Abstract
The Sauropsida includes the extant crocodiles, birds, turtles, lizards and snakes. With roughly 30,000 described species, it is not only the largest phylogenetic group within Amniota, but the largest extant group within all tetrapods. Like many other tetrapod branches, sauropsids have evolved many adaptations to aquatic lifestyles: from species that occasionally feed in aquatic habitats to fully aquatic forms that only rise to the water’s surface for breathing. As amniotes, sauropsids can safely be considered primarily terrestrial vertebrates and any adaptations to aquatic life and feeding can be regarded as secondary features. Sauropsids show a very broad spectrum of convergently-evolved adaptations for aquatic feeding, from crocodylian apex predators to high-performance suspension feeding birds, suction feeding in turtles and alga-scraping in marine iguanas. Adaptations for aquatic feeding in sauropsids have evolved multiple times independently, both between and within groups. For example, suction feeding has evolved independently in turtles and birds; extremely fast forward strikes by straightening of the curved postcranial vertebral column in birds and snakes; and suspension feeding in mallards, flamingoes and sea-birds. In the following sections, we summarize the diverse adaptations to aquatic feeding in crocodylians, birds, lepidosaurs and turtles and highlight convergence and homologies where appropriate.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aquatic feeding
- Bidirectional flow system
- Biomechanical modelling
- Bite force
- Finite element analysis
- Inertial feeding
- Jaw prehension
- Ram feeding
- Suction feeding
- Suspension feeding
7.1 Crocodylia
7.1.1 Introduction
Today, crocodylians (Archosauria: Crocodylia) are medium to large-bodied, semi-aquatic reptiles with snap-trap jaws and impressive bite-force capacities, inhabiting tropical zones around the world (Gignac et al., 2019). Long considered to be “living fossils”, it is now understood that crocodylians, along with their crocodyliform precursors, previously exhibited a wide range of body plans and skull shapes corresponding to diverse locomotor, dietary, and habitat specializations, including adaptations to terrestrial and marine niches (Stubbs et al., 2013; Mannion et al., 2015). Numerous studies have demonstrated that crocodyliforms have been shaped extensively by convergent evolution (von Huene, 1933; Buffetaut, 1982; Brochu, 2001; Wroe & Milne, 2007; Jones, 2008; Pierce et al., 2009; Wilberg et al., 2019). These morphological shifts have helped this group invade new adaptive zones (Erwin, 1992; Wainwright & Price, 2016) and dominate predatory niches in and around the water for the past 200 million years (Wilberg et al., 2019).
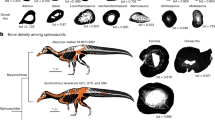
Fish-eating, or piscivory, is a common behavior of crocodylians, owing to the abundance of such prey in the world’s rivers, lakes and oceans. One species in particular, the Indian gharial (Gavialis gangeticus), is routinely singled out as the most piscivorous of extant crocodylians (Pooley, 1989; Whitaker & Basu, 1982; Thorbjarnarson, 1990; Trutnau & Sommerlad, 2006; but also see Forsyth, 1910; Shortt, 1921; Biswas, 1970), capable of securing agile aquatic prey with its elongated jaws and > 100 needle-like teeth (Taylor, 1987; Singh, 2015; Ballell et al., 2019). Several other extant crocodylians, including the semi-piscivorous Malay (“false”) gharial (Tomistoma schlegelii), the African slender-snouted crocodile (Mecistops cataphractus), the Orinoco crocodile (Crocodylus intermedius), and the Australian freshwater crocodile (C. johnstoni) (Pooley & Gans, 1976; Pooley, 1989; Webb & Manolis, 1989), while not strictly piscivorous, do consume an abundance of fish (Brochu, 2001; Erickson et al., 2012). Notably, all share a series of independently derived features, including slender snouts (see Fig. 7.1), that appear to permit the capture of highly elusive aquatic prey, especially fish (Brochu, 2001; Erickson et al., 2012), by enabling wide head sweeps encompassing large strike zones (Erickson et al., 2012; Ballell et al., 2019) and rapid jaw closure under water (McHenry et al., 2006).
Images of adult skulls in dorsal view exemplifying snout proportions in Paleosuchus trigonatus (Field Museum of Natural History specimen no. [FMNH] 68,879; upper right), which has a snout aspect ratio—mid-rostral width [white dashed line] divided by snout length [black dashed line]—of 0.51, near the interspecific mean of 0.53 for the clade Crocodylia (Erickson et al., 2012). The five semi- and highly-piscivorous species with narrow snouts are ordered from left to right by ascending value of snout aspect ratio: Gavialis gangeticus (FMNH 82861) and Tomistoma schlegelii (University of California Museum of Paleontology specimen no. 81702) are considered longirostrine (Brochu, 2001), whereas Crocodylus johnstoni (Texas Memorial Museum specimen no. [TMM] M-6807), Mecistops cataphractus (TMM M-3529), and C. intermedius (FMNH 75658) are considered to be slender-snouted. All skull images are scaled to the same head width for easier comparison, following O’Brien et al. (2019). Photographs taken by P.M.G.; TMM M-6807 and M-3529 are dorsal view, 3D projections based on computed tomography scans completed by the University of Texas at Austin
Such rostrodental traits are shared by other secondarily aquatic fish-eating specialists, including river dolphins (e.g., Inia geoffrensis; Walmsley et al., 2013; McCurry et al., 2017a), suggesting that piscivorous crocodylian taxa are excellent modern analogs for addressing the convergent evolution of fish-eating and the re-invasion of freshwater and marine niches by now extinct tetrapods, especially reptiles (Massare, 1987; Hua & De Buffrenil, 1996; McHenry et al., 2006; Pierce & Benton, 2006; Pierce et al., 2009; Young et al., 2010; Salas-Gismondi, 2016; McCurry et al., 2017a, b; Ballell et al., 2019). In fact, crocodyliforms from the Jurassic and Cretaceous exhibit multiple independent forays into freshwater and marine environments, as evidenced by characteristic rostral, dental, and body-shape features found in fossils preserved within aquatic and marine depositional environments (Young et al., 2010; Bronzati et al., 2015; Ballell et al., 2019; Wilberg et al., 2019). Additionally, the appearance of conspicuously long-snouted (longirostrine) morphologies is linked to increases in body size in aquatic taxa (Godoy, 2020). Paleobiogeographic reconstructions indicate that members of the Thalattosuchia, Pholidosuridae, and Dyrosauridae share comparable, or more extreme, adaptations to aquatic and marine lifestyles than those observed in extant crocodylians (Wilberg et al., 2019). The most highly derived examples occurred among thalattosuchians, a group of fully pelagic, marine crocodyliforms featuring tail flukes, manual and pedal flippers, and hydrodynamic body shapes (Young et al., 2010; Foffa et al., 2018; Ballell et al., 2019; Schwab et al., 2020). Across many of these groups, both extant and extinct, shifts between freshwater and marine environments appear to have been common, indicating that such specializations were essential for land-to-water transitions (Wilberg et al., 2019).
Studying the aquatic feeding mechanics and cranial sensory systems of crocodylians has led to major advances in our understanding of how adaptation to aquatic niches can shape reptile bauplans (Massare, 1987; Hua & De Buffrenil, 1996; Pierce et al., 2009; Young et al., 2010; Schwab et al., 2020). Below, we detail these specializations as well as discuss how crocodylians detect, capture, and consume aquatic prey, including how this understanding can help to further advance the study of extreme convergence in the crocodyliform fossil record.
7.1.2 Food Detection
Semi-aquatic taxa commonly possess visual acuity in subaerial and subaquatic environments (Howland & Sivak, 1984; Underwood, 1970). Extant crocodylians, however, have visual systems equipped for functional focus and accommodation in subaerial environments only. Like many birds, they possess nasotemporally elongate foveae, five types of photoreceptors (one rod, one double-cone, and three single cones), and tapeta lucida (Nagloo et al., 2016; Soares & Bierman, 2018). These traits are advantageous for focusing along the length of shorelines, seeing in trichromatic color vision, and maintaining low-light vision by increasing the light available to stimulate photoreceptors. As a result, visual acuity is generally good in air (Nagloo et al., 2016); however, this is not the case underwater (Fleishman et al., 1988). Subaquatic vision is highly reduced in all species studied to date, including G. gangeticus (Fleishman et al., 1988). Although crocodylians reduce pupil size when they dive to adjust focal depth, they do not achieve the wide accommodation range necessary for subaquatic visual acuity (Fleishman et al., 1988). They likewise lack the flattened corneas, typical of water fowl (Sivak, 1980), needed to reduce refraction underwater. Thus, even highly piscivorous taxa are severely farsighted (hyperopia) when hunting aquatic prey (Fleishman et al., 1988).
However, crocodylians are highly capable subaquatic hunters, even in the absence of subaquatic visual acuity (Neill, 1971; Schaller & Crawshaw Jr., 1982). Instead of relying on vision, crocodylians detect prey in the water using integumentary sensory organs (a.k.a., dome pressure receptors; Brazaitis, 1987; Soares, 2002; Leitch & Catania, 2012). These neurosensory structures evolved independently from the lateral line systems of fishes and amphibians (e.g., Coombs et al., 2012) but share broadly similar roles for mechanically interpreting the direction of pressure waves in the water column (Soares, 2002). Remarkably, these integumentary sensory organs enable greater high-resolution mechanosensitivity than is achievable by the primate fingertip (Leitch & Catania, 2012), thus providing the dense sensory input necessary for precise orienting behaviors, even in the absence of light (Singh, 1976; Soares, 2002). Alligators and caimans restrict integumentary sensory organs to the head, whereas other crocodylians harbor them on scales across most of the body surface (Leitch & Catania, 2012). Presumably, this broader sensory field enables more accurate directional assessment for the source of water displacement caused by potential prey, conspecifics, and abiotic factors (also see Jackson et al., 1996; Jackson & Brooks, 2007).
7.1.3 Food Capture
Crocodylians are jaw-prehension feeders, and most species are mesorostrine, having medium-length snouts (McCurry et al., 2017b). Species such as Crocodylus rhombifer and Paleosuchus trigonatus (see Fig. 7.1) exemplify the mean crocodylian snout aspect ratio (snout width divided by snout length; Erickson et al., 2012) of approximately 0.53 with most other taxa falling within one standard deviation of this value. These species have snouts that are relatively longer than those of other reptiles but are of average length for crocodylians. A few forms have slender snouts that are not exceptionally long but are notably narrow, represented today by C. crocodylus, C. intermedius, and Mecistops cataphractus (Brochu, 2001; McHenry et al., 2006; Erickson et al., 2012). Two other species, Gavialis gangeticus and Tomistoma schlegelii, are characterized by exceptionally narrow and elongate jaws (i.e., a longirostrine morphology), giving their pre-orbital skulls a tubular appearance (Brochu, 2001). All of these species are comparably slender-snouted (Fig. 7.1), but C. crocodylus, C. intermedius, and M. cataphractus have snout aspect ratios of 0.35–0.24, whereas G. gangeticus and T. schlegelii have ratios of 0.10 and 0.18, respectively (Erickson et al., 2012).
When capturing and consuming fish, generalist semi-aquatic crocodylians, such as Alligator mississippiensis and Crocodylus niloticus employ lateral swipes of the head and rapid jaw closure (Taylor, 1987). This approach appears to minimize pressure drag induced by broad rostra by moving the snout mediolaterally, along the axis of its lowest profile (Liem et al., 2001). This behavior displaces less water than would be the case by attempting to raise or lower the snout within the water column, thereby incurring lower resistance to motion (McHenry et al., 2006; Pierce et al., 2008; McCurry et al., 2017b). Slender-snouted forms appear to be adapted for minimizing drag along the mediolateral as well as the dorsoventral axis, enabling opportunities to elevate and depress the jaw while also moving the rostrum from side-to-side without eliciting substantial additional pressure drag. Gavialis gangeticus, for example, routinely makes rapid, 180° sweeps with its head underwater and is highly successful at lateral strikes that are ≤90° (Thorbjarnarson, 1990). In addition, longirostrine taxa are proposed to achieve the most rapid closure at the distal end of the jaws (Alexander, 1983; Taylor, 1987; McHenry et al., 2006), further improving their chances of prey capture success.
Jaw elongation, however, may come with trade-offs. McHenry et al. (2006) calculated drag moments for crocodylian jaws based on standardized skull dimensions and found that longirostrine ecomorphs incurred substantially greater drag moments than shorter, narrow-snouted forms. This is because the more rapidly a rigid beam rotates through water, the more pressure drag it incurs, and longer skulls distribute more material further from the center of rotation (e.g., cranial cervical joint, quadrate articular joint; McHenry et al., 2006). Lever mechanics dictate that the distal end of a longer snout necessarily moves more rapidly than that of a shorter snout, all else being equal (Cochran, 1982). Thus, pressure drag increases greatly (i.e., as a quadratic function; McHenry et al., 2006) along with rostral elongation. This drag disadvantage is presumably balanced somewhat by the narrower snout aspect ratios of longirostrine ecomorphs (McHenry et al., 2006; Erickson et al., 2012; Fig. 7.1), causing the mechanical benefits of narrowing and elongation to be common contributors to convergent skull evolution among highly aquatic and pelagic crocodyliforms (McHenry et al., 2006; Pierce & Benton, 2006; Pierce et al., 2009; Young et al., 2010; Walmsley et al., 2013; McCurry et al., 2017a, b; Ballell et al., 2019). These findings help elucidate why the highly plastic rostra of crocodyliforms (Iordansky, 1973; Langston, 1973; Brochu, 2001) converge in predominantly aquatic environments. Indeed, the evolution of superficially similar longirostrine phenotypes even appears attainable via multiple potential developmental pathways (Morris et al., 2019).
In addition to slender and sometimes elongate jaws, piscivorous crocodyliforms have tended to evolve relatively long retroarticular processes (e.g., dyrosaurs, thalattosuchians, gavialoids; Gignac & O’Brien, 2016). As an in-lever for the two largest jaw elevator muscles (Musculus pterygoideus dorsalis and ventralis; Holliday & Witmer, 2007), this feature might be taken to be an adaptation for higher maximum bite forces (Gignac & Erickson, 2016). However, the retroarticular process also serves as the in-lever for the M. depressor mandibulae, the sole jaw-opening muscle (Holliday & Witmer, 2007). As a result, convergently elongate retroarticular processes among aquatic and marine crocodyliforms may serve to enhance the force of subaquatic jaw opening, which is otherwise resisted by drag forces and the viscosity of water (Gignac et al., 2019). This hypothesis requires further evaluation but may represent an important potential functional integration within the cranio-dental complex of highly-piscivorous crocodyliforms (Gignac & O’Brien, 2016; Gignac et al., 2019).
Elongation of the jaws also impacts the mechanical response of the skull to loads imposed during prey capture, both by elevator muscles acting to close the jaws as well as reaction forces at bite points and jaw joints. Bone-strain experiments and biomechanical modeling of mesorostrine species (particularly for the model taxon Alligator mississippiensis) have demonstrated that during biting the snout is subjected to upward bending and twisting (Metzger et al., 2005). Interestingly, the upper jaw does not appear to be optimized for resisting the high feeding forces imposed by the jaw muscles and bite forces, suggesting competing functional demands (e.g., streamlining for stealth and lateral snapping movements in water) also impact skull shape. The lower jaws experience primarily dorsoventral bending and twisting about their long axes (Porro et al., 2013) with mediolateral bending constrained by the presence of enlarged pterygoid flanges (Porro et al., 2011).
