Abstract
This chapter describes preoperative work-up, operative planning, and the technique of peroral endoscopic myotomy for achalasia, as well as utilizing EndoFLIPTM and troubleshooting difficult situations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Peroral endoscopic myotomy
- Achalasia
- Timed barium esophagram
- High-resolution impedance manometry
- EndoFLIP
This chapter describes preoperative work-up, operative planning, and the technique of peroral endoscopic myotomy for achalasia, as well as utilizing EndoFLIPTM and troubleshooting difficult situations.
9.1 Clinical History
A 56-year-old woman presents in the clinic with a 5-year history of chest pain, heartburn, and dysphagia. She was initially diagnosed with gastroesophageal reflux disease and put on a proton-pump inhibitor. Her symptoms did not resolve with anti-acid medication, and a subsequent esophagogastroduodenoscopy (EGD) was negative for esophagitis.
As her dysphagia progressed from solids to liquids, a complete work-up for esophageal motility disorder is performed:
-
Repeat EGD: dilated and tortuous esophagus without mucosal-based lesions, suspicious for achalasia (Fig. 9.1).
-
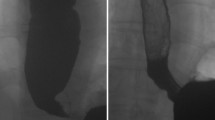
Timed barium esophagram: smooth tapering of the distal esophagus and a persistent contrast column of 10.2 cm at 5 min, indicative of bolus retention (Fig. 9.2).
-
High-resolution impedance manometry (HRIM): elevated mean 4-s integrated relaxation pressure (IRP) of 32.4 mmHg, 10 failed swallows, and panesophageal pressurization consistent with type II achalasia according to the Chicago Classification (Fig. 9.3).
She is presented with three treatment options: pneumatic dilation, laparoscopic Heller myotomy, and peroral endoscopic myotomy (POEM). She chose to undergo POEM.
9.2 Operation
9.2.1 Patient Preparation and Operative Setup
Prior to surgery, the patient is kept on a clear liquid diet for 48 h and completes a 5-day course of oral nystatin or fluconazole for Candida prophylaxis.
The patient is positioned supine with the right arm abducted and the left arm tucked at the side, with the abdomen prepped in case needle decompression is required for the presence of capnoperitoneum. The bed is lowered and step stools positioned as needed to minimize strain and fatigue of the operator (Fig. 9.4). Sequential compression devices are utilized for thromboprophylaxis and a second-generation cephalosporin or comparable preoperative antibiotic is given. POEM is performed under general anesthesia with endotracheal intubation and skeletal muscle paralysis. Special care with rapid sequence induction and intubation is utilized to prevent aspiration.
The EndoFLIP machine is positioned adjacent to the endoscopy tower to allow easy serial placement of the EndoFLIP catheter. The catheter is primed and purged prior to the start of the procedure.
9.2.2 Operative Procedure
9.2.2.1 Step 1: Initial Endoscopy and Post-induction EndoFLIP Measurement
Endoscopy is performed using a single-channel, high-definition gastroscope with CO2 insufflation. The esophagus and stomach are aspirated of any retained fluid and food, the stomach is desufflated, and the esophageal body is examined for Candida or stasis esophagitis.
Once the esophagus is evaluated and cleared of any residual debris, the EndoFLIP catheter is guided into position across the lower esophageal sphincter under direct visual guidance with the endoscope. An initial, post-induction/pre-myotomy distensibility index (DI) is obtained and recorded (Fig. 9.5).
The endoscope is then fitted with an oblique transparent dissection cap (longer end of bevel posterior), and the distance from the squamo-columnar junction to the incisors is measured on the scope shaft (Fig. 9.6).
