Abstract
Research in the past 5 years has provided growing evidence supporting the view that the posterior cerebellum is involved in social mentalizing (i.e., theory of mind). We investigated the hypothesis that the posterior cerebellum builds internal action models of our social interactions to predict how other people’s actions will be executed, and what our most likely responses to these actions will be. We developed novel social sequencing tasks that involved a combination of (a) learning or generating chronological sequences of social actions either in an explicit or implicit manner, which (b) require social mentalizing on another person’s mental state such as goals, beliefs, and implied traits. Together, the fMRI results unequivocally confirm the central role of the posterior cerebellar Crus 2 in identifying and automatizing action sequencing during social mentalizing, and in predicting future action sequences based on social mentalizing inferences about others. These findings provided the incentive to investigate non-invasive neurostimulation with healthy participants targeting the posterior cerebellum. Both transcranial magnetic stimulation (TMS) as well as transcranial direct current stimulation (tDCS) showed beneficial effects on social sequencing tasks, as participants generated correct social action sequences quicker. These stimulation techniques guided by novel cerebellar social sequencing insights might have the potential to increase posterior cerebellar plasticity and alleviate social impairments in mental disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Posterior cerebellum
- Action sequencing
- Social mentalizing
- False beliefs
- Trait inferences
- Implicit learning
- Action prediction
- Non-invasive neurostimulation
- Transcranial magnetic stimulation (TMS)
- Transcranial direct current stimulation (tDCS)
Human interaction is highly dependent on the ability to correctly process, interpret, and react to other persons’ behaviors in social situations. A key ability in interpreting social interactions is understanding the mental state of other persons, such as their intentions, beliefs, preferences, and personality traits. This is called mentalizing or theory of mind (for meta-analyses, see Schurz et al. 2014; Van Overwalle 2009). Engaging in successful social encounters relies on learning from our past experiences, which prepares us to anticipate similar social interactions in the future. Social learning is in many respects similar to other types of learning such as the coordination of movements or cognitive procedures (Brown and Brüne 2012; Rushworth et al. 2009). It is an ongoing process that creates and strengthens internal neural representations of frequently processed sequences of observations, movements, or actions. The cerebellum is a key region in implicitly (i.e., with little awareness) automatizing and fine-tuning of such internal representations of recurring event sequences in time and space (Leggio and Molinari 2015). These representations allow us to recognize familiar events sequences so that we immediately know how to move and act appropriately. Importantly, it also allows to identify discrepant event sequences which are atypical or new (Stoodley and Tsai 2021). When such unanticipated event sequences occur, the cerebellum issues error signals that motivate the rapid adjustment of ongoing behavior. If these discrepant events recur, this leads to the creation of novel or adjusted cerebellar internal representations (Heleven et al. 2019; Ferrari et al. 2018; Pu et al. 2020). In a social context, for example, the observation of unfriendly behavior performed by a person to whom we thought was ‘friendly,’ can lead to the adjustment of our trait representation of this person, and the anticipation of more unfriendly behaviors by this person in the future. Or, if we realize that this person’s reaction was triggered by a remark, we might add to our representation that the person is also ‘touchy,’ and adjust our social interaction sequences with this person by adding soothing behaviors, which leads to more desirable social outcomes.
The cerebellum is highly connected to the cerebral cortex (Buckner et al. 2011). The relatively uniform anatomy and physiology of the cerebellum has given rise to the idea that this structure performs the same computational function across diverse domains (Diedrichsen et al. 2019). While the cortical brain areas are considered to be involved in the initial processing of information along seven major domains or networks (Yeo et al. 2011), the cerebellar areas connected to them are considered to be involved in the sequential processing and coordination of this domain-specific information, leading to automatization in time and space. This cerebello-cortical connectivity is accomplished via closed-loop links, starting from a specific location in the cerebral cortex to a specific area in the cerebellum, and back to the same cortical location. The cerebellum thus borrows its functional specialization from its specific connectivity with the cerebral cortex (Buckner et al. 2011).
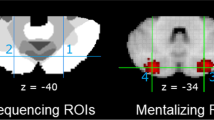
In this chapter, we focus on the cerebellar network involved in social mentalizing. Researchers demonstrated that the posterior cerebellum is mainly involved in processing social information (meta-analyses by Van Overwalle et al. 2014, 2015a). In particular, the posterior part of the cerebellum known as Crus 2 is preferentially engaged during social mentalizing (meta-analysis by Van Overwalle et al. 2020; see Fig. 78.1). Neuroscientists have convincingly shown that there are functional connections between Crus 2 and the mentalizing network in the cortex. Specifically, social mentalizing recruits a circuitry of closed-loops between the posterior cerebellar Crus 2 and the temporo-parietal junction, and via the latter to the medial prefrontal cortex, two key areas in the cortical mentalizing network (Van Overwalle et al. 2015b).
Top (transversal) view of the cerebellum with the mentalizing network marked in white. The centers of the areas in the bilateral Crus 2, which are preferentially engaged during social sequencing tasks, are marked in blue as 1 and 2 with MNI coordinates ±25−75−40. Adapted from Van Overwalle et al. (2020)
1 The Social Sequencing Function of the Posterior Cerebellar Crus 2
But what is the specific function of posterior cerebellar Crus 2? One major hypothesis is that, in line with the uniform function of the cerebellum in (implicit) learning and automatizing of event sequences, Crus 2 is involved in identifying sequences of social events that require mentalizing about other persons. In order to demonstrate this domain-specific mentalizing functionality, new mentalizing tasks have been developed that allowed us to directly investigate social sequencing for different aspects of mentalizing.
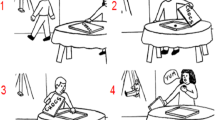
The first tasks that were developed for this purpose were the Picture and Verbal Sequencing tasks, based on earlier work (Langdon and Coltheart 1999; Baron-Cohen et al. 1986, 1999). In both tasks, participants view four events of a story (presented as cartoon-like pictures, see Fig. 78.2; or sentences) in a random order and are instructed to put them in their correct chronological order. A particular aspect of these stories is that they involve others’ false beliefs, this is, when an agent has a different interpretation and representation of reality because this reality changed unbeknownst to them (for an example, see Fig. 78.2). In contrast, in true belief stories an agent is aware of this changed reality. Understanding false beliefs requires distinguishing others’ beliefs from one’s own (Kampis et al. 2017; Wimmer and Perner 1983).
Picture sequencing task. An example of a false-belief sequence. (The correct order is 2—1—4—3: a boy checks the last chocolate in a box—goes playing and leaves the box unattended—meanwhile a girl discovers the last chocolate and eats it—on his return, the boy is surprised to find the box empty; from Langdon and Coltheart 1999; see also Baron-Cohen et al. 1986; Heleven et al. 2019)
An fMRI study by Heleven et al. (2019) demonstrated that healthy participants show more posterior cerebellar activation when putting false or true belief stories in their correct order as compared to social scripts or mechanical stories, suggesting that processing of non-routine social belief sequences recruits more cerebellar activation as compared to routine (social or non-social) sequences. Moreover, story sequencing resulted in more cerebellar activation than a non-sequencing control task in which the story events were presented in the correct chronological order. This is in line with a previous study by Van Overwalle et al. (2019) which demonstrated that cerebellar patients perform less accurately than healthy control participants on the Picture Sequencing task when putting false belief event sequences in their correct order as compared to social scripts or mechanical stories. Both studies highlight the involvement of the cerebellum in mentalizing-related sequence processing regarding other persons’ beliefs.
In order to further investigate the social sequential functionality of the posterior cerebellum, a series of novel sequencing tasks were developed. In these tasks social sequencing was compared against two control conditions involving either (1) social non-sequencing and (2) non-social sequencing. Thus, fleshing out both the sequencing and social functionality of the posterior cerebellum. Figure 78.3 depicts a number of these novel tasks involving the combination of sequencing and a variety of mentalizing aspects such as others’ goal-directed intentions (Li et al. 2021), traits of others (Pu et al. 2020), and trait-related predictions (Haihambo et al. 2021; not shown), not only at an explicit level, but also at an implicit level (Ma et al. 2021a). These tasks were explored in a series of neuroimaging studies on healthy participants, using fMRI.
-
A first aspect of social mentalizing that was investigated is goal-directed navigation (Li et al. 2021; Fig. 78.3a). In this task, participants observed a human-like agent (i.e., smurf) navigating through a grid towards a desired goal. They were instructed to memorize and subsequently replicate the trajectory of the agent. In the control conditions, they merely observed this without memorizing the trajectory (non-sequencing control) or they observed a ball rolling down and randomly following the same trajectory due to the uneven terrain (non-social control).
-
Trait-implying action processing is a second aspect of social mentalizing that was investigated (Pu et al. 2020; Fig. 78.3b). In this task, participants viewed a series of behavioral descriptions that implied a personality trait about a person, and were instructed to memorize and recall the temporal order of these behaviors, and to infer the implied trait. For instance, giving first a compliment, then buying a present, and next picking up a book for someone; these distinct actions all imply kindness as a trait. In control conditions, the order of behaviors had not to be memorized (non-sequential control), or objects were described that implied the same characteristic (non-social control).
-
A third aspect investigated was trait-related prediction. Haihambo et al. (2021) explored this question by reversing the task logic of the trait study by Pu et al. (2020) described above. Participants were first given the trait of a protagonist (e.g., Fumak is dishonest), and then they had to select four out of six possible actions that were consistent with the trait information and put them in the correct chronological order.
-
A fourth investigated aspect is implicit sequence processing (Ma et al. 2021a). Testing implicit social learning is important, because as we stated in our introduction, the cerebellum is necessary for implicit learning and automatization. In the novel Belief Serial Response Time (SRT) task developed by Ma et al. (2021a), participants were requested to identify true and false beliefs of two human-like agents. Participants saw one of two smurfs, receiving flowers at four fixed locations on top of the screen (Fig. 78.3c), and were requested to report how many flowers the smurf thought to have received. The smurf held a true belief when he or she was oriented toward the flowers and a potential false belief when oriented away from the flowers. In the latter case, the correct answer was the number of flowers the smurf thought to have received the last time he or she held a true belief. Unbeknownst to the participants, there was a standard sequence of these true–false beliefs which was repeated over the course of the experiment. This is a crucial aspect of an SRT task (Nissen and Bullemer 1987), because repeating the same sequence of stimuli over time typically shows faster responding over time, demonstrating implicit learning. Moreover, when the standard sequence is interrupted by random orders, the response time goes up again.
(a) Goal-directed trajectories: An example of a trial depicting a smurf moving along a trajectory from start (denoted by “S”) to end (“E”) shown in gray (characters and gray not shown to the participants; Li et al. 2021). (b) Trait-implying Action Sequences: An example of a trial with six sentences implying a trait of which the order had to be memorized (Pu et al. 2020). (c) Implicit Belief Serial Response Time: Participants had to answer as fast as possible how many flowers the smurf could see when oriented to the flowers; and when not, how many flowers he or she had seen previously. Unbeknownst to them, there was a fixed order of true and false beliefs held by the smurfs (Ma et al. 2021b)
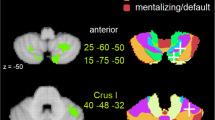
Figure 78.4 depicts the main neuroimaging results of these novel tasks. As can be seen, the critical social sequencing condition showed stronger recruitment in the posterior cerebellar Crus for goal-directed intentions (Li et al. 2021; Fig. 78.4a), trait-implying actions of others (Pu et al. 2020; Fig. 78.4b), and trait-based action prediction (Haihambo et al. 2021; Fig. 78.4c) in comparison with non-sequencing control conditions where participants only observed trajectories or read trait sentences, without memorizing or generating their order. Comparisons against the non-social control conditions showed similar activation of the posterior cerebellar Crus. In the Belief SRT task (Ma et al. 2021b; Fig. 78.4d), a comparison of standard sequences at the later “test” phase (with occasional interruptions by random sequences so that the standard sequence had to be reinstated) versus the earlier “training” phase (with only standard sequences) showed similar recruitment of the posterior cerebellar Crus. Taken together, this series of fMRI studies confirms the posterior cerebellar involvement in different aspects of social mentalizing-related sequence processing. These findings are in line with the hypothesized function of the posterior cerebellum: learning, automatizing, and fine-tuning of internal representations of social sequence information that requires mentalizing.
fMRI contrasts for various mentalizing sequencing tasks revealing activation in the posterior cerebellum. (a) Goal-directed Trajectories (Li et al. 2021). (b) Trait-implying Action Sequences (Pu et al. 2020). (c) Trait-based Action Prediction (Haihambo et al. 2021). (d) Implicit Belief Serial Response Time (Ma et al. 2021b)
2 Clinical Implications
The importance of research on social mentalizing-related sequence processing becomes apparent when we consider the clinical implications of these studies. It has been demonstrated that deficits in the posterior cerebellum may play a crucial role in the onset and maintenance of psychiatric disorders related to mentalizing deficits, such as autism spectrum disorder (D’Mello et al. 2015; Olivito et al. 2018; Velikonja et al. 2019), depression (Bora and Berk 2016; Schutter 2016), bipolar disorder(Lupo et al. 2021), obsessive compulsive disorder (Jansen et al. 2020; Narayanaswamy et al. 2016; Xu et al. 2019; Zhang et al. 2019), addiction (Kornreich et al. 2013; Maurage et al. 2011; Miquel et al. 2016; Onuoha et al. 2016) and schizophrenia (Bernard and Mittal 2015; Brady et al. 2020; Moberget et al. 2018, 2019; Pinkham 2014). The results of the novel social sequencing tasks suggest that the posterior cerebellum may potentially play a central role in social dysfunctions observed in many clinical pathologies, due to impairment in social sequencing that requires mentalizing about others (Van Overwalle et al. 2021). For each of these disorders, we can investigate which aspects of social mentalizing-related sequence processing is key. It might help clinicians to identify and understand social problems in detail, leading to a more personal diagnoses and treatment. The newly developed mentalizing sequencing tasks have the potential to be helpful tools for diagnosing or treating people with cerebellar problems in a clinical setting.
3 Non-invasive Brain Stimulation
As demonstrated earlier, neuroimaging and patient studies have consistently demonstrated that the posterior cerebellar Crus 2 is essential in sequencing social mentalizing information, and thus suggest this cerebellar area as potential target for treatment to improve social functioning. One treatment directly targeting the brain is non-invasive neurostimulation such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). These techniques seem to have beneficial effects on motor, cognitive, and affective problems related to the cerebellum (for a review see Van Dun et al. 2017). In two neurostimulation studies, the potential benefits for social processing were explored using cerebellar TMS (Heleven et al. 2021) and cerebellar tDCS (Heleven et al. 2022). We investigated the effects of these stimulation techniques on healthy participants’ performances on the Picture and Verbal Sequencing tasks. The results in Fig. 78.5 show that in each task, neurostimulation resulted in faster response times for putting the events in their correct order. No such improvements were found after sham (i.e., control) stimulation. However, these studies did not show differential effects of cerebellar neurostimulation for different types of sequences involving mechanical, social script, or social beliefs, except for a surprising lack of effect for false-belief sequences in the tDCS study. A number of parameters of the studies such as the focus of the stimulation and statistical power can explain these non-specific or unexpected results. Follow-up research taking into account limitations of these preliminary studies is needed in order to obtain more convincing results. Moreover, we would expect differential neurostimulation effects for different types of sequences (i.e., mechanical, social script, or social beliefs) when investigating different psychiatric populations with distinct cerebellar problems related to social mentalizing. These groups should therefore be included in future studies.
(Left) Logged response times pre (blue) and post (orange) TMS per story type for the Picture Sequencing task (Heleven et al. 2021). (Right) Response times pre (blue) and post (orange) anodal tDCS for Picture Sequencing (preliminary data). Asterisks indicate significant differences from pre to post stimulation using a two-sided t-test with ∗ p < 0.05, ∗∗∗ p < 0.001. In both sham conditions, pre to post differences were almost all non-significant
Taken together, neuroscientific research has convincingly shown a role for the cerebellum in social mentalizing sequencing. Newly developed tasks allow us to investigate different aspects of mentalizing-related sequences such as goal-directed navigation, trait processing, trait-based prediction, and implicit automatization of belief processing. Research on this topic is ongoing and promising, and will continue to contribute to our understanding and treatment of human interaction related to the social cerebellum. As always, future investigation is needed to increase our insight in the underlying cerebellar mechanisms and potential applications for improving successful social interaction.
References
Baron-Cohen S, Leslie AM, Frith U (1986) Mechanical, behavioural and Intentional understanding of picture stories in autistic children. Br J Dev Psychol 4(2):113–125. https://doi.org/10.1111/j.2044-835x.1986.tb01003.x
Baron-Cohen S, O’Riordan M, Stone V, Jones R, Plaisted K (1999) Recognition of faux pas by normally developing children and children with asperger syndrome or high-functioning autism. J Autism Dev Disord 29(5):407–418
Bernard JA, Mittal VA (2015) Dysfunctional activation of the cerebellum in schizophrenia. Clin Psychol Sci 3(4):545–566. https://doi.org/10.1177/2167702614542463
Bora E, Berk M (2016) Theory of mind in major depressive disorder: a meta-analysis. J Affect Disord 191:49–55. https://doi.org/10.1016/j.jad.2015.11.023
Brady RO, Beermann A, Nye M, Eack SM, Mesholam-Gately R, Keshavan MS, Lewandowski KE (2020) Cerebellar-cortical connectivity is linked to social cognition trans-diagnostically. Front Psych 11:573002. https://doi.org/10.3389/fpsyt.2020.573002
Brown EC, Brüne M (2012) The role of prediction in social neuroscience. Front Hum Neurosci 6:147. https://doi.org/10.3389/fnhum.2012.00147
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Thomas Yeo BT (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106(5):2322–2345. https://doi.org/10.1152/jn.00339.2011
D’Mello AM, Crocetti D, Mostofsky SH, Stoodley CJ (2015) Cerebellar gray matter and lobular volumes correlate with core autism symptoms. NeuroImage 7:631–639. https://doi.org/10.1016/j.nicl.2015.02.007
Diedrichsen J, King M, Hernandez-Castillo C, Sereno M, Ivry RB (2019) Universal transform or multiple functionality? Understanding the contribution of the human cerebellum across task domains. Neuron 102(5):918–928. https://doi.org/10.1016/j.neuron.2019.04.021
Ferrari C, Oldrati V, Gallucci M, Vecchi T, Cattaneo Z (2018) The role of the cerebellum in explicit and incidental processing of facial emotional expressions: a study with transcranial magnetic stimulation. NeuroImage 169:256–264. https://doi.org/10.1016/j.neuroimage.2017.12.026
Haihambo N, Ma Q, Baeken C, Deroost N, Baetens K, Heleven E, Van Overwalle F (2021) Social thinking is for doing: the posterior cerebellum supports prediction of social actions based on personality traits. Soc Cogn Affect Neurosci 17(2):241–251. https://doi.org/10.1093/scan/nsab087
Heleven E, van Dun K, Van Overwalle F (2019) The posterior cerebellum is involved in constructing social action sequences: an fMRI study. Sci Rep 9(1):11110. https://doi.org/10.1038/s41598-019-46962-7
Heleven E, van Dun K, De Witte S, Baeken C, Van Overwalle F (2021) The role of the cerebellum in social and non-social action sequences: a preliminary LF-rTMS study. Front Hum Neurosci 15:593821
Heleven E, Ma Q, Daineffe LB, Vazquez MG, Deroost N, Baetens K, Baeken C (2022) The effect of Cerebellar tDCS on Picture and Verbal Action Sequencing. Unpublished data
Jansen M, Overgaauw S, De Bruijn ERA (2020) Social cognition and obsessive-compulsive disorder: a review of subdomains of social functioning. Front Psych 11:118. https://doi.org/10.3389/fpsyt.2020.00118
Kampis D, Fogd D, Kovács ÁM (2017) Nonverbal components of Theory of Mind in typical and atypical development. Infant Behav Dev 48:54–62. https://doi.org/10.1016/j.infbeh.2016.11.001
Kornreich C, Brevers D, Canivet D, Ermer E, Naranjo C, Constant E et al (2013) Impaired processing of emotion in music, faces and voices supports a generalized emotional decoding deficit in alcoholism. Addiction 108(1):80–88. https://doi.org/10.1111/j.1360-0443.2012.03995.x
Langdon R, Coltheart M (1999) Mentalising, schizotypy, and schizophrenia. Cognition 71:43–71
Leggio M, Molinari M (2015) Cerebellar sequencing: a trick for predicting the future. Cerebellum 14(1):35–38. https://doi.org/10.1007/s12311-014-0616-x
Li M, Ma Q, Baetens K, Pu M, Deroost N, Baeken C et al (2021) Social cerebellum in goal-directed navigation. Soc Neurosci 16(5):467–485. https://doi.org/10.1080/17470919.2021.1970017
Lupo M, Olivito G, Gragnani A, Saettoni M, Siciliano L, Pancheri C et al (2021) Comparison of cerebellar grey matter alterations in bipolar and cerebellar patients: evidence from voxel-based analysis. Int J Mol Sci 22(7):3–7. https://doi.org/10.3390/ijms22073511
Ma Q, Heleven E, Funghi G, Pu M, Baetens K, Deroost N, Van Overwalle F (2021a) Implicit learning of true and false belief sequences. Front Psychol 12:643594. https://doi.org/10.3389/fpsyg.2021.643594
Ma Q, Pu M, Heleven E, Haihambo NP, Baetens K, Baeken C et al (2021b) The posterior cerebellum supports implicit learning of social belief sequences. Cogn Affect Behav Neurosci 21(5):970–992. https://doi.org/10.3758/s13415-021-00910-z
Maurage P, Grynberg D, Noël X, Joassin F, Philippot P, Hanak C et al (2011) Dissociation between affective and cognitive empathy in alcoholism: a specific deficit for the emotional dimension. Alcohol Clin Exp Res 35(9):1662–1668. https://doi.org/10.1111/j.1530-0277.2011.01512.x
Miquel M, Vazquez-Sanroman D, Carbo-Gas M, Gil-Miravet I, Sanchis-Segura C, Carulli D et al (2016) Have we been ignoring the elephant in the room? Seven arguments for considering the cerebellum as part of addiction circuitry. Neurosci Biobehav Rev 60:1–11. https://doi.org/10.1016/j.neubiorev.2015.11.005
Moberget T, Doan NT, Alnæs D, Kaufmann T, Córdova-Palomera A, Lagerberg TV et al (2018) Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite mega-analysis of 983 patients and 1349 healthy controls. Mol Psychiatry 23(6):1512–1520. https://doi.org/10.1038/mp.2017.106
Moberget T, Alnæs D, Kaufmann T, Doan NT, Córdova-Palomera A, Norbom LB et al (2019) Cerebellar gray matter volume is associated with cognitive function and psychopathology in adolescence. Biol Psychiatry 86(1):65–75. https://doi.org/10.1016/j.biopsych.2019.01.019
Narayanaswamy JC, Jose D, Kalmady SV, Agarwal SM, Venkatasubramanian G, Janardhan Reddy YC (2016) Cerebellar volume deficits in medication-naïve obsessive compulsive disorder. Psychiatry Res Neuroimaging 254:164–168. https://doi.org/10.1016/j.pscychresns.2016.07.005
Nissen MJ, Bullemer P (1987) Attentional requirements of learning: evidence from performance measures. Cogn Psychol 32(1):1–32. https://doi.org/10.1016/0010-0285(87)90002-8
Olivito G, Lupo M, Laghi F, Clausi S, Baiocco R, Cercignani M et al (2018) Lobular patterns of cerebellar resting-state connectivity in adults with Autism Spectrum Disorder. Eur J Neurosci 47(6):729–735. https://doi.org/10.1111/ejn.13752
Onuoha RC, Quintana DS, Lyvers M, Guastella AJ (2016) A meta-analysis of theory of mind in alcohol use disorders. Alcohol Alcohol 51(4):410–415. https://doi.org/10.1093/alcalc/agv137
Pinkham AE (2014) Social cognition in schizophrenia. J Clin Psychiatry 75(SUPPL. 2):14–19. https://doi.org/10.4088/JCP.13065su1.04
Pu M, Heleven E, Delplanque J, Gibert N, Ma Q, Funghi G, Van Overwalle F (2020) The posterior cerebellum supports the explicit sequence learning linked to trait attribution. Cogn Affect Behav Neurosci 20(4):798–815
Rushworth MF, Mars RB, Summerfield C (2009) General mechanisms for making decisions? Curr Opin Neurobiol 19(1):75–83. https://doi.org/10.1016/j.conb.2009.02.005
Schurz M, Radua J, Aichhorn M, Richlan F, Perner J (2014) Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci Biobehav Rev 42:9–34. https://doi.org/10.1016/j.neubiorev.2014.01.009
Schutter DJLG (2016) A cerebellar framework for predictive coding and homeostatic regulation in depressive disorder. Cerebellum 15(1):30–33. https://doi.org/10.1007/s12311-015-0708-2
Stoodley CJ, Tsai PT (2021) Adaptive prediction for social contexts: the cerebellar contribution to typical and atypical social behaviors. Annu Rev Neurosci 44:475–493. https://doi.org/10.1146/annurev-neuro-100120-092143
Van Dun K, Bodranghien F, Manto M, Mariën P (2017) Targeting the cerebellum by noninvasive neurostimulation: a Review. Cerebellum 16(3):695–741. https://doi.org/10.1007/s12311-016-0840-7
Van Overwalle F (2009) Social cognition and the brain: a meta-analysis. Hum Brain Mapp 30(3):829–858. https://doi.org/10.1002/hbm.20547
Van Overwalle F, Baetens K, Mariën P, Vandekerckhove M (2014) Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. NeuroImage 86:554–572. https://doi.org/10.1016/j.neuroimage.2013.09.033
Van Overwalle F, Baetens K, Mariën P, Vandekerckhove M (2015a) Cerebellar areas dedicated to social cognition? A comparison of meta-analytic and connectivity results. Soc Neurosci 10(4):337–344. https://doi.org/10.1080/17470919.2015.1005666
Van Overwalle F, D’aes T, Mariën P (2015b) Social cognition and the cerebellum: a meta-analytic connectivity analysis. Hum Brain Mapp 36(12):5137–5154. https://doi.org/10.1002/hbm.23002
Van Overwalle F, De Coninck S, Heleven E, Perrotta G, Taib NOB, Manto M, Mariën P (2019) The role of the cerebellum in reconstructing social action sequences: a pilot study. Soc Cogn Affect Neurosci 14(5):549–558. https://doi.org/10.1093/scan/nsz032
Van Overwalle F, Ma Q, Heleven E (2020) The posterior crus II cerebellum is specialized for social mentalizing and emotional self-experiences: a meta-analysis. Soc Cogn Affect Neurosci 15(9):905–928. https://doi.org/10.1093/scan/nsaa124
Van Overwalle F, Baeken C, Campanella S, Crunelle CL, Heleven E, Kornreich C, Baetens K (2021) The role of the posterior cerebellum in dysfunctional social sequencing. Cerebellum (0123456789). https://doi.org/10.1007/s12311-021-01330-y
Velikonja T, Fett AK, Velthorst E (2019) Patterns of nonsocial and social cognitive functioning in adults with autism spectrum disorder: a systematic review and meta-analysis. JAMA Psychiat 76(2):135–151. https://doi.org/10.1001/jamapsychiatry.2018.3645
Wimmer H, Perner J (1983) Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition 13:103–128
Xu T, Zhao Q, Wang P, Fan Q, Chen J, Zhang H et al (2019) Altered resting-state cerebellar-cerebral functional connectivity in obsessive-compulsive disorder. Psychol Med 49(07):1156–1165. https://doi.org/10.1017/S0033291718001915
Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M et al (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106(3):1125–1165. https://doi.org/10.1152/jn.00338.2011
Zhang H, Wang B, Li K, Wang X, Li X, Zhu J et al (2019) Altered functional connectivity between the cerebellum and the cortico-striato-thalamo-cortical circuit in obsessive-compulsive disorder. Front Psych 10:1–8. https://doi.org/10.3389/fpsyt.2019.00522
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Heleven, E., Overwalle, F.V. (2023). The Social Cerebellum and Human Interactions. In: Gruol, D.L., Koibuchi, N., Manto, M., Molinari, M., Schmahmann, J.D., Shen, Y. (eds) Essentials of Cerebellum and Cerebellar Disorders. Springer, Cham. https://doi.org/10.1007/978-3-031-15070-8_78
Download citation
DOI: https://doi.org/10.1007/978-3-031-15070-8_78
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15069-2
Online ISBN: 978-3-031-15070-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)