Abstract
Neurotransplantation is as a potential therapeutic method for diseases of the nervous system, including the cerebellum. Experiments on laboratory animals have shown many promising results, but also potential risks and limitations, with many questions remaining unanswered. We discuss the main goals of neurotransplantation as a treatment of cerebellar disorders, potential graft effect mechanisms, types of grafts with their advantages and disadvantages, and problems of graft development and functional integration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cerebellar pathologies are manifested by motor, cognitive, behavioral, and emotional disorders. In severe cases, they lead to complete disability of the patient or even premature death. For some cerebellar diseases, such as immune-mediated cerebellar ataxias, effective therapy is available, particularly if administered early upon correct and timely diagnosis at a stage when the cerebellar reserve is sufficient. On the other hand, some pathologies, e.g., trauma, cerebrovascular disorders, tumors, and their surgical treatment lead to irreversible damage to the cerebellar tissue occurring suddenly or when in an advanced stage at the time of diagnosis. Other cases are the slowly progressive cerebellar degenerative diseases, for which, however, usually no causal therapy capable of substantial slowing down of the process is currently available in the clinic, although animal studies have opened novel perspectives (see Chap. 108).
These pathologies lead to the loss of all types of cerebellar neurons focally (e.g., traumas, cerebrovascular diseases, and tumors), or to the rather diffuse extinction of specific neuronal population (e.g., degenerative processes, untreated immune-mediated cerebellitis). Thereby they reduce cerebellar reserve, induce a non-restorable state, and may lead to irreversible deterioration of cerebellar functions (Mitoma and Manto 2016). Because of the problematic treatment of many of the cerebellar diseases and their severe functional consequences, new therapeutic approaches are being investigated. These include, first of all, new or repurposed neuroprotective medicaments, pharmacotherapy or genetic therapy, targeting specifically the pathogenesis of the disease, or non-invasive cerebellar stimulation (see Chap. 106) promoting residual cerebellar functions (Mitoma and Manto 2016; Gandini et al. 2020).
Neurotransplantation also belongs among the experimental therapies for cerebellar damage. It has been investigated as a promising and hopeful approach for decades. Despite intensive research and the quantity of new knowledge, there are still many questions and doubts. Neurotransplantation is not yet a safe, effective, and routine therapy, but remains mostly in the stage of experiments on laboratory animals (Cendelin et al. 2019).
2 Goal of Neurotransplantation Therapy
Cerebellar transplantation goals include (1) substitution of lost cells, (2) promotion of function of residual cerebellar tissue via plasticity stimulation, and (3) prevention (or delay) of degeneration of intrinsic cerebellar neurons. The problem is that proper cerebellar function is dependent on specific cerebellar circuitries, consisting of projection Purkinje neurons, many types of cerebellar cortex interneurons, deep cerebellar nuclei, as well as on multiple afferent and efferent connections with many neural structures. Effective restoration of these complex circuitries by grafted neurons would be extremely difficult since it requires survival and appropriate differentiation, and adequate synaptic integration of a sufficient number of grafted cells. Nevertheless, it would be the only solution if most of the intrinsic neurons of a certain type or a substantial part of the cerebellar tissue is damaged. In the case of cerebellar function support, the sufficient cerebellar reserve (see Chap. 110) must still be preserved to maintain a restorable state (Mitoma and Manto 2016; Cendelin et al. 2018a). If a small amount of tissue (neurons of a certain type) remains, it is not capable of compensating for the loss of function of extinct cells and to maintain cerebellar function on an acceptable level. Analogously, prevention of neuronal degeneration can have a substantial effect only if started before the massive neuronal loss has developed. From this point of view, different goals and mechanisms of graft effect are of importance in slowly developing early-stage diseases, fully developed mild damage and in fully developed massive damage to cerebellar tissue, or advanced stages of progressive uncontrollable cerebellar degeneration (Cendelin et al. 2018a, 2019).
3 Graft Types and Mechanisms of Their Effect
Because grafting mature neural tissue is not possible, immature cerebellar cells, neural precursors or stem cells are used. Individual types of grafts have their advantages and can act via different, in some cases multiple mechanisms to meet one or more of the transplantation goals as described above. Indeed, they also have disadvantages and serious limitations that are of a biological, technical, and, in some cases, ethical nature.
