s disease
- ER:
-
Endoplasmic reticulum
- GWAs:
-
Genome-wide association studies
- HSV1:
-
Herpes simplex virus 1
- LTP:
-
Long-term potentiation
- MAPT:
-
Microtubule-associated protein tau
- MCI:
-
Mild cognitive impairment
- NFT:
-
Neurofibrillary tangles
- NHD:
-
Nasu-Hakola disease
- NREM:
-
Non-rapid eye movement
- OR:
-
Odds ratio
- PSEN1:
-
Presenilin 1
- PSEN2:
-
Presenilin 2
- ROS:
-
Reactive oxygen species
- TREM2:
-
Triggering Receptor Expressed on Myeloid cells 2
- WGS:
-
Whole-genome sequencing
References
Alzheimer, A. (1907). Uber eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeits Psychiat PsychischYGerichtlich Med, 64, 146–148.
Arboleda-Velasquez, J. F., Lopera, F., O’Hare, M., Delgado-Tirado, S., Marino, C., Chmielewska, N., Saez-Torres, K. L., Amarnani, D., Schultz, A. P., Sperling, R. A., Leyton-Cifuentes, D., Chen, K., Baena, A., Aguillon, D., Rios-Romenets, S., Giraldo, M., Guzmán-Vélez, E., Norton, D. J., Pardilla-Delgado, E., … Quiroz, Y. T. (2019). Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: A case report. Nature Medicine, 25, 1680–1683.
Arnold, S. E., Hyman, B. T., Flory, J., Damasio, A. R., & Van Hoesen, G. W. (1991). The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral Cortex, 1, 103–116.
Baki, L., Marambaud, P., Efthimiopoulos, S., Georgakopoulos, A., Wen, P., Cui, W., Shioi, J., Koo, E., Ozawa, M., Friedrich, V. L., Jr., & Robakis, N. K. (2001). Presenilin-1 binds cytoplasmic epithelial cadherin, inhibits cadherin/p120 association, and regulates stability and function of the cadherin/catenin adhesion complex. Proceedings of the National Academy of Sciences of the United States of America, 98, 2381–2386.
Benilova, I., Karran, E., & De Strooper, B. (2012). The toxic Abeta oligomer and Alzheimer’s disease: An emperor in need of clothes. Nature Neuroscience, 15, 349–357.
Berg, L., Mckeel, D. W., Jr., Miller, J. P., Storandt, M., Rubin, E. H., Morris, J. C., Baty, J., Coats, M., Norton, J., Goate, A. M., Price, J. L., Gearing, M., Mirra, S. S., & Saunders, A. M. (1998). Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: Relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Archives of Neurology, 55, 326–335.
Blessed, G., Tomlinson, B. E., & Roth, M. (1968). The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. The British Journal of Psychiatry, 114, 797–811.
Castellani, R. J., Nunomura, A., Lee, H. G., Perry, G., & Smith, M. A. (2008). Phosphorylated tau: Toxic, protective, or none of the above. Journal of Alzheimer’s Disease, 14, 377–383.
Castellani, R. J., Lee, H. G., Siedlak, S. L., Nunomura, A., Hayashi, T., Nakamura, M., Zhu, X., Perry, G., & Smith, M. A. (2009). Reexamining Alzheimer’s disease: Evidence for a protective role for amyloid-beta protein precursor and amyloid-beta. Journal of Alzheimer’s Disease, 18, 447–452.
Castellani, R. J., Rolston, R. K., & Smith, M. A. (2010). Alzheimer disease. Disease-a-Month, 56, 484–546.
Citron, M., Westaway, D., Xia, W., Carlson, G., Diehl, T., Levesque, G., Johnson-Wood, K., Lee, M., Seubert, P., Davis, A., Kholodenko, D., Motter, R., Sherrington, R., Perry, B., Yao, H., Strome, R., Lieberburg, I., Rommens, J., Kim, S., … Selkoe, D. J. (1997). Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nature Medicine, 3, 67–72.