Experimental and modeling results from Alligator mississippiensis form a baseline against which skull mechanical behavior during biting in other, rarer taxa can be compared. For example, even though geometric morphometric analyses show that the longirostrine taxa Gavialis gangeticus and Tomistoma schlegelii occupy disparate areas of morphospace—implying major skull-shape differences despite the fact that both are superficially “long snouted” (Brochu, 2001; Pierce et al., 2008)—two-dimensional finite element analysis (FEA) indicates that the upper jaws of both experience substantially higher stresses under feeding loads compared to shorter-snouted crocodylians (Pierce et al., 2008). Results from comparative three-dimensional FEA of the skulls of multiple crocodylian taxa also support these findings. McHenry et al. (2006) observed that the narrow snout of adult Crocodylus johnstoni experienced higher strains during biting than those of five blunt-snouted (brevirostrine) taxa. Similar patterns were exhibited by the lower jaws of crocodylians across a range of snout aspect ratios: the mandibles of blunt-snouted forms exhibited lower strains when subjected to simulated biting, shaking, and torsional loads compared to the mandibles of narrow- and long-snouted species (Walmsley et al., 2013).
Biomechanical modeling also reveals broad similarities in skull mechanical behavior across other clades exhibiting longirostrine morphologies (and piscivorous diets either documented or inferred based on convergent feeding functional morphology and habitat). For example, comparable strain differences were reported for short and long-snouted taxa of crocodylians and odontocetes during biting, shaking, and twisting, particularly at anterior bite points. Regardless of phylogenetic affinity, brevirostrine taxa experience lower strains than longirostrine ones, suggesting analogous form-function relationships even among unrelated clades (McCurry et al., 2017a). Convergence of skull shape and performance with those of longirostrine crocodylians has also been reported for Triassic phytosaurs, Jurassic thalattosuchians, and Cretaceous dinosaurs (Rayfield et al., 2007; Stubbs et al., 2013; Lemanis et al., 2019). This phylogenetic menagerie has also allowed researchers to probe how subtle shape differences and inferred loading behaviors between longirostrine species can result in variations in mechanical performance. For instance, initial simulation studies on the elongate snout of the spinosaurid dinosaur Baryonyx indicated that it performed more similarly to that of Gavialis gangeticus than Alligator mississippiensis in terms of its mechanical response to feeding loads (Rayfield et al., 2007). Further analyses, however, suggest that the response of the snout of the spinosaurid Spinosaurus seems to resemble that of G. gangeticus more so, whereas the mechanical performance of Baryonyx diverged from both that of the gharial and the closely related Spinosaurus (Cuff & Rayfield, 2013). Similarly, the long-snouted thalattosuchian Pelagosaurus typus, while sharing a skull morphology and general stress distribution patterns with G. gangeticus, exhibited lower mechanical resistance to simulated feeding forces, suggesting this fossil taxon may have specialized further for the consumption of compliant prey (Ballell et al., 2019).
As with their jaws, the teeth of slender-snouted crocodylians are also elongate. Tall, narrow, and conical teeth are thought to allow these ecomorphs to more readily seize elusive prey by spearing them during underwater jaw closure, as compared to their blunter-toothed, brevirostrine counterparts (Pooley, 1989; Grenard, 1991; Grigg et al., 2001). Although the posterior teeth are the most robust in the jaw, even in Gavialis gangeticus, they are considerably less molarifom than those of other crocodylians, which seize prey items with the anterior teeth and crush them posteriorly (Erickson et al., 2012). Because aquatic prey is typically swallowed whole, employing crushing bites via the posterior tooth row is rarely observed (Taylor, 1987). As a result, slender-snouted species are capable of using almost their entire jawline to spear prey (e.g., Thorbjarnarson, 1990), making them highly effective underwater hunters.
It was long suspected that semi- and highly-piscivorous taxa are bite-force limited and that evolution of their narrow and elongate jaws favored a trade-off from high-force to high-velocity biting (see e.g., McHenry et al., 2006). The latter, it is reasoned, provides advantages for rapid jaw closure to enable the securing of elusive prey that is capable of swimming off in any direction (Gignac et al., 2019). In 2012, Erickson and colleagues tested whether bite forces are correlated with dietary preferences. Of the five recognized slender-snouted crocodylians alive today (Crocodylus johnstoni, C. intermedius, Gavialis gangeticus, Mecistops cataphracus, and Tomistoma schlegelii), only G. gangeticus was an outlier for the clade (i.e., a quantitatively low-force biter; Erickson et al., 2012). Adults of the other four species reliably generate bite forces indistinguishable from those of all other remaining adult crocodylians, relative to their body size (Erickson et al., 2012, 2014). Notably, these four slender-snouted species maintain the pennate muscle-fiber arrangement and lateralized jaw insertion of the largest jaw elevator muscle (e.g., Musculus pterygoideus ventralis; Endo et al., 2002) common to all other crocodylians (Iordansky, 1964; Endo et al., 2002; Holliday & Witmer, 2007). By contrast, the lineage of G. gangeticus evolved fusiform-fibered jaw muscles with medially shifted insertion points (e.g., M. pterygoideus ventralis; Endo et al., 2002), which are advantageous for rapid—at the expense of forceful—jaw closure (Porro et al., 2011). In addition, the jaw musculature of G. gangeticus features a remarkably well-developed M. pseudotemporalis superficialis when compared to other slender-snouted crocodylians (Endo et al., 2002). Hypertrophy of this muscle suggests that it plays a greater role in jaw elevation in G. gangeticus, potentially compensating, to some extent, for the evolutionary shift away from the high-force generating, plesiomorphic arrangement of crocodylian jaw-closing musculature (Holliday & Witmer, 2009).
Exceptional bite-force capabilities would seem to be a peculiar feature of fish-eating taxa, not only because of their apparently delicate jaws and teeth but also because it allows them to generate exceptionally high tooth contact pressures (= maximum bite force/tooth contact area) when they engage with prey. Coupling high bite-force capacities with narrow tooth cross sections causes these pressures routinely to be more than ten-fold greater than those required to indent cortical bone, which is among the stiffest of vertebrate tissues (Carter & Beaupré, 2001; Turner et al., 2001; Erickson et al., 2012). That needle-toothed fish-eaters are capable of generating tooth pressures far in excess of those necessary to puncture their aquatic and marine prey (Erickson et al., 2012) suggests that semi- and highly-piscivorous crocodylians are mechanically over-capable for their feeding niches (Erickson et al., 2012, 2014; Gignac et al., 2019). Theoretically, this places their jaws and teeth closer to rupture when feeding on large prey, as demonstrated by beam theory and finite element analyses (Busbey, 1995; McHenry et al., 2006; Porro et al., 2011, 2013; Ballell et al., 2019). Why this should be the case likely has more to do with the phylogenetic inertia of evolving from large-bodied, generalist ancestors than it does with the functional value of driving delicate teeth through the compliant bodies of aquatic prey (Erickson et al., 2012). These forms seem to avoid damaging their svelte rostrodental features by behaviorally electing to consume relatively smaller and more compliant food resources (Erickson et al., 2012, 2014). Thus, although their slender and elongate rostrodental morphologies appear to have been shaped by their environments, convergence did not necessarily alter the full suite of their feeding capabilities (Erickson et al., 2012, 2014; Gignac & O’Brien, 2016).
What constitutes convergence of the crocodyliform jaw apparatus upon principally aquatic feeding modes? The relationships outlined above support phylogenetically broad, deep-time sampling that suggest rostra and dentitions have been plesiomorphically decoupled from the post-orbital region of the skull of crocodylians (Gignac & O’Brien, 2016) and their evolutionary precursors (Felice et al., 2019; Gignac et al., 2019; O’Brien et al., 2019). If generally applicable, then a common evolutionary sequence—pieced together from extant trait combinations—emerges: crocodyliforms may first converge upon needle-toothed and slender or long-snouted prey-capture morphologies without similarly extreme alteration of their musculoskeletal apparatus (e.g., Crocodylus johnstoni, Mecistops cataphractus, Tomistoma schlegelii; Iordansky, 1964; Endo et al., 2002); this may be followed by retroarticular process elongation and jaw elevator muscle evolution in the most specialized lineages (e.g., Gavialoidea; Brochu, 2001; Endo et al., 2002) as the trade-off of plesiomorphic, high bite-force capacities gives way to apomorphic, rapid jaw-closing velocities. Gavialis gangeticus is unlike all other crocodylians in that it possesses the most extreme of these character states (Iijima, 2017). A future focus on addressing the development, function, and evolution of gavialoid feeding biomechanics will go a long way to further clarifying convergence in the aquatic feeding of crocodyliforms, foremost, and secondarily aquatic tetrapods more generally.
7.1.4 Intraoral Transport, Processing and Swallowing
After a prey item is seized in the anterior part of the jaws, it is shifted posteriorly in the oral cavity using inertial feeding. Gravity alone, or the use of rapid, jerking head movements in coordination with jaw elevation and depression (Cleuren & De Vree, 1992), accelerates the prey item, harnessing its inertia to shift it backwards in the oral cavity (Taylor, 1987). The tongue is affixed to the floor of the oral cavity and does not participate substantially in intraoral prey transport (Busbey, 1989; Cleuren & De Vree, 1992). While feeding in water, crocodylians elevate the head above the water line to employ inertial feeding (Abercromby, 1913; Johnson, 1973; Taylor, 1987). A prey item may first be shifted to the posterior region of the dentition for the application of additional, more forceful bites, but ultimately it is moved towards the gular valve and esophagus for swallowing (Gans, 1969; Pooley & Gans, 1976).
Crocodylians are capable of capturing submerged prey without swallowing water owing to a soft palatine flap that forms a seal against the tongue (Fleming & Fontenot, 2015). This gular valve excludes water from entering the esophagus and trachea. It is assumed that for most crocodylians the valve also prevents swallowing of prey while submerged because even semi- and highly piscivorous species are seen to elevate their heads above the water line to achieve deglutition (Taylor, 1987). However, subaquatic swallowing is routinely and directly observed in captive individuals (e.g., Crocodylus johnstoni, C. porosus, Tomistoma schelegelli; St. Augustine Alligator Farm Zoological Park, 2020). Deglutition occurs by using cyclical muscular contractions of the gular region (Cleuren & De Vree, 1992). Prey items are pushed past the gular valve and into the esophagus using active protraction and retraction of the hyoid apparatus in small, repetitive orbits (Busbey, 1989; Cleuren & De Vree, 1992). This behavior is often assisted by forward thrusts of the head (especially underwater), gravity, or jaw depression and elevation to exaggerate the range of hyoid motion (Taylor, 1987; Cleuren & De Vree, 1992). Fish are typically, but not always, maneuvered so that they enter the esophagus head first (Thorbjarnarson, 1990; Sharma et al., 2013).
7.2 Birds
7.2.1 Introduction
Birds are characterized by the presence of feathers, the modification of the forelimbs to wings, toothless beaked jaws, hard-shelled eggs, a high metabolic rate along with a specialized, high-performance respiratory system, and a four-chambered heart. Birds comprise the most diverse terrestrial vertebrate group, with more than 18,000 described species (Barrowclough et al., 2016). Based on fossil, morphological, physiological and molecular biological evidence, birds are considered extant theropod dinosaurs–and accordingly, represent the extant sister group of crocodylians (Janke & Arnason, 1997; Prum, 2002). Birds are primarily terrestrial amniotes and most have retained the ability to fly, but some branches have adapted to aquatic lifestyles to different degrees and have evolved, on multiple independent occasions, strategies for exploiting aquatic food sources (Schwenk & Rubega, 2005; Rico-Guevara et al., 2019), including mechanisms as diverse as suspension feeding (filter feeding), surface skimming, scything, pursuit fishing, spearing of prey and, in at least one case, suction feeding.
7.2.2 Food Detection
Birds primarily use vision to detect food (Goldsmith, 1990). In fact, birds might be the most visually dependent group amongst vertebrates, reflected by the relatively large size of their eyes compared to those of other vertebrates of similar mass (Zeigler & Bischof, 1993). Most birds are tetrachromatic, with green, red, blue and ultraviolet (UV) sensitive cone photoreceptors in the retina (Wilkie et al., 1998). The photoreceptors of birds bear colored oil droplets which narrow spectral sensitivity and reduce the overlap in sensitivity between cone types, which in turn is hypothesized to improve color discrimination (Govardovskii, 1983; Vorobyev, 2003; Olsson et al., 2015). Bird eyes have adapted to a wide spectrum of functional demands (Zeigler & Bischof, 1993). Many aquatic birds, for instance, have evolved very flexible lenses that allow accommodation of their eyes to air and water (Gill, 1995). Next to vision, tactile cues play a central role for food detection in many aquatic feeding birds, and the number and distribution of mechanoreceptors on the beak and tongue are correlated with the respective feeding behavior (Gottschaldt, 1985). For example, mechanoreceptors are concentrated on the beak tip in shorebirds that engage in probing (von Bolze, 1968; Pettigrew & Frost, 1985). By contrast, mechanoreceptors are concentrated on the tip and the lateral ridges of the beak, as well as on the fleshy tongue in mallards that use suspension feeding (Berkhoudt, 1979). A correlation of the distribution of mechanoreceptors and feeding mode allows fast feedback-responses to fine-tune the feeding behavior to a given situation. The role of olfaction has, for a long time, been underestimated in birds, but empirical studies have shown that the sense of smell plays an important role in food detection in many avian groups (Roper, 1999). Olfactory information is processed in the olfactory bulbs of the central nervous system and anatomical investigations have revealed that the olfactory bulbs are significantly enlarged in birds using olfactory cues to detect prey compared to non-olfactory-oriented birds (Bang & Cobb, 1968), a trait that has evolved several times independently in different bird branches. For example, some vultures, such as the turkey vulture, have large olfactory bulbs and are known to localize food by smell (Smith & Paselk, 1986). Similarly, it was shown experimentally that odoriferous baits attract various sea-bird species. For instance, cod-liver oil slicks deployed on the water surface induced specific search behaviors in storm-petrels and other sea-birds (Lequette et al., 1989; Verheyden & Jouventin, 1994). Acoustic location of prey has been shown to be employed by (mostly nocturnal) hawks and owls (Rice, 1982). Although the auditory systems also seem to be well-developed in aquatic feeding birds and acoustic communication is important to them, prey detection by acoustic cues seems to play a minor role. A sonar system for prey detection was once hypothesized for penguins (Poulter, 1969), but no morphological or physiological evidence has been found to support this idea (Wever et al., 1969).
7.2.3 Food Capture
Convergently with other groups of aquatic and marine vertebrates, such as cetaceans, tadpoles, actinopterygians, chondrichthyans and cyclostomes, birds have evolved elaborate mechanisms for filtering small food particles from the water by suspension feeding. Suspension feeding birds, such as mallards, flamingoes and some sea-birds, are equipped with rows of fine keratinized lamellae along the margins of their bill, which can strain small particles from the water (Jenkin, 1957; Zweers & Wouterlood, 1973; Kooloos et al., 1989; Sanderson & Wassersug, 1990). To induce water flow into the mouth, mallards and flamingoes oscillate their piston-like tongue in an anterior-posterior direction as the mouth opens and closes with each tongue cycle. Ingested water is released through the lateral lamellae of the beak where food particles are retained (Fig. 7.2). Keratinized spines located on the posterior margin of the tongue finally dislodge the entrapped food particles from the lamellae and draw them toward the esophagus as the tongue is retracted (Jenkin, 1957; Zweers et al., 1977; Kooloos et al., 1989; Sanderson & Wassersug, 1990). Similarly, some prions (Pachyptila desolata, P. vittata, P. salvini) are likely to use cyclic movements of their large muscular tongue to induce water flows across their filter apparatus (Klages & Cooper, 1992). Additionally, prions, along with the short-tailed shearwater (Puffinus tenuirostris), have also been reported to use a method whereby they swim with their head and gaping beak patially submerged, allowing water to continuously flow in through the anterior region of the gape and leave at the posterior corners of the beak with food objects being retained at the palatal papillae in prions, or within the filter apparatus consisting of lateral lingual papillae that overlap with the lateral palatal papillae in the short-tailed shearwater (Prince, 1980; Morgan & Ritz, 1982; Klages & Cooper, 1992). The suspension feeding mode in prions shows striking convergence of both form and function with the mode used by right whales, Eubalena sp., shedding light on the origin of their trivial name: “whale-birds” (Sanderson & Wassersug, 1990).