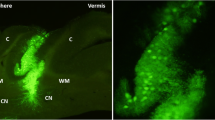
Fetal (embryonic) cerebellar tissue transplantation is a classic approach that has been used in many mouse models of cerebellar degenerations since the 1980s (Sotelo and Alvarado-Mallart 1987; Kohsaka et al. 1988; Triarhou et al. 1996; Kaemmerer and Low 1999; Fuca et al. 2017; Purkartova et al. 2019) (Fig. 109.1). The graft can be injected either as a solid piece of tissue or as a cell suspension, surviving for many weeks or months in both healthy and mutant cerebellum (Kaemmerer and Low 1999; Fuca et al. 2017; Cendelin et al. 2018b; Purkartova et al. 2019). Fetal cerebellar tissue is a good source of Purkinje cells (Fig. 109.1b) and thus could potentially be used to replenish a deficient population of these projection neurons of the cerebellar cortex (Sotelo et al. 1990; Carletti and Rossi 2005; Fuca et al. 2017; Cendelin et al. 2018b; Purkartova et al. 2019). It could also be effective in the substitution of granule cells (Kohsaka et al. 1988).
Nevertheless, there are controversial results regarding the integration of fetal cerebellar grafts into the host’s cerebellum (Kohsaka et al. 1988; Sotelo et al. 1990; Carletti et al. 2008; Cendelin et al. 2018b; Purkartova et al. 2019). There may be differences in intensity of colonization of the host’s cerebellum by grafted Purkinje cells and axons sprouting from the graft into the host’s tissue not only between healthy and mutant mice but also between different types of cerebellar mutants (Purkartova et al. 2019). Alleviation of motor deficits after fetal cerebellar cell transplantation has been found only in some cerebellar mutants (Triarhou et al. 1996; Kaemmerer and Low 1999), while other studies reported no or only weak effects (Fuca et al. 2017; Cendelin et al. 2018b; Purkartova et al. 2019).
As in the neurotransplantation treatment of Parkinson’s disease by fetal dopaminergic neurons, the only available source of fetal cerebellum would be aborted human fetuses. This is a problematic source, because of difficult quality standardization, risk of infection transmission, and also from an ethical point of view.
Neural stem cells can be isolated from donor brain, particularly from its neurogenic zones (e.g., subventricular zone) and immature (fetal) brain. The advantage is the possibility of in vitro expansion of these stem cells, which is, however, limited. Therefore, the source is dependent on donor brain availability. There are similar technical and ethical problems, as in fetal cerebellar tissue. Neural stem cells do not show a strong tendency to differentiate into cerebellar-specific neuronal phenotypes (Chintawar et al. 2009; Rolando et al. 2010; Mendonca et al. 2015); in particular, differentiation into Purkinje cells is rare (Rosario et al. 1997; Lee et al. 2005). On the other hand, grafted neural stem cells have been shown to contribute to the survival of degenerating intrinsic cells by metabolic support via gap junctions (Jaderstad et al. 2010).
Embryonic stem cells can be maintained and expanded effectively in vitro and show a capacity to differentiate into various cell types or cerebellar organoids (Muguruma et al. 2015). Nevertheless, human embryonic cells are ethically problematic and their manipulation is strictly regulated by national legislation.
Carcinoma stem cells are derived from carcinoma (teratocarcinoma) and, as such, they are easy to maintain and expand in vitro. Neural progenitors derived from carcinoma stem cells grafted into the cerebellum of normal and cerebellar mutant mice did not differentiate into local specific neuronal phenotypes and overall morphology of the graft did not promise any positive effect (Houdek et al. 2012). On the other hand, these cells had a therapeutic effect in animal models of amyotrophic lateral sclerosis (Garbuzova-Davis et al. 2002), spinal cord injury (Saporta et al. 2002), or stroke (Hara et al. 2007). Nevertheless, carcinoma stem cells are considered dangerous for human use, although their tumorigenic capacity might be reduced by advanced neurodifferentiation (Pleasure et al. 1992; Garbuzova-Davis et al. 2002).
Adult stem cells can be isolated from various tissues without any significant damage to the donor. Advanced technologies even allow the generation of induced pluripotent stem cells from somatic cells (iPSCs) (Takahashi and Yamanaka 2006). Their features and therapeutic potential are not yet fully explored. Nevertheless, Purkinje cells and cerebellar organoids have already been generated from iPSCs (Wang et al. 2015; Watson et al. 2018; Nayler et al. 2021), suggesting that they can become an effective tool for cerebellar cell therapy. This type of graft would be available for autotransplantation as well (considering in vitro gene therapy in the case of hereditary cerebellar degeneration).
