Abstract
Background. There still exists limitations in the recovery of severe upper limb impairment after stroke, and brain computer interface maybe a hopeful therapy. Methods. A 76-year-old male hemiplegic patient with severe paretic upper limb was admitted. In the first four weeks, 20 sessions classic motor imagery was added in addition to routine treatments. Then, 20 sessions brain-computer interface training was added over the next four weeks. Behavioral characteristics, neuroelectrophysiology and neuroimaging were assessed at multiple time, such as the FuglMeyer Assessment Upper Extremity, the Motor Status Scale (MSS), the Action Research Arm Test (ARAT), Active range of motion of the paretic wrist and Modified Barthel Index (MBI). Functional magnetic resonance imaging (fMRI) was used to investigate the effect of the above interventions on the recovery of brain and its structural plasticity. Results. The patient's upper limb motor function improved after two different therapy interventions, however, the efficacy of BCI training was more obvious: after classic motor imagery, the paretic wrist could actively flex, but extension is still irrealizable. However, after BCI training, the paretic wrist was able to extend proactively. The fMRI findings revealed positive and dynamic changes on brain structure and function. Conclusion. BCI training could effectively promote the movement recovery after stroke than traditional motor imagery even if they showed apparent initial paralysis. An association between functional improvement and brain structure remodeling was observed. These findings serve as a conceptual investigations to encourage further relevant research.
Y.-Q. Hu, R.-R. Lu and T.-H. Gao—Contributed equally to this work.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Globally, stroke is still the third leading cause of disability [1]. Stroke patients mostly left different functional disorders, such as motor dysfunction, cognitive impairment, speech disorder, dysphagia, etc., among which motor dysfunction is the most common [2].
Although a proportion of patients can obtain a certain degree of functional recovery through rehabilitation training, such as constrained induced movement therapy [3], task-oriented training [4]. However, existing rehabilitation interventions have shown very little efficacy for those chronic stroke patients with severe motor impairment [5], which cause great economic and mental burden on the families of stroke patients and society [6]. Therefore, it is urgent to find more effective treaments. Previous studies have proved that classical motor imagery could promote the motor recovery, which has a lower requirements for patients' actual retained motor function [7, 8]. Intensive training could be avoided which might cause abnormal movement patterns and compensatory movements, meanwhile, there was an internal stimulation to their brain which could increase the familiarity of movement [9]. However, classical motor imagery also have some limitations. During training, patients are required to have inact cognitive function to match with motor imagery activities, and maintain attention simultaneously. This is difficult in practical training. Therefore, the researches about it were heterogeneous and had different clinical efficacy [10,11,12]. Brain-computer interface (BCI) is a new rehabilitation technology, which can directly convert the signals generated by brain activities into computer commands to interact with the surrounding environment without the participation of peripheral nerves and muscles [13]. This stimulation process may have an impact on brain plasticity, thus promoting the motor function recovery for stroke patients [14]. This is a good supplement to the current clinical treatment. The purpose of this study was to compare the clinical efficacy of classical motor imagery and brain-computer interface on severe upper limb impairment in stroke patients. Functional Magnetic Resonance Imaging (fMRI) was applied to observe brain functional and structural plasticity to explore the potential mechanism of brain-computer interface to promote functional recovery.
2 Methodology
2.1 Patients
A 76-year-old man with left hemiplegia caused by cerebral infarction in the right lateral paraventricular and basal ganglia was recruited. He had severe left hemiplegia—there was no active movement on his wrist, his left finger could only co-flex within a range of 1.5 cm and the initiation of flexion was slow. He has significantly limitations for activities of daily living. He depended on assistance for his personal hygiene and dressing and he walked slowly with the aid of a cane. Moreover, the paretic hand prevented him from lifting his arm. The absence of speech and cognitive impairments allowed him to perform motor imagery and BCI training accurately under guidance. The patient had hypertension in the past, which was controlled by oral drugs and was stable. He received regular rehabilitation immediately after stroke, but there was no substantial improvement in the left wrist and hand more than 4 months later (P1). Before entering the study, we had fully communicated with the patient and signed the informed consent form, which was approved by the Ethics Committee of Huashan Hospital affiliated to Fudan University.