Foraging flamingoes (Phoenicopteridae). Flamingoes typically forage by partly submerging their beak which is equipped with rows of fine keratinized lamellae along the upper bill. Oscillations of their piston-like tongue induce water flow across the fine lamellae whereupon food particles are entrapped. Photo by David Hensley on Unsplash
Suspension feeding does not rely on visual detection of prey, but rather on mechanosensation (and possibly gustation, see Berkhoudt, 1985), where sensitive beaks and tongues allow fast feedback responses during feeding bouts (Berkhoudt, 1979). Similarly, scything and skimming rely on tactile cues, but in contrast to suspension feeding, these feeding modes are used to catch larger prey (Becker et al., 2002; Swennen & Yu, 2005). Scything has evolved independently in a few branches of wading birds, including spoonbills (Platalea sp.) and avocets (Recurvirostra sp.), that typically feed in shallow waters (Becker et al., 2002). Although spoonbills and avocets have very different beak morphologies, their scything mechanism shows striking similarities. Scything birds submerge their slightly opened elongated beaks and sweep their heads from side to side while wading through the water (Becker et al., 2002). As the slightly opened beak contacts potential prey objects, mostly fish and crustaceans, they are quickly captured by the closing beak and swallowed (Swennen & Yu, 2005).
Skimming is a unique feeding behavior where the skimmer flies straight and close over the water surface with the mouth slightly open and the lower beak partially submerged. Food objects that are contacted by the lower beak are seized as a result of a fast reflex beak closure (Tomkins, 1951; Martin et al., 2007). Skimming is found in the scissorbills (Rynchops sp.) that are characterized by a special morphological adaptation for skimming, a substantially-elongated lower beak, which might largely prevent feeding methods other than skimming (Tomkins, 1951; Black & Harris, 1983).
In contrast to suspension feeding, scything and skimming, vision becomes essential for birds that target and strike at individual prey items. Herons (Ardeidae), for example, are mostly ambush predators and target their prey (usually fish, crustaceans or amphibians) while standing still in shallow water, or standing on platforms close to water (Kushlan, 1976). After estimating the position of the prey accurately, they strike by a sudden, rapid straightening of the long neck that thrusts the head forwards and downwards towards the prey (Katzir & Intrator, 1987; Lotem et al., 1991). The head can reach mean velocities of over 270 cm/s and, immediately before the bill contacts the prey, the beak is slightly opened to seize it (Katzir & Intrator, 1987). While smaller prey items are usually grasped by the closing beak, larger prey items are often stabbed and speared by the sharply pointed beak tips (Forbes, 1982).
Similarly (and convergently evolved) to herons, snakebirds (Anhinga sp.) possess long necks, elongated sharply pointed beaks and also capture aquatic prey by rapidly straightening their neck to grasp–or more commonly–spear it (Owre, 1967) (Fig. 7.3). Just like herons, snakebirds strike at prey with a slightly open beak. However, in contrast to herons and egrets, snakebirds do not strike from an emergent position relative to their aquatic prey, but are instead skilled foot-propelled divers that quietly ambush their prey (usually fish) underwater to catch it using a sudden strike (Owre, 1967).
Snakebird (Anhinga sp.) with speared prey. Snakebirds are skilled divers and are equipped with a long neck and elongated, sharply pointed beak. After stalking prey, it is targeted and captured by rapidly straightening the curved neck to finally spear it. Photo by R. Mac Wheeler on Unsplash
Probably the most spectacular prey capture mode amongst aquatic feeding birds is the plunge-dive. In short, a plunge-dive is a hunting strategy whereby birds dive head-first from the air into water (Duffy et al., 1986; Carl, 1987; Chang et al., 2016), and adaptations to it have convergently evolved in sulids (with gannets and boobies), terns (Sterninae), kingfishers (Alcedinidae), pelicans (Pelecanidae) and cormorants (Phalacrocoracidae). The northern gannet (Morus bassanus), for example, can dive from heights of up to 45 m, attaining speeds at the impact with water of more than 20 m/s. Such a dive would most probably be lethal to humans, but plunge-divers exhibit kinematic and morphological adaptations, such as a sharp, arrow-like body posture with a long and slender beak that, together, minimize drag and keep impact forces relatively low (Chang et al., 2016; Crandell et al., 2019). In fact, the sum of adaptations in plunge-divers results in only very low decelerations when hitting the water surface (Ropert-Coudert et al., 2004). Once submerged, the birds immediately grasp the targeted prey with their beak or use their momentum to travel underwater to attain a desired depth of up to 10 m (Ropert-Coudert et al., 2004). If the initial plunge-dive does not immediately result in successful prey capture, the birds can actively pursue the targeted prey item for a short time by using their feet or wings for propulsion (Ropert-Coudert et al., 2004). After a prey item is captured, the birds rise to the water surface, mostly passively due to their high buoyancy.
Other aquatic birds actively hunt prey underwater without plunge-diving. In fact, pursuit feeding is one of the most common feeding modes and has evolved many times independently in different branches of birds (Shealer, 2002). In pursuit hunts, prey (usually fish or crustaceans) is typically detected visually, stalked and grasped by the beak after a short chase. Pursuit hunters are fast and skilled divers that, depending on the species, use paddling by webbed feet, wing beats, or a combination thereof to propel themselves forwards (Townsend, 1909; Owre, 1967; Raikow et al., 1988). Many pursuit hunters show morphological adaptations to efficiently seize slippery prey, such as a terminal hook on the upper beak (Owre, 1967; Anderson et al., 1974; Shealer, 2002) or keratinous papillae and spines on beaks, palate and tongue (Kobayashi et al., 1998; Matsumoto & Evans, 2017). However, fast approaches to a prey might induce a positive pressure gradient in front of the birds’ head that could push floating prey away (i.e. a “bow wave”) or at least alert the prey organism to the approach of a predator (Taylor, 1987). How pursuit hunting birds circumvent hydrodynamic effects imposed by fast accelerations towards aquatic prey has not yet been studied in detail, but the generally streamlined body posture, in combination with a long and slender beak, might keep negative hydrodynamic effects low (Parfitt & Vincent, 2005; Crandell et al., 2019). Other aquatic vertebrates often use suction feeding to overcome negative hydrodynamic effects (see the section on turtles in this chapter). As of yet, only one bird species has been reported to use suction feeding: the little auk, Alle alle, a 150 g diving seabird of the North Atlantic (Enstipp et al., 2018), which actively chases small prey (copepods) underwater and engulfs them in the final stage by actively induced suction flows. Their suction feeding mechanism has not yet been studied in detail, but video recordings show that gular depression (sub-lingual pouch extension) shortly follows beak opening and induces prey ingestion (Enstipp et al., 2018). This movement pattern is very similar to the general pattern observed in other suction feeding vertebrates, including chondrichthyans, actinopterygians, dipnoans, lissamphibians, turtles and mammals: jaw opening is followed by gular (hyobranchial) depression (Lauder, 1980; Bemis & Lauder, 1986; Deban & Wake, 2000; Lemell et al., 2002; Wilga & Sanford, 2008; Kane & Marshall, 2009). Accordingly, the little auk nicely shows once more that suction feeding has evolved multiple times independently by convergence upon particular motion patterns.
Although suction feeding seems to be an important adaptation for aquatic predators, the masters of underwater pursuit hunting in birds, the penguins (Spheniscidae), are unlikely to use suction feeding (Charrassin et al., 2001; Takahashi et al., 2004). Penguins are capable of long and deep dives during which they catch prey such as shrimp and fish that are seized by the beak. Not much is known of the biomechanics of penguin feeding, but experiments with transponders have shown that penguins not only catch prey during their dives but also swallow prey under water (Charrassin et al., 2001; Takahashi et al., 2004).
7.2.4 Food Transport, Processing and Swallowing
After successful food capture, most aquatic-feeding birds must raise their head above the water level and use terrestrial transport mechanisms, such as fast dorsally directed head rotation while loosening the grip upon the prey item to throw it from the beak tip to the back of the oral cavity for swallowing (Owre, 1967; Forbes, 1982; Swennen & Yu, 2005) (i.e. inertial transport, see also the parts of this chapter dealing with lizards and crocodylians). Extensive intraoral processing is rare in birds, given that most of the physical processing action is performed in the gizzard (Van Gils et al., 2003; Fritz et al., 2011). Still, some aquatic feeding birds do direct a series of bites to the prey held in the beak. For example, herons have been reported to “chew” fishes so as to break their spines, scales and other protective mechanical adaptations (Forbes, 1982). Similarly, the high frequency beak movements of mallards might, next to their role in suspension feeding, be used to mechanically reduce food items before swallowing. However, all these processing functions occur with the head raised out of the water. Only a few birds are known to intraorally transport and swallow food underwater. The little auk catches large numbers of copepods individually, but how aquatic transport and swallowing is accomplished remains unknown. Penguins are exceptional as they might, next to auks, be the only birds that can intraorally transport and swallow underwater. Key to allowing intraoral transport in penguins is the interplay between the tongue and the palate (Matsumoto & Evans, 2017). Both palate and tongue are studded with large, sharply pointed keratinous papillae pointing rearwards and once prey is seized by the beak, cyclic pro- and retraction of the tongue moves any food object posteriorly (Kobayashi et al., 1998; Matsumoto & Evans, 2017).
7.3 Lepidosauria
7.3.1 Introduction
The taxon Lepidosauria contains over 9900 species (Uetz, 2010) and includes two orders, the Squamata and Rhynchocephalia. The Squamata comprises lizards, snakes and amphisbaenids, while the Rhynchocephalia is represented solely by the extant genus Sphenodon. Although body size, shape, and lifestyle varies significantly within lepidosaurs, they all possess overlapping keratinous scales. Some lepidosaurs are ferocious predators that chase prey larger than themselves, while others are ambush predators, insectivores, scavengers, omnivores or herbivores (Schwenk, 2000a). Some lepidosaurs are fast runners and skilled climbers, others live a fossorial lifestyle. Limbs can be well-developed, but have been lost independently in many groups, such as in snakes, amphisbaenids, anguids and pygopodids. The ancestral lifestyle of lepidosaurs is certainly terrestrial, but some groups live close to water, are semiaquatic or have evolved fully aquatic lifestyles.
7.3.2 Food Detection
Lepidosaurs have a full arsenal of sensory systems for detecting food at their disposal (Schwenk, 2000a) but vision might be the major sensory system for detecting food sources in most cases. Diurnal lepidosaurs are assumed to be capable of color vision. In fact, some studies show preferences of some lizards for food items of a certain color (Benes, 1969; McGovern et al., 1984).
Next to vision, most lepidosaurs rely heavily on chemosensory cues to detect food (Schwenk, 2000a). Chemosensation is achieved via three main systems: (1) gustation, (2) olfaction, and (3) the vomeronasal system. Gustation is mostly used for the discrimination of food once items have been seized (Berkhoudt, 1985; Schwenk, 1985). Gustatory cues are transmitted by taste buds which are located in the oropharyngeal cavity, including on the tongue of many species. By contrast, olfaction is used to detect more volatile chemicals; that is, to detect food from a distance (Kratzing, 1975; Bull et al., 1999). Olfaction is mediated by the olfactory epithelia that cover the nasal cavities. The vomeronasal system consists of the paired vomeronasal organs that lie dorsal to the anterior portion of the palate. Each vomeronasal organ houses a cavity that opens into the oral cavity and is lined with a chemosensory epithelium (Parsons, 1970), and it is this epithelium that is stimulated by environmental chemicals gathered by the tongue during a behavior known as tongue flicking (Halpern & Kubie, 1980; Schwenk, 1995, 2000a). Tongue flicking is best known for snakes and some lizards, but it is employed by virtually all lepidosaurs (Schwenk, 1995, 2000a). Infrared organs are known from snakes and are used for the perception of electromagnetic waves with a length of 8000–12,000 nm (Grace et al., 1999), which corresponds with the wavelength radiated from the surface of endothermic animals, such as mammals and birds (Goris, 2011). However, such infrared “vision” is so far known only for terrestrially-feeding snakes.
Aquatic snakes possess specialized mechanoreceptors (scale sensilla) that detect water motion and are likely used for prey detection (Van Der Kooij & Povel, 1996; Westhoff et al., 2005; Catania et al., 2010; Crowe-Riddell et al., 2016). Scale sensilla of aquatic snakes and the lateral line system of fishes and aquatic lissamphibians might be regarded as convergently evolved mechanosensitive systems. Although lepidosaurs show an impressive range of sensory systems available for food detection, mechanoreception, vision, and chemoreception are likely the most important sensory systems for detecting and localizing food under aquatic conditions (Drummond, 1983; Kutsuma et al., 2018).
7.3.3 Food Capture
Aquatic feeding is exhibited by many squamate clades. Snakes, in particular, are known to have secondarily evolved aquatic or semiaquatic lifestyles and aquatic food uptake (Cundall & Greene, 2000; Moon et al., 2019), but several lizards have convergently acquired semiaquatic lifestyles and are capable of aquatic feeding (Carpenter, 1966; Mayes et al., 2005; Mesquita et al., 2006; Langner, 2017).
Aquatic feeding has evolved multiple times, and independently in most major snake groups. It is found among boas, pythons, elapids, viperids and colubrids (Young, 1991; Cundall & Greene, 2000; Bilcke et al., 2006). Accordingly, snakes show a broad spectrum of convergent morphological, behavioral and physiological adaptations for catching prey under water. Several levels of aquatic commitment are known among snakes: from snakes that occasionally strike aquatic prey from land, to semiaquatic snakes that regularly enter aquatic habitats in search of prey, to permanently aquatic snakes that forage exclusively under water (Drummond, 1983). Regardless, whether a snake is semi- or fully aquatic, it has to overcome the same functional challenge: because of their morphological constraints, snakes, in general, are not capable of suction feeding. Such morphological constraints include a reduced hyobranchial skeleton and associated musculature (McDowell, 1972; Alfaro, 2002; Herrel et al., 2008) that is used in other aquatic vertebrates for rapid oropharyngeal volume expansion in suction feeding. As observed in other tetrapods that forage on elusive aquatic prey, at least some amount of suction feeding has been hypothesized to be advantageous for avoiding the bow wave generated in front of the accelerating head that would push prey away or alert prey of the approaching predator (Taylor, 1987). Nonetheless, the evolutionary success of aquatic feeding in snakes implies that they are capable of efficient aquatic feeding in the absence of being able to suction feed. So how do aquatic snakes strike prey and how do they cope with the physical constraints imposed by a medium that is about 850 times as dense and 50 times as viscous as air?
The ability of some snakes to execute very fast strikes underwater suggests that bow waves do not impose a universal constraint on aquatic feeding behavior (Alfaro, 2002). In other words, snakes have not evolved strategies to completely avoid bow waves during prey strikes, but they have evolved strategies to limit negative hydrodynamic effects to small enough values to permit the successful catching of prey (Herrel et al., 2008; Van Wassenbergh et al., 2009; Segall et al., 2019). Two main strategies are used by aquatic-feeding snakes to catch elusive prey: lateral and frontal strikes (Figs. 7.4 and 7.5). Lateral strikes are achieved by swinging the head to the side. Sideways movements can be continued until the head is directed 180° from its original orientation (Drummond, 1983). Depending on the snake species and the foraging situation, lateral strikes can be (1) slow and repetitive or (2) sudden and fast (Young, 1991; Alfaro, 2003). Repetitive sideways movements of the head with the gape open, referred to as “lateral head sweeping“, are mostly used by snakes that feed in water with high prey densities and use tactile cues to search for prey (Drummond, 1983; Alfaro, 2002, 2003). In contrast, fast lateral strikes are used if the snake directs its attack to a specific prey item and can achieve peak velocities comparable to those of high-performance terrestrially-striking snakes (Smith et al., 2002; Alfaro, 2003; Catania, 2009). As the lateral strike is performed with the mouth gaping, the hydrodynamic disadvantage (e.g., pressure drag and bow wave) is reduced (Fig. 7.5c) compared to the situation with the mouth closed (Young, 1991; Braun & Cundall, 1995). Additionally, as surface area exposed to the fluid consists only of the lateral head area in lateral strikes, this capture mode is also feasible for snakes with relatively large and wide heads (Young, 1991; Vincent et al., 2009). Compared to frontal strikes, lateral strikes are usually not particularly accurate, probably because of the lack of visual overlap between the left and right eye, which makes estimates of prey distance difficult (Herrel et al., 2008). However, a particularly elaborate and effective lateral strike mechanism has been reported for the tentacled snake, Erpeton tentaculatum, which is a typical ambush predator and exploits the typical escape response (C-start maneuver) of fish to catch them efficiently. To do so, the tentacled snake feints with its trunk to elicit a C-start-response in a nearby fish, which startles if towards the snakes’ approaching jaws, or a position the snake anticipates and strikes toward (Smith et al., 2002; Catania, 2009, 2010).
Computational fluid dynamic (CFD) simulation of a frontal (a, b) and a lateral aquatic strike of Natrix tessellata along the posterior to anterior axis of the midsagittal plane (a), and for a series of frontal view planes (4 mm interval) at one time instant, showing anterior–posterior flow velocity (b) and right–left flow velocity (c). In this CFD simulation, the snake model translated with a forward velocity of 1 m/s and started to close its mouth at time = 40 ms. The prey contacted the lower jaw at time = 53 ms. The velocity scale is the same for (a) and (b). Modified from Van Wassenbergh et al. (2009)
Frontal strikes in aquatic snakes are based on a fast forward acceleration of the head with the jaws open (Drummond, 1983; Van Wassenbergh et al., 2009). The forward acceleration of the head results from fast straightening of the curved trunk and neck, and prey is captured by the closing jaws (Drummond, 1983; Alfaro, 2003) (Fig. 7.4). Previously, it had been hypothesized that underwater strikes with open jaws may be hindered by drag and may generate bow waves that displace prey, making this method of capture more challenging (Young, 1991; Vincent et al., 2005; Moon et al., 2019). However, in silico (Van Wassenbergh et al., 2009) (Fig. 7.5b, c) and experimental (Segall et al., 2019) studies have shown that the hydrodynamic disadvantages are limited and that the head shapes of forward-striking snakes minimize the hydrodynamic constraints. Van Wassenbergh et al. (2009) showed that hydrodynamic drawbacks are minimized when snakes strike at large prey, as the inertia of large prey reduces the effect of the bow wave. Furthermore, precise aiming prevents the prey from deviating to a path that eludes the corners of the mouth. Indeed, most aquatic snakes forage for relatively large prey organisms (e.g., fish or amphibians) and frontal strikers have excellent underwater vision that allows precise aiming (Schaeffel & de Queiroz, 1990; Alfaro, 2002). Segall et al. (2019) showed that hydrodynamic drawbacks of frontal striking snakes can be further minimized by morphological adaptations, such as having narrow (Van Wassenbergh et al., 2009) or short (Segall et al., 2020) heads, if such modifications decrease the area exposed to the fluid. In fact, aquatic snakes have evolved, independently and on multiple occasions, narrower anterior regions of the head (Segall et al., 2016) and a shorter head (Segall et al., 2020).
Other strategies employed by aquatic snakes to overcome hydrodynamic effects imposed by the inability to perform suction feeding comprise trapping burrowing fishes in their burrows or crevices (Voris & Voris, 1983; Young, 1991) or very slow feeding modes, as for example found in the turtle-headed snake that feeds on fish eggs (Shine et al., 2004).
In contrast to snakes, aquatic feeding in lizards remains only superficially studied. The best-known example of aquatic feeding lizards is probably the marine iguana, Amblyrhynchus cristatus, from the Galapagos Islands. Marine iguanas spend most of their time on land but undertake prolonged dives to scrape algae from submerged rocks (Carpenter, 1966; Wikelski & Trillmich, 1994). The mechanics of its scraping behavior have not been studied in detail but based on the high inertia of algae tightly fixed to the substrate, it might be assumed that scraping algae from submerged rocks is not fundamentally different from grazing on land. Varanids, in contrast, are largely carnivorous and a few species are able to exploit aquatic food sources, including elusive prey such as fish, shrimps, or crabs (Mayes et al., 2005; Kulabtong & Mahaprom, 2014). These varanids locate aquatic prey by chemical cues and actively chase them (Mayes et al., 2005). The aquatic ingestion mode of varanids is unknown, but due to adaptations of the hyobranchial musculoskeletal system, it probably does not involve suction feeding (Smith, 1986). Instead, varanids might use laterally- or frontally-directed strikes similar to those of aquatic snakes or crocodylians (see the respective sections of this chapter), or a modified mechanism of suction feeding based on fast mouth opening, analogous to the method used by the Chinese giant salamander (Heiss et al., 2013). The earless monitor, Lanthanotus borneensis, is largely terrestrial, but it is known to be a skilled swimmer that regularly visits creeks to prey on fish and crustaceans (Harrisson, 1961; Harrisson & Haile, 1961; Langner, 2017). Its feeding mechanism has, to date, not been studied in any detail, but Lanthanotus might use similar strategies to the closely related varanids. Other aquatic foraging lizards include the teiids Crocodilurus amazonicus and Dracaena guianensis (Mesquita et al., 2006), the scincids Tropidophorus hainanus and Sphenomorphus cryptotis and the shinisaurid Shinisaurus crocodilurus (Fig. 7.6) (Ziegler et al., 2008), but their aquatic feeding mechanisms have not yet been studied. However, lizards might have evolved multiple ways of feeding underwater. For example, it has been shown that sometimes only small alterations of a terrestrial behavioral repertoire are necessary for exploiting aquatic food sources, as exemplified by Hawaiian Anolis lizards that have learned to use fast forward lunges to catch guppies that swim to the water surface of artificial fish tanks (Hawaii Hobbyist, 2019). In theory, the behavior exemplified by the Hawaiian Anolis lizards might be just a few functional steps away from more elaborate aquatic prey capture strategies.
7.3.4 Food Transport, Processing and Swallowing
Following prey capture and subjugation, snakes usually use a mechanism referred to as the “pterygoid walk”, where alternate pro- and retraction of left and right jaws, plus the respective pterygoid bone, pull the prey item posteriorly (Kardong, 1977; Moon, 2000). The pterygoid walk can be used equally well in terrestrial and aquatic conditions. This snake-specific intraoral transport mechanism is more efficient in animals with relatively longer quadrate bones as the width and height of the posterior part of the head impacts the length of the lever arm involved in the pterygoid walk: the wider the head, the more efficient are intraoral transport and swallowing (Young, 1991; Vincent et al., 2009). Accordingly, aquatic snakes seem to be subject to a functional trade-off between prey capture and intraoral transport: narrow or short heads might reduce hydrodynamic drawbacks during a frontal strike, but render intraoral transport and swallowing slow (Vincent et al., 2009). On the other hand, broad or elongated heads, with longer quadrate bones, allow rapid intraoral transport and swallowing, but make frontal strikes hydrodynamically more challenging. Aquatic snakes might have solved this trade-off in two ways: lateral strikers can possess a wide head without suffering hydrodynamic drawbacks (Vincent et al., 2009) and frontally striking snakes have a streamlined, narrow anterior, but a wider posterior region of the head and/or longer quadrate bones (Segall et al., 2016; Rhoda et al., 2020). Anyway, we might still be far from fully understanding the form-function relationships and functional trade-offs characteristic of aquatic feeding snakes. Specifically, while many studies have focused on semiaquatic snakes, such as natricines (e.g. Bilcke et al., 2006; Van Wassenbergh et al., 2009; Vincent et al., 2009), others have taken a wider phylogenetic approach and have included semiaquatic and fully aquatic species (e.g. Segall et al., 2016, 2019, 2020). The functional demands imposed on the semiaquatic species might differ fundamentally from those acting on the fully aquatic ones. In fact, the fully aquatic species tend to have short heads and the more semiaquatic species have long and narrow heads, suggesting that, in addition to the hydrodynamic constraints, semi-terrestrial habits induce additional constraints (M. Segall, pers. comm., June 2021). Phylogenetic mapping of morphological and functional solutions for overcoming the functional trade-off between aquatic strike and intraoral transport show that they have evolved multiple times independently (Segall et al., 2016).
Aquatic-feeding lizards probably use slightly modified terrestrial intraoral transport mechanisms with a submerged or emergent head, and move food posteriorly towards the esophagus by employing cyclic tongue loops (Schwenk, 2000a). Similar terrestrial feeding styles have been shown to be used by some semiaquatic turtles for intraoral transport (Natchev et al., 2010). Alternatively, lizards might raise their heads above the water line to make use of inertial transport (Smith, 1982, 1986) (Fig. 7.6) by quick dorsal or lateral head movements while temporarily releasing the grip on the prey, for example, literally throwing the prey through the mouth to the esophagus–analogous to mechanisms employed by crocodylians (Cleuren & De Vree, 1992, 2000). Hypotheses on aquatic transport in lizards remain speculative at this point, signaling the urgent need for empirical studies.
7.4 Testudines
7.4.1 Introduction
Turtles are one of the oldest known reptile orders, appearing about 240 million years ago, shortly after the Permian–Triassic extinction event. The phylogenetic position of turtles is still not fully resolved because morphological, developmental and genetic studies have been unable to reach a consensus on the relationships of the group within sauropsids (e.g., Anquetin, 2011; Bever et al., 2015). Eunotosaurus africanus is generally accepted as a stem turtle (Bever et al., 2015), followed by Pappochelys rosinae (Schoch & Sues, 2015) and Odontochelys semitestatcea (Li et al., 2008). In Pappochelys the plastron is not yet evident, but robust gastralia are present, indicating the origin of the plastron through fusion of the ventral ribs (Gilbert et al., 2001). Odontochelys represents the next step in shell evolution and has a fully developed plastron, with the dorsal shell consisting of neural plates and expanded ribs. This kind of evolutionary step (broadening of dorsal ribs) is also recognizable in the embryonic development of extant turtles (e.g., Sheil & Greenbaum, 2005; Scheyer et al., 2013).
Also unresolved is the question of what was the original habitat of the stem turtles. Eunotosaurus probably lived in terrestrial habitats (Bever et al., 2015), Pappochelys and Odontochelys have been described as being semiaquatic, living along lake shores with frequent visits to water (Schoch & Sues, 2015). However, Joyce (2015) argued that Odontochelys was likely a fully terrestrial stem turtle, and, at most, an inhabitant of swampy freshwater environments. The oldest known completely-shelled turtles (i.e. the Upper Triassic Proterochersis and Proganochelys), were likely semiaquatic (Gaffney, 1990); however, Joyce and Gauthier (2004) and Scheyer and Sander (2007) argued for a terrestrial habitat preference for Proterochersis and Proganochelys because of their forelimbs bearing short hands (Joyce & Gauthier, 2004) and because of similarities in shell bone histology to that of extant terrestrial turtles (Scheyer & Sander, 2007). In sum, turtles most likely had a terrestrial origin, thus representing an important taxon for the study of feeding in secondarily aquatic vertebrates.
Turtles are among the most morphologically specialized vertebrates. They have evolved an unusual body plan, with most of their body encased in a protective box of bone and keratin. Collectively, within the two testudinian suborders, Pleurodira (side-necked turtles) and Cryptodira (hidden-necked turtles), there are 14 extant families and around 470 species (Rhodin et al., 2017). These show adaptations to different lifestyles and are found from marine to freshwater to terrestrial habitats, as well as from temperate to tropical regions. All pleurodirans and many cryptodirans are fully aquatic, and only the cryptodiran superfamily Testudinoidea (Emydidae, Geoemydidae, and Testudinidae) has successfully reconquered terrestrial habitats (Fig. 7.7). Dietary preferences range from completely carnivorous to completely herbivorous, but most extant species are omnivorous. The two major foraging methods are sit-and-wait foraging and active foraging; in water typical aquatic feeding modes range from ram feeding to suction feeding.
Cladogram modified from Joyce and Gauthier (2004) illustrating the assumed habitat preferences of the major clades of crown turtles and their hypothetical ancestors. Joyce and Gauthier (2004) advocated terrestriality as the ancestral lifestyle for the entire clade, with convergent acquisition of aquatic habits in sauropterygians and crown turtles, and a subsequent reversal to terrestrial habits in the Testudinidae
7.4.2 Food Detection
Among turtles, visual, chemical, and tactile cues may be involved in food detection, with visual and olfactory senses being predominantly used in water. Turtles have photoreceptors containing colored oil droplets that appear to play a role in contrast enhancement and in protection from glare. The turtle eye is especially sensitive to red light (Granda & Dvorak, 1977). Similarly to tadpoles, aquatic turtles have retinal visual pigments (porphyropsins), which leads to a red shift and a considerable improvement of sensitivity in water (Reuter & Peichl, 2008). Marine turtles are emmetropic (normal sighted) in water and myopic (nearsighted) in air. Freshwater turtles show the opposite trend, being emmetropic in air but their eyes have a sufficiently developed accommodative range to be able to fully compensate for the lack of refraction at the cornea in water (Kröger & Katzir, 2008). Accordingly, the turtle eye is well suited for food detection under aquatic conditions.
Chemical cues can be detected via olfaction, vomeronasal chemoreception and gustation. Underwater nasal chemoreception is in general used for exploratory behavior, food location and discrimination, as well as reproductive behavior (Schwenk, 2008). The nasal cavity in turtles typically consists of a dorsal chamber containing the olfactory epithelium and a ventral “intermediate region” in which the vomeronasal epithelium lies (Tucker, 1971). Sea turtles (Cheloniidae and Dermochelyidae) are known to rely heavily on chemical cues to detect food sources and, in contrast to most marine animals, they surface to breathe and thus potentially have access to olfactory cues in both air and in water (Endres et al., 2009). Gustation is mediated by taste buds that are located within the oropharyngeal cavity. Taste buds are developed to a variable degree in the various turtle branches. For example, semiaquatic or terrestrial species are well equipped with taste buds (Heiss et al., 2008, 2011; Lintner et al., 2012), whereas highly aquatic species lack them on their poorly developed tongue (Lemell et al., 2002; Beisser et al., 2004) and sea turtles appear to lack taste buds completely (Iwasaki et al., 1996a, b). When present, taste buds can be distributed randomly throughout the oropharyngeal cavity (Heiss et al., 2011) or may show patterns of regional concentration that are correlated with the respective mode of food prehension. For instance, the semiaquatic turtle Cuora amboinensis shows aggregations of taste buds on the praechoanal palate and grasps food by its jaws (Heiss et al., 2008). Accordingly, the first contact with the food item occurs at the tip of the beak (i.e. the praechoanal region) that has high taste bud densities, allowing rapid feedback response and the avoidance of unpalatable items.
Next to vision and chemosensation, some aquatic turtles use mechanosensitive elements, such as mechanosensitive skin flaps and barbels on the anteroventral areas of the neck and head, to detect prey. Such mechanosensitive elements are sensitive to water motions and are, for instance, found in Chelus fimbriatus, a typical aquatic ambush predator (Wise et al., 1989). The skin flaps on the ventral region of the neck and the barbels (ventral to the mandibles) are innervated by peripheral nerve fibers and have been reported to be sensitive to small disturbances (Hartline, 1967).
7.4.3 Food Capture
When feeding in water, suction feeding is the predominant mode employed. Although most fish and larval salamanders use a suction feeding mechanism with uni-directional flow (water flows in through the mouth opening and out through the gill openings), reptiles and other secondarily aquatic vertebrates rely on a bidirectional flow system, wherein water flows in through the mouth but is then expelled through the nostrils or slightly opened jaws (Lauder & Prendergast, 1992). Such a bidirectional system implies a decrease in feeding performance due to lower negative pressure generated within the buccal cavity (Lauder & Shaffer, 1986). This impairment must be overcome by morphological specializations in secondarily aquatic lineages that lack gill openings. Such specializations in turtles comprise a flat and streamlined skull, as found in trionychids and chelids, which is very effective for fast forward movement of the head during capture as a bow wave can be minimized. Another trait of aquatic feeders is the enlarged supraoccipital bone which supports the jaw adductor musculature, along with an enlargement of the upper temporal area. Such a configuration can be used for generating the high bite forces (Herrel et al., 2002) typical of durophagous or carnivorous biting specialists. Additionally, such traits are advantageous for closing the mouth against water resistance during rapid forward movement of the head (e.g., in Chelus fimbriatus, Lemell et al., 2002). Further, morphological features typical of vertebrates related to feeding in aquatic habitats (Bramble & Wake, 1985) include: a large, rigid and well-ossified hyoid skeleton with massive hyoid musculature for rapid depression of the hyoid apparatus during the oropharyngeal expansion phase of the suction strike; a flat and smooth palate; a small tongue; and a short gape with labial folds for stabilizing the water flow inside the mouth cavity. All of these features are exhibited by freshwater turtles. However, marine turtles do not feature such morphologies, which is related to their feeding preferences. The skulls of marine turtles are more suited for forceful biting while feeding on relatively slow moving and sometimes armored prey (Jones et al., 2012). The tongue usually does not play a role during food manipulation in purely aquatic feeders. In such turtles the tongue tends to be reduced in size and in surface structure so as not to obstruct suction kinematics. Chelus fimbriatus is a good example of this configuration (Lemell et al., 2010) because its tongue is reduced to a tiny evagination anterior to the larynx and lacks any dorsal morphological differentiation. In more generalist aquatic to semiaquatic species, the tongue plays no role during prey capture but is used in some cases for further manipulation phases, similarly to more terrestrial species (Fig. 7.8).
Scanning electron microscopic images of the tongue surface of strictly aquatic (top) to semiaquatic to terrestrial (bottom) turtles. From left to right: top row Chelus fimbriatus (Lemell et al., 2010), Acanthochelys pallidipectoris (Beisser et al., 1995), Pelusios castaneus (Lemell et al., 2000); bottom row Cuora amboinensis (provided by CJ Beisser), Rhinoclemmys pulcherrima (Beisser et al., 2004), Cuora galbinifrons (Natchev et al., 2010), Manouria emys emys (Heiss et al., 2011). Compare the increase of size and number of dorsal papillary structures from highly aquatic to terrestrial
In semi-aquatic species, skull design more closely resembles that of terrestrial species, being relatively tall and narrow. But some features indicate adaptation to an aquatic medium; for example, many of these taxa are moderate suction feeders, using suction to compensate for the bow wave forming in front of the head as it is accelerated forwards towards the prey (Natchev et al., 2009). Aquatically-adapted features include a flat palate, as in the predominantly aquatic Cuora amboinensis, in contrast to a vaulted one, as in the predominantly terrestrial Cuora galbinifrons (Natchev et al., 2009, 2010). The tongue of amphibious species is simple and poorly endowed with glands, and bears low to moderately high papillae that lack intrinsic muscles. These papillae are longer in more terrestrial species. Such traits are also obvious in salamanders, in which the surface topography of the tongue changes seasonally with the shift from an aquatic to a terrestrial lifestyle (Heiss et al., 2017).
When turtles feed under water, the kinematics (such as the timing of gaping and hyobranchial depression) show many similarities with other suction feeding vertebrates. A typical feeding sequence requires multiple gape cycles from ingestion to swallowing, so there is always some variation in kinematic patterns during prey capture and manipulation phases. Although these movements are probably driven by a central pattern generator, sensory feedback is important for any adjustments associated with the respective feeding phase and the type and properties of the food (hard, soft) (Schwenk, 2000b). An absolutely necessary feature for successful ingestion is the anteroposterior sequence in peak excursions of head elements, which are mouth opening, hyoid depression and esophageal expansion. In aquatic-feeding turtles the hyoid apparatus has become enlarged to increase the volume of the mouth cavity during the expansion phase, as occurs with the suspensorium of teleost fish; the tongue, in contrast, has become reduced in size to minimize turbulence during prey uptake. Esophageal expansion appears to be convergent with the expansion of the opercular cavity of teleost fish, and serves to maintain the unidirectional flow posteriorly until the jaws have closed. This esophageal bulging commences via lateral dilatation of the second ceratobranchials, with further expansion achieved passively by the force of the incoming water. Pipid frogs show a similar modification for temporarily storing water sucked in during prey capture. The post-glottal pharynx of Hymenochirus boettgeri (Sokol, 1969) and the highly extensible buccopharyngeal region of Pipa pipa (Fernandez et al., 2017) serve as a temporary reservoir for a large volume of water.
The most specialized prey capture strategy in aquatic turtles may be that of the luring mechanism employed by Macrochelys temminckii (Drummond & Gordon, 1979; Spindel et al., 1987). Macrochelys has developed an unusual sit and wait feeding strategy: while remaining motionless they present a red, wiggling lure situated at the center of their widely opened jaws. This lure is a highly mobile, vermiform appendage of the tongue. If curious potential prey animals (usually fish or other turtles) approach closely enough they are caught by a sudden and violent strike.
7.4.4 Intraoral Transport, Processing and Swallowing
Further prey processing stages of aquatic turtles, such as intraoral transportation and swallowing, have not yet been sufficiently investigated. In aquatic feeding specialists, the prey is either reduced or sucked further backwards, or it is transported from the esophagus directly to the stomach by waves of contraction. The kinematic pattern of manipulation and intraoral transport phases is essentially identical to that typical of the capture phase, albeit much slower. Inertial feeding (sensu Gans, 1969) is typically used for intraoral transport (Lemell & Weisgram, 1997; Van Damme & Aerts, 1997). During transport, the jaws release the object and the head shifts anteriorly while the inertia of the object restricts its propensity to move. Hyobranchial depression supports these head movements by holding the prey in place or sucking it further backwards. During these manipulation cycles, reduced material, such as cracked shells of mollusks, can be expelled. The jaws are held slightly open to allow flushing of the residual water, with or without any content. Lemell et al. (2002) described two transport modes for Chelus, based upon the analysis of X-ray videos. They distinguished between two suction mechanisms: in the first the complete hyobranchial system undergoes several slight movements to carry the prey further backwards toward the esophagus. The second transport mode is characterized by a single, massive hyobranchial depression with much slower velocity, resulting in the prey item being sucked further in, as far as the posterior end of the pharynx, where it is held in place by the horns of the second branchial arch while the water is expelled. The tongue usually does not play a role during manipulation in purely aquatic feeders, but in semiaquatic species it does. Entirely lingually-based aquatic transport, with patterns of jaw, head and hyolingual movements resembling those of terrestrial prey positioning/transport, likely driven by the same motor-program, is executed in the manner described for the predominantly terrestrial C. galbinifrons (Bels et al., 2008; Natchev et al., 2010). Bels et al. (1998) compared food ingestion of the estuarine Malaclemys terrapin with that of the truly marine Dermochelys coriacea. Whereas Malaclemys is able to modulate at least the manipulation phases according to the particular prey type by using the tongue for further transport, Dermochelys uses actively generated water flow along with tongue movements. Rhythmic movements of the hyolingual apparatus carry food to the posterior end of the pharynx where it is swallowed by the action of the pharyngeal constrictors and moved to the stomach by peristaltic contractions of the esophageal musculature.
7.5 Conclusions
With numerous independent invasions of aquatic environments and trophic niches, sauropsids exemplify successful alternatives to a life on land. Retaining predominantly jaw-prehension feeding modes, aquatic lizards, snakes, crocodilians, and birds tend to use grasping as the means of food acquisition. On one hand, evolution of specializations that enable subaquatic head acceleration (especially in piscivorous reptiles) such as expansive necks and slender jaws seem to dominate convergent predatory phenotypes. On the other hand, many aquatic bird species have modified their approach to jaw prehension by harvesting small objects suspended in the water column via filter feeding. To induce a flow of water for filtration, these sauropsids evolved piston-like tongues that oscillate as the jaws open and close, enabling beak lamellae to capture food particles as water is expelled. Turtles deserve special attention as a stand-out reptile group because of their use of suction feeding, which is achieved by expansion of oropharyngeal volume—a feature shared among other sauropsids only by the little Auk.
The feeding strategies and functional morphologies explored in this chapter, however, are certainly not unique to sauropsids. For example, highly elaborate mechanisms for generating suction flows by rapid oropharyngeal volume expansion have evolved independently in such diverse groups as cartilaginous and bony fishes, salamanders, caecilians, anurans, cetaceans, and pinnipeds. Similarly, suspension feeding using complex food-particle trapping systems can be found in agnathans, cartilaginous and bony fishes, anuran tadpoles, and cetaceans. What seems so remarkable is not necessarily that sauropsids have converged on these tried-and-true feeding systems, but that such phylogenetically diverse groups have evolved similar approaches to food capture so regularly. In this regard, sauroposids are yet another attestation of the role that aquatic environments have played in shaping the body systems of even distantly related vertebrates.
References
Abercromby, A. F. (1913). How a crocodile feeds. Spolia Zeylanica, 9, 147–148.
Alexander, R. M. N. (1983). Animal mechanics (2nd ed.). Blackwell Scientific Publications.
Alfaro, M. E. (2002). Forward attack modes of aquatic feeding garter snakes. Functional Ecology, 16, 204–215.
Alfaro, M. E. (2003). Sweeping and striking: A kinematic study of the trunk during prey capture in three thamnophiine snakes. The Journal of Experimental Biology, 206, 2381–2392.
Anderson, B. W., Reeder, M. G., & Timken, R. L. (1974). Notes on the feeding behavior of the Common Merganser (Mergus merganser). Condor, 76, 472–476.
Anquetin, J. (2011). Reassessment of the phylogenetic interrelationships of basal turtles (Testudinata). Journal of Systematic Palaeontology, 10(1), 3–45.
Ballell, A., Moon, B. C., Porro, L. B., Benton, M. J., & Rayfield, E. J. (2019). Convergence and functional evolution of longirostry in crocodylomorphs. Palaeontology, 62(6), 867–887.
Bang, B. G., & Cobb, S. (1968). The size of the olfactory bulb in 108 species of birds. Auk, 85, 55–61.
Barrowclough, G. F., Cracraft, J., Klicka, J., & Zink, R. M. (2016). How many kinds of birds are there and why does it matter? PLoS One, 11, e0166307.
Becker, M., Rubega, M. A., & Oring, L. (2002). Development of feeding mechanics in growing birds: scything behavior in juvenile American avocets (Recurvirostra americana). Bird Behavior, 15, 1–10.
Beisser, C. J., Weisgram, J., & Splechtna, H. (1995). Dorsal lingual epithelium of Platemys pallidipectoris (Pleurodira, Chelidae). Journal of Morphology, 226, 267–276.
Beisser, C. J., Lemell, P., & Weisgram, J. (2004). The dorsal lingual epithelium of Rhinoclemmys pulcherrima incisa (Chelonia, Cryptodira). The Anatomical Record, 277A, 227–235.
Bels, V. L., Davenport, J., & Renous, S. (1998). Food ingestion in the estuarine turtle Malaclemys terrapin: Comparison with the marine leatherback turtle Dermochelys coriacea. Journal of the Marine Biological Association of the UK, 78, 953–972.
Bels, V. L., Baussart, S., Davenport, J., Shorten, M., O’Riordan, R. M., Renous, S., & Devneport, J. (2008). Functional evolution of feeding behaviour in turtles. In J. Wyneken, M. H. Godfrey, & V. Bels (Eds.), Biology of turtles (pp. 189–212). CRC Press Taylor & Francis Group.
Bemis, W., & Lauder, G. V. (1986). Morphology and function of the feeding apparatus of the lungfish, Lepidosiren paradoxa (Dipnoi). Journal of Morphology, 187, 81–108.
Benes, E. S. (1969). Behavioral evidence for color discrimination by the whiptail lizard, Cnemidophorus tigris. Copeia, 1969(4), 707–722.
Berkhoudt, H. (1979). The morphology and distribution of cutaneous mechanoreceptors (Herbst and Grandry corpuscles) in bill and tongue of the mallard (Anas platyrhynchos L.). Netherlands Journal of Zoology, 30, 1–34.
Berkhoudt, H. (1985). Structure and function of avian taste receptors. In A. S. King & J. McLelland (Eds.), Form and function in birds (pp. 463–496). Academic.
Bever, G. S., Lyson, T. R., Field, D. J., & Bhullar, B. S. (2015). Evolutionary origin of the turtle skull. Nature, 525, 239–242.
Bilcke, J., Herrel, A., & Van Damme, R. (2006). Correlated evolution of aquatic prey-capture strategies in European and American natricine snakes. Biological Journal of the Linnean Society, 88, 73–83.
Biswas, S. (1970). A preliminary survey of gharial in the Koshi River. Indian Forester, 96(9), 705–710.
Black, B. B., & Harris, L. D. (1983). Feeding habitat of Black Skimmers wintering on the Florida Gulf coast. Wilson Bulletin, 95(3), 404–415.
Bramble, D. M., & Wake, D. B. (1985). Feeding mechanisms of lower tetrapods. In M. Hildebrand, D. M. Bramble, K. F. Liem, & D. B. Wake (Eds.), Functional vertebrate morphology (Vol. 13, pp. 230–261). Harvard University Press.
Braun, T., & Cundall, D. (1995). Hydrodynamics of fishing in snakes (Nerodia). American Zoologist, 35, 105A.
Brazaitis, P. (1987). Identification of crocodilian skins and products. In G. J. Webb, S. C. Manolis, & P. J. Whitehead (Eds.), Wildlife Management: Crocodiles and Alligators (pp. 373–386). Surrey Beatty and Sons Pty Ltd.
Brochu, C. A. (2001). Crocodylian snouts in space and time: Phylogenetic approaches toward adaptive radiation. American Zoologist, 41(3), 564–585.
Bronzati, M., Montefeltro, F. C., & Langer, M. C. (2015). Diversification events and the effects of mass extinctions on Crocodyliformes evolutionary history. Royal Society Open Science, 2(5), 140385.
Buffetaut, E. (1982). Radiation évolutive, paléoécologie et biogéographie des crocodiliens mésosuchiens. Mémoires de la Société Géologique de France, 142, 1–88.
Bull, C. M., Griffin, C. L., & Johnston, G. R. (1999). Olfactory discrimination in scat-piling lizards. Behavioral Ecology, 10, 136–140.
Busbey, A. B. I. I. I. (1989). Form and function of the jaw musculature of Alligator mississippiensis. Journal of Morphology, 202, 99–127.
Busbey, A. B. (1995). The structural consequences of skull flattening in crocodilians. In J. J. Thomas (Ed.), Functional morphology in vertebrate paleontology (pp. 173–192). Cambridge University Press.
Carl, R. A. (1987). Age-class variation in foraging techniques by Brown Pelicans. Condor, 89(3), 525–533.
Carpenter, C. C. (1966). The marine iguana of the Galapagos Islands: Its behavior and ecology. Proceedings of the California Academy of Sciences, 34, 329–376.
Carter, D. R., & Beaupré, G. S. (2001). Skeletal function and form: Mechanobiology of skeletal development, aging, and regeneration. Cambridge University Press.
Catania, K. C. (2009). Tentacled snakes turn C-starts to their advantage and predict future prey behavior. Proceedings of the National Academy of Sciences of the United States of America, 106, 11183–11187.
Catania, K. C. (2010). Born knowing: Tentacled snakes innately predict future prey behavior. PLoS One, 5, e10953.
Catania, K. C., Leitch, D., & Gauthier, D. (2010). Function of the appendages in tentacled snakes (Erpeton tentaculatus). The Journal of Experimental Biology, 213, 359–367.
Chang, B., Croson, M., Straker, L., Gart, S., Dove, C., Gerwin, J., & Jung, S. (2016). How seabirds plunge-dive without injuries. Proceedings of the National Academy of Sciences of the United States of America, 113, 12006–12011.
Charrassin, J.-B., Kato, A., Handrich, Y., Sato, K., Naito, Y., Ancel, A., Bost, C.-A., Gauthier-Clerc, M., Ropert-Coudert, Y., & Le Maho, Y. (2001). Feeding behaviour of free–ranging penguins determined by oesophageal temperature. Proceedings of the Royal Society London B, 268, 151–157.
Cleuren, J., & De Vree, F. (1992). Kinematics of the jaw and hyolingual apparatus during feeding in Caiman crocodilus. Journal of Morphology, 212, 141–154.
Cleuren, J., & De Vree, F. (2000). Feeding in crocodilians. In K. Schwenk (Ed.), Feeding: Form, function, and evolution in tetrapod vertebrates (pp. 337–358). Academic.
Cochran, G. V. B. (1982). A primer of orthopaedic biomechanics. Churchill Livingstone.
Coombs, S., Görner, P., & Münz, H. (2012). The mechanosensory lateral line: Neurobiology and evolution. Springer.
Crandell, K., Howe, R., & Falkingham, P. (2019). Repeated evolution of drag reduction at the air–water interface in diving kingfishers. Journal of the Royal Society Interface, 16, 20190125.
Crowe-Riddell, J. M., Snelling, E. P., Watson, A. P., Suh, A. K., Partridge, J. C., & Sanders, K. L. (2016). The evolution of scale sensilla in the transition from land to sea in elapid snakes. Open Biology, 6, 160054.
Cuff, A. R., & Rayfield, E. J. (2013). Feeding mechanics in spinosaurid theropods and extant crocodilians. PLoS One, 8(5), e65295.
Cundall, D., & Greene, H. W. (2000). Feeding in snakes. In K. Schwenk (Ed.), Feeding: Form, function, and evolution in tetrapod vertebrates (pp. 293–333). Academic.
Deban, S., & Wake, D. (2000). Aquatic feeding in salamanders. In K. Schwenk (Ed.), Feeding: Form, function and evolution in tetrapod vertebrates (pp. 65–94). Academic.
Drummond, H. (1983). Aquatic foraging in garter snakes: A comparison of specialists and generalists. Behaviour, 86, 1–30.
Drummond, H., & Gordon, E. R. (1979). Luring in the neonate alligator snapping turtle (Macroclemys temmincki): Description and experimental analysis. Zeitschrift für Tierpsychologie, 50, 136–152.
Duffy, D. C., Wilson, R. P., Wilson, M. P., & Velasquez, C. R. (1986). Plunge-diving by olivaceous cormorants in Chile. Wilson Bulletin, 98, 607–608.
Endo, H., Aoki, R., Taru, H., Kimura, J., Sasaki, M., Yamamoto, M., Arishima, K., & Hayashi, Y. (2002). Comparative functional morphology of the masticatory apparatus in the long-snouted crocodiles. Anatomia, Histologia, Embryologia, 31(4), 206–213.
Endres, C. S., Putman, N. F., & Lohmann, K. J. (2009). Perception of airborne odors by loggerhead sea turtles. The Journal of Experimental Biology, 212, 3823–3827.
Enstipp, M. R., Descamps, S., Fort, J., & Grémillet, D. (2018). Almost like a whale–first evidence of suction feeding in a seabird. The Journal of Experimental Biology, 221, jeb182170.
Erickson, G. M., Gignac, P. M., Steppan, S., Lappin, A. K., Vliet, K. A., Brueggen, J. D., Inouye, B. D., Kledzik, D., & Webb, G. J. W. (2012). Insights into the ecology and evolutionary success of crocodilians revealed through bite force and tooth pressure experimentation. PLoS One, 7, e31781.
Erickson, G. M., Gignac, P. M., Lappin, A. K., Vliet, K. A., Brueggen, J. D., & Webb, G. J. W. (2014). A comparative analysis of ontogenetic bite-force scaling among Crocodylia. The Journal of Zoology London, 292(1), 48–55.
Erwin, D. H. (1992). A preliminary classification of evolutionary radiations. Historical Biology, 6(2), 133–147.
Felice, R. N., Watanabe, A., Cuff, A. R., Noirault, E., Pol, D., Witmer, L. M., Norell, M. A., O'Connor, P. M., & Goswami, A. (2019). Evolutionary integration and modularity in the archosaur cranium. Integrative and Comparative Biology, 59(2), 371–382.
Fernandez, E., Irish, F., & Cundall, D. (2017). How a frog, Pipa pipa, succeeds or fails in catching fish. Copeia, 105(1), 108–119.
Fleishman, L. J., Howland, H. C., Howland, M. J., Rand, A. S., & Davenport, M. L. (1988). Crocodiles don’t focus underwater. The Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology, 163(4), 441–443.
Fleming, G. J., & Fontenot, D. K. (2015). Crocodilians (crocodiles, alligators, caiman, gharial). In R. E. Miller & M. E. Fowler (Eds.), Fowler’s zoo and wild animal medicine (Vol. 8, pp. 38–49). Elsevier.
Foffa, D., Young, M. T., Stubbs, T. L., Dexter, K. G., & Brusatte, S. L. (2018). The long-term ecology and evolution of marine reptiles in a Jurassic seaway. Nature Ecology and Evolution, 2(10), 1548–1555.
Forbes, L. (1982). Prey manipulation in the great blue heron. The Murrelet, 63, 89–91.
Forsyth, H. W. (1910). The food of crocodiles. The Journal of the Bombay Natural History Society, 20, 1160.
Fritz, J., Hummel, J., Kienzle, E., Wings, O., Streich, W. J., & Clauss, M. (2011). Gizzard vs. teeth, it’s a tie: Food-processing efficiency in herbivorous birds and mammals and implications for dinosaur feeding strategies. Paleobiology, 37, 577–586.
Gaffney, E. S. (1990). The comparative osteology of the triassic turtle Proganochelys. Bulletin of the American Museum of Natural History, 194, 1–268.
Gans, C. (1969). Comments on inertial feeding. Copeia, 1969(4), 855–857.
Gignac, P. M., & Erickson, G. M. (2016). Ontogenetic bite-force modeling of Alligator mississippiensis: Implications for dietary transitions in a large-bodied vertebrate and the evolution of crocodylian feeding. Journal of Zoology, 299(4), 229–238.
Gignac, P. M., & O’Brien, H. D. (2016). Suchian feeding success at the interface of ontogeny and macroevolution. Integrative and Comparative Biology, 56(3), 449–458.
Gignac, P. M., O’Brien, H. D., Turner, A. H., & Erickson, G. M. (2019). Feeding in crocodylians and their relatives: Functional insights from ontogeny and evolution. In V. Bels & I. Q. Wishaw (Eds.), Feeding in vertebrates: Evolution, morphology, behavior, biomechanics (pp. 575–610). Springer.
Gilbert, S. F., Loredo, G. A., Brukman, A., & Burke, A. C. (2001). Morphogenesis of the turtle shell: The development of a novel structure in tetrapod evolution. Evolution and Development, 3, 47–58.
Gill, F. B. (1995). Ornithology. Macmillan.
Godoy, P. L. (2020). Crocodylomorph cranial shape evolution and its relationship with body size and ecology. Journal of Evolutionary Biology, 33(1), 4–21.
Goldsmith, T. H. (1990). Optimization, constraint, and history in the evolution of eyes. The Quarterly Review of Biology, 65, 281–322.
Goris, R. C. (2011). Infrared organs of snakes: An integral part of vision. Journal of Herpetology, 45, 2–14.
Gottschaldt, K. (1985). Structure and function of avian somatosensory receptors. In A. S. King & J. McLelland (Eds.), Form and function in birds (Vol. 3, pp. 375–461). Academic.
Govardovskii, V. I. (1983). On the role of oil drops in colour vision. Vision Research, 23, 1739–1740.
Grace, M. S., Church, D. R., Kelly, C. T., Lynn, W. F., & Cooper, T. M. (1999). The Python pit organ: imaging and immunocytochemical analysis of an extremely sensitive natural infrared detector. Biosensors & Bioelectronics, 14, 53–59.
Granda, A. M., & Dvorak, C. A. (1977). Vision in turtles. The visual system in vertebrates. In F. Crescitelli (Ed.), Handbook of sensory physiology (Vol. 7/5). Springer.
Grenard, S. (1991). Handbook of alligators and crocodiles. Krieger Publishing.
Grigg, G. C., Seebacher, F., & Franklin, C. E. (2001). Crocodilian biology and evolution. Surrey Beatty.
Halpern, M., & Kubie, J. L. (1980). Chemical access to the vomeronasal organs of garter snakes. Physiology & Behavior, 24, 367–371.
Harrisson, B. (1961). Lanthanotus borneensis-habits and observations. The Sarawak Museum Journal, 10, 286–292.
Harrisson, T., & Haile, N. (1961). A rare earless monitor lizard from Borneo. Nature, 190, 1213–1213.
Hartline, P. (1967). The unbelievable fringed turtle. International Tortoise and Turtle Society Journal, 1, 24–29.
Hawaii Hobbyist. (2019, September 14). Lizard eats guppy for lunch [video file]. YouTube. Accessed December 2, 2020, from https://www.youtube.com/watch?v=pWq3r20jjIM
Heiss, E., Plenk, H. J., & Weisgram, J. (2008). Microanatomy of the palatal mucosa of the semiaquatic Malayan box turtle, Cuora amboinensis, and functional implications. The Anatomical Record, 291, 876–885.
Heiss, E., Natchev, N., Schwaha, T., Salaberger, D., Lemell, P., Beisser, C., & Weisgram, J. (2011). Oropharyngeal morphology in the basal tortoise Manouria emys emys with comments on form and function of the testudinid tongue. Journal of Morphology, 272, 1217–1229.
Heiss, E., Natchev, N., Gumpenberger, M., Weissenbacher, A., & Van Wassenbergh, S. (2013). Biomechanics and hydrodynamics of prey capture in the Chinese giant salamander reveal a high-performance jaw-powered suction feeding mechanism. Journal of the Royal Society Interface, 10(82), 20121028.
Heiss, E., Handschuh, S., Aerts, P., & Van Wassenbergh, S. (2017). A tongue for all seasons: Extreme phenotypic flexibility in salamandrid newts. Scientific Reports, 7, 1006.
Herrel, A., O’Reilly, J. C., & Richmond, A. M. (2002). Evolution of bite performance in turtles. Journal of Evolutionary Biology, 15, 1083–1094.
Herrel, A., Vincent, S., Alfaro, M., Van Wassenbergh, S., Vanhooydonck, B., & Irschick, D. (2008). Morphological convergence as a consequence of extreme functional demands: Examples from the feeding system of natricine snakes. Journal of Evolutionary Biology, 21, 1438–1448.
Holliday, C. M., & Witmer, L. M. (2007). Archosaur adductor chamber evolution: integration of musculoskeletal and topological criteria in jaw muscle homology. Journal of Morphology, 268(6), 457–484.
Holliday, C. M., & Witmer, L. M. (2009). The epipterygoid of crocodyliforms and its significance for the evolution of the orbitotemporal region of eusuchians. The Journal of Vertebrate Paleontology, 29(3), 715–733.
Howland, H. C., & Sivak, J. (1984). Penguin vision in air and water. Vision Research, 24(12), 1905–1909.
Hua, S., & De Buffrenil, V. (1996). Bone histology as a clue in the interpretation of functional adaptations in the Thalattosuchia (Reptilia, Crocodylia). The Journal of Vertebrate Paleontology, 16(4), 703–717.
Iijima, M. (2017). Assessment of trophic ecomorphology in non-alligatoroid crocodylians and its adaptive and taxonomic implications. Journal of Anatomy, 231(2), 192–211.
Iordansky, N. N. (1964). The jaw muscles of the crocodiles and some relating structures of the crocodilian skull. Anatomischer Anzeiger, 115, 256–280.
Iordansky, N. N. (1973). The skull of the Crocodilia. In C. Gans & T. S. Parson (Eds.), The biology of the Reptilia (Vol. 4, pp. 210–262). Academic.
Iwasaki, S. I., Asami, T., & Wanichanon, C. (1996a). Fine structure of the dorsal lingual epithelium of the juvenile hawksbill turtle, Eretmochelys imbricata bissa. The Anatomical Record, 244, 437–443.
Iwasaki, S. I., Wanichanon, C., & Asami, T. (1996b). Histological and ultrastructural study of the lingual epithelium of the juvenile Pacific ridley turtle, Lepidochelys olivacea (Chelonia, Cheloniidae). Annals of Anatomy, 178, 243–250.
Jackson, K., & Brooks, D. R. (2007). Do crocodiles co-opt their sense of “touch” to “taste”? A possible new type of vertebrate sensory organ. Amphibia-Reptilia, 28(2), 277–285.
Jackson, K., Butler, D. G., & Youson, J. H. (1996). Morphology and ultrastructure of possible integumentary sense organs in the estuarine crocodile Crocodylus porosus. Journal of Morphology, 229(3), 315–324.
Janke, A., & Arnason, U. (1997). The complete mitochondrial genome of Alligator mississippiensis and the separation between recent archosauria (birds and crocodiles). Molecular Biology and Evolution, 14, 1266–1272.
Jenkin, P. M. (1957). The filter-feeding and food of flamingoes (Phoenicopteri). Philosophical Transactions of the Royal Society London B Biological Sciences, 240, 401–493.
Johnson, C. R. (1973). Behaviour of the Australian crocodiles, Crocodylus johnstoni and C. porosus. Zoological Journal of the Linnean Society, 52(4), 315–336.
Jones, M. E. H. (2008). Skull shape and feeding strategy in Sphenodon and other Rhynchocephalia (Diapsida: Lepidosauria). Journal of Morphology, 269(8), 945–966.
Jones, M. E. H., Werneburg, I., Curtis, N., Penrose, R., O’Higgins, P., Fagan, M. J., & Evans, S. E. (2012). The head and neck anatomy of sea turtles (Cryptodira: Chelonioidea) and skull shape in testudines. PLoS One, 7(11), e47852.
Joyce, W. G. (2015). The origin of turtles: a paleontological perspective. The Journal of Experimental Zoology, 324(3), 181–193.
Joyce, W. G., & Gauthier, J. A. (2004). Palaeoecology of Triassic stem turtles sheds new light on turtle origins. Proceedings of the Royal Society of London B, 271, 1–5.
Kane, E., & Marshall, C. (2009). Comparative feeding kinematics and performance of odontocetes: Belugas, Pacific white-sided dolphins and long-finned pilot whales. The Journal of Experimental Biology, 212, 3939–3950.
Kardong, K. V. (1977). Kinesis of the jaw apparatus during swallowing in the cottonmouth snake, Agkistrodon piscivorus. Copeia, 1977, 338–348.
Katzir, G., & Intrator, N. (1987). Striking of underwater prey by a reef heron, Egretta gularis schistacea. Journal of Comparative Physiology A, 160, 517–523.
Klages, N. T., & Cooper, J. (1992). Bill morphology and diet of a filter-feeding seabird: The broad-billed prion Pachyptila vittata at South Atlantic Gough Island. Journal of Zoology, 227, 385–396.
Kobayashi, K., Kumakura, M., Yoshimura, K., Inatomi, M., & Asami, T. (1998). Fine structure of the tongue and lingual papillae of the penguin. Archives of Histology and Cytology, 61, 37–46.
Kooloos, J. G. M., Kraaijeveld, A. R., Langenbach, G. E. J., & Zweers, G. A. (1989). Comparative mechanics of filter feeding in Anas platyrhynchos, Anas clypeata and Aythya fuligula (Aves, Anseriformes). Zoomorphology, 108, 269–290.
Kratzing, J. E. (1975). The fine structure of the olfactory and vomeronasal organs of a lizard (Tiliqua scincoides scincoides). Cell and Tissue Research, 156, 239–252.
Kröger, R. H. H., & Katzir, G. (2008). Comparative anatomy and physiology of vision in aquatic tetrapods. In J. G. M. Thewissen (Ed.), Sensory evolution on the threshold – Adaptations in secondarily aquatic vertebrates. University of California Press.
Kulabtong, S., & Mahaprom, R. (2014). Observation on food items of Asian water monitor, Varanus salvator (Laurenti, 1768) (Squamata Varanidae), in urban eco-system, Central Thailand. Biodiversity Journal, 6, 695–698.
Kushlan, J. A. (1976). Feeding behavior of North American herons. Auk, 93, 86–94.
Kutsuma, R., Sasai, T., & Kishida, T. (2018). How snakes find prey underwater: Sea snakes use visual and chemical cues for foraging. Zoological Science, 35, 483–486.
Langner, C. (2017). Of an enigmatic lizard: First records of habitat use, behavior, and food items of Lanthanotus borneensis Steindachner, 1878 in its natural habitat. Russian Journal of Herpetology, 24, 1–10.
Langston, W. J. (1973). The crocodilian skull in historical perspective. In C. Gans & T. S. Parson (Eds.), The biology of the Reptilia (Vol. 4, pp. 263–284). Academic.
Lauder, G. V. (1980). Evolution of the feeding mechanism in primitive actionopterygian fishes: A functional anatomical analysis of Polypterus, Lepisosteus, and Amia. Journal of Morphology, 163, 283–317.
Lauder, G. V., & Prendergast, T. (1992). Kinematics of aquatic prey capture in the snapping turtle Chelydra serpentina. The Journal of Experimental Biology, 164, 55–78.
Lauder, G. V., & Shaffer, H. B. (1986). Functional design of the feeding mechanism in lower vertebrates: Unidirectional and bidirectional flow systems in the tiger salamander. Zoological Journal of the Linnean Society, 88, 277–290.
Leitch, D. B., & Catania, K. C. (2012). Structure, innervation and response properties of integumentary sensory organs in crocodilians. Journal of Experimental Biology, 215(23), 4217–4230.
Lemanis, R., Jones, A. S., Butler, R. J., Anderson, P. S., & Rayfield, E. J. (2019). Comparative biomechanical analysis demonstrates functional convergence between slender-snouted crocodilians and phytosaurs. PeerJ Preprints, 7, e27476v1.
Lemell, P., & Weisgram, J. (1997). Feeding patterns of Pelusios castaneus. Netherlands Journal of Zoology, 47(4), 429–441.
Lemell, P., Beisser, C. J., & Weisgram, J. (2000). Morphology and function of the feeding apparatus of Pelusios castaneus (Chelonia; Pleurodira). Journal of Morphology, 244, 127–135.
Lemell, P., Lemell, C., Snelderwaard, P., Gumpenberger, M., Wochesländer, R., & Weisgram, J. (2002). Feeding patterns of Chelus fimbriatus (Pleurodira: Chelidae). The Journal of Experimental Biology, 205, 1495–1506.
Lemell, P., Beisser, C. J., Gumpenberger, M., Snelderwaard, P., Gemel, R., & Weisgram, J. (2010). The feeding apparatus of Chelus fimbriatus (Pleurodira; Chelidae) – Adaptation perfected? Amphibians and Reptiles, 31, 97–107.
Lequette, B., Verheyden, C., & Jouventin, P. (1989). Olfaction in subantarctic seabirds: Its phylogenetic and ecological significance. Condor, 91, 732–735.
Li, C., Wu, X. C., Rieppel, O., Wang, L., & Zhao, L. (2008). An ancestral turtle from the Late Triassic of southwestern China. Nature, 456, 497–501.
Liem, K. R., Bemis, W. E., Walker, W. F., Jr., & Grande, L. (2001). Functional anatomy of the vertebrates: An evolutionary perspective. Thomson and Brooks/Cole.
Lintner, M., Weissenbacher, A., & Heiss, E. (2012). The oropharyngeal morphology in the semiaquatic giant Asian pond turtle, Heosemys grandis, and its evolutionary implications. PLoS One, 7(9), e46344.
Lotem, A., Schechtman, E., & Katzir, G. (1991). Capture of submerged prey by little egrets, Egretta garzetta garzetta: strike depth, strike angle and the problem of light refraction. Animal Behaviour, 42, 341–346.
Mannion, P. D., Benson, R. J., Carrano, M. T., Tennant, J. P., Judd, J., & Butler, R. J. (2015). Climate constrains the evolutionary history and biodiversity of crocodylians. Nature Communications, 6(1), 8438.
Martin, G. R., Mcneil, R., & Rojas, L. M. (2007). Vision and the foraging technique of skimmers (Rynchopidae). Ibis, 149, 750–757.
Massare, J. A. (1987). Tooth morphology and prey preference of Mesozoic marine reptiles. The Journal of Vertebrate Paleontology, 7(2), 121–137.
Matsumoto, R., & Evans, S. E. (2017). The palatal dentition of tetrapods and its functional significance. Journal of Anatomy, 230, 47–65.
Mayes, P., Thompson, G., & Withers, P. (2005). Diet and foraging behaviour of the semi-aquatic Varanus mertensi (Reptilia: Varanidae). Wildlife Research, 32, 67–74.
McCurry, M. R., Evans, A. R., Fitzgerald, E. M. G., Adams, J. W., Clausen, P. D., & McHenry, C. R. (2017a). The remarkable convergence of skull shape in crocodilians and toothed whales. Proceedings of the Royal Society B, 284(1850), 20162348.
McCurry, M. R., Walmsley, C. W., Fitzgerald, E. M. G., & McHenry, C. R. (2017b). The biomechanical consequences of longirostry in crocodilians and odontocetes. Journal of Biomechanics, 56, 61–70.
McDowell, S. B. (1972). The evolution of the tongue of snakes, and its bearing on snake origins. In T. Dobzhansky, M. K. Hecht, & W. C. Steere (Eds.), Evolutionary biology (pp. 191–273). Springer.
McGovern, G. M., Mitchell, J. C., & Knisley, C. B. (1984). Field experiments on prey selection by the whiptail lizard, Cnemidophorus inornatus, in Arizona. Journal of Herpetology, 18, 347–349.
McHenry, C. R., Clausen, P. D., Daniel, W. J. T., Meers, M. B., & Pendharkar, A. (2006). Biomechanics of the rostrum in crocodilians: A comparative analysis using finite-element modeling. The Anatomical Record Part A, 288A, 827–849.
Mesquita, D. O., Colli, G. R., Costa, G. C., França, F. G., Garda, A. A., & Péres, A. K., Jr. (2006). At the water’s edge: Ecology of semiaquatic teiids in Brazilian Amazon. Journal of Herpetology, 40, 221–229.
Metzger, K. A., Daniel, W. J. T., & Ross, C. F. (2005). Comparison of beam theory and finite-element analysis with in vivo bone strain data from the alligator cranium. The Anatomical Record, 283A(2), 331–348.
Moon, B. R. (2000). The mechanics of swallowing and the muscular control of diverse behaviours in gopher snakes. The Journal of Experimental Biology, 203, 2589–2601.
Moon, B. R., Penning, D. A., Segall, M., & Herrel, A. (2019). Feeding in snakes: Form, function, and evolution of the feeding system. In V. Bels & I. Q. Whishaw (Eds.), Feeding in vertebrates (pp. 527–574). Springer.
Morgan, W. L., & Ritz, D. A. (1982). Comparison of the feeding apparatus in the muttonbird, Puffinus tenuirostris (Temminck) and the fairy prion, Pachyptila turtur (Kuhl) in relation to the capture of the krill, Nyctiphanes australis Sars. Journal of Experimental Marine Biology and Ecology, 59, 61–75.
Morris, Z. S., Vliet, K. A., Abzhanov, A., & Pierce, S. E. (2019). Heterochronic shifts and conserved embryonic shape underlie crocodylian craniofacial disparity and convergence. Proceedings of the Royal Society B, 286(1897), 20182389.
Nagloo, N., Collin, S. P., Hemmi, J. M., & Hart, N. S. (2016). Spatial resolving power and spectral sensitivity of the saltwater crocodile, Crocodylus porosus, and the freshwater crocodile, Crocodylus johnstoni. Journal of Experimental Biology, 219(9), 1394–1404.
Natchev, N., Heiss, E., Lemell, P., Stratev, D., & Weisgram, J. (2009). Analysis of prey capture and food transport kinematics in two Asian box turtles, Cuora amboinensis and Cuora flavomarginata (Chelonia, Geoemydidae), with emphasis on terrestrial feeding patterns. Zoology, 112(2), 113–127.
Natchev, N., Lemell, P., Heiss, E., Beisser, C. J., & Weisgram, J. (2010). Aquatic feeding in a terrestrial turtle: a functional-morphological study of the feeding apparatus in the Indochinese box turtle Cuora galbinifrons (Testudines, Geoemydidae). Zoomorphology, 129, 111–119.
Neill, W. T. (1971). The Last of the Ruling Reptiles: Alligators. Columbia University Press, Ithaca.
O’Brien, H. D., Lynch, L. M., Vliet, K. A., Brueggen, J., Erickson, G. M., & Gignac, P. M. (2019). Crocodylian head width allometry and phylogenetic prediction of body size in extinct crocodyliforms. Integrative Organismal Biology, 1(1), obz006.
Olsson, P., Lind, O., & Kelber, A. (2015). Bird colour vision: Behavioural thresholds reveal receptor noise. The Journal of Experimental Biology, 218, 184–193.
Owre, O. T. (1967). Adaptations for locomotion and feeding in the Anhinga and the Double-crested Cormorant. Ornithological Monographs, 1967, 1–138.
Parfitt, A. R., & Vincent, J. F. V. (2005). Drag reduction in a swimming Humboldt Penguin, Spheniscus humboldtii, when the boundary layer is turbulent. Journal of Bionic Engineering, 2(2), 57–62.
Parsons, T. S. (1970). The nose and Jacobson’s organ. In C. Gans & T. S. Parsons (Eds.), Biology of the reptilia (Vol. 2, pp. 99–191). Academic Press.
Pettigrew, J., & Frost, B. (1985). A tactile fovea in the Scolopacidae? Brain, Behavior and Evolution, 26, 185–195.
Pierce, S. E., & Benton, M. J. (2006). Pelagosaurus typus Bronn, 1841 (Mesoeucrocodylia: Thalattosuchia) from the Upper Lias (Toarcian, Lower Jurassic) of Somerset, England. The Journal of Vertebrate Paleontology, 26(3), 621–635.
Pierce, S. E., Angielczyk, K. D., & Rayfield, E. J. (2008). Patterns of morphospace occupation and mechanical performance in extant crocodilian skulls: A combined geometric morphometric and finite element modeling approach. Journal of Morphology, 269(7), 840–864. https://doi.org/10.1002/jmor.10627
Pierce, S. E., Angielczyk, K. D., & Rayfield, E. J. (2009). Shape and mechanics in thalattosuchian (Crocodylomorpha) skulls: Implications for feeding behaviour and niche partitioning. Journal of Anatomy, 215(5), 555–576.
Pooley, A. C. (1989). Food and feeding habits. In C. A. Ross (Ed.), Crocodiles and alligators (pp. 76–91). Facts on File.
Pooley, A. C., & Gans, C. (1976). The Nile crocodile. Scientific American, 234(4), 114–125.
Porro, L. B., Holliday, C. M., Anapol, F., Ontiveros, L. C., Ontiveros, L. T., & Ross, C. F. (2011). Free body analysis, beam mechanics, and finite element modeling of the mandible of Alligator mississippiensis. Journal of Morphology, 272(8), 910–937.
Porro, L. B., Metzger, K. A., Iriarte-Diaz, J., & Ross, C. F. (2013). In vivo bone strain and finite element modeling of the mandible of Alligator mississippiensis. Journal of Anatomy, 223(3), 195–227.
Poulter, T. C. (1969). Sonar of penguins and fur seals. Proceedings of the California Academy of Sciences, 3, 363–380.
Prince, P. A. (1980). The food and feeding ecology of blue petrel (Halobaena caerulea) and dove prion (Pachyptila desolata). Journal of Zoology, 190, 59–76.
Prum, R. O. (2002). Why ornithologists should care about the theropod origin of birds. Auk, 119, 1–17.
Raikow, R. J., Bicanovsky, L., & Bledsoe, A. H. (1988). Forelimb joint mobility and the evolution of wing-propelled diving in birds. Auk, 105, 446–451.
Rayfield, E. J., Milner, A. C., Xuan, V. B., & Young, P. G. (2007). Functional morphology of spinosaur ‘crocodile-mimic’ dinosaurs. The Journal of Vertebrate Paleontology, 27(4), 892–901.
Reuter, T., & Peichl, L. (2008). Structure and function of the retina in aquatic tetrapods. In J. G. M. Thewissen (Ed.), Sensory evolution on the threshold – Adaptations in secondarily aquatic vertebrates. University of California Press.
Rhoda, D., Polly, P. D., Raxworthy, C., & Segall, M. (2020). Morphological integration and modularity in the hyperkinetik feeding system of aquatic-foraging snakes. Evolution, 75(1), 56–72.
Rhodin, A. G. J., Iverson, J. B., Bour, R., Fritz, U., Georges, A., Shaffer, H. B., & van Dijk, P. P. (2017). Turtles of the World: Annotated checklist and atlas of taxonomy, synonymy, distribution, and conservation status (8th Ed.). In Rhodin, A. G. J., Iverson, J. B., van Dijk, P. P., Saumure, R. A., Buhlmann, K. A., Pritchard, P. C. H., & Mittermeier, R. A. (Eds.). Conservation biology of freshwater turtles and tortoises: A compilation project of the IUCN/SSC tortoise and freshwater turtle specialist group (Vol. 7, pp. 1–292). Chelonian Research Foundation and Turtle Conservancy.
Rice, W. R. (1982). Acoustical location of prey by the marsh hawk: Adaptation to concealed prey. The Auk, 99, 403–413.
Rico-Guevara, A., Sustaita, D., Gussekloo, S., Olsen, A., Bright, J., Corbin, C., & Dudley, R. (2019). Feeding in birds: Thriving in terrestrial, aquatic, and aerial niches. In V. Bels & I. Q. Whishaw (Eds.), Feeding in vertebrates (pp. 643–693). Springer.
Roper, T. J. (1999). Olfaction in birds. Advances in the Study of Behavior, 28, 247–332.
Ropert-Coudert, Y., Grémillet, D., Ryan, P., Kato, A., Naito, Y., & Le Maho, Y. (2004). Between air and water: the plunge dive of the Cape Gannet Morus capensis. Ibis, 146, 281–290.
Salas-Gismondi, R. (2016) The evolution of orbital shape in gavialoid crocodylians is associated with habits and visual foraging strategies. 76th Annual Meeting for the Society of Vertebrate Paleontology Abstracts, 32.
Sanderson, S. L., & Wassersug, R. (1990). Suspension-feeding vertebrates. Scientific American, 262, 96–102.
Schaeffel, F., & de Queiroz, A. (1990). Alternative mechanisms of enhanced underwater vision in the garter snakes Thamnophis melanogaster and T. couchii. Copeia, 1990, 50–58.