Mesenchymal stem cells (MSCs) can be isolated from, e.g., bone marrow, umbilical cord, or adipose tissue, without injuring the donor substantially. Autotransplantation is also possible. MSCs have been grafted in many mouse models of cerebellar diseases, showing mostly positive results. They usually do not differentiate intensively into cerebellar-specific neurons (Bae et al. 2007; Jones et al. 2010; Chang et al. 2011; Matsuura et al. 2014) and thus do not appear to be an optimum graft for cell replacement therapy.
Effects are rather based on degenerating cell rescue, amelioration of neuropathology in the cerebellum, anti-inflammatory effects, and support of residual cerebellar tissue plasticity (Jones et al. 2010; Matsuura et al. 2014; Huda et al. 2016). These activities are mediated by the production of various bioactive compounds, such as neurotrophic factors and other substances contained in extracellular vesicles (Jones et al. 2010; Nakano and Fujimiya 2021). Cell rescue can also be achieved by fusion (Bae et al. 2007; Diaz et al. 2012; Huda et al. 2016; Kemp et al. 2018). Cell pathology seems to increase the frequency of fusion events compared to healthy tissue (Diaz et al. 2012; Huda et al. 2016; Kemp et al. 2018). Fusion between grafted MSCs and degenerating Purkinje cells generated functioning Purkinje cells, whereas the MSCs provide a normal nucleus that is reprogrammed to maintain Purkinje neuron phenotype of the heterokaryon cell (Bae et al. 2007; Kemp et al. 2018). Intravenous, i.e., systemic administration of MSCs is also possible. In SCA2 mice, it has been shown to be even more effective than local intracerebellar injection (Chang et al. 2011). In aggressive cerebellar degeneration, repeated systemic injection of bone marrow cells might be necessary (Díaz et al. 2019).
4 Host Tissue Factors Determining Graft Survival, Development and Functional Integration
Survival, appropriate differentiation, migration, and synaptic integration of grafted cells would be essential, namely if their specific therapeutic effect based on lost cell substitution is required. These processes depend not only on the properties of the grafted cells (graft type, differentiation stage, in vitro pre-treatment), but also on signals from the host’s tissue milieu. The cerebellum, however, belongs to less neurogenic structures that do not stimulate differentiation of neural stem cells into local specific neurons (Suhonen et al. 1996). Another obstacle can be the granular layer of adult mice that acts as a barrier preventing grafted Purkinje cell axons to grow from the molecular layer toward the deep cerebellar nuclei (Keep et al. 1992; Carletti et al. 2008). Therefore, establishing corticonuclear projections is problematic if grafted Purkinje cells occupy the molecular layer.
From the point of view of the clinical use of cerebellar transplantation, it is important to consider grafting into a pathologically changed tissue providing potentially different signals from the healthy one. These signals can either be of a positive or a negative nature. For instance, the Purkinje cell deficient cortex of Purkinje cell degeneration (pcd) mice has been reported to exert a selective neurotrophic effect on grafted Purkinje cells that colonized the molecular layer (Sotelo and Alvarado-Mallart 1987). In addition, the cerebellum of these mice provides stronger signals that promote the survival of grafted Purkinje cells, compared to the healthy cerebellum (Carletti and Rossi 2005). In contrast, in the cerebellum of Lurcher mice, embryonic cerebellar grafts maintain a rather delimited structure, with a low tendency for cell migration and fiber sprouting, unlike in healthy and pcd mice (Cendelin et al. 2018b; Purkartova et al. 2019). It is not yet clear what factors are responsible for such differences in graft integration between the normal cerebellum and various cerebellar pathologies. In cerebellar mutants, there are differences in microvascular bed (Kolinko et al. 2016), trophic factor levels (Salomova et al. 2020), distribution and activation of glial cells (Baltanas et al. 2013; Salomova et al. 2020), immune system and inflammatory parameters (Mandakova et al. 2005; Vernet-der Garabedian et al. 2013), or corticosterone level reactivity (Hilber et al. 2004) that can potentially influence the levels of cerebellar tissue neurogenicity.