2.2 Intervention
Classical Motor Imagery. In the first four weeks, the patient underwent classical motor imagery (P2) [15]. The treatment was carried out in a relatively quiet and comfortable environment. Firstly, the patient was told to keep in a comfortable sitting position, then closed his eyes and imagined that he was in a familiar environment under the guidance of the therapist. Secondly, the therapist helped him to relax the body, and start instructing him to imagine the relevant movements. The content of the imagery task was adjusted by the therapist which could be combined with occupational therapy. For example, “extending slowly the paretic arm to touch the red apple placed in front of him, and then withdrawing it slowly”, “imaging the active flexion and extension of the paretic wrist and fingers” or “stretching out the affected side arm, picking up the water cup on the table to drink water” and so on. Each imagery task could be repeated several times. After the imagery task, patient was asked to lift both hands for 10 times. Each training consisted of 3–4 sessions, and there was 2 min to break between each session. The whole last approximately 30 min, 5 times a week last for 4 weeks. In the process of training, the therapist should pay attention to keep the patient focused and avoid interference. After each session, the patient underwent routine rehabilitation therapy, including physical therapy, occupational therapy, etc.
BCI Training.
Over the next four weeks, the patient underwent brain-computer interface (P3). In this study, a new brain-computer interface technology based on motor imagery was applied, combined with light touch stimulation and visual feedback from VR which we call it “multimodal perceptual feedback training”. Before the treatment, the patient was given an EEG cap and connected to the electrode with a conductive ointment. After the training, the patient first received light touch input from both hands (completed by a brush), at the same time, the patient wore a pair of VR glasses which could see the movement of the virtual hands. After the sensory stimulation stopped, the patient conducted an imagery task about extension of the left or right wrist according to the prompt in the screen, meanwhile, the screen would give a feedback on the degree of imagery task completion through the action of the virtual hands, during which the patient continuously received visual stimulation and feedback. Each training session was 30 min with four cycles, each containing 20 random left or right hand imagery tasks lasting approximately 6 min with a 2-min rest between cycles. Five times a week, for a total of 20 training sessions. Similarly, the patient would undergo the same routine treatment after BCI training.
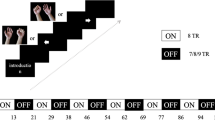
The brief procedure is listed in Fig. 1.
2.3 Functional Magnetic Resonance Imaging
fMRI scans were performed before and after BCI training. The task was designed as the motor imagery of both hands, consisting of three types of tasks which were named left hand grip, right hand grip and rest respectively. A, B, and C were used to represent the three tasks, each performed for 20 s with no interval between task. In sequence of ABC, BCA, CAB and repeated three times.
Echo planer imaging (EPI) was used to acquire functional MR images data: TR (repetition Time) = 2000 ms, TE(Echo Time) = 30 ms, FOV(Field of view) = 192 mm × 192 mm, flip angle = 90, voxel size = 3 mm × 3 mm × 3mm, matrix size = 64 × 64. slice thickness = 3 mm, and slice gap = 0 mm. Forty-two slices of axial planer images were acquired, including the whole cerebral cortex and the cerebellum.
Preprocessing of fMRI data was performed using the SPM12 software, the main steps included: ① Time difference correction between all scan layers was performed. ② The interlayers of the rigid body were rearranged for head movement correction. ③ The structural images of T1 are divided into different components such as gray matter and white matter and matched into the functional images, which the structural and functional images are converted into the Montreal Neurological Institute (MNI) standard space. ④ Finally, spatial smoothing was performed to form a spatial smoothing of a half-height full-width 6 mm Gaussian smoothing core. Statistical significance was considered at p < 0.001 (after FWE correction) to observe the changes in the activated brain regions.
3 Results
All assessments were conducted by the same physician who did not participate the study before and after treatment.
Fugl-Meyer Assessment Upper Extremity (FMA-UE), Motor Status Scale (MSS), Action Research Arm Test (ARAT) and the active motion range of paretic wrist were observed during P1, P2 and P3 to assess the arm and hand motor function. Modified Barthel index (MBI) was used to assess the daily living ability. Functional magnetic resonance imaging (fMRI) was applied to assess the brain change of structure and function.
3.1 Clinical Outcome
The parameters were improved in both different treatment options (Table 1). After classic motor imagery, the motor range and strength of the left shoulder was increased. Moreover, the patient was able to actively flex his wrist (flexion angle was about 10°), the active range co-flexion of the fingers increased from 1.5 cm to about 2.5 cm, and the muscle strength increased in the flexion of fingers, but he still could not extend his paretic wrist or finger. However, after BCI training, the patient could actively extend his paretic wrist (extension angle was about 15–20°) and the flexion angle of wrist also increased to about 60°. He could pinch a piece of A4 paper by his thumb and index finger and maintain a certain strength. The sEMG results also improved, with synergistic contraction rates (extensor/extensor + flexor * 100%) calculated for both treatments.