9.2.2.2 Step 2: Mucosotomy and Entry into the Submucosal Space
A submucosal bleb is created in the esophageal wall 12 cm proximal to the esophagogastric junction (EGJ) at the 1 o’clock position, using a sclerotherapy needle containing a solution of 0.9% saline and 10 mL of indigo carmine (0.2 mg/mL) (Fig. 9.7). An endoscopic cautery knife was used to create a 2-cm longitudinal mucosotomy over the submucosal bleb (Fig. 9.8). The submucosal areolar tissue was cleared to expose the circular muscle layer. The endoscope was then bluntly maneuvered into the submucosal space, utilizing the oblique scope cap.
9.2.2.3 Step 3: Creation of the Submucosal Tunnel and Post-Tunnel EndoFLIP Measurement
Creation of the anterior submucosal tunnel requires a combination of electrocautery (Fig. 9.9) and hydro-dissection using a solution of saline and indigo carmine (Fig. 9.10). The orientation is checked periodically by ensuring the meniscus of injected fluid within the tunnel is lying in a posterior and dependent nature. It is important to ensure the myotomy tunnel is not spiraling posteriorly. The tunnel is extended 3 cm distal to the EGJ. Narrowing of the submucosal tunnel and the appearance of palisading vessels and aberrant muscle bundles (Fig. 9.11), as well as markings of the shaft of the endoscope, signify transition beyond the EGJ. As this area is highly vascular, the patient’s systolic blood pressure is kept under 120 mmHg to minimize bleeding. After completion of the submucosal tunnel (Fig. 9.12), the scope is withdrawn from the tunnel and advanced through the true lumen of the esophagus into the stomach.
Following confirmation of adequate tunnel length, the EndoFLIP catheter is then re-introduced and positioned across the EGJ under direct visualization with the endoscope. A post-tunnel distensibility index (DI) is then obtained and recorded.
9.2.2.4 Step 4: Myotomy and Post-myotomy EndoFLIP Measurement
A selective anterior myotomy of the inner circular muscle layer is started at 6 cm above the EGJ, using electrocautery to divide individual fibers and is extended to the end of the submucosal tunnel (Fig. 9.13). Special attention is taken to protect the mucosa during myotomy progression (Fig. 9.14); it is important to watch for splaying of longitudinal muscle fibers. The scope is withdrawn from the tunnel and advanced into the gastric lumen to observe the patency of the EGJ.
Following completion of the myotomy, the EndoFLIP catheter is re-introduced and placed across the EGJ under direct visual guidance with the endoscope. A post-myotomy distensibility index (DI) is then obtained and recorded (Fig. 9.15). The post-myotomy DI is then compared to the pre-myotomy/post-induction DI in order to help the operator determine if additional myotomy needs to be performed. The decision to perform additional myotomy should be based on both EndoFLIP measurement and direct visual assessment of the myotomy. General guidelines for an adequate myotomy include a final distensibility of >4, at least a doubling of DI compared with the initial and/or intermediate measurement and a post-myotomy minimal EJG diameter of at least 12.5 mm. These measurements are done at the 40 ml fill volume for the 8 cm catheter or 60 ml fill volume for the 16 cm catheter. All measurements should ideally be performed with the patient fully paralyzed and stomach completely desufflated.
9.2.2.5 Step 5: Mucosotomy Closure
Finally, the scope is re-advanced into the tunnel, and a solution of bacitracin is used to irrigate the tunnel. The scope is withdrawn into the true esophageal lumen and the mucosotomy was closed with seven endoscopic clips (Figs. 9.16 and 9.17). A Veress needle is used to relieve capnoperitoneum (only if present). The stomach is intubated one last time, aspirated, and desufflated before withdrawal of the endoscope.
9.3 Troubleshooting
POEM is a safe surgical option for the treatment of achalasia, but it requires advanced endoscopic and surgical skills. Initial endoscopy can be difficult in the context of food impaction or retention. This situation can be prevented by giving a clear liquid diet 48 h prior to surgery. In cases of severe esophageal stasis, extensive lavage and aspiration are often required before mucosotomy.