5 Conclusion
It is important to consider that neurotransplantation is an invasive method with many risks, including the classic complications of surgery or infection introduction into the cranial cavity. In addition, the potential tumorigenic capacity of grafted cells should be considered (Garbuzova-Davis et al. 2002; Amariglio et al. 2009). Currently, there have been only a few clinical trials on cerebellar patients (Wu et al. 1991; Tian et al. 2009). Therefore, valid information on the efficiency, long-term persistence of the effect and the safety of neurotransplantation as a therapy of cerebellar diseases is still missing. Although many studies in animal models show the promising efficiency of cerebellar transplantation (Triarhou et al. 1996; Kaemmerer and Low 1999; Bae et al. 2007; Chintawar et al. 2009; Mendonca et al. 2015), other studies are rather pessimistic or controversial (Fuca et al. 2017; Cendelin et al. 2018b; Purkartova et al. 2019). Furthermore, there is a wide spectrum of cerebellar pathologies. Hereditary cerebellar degenerations themselves represent a highly heterogeneous group. Each disease might determine different specific conditions in the patient’s cerebellum that could lead to a different response to grafted cells. Thus, cerebellar transplantation can present different positive as well as negative effects (cost/benefit ratio) in individual diseases and pathologies. Tailored approaches might be considered.
References
Amariglio N, Hirshberg A, Scheithauer BW, Cohen Y, Loewenthal R, Trakhtenbrot L, Paz N, Koren-Michowitz M, Waldman D, Leider-Trejo L, Toren A, Constantini S, Rechavi G (2009) Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med 6(2):e1000029
Bae JS, Han HS, Youn DH, Carter JE, Modo M, Schuchman EH, Jin HK (2007) Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells 25(5):1307–1316
Baltanas FC, Berciano MT, Valero J, Gomez C, Diaz D, Alonso JR, Lafarga M, Weruaga E (2013) Differential glial activation during the degeneration of Purkinje cells and mitral cells in the PCD mutant mice. Glia 61(2):254–272
Carletti B, Rossi F (2005) Selective rather than inductive mechanisms favour specific replacement of Purkinje cells by embryonic cerebellar cells transplanted to the cerebellum of adult Purkinje cell degeneration (pcd) mutant mice. Eur J Neurosci 22(5):1001–1012
Carletti B, Williams IM, Leto K, Nakajima K, Magrassi L, Rossi F (2008) Time constraints and positional cues in the developing cerebellum regulate Purkinje cell placement in the cortical architecture. Dev Biol 317(1):147–160
Cendelin J, Mitoma H, Manto M (2018a) Neurotransplantation therapy and cerebellar reserve. CNS Neurol Disord Drug Targets 17(3):172–183
Cendelin J, Purkartova Z, Kubik J, Ulbricht E, Tichanek F, Kolinko Y (2018b) Long-term development of embryonic cerebellar grafts in two strains of Lurcher mice. Cerebellum (London, England) 17(4):428–437
Cendelin J, Buffo A, Hirai H, Magrassi L, Mitoma H, Sherrard R, Vozeh F, Manto M (2019) Task force paper on cerebellar transplantation: are we ready to treat cerebellar disorders with cell therapy? Cerebellum (London, England) 18(3):575–592
Chang YK, Chen MH, Chiang YH, Chen YF, Ma WH, Tseng CY, Soong BW, Ho JH, Lee OK (2011) Mesenchymal stem cell transplantation ameliorates motor function deterioration of spinocerebellar ataxia by rescuing cerebellar Purkinje cells. J Biomed Sci 18:54
Chintawar S, Hourez R, Ravella A, Gall D, Orduz D, Rai M, Bishop DP, Geuna S, Schiffmann SN, Pandolfo M (2009) Grafting neural precursor cells promotes functional recovery in an SCA1 mouse model. J Neurosci 29(42):13126–13135
Diaz D, Recio JS, Weruaga E, Alonso JR (2012) Mild cerebellar neurodegeneration of aged heterozygous PCD mice increases cell fusion of Purkinje and bone marrow-derived cells. Cell Transplant 21(7):1595–1602
Díaz D, Del Pilar C, Carretero J, Alonso JR, Weruaga E (2019) Daily bone marrow cell transplantations for the management of fast neurodegenerative processes. J Tissue Eng Regen Med 13(9):1702–1711