3.2 fMRI
No significant brain region activation was observed during the motor imagery task of the paretic hand before the BCI training. While, after 20 sessions of BCI training, extensive regional activation occurred in both cerebral hemispheres, including bilateral motor cortex, superior frontal gyrus, middle frontal gyrus and posterior-temporal lobe among which the strongest activation was the bilateral motor cortex.
Combined with the results of the clinical evaluation in the previous chapter (Table 1), the patient showed a more pronounced improvement in upper limb motor function after BCI training. The positive result suggested that the activation of bilateral motor cortex may play a meaningful role in functional recovery, which might achieve functional remodeling after injury.
In the motor imagery of the unaffected hand, the differences all appeared in the motor cortex of the left hemisphere both before and after intervention, but there were significant differences in the specific brain regions of activation.
Prior to the intervention, standard left motor cortex activation was performed during the right-hand motor imagery task, while the activation area changed after the intervention and began to move downward (although it was still in the motor cortex, but closer to the facial muscles area). The change seemed to suggest a functional remodeling occurred in the left motor cortex after intervention, in which a part of the left motor cortex was “seconded” to dominate the motor function of the paretic hand to promote the functional recovery (Fig. 2).
4 Discussion
According to our study, after 20 sessions intervention, brain-computer interface based on motor imagery showed a better rehabilitation superiority than classical motor imagery that change value of each evaluation index showed more obvious improvement (Table 1), especially functional recovery in wrist and hand. Those previous stroke patients with severe hemiplegia rarely or even cannot obtain the functional recovery of wrist and hand, which also strikes the patients’ enthusiasm and confidence in recovery. Therefore, we believe that this finding is a great encouragement and has great significance for the existing clinical rehabilitation. As we know, stroke patients with severe paretic upper limb rarely or even unable to recover their wrist and hand functions, which greatly struck the patients’ enthusiasm and confidence in recovery. Therefore, we believed that what we found in this study had a massive inspiration and important significance to the current clinical rehabilitation. Pichiorri had demonstrated that the rehabilitation effects of motor imagery could be enhanced in BCI system using a randomized controlled trial [16], which also indicated the clinical feasibility and effectiveness of this study from the side. Recently, our research group has also carried out relevant clinical trials, and the results showed that compared with classical motor imagery, the BCI system based on motor imagery had a better trend of promoting the recovery of upper limb motor function [17].
Of course, we needed to recognize that this was just a case report and may be with some contingency. Refining and expanding studies need be done in future, preferably to conduct randomized controlled trials to obtain more reliable conclusions. The results of this study still indicate huge promise for patients with severe paretic upper limb.
From the fMRI results, corresponding structural changes occurred in both bilateral brains after BCI training. Motor cortex of the ipsilateral hemisphere showed obviously enhanced activity which suggested that BCI training might help to enhanced the activity in motion regions of the ipsilateral hemisphere to achieve functional recovery. Meanwhile, It should also be noted that the motor cortex of the contralateral hemisphere also showed corresponding changes. The brain area activated during the task of right-hand motor imagery was shifted downward from the standard M1 region to the facial muscle group region. The dynamic change might suggest that the contralateral hemisphere might “second” some part of motor region to assist the ipsilateral hemisphere to complete the control to motion function. This was different from the previous theory of contralateral compensation or ipsilateral compensation [18, 19]. This might suggest that function recovery after stroke do not depend on a simple brain structural change, but might be related to the lesion location and severity, thus showing changeable structural restructuring and functional improvement. These above still need to be verified by more rigorous randomized controlled trials.
In addition, the ability of daily life did not show visible changes, considering that might be related to the choice of assessment scale, and we also should realize that the improvement of motor function did not directly represent the improvement of life ability because of the transformation from motor function to life ability still need many aspects to cooperate which also suggested that we need to consider more in the future research.
5 Conclusion
As an exploratory case-report, this study has a suggestive and encouraging effect that BCI training may be effective in promoting functional recovery in chronic stroke patients with severe hemiplegic upper limb, especially in those with severely paretic wrist. The fMRI results also suggest that BCI training may facilitate brain structural remodeling.