Cruchaga, C., Haller, G., Chakraverty, S., Mayo, K., Vallania, F. L., Mitra, R. D., Faber, K., Williamson, J., Bird, T., Diaz-Arrastia, R., Foroud, T. M., Boeve, B. F., Graff-Radford, N. R., St Jean, P., Lawson, M., Ehm, M. G., Mayeux, R., & Goate, A. M. (2012). Rare variants in APP, PSEN1 and PSEN2 increase risk for AD in late-onset Alzheimer’s disease families. PLoS One, 7, e31039.
De Strooper, B., Annaert, W., Cupers, P., Saftig, P., Craessaerts, K., Mumm, J. S., Schroeter, E. H., Schrijvers, V., Wolfe, M. S., Ray, W. J., Goate, A., & Kopan, R. (1999). A presenilin-1-dependent gamma-secretase-like protease mediates release of notch intracellular domain. Nature, 398, 518–522.
Duff, K., Eckman, C., Zehr, C., Yu, X., Prada, C. M., Perez-Tur, J., Hutton, M., Buee, L., Harigaya, Y., Yager, D., Morgan, D., Gordon, M. N., Holcomb, L., Refolo, L., Zenk, B., Hardy, J., & Younkin, S. (1996). Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature, 383, 710–713.
Emrani, S., Arain, H. A., Demarshall, C., & Nuriel, T. (2020). APOE4 is associated with cognitive and pathological heterogeneity in patients with Alzheimer’s disease: A systematic review. Alzheimer’s Research & Therapy, 12, 141.
Everett, J., Collingwood, J. F., Tjendana-Tjhin, V., Brooks, J., Lermyte, F., Plascencia-Villa, G., Hands-Portman, I., Dobson, J., Perry, G., & Telling, N. D. (2018). Nanoscale synchrotron X-ray speciation of iron and calcium compounds in amyloid plaque cores from Alzheimer’s disease subjects. Nanoscale, 10, 11782–11796.
Giannakopoulos, P., Herrmann, F. R., Bussiere, T., Bouras, C., Kovari, E., Perl, D. P., Morrison, J. H., Gold, G., & Hof, P. R. (2003). Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology, 60, 1495–1500.
Hardy, J. A., & Higgins, G. A. (1992). Alzheimer’s disease: The amyloid cascade hypothesis. Science, 256, 184–185.
Holmes, C., Boche, D., Wilkinson, D., Yadegarfar, G., Hopkins, V., Bayer, A., Jones, R. W., Bullock, R., Love, S., Neal, J. W., Zotova, E., & Nicoll, J. A. (2008). Long-term effects of Abeta42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet, 372, 216–223.
Holth, J. K., Fritschi, S. K., Wang, C., Pedersen, N. P., Cirrito, J. R., Mahan, T. E., Finn, M. B., Manis, M., Geerling, J. C., Fuller, P. M., Lucey, B. P., & Holtzman, D. M. (2019). The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science, 363, 880–884.
Jamieson, G. A., Maitland, N. J., Wilcock, G. K., Craske, J., & Itzhaki, R. F. (1991). Latent herpes simplex virus type 1 in normal and Alzheimer’s disease brains. Journal of Medical Virology, 33, 224–227.
Jansen, I. E., Savage, J. E., Watanabe, K., Bryois, J., Williams, D. M., Steinberg, S., Sealock, J., Karlsson, I. K., Hägg, S., Athanasiu, L., Voyle, N., Proitsi, P., Witoelar, A., Stringer, S., Aarsland, D., Almdahl, I. S., Andersen, F., Bergh, S., Bettella, F., … Posthuma, D. (2019). Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nature Genetics, 51, 404–413.
Kamal, A., Almenar-Queralt, A., Leblanc, J. F., Roberts, E. A., & Goldstein, L. S. (2001). Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature, 414, 643–648.
Kunkle, B. W., Grenier-Boley, B., Sims, R., Bis, J. C., Damotte, V., Naj, A. C., Boland, A., Vronskaya, M., Van Der Lee, S. J., Amlie-Wolf, A., Bellenguez, C., Frizatti, A., Chouraki, V., Martin, E. R., Sleegers, K., Badarinarayan, N., Jakobsdottir, J., Hamilton-Nelson, K. L., Moreno-Grau, S., et al. (2019). Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nature Genetics, 51, 414–430.