Fuca E, Guglielmotto M, Boda E, Rossi F, Leto K, Buffo A (2017) Preventive motor training but not progenitor grafting ameliorates cerebellar ataxia and deregulated autophagy in tambaleante mice. Neurobiol Dis 102:49–59
Gandini J, Manto M, Bremova-Ertl T, Feil K, Strupp M (2020) The neurological update: therapies for cerebellar ataxias in 2020. J Neurol 267(4):1211–1220
Garbuzova-Davis S, Willing AE, Milliken M, Saporta S, Zigova T, Cahill DW, Sanberg PR (2002) Positive effect of transplantation of hNT neurons (NTera 2/D1 cell-line) in a model of familial amyotrophic lateral sclerosis. Exp Neurol 174(2):169–180
Hara K, Matsukawa N, Yasuhara T, Xu L, Yu G, Maki M, Kawase T, Hess DC, Kim SU, Borlongan CV (2007) Transplantation of post-mitotic human neuroteratocarcinoma-overexpressing Nurr1 cells provides therapeutic benefits in experimental stroke: in vitro evidence of expedited neuronal differentiation and GDNF secretion. J Neurosci Res 85(6):1240–1251
Hilber P, Lorivel T, Delarue C, Caston J (2004) Stress and anxious-related behaviors in Lurcher mutant mice. Brain Res 1003(1–2):108–112
Houdek Z, Cendelin J, Kulda V, Babuska V, Cedikova M, Kralickova M, Pachernik J, Stefano GB, Vozeh F (2012) Intracerebellar application of P19-derived neuroprogenitor and naive stem cells to Lurcher mutant and wild type B6CBA mice. Med Sci Monit 18(5):Br174–Br180
Huda F, Fan Y, Suzuki M, Konno A, Matsuzaki Y, Takahashi N, Chan JK, Hirai H (2016) Fusion of human fetal mesenchymal stem cells with “degenerating” cerebellar neurons in spinocerebellar ataxia type 1 model mice. PLoS One 11(11):e0164202
Jaderstad J, Jaderstad LM, Li J, Chintawar S, Salto C, Pandolfo M, Ourednik V, Teng YD, Sidman RL, Arenas E, Snyder EY, Herlenius E (2010) Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host. Proc Natl Acad Sci U S A 107(11):5184–5189
Jones J, Jaramillo-Merchan J, Bueno C, Pastor D, Viso-Leon M, Martinez S (2010) Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model of cerebellar ataxia. Neurobiol Dis 40(2):415–423
Kaemmerer WF, Low WC (1999) Cerebellar allografts survive and transiently alleviate ataxia in a transgenic model of spinocerebellar ataxia type-1. Exp Neurol 158(2):301–311
Keep M, Alvarado-Mallart RM, Sotelo C (1992) New insight on the factors orienting the axonal outgrowth of grafted Purkinje cells in the pcd cerebellum. Dev Neurosci 14(2):153–165
Kemp KC, Dey R, Verhagen J, Scolding NJ, Usowicz MM, Wilkins A (2018) Aberrant cerebellar Purkinje cell function repaired in vivo by fusion with infiltrating bone marrow-derived cells. Acta Neuropathol 135(6):907–921
Kohsaka S, Takayama H, Ueda T, Toya S, Tsukada Y (1988) Reorganization of cerebellar cell suspension transplanted into the weaver mutant cerebellum and immunohistochemical detection of synaptic formation. Neurosci Res 6(2):162–166
Kolinko Y, Cendelin J, Kralickova M, Tonar Z (2016) Smaller absolute quantities but greater relative densities of microvessels are associated with cerebellar degeneration in Lurcher mice. Front Neuroanat 10:35
Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ (2005) Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci 8(6):723–729
Mandakova P, Sinkora J, Sima P, Vozeh F (2005) Reduced primary T lymphopoiesis in 3-month-old lurcher mice: sign of premature ageing of thymus? Neuroimmunomodulation 12(6):348–356
Matsuura S, Shuvaev AN, Iizuka A, Nakamura K, Hirai H (2014) Mesenchymal stem cells ameliorate cerebellar pathology in a mouse model of spinocerebellar ataxia type 1. Cerebellum (London, England) 13(3):323–330
Mendonca LS, Nobrega C, Hirai H, Kaspar BK, Pereira de Almeida L (2015) Transplantation of cerebellar neural stem cells improves motor coordination and neuropathology in Machado-Joseph disease mice. Brain 138(Pt 2):320–335
Mitoma H, Manto M (2016) The physiological basis of therapies for cerebellar ataxias. Ther Adv Neurol Disord 9(5):396–413
Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y (2015) Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep 10(4):537–550