References
GBD 2019 Stroke Collaborators: Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20(10), 795–820 (2021). https://doi.org/10.1016/S1474-4422(21)00252-0
Feigin, V.L.: Stroke: practical management. JAMA 300(19), 2311–2312 (2008). https://doi.org/10.1001/jama.2008.633
Wolf, S.L., Winstei, C.J., Mille, J.P., et al.: Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 296(17), 2095–2104 (2006). https://doi.org/10.1001/jama.296.17.2095
Winstein, C.J., Wolf, S.L., Dromerick, A.W., et al.: Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: the ICARE randomized clinical trial. JAMA 315(6), 571–581 (2016). https://doi.org/10.1001/jama.2016.0276
Caria, A., Weber, C., Brötz, D., et al.: Chronic stroke recovery after combined BCI training and physiotherapy: a case report. Psychophysiology 48(4), 578–582 (2011). https://doi.org/10.1111/j.1469-8986.2010.01117.x
Pandian, J.D., Sebastian, I.A.: Integrated approach to stroke burden: are we doing enough? Lancet Neurol. 20(10), 774–775 (2021). https://doi.org/10.1016/S1474-4422(21)00287-8
Page, S.J., Levine, P., Leonard, A.: Mental practice in chronic stroke: results of a randomized, placebo-controlled trial. Stroke 38(4), 1293–1297 (2007). https://doi.org/10.1161/01.STR.0000260205.67348.2b
Wang, H., Xu, G., Wang, X., et al.: The Reorganization of resting-state brain networks associated with motor imagery training in chronic stroke patients. IEEE Trans. Neural Syst. Rehabil. Eng. 27(10), 2237–2245 (2019). https://doi.org/10.1109/TNSRE.2019.2940980
Hétu, S., Grégoire, M., Saimpont, A., et al.: The neural network of motor imagery: an ALE meta-analysis. Neurosci. Biobehav. Rev. 37(5), 930–949 (2013). https://doi.org/10.1016/j.neubiorev.2013.03.017
Liu, H., Song, L.P., Zhang, T.: Mental practice combined with physical practice to enhance hand recovery in stroke patients. Behav. Neurol. 2014, 876416 (2014). https://doi.org/10.1155/2014/876416
Rayegani, S.M., Raeissadat, S.A., Sedighipour, L., et al.: Effect of neurofeedback and electromyographic-biofeedback therapy on improving hand function in stroke patients. Top Stroke Rehabil. 21(2), 137 (2014). https://doi.org/10.1310/tsr2102-137
Schuster, C., Butler, J., Andrews, B., et al.: Comparison of embedded and added motor imagery training in patients after stroke: results of a randomised controlled pilot trial. Trials 13, 11 (2012). https://doi.org/10.1186/1745-6215-13-11.PMID: 22269834
Nicolas-Alonso, L.F., Gomez-Gil, J.: Brain computer interfaces, a review. Sensors (Basel) 12(2), 1211–1279 (2012). https://doi.org/10.3390/s120201211
Daly, J.J., Cheng, R., Rogers, J., et al.: Feasibility of a new application of noninvasive Brain Computer Interface (BCI): a case study of training for recovery of volitional motor control after stroke. J. Neurol. Phys. Ther. 33(4), 203–211 (2009). https://doi.org/10.1097/NPT.0b013e3181c1fc0b
Page, S.J., Levine, P., Sisto, S.A., Johnston, M.V.: Mental practice combined with physical practice for upper-limb motor deficit in subacute stroke. Phys. Ther. 81(8), 1455–1462 (2001). https://doi.org/10.1093/ptj/81.8.1455
Pichiorri, F., Morone, G., Petti, M., et al.: Brain-computer interface boosts motor imagery practice during stroke recovery. Ann. Neurol. 77(5), 851–865 (2015). https://doi.org/10.1002/ana.24390
Hu, Y.Q., Gao, T.H., Li, J., et al.: Motor imagery-based brain-computer interface combined with multimodal feedback to promote upper limb motor function after stroke: a preliminary study. Evid Based Complement Alternat. Med. 2021, 1116126 (2021). https://doi.org/10.1155/2021/1116126
Johansen-Berg, H., Rushworth, M.F., Bogdanovic, M.D., et al.: The role of ipsilateral premotor cortex in hand movement after stroke. Proc. Natl. Acad. Sci. USA 99(22), 14518–14523 (2002). https://doi.org/10.1073/pnas.222536799
Schaechter, J.D., Perdue, K.L.: Enhanced cortical activation in the contralesional hemisphere of chronic stroke patients in response to motor skill challenge. Cereb Cortex. 18(3), 638–647 (2008). https://doi.org/10.1093/cercor/bhm096
Acknowledgements
This work was supported by Natural Science Foundation of China (NSFC) [grant number 81902280].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Hu, YQ., Lu, RR., Gao, TH., Zhuang, J., Bai, YL. (2022). Effects of Brain-Computer Interface and Classical Motor Imagery for Upper Limb Impairment After Stroke: A Case Report. In: Liu, H., et al. Intelligent Robotics and Applications. ICIRA 2022. Lecture Notes in Computer Science(), vol 13457. Springer, Cham. https://doi.org/10.1007/978-3-031-13835-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-13835-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-13834-8
Online ISBN: 978-3-031-13835-5
eBook Packages: Computer ScienceComputer Science (R0)