Lucey, B. P., Mccullough, A., Landsness, E. C., Toedebusch, C. D., Mcleland, J. S., Zaza, A. M., Fagan, A. M., Mccue, L., Xiong, C., Morris, J. C., Benzinger, T. L. S., & Holtzman, D. M. (2019). Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Science Translational Medicine, 11, eaau6550.
Maiorini, A. F., Gaunt, M. J., Jacobsen, T. M., Mckay, A. E., Waldman, L. D., & Raffa, R. B. (2002). Potential novel targets for Alzheimer pharmacotherapy: I. Secretases. Journal of Clinical Pharmacy and Therapeutics, 27, 169–183.
Masters, C. L., Simms, G., Weinman, N. A., Multhaup, G., Mcdonald, B. L., & Beyreuther, K. (1985). Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proceedings of the National Academy of Sciences of the United States of America, 82, 4245–4249.
Niwa, M., Sidrauski, C., Kaufman, R. J., & Walter, P. (1999). A role for presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded protein response. Cell, 99, 691–702.
Norton, S., Matthews, F. E., Barnes, D. E., Yaffe, K., & Brayne, C. (2014). Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurology, 13, 788–794.
Nunomura, A., Castellani, R. J., Zhu, X., Moreira, P. I., Perry, G., & Smith, M. A. (2006). Involvement of oxidative stress in Alzheimer disease. Journal of Neuropathology and Experimental Neurology, 65, 631–641.
Pietrzik, C. U., Yoon, I. S., Jaeger, S., Busse, T., Weggen, S., & Koo, E. H. (2004). FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. The Journal of Neuroscience, 24, 4259–4265.
Pisa, D., Alonso, R., Rábano, A., Rodal, I., & Carrasco, L. (2015). Different brain regions are infected with fungi in Alzheimer’s disease. Scientific Reports, 5, 15015.
Plascencia-Villa, G., & Perry, G. (2021). Preventive and therapeutic strategies in Alzheimer’s disease: Focus on oxidative stress, redox metals, and ferroptosis. Antioxidants & Redox Signaling, 34, 591–610.
Plascencia-Villa, G., Ponce, A., Collingwood, J. F., Arellano-Jiménez, M. J., Zhu, X., Rogers, J. T., Betancourt, I., José-Yacamán, M., & Perry, G. (2016). High-resolution analytical imaging and electron holography of magnetite particles in amyloid cores of Alzheimer’s disease. Scientific Reports, 6, 24873.
Rajan, K. B., Barnes, L. L., Wilson, R. S., Mcaninch, E. A., Weuve, J., Sighoko, D., & Evans, D. A. (2017). Racial differences in the association between apolipoprotein E risk alleles and overall and total cardiovascular mortality over 18 years. Journal of the American Geriatrics Society, 65, 2425–2430.
Readhead, B., Haure-Mirande, J. V., Funk, C. C., Richards, M. A., Shannon, P., Haroutunian, V., Sano, M., Liang, W. S., Beckmann, N. D., Price, N. D., Reiman, E. M., Schadt, E. E., Ehrlich, M. E., Gandy, S., & Dudley, J. T. (2018). Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron, 99, 64-82.e7.
Reiman, E. M., Arboleda-Velasquez, J. F., Quiroz, Y. T., Huentelman, M. J., Beach, T. G., Caselli, R. J., Chen, Y., Su, Y., Myers, A. J., Hardy, J., Paul Vonsattel, J., Younkin, S. G., Bennett, D. A., De Jager, P. L., Larson, E. B., Crane, P. K., Keene, C. D., Kamboh, M. I., Kofler, J. K., et al. (2020). Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nature Communications, 11, 667.
Santacruz, K., Lewis, J., Spires, T., Paulson, J., Kotilinek, L., Ingelsson, M., Guimaraes, A., Deture, M., Ramsden, M., Mcgowan, E., Forster, C., Yue, M., Orne, J., Janus, C., Mariash, A., Kuskowski, M., Hyman, B., Hutton, M., & Ashe, K. H. (2005). Tau suppression in a neurodegenerative mouse model improves memory function. Science, 309, 476–481.