Nakano M, Fujimiya M (2021) Potential effects of mesenchymal stem cell derived extracellular vesicles and exosomal miRNAs in neurological disorders. Neural Regen Res 16(12):2359–2366
Nayler S, Agarwal D, Curion F, Bowden R, Becker EBE (2021) High-resolution transcriptional landscape of xeno-free human induced pluripotent stem cell-derived cerebellar organoids. Sci Rep 11(1):12959
Pleasure SJ, Page C, Lee VM (1992) Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J Neurosci 12(5):1802–1815
Purkartova Z, Tichanek F, Kolinko Y, Cendelin J (2019) Embryonic cerebellar graft morphology differs in two mouse models of cerebellar degeneration. Cerebellum (London, England) 18(5):855–865
Rolando C, Gribaudo S, Yoshikawa K, Leto K, De Marchis S, Rossi F (2010) Extracerebellar progenitors grafted to the neurogenic milieu of the postnatal rat cerebellum adapt to the host environment but fail to acquire cerebellar identities. Eur J Neurosci 31(8):1340–1351
Rosario CM, Yandava BD, Kosaras B, Zurakowski D, Sidman RL, Snyder EY (1997) Differentiation of engrafted multipotent neural progenitors towards replacement of missing granule neurons in meander tail cerebellum may help determine the locus of mutant gene action. Development 124(21):4213–4224
Salomova M, Tichanek F, Jelinkova D, Cendelin J (2020) Abnormalities in the cerebellar levels of trophic factors BDNF and GDNF in pcd and lurcher cerebellar mutant mice. Neurosci Lett 725:134870
Saporta S, Makoui AS, Willing AE, Daadi M, Cahill DW, Sanberg PR (2002) Functional recovery after complete contusion injury to the spinal cord and transplantation of human neuroteratocarcinoma neurons in rats. J Neurosurg 97(1 Suppl):63–68
Sotelo C, Alvarado-Mallart RM (1987) Embryonic and adult neurons interact to allow Purkinje cell replacement in mutant cerebellum. Nature 327(6121):421–423
Sotelo C, Alvarado-Mallart RM, Gardette R, Crepel F (1990) Fate of grafted embryonic Purkinje cells in the cerebellum of the adult “Purkinje cell degeneration” mutant mouse. I. Development of reciprocal graft-host interactions. J Comp Neurol 295(2):165–187
Suhonen JO, Peterson DA, Ray J, Gage FH (1996) Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature 383(6601):624–627
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Tian ZM, Chen T, Zhong N, Li ZC, Yin F, Liu S (2009) Clinical study of transplantation of neural stem cells in therapy of inherited cerebellar atrophy. Beijing Da Xue Xue Bao Yi Xue Ban 41(4):456–458
Triarhou LC, Zhang W, Lee WH (1996) Amelioration of the behavioral phenotype in genetically ataxic mice through bilateral intracerebellar grafting of fetal Purkinje cells. Cell Transplant 5(2):269–277
Vernet-der Garabedian B, Derer P, Bailly Y, Mariani J (2013) Innate immunity in the Grid2Lc/+ mouse model of cerebellar neurodegeneration: glial CD95/CD95L plays a non-apoptotic role in persistent neuron loss-associated inflammatory reactions in the cerebellum. J Neuroinflammation 10:65
Wang S, Wang B, Pan N, Fu L, Wang C, Song G, An J, Liu Z, Zhu W, Guan Y, Xu ZQ, Chan P, Chen Z, Zhang YA (2015) Differentiation of human induced pluripotent stem cells to mature functional Purkinje neurons. Sci Rep 5:9232
Watson LM, Wong MMK, Vowles J, Cowley SA, Becker EBE (2018) A simplified method for generating Purkinje cells from human-induced pluripotent stem cells. Cerebellum (London, England) 17(4):419–427
Wu CY, Bao XF, Zhang C, Zhang QL (1991) Fetal tissue grafts for cerebellar atrophy. Chin Med J (Engl) 104(3):198–203
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Cendelin, J., Purkartova, Z. (2023). Grafting. In: Gruol, D.L., Koibuchi, N., Manto, M., Molinari, M., Schmahmann, J.D., Shen, Y. (eds) Essentials of Cerebellum and Cerebellar Disorders. Springer, Cham. https://doi.org/10.1007/978-3-031-15070-8_109
Download citation
DOI: https://doi.org/10.1007/978-3-031-15070-8_109
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15069-2
Online ISBN: 978-3-031-15070-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)