Abstract
Targeted management and therapy for acute kidney injury (AKI) remains challenging. Much of the care for pediatric AKI to date remains supportive in nature. Earlier detection of AKI may allow intervention with fluid management measures before AKI is fully established, thus allowing the clinician to move beyond supportive care and improve outcomes. Early identification of patients at risk to develop AKI, optimizing fluid and electrolyte status, and avoidance of nephrotoxins are key points in early goal directed management of AKI. Fluid overloaded states may develop in AKI and early renal replacement therapy should be strongly considered to ameliorate and/or allow for correction of volume disturbance and metabolic derangement and permit adequate nutrition to the critically ill pediatric patient.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Continuous renal replacement therapy
- Peritoneal dialysis
- Hemodialysis
- Electrolyte disturbance
- Fluid overload
- Renal angina
Introduction
Acute kidney injury (AKI) affects an increasing proportion of critically ill patients who now survive medical and surgical complications that were once often fatal. Despite increased efforts to recognize and prevent AKI, progression to kidney failure continues to occur with alarming frequency. The treatment of AKI in critically ill and injured children requires understanding of medical management of disease with ready availability of renal replacement therapy (RRT) as well as adaptability for use in pediatric patients of all ages and sizes. In this chapter, we review medical management of AKI as well as traditional and emerging RRT modalities.
Medical Management of AKI
Medical management of AKI includes optimizing renal perfusion, preventing or reducing fluid overload, correcting electrolyte abnormalities and acid-base disturbances, supporting patient nutrition, and closely monitoring administration of nephrotoxic medications all while considering the patient’s need for renal replacement if medical management proves ineffective [1,2,3]. The prevention of AKI is the foremost means of management. The clinician must pay close attention to subtle changes in serum creatinine and corresponding urine output as creatinine increase is a late marker of AKI [4]. Chapter 46 discusses this topic in detail.
Renal Perfusion
Intravenous (IV) fluid is used to treat hypovolemia in an attempt to maintain end organ perfusion, but overly aggressive resuscitation may lead to fluid overload. Both crystalloid and colloid (typically albumin) are utilized in fluid resuscitation; however, two major studies in adults have failed to demonstrate a clear benefit on AKI outcomes or survival difference for colloid versus crystalloid infusions [5, 6]. However, colloid may be advantageous over crystalloid in patients requiring large amounts of fluid resuscitation in the setting of sepsis or burn injury [7].
Vasopressors in conjunction with IV fluid resuscitation for vasomotor shock may improve kidney perfusion and are recommended in patients who have or are at risk of AKI [7]. Agents recommended are norepinephrine, vasopressin, and dopamine. Vasopressin and norepinephrine use has increased due to favorable side effect profiles versus the arrhythmic abnormalities noted with dopamine [8, 9].
The use of renal vasodilators to increase renal perfusion does not improve outcomes. Specifically, dopamine has been employed at low dosages in an effort to improve renal perfusion by promoting vasodilatation. Adult studies of “renal dose” dopamine show no benefit and may even suggest harm [10,11,12,13]. More recently the selective dopamine agonist fenoldopam has been utilized to augment renal blood flow. Adult literature from single-center studies suggests a decline in both mortality and the need for RRT [14]. Furthermore, meta-analysis of 16 trials of fenoldopam in adults concluded that fenoldopam decreased the incidence of AKI, the need for RRT, intensive care unit (ICU) stay and death from any cause [15]. To date, one available randomized controlled trial has suggested that use of high-dose fenoldopam in pediatric patients on cardiac bypass significantly reduced the use of diuretics and vasodilators during bypass [16]. Current Kidney Disease Improving Global Outcomes (KDIGO) recommendations are against the use of fenoldopam to prevent or treat AKI given the high risk for hypotension associated with its use in ICU patients in comparison to the relatively sparse data available regarding its efficacy [7]. Emerging data from randomized, blinded trials suggest that fenoldopam may be beneficial in complex cardiac surgeries to improve the quality of perfusion during cardiopulmonary bypass and prevent AKI in both adults and children [16, 17]. The role for fenoldopam in AKI requires further clarification.
Volume Status
Awareness of volume status is critical in a patient with AKI since patients may have hypovolemia, euvolemia, or hypervolemia (fluid overload). Volume status should be continually reassessed and correlated with patient intake and output as well as daily weights. Heart rate, blood pressure, capillary refill, and skin turgor are all key components of assessing volume status.
Hypovolemia should be addressed with isotonic fluid to restore intravascular volume, and if necessary, inotropic support. AKI secondary to prerenal azotemia is likely in a severely volume depleted child. Aggressive fluid resuscitation with 10–20 mL/kg normal saline boluses to re-establish intravascular volume is recommended, and if there is no urine output once volume status is restored, a significant dose of furosemide (2 mg/kg IV) may be given. It is important to recognize that administration of furosemide and subsequent diuresis may simply result in conversion of the AKI from oliguric to non-oliguric and will not alter the course of the renal injury itself [18]. If a furosemide trial is used, a single high dose should be administered with observation for response. Furosemide should not be continued if there is ongoing oliguria [19].
Fluid overload has been associated with increased morbidity and mortality in pediatric critical care patients; however, the pathophysiology leading to poor outcome is not fully delineated [20, 21]. Examples of AKI with hypervolemia include following aggressive fluid resuscitation in septic patients and in patients with left ventricular cardiac dysfunction. The use of excessive normal saline for fluid resuscitation is associated with hyperchloremia, which has been demonstrated to diminish renal sodium excretion [22, 23] and impair renal blood flow [24], thus heightening the risk for AKI. Congestive heart failure due to poor left ventricular function can result in poor renal perfusion secondary to deficits in forward flow, which further promotes edema formation through activation of the renin-angiotensin-aldosterone axis and subsequent sodium retention [25]. Diuretic use in the adult ICU patient with AKI is associated with heightened risk of both death and non-recovery of renal function [19]. A trial of high-dose furosemide may be used to relieve hypervolemia given evidence that persistent, positive fluid balance is associated with increasing mortality in adult patients who develop AKI [26]. It is not advisable, however, to rely on diuretic therapy and fluid and nutritional restriction to avoid RRT given that such use of diuretics for either AKI “prophylaxis” or therapy does not improve outcomes [27, 28]. Patients with fluid overload not responsive to diuretics require consideration of RRT.
Cumulative percent fluid overload may be calculated as follows:
fluid input (liters) - fluid output (liters) / ICU admission weight (kg) * 100
Evaluation for RRT should occur in the setting of >10% cumulative fluid overload. Initiation of RRT is advised if cumulative fluid overload is >20% [29].
Critically ill pediatric patients presenting with euvolemia but oliguria that is unresponsive to fluid resuscitation may have intrinsic AKI. In this population, continued fluid resuscitation may be detrimental if the patient remains oliguric; thus, guidelines suggest restricting fluid to insensible losses plus replacement of urine output plus extra renal losses [30]. However, nutrition needs may require early RRT to prevent volume overload and electrolyte disturbances.
Electrolyte Abnormalities
Electrolyte abnormalities occur commonly in AKI and may develop rapidly, necessitating vigilant monitoring. Hyperkalemia is common, particularly in oliguric patients, and is potentially life-threatening due to ventricular tachycardia and fibrillation. Medical management of hyperkalemia is directed towards removal of potassium from the body (sodium polystyrene sulfonate, loop diuretic if responsive) and preventing arrhythmias via driving potassium into the cells (albuterol, sodium bicarbonate, insulin + glucose) and stabilizing the cardiac membrane (calcium), as summarized in Table 52.1. Dialysis may be necessary if moderate to severe hyperkalemia is refractory to medical management (Table 52.1).
Hyponatremia occurs more often than hypernatremia in AKI [30]. Hyponatremia is often due to water retention and may be exacerbated by intake of hypotonic fluids. Hyponatremia due to water retention secondary to volume depletion may respond to isotonic fluid. In contrast, fluid restriction is effective if due to free water excess. RRT may be necessary if renal dysfunction prevents excretion of excess free water. Sodium levels less than <120 mEq/L are associated with a high risk for cerebral edema and seizures. Sodium correction with hypertonic saline solution over several hours should be initiated. Further correction of hyponatremia can be achieved by free water restriction.
Hyperphosphatemia in AKI is secondary to reduction in glomerular filtration rate (GFR). Hyperphosphatemia may be treated with phosphate binders and dietary phosphorus restriction. Selection of a phosphate binder should consider the patient’s calcium level. In patients with low ionized calcium, a calcium-containing phosphate binder (e.g. calcium carbonate) should be used; by contrast, in patients with hypercalcemia, a non-calcium phosphate binder, such as sevelamer, is recommended.
Hypocalcemia occurs in AKI secondary to high serum phosphorus. Both total and ionized calcium levels should be monitored since total calcium may be inaccurate due to decreased total calcium secondary to hypoalbuminemia and changes in calcium binding to albumin based on the patient’s acid-base status. Initial treatment for mild hypocalcemia in AKI and phosphate retention is correction of hyperphosphatemia, typically using oral phosphate binders. Symptomatic hypocalcemia requires correction with IV calcium. This replacement should be provided with caution in the severely hyperphosphatemic patient given the possibility of systemic calcium phosphate precipitation, potentially worsening existing AKI with calcium phosphate deposition in the renal tubules. Inability to correct hypocalcemia in a symptomatic patient (e.g. tetany and/or seizures) secondary to severe hyperphosphatemia is an indication for dialysis.
For children who are not receiving RRT, several measures can prevent severe metabolic and electrolyte disturbances. First, no supplemental phosphorus or potassium should be provided to the patient unless symptomatic or there is significant hypophosphatemia or hypokalemia. Second, to prevent worsening hypertension and fluid overload, sodium should be restricted to 2–3 mEq/kg/day. Third, parenteral or enteral nutrition should be considered early in the patient’s course, as described below, to replete electrolyte abnormalities. If adequate nutrition cannot be provided due to fluid overload, RRT should be initiated. Serum electrolytes, phosphorus, calcium, and albumin should be regularly monitored as dictated by the patient’s clinical status.