Preoperative treatment with antifungals (nystatin or fluconazole) is recommended to prevent Candida esophagitis, which has a high prevalence in patients with achalasia.
Esophageal muscle circular thickening, sigmoid esophagus, and severe esophageal dilatation can add technical difficulty to POEM. The use of an esophageal overtube for these cases may help with scope bowing and facilitate tunnel creation. A decision to proceed with POEM in a patient with these findings should be at the discretion of the operating surgeon.
After longitudinal mucosotomy, the endoscope is bluntly maneuvered into the submucosal space. In patients with type III achalasia, Jackhammer esophagus, or esophageal spasms, this step can be technically challenging.
Additionally, bleeding can occur in the submucosal tunnel, particularly when approaching the EGJ, because of the robust blood supply in this area. Bleeding can be controlled by directly coagulating injured vessels (away from mucosa) or applying indirect pressure onto the tunnel with the endoscope positioned in the esophageal true lumen. A systolic blood pressure goal under 120 mmHg is also recommended during this step of the procedure.
Mucosal injury can occur during initial endoscopy or submucosal tunnel creation, but it usually can be repaired by clips. Depending on the extent and location of the mucosal defect, the procedure can be aborted, continued, or performed at an orientation other than the anterior position.
The current recommendation for spastic achalasia is an extended proximal myotomy ablating the entire spastic segment. In these patients, the submucosal tunnel and myotomy are started more proximally in the esophageal body.
Sparing of longitudinal muscle fibers is not always possible. Limited areas of full-thickness myotomy are acceptable in POEM.
Capnoperitoneum may require decompression with a Veress needle. Capnoperitoneum should not be considered as a complication, but rather as a normal consequence of the procedure, given that CO2 can easily dissect through tissue planes.
Finally, mucosal redundancy and infoldings can add to the difficulty of mucosotomy clip closure. Various types of clips can be used to facilitate this last step.
The EndoFLIP catheter is utilized to obtain a distensibility index both before and after myotomy in order to provide the operator with objective data related to the quality of their myotomy. Data from the EndoFLIP should be used in conjunction with direct inspection of the submucosal tunnel and myotomy to assess adequacy.
9.4 Postoperative Course
Postoperatively, the patient is extubated and transferred to the postanesthesia care unit for observation. She stayed overnight and was kept on a liquid diet. She received standing intravenous (IV) antiemetics, pain medication, and IV narcotics as needed.
She was started on clear liquids, advanced to full liquids, and discharged on the afternoon of postoperative day 1 on oral anti-acid medication. The full liquid diet is maintained for 2 weeks and then gradually increased to include soft foods.
She was seen 2 weeks postoperatively and reported great improvement in her symptoms. Subsequently, she was advanced to a regular diet and scheduled for long-term physiologic follow-up studies.
At 9 months, she was complaining of minimal heartburn on anti-acid medication. The EGD showed Los Angeles grade A reflux esophagitis. The HRIM showed absent peristalsis with eight failed swallows without panesophageal pressurization, and an IRP of 11 mmHg. At 18 months after the POEM procedure, her Eckardt score had decreased to 0 from a preoperative score of 5.
It should be noted that select patients are able to discharge the same day provided they are able to tolerate a liquid diet with adequate pain control and reliable means of transportation should they need to return to the hospital for any reason.
Suggested Reading
Blatnik JA, Ponsky JL. Advances in the treatment of achalasia. Curr Treat Options Gastroenterol. 2014;12:49–58.
Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ, International High Resolution Manometry Working Group. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24 Suppl 1:57–65.
Campagna RAJ, Cirera A, Holmstrom AL, Triggs JR, Teitelbaum EN, Carlson DA, Pandolfino JE, Hungness ES. Outcomes of 100 patients more than 4 years after POEM for Achalasia. Ann Surg. 2021;273(6):1135–40.
Carlson DA. Functional lumen imaging probe: the FLIP side of esophageal disease. Curr Opin Gastroenterol. 2016;32(4):310–8.
Eleftheriadis N, Inous H, Ikeda H, Onimaru M, Yoshida A, Hosoya T, et al. Training in peroral endoscopic myotomy (POEM) for esophageal achalasia. Ther Clin Risk Manag. 2012;8:329–42.
Holmstrom AL, Campagna RAJ, Alhalel J, Carlson DA, Pandolfino JE, Hungness ES, Teitelbaum EN. Intraoperative FLIP distensibility during POEM varies according to achalasia subtype. Surg Endosc. 2021;35(6):3097–103.
Hungness ES, Teitelbaum EN, Santos BF, Arafat FO, Pandolfino JE, Kahrilas PJ, Soper NJ. Comparison of perioperative outcomes between peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy. J Gastrointest Surg. 2013;17:228–35.
Inoue H, Minami H, Kobayashi Y, Sato Y, Kaga M, Suzuki M, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–71.
Inoue E, Tianle KM, Ikeda H, Hosoya T, Onimaru M, Yoshida A, et al. Peroral endoscopic myotomy for esophageal achalasia: technique, indication, and outcomes. Thorac Surg Clin. 2011;21:519–25.
NOSCAR POEM White Paper Committee, Stavropoulos SN, Desilets DJ, Fuchs KH, Gostout CJ, Haber G, et al. Per-oral endoscopic myotomy white paper summary. Gastrointest Endosc. 2014;80:1–15.
Ren Z, Zhong Y, Zhou P, Xu M, Cai M, Li L, et al. Perioperative management and treatment for complications during and after peroral endoscopic myotomy (POEM) for esophageal achalasia (EA) (data from 119 cases). Surg Endosc. 2012;26:3267–72.
Roman S, Gyawali CP, Xiao Y, Pandolfino JE, Kahrilas PJ. The chicago classification of motility disorders: an update. Gastrointest Endosc Clin N Am. 2014;24:545–61.
Sharata AM, Dunst CM, Pescarus R, Shlomovitz E, Wille AJ, Reavis KM, Swanström LL. Peroral endoscopic myotomy (POEM) for esophageal primary motility disorders: analysis of 100 consecutive patients. J Gastrointest Surg. 2015;19:161–70.
Su B, Callahan ZM, Novak S, Kuchta K, Ujiki MB. Using impedance planimetry (EndoFLIP) to evaluate myotomy and predict outcomes after surgery for achalasia. J Gastrointest Surg. 2020;24(4):964–71.
Teitelbaum EN, Rajeswaran S, Zhang R, Sieberg RT, Miller FH, Soper NJ, Hungness ES. Peroral esophageal myotomy (POEM) and laparoscopic Heller myotomy produce a similar short-term anatomic and functional effect. Surgery. 2013;154:885–91; discussion 891–2.
Teitelbaum EN, Soper NJ, Arafat FO, Santos BF, Kahrilia PJ, Pandolfino JE, Hungness ES. Analysis of a learning curve and predictors of intraoperative difficulty for peroral esophageal myotomy (POEM). J Gastrointest Surg. 2014a;18:92–8; discussion 98–9.
Teitelbaum EN, Soper NJ, Santos BF, Arafat FO, Pandolfino JE, Kahrilas PJ, et al. Symptomatic and physiologic outcomes one year after peroral esophageal myotomy (POEM) for treatment of achalasia. Surg Endosc. 2014;28:3359–65.
Acknowledgements
The authors gratefully acknowledge the contribution of Rym El Khoury, MD, to this chapter in the first edition.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hungness, E.S., Snyder, M.M. (2022). Peroral Endoscopic Myotomy (POEM). In: Herbella, F.A.M., Patti, M.G. (eds) Atlas of Esophageal Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-12790-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-12790-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-12789-2
Online ISBN: 978-3-031-12790-8
eBook Packages: MedicineMedicine (R0)