Schaller, G. B., & Crawshaw, P. G., Jr. (1982). Fishing behavior of paraguayan caiman (Caiman crocodilus). Copeia, 1982(1), 66–72.
Scheyer, T. M., & Sander, P. M. (2007). Shell bone histology indicates terrestrial palaeoecology of basal turtles. Proceedings of the Royal Society of London Series B, 274, 1885–1893.
Scheyer, T. M., Werneburg, I., Mitgutsch, C., Delfino, M., & Sanchez-Villagra, R. (2013). Three ways to tackle the turtle: Integrating fossils, comparative embryology, and microanatomy. In D. B. Brinkman, P. A. Holroyd, & J. D. Garder (Eds.), Morphology and evolution of turtles (pp. 63–70). Springer.
Schoch, R. R., & Sues, H. D. (2015). A Middle Triassic stem-turtle and the evolution of the turtle body plan. Nature, 523, 584–587.
Schwab, J. A., Young, M. T., Neenan, J. M., Walsh, S. A., Witmer, L. M., Herrera, Y., Allain, R., Brochu, C. A., Choiniere, J. N., Clark, J. M., Dollman, K. N., Etches, S., Fritsch, G., Gignac, P. M., Ruebenstahl, A., Sachs, S., Turner, A. H., Vignaud, P., Wilberg, E. W., Xu, X., Zanno, L. E., & Brusatte, S. L. (2020). Inner ear sensory system changes as extinct crocodylomorphs transitioned from land to water. Proceedings of the National Academy of Sciences of the United States of America, 117(19), 10422–10428.
Schwenk, K. (1985). Occurrence, distribution and functional significance of taste buds in lizards. Copeia, 1985, 91–101.
Schwenk, K. (1995). Of tongues and noses: Chemoreception in lizards and snakes. Trends in Ecology & Evolution, 10, 7–12.
Schwenk, K. (2000a). Feeding in lepidosaurs. In K. Schwenk (Ed.), Feeding: Form, function and evolution in tetrapod vertebrates (pp. 175–291). Academic.
Schwenk, K. (2000b). An introduction to tetrapod feeding. In K. Schwenk (Ed.), Feeding: Form, function and evolution in tetrapod vertebrates (pp. 21–61). Academic.
Schwenk, K. (2008). Comparative anatomy and physiology of chemical senses in nonavian aquatic reptiles. In J. G. M. Thewissen (Ed.), Sensory evolution on the threshold – Adaptations in secondarily aquatic vertebrates (pp. 65–81). University of California Press.
Schwenk, K., & Rubega, M. (2005). Diversity of vertebrate feeding systems. In J. M. Starck & T. Wang (Eds.), Physiological and ecological adaptations to feeding in vertebrates (pp. 1–41). Science Publishers.
Segall, M., Cornette, R., Fabre, A.-C., Godoy-Diana, R., & Herrel, A. (2016). Does aquatic foraging impact head shape evolution in snakes? Proceedings of the Royal Society of London. Series B, 283, 20161645.
Segall, M., Herrel, A., & Godoy-Diana, R. (2019). Hydrodynamics of frontal striking in aquatic snakes: Drag, added mass, and the possible consequences for prey capture success. Bioinspiration & Biomimetics, 14, 1–9.
Segall, M., Cornette, R., Godoy-Diana, R., & Herrel, A. (2020). Exploring the functional meaning of head shape disparity in aquatic snakes. Ecology and Evolution, 10(14), 6993–6700.
Sharma, B. K., Kulshreshtha, S., & Rahmani, A. R. (2013). Faunal heritage of Rajasthan, India: Conservation and management of vertebrates (Vol. 2). Springer.
Shealer, D. A. (2002). Foraging behavior and food of seabirds. In E. A. Schreiber & J. Burger (Eds.), Biology of marine birds (pp. 137–177). CRC Press.
Sheil, C. A., & Greenbaum, E. (2005). Reconsideration of skeletal development of Chelydra serpentina (Reptilia: Testudinata: Chelydridae): Evidence for intraspecific variation. Journal of Zoology, 265, 235–267.
Shine, R., Bonnet, X., Elphick, M., & Barrott, E. (2004). A novel foraging mode in snakes: Browsing by the sea snake Emydocephalus annulatus (Serpentes, Hydrophiidae). Functional Ecology, 18, 16–24.
Shortt, W. H. O. (1921). A few hints on crocodile shooting. Journal, Bombay Natural History Society, 29, 77.
Singh, L. A. K. (1976). Rearing gharial in captivity. Hamadryad, 1(2), 5–6.
Singh, A. (2015). Save the gharials. Science India, 18, 33–35.
Sivak, J. G. (1980). Avian mechanisms for vision in air and water. Trends in Neurosciences, 3(12), 314–317. https://doi.org/10.1016/S0166-2236(80)80182-2
Smith, K. K. (1982). An electromyographic study of the function of the jaw adducting muscles in Varanus exanthematicus (Varanidae). Journal of Morphology, 173, 137–158.
Smith, K. K. (1986). Morphology and function of the tongue and hyoid apparatus in Varanus (Varanidae, Lacertilia). Journal of Morphology, 187, 261–287.
Smith, S. A., & Paselk, R. A. (1986). Olfactory sensitivity of the turkey vulture (Cathartes aura) to three carrion-associated odorants. Auk, 103, 586–592.
Smith, T. L., Povel, G. D. E., & Kardong, K. V. (2002). Predatory strike of the tentacled snake (Erpeton tentaculatum). Journal of Zoology, 256, 233–242.
Soares, D. (2002). An ancient sensory organ in crocodilians. Nature, 417(6886), 241–242.
Soares, D., & Bierman, H. (2018). Crocodilian sensory systems. In J. Vonk & T. Shackelford (Eds.), Encyclopedia of animal cognition and behavior (pp. 1–6). Springer International Publ.
Sokol, O. M. (1969). Feeding in the pipid frog Hymenochirus boettgeri (Tornier). Herpetologica, 25(1), 9–24.
Spindel, E. L., Dobie, J. L., & Buxton, D. F. (1987). Functional mechanism and histological composition of the lingual appendage in the Alligator Snapping Turtle, Macroclemys temminckii (Troost) (Testudines: Chelydridae). Journal of Morphology, 194, 287–301.
St. Augustine Alligator Farm Zoological Park. (2020). Crocodilians Swallowing Underwater [Online]. Accessed December 21, 2020, from https://www.alligatorfarm.com/blog-item/crocodilians-swallowing-underwater/
Stubbs, T. L., Pierce, S. E., Rayfield, E. J., & Anderson, P. S. L. (2013). Morphological and biomechanical disparity of crocodile-line archosaurs following the end-Triassic extinction. Proceedings of the Royal Society B, 280(1770), 20131940.
Swennen, C. K., & Yu, Y.-T. (2005). Food and feeding behavior of the black-faced spoonbill. Waterbirds, 28, 19–27.
Takahashi, A., Dunn, M., Trathan, P., Croxall, J., Wilson, R. P., Sato, K., & Naito, Y. (2004). Krill-feeding behaviour in a chinstrap penguin compared to fish-eating in Magellanic penguins: a pilot study. Marine Ornithology, 32, 47–54.
Taylor, M. A. (1987). How tetrapods feed in water: A functional analysis by paradigm. Zoological Journal of the Linnean Society London, 91(2), 171–195.
Thorbjarnarson, J. B. (1990). Notes on the feeding behavior of the gharial (Gavialis gangeticus) under semi-natural conditions. Journal of Herpetology, 24(1), 99–100.
Tomkins, I. T. (1951). Method of feeding of the black skimmer, Rynchops nigra. Auk, 68, 236–239.
Townsend, C. W. (1909). The use of the wings and feet by diving birds. Auk, 26, 234–248.
Trutnau, L., & Sommerlad, R. (2006). Crocodilians: Their natural history and captive husbandry. Edition Chimaira.
Tucker, D. (1971). Nonolfactory responses from the nasal cavity: Jacobson’s organ and the trigeminal system. In L. M. Beidler (Ed.), Handbook of sensory physiology, Vol. IV, Chemical senses, Part 1, Olfaction (pp. 151–181). Springer.
Turner, C. H., Wang, T., & Burr, D. B. (2001). Shear strength and fatigue properties of human cortical bone determined from pure shear tests. Calcified Tissue International, 69(6), 373–378.
Uetz, P. (2010). The original descriptions of reptiles. Zootaxa, 2334, 59–68.
Underwood, G. (1970). The eye. In C. Gans (Ed.), Biology of the Reptilia (Vol. 2, pp. 1–79). Academic.
Van Damme, J., & Aerts, P. (1997). Kinematics and functional morphology of aquatic feeding in Australian snake-necked turtles (Pleurodira; Chelodina). Journal of Morphology, 233, 113–125.
Van Der Kooij, J., & Povel, D. (1996). Scale sensillae of the file snake (Serpentes: Acrochordidae) and some other aquatic and burrowing snakes. Netherlands Journal of Zoology, 47, 443–456.
Van Gils, J. A., Piersma, T., Dekinga, A., & Dietz, M. W. (2003). Cost-benefit analysis of mollusc-eating in a shorebird II. Optimizing gizzard size in the face of seasonal demands. The Journal of Experimental Biology, 206, 3369–3380.
Van Wassenbergh, S., Brecko, J., Aerts, P., Stouten, I., Vanheusden, G., Camps, A., Van Damme, R., & Herrel, A. (2009). Hydrodynamic constraints on prey-capture performance in forward-striking snakes. Journal of the Royal Society Interface, 7, 773–785.
Verheyden, C., & Jouventin, P. (1994). Olfactory behavior of foraging procellariiforms. Auk, 111, 285–291.
Vincent, S. E., Herrel, A., & Irschick, D. J. (2005). Comparisons of aquatic versus terrestrial predatory strikes in the pitviper, Agkistrodon piscivorus. Journal of Experimental Zoology Part A, 303, 476–488.
Vincent, S. E., Brandley, M., Herrel, A., & Alfaro, M. (2009). Convergence in trophic morphology and feeding performance among piscivorous natricine snakes. Journal of Evolutionary Biology, 22, 1203–1211.
von Bolze, G. (1968). Anordnung und Bau der herbstschen Körperchen in Limicolenschnabeln im Zusammenhang mit Nahrungsfindung. Zoologischer Anzeiger, 181, 313–355.
von Huene, F. (1933). Ein Versuch zur Stammesgeschichte der Krokodile. Centralblatt für Mineralogie, Geologie und Paläontologie, Abteilung B, 11, 577–585.
Voris, H. K., & Voris, H. H. (1983). Feeding strategies in marine snakes: An analysis of evolutionary, morphological, behavioral and ecological relationships. American Zoologist, 23, 411–425.
Vorobyev, M. (2003). Coloured oil droplets enhance colour discrimination. Proceedings of the Royal Society of London Series B, 270, 1255–1261.
Wainwright, P. C., & Price, S. A. (2016). The impact of organismal innovation on functional and ecological diversification. Integrative and Comparative Biology, 56(3), 479–488.
Walmsley, C. W., Smits, P. D., Quayle, M. R., McCurry, M. R., Richards, H. S., Oldfield, C. C., Wroe, S., Clausen, P. D., & McHenry, C. R. (2013). Why the long face? The mechanics of mandibular symphysis proportions in crocodiles. PLoS One, 8, e53873.
Webb, G., & Manolis, C. (1989). Crocodiles of Australia. Reed.
Westhoff, G., Fry, B. G., & Bleckmann, H. (2005). Sea snakes (Lapemis curtus) are sensitive to low-amplitude water motions. Zoology, 108, 195–200.
Wever, E. G., Herman, P. N., Simmons, J. A., & Hertzler, D. R. (1969). Hearing in the blackfooted penguin, Spheniscus demersus, as represented by the cochlear potentials. Proceedings of the National Academy of Sciences of the United States of America, 63, 676–680.
Whitaker, R., & Basu, D. (1982). The gharial (Gavialis gangeticus): A review. Journal Bombay Natural History Society, 79(3), 531–548.
Wikelski, M., & Trillmich, F. (1994). Foraging strategies of the Galapagos marine iguana (Amblyrhynchus cristatus): Adapting behavioral rules to ontogenetic size change. Behaviour, 128, 255–279.
Wilberg, E. W., Turner, A. H., & Brochu, C. A. (2019). Evolutionary structure and timing of major habitat shifts in Crocodylomorpha. Scientific Reports, 9(1), 1–10.
Wilga, C. D., & Sanford, C. P. (2008). Suction generation in white-spotted bamboo sharks Chiloscyllium plagiosum. The Journal of Experimental Biology, 211, 3128–3138.
Wilkie, E. S., Vissers, P. M., Das, D., DeGrip, J. W., Bowmaker, K. J., & Hunt, M. D. (1998). The molecular basis for UV vision in birds: Spectral characteristics, cDNA sequence and retinal localization of the UV-sensitive visual pigment of the budgerigar (Melopsittacus undulatus). The Biochemical Journal, 330, 541–547.
Wise, S. C., Formanovic, D. R., & Brodie, E. D. (1989). Matamata turtles ambush but do not herd prey. Journal of Herpetology, 23, 297–299.
Wroe, S., & Milne, N. (2007). Convergence and remarkably consistent constraint in the evolution of carnivore skull shape. Evolution, 61(5), 1251–1260.
Young, B. (1991). The influences of the aquatic medium on the prey capture system of snakes. Journal of Natural History, 25, 519–531.
Young, M. T., Brusatte, S. L., Ruta, M., & de Andrade, M. B. (2010). The evolution of Metriorhynchoidea (Mesoeucrocodylia, Thalattosuchia): an integrated approach using geometric morphometrics, analysis of disparity, and biomechanics. Zoological Journal of the Linnean Society, 158(4), 801–859.
Zeigler, H. P., & Bischof, H.-J. (1993). Vision, brain, and behavior in birds. MIT Press.
Ziegler, T., Quyet, L. K., Thanh, V. N., Hendrix, R., & Böhme, W. (2008). A comparative study of crocodile lizards (Shinisaurus crocodilurus AHL, 1930) from Vietnam and China. The Raffles Bulletin of Zoology, 56, 181–187.
Zweers, G., & Wouterlood, F. (1973). Functional anatomy of the feeding apparatus of the mallard (Anas platyrhynchos L.). In Proceedings of the Third European Anatomical Congress. Manchester (pp. 88–89).
Zweers, G., Gerritsen, A. C., & van Kranenburg-Voogd, P. (1977). Mechanics of feeding of the mallard (Anas playrhyncos L., Aves, Anseriformes). Contributions to Vertebrate Evolution, 3, 1–109.
Acknowledgements
E.H. thanks R. Mac Wheeler, D. Hensley, M. Mulder, S. Van Wassenbergh and J. Brecko for providing the material used in Figs. 7.2, 7.3, 7.4, 7.5 and 7.6. P.M.G. and L.B.P. thank A. Resetar, P. Makovicky, and the Field Museum of Natural History for collections access. They also thank H. O’Brien, G. Erickson, K. Vliet, G. Webb, C. Ross, C. Holliday, K. Sellers, E. Rayfield, C. Brochu, A. Turner, J. Brueggen, E. Wilberg, A. Watanabe, S. Steppan and A. Lappin for more than a decade of thoughtful feedback about the evolution of crocodyliform feeding biomechanics. Support was provided to P.M.G. by the Department of Anatomy and Cell Biology at the Oklahoma State University Center for Health Sciences and the National Science Foundation (DEB 1754659).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Heiss, E., Gignac, P.M., Porro, L.B., Lemell, P. (2023). Convergence of Aquatic Feeding Modes in the Sauropsida (Crocodiles, Birds, Lizards, Snakes and, Turtles). In: Bels, V.L., Russell, A.P. (eds) Convergent Evolution. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-031-11441-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-11441-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-11440-3
Online ISBN: 978-3-031-11441-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)