Acid-Base Disturbances
Metabolic acidosis in AKI is due to renal dysfunction and systemic disease (e.g. sepsis, trauma, burns). Severe acidosis can be treated with IV or oral sodium bicarbonate, with careful monitoring for fluid overload and worsening of hypertension due to the sodium load. Patients with AKI who develop severe, refractory metabolic acidosis may require dialysis therapy, especially in the setting of oligoanuria. It is important to measure the serum total and ionized calcium prior to bicarbonate treatment due to potential for symptomatic hypocalcemia given increased pH-dependent binding of calcium to proteins, which decreases the ionized calcium.
Nutritional Interventions
Patients with AKI are in a hypercatabolic state and should have nutritional support to ensure full calorie, protein, and micronutrient delivery. Nutritional goals include preservation of lean body mass and avoidance of metabolic derangements. Potential benefits include improved wound healing, immune function, and scavenging of oxygen free radicals, with the goal of decreasing patient mortality [32].
Delivery
Adequate nutrition may require supplemental enteral and/or parenteral nutrition if oral intake cannot meet nutritional requirements. Enteral nutrition is preferred if the gastrointestinal tract is functioning given the ease of administration and lower rates of infection. Conversely, parenteral nutrition should be employed when the gastrointestinal tract cannot be utilized and/or enteral feeding cannot provide sufficient nutrition [33]. See Fig. 52.1 for a decision tree illustrating mode of nutritional support in AKI [34].
Decision tree for nutritional support in AKI patients with protein energy wasting (PEW) or at risk of PEW. (Used with permission of John Wiley and Sons from Fiaccadori et al. [34])
Protein
Current evidence suggests that patients with AKI require increased protein, particularly when receiving RRT [35]. Protein-energy wasting (PEW) is loss of lean body mass and fat mass and may occur in patients with AKI d [36]. PEW is an independent predictor for patient mortality and is also directly associated with the length of hospital stay and risk of complications [37]. The adult literature recommends an increased protein intake goal of 1.5–2 g/kg per day for hypercatabolic patients or for patients receiving RRT that utilizes high-flux and/or highly efficient filters, which are associated with amino acid losses [38].
Calories and Lipid
General guidelines suggest that critically ill children should receive from 1 to 1.3 times basal metabolic needs in calories [39]; however, additional calories for hypercatabolic states should be provided given the patient’s pre-existing state of nutrition. To optimize enteral nutrition, “renal-specific” formulas are an option for patients being medically managed with AKI or on RRT. These formulas may be beneficial given high caloric and protein density with low electrolyte levels [40]. Adequate caloric intake is needed to prevent catabolism, to promote protein synthesis and to offset heat-dependent caloric losses [41]. Parenteral nutrition, including IV lipids, is necessary if enteral nutrition cannot be provided [38, 41].
Nutrients
Levels of vitamin C and water-soluble vitamins, including thiamine and folic acid, may be low in patients with AKI [42]. Use of RRT can exacerbate nutrient and trace element losses due to the very efficient removal of small molecular weight substances [43]. Appropriate replacement is indicated.
Glycemic Control
Stress hyperglycemia is a notable feature of critical illness [44]. Several studies have investigated the impact of conservative versus intensive insulin therapy on patient mortality, with secondary analyses investigating impact of glycemic control on incidence of AKI [45,46,47]. Tight glycemic control has decreased the incidence of AKI in adults [48]. Insulin is recommended to correct hyperglycemia in AKI, with typical glucose goals between 110 and 150 mg/dL [49].
Avoidance of Nephrotoxins
Many commonly used medications are metabolized and/or excreted by the kidneys. Nephrotoxic medications in the ICU contribute to nearly 25% of AKI cases [50, 51]. Prevention of drug-induced AKI is more effective than any available therapy; recognition of high-risk patients is therefore necessary. The dose and frequency of administration of any potentially nephrotoxic medication should be adjusted (or avoided) based on the patient’s GFR.
Common nephrotoxins include aminoglycosides, nonsteroidal anti-inflammatory drugs (NSAIDs), contrast agents, and chemotherapeutic and immunosuppressant medications [52]. AKI induced by drugs occurs by two predominant mechanisms: direct toxicity to renal tubular epithelium, as is seen with aminoglycosides and amphotericin, and interference with autoregulatory mechanisms, leading to unrestricted vasoconstriction and reduced renal blood flow, as is seen with NSAID toxicity. NSAIDs are a common cause of AKI in children, even when ingested at recommended doses; the incidence of AKI may be underestimated both in the inpatient and outpatient settings [53]. Aminoglycosides are widely utilized in pediatric patients. Repeat administration of these agents may lead to renal interstitial and tubular epithelial cell accumulation, and as such, recommendations have been made to administer aminoglycosides every 24 h (or less) to minimize toxicity, with drug levels obtained daily [7].
Contrast-induced AKI (CI-AKI) in adults is associated with an increased risk of mortality in the year following the episode of AKI [54]. The incidence in children is not well-characterized. Patients with chronic kidney disease or diabetes are at increased risk of CI-AKI [54]. Volume and osmolality of contrast administered is directly associated with risk of AKI [55, 56], and nonionic agents are thought to be safer, especially in patients with chronic kidney disease [57]. The pathophysiology of CI-AKI is still largely unknown. Severe vasoconstriction following contrast administration has been implicated [58, 59], as has direct cytotoxicity via oxygen free radical generation. Serum creatinine rises 1–2 days after the imaging procedure and is usually not accompanied by oligoanuria [60]. Dialysis is required in a minority of patients. No treatment exists other than support if CI-AKI occurs. However, in recent years, attention has focused on prevention [61, 62]. A meta-analysis of prevention strategies recommends pre- and post-contrast IV volume expansion with bicarbonate-containing fluids and use of low or iso-osmolar contrast agents in the smallest volume possible in patients with pre-existing kidney disease who are at increased risk. N-acetylcysteine and ascorbic acid have also been suggested for use as free radical scavengers in the higher risk populations [57]. N-acetylcysteine is indicated for the prevention of contrast-induced AKI per the 2012 KDIGO guidelines [7]. While older studies have suggested a benefit [63], controversy regarding its use remains as the effect of N-acetylcysteine to prevent AKI can be variable, and several studies have failed to show a significant benefit. There is no significant benefit of either N-acetylcysteine or bicarbonate infusion for prevention of AKI, even among populations at high risk for kidney complications [64]. There have been no randomized trials in children.
Other Medical Therapies
Growth Factors
Renal tubular injury is a major component in the pathogenesis of AKI, and renal tubular repair is a required step for recovery. Growth factors play an important role in the regeneration of epithelial cells. In animal models, several growth factors have been shown to accelerate recovery from renal injury such as erythropoietin, insulin-like growth factor, hepatocyte growth factor, and epidermal growth factor [65]. In cellular and animal models, erythropoietin appears to reduce necrosis and apoptosis in renal epithelial cells while also promoting cell proliferation [66]; however, human studies have failed to yield AKI-related benefit from use of erythropoietin for primary AKI prevention [67]. Similarly, hepatocyte growth factor and insulin-like growth factor-1 appear to limit apoptosis in animal models of renal injury [68, 69], but again, preliminary clinical trials with IGF-1 have not shown benefit to patients. The KDIGO work group recommends against use of IGF-1 to prevent or treat AKI [7].
Adenosine Receptor Antagonists
Theophylline is a non-selective adenosine receptor antagonist that is thought to increase renal blood flow at the level of the afferent arteriole [70]. KDIGO recommendations suggest a single dose of theophylline in high-risk neonates with perinatal asphyxia at risk of AKI [7]. Research to date supports an initial renoprotective effect following theophylline in asphyxiated infants; however, long-term impact on renal function is unclear [71, 72].
Renal Replacement Therapies in AKI
The lack of evidence-based guidelines regarding the definition of pediatric AKI (see Chap. 46) has resulted in much uncertainty and discussion regarding the indications and timing for initiation of RRT as well as the optimal modality to employ. Retrospective reviews in critically ill children demonstrate that those who develop AKI early in a hospital course have greater morbidity and mortality [73, 74]. Use of RRT may prevent and correct life-threatening complications of AKI refractory to medical management. Indications for initiation of RRT in pediatric AKI have traditionally been those used for end-stage renal disease (and are not necessarily easily juxtaposed in the acute setting), including metabolic/electrolyte abnormalities refractory to medical therapy, symptomatic fluid overload, and/or symptomatic uremia; however, metabolic derangement and fluid overload are often late findings in severe renal injury [75]. In the acute setting, consideration of RRT must account for the patient’s clinical situation in the context of the aforementioned laboratory abnormalities.
Adult literature suggests that earlier initiation of RRT, for example before the appearance of florid metabolic derangements and symptomatic fluid overload, may yield better patient outcomes, including decreased risk of death, shorter duration of RRT, and shorter hospital stays [76, 77]. For example, volume overload in the setting of AKI refractory to medical management is an indication for RRT even without significant azotemia or elevation in creatinine. Additionally, the presence of multisystem organ dysfunction in the presence of AKI refractory to medical management is a strong indication for initiation of RRT.
More recently, the concept of renal angina [78] has been suggested whereby likelihood of developing AKI is informed by baseline and contextual factors as well as objective evidence to identify those patients at greatest risk for renal injury. Additionally, renal angina criteria stratify patients into moderate-, high-, and very-high risk categories for AKI based on their underlying clinical condition (Table 52.2). Renal angina can be thought of mathematically as “signs of injury” (i.e., fluid overload, estimated creatinine clearance) multiplied by presence of AKI and is comparable to assessment of risk for a myocardial infarction in an adult patient presenting with chest pain. Given that there are currently no reliable biomarkers for establishing the severity or prognosis of a patient’s AKI, renal angina criteria may help the clinician to predict risk for AKI early in the clinical course and intervene, if needed, with RRT before reaching a state of fluid and metabolic derangement. In one study, the renal angina index obtained on Day 0 of pediatric ICU admission was predictive for progression to AKI on ICU Day 3 [79].
Greater than 20% fluid overload is a significant independent risk factor for increased morbidity and mortality [80]. Other less concrete indications for initiation of RRT include oliguria not responsive to diuretics, escalating ventilatory requirements (especially if pulmonary edema is secondary to fluid overload), need for a large volume of medications/blood products in a currently fluid overloaded patient (>10% overload), and/or when ability to provide adequate nutrition is compromised by fluid restriction secondary to fluid overload and oliguria.
Literature exists regarding when to stop RRT in the patient with AKI. In stopping RRT, the nephrologist may decrease the frequency of therapy from daily to every other day or change modality (e.g. conversion from CRRT to acute hemodialysis [HD]). The decision to stop should be based on evidence for improvement in the underlying disease pathology that led to RRT initiation and improvement in renal function (i.e., increased urine output, diminished azotemia, and decreased fluid overload).
Modality Choice
When initiating RRT for AKI, the clinician should first identify the goals of dialytic therapy. The patient’s size, hemodynamic stability, and potential for vascular access also inform modality selection. Choice of therapy will also depend on clinician preference, available resources (e.g. dialysis equipment, nursing staff), and even capabilities for placement of dialysis access.
Acute Peritoneal Dialysis
Acute peritoneal dialysis (PD) provides gradual solute and water clearance through both convective and diffusion-based mechanisms. Although the use of continuous renal replacement therapy (CRRT) has dramatically increased in the past decade, there remains an important role for acute PD in preterm neonates with limited vascular access and patients admitted to the ICU following surgery for congenital heart defects [81, 82]. Acute PD is still the modality of choice in many countries, especially in the developing world [83,84,85] as it is a relatively inexpensive form of dialysis that does not require sophisticated technical expertise or equipment. It avoids the risks and complications associated with extracorporeal perfusion, including the possible need for blood-product exposure and systemic anticoagulation. Additionally, large volumes of fluid can be removed slowly over a prolonged period, maintaining hemodynamic stability. Due to the relatively slower solute clearance, including that of nitrogenous waste products, it is not associated with dialysis disequilibrium, which may occur in acute intermittent HD.
Initiation
An in-depth discussion regarding PD access is provided in Chap. 63. In brief, PD does not require vascular access, which can be a challenge in infants and small children, and provides a means for critically ill patients to be dialyzed with preservation of vascular access, thus allowing for rapid institution of therapy even in the less hemodynamically stable patient. Access can be obtained using semi-rigid stylet catheters requiring a trochar and canula method of insertion, the main advantage of which is the ease of bedside insertion by the pediatric nephrologist without surgical intervention and general anesthesia [86]. In patients who can tolerate a surgical procedure, placement of a tunneled permanent catheter is preferred to semi-rigid, temporary catheters to reduce technical complications such as leaks and catheter obstruction [87]. Newer acute placement techniques are performed with soft catheters, often where a Seldinger technique is utilized to insert the catheter over a guidewire (Fig. 52.2). This technique can be well-utilized in infants as it carries a minimal risk of dialysate leakage since no incision is required for the catheter insertion. Consequently, the risk of peritonitis is less, and these catheters can be kept for up to 5 days without complications [87].
Prescription and Technique
The dialysis prescription for acute PD comprises four major components: the exchange volume, dialysate composition, individual cycle time, consisting of fill, dwell and drain, and total length of the dialysis session. For acute PD, the target exchange fill volume for adequate dialysis in terms of fluid and solute clearance, without the risk of leakage, is 30 mL/kg. However, smaller initial volumes of 10 mL/kg may be used for at least 24–48 h, if there is a risk of leakage from a wide incision site or if a tunneled cuffed catheter is used. Initially, short dwells (e.g. 30 min or hourly exchanges) for 48–72 h are required to remove accumulated solutes and excess fluid. Volume and metabolic control may best be achieved with exchanges performed around-the-clock. Subsequently, in maintenance dialysis, the dwell times can be extended, and total daily therapy time may be reduced, similar to chronic PD, with increasing volumes up to 40–45 mL/kg if a cuffed catheter has been used. PD should be continued until urine output improves, indicating recovering renal function.
Commercial dialysate solutions are available differing in osmolality, osmotic agent, and buffer. The osmotic agent is typically dextrose in a variety of pre-prepared concentrations. The hypertonic dextrose solutions utilized in PD provide a source of additional calories that may be beneficial in the critically ill child where IV access for nutrition and maintenance of glycemia may be limited; conversely, hyperglycemia may result from dextrose used in dialysate and insulin therapy may be required [88].
Complications
PD is contraindicated in patients with diaphragmatic defects, omphalocele, gastroschisis, and bladder exstrophy [89]. Complications with PD include catheter malfunction, peritonitis, and poor ultrafiltration. Neonates may have poor drainage due to catheter malposition or kinking, omental wrapping or a fibrin clot, which is exacerbated by the relatively small-bore peritoneal catheters required in the smallest patients. Inadequate drainage may also occur due to constipation. Bedside PD catheter placement with a semi-rigid trochar is associated with a risk of viscus perforation, especially in neonates, both at the time of insertion and with increasing dialysis duration. Severe abdominal pain and shock may occur, and the catheter must be removed for bowel repair and treatment of sepsis. The incidence of peritonitis is highest with the semi-rigid catheter, particularly if it has been kept in place for longer than 72 h [86]. In some cases, patients may develop a diaphragmatic pleuroperitoneal communication following cardiothoracic procedures, which results in a large pleural effusion once PD is initiated. Hypothermia is a complication of PD, particularly in neonates and small children, if dialysate is not warmed prior to infusion into the peritoneal cavity.
Poor ultrafiltration may occur, especially in critically ill infants due to the low fill volume with inadequate fluid reservoir intraperitoneally. Often it is difficult to increase the dwell to the desired volume in the setting of infant acute respiratory distress and low lung volumes as the increasing peritoneal fluid fill volumes results in splinting of the diaphragm. In some cases, during the inflow phase, critically ill infants may desaturate, and require transient compensation in ventilatory pressures. As a result, there is poor ultrafiltration, which increases the fluid retention, thus worsening the respiratory distress. These ill patients are often hypotensive requiring multi-agent inotropic and pressor support. The resultant decrease in bowel perfusion due to vasoconstriction of the mesenteric vessels secondary to pressor support may also contribute to poor ultrafiltration. Additionally, in neonates there is a decrease in the osmotic gradient, because of increased absorption of dextrose from the dialysate, resulting in poor ultrafiltration. With the use of higher dialysate dextrose concentrations to facilitate ultrafiltration, hyperglycemia may occur and necessitate insulin administration.
Electrolyte abnormalities may occur with PD. Hypokalemia may occur, and if noted, potassium should preferably be added to the IV fluid if the patient is not feeding, rather than adding to the dialysate, to avoid frequent bag changes due to changing orders. Potassium can be added to the dialysate if the hypokalemia is severe enough such that the maximum safe concentration of potassium infusion will be exceeded.
Acute Intermittent Hemodialysis
In many countries, acute intermittent HD is the mainstay of dialysis for AKI, particularly in older children. Its main advantage is rapid ultrafiltration and solute removal. It is therefore indicated in AKI that requires rapid fluid removal (acute fluid overload) or rapid solute removal such as hyperkalemia, tumor lysis syndrome, toxic poisonings and other profound metabolic abnormalities. Acute intermittent HD is ideal for hemodynamically stable patients who can tolerate rapid fluid shifts. It is a versatile modality as it allows for ultrafiltration without solute removal, as well as adjustment of the dialysate bath to treat electrolyte abnormalities such as hypernatremia. Moreover, because of the intermittent nature of the dialysis, patients can be mobilized for other procedures. Systematic reviews have suggested that in hemodynamically stable adult patients the continuous forms of RRT do not appear to have a survival advantage over intermittent HD [90,91,92].
Initiation
When initiating acute HD, one must consider vascular access, HD prescription, and type of dialyzer membrane. Other factors include the patient’s ability to tolerate rapid fluid shifts, the need for vasoactive substances to maintain blood pressure, and total fluid removal goals.
Vascular access for HD is discussed in detail in Chap. 65. As with all extracorporeal therapies, treatment success is dependent on the quality of the vascular access. Adequate blood flow (Qb) is essential to providing optimal therapy with minimal interruption. In pediatric patients, the choice of vascular access, catheter size, and insertion site is critical. Short, large bore catheters provide improved performance due to lower resistance to flow [93]; conversely, longer, smaller-bore catheters (e.g. Broviac catheters) are unsuitable due to their high flow resistance.
The HD prescription in AKI must be individualized to provide adequate solute clearance and fluid removal. The prescription for acute intermittent HD is comprised of the dialysis dose delivered per session and the frequency of the sessions. Additional factors affecting the individual HD prescription include the extracorporeal circuit volume, the dialyzer size, blood flow rate, dialysate flow rate, ultrafiltration required, dialysate composition, anticoagulation, and length of session. Blood flow rate is determined by vascular access and determines solute clearance, with higher blood flow increasing solute clearance by optimizing diffusion and convection. Dialysate flow rate is also a determinant of solute clearance [94]. Dialysate flow rate should be at least 1.5 times greater than the blood flow rate to maximize diffusion gradients of solutes. In pediatric patients, HD equipment and prescription require modifications for smaller children (i.e., infants less than 3 kg).
When initiating acute HD for AKI, dialysis dose delivered may change frequently with a need for greater renal support during initial therapy for AKI, as compared to the relatively stable initial dialysis doses provided in initiating chronic HD for end-stage renal disease [95]. Similarly, in patients receiving chronic HD, urea kinetic modeling (Kt/V) is utilized as a measure of dialysis adequacy; however, due to the rapidity of fluid shifts with therapy and frequently changing renal function, Kt/V may not be as reliable in the patient with AKI receiving HD [96]. A review of dialysis dosage in adults concluded that in AKI a Kt/Vurea greater than 1.2 from thrice weekly intermittent HD is associated with improved survival in patients with intermediate severity of illness but does not influence outcomes in more severely ill patients [97]. Increased acute HD treatment frequency may be required despite reaching “adequate” Kt/V to achieve daily fluid removal goals. Although Kt/V recommendations exist, dialysis dosage must be individualized and higher doses of therapy for various metabolic derangements may be required in AKI [97].
The total volume of the extracorporeal circuit includes the volume of the tubing and the dialyzer and should not exceed more than 10% of the patient’s blood volume, calculated as 75 mL/kg for older children and 80 mL/kg for infants. If the extracorporeal blood volume exceeds 10–15% of the patient’s total blood volume, or the patient has a low hematocrit and/or hemodynamic instability, a blood prime is recommended [98]. When initiating a blood prime, using buffered packed red blood cells (PRBC) or transfusing the PRBC post-membrane in conjunction with a saline prime have been shown to reduce risk of “bradykinin release syndrome” (BRS) [99]. BRS, characterized by a precipitous decline in blood pressure 5–10 min after initiating both acute HD and continuous RRT, has been associated with the use of the AN-69 polyacrylonitrile membrane [99, 100]. Exposure of the primed blood to the negatively charged AN-69 membrane co-activates pre-kallikrein and Hageman factor, resulting in the release of bradykinin, a potent vasodilator. The reaction is potentiated by exposure to blood with an acid pH, which is typical of banked blood used for blood priming the circuit. Thus, the use of a blood prime with an AN-69 membrane can result in profound hypotension. PRBCs to be used in a blood prime should be diluted with normal saline to produce a hematocrit of approximately 35–40%. Buffering the banked blood for priming to physiologic pH prior to priming the circuit or infusing the blood post-filter at the same rate as a saline prime have been shown to be effective in minimizing the BRS, as has avoidance of the AN69 membrane [101, 102].
Dialyzer selection considers the type of membrane desired, the need for a blood prime, the dialyzer membrane surface area, and the ultrafiltration coefficient [103]. The membrane properties of the dialyzer, such as membrane thickness, pore size, and pore density affect dialysis efficiency, with varying clearances for small and middle molecular weight solutes. Dialyzer membrane biocompatibility should be considered when initiating acute HD. See Chap. 66 for additional information on this topic. The use of biocompatible synthetic membranes does not appear to confer any significant clinical advantage either in terms of mortality or AKI recovery when compared to substituted cellulosic membranes [104, 105]. High-flux membranes have larger pores resulting in greater clearances of higher molecular weight solutes but have the risk of back transport from the dialysate of water-borne solute contaminants. In a systematic review comparing the use of high-flux and low-flux membranes in AKI in adults, there was no difference in the risk of mortality or dialysis dependence in survivors [105]. However, in another meta-analysis, there appeared to be a significantly improved renal function recovery with the use of high-flux membranes [104]. High-cut-off-point membranes made from polyamide/polyarylethersulfone, polysulfone, or cellulose triacetate, have greater cytokine clearance and enhanced adsorption properties than conventional high-flux dialyzers [106], and have been developed for use in septic patients with AKI [107, 108]. Treatment using high-cut-off-point membranes has been shown in animal models of sepsis to have beneficial effects on immune cell function and survival [109]. Preliminary clinical studies show that use of these membranes in adult patients with AKI was associated with decreased need for vasopressor therapy, with no reports of serious adverse effects [108].
The smaller blood volumes in infants and young children place them at risk for blood loss due to clotting of the dialyzer; thus, anticoagulation is required with acute intermittent HD for pediatric AKI. Heparin is the most commonly used anticoagulant for intermittent HD. A loading dose of heparin may be given at the start of dialysis followed by intermittent bolus heparin doses. To monitor therapy, the activated partial thromboplastin time (aPTT) or activated clotting time (ACT) may be used. The aPTT should be kept at 1.2–1.5 times the baseline, and the ACT between 120 and 180 s. When heparin is utilized, platelet count should be monitored frequently to assess for development of heparin-induced thrombocytopenia [110]. In coagulopathic patients, heparin-free dialysis can be performed by intermittently flushing the circuit with 0.9% saline; unfortunately, this method increases the ultrafiltration target and decreases dialysis efficiency [111,112,113]. When using saline anticoagulation, the filter pressure should be monitored, and dialyzer inspected for early clot formation. Regional citrate anticoagulation is also an alternative to systemic heparin anticoagulation in the coagulopathic patient [114] requiring HD for AKI.
Complications
HD in young children can be challenging due to the smaller patient blood volume. This problem is accentuated in the critically ill child, where pressor infusion may be required to support the systemic blood pressure. Moreover, these children may have acute respiratory distress syndrome with hypoxemia or other associated clinical problems such as congestive heart failure or cerebral edema. Therefore, maintenance of an adequate blood pressure in these children is critical to alleviate tissue hypoxia.
Rapid HD using dialyzers with larger surface areas in patients with very high plasma blood urea nitrogen (BUN) concentrations may result in the dialysis disequilibrium syndrome, characterized by neurological symptoms such as fatigue, headache, nausea, vomiting, altered consciousness, convulsions, and coma [115]. Patients with AKI may be at increased risk due to catabolism (high BUN) and pre-existing neurological compromise related to the acute illness. Measures to prevent the disequilibrium syndrome include decreasing the initial dialysis dose, increasing dialysate sodium concentration (143–146 mmol/L), and administration of osmotically active substances such as IV mannitol (0.5–1 g/kg) to prevent rapid osmolar shifts that can cause cerebral edema [116].
Hypotension is one of the most common complications with acute intermittent HD and occurs in part due to rapid fluid and solute removal. Technical advances in the delivery of HD have dramatically reduced the propensity for intradialytic hypotension. The use of volume-controlled dialysis machines and biocompatible synthetic dialysis membranes helped decrease the incidence of intradialytic hypotension. In adult studies, it has been demonstrated that priming the circuit with isotonic saline, discontinuing vasodilator therapy, keeping the dialysate sodium greater than 145 mmol/L and setting the dialysate temperature to below 37 °C result in lesser hemodynamic instability and better outcomes [117]. See Table 52.3 for a summary of recommendations to minimize hemodynamic instability with acute HD. Additionally, use of in-line noninvasive blood volume monitoring to minimize abrupt changes in extracellular volume is useful in young children with hemodynamic instability where large acute changes in extracellular volume are not well tolerated [118]. This method of performing intradialytic noninvasive blood volume monitoring indicates intravascular blood volume change during the dialysis session (Fig. 52.3).
Catheter site complications are possible in acute HD and include infection at the catheter exit site, catheter malfunction, and risk of hematologic disturbance (e.g. bleeding, clot). In the event of signs of infection such as fever, line blood cultures should be obtained, and empiric antibiotics started. There is also risk for clotting of the extracorporeal system in acute HD; this can place patients at risk for acute blood loss if the blood is unable to be returned to the patient.
Continuous Renal Replacement Therapies
CRRT is now widely available in pediatric centers throughout the world, and in some has become the preferred method of RRT. CRRT in AKI offers several advantages over traditional dialysis methods when used in critically ill, unstable patients. Because CRRT is continuous, removal of solutes and modification of the volume and composition of the extracellular fluid occur gradually. Unstable patients, who are often intolerant of the abrupt fluid volume and solute concentration changes that accompany standard HD treatments, can be successfully treated with CRRT. The precision and stability with which fluid and electrolyte balance can be maintained using CRRT is unmatched by any currently available dialysis therapies, except perhaps the extended HD techniques mentioned in the following section.
The basic principles of CRRT are similar for adults and children. However, the application of these modalities in children requires attention to several important details unique to therapy in pediatric patients. For example, extracorporeal blood volume may be large compared to the patient blood volume, necessitating blood circuit priming in the very small child as described in the section on acute HD (see “acute intermittent hemodialysis,” above). The most demanding considerations arising in pediatric CRRT are related to the need to adapt equipment and prescriptions designed for adult-size patients in order to meet the special needs of the smallest of pediatric patients requiring renal support.
Initiation
The indications for initiating CRRT in children and adults are similar and most often involve the treatment of AKI and fluid overload in a critically ill patient [20, 119]. CRRT may be combined with extracorporeal membrane oxygenation (ECMO) and plasmapheresis circuits.
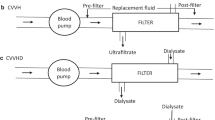
CRRT refers to a variety of modalities that use one or both solute clearance mechanisms. In continuous venovenous hemofiltration (CVVH), blood flows through the hemofilter, generating large volumes of ultrafiltrate, which is replaced by a physiologic “replacement fluid,” either before (pre-dilution) or after (post-dilution) the hemofilter (Fig. 52.4a). Clearance is thus exclusively convective. If a dialysate is infused into the hemofilter, clearance is primarily diffusive, as in HD. Hence, this CRRT modality is called continuous venovenous HD (CVVHD, Fig. 52.4b). When both replacement fluid and dialysate are used, permitting convective and diffusive clearance, the therapy is known as continuous venovenous hemodiafiltration (CVVHDF, Fig. 52.4c).
(a) Diagram of a convective based (CVVH) continuous renal replacement therapy (RRT). Note use of either pre- or post-filter replacement fluid rather than dialysate. (b) Diagram of diffusion based (CVVHD) continuous RRT. Note use of dialysate rather than replacement fluid. Dialysate flow is countercurrent to blood flow. (c) Diagram of combined convective and diffusive based (CVVHDF) continuous RRT. Note use of both dialysate and replacement fluids
Initiation of CRRT also requires adequate vascular access, as discussed previously and in Chap. 65.
Blood Flow Rates
With a well-functioning vascular access, it is possible to adjust Qb based on to the size of the child and the clinical setting. Higher Qb may support longer filter life by reducing the likelihood of filter fiber clotting. Higher Qb also facilitates increased patient fluid removal by providing greater filter plasma flow rates and reduces the loss of clearance efficiency from pre-dilution mode CVVH or CVVHDF. However, not all patients will tolerate a higher Qb, especially at initiation of CRRT. Hence, we suggest initiating CRRT with lower Qb and advance to the targeted rate over the first 30 min of therapy as tolerated. In contrast to pediatric HD where initial Qb can be readily extrapolated from patient body weight, there are no true “body-weight” recommendations for Qb in any form of pediatric CRRT. The Qb chosen should provide adequate clearance for the size of the patient, with consideration of access limitations and device requirements. Recommendations for Qb range from 4 to 10 mL/kg/min; consequently, Qb may vary widely in CRRT. Depending on the patency of the access, the Qb may need to be higher to maintain flow. For example, 10–12 mL/kg/min may be necessary to accommodate technical requirements (access) and clearance in extremely low birth-weight neonates while 2–4 mL/kg/min may be appropriate in larger adolescents.
Solutions
The tolerability of CRRT has been greatly improved with the introduction of bicarbonate-based CRRT solutions. In the past, with lactate as the buffer, worsening lactic acidosis was common, leading to hypotension and depression of cardiac function [120]. A series of comparative clinical trials of lactate- and bicarbonate-based CRRT fluids in adults [121, 122] and children [123] have demonstrated the superiority of bicarbonate as a buffer; consequently, bicarbonate-based CRRT solutions are now the standard of care, although trace amounts of lactate may be used in solutions to maintain stability.
CRRT solutions also contain sodium, potassium, chloride, glucose, calcium, phosphate, and magnesium. Bicarbonate-based CRRT solutions are available from several manufacturers in a wide array of electrolyte formulations. Most hospital pharmacies stock only a single brand and in only a few formulations. A feature of CRRT, especially in small patients, is the tendency over time for the composition of the CRRT fluids to determine the electrolyte composition of the patient. A fluid low in potassium, phosphorous and magnesium may be appropriate at initiation of CRRT when concentrations of these electrolytes in AKI patients are often elevated. However, depending on the CRRT prescription, within a short time the patient may become deficient in these electrolytes, which can complicate management. Thus, while a “starter” fluid with reduced potassium, phosphorous and magnesium is needed, a fluid that includes these electrolytes in physiologic concentrations should follow. Rather than stocking multiple formulations, some pharmacies may prefer to add potassium, phosphorous, magnesium and even additional bicarbonate to the “starter” solutions as needed, a practice that may add the risk of pharmacy errors and increase costs. Calcium is always left out of solutions when phosphate is present to avoid precipitation. Calcium has usually, but not always, been left out of CRRT solutions used with citrate anticoagulation, as will be discussed below.
Prescription
The optimal “dose” of RRT is not known. Adult AKI studies by Ronco and colleagues using CVVH established a total convective clearance (replacement fluid plus patient fluid removal) target of 35 mL/kg/h as a threshold below which survival was significantly worse [124]. In a subset of these patients with sepsis, there was a trend in favor of improved survival with total convective clearance ≥45 mL/kg/h. Despite theoretical considerations that seemed to favor high clearance targets in cytokine-driven illnesses like sepsis [125] and preliminary results in septic adults treated with very high flow CRRT [126], available evidence does not support the use of clearance targets above 20–35 mL/kg/h. For pediatric patients, this translates to 2–3 L/1.73 m2/h, rates that are reasonably easy to achieve.
Anticoagulation
Effective CRRT requires optimal anticoagulation. Activation of the clotting cascade occurs in CRRT circuits due to contact of the circulating blood with artificial surfaces. Low blood flow rates, turbulent flow, small catheters and high hematocrits hasten clotting. Anticoagulation regimens using mixed molecular weight heparin or sodium citrate are the most commonly used in pediatric CRRT, and either can be effective. An early comparison in pediatric centers showed equal filter life span with heparin and citrate, but more hemorrhagic events in the heparin group [127].
Heparin has been the mainstay of HD anticoagulation for decades. Many pediatric CRRT programs continue to rely on heparin. Heparin is infused in the CRRT circuit pre-filter and titrated to achieve a targeted post-filter activated aPTT 1.5–2 times normal, or an ACT between 180 and 220 s. This is usually accomplished by giving an initial heparin bolus of 20–30 units/kg, followed by a continuous infusion of 10–20 units/kg/h. Alternatively, the circuit may be rinsed and primed with 1–2 L of normal saline to which has been added 2500–5000 units/L of heparin, followed by the pre-filter heparin infusion.
Sodium citrate anticoagulation is widely used in pediatric CRRT programs due to its ease of administration and decreased bleeding risk [128]. By infusing citrate into the arterial limb of the CRRT tubing as it leaves the catheter, calcium ions are bound to the citrate, reducing available calcium and thereby inhibiting coagulation within the circuit, since normal coagulation is calcium-dependent. Systemic hypocalcemia is prevented by infusion of either calcium gluconate or calcium chloride into the patient at a central site. Thus, citrate anticoagulation achieves regional anticoagulation by affecting only the circuit, thereby eliminating the increased risk of bleeding with heparin. Since the original citrate protocol employing 4% trisodium citrate (440 mEq/L sodium), newer modifications utilize anticoagulant citrate dextrose “A” (ACD-A), a less-concentrated formulation, which is also commonly used as the anticoagulant in apheresis procedures.
Adverse effects of citrate anticoagulation include acid-base disturbances, citrate excess and hyperglycemia in infants when ACD-A is used. Patients receiving citrate anticoagulation may develop metabolic alkalosis; fortunately, citrate is readily cleared by dialysis [129]. Citrate excess may be diagnosed by monitoring the ratio of the total calcium to the ionized calcium levels [130]. If hepatic metabolism of citrate is insufficient, citrate accumulates; thus, patients with diminished liver function are at increased risk for citrate excess. Citrate excess can occur when citrate clearance is less than citrate delivery. Citrate is not inherently toxic, but citrate excess causes systemic hypocalcemia. Total calcium levels rise and the ratio of total calcium to systemic ionized calcium levels rises precipitously. As citrate accumulation progresses, it becomes more difficult to maintain the declining systemic ionized calcium levels within normal ranges. Monitoring of ionized calcium is the most sensitive way to detect citrate accumulation [131]. Treatment often requires increasing the removal of citrate by increasing clearance within the circuit (i.e., increased dialysate and/or replacement fluid rate); this assures ongoing anticoagulation while balancing the build-up of citrate in the patient. An initial citrate infusion rate of 50–70% of the usual rate is also recommended in patients with hepatic insufficiency who are at increased risk for citrate toxicity. A pediatric citrate anticoagulation protocol using bicarbonate dialysate has been published [132].
It is also possible in certain situations to use no anticoagulation, relying on periodic saline flushes of the circuit. This approach is typically considered in larger patients with evidence of a sustained coagulopathy due to disseminated intravascular coagulopathy or hepatic failure. However, many of these patients are receiving periodic fresh frozen plasma and platelet infusions to correct the underlying coagulopathy; these infusions will clot a CRRT system when no anticoagulation is used. Moreover, patients with hepatic failure may have a paradoxical hypercoagulable state. An uncontrolled study demonstrated that the no coagulation/saline flushes approach was associated with an inferior circuit life span compared to heparin or citrate anticoagulation [127].
CRRT Use in Combination Therapies
Extra-corporeal Membrane Oxygenation
The widespread use of ECMO in neonatal and pediatric critical care units along with the common occurrence of AKI in these patients with multi-organ dysfunction has led to the need to incorporate CRRT into the ECMO circuit. Fluid overload at CRRT initiation has been shown to be a consistent factor associated negatively with survival in ECMO and preventing the development of significant fluid overload at the outset of ECMO may be more clinically effective than attempting fluid removal later in therapy [133].
The ECMO circuit is fully heparinized, eliminating the need for anticoagulation of the CRRT circuit. Blood flow in the ECMO circuit is often 20–30 times that required for optimal CRRT. Newer ECMO circuits with multiple access phalanges allow the insertion of the CRRT circuit in an entirely pre-oxygenator location, avoiding shunt of oxygenated blood from the patient when the CRRT circuit is placed in a post- to pre-oxygenator position. Close collaboration between CRRT and ECMO teams is required to find the best location for the CRRT circuit and to coordinate therapy goals [134].
Plasma Therapy
Patients with AKI secondary to immune complex–mediated disease and sepsis-associated thrombotic microangiopathy may require both CRRT and plasma therapies (i.e., plasmapheresis, plasma exchange) [135]. CRRT is readily combined concurrently with plasma therapy procedures without interrupting the CRRT circuit. The placement of a three-way stopcock at both arterial and venous limbs of the CRRT circuit at the connection to the double lumen catheter allows diversion of blood through the centrifugation plasmapheresis machine [136].
Plasma exchange removes inflammatory mediators and replaces the volume with fresh frozen plasma in attempt to correct underlying homeostatic abnormalities; conversely, plasmapheresis removes plasma with inflammatory mediators, but replaces the volume with a non-plasma solution (usually albumin) [137]. Additionally, CRRT is believed to have an immunomodulatory effect on inflammatory cytokines in sepsis; however, the impact on patient outcome in sepsis remains unclear and the primary role remains management of fluid overload [138]. CRRT may downregulate the inflammatory response through nonselective extracorporeal removal, mainly by absorption, of cytokines and other mediators, restoring hemodynamic and immunologic homeostasis [139]. One retrospective study demonstrated benefit of isovolemic hemofiltration followed by conventional continuous venovenous hemofiltration in patients with septic shock and oliguric AKI with subsequent improvement in oxygenation and mean arterial pressure as well as significant improvement in survival at 28 days versus those receiving conventional supportive therapy [140].
Complications
CRRT requires the patient to remain relatively immobilized while connected to the CRRT circuit for prolonged periods. As a result, small children typically require sedation and occasionally even pharmacological paralysis to prevent small movements that may disrupt flow in the CRRT circuit. Additionally, a relatively large fraction of total circulating blood volume is in the extracorporeal circuit, placing the child at substantial risk for hypothermia during CRRT. Careful temperature monitoring is required during RRT, particularly when combination therapies are utilized. In-line fluid warmers can be used but increase priming volume. Line warmers that can be applied to the return line offer the best results.
Outcome
The Prospective Pediatric CRRT (ppCRRT) Registry reports an overall pediatric CRRT survival rate of 58% [80]. Survival of pediatric patients treated with CRRT has been reported in single center studies to vary widely by disease and modality [20, 141, 142]. A single center study initially demonstrated that the degree of fluid overload was an independent determinant of outcome in pediatric patients treated with CRRT [20], and that was confirmed by a large multicenter study from the ppCRRT Registry [80]. Patient survival was inversely correlated with percentage fluid overload at initiation of CRRT: survivors had a mean fluid overload of 14.2% while in non-survivors had a mean fluid overload of 25.4%, a difference that was highly significant and independent of diagnosis or severity of illness [20]. Further analysis of the ppCRRT Registry data demonstrated that 20% fluid overload was associated with four times the mortality of pediatric patients receiving CRRT when compared to patients with less than 10% fluid overload at initiation of CRRT [80]. These data suggest that earlier initiation of measures to control fluid accumulation, including CRRT, may improve survival.
Extended Hemodialysis Techniques
Extended hemodialysis techniques, also known as hybrid therapies, utilize intermittent hemodialysis machine technology while providing the slower solute and fluid removal associated with continuous RRT for use in less stable patients with AKI [143, 144]. The terms for these modalities include sustained low-efficiency daily dialysis (SLEDD) or extended daily dialysis (EDD) or slow continuous dialysis (SCD) [145].
SLEDD is a dialytic modality that allows for flexible options in treatment duration, prolonged or even continuous treatments, using conventional dialysis machines with varying pump speeds for 6–18 h daily [146]. Variants such as sustained low-efficiency daily diafiltration (SLEDD-f), aimed at improving clearance of middle molecular inflammatory mediators of the systemic inflammatory response associated with sepsis, have been developed for clinical use [92, 147]. Advantages of SLEDD-f over CVVHDF include faster clearance of small solutes and fluid removal yet maintaining hemodynamic stability [148]. It allows flexible therapeutic schedules so that patients are accessible and can be mobilized for other medical treatments.
Hybrid therapies also have lower heparin requirement than CRRT, but less frequent clotting. The reported incidence of clotting is 17–26% with heparin, while the reported incidence of circuit clotting without anticoagulation is 24–26% using single pass machines and lower using batch systems [149]. Hybrid therapies, with the high diffusive capacity for solutes, are able to correct alkalosis or hypernatremia, while at the same time removing the calcium chelated citrate complexes in citrate anticoagulation, an advantage in patients with liver failure [150]. With hybrid therapies, phosphate removal can be very extensive. Hypophosphatemia and metabolic alkalosis is easily induced in a critically ill patient, especially those on prolonged parenteral nutrition; therefore, preemptive fluid correction with phosphorous and reduction in dialysate bicarbonate are often warranted.
Conclusion
AKI is common in hospitalized children and associated with high risk of mortality and long-term morbidity. Recent advances in the understanding of the pathophysiology of AKI have pointed to newer diagnostic and therapeutic strategies that focus on early recognition and treatment. Exciting developments in technology have made RRT more accessible and more easily applied in the pediatric setting. Yet, despite these advances, mortality rates among children with AKI remain disturbingly high. Hopefully, future developments will improve outcomes for children with AKI.
References
Bunchman TE. Treatment of acute kidney injury in children: from conservative management to renal replacement therapy. Nat Clin Pract Nephrol. 2008;4(9):510–4.
Himmelfarb J, Joannidis M, Molitoris B, Schietz M, Okusa MD, Warnock D, et al. Evaluation and initial management of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(4):962–7.
Andreoli SP. Acute kidney injury in children. Pediatr Nephrol. 2009;24(2):253–63.
Zappitelli M, Bernier PL, Saczkowski RS, Tchervenkov CI, Gottesman R, Dancea A, et al. A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int. 2009;76(8):885–92.
Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–56.
Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–53.
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2(Suppl):1–138.
De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–89.
Delmas A, Leone M, Rousseau S, Albanese J, Martin C. Clinical review: vasopressin and terlipressin in septic shock patients. Crit Care. 2005;9(2):212–22.
Venkataraman R, Kellum JA. Prevention of acute renal failure. Chest. 2007;131(1):300–8.
Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356(9248):2139–43.
Friedrich JO, Adhikari N, Herridge MS, Beyene J. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med. 2005;142(7):510–24.
Lauschke A, Teichgraber UK, Frei U, Eckardt KU. ‘Low-dose’ dopamine worsens renal perfusion in patients with acute renal failure. Kidney Int. 2006;69(9):1669–74.
Cogliati AA, Vellutini R, Nardini A, Urovi S, Hamdan M, Landoni G, et al. Fenoldopam infusion for renal protection in high-risk cardiac surgery patients: a randomized clinical study. J Cardiothorac Vasc Anesth. 2007;21(6):847–50.
Landoni G, Biondi-Zoccai GG, Tumlin JA, Bove T, De Luca M, Calabro MG, et al. Beneficial impact of fenoldopam in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials. Am J Kidney Dis. 2007;49(1):56–68.
Ricci Z, Luciano R, Favia I, Garisto C, Muraca M, Morelli S, et al. High-dose fenoldopam reduces postoperative neutrophil gelatinase-associated lipocaline and cystatin C levels in pediatric cardiac surgery. Crit Care. 2011;15(3):R160.
Ranucci M, De Benedetti D, Bianchini C, Castelvecchio S, Ballotta A, Frigiola A, et al. Effects of fenoldopam infusion in complex cardiac surgical operations: a prospective, randomized, double-blind, placebo-controlled study. Minerva Anestesiol. 2010;76(4):249–59.
Cantarovich F, Rangoonwala B, Lorenz H, Verho M, Esnault VL, High-Dose Flurosemide in Acute Renal Failure Study Group. High-dose furosemide for established ARF: a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Am J Kidney Dis. 2004;44(3):402–9.
Mehta RL, Pascual MT, Soroko S, Chertow GM, Group PS. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288(20):2547–53.
Goldstein SL, Currier H, Graf C, Cosio CC, Brewer ED, Sachdeva R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309–12.
Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Patil N, Ambalavanan N. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr Nephrol. 2013;28(4):661–6.
Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer’s solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesth Analg. 1999;88(5):999–1003.
Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab)normal saline and physiological Hartmann’s solution: a randomized double-blind crossover study. Clin Sci. 2003;104(1):17–24.
Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71(3):726–35.
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527–39.
Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD, National Heart Lung, and Blood Institute Acute Respiratory Distress Syndrome Network, et al. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;6(5):966–73.
Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia. 2010;65(3):283–93.
Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ. 2006;333(7565):420.
Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32(8):1771–6.
Andreoli SP. Management of acute kidney injury in children: a guide for pediatricians. Paediatr Drugs. 2008;10(6):379–90.
Andreoli SP. Acute and chronic renal failure in children. In: Gearhart JP, Rink RC, Mouriquand PDE, editors. Pediatric urology. Philadelphia: Saunders; 2001. p. 777–89.
Fiaccadori E, Cremaschi E. Nutritional assessment and support in acute kidney injury. Curr Opin Crit Care. 2009;15(6):474–80.
McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN Parenter Enteral Nutr. 2009;33(3):277–316.
Fiaccadori E, Cremaschi E, Regolisti G. Nutritional assessment and delivery in renal replacement therapy patients. Semin Dial. 2011;24(2):169–75.
Zappitelli M, Goldstein SL, Symons JM, Somers MJ, Baum MA, Brophy PD, et al. Protein and calorie prescription for children and young adults receiving continuous renal replacement therapy: a report from the Prospective Pediatric Continuous Renal Replacement Therapy Registry Group. Crit Care Med. 2008;36(12):3239–45.
Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–8.
Fiaccadori E, Lombardi M, Leonardi S, Rotelli CF, Tortorella G, Borghetti A. Prevalence and clinical outcome associated with preexisting malnutrition in acute renal failure: a prospective cohort study. J Am Soc Nephrol. 1999;10(3):581–93.
Cano NJ, Aparicio M, Brunori G, Carrero JJ, Cianciaruso B, Fiaccadori E, et al. ESPEN guidelines on parenteral nutrition: adult renal failure. Clin Nutr. 2009;28(4):401–14.
Briassoulis G, Tsorva A, Zavras N, Hatzis T. Influence of an aggressive early enteral nutrition protocol on nitrogen balance in critically ill children. J Nutr Biochem. 2002;13(9):560.
Fiaccadori E, Maggiore U, Giacosa R, Rotelli C, Picetti E, Sagripanti S, et al. Enteral nutrition in patients with acute renal failure. Kidney Int. 2004;65(3):999–1008.
Fiaccadori E, Maggiore U, Rotelli C, Giacosa R, Picetti E, Parenti E, et al. Effects of different energy intakes on nitrogen balance in patients with acute renal failure: a pilot study. Nephrol Dial Transplant. 2005;20(9):1976–80.
Story DA, Ronco C, Bellomo R. Trace element and vitamin concentrations and losses in critically ill patients treated with continuous venovenous hemofiltration. Crit Care Med. 1999;27(1):220–3.
Nakamura AT, Btaiche IF, Pasko DA, Jain JC, Mueller BA. In vitro clearance of trace elements via continuous renal replacement therapy. J Renal Nutr. 2004;14(4):214–9.
Van Cromphaut SJ. Hyperglycaemia as part of the stress response: the underlying mechanisms. Best Pract Res Clin Anaesthesiol. 2009;23(4):375–86.
Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–61.
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–67.
Hermans G, Wilmer A, Meersseman W, Milants I, Wouters PJ, Bobbaers H, et al. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med. 2007;175(5):480–9.
Schetz M, Vanhorebeek I, Wouters PJ, Wilmer A, Van den Berghe G. Tight blood glucose control is renoprotective in critically ill patients. J Am Soc Nephrol. 2008;19(3):571–8.
Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:S1–138.
Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66(4):1613–21.
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8.
Kellum JA, Leblanc M, Venkataraman R. Acute renal failure. Clin Evid. 2008;2008:pii 2001.
Misurac JM, Knoderer CA, Leiser JD, Nailescu C, Wilson AC, Andreoli SP. Nonsteroidal anti-inflammatory drugs are an important cause of acute kidney injury in children. J Pediatr. 2013;162(6):1153–9, 9 e1.
Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105(19):2259–64.
Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993;188(1):171–8.
Solomon R. The role of osmolality in the incidence of contrast-induced nephropathy: a systematic review of angiographic contrast media in high risk patients. Kidney Int. 2005;68(5):2256–63.
Pannu N, Wiebe N, Tonelli M. Prophylaxis strategies for contrast-induced nephropathy. JAMA. 2006;295(23):2765–79.
Cantley LG, Spokes K, Clark B, McMahon EG, Carter J, Epstein FH. Role of endothelin and prostaglandins in radiocontrast-induced renal artery constriction. Kidney Int. 1993;44(6):1217–23.
Pflueger A, Larson TS, Nath KA, King BF, Gross JM, Knox FG. Role of adenosine in contrast media-induced acute renal failure in diabetes mellitus. Mayo Clin Proc. 2000;75(12):1275–83.
Rudnick MR, Berns JS, Cohen RM, Goldfarb S. Nephrotoxic risks of renal angiography: contrast media-associated nephrotoxicity and atheroembolism – a critical review. Am J Kidney Dis. 1994;24(4):713–27.
Taber SS, Mueller BA. Drug-associated renal dysfunction. Crit Care Clin. 2006;22(2):357–74, viii.
Peixoto AJ. Critical issues in nephrology. Clin Chest Med. 2003;24(4):561–81.
McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51(15):1419–28.
Weisbord SD, Gallagher M, Jneid H, et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. NEJM. 2018;378:603–14.
Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7(4):189–200.
Bernhardt WM, Eckardt KU. Physiological basis for the use of erythropoietin in critically ill patients at risk for acute kidney injury. Curr Opin Crit Care. 2008;14(6):621–6.
Endre ZH, Walker RJ, Pickering JW, Shaw GM, Frampton CM, Henderson SJ, et al. Early intervention with erythropoietin does not affect the outcome of acute kidney injury (the EARLYARF trial). Kidney Int. 2010;77(11):1020–30.
Miller SB, Martin DR, Kissane J, Hammerman MR. Insulin-like growth factor I accelerates recovery from ischemic acute tubular necrosis in the rat. Proc Natl Acad Sci U S A. 1992;89(24):11876–80.
Vijayan A, Martin DR, Sadow JL, Kissane J, Miller SB. Hepatocyte growth factor inhibits apoptosis after ischemic renal injury in rats. Am J Kidney Dis. 2001;38(2):274–8.
Gouyon JB, Guignard JP. Theophylline prevents the hypoxemia-induced renal hemodynamic changes in rabbits. Kidney Int. 1988;33(6):1078–83.
Bhat MA, Shah ZA, Makhdoomi MS, Mufti MH. Theophylline for renal function in term neonates with perinatal asphyxia: a randomized, placebo-controlled trial. J Pediatr. 2006;149(2):180–4.
Bakr AF. Prophylactic theophylline to prevent renal dysfunction in newborns exposed to perinatal asphyxia – a study in a developing country. Pediatr Nephrol. 2005;20(9):1249–52.
Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38(3):933–9.
Proulx F, Gauthier M, Nadeau D, Lacroix J, Farrell CA. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994;22(6):1025–31.
Flynn JT. Choice of dialysis modality for management of pediatric acute renal failure. Pediatr Nephrol. 2002;17(1):61–9.
Liu KD, Himmelfarb J, Paganini E, Ikizler TA, Soroko SH, Mehta RL, et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1(5):915–9.
Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, et al. Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care. 2009;24(1):129–40.
Basu RK, Chawla LS, Wheeler DS, Goldstein SL. Renal angina: an emerging paradigm to identify children at risk for acute kidney injury. Pediatr Nephrol. 2012;27(7):1067–78.
Basu RK, Zappitelli M, Brunner L, Wang Y, Wong HR, Chawla LS, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014;85(3):659–67.
Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316–25.
Bonilla-Felix M. Peritoneal dialysis in the pediatric intensive care unit setting: techniques, quantitations and outcomes. Blood Purif. 2013;35(1–3):77–80.
Warady BA, Bunchman T. Dialysis therapy for children with acute renal failure: survey results. Pediatr Nephrol. 2000;15(1–2):11–3.
Gong WK, Tan TH, Foong PP, Murugasu B, Yap HK. Eighteen years experience in pediatric acute dialysis: analysis of predictors of outcome. Pediatr Nephrol. 2001;16(3):212–5.
Anochie IC, Eke FU. Paediatric acute peritoneal dialysis in southern Nigeria. Postgrad Med J. 2006;82(965):228–30.
Phadke KD, Dinakar C. The challenges of treating children with renal failure in a developing country. Perit Dial Int. 2001;21(Suppl 3):S326–9.
Wong SN, Geary DF. Comparison of temporary and permanent catheters for acute peritoneal dialysis. Arch Dis Child. 1988;63(7):827–31.
Chadha V, Warady BA, Blowey DL, Simckes AM, Alon US. Tenckhoff catheters prove superior to cook catheters in pediatric acute peritoneal dialysis. Am J Kidney Dis. 2000;35(6):1111–6.
Reznik VM, Griswold WR, Peterson BM, Rodarte A, Ferris ME, Mendoza SA. Peritoneal dialysis for acute renal failure in children. Pediatr Nephrol. 1991;5(6):715–7.
Schaefer F, Warady BA. Peritoneal dialysis in children with end-stage renal disease. Nat Rev Nephrol. 2011;7(11):659–68.
Rabindranath K, Adams J, Macleod AM, Muirhead N. Intermittent versus continuous renal replacement therapy for acute renal failure in adults. Cochrane Database Syst Rev. 2007;3:CD003773.
Bagshaw SM, Berthiaume LR, Delaney A, Bellomo R. Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: a meta-analysis. Crit Care Med. 2008;36(2):610–7.
Abi Antoun T, Palevsky PM. Selection of modality of renal replacement therapy. Semin Dial. 2009;22(2):108–13.
Bunchman T, Gardner J, Kershaw D, Maxvold N. Vascular access for hemodialysis or CVVH(D) in infants and children. Nephrol Dial Transplant. 1994;23:314–7.
Muller D, Goldstein SL. Hemodialysis in children with end-stage renal disease. Nat Rev Nephrol. 2011;7(11):650–8.
Mehta RL. Indications for dialysis in the ICU: renal replacement vs. renal support. Blood Purif. 2001;19(2):227–32.
Mehta RL, Bouchard J. Dialysis dosage in acute kidney injury: still a conundrum? J Am Soc Nephrol. 2008;19(6):1046–8.
Palevsky PM. Renal replacement therapy in acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):76–84.
Goldstein SL. Overview of pediatric renal replacement therapy in acute renal failure. Artif Organs. 2003;27(9):781–5.
Brophy PD, Mottes TA, Kudelka TL, McBryde KD, Gardner JJ, Maxvold NJ, et al. AN-69 membrane reactions are pH-dependent and preventable. Am J Kidney Dis. 2001;38(1):173–8.
Parshuram CS, Cox PN. Neonatal hyperkalemic-hypocalcemic cardiac arrest associated with initiation of blood-primed continuous venovenous hemofiltration. Pediatr Crit Care Med. 2002;3(1):67–9.
Pasko DA, Mottes TA, Mueller BA. Pre dialysis of blood prime in continuous hemodialysis normalizes pH and electrolytes. Pediatr Nephrol. 2003;18(11):1177–83.
Hackbarth RM, Eding D, Gianoli Smith C, Koch A, Sanfilippo DJ, Bunchman TE. Zero balance ultrafiltration (Z-BUF) in blood-primed CRRT circuits achieves electrolyte and acid-base homeostasis prior to patient connection. Pediatr Nephrol. 2005;20(9):1328–33.
Fischbach M, Edefonti A, Schroder C, Watson A. Hemodialysis in children: general practical guidelines. Pediatr Nephrol. 2005;20(8):1054–66.
Alonso A, Lau J, Jaber BL. Biocompatible hemodialysis membranes for acute renal failure. Cochrane Database Syst Rev. 2008;1:CD005283.
Pannu N, Klarenbach S, Wiebe N, Manns B, Tonelli M. Renal replacement therapy in patients with acute renal failure: a systematic review. JAMA. 2008;299(7):793–805.
Uchino S, Bellomo R, Morimatsu H, Goldsmith D, Davenport P, Cole L, et al. Cytokine dialysis: an ex vivo study. ASAIO J. 2002;48(6):650–3.
Haase M, Bellomo R, Baldwin I, Haase-Fielitz A, Fealy N, Davenport P, et al. Hemodialysis membrane with a high-molecular-weight cutoff and cytokine levels in sepsis complicated by acute renal failure: a phase 1 randomized trial. Am J Kidney Dis. 2007;50(2):296–304.
Haase M, Bellomo R, Morgera S, Baldwin I, Boyce N. High cut-off point membranes in septic acute renal failure: a systematic review. Int J Artif Organs. 2007;30(12):1031–41.
Rimmele T, Assadi A, Cattenoz M, Desebbe O, Lambert C, Boselli E, et al. High-volume haemofiltration with a new haemofiltration membrane having enhanced adsorption properties in septic pigs. Nephrol Dial Transplant. 2009;24(2):421–7.
Warkentin TE, Greinacher A, Koster A, Lincoff AM, American College of Chest Physicians. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 Suppl):340S–80.
Stamatiadis DN, Helioti H, Mansour M, Pappas M, Bokos JG, Stathakis CP. Hemodialysis for patients bleeding or at risk for bleeding, can be simple, safe and efficient. Clin Nephrol. 2004;62(1):29–34.
Caruana RJ, Raja RM, Bush JV, Kramer MS, Goldstein SJ. Heparin free dialysis: comparative data and results in high risk patients. Kidney Int. 1987;31(6):1351–5.
Sanders PW, Taylor H, Curtis JJ. Hemodialysis without anticoagulation. Am J Kidney Dis. 1985;5(1):32–5.
Lohr JW, Slusher S, Diederich D. Safety of regional citrate hemodialysis in acute renal failure. Am J Kidney Dis. 1989;13(2):104–7.
Patel N, Dalal P, Panesar M. Dialysis disequilibrium syndrome: a narrative review. Semin Dial. 2008;21(5):493–8.
Rees L. Hemodialysis. In: Avner ED, Harmon WE, Niaudet P, editors. Pediatric nephrology. 5th ed. Philadelphia: Lippincott, Williams & Wilkins; 2004.
Schortgen F, Soubrier N, Delclaux C, Thuong M, Girou E, Brun-Buisson C, et al. Hemodynamic tolerance of intermittent hemodialysis in critically ill patients: usefulness of practice guidelines. Am J Respir Crit Care Med. 2000;162(1):197–202.
Dheu C, Terzic J, Menouer S, Fischbach M. Importance of the curve shape for interpretation of blood volume monitor changes during haemodiafiltration. Pediatr Nephrol. 2009;24(7):1419–23.
Bunchman TE, Maxvold NJ, Kershaw DB, Sedman AB, Custer JR. Continuous venovenous hemodiafiltration in infants and children. Am J Kidney Dis. 1995;25(1):17–21.
Davenport A, Will EJ, Davison AM. Hyperlactataemia and metabolic acidosis during haemofiltration using lactate-buffered fluids. Nephron. 1991;59(3):461–5.
Thomas AN, Guy JM, Kishen R, Geraghty IF, Bowles BJ, Vadgama P. Comparison of lactate and bicarbonate buffered haemofiltration fluids: use in critically ill patients. Nephrol Dial Transplant. 1997;12(6):1212–7.
Zimmerman D, Cotman P, Ting R, Karanicolas S, Tobe SW. Continuous veno-venous haemodialysis with a novel bicarbonate dialysis solution: prospective cross-over comparison with a lactate buffered solution. Nephrol Dial Transplant. 1999;14(10):2387–91.
Maxvold N, Flynn J, Smoyer W, et al. Prospective crossover comparison of bicarbonate vs lactate-based dialysate for pediatric CVVHD. Blood Purif. 1999;17:27–9.
Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;356(9223):26–30.
Di Carlo JV, Alexander SR. Hemofiltration for cytokine-driven illnesses: the mediator delivery hypothesis. Int J Artif Organs. 2005;28(8):777–86.
Honore PM, Joannes-Boyau O, Boer W, Collin V. High-volume hemofiltration in sepsis and SIRS: current concepts and future prospects. Blood Purif. 2009;28(1):1–11.
Brophy PD, Somers MJ, Baum MA, Symons JM, McAfee N, Fortenberry JD, et al. Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT). Nephrol Dial Transplant. 2005;20(7):1416–21.
Mehta RL, McDonald BR, Aguilar MM, Ward DM. Regional citrate anticoagulation for continuous arteriovenous hemodialysis in critically ill patients. Kidney Int. 1990;38(5):976–81.
Chadha V, Garg U, Warady BA, Alon US. Citrate clearance in children receiving continuous venovenous renal replacement therapy. Pediatr Nephrol. 2002;17(10):819–24.
Meier-Kriesche HU, Gitomer J, Finkel K, DuBose T. Increased total to ionized calcium ratio during continuous venovenous hemodialysis with regional citrate anticoagulation. Crit Care Med. 2001;29(4):748–52.
Oudemans-van Straaten HM. Citrate anticoagulation for continuous renal replacement therapy in the critically ill. Blood Purif. 2010;29(2):191–6.
Bunchman TE, Maxvold NJ, Barnett J, Hutchings A, Benfield MR. Pediatric hemofiltration: normocarb dialysate solution with citrate anticoagulation. Pediatr Nephrol. 2002;17(3):150–4.
Selewski DT, Cornell TT, Blatt NB, Han YY, Mottes T, Kommareddi M, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 2012;40(9):2694–9.
Ricci Z, Ronco C, Picardo S. CRRT in series with extracorporeal membrane oxygenation in pediatric patients. Kidney Int. 2010;77(5):469–70; author reply 71.
Peng ZY, Kiss JE, Cortese-Hasset A, Carcillo JA, Nguyen TC, Kellum JA. Plasma filtration on mediators of thrombotic microangiopathy: an in vitro study. Int J Artif Organs. 2007;30(5):401–6.
Yorgin PD, Eklund DK, al-Uzri AA, Whitesell L, Theodorou AA. Concurrent centrifugation plasmapheresis and continuous venovenous hemodiafiltration. Pediatr Nephrol. 2000;14(1):18–21.
House AA, Ronco C. Extracorporeal blood purification in sepsis and sepsis-related acute kidney injury. Blood Purif. 2008;26(1):30–5.
Servillo G, Vargas M, Pastore A, Procino A, Iannuzzi M, Capuano A, et al. Immunomodulatory effect of continuous venovenous hemofiltration during sepsis: preliminary data. Biomed Res Int. 2013;2013:108951.
Grootendorst AF. The potential role of hemofiltration in the treatment of patients with septic shock and multiple organ dysfunction syndrome. Adv Ren Replace Ther. 1994;1(2):176–84.
Piccinni P, Dan M, Barbacini S, Carraro R, Lieta E, Marafon S, et al. Early isovolaemic haemofiltration in oliguric patients with septic shock. Intensive Care Med. 2006;32(1):80–6.
Symons JM, Brophy PD, Gregory MJ, McAfee N, Somers MJ, Bunchman TE, et al. Continuous renal replacement therapy in children up to 10 kg. Am J Kidney Dis. 2003;41(5):984–9.
Flores FX, Brophy PD, Symons JM, Fortenberry JD, Chua AN, Alexander SR, et al. Continuous renal replacement therapy (CRRT) after stem cell transplantation. A report from the prospective pediatric CRRT Registry Group. Pediatr Nephrol. 2008;23(4):625–30.
Kudoh Y, Iimura O. Slow continuous hemodialysis – new therapy for acute renal failure in critically ill patients – Part 1. Theoretical consideration and new technique. Jpn Circ J. 1988;52(10):1171–82.
Kudoh Y, Shiiki M, Sasa Y, Hotta D, Nozawa A, Iimura O. Slow continuous hemodialysis – new therapy for acute renal failure in critically ill patients – Part 2. Animal experiments and clinical implication. Jpn Circ J. 1988;52(10):1183–90.
Tam PY, Huraib S, Mahan B, LeBlanc D, Lunski CA, Holtzer C, et al. Slow continuous hemodialysis for the management of complicated acute renal failure in an intensive care unit. Clin Nephrol. 1988;30(2):79–85.
Cruz D, Bobek I, Lentini P, Soni S, Chionh CY, Ronco C. Machines for continuous renal replacement therapy. Semin Dial. 2009;22(2):123–32.
Fliser D, Kielstein JT. Technology insight: treatment of renal failure in the intensive care unit with extended dialysis. Nat Clin Pract Nephrol. 2006;2(1):32–9.
Marshall MR, Ma T, Galler D, Rankin AP, Williams AB. Sustained low-efficiency daily diafiltration (SLEDD-f) for critically ill patients requiring renal replacement therapy: towards an adequate therapy. Nephrol Dial Transplant. 2004;19(4):877–84.
Kumar VA, Craig M, Depner TA, Yeun JY. Extended daily dialysis: a new approach to renal replacement for acute renal failure in the intensive care unit. Am J Kidney Dis. 2000;36(2):294–300.
Morath C, Miftari N, Dikow R, Hainer C, Zeier M, Morgera S, et al. Sodium citrate anticoagulation during sustained low efficiency dialysis (SLED) in patients with acute renal failure and severely impaired liver function. Nephrol Dial Transplant. 2008;23(1):421–2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Harshman, L.A., Brophy, P.D., Symons, J.M. (2023). Management of Pediatric Acute Kidney Injury. In: Schaefer, F., Greenbaum, L.A. (eds) Pediatric Kidney Disease. Springer, Cham. https://doi.org/10.1007/978-3-031-11665-0_52
Download citation
DOI: https://doi.org/10.1007/978-3-031-11665-0_52
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-11664-3
Online ISBN: 978-3-031-11665-0
eBook Packages: MedicineMedicine (R0)