Scheuner, D., Eckman, C., Jensen, M., Song, X., Citron, M., Suzuki, N., Bird, T. D., Hardy, J., Hutton, M., Kukull, W., Larson, E., Levy-Lahad, E., Viitanen, M., Peskind, E., Poorkaj, P., Schellenberg, G., Tanzi, R., Wasco, W., Lannfelt, L., … Younkin, S. (1996). Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nature Medicine, 2, 864–870.
Selkoe, D. J. (2008). Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behavioural Brain Research, 192, 106–113.
Sienski, G., Narayan, P., Bonner, J. M., Kory, N., Boland, S., Arczewska, A. A., Ralvenius, W. T., Akay, L., Lockshin, E., He, L., Milo, B., Graziosi, A., Baru, V., Lewis, C. A., Kellis, M., Sabatini, D. M., Tsai, L.-H., & Lindquist, S. (2021). APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Science Translational Medicine, 13, eaaz4564.
Smine, A., Xu, X., Nishiyama, K., Katada, T., Gambetti, P., Yadav, S. P., Wu, X., Shi, Y. C., Yasuhara, S., Homburger, V., & Okamoto, T. (1998). Regulation of brain G-protein go by Alzheimer’s disease gene presenilin-1. The Journal of Biological Chemistry, 273, 16281–16288.
Smith, P. J., Blumenthal, J. A., Hoffman, B. M., Cooper, H., Strauman, T. A., Welsh-Bohmer, K., Browndyke, J. N., & Sherwood, A. (2010). Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine, 72, 239–252.
Spires-Jones, T. L., Stoothoff, W. H., De Calignon, A., Jones, P. B., & Hyman, B. T. (2009). Tau pathophysiology in neurodegeneration: A tangled issue. Trends in Neurosciences, 32, 150–159.
Tanzi, R. E. (2005). The synaptic Abeta hypothesis of Alzheimer disease. Nature Neuroscience, 8, 977–979.
Vetrivel, K. S., & Thinakaran, G. (2006). Amyloidogenic processing of beta-amyloid precursor protein in intracellular compartments. Neurology, 66, S69–S73.
Walsh, D. M., Klyubin, I., Fadeeva, J. V., Cullen, W. K., Anwyl, R., Wolfe, M. S., Rowan, M. J., & Selkoe, D. J. (2002). Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature, 416, 535–539.
Wilquet, V., & De Strooper, B. (2004). Amyloid-beta precursor protein processing in neurodegeneration. Current Opinion in Neurobiology, 14, 582–588.
Wingo, A. P., Liu, Y., Gerasimov, E. S., Gockley, J., Logsdon, B. A., Duong, D. M., Dammer, E. B., Robins, C., Beach, T. G., Reiman, E. M., Epstein, M. P., De Jager, P. L., Lah, J. J., Bennett, D. A., Seyfried, N. T., Levey, A. I., & Wingo, T. S. (2021). Integrating human brain proteomes with genome-wide association data implicates new proteins in Alzheimer’s disease pathogenesis. Nature Genetics, 53, 143–146.
Yu, G., Nishimura, M., Arawaka, S., Levitan, D., Zhang, L., Tandon, A., Song, Y. Q., Rogaeva, E., Chen, F., Kawarai, T., Supala, A., Levesque, L., Yu, H., Yang, D. S., Holmes, E., Milman, P., Liang, Y., Zhang, D. M., Xu, D. H., … St George-Hyslop, P. (2000). Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature, 407, 48–54.
Acknowledgments
This research effort was possible by the generous support from the Alzheimer’s Association (AARF-17-529742), NIH National Institute on Aging (R01AG066749), The Lowe Foundation, Semmes Foundation, and Kleberg Foundation.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this entry
Cite this entry
Castellani, R.J., Plascencia-Villa, G., Perry, G. (2022). Pathogenesis of Alzheimer’s Disease. In: Kostrzewa, R.M. (eds) Handbook of Neurotoxicity. Springer, Cham. https://doi.org/10.1007/978-3-031-15080-7_162
Download citation
DOI: https://doi.org/10.1007/978-3-031-15080-7_162
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15079-1
Online ISBN: 978-3-031-15080-7
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences