Abstract
To resist hydrodynamic forces, two main underwater attachment strategies have evolved multiple times in aquatic animals: glue-like “bioadhesive secretions” and pressure-driven “suction attachment”. In this chapter, we use a multi-level approach to highlight convergence in underwater attachment mechanisms across four different length-scales (organism, organ, microscopic and molecular). At the organism level, the ability to attach may serve a variety of functions, the most important being: (i) positional maintenance, (ii) locomotion, (iii) feeding, (iv) building, and (v) defense. Aquatic species that use bioadhesive secretions have been identified in 28 metazoan phyla out of the 34 currently described, while suction organs have a more restricted distribution and have been identified in five phyla. Although biological adhesives are highly diverse, it is possible to categorize them into four main types according to the time scale of operation: permanent, temporary, transitory, and instantaneous adhesion. At the organ level some common principles have independently evolved in different biological lineages: for example, animals with single-unit attachment organs can be distinguished from those with multi-unit organs. Fundamental design elements can also be recognized for both types of attachment mechanisms. Suction attachment systems comprise a circular or elliptical attachment disc, a sealing rim to prevent leakage and a mechanism to lower the internal pressure. Bioadhesive-producing organs, on the other hand, usually contain a glandular tissue associated with connective tissues or other types of load-bearing support structures and muscles that facilitate locomotion or mechanical detachment. At the microscopic level, similar designs and organizations appear once again to have emerged independently in different phylogenetic lineages. Independent of the taxon and type of adhesion, there are species in which the biosynthesis, packaging and release of adhesive secretions takes place at the level of a single type of secretory cell, whereas in others these secretions are produced by two or more secretory cell types. Duo-gland adhesive systems involved in temporary adhesion present an additional level of complexity as they also exhibit de-adhesive secretory cells. Yet, strikingly similar cellular organizations have been reported in highly disparate species. In the case of biological suction organs, regions of the organ that contact the substratum are highly textured with stiff microstructures. Although clearly non-homologous in different animals, these microstructures are thought to enhance friction on rough surfaces. At the molecular level, proteins are the main organic constituent of adhesive secretions in aquatic animals. We compared the global amino acid compositions of bioadhesives using principal component analysis to show that homologous adhesives from phylogenetically related species cluster together, and there is little overlap between taxonomic groups. However, several non-permanent adhesives are grouped together even though they belong to disparate phyla, indicating convergence in amino acid composition. We also investigated relatedness among individual adhesive proteins using a sequence similarity-based clustering analysis. While many proteins appear taxon-specific, some have clear sequence homologies based on shared protein domains between phylogenetically distant organisms. However, it is highly probable that these domains, which are also present in many non-adhesive proteins, were convergently acquired from ancestral proteins with unrelated general functions. We herein present morphological, structural, and molecular convergences between different attachment mechanisms in aquatic animals that likely arose in response to shared functional and selective pressures.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
16.1 Introduction
The physical environment in our oceans and freshwater systems is drastically different from that on land (Ditsche & Summers, 2014). Terrestrial organisms must contend with gravity on a daily basis, and it is easy to feel the direct consequence of gravity when we lift heavy objects or do a pull-up for exercise. Conversely, due to the low density of our atmosphere, we are able to walk and run without undue effort, unless confronted by extreme conditions such as severe storms. The density of water, on the other hand, denies gravity the power to hold aquatic animals to the bottom, and buoyancy forces need to be balanced to control sinking or floating. In addition, in aquatic environments, forces exerted by flowing water (hydrodynamic forces) can be strong and directionally unpredictable, requiring specific mechanisms, sturdy in all directions, to counteract them. Indeed, many aquatic animals lack grasping limbs to grip onto solid objects. To survive in such conditions animals from multiple phyla have evolved the ability to attach to various substrates underwater, often using specialized appendages or regions of their bodies called attachment organs (Gorb, 2008; Ditsche & Summers, 2014). Such adaptations allow animals to stick to substrates in order to move (e.g., limpets, sea stars, and octopuses), to maintain position (e.g., barnacles, mussels, and remora fish), to feed (e.g., cephalopods and comb jellies), or to build shelter (e.g., sandcastle worms and caddisfly larvae).
Interestingly, despite the diversity in the morphology and function of metazoan attachment organs (Nachtigall, 1974; Gorb, 2008), aquatic animals from multiple phyla mainly rely on two strategies for underwater attachment: either glue-like ‘bioadhesive secretions’ or pressure-driven ‘suction attachment.’ Bioadhesive secretions are complex mixtures of proteins, sugars and lipids and are most often used for attaching an organism to a non-living surface, including dynamic attachment during locomotion and permanent fixation (Nachtigall, 1974; Hennebert et al., 2015; Davey et al., 2021). Some well-known examples of aquatic animals that use bioadhesive secretions are echinoderms (e.g., sea stars, sea urchins), barnacles, and mussels. Conversely, suction requires muscular contraction for the generation of the pressure difference required for attachment (although glandular secretions can help with sealing) and is strictly used for temporary attachment (Nachtigall, 1974). Animals that rely on suction include cephalopods (e.g., octopuses and squids), numerous fishes (e.g., remora fish, clingfish, gobies), and insects (e.g., net-winged midge larvae and diving beetles).
As with any effort to categorize and characterize form and function in biology, there will be exceptions that are not adequately captured by these two mechanisms. Moreover, it is at times difficult to distinguish the two strategies: sea star and sea urchin tube feet were long considered to be suction organs before it was shown that they rely solely on adhesive secretions for attachment (Hennebert et al., 2012). Finally, an organism may use both suction and adhesive attachments (e.g., lottiid limpets; see Sect. 16.3). Nevertheless, suction attachment and bioadhesive secretion represent the two most common approaches to biological attachment in wet environments (Ditsche & Summers, 2014). It is likely that the diverse aquatic animals that employ these approaches have repeatedly arrived at similar forms and strategies in response to overlapping physical conditions and demands. In other words, there appear to be many examples of convergence in the attachment strategies of aquatic organisms. How widespread is evolutionary convergence in suction attachment organs or bioadhesive-secreting organs? What additional insights can we gain from identifying characteristics that have repeatedly emerged in unrelated taxa? These are some of the questions that are explored in this chapter. As pointed out by Tyler (1988), convergence should be reflected in a lack of correspondence in the functional hierarchy of components. Hence, we use a multi-scale approach to highlight convergence in underwater attachment mechanisms at four different length-scales: (1) individual organisms, (2) organs, (3) cells and microscopic structures, (4) molecules (Fig. 16.1). At the largest length-scale it is expected that two morphologically similar structures, either homologous or analogous, are also functionally similar. However, differences between both structures in the way functions are performed at lesser length-scales are indications that the probability of convergence between them is high. The adhesive organs of many interstitial invertebrates, for example, have the same general function, namely temporary maintenance of position on sand grains, but the finer levels of the functional hierarchy are performed by different molecular components (Tyler, 1988).
Example of an intertidal community illustrating the multi-scale approach used in the present review. (a) At the macroscopic scale, many organisms inhabiting this hydrodynamically stressful environment rely on different attachment mechanisms for maintenance of position as well as for various other functions. Sea stars, for example, use temporary adhesion to the substratum for static sustained attachment to withstand the action of waves, for dynamic attachment during locomotion, or to grip and pry open the mussels on which they feed. (b) At the centi−/milliscopic scale their adhesion relies on a multitude of small appendages, the tube feet, each acting as an individual tether connecting the animal to the substratum. (continued overleaf) (c) At the microscopic scale the tube foot epidermis encloses a duo-gland adhesive system comprising two types of adhesive cells (in grey) that co-secrete the adhesive layer joining the tube foot to the substratum, and de-adhesive cells (in black) that produce a releasing secretion that allows detachment. (d) At the nanoscopic scale the adhesive material consists of a mixture of proteins and glycoproteins, some of which are involved in interfacial adhesive interactions and others in bulk cohesive function
16.2 An Organism-Level Approach to Attachment Mechanisms in Aquatic Organisms
An impressive diversity of aquatic organisms uses attachment mechanisms at one or more stages of their life cycle. Of the most current list of metazoan phyla (34 according to Giribet & Edgecombe, 2020), the vast majority contain species that attach using suction organs, bioadhesive secretions, or both (Fig. 16.2). Species that use bioadhesive secretions, or are strongly suspected to do so, have been identified in 29 phyla (28 if only aquatic organisms are considered), whereas suction organs have a more restricted distribution (at least if we are only considering this at the phylum level) and have been identified in five phyla (Craniata, Arthropoda, Platyhelminthes, Mollusca and Annelida). The widespread distribution of bioadhesive secretions within the animal kingdom may erroneously suggest that all metazoan adhesive organs are homologous; however, homology cannot be inferred from the simple presence/absence of an adhesive system. Instead, a detailed analysis of adhesive systems is needed to understand if and how they are interrelated. Moreover, the high proportion of taxa using bioadhesives at the phylum level can be explained, in part, by the fact that this attachment mechanism was considered to be present even when it has only been described for a very limited subset of species in the phylum. It may not be reflected, therefore, at lower taxonomic levels, indicating several independent evolutionary events. As a representative example, most chaetognaths are pelagic (i.e., free-swimming organisms never relying on attachment mechanisms), but a few species are benthic and can adhere to surfaces (John, 1933; Feigenbaum, 1976). Moreover, although suction organs (also commonly referred to as suckers) are predominantly found in aquatic animals (including parasites living in the fluids of other animals), adhesive secretions are generated by both aquatic and terrestrial animals. For the sake of completeness, terrestrial organisms producing adhesive secretions were included in the total count of phyla. Terrestrial animals often use adhesives for prey capture or defense (e.g., spiders and velvet worms), although some use adhesive secretions for locomotion (e.g., snails and slugs) (Hennebert et al., 2015).
Phylogenetic distribution of metazoans that use suction organs or bioadhesive secretions. The metazoan phylogenetic tree is based on Giribet and Edgecombe (2020). Biological functions associated with the attachment strategies are highlighted at the phylum-level. The names of taxa are shown in grey when no adhesion system is known; in black when adhesion systems are known and in bold when they have been studied to some extent (see Supplementary Table 16.1 for details). The species icons represent the taxa for which molecular data are available and which have been used in the molecular analyses that follow
In metazoans, attachment mechanisms may serve a variety of functions, the most important being: (1) position maintenance, (2) locomotion, (3) feeding, (4) building shelter, and (5) defense (Fig. 16.2; Nachtigall, 1974; Hennebert et al., 2015). As expected, trying to define a strict terminology for the biological functions associated with attachment mechanisms is educatively useful but potentially problematic because these functions may be ecologically interconnected (e.g., a shelter can also be used to carry out feeding and for defense). As mentioned in the introduction, aquatic animals must resist hydrodynamic stresses and, therefore, many benthic species rely on bioadhesives or suction organs to attach to non-living surfaces or to other organisms to maintain their position. Depending on the biology of the species, this attachment may be long- or short-term. Dynamic, short-term attachment also allows for locomotion in turbulent environments. For some species, attachment mechanisms (i.e., bioadhesives or suction organs) also allow prey capture and, in the case of bioadhesives, the collection of food particles from the water column or from the bottom. Many filter feeding organisms that rely on adhesive mucus to trap particles therefore fall into this latter food collection category (e.g., some cnidarians, molluscs, annelids or brachiopods). The latter two functions, building and defense, only concern bioadhesives. Building involves the gluing of exogenous materials together for the construction of tubes, nests or burrows (e.g., sandcastle worms, caddisfly larvae, three-spined sticklebacks), and defense pertains to the release of a sticky material as a protective reaction against predators (e.g., sea cucumbers, centipedes, salamanders) (von Byern et al., 2017).
Although the diversity of biological adhesives is vast in terms of components, interactions and functions, some common principles have evolved independently in different biological lineages. In aquatic organisms, biological adhesives can be grouped together into four main types according to the time scale of operation: permanent, temporary, transitory and instantaneous adhesion (Fig. 16.2; Flammang, 1996; Whittington & Cribb, 2001; Flammang et al., 2005). Permanent adhesion, represented in 12 phyla, involves the secretion of a bioadhesive that hardens with time and forms a durable cement. As observed in barnacles, for example, this type of adhesion is seen in a number of phyla that include sessile benthic organisms that remain firmly fixed at the same place throughout their lifetime (e.g., Porifera, Cnidaria, Tunicata, Arthropoda, Mollusca, Bryozoa; Fig. 16.2). By definition, permanent adhesion is used exclusively for position maintenance or building. Temporary and transitory adhesion both correspond to a non-permanent type of adhesion and permit, for example, the combination of adhesion and locomotion at the same time, thus allowing adult organisms to graze, hunt or search for a mate, and larval forms to explore surfaces prior to metamorphosis. Transitory adhesion is used by many benthic and vagile organisms that creep on the substratum. It allows simultaneous adhesion and movement along a substratum, whereby the animals attach using a thin layer of secretion that is often left behind them as they move. This type of adhesion is characteristic of invertebrates that move along the substratum by ciliary gliding—mostly small soft-bodied invertebrates from the phyla Platyhelminthes, Nemertea, Gastrotricha and Annelida (Martin, 1978a, b). Larger animals, like sea anemones and gastropod molluscs, also use transitory adhesion, moving by means of waves of muscular contractions running along their attachment organ (the pedal disc in sea anemones, and the foot in gastropods) (Jones & Trueman, 1970; Edmunds et al., 1976). In limpets (Phylum Mollusca), the term transitory adhesion has recently been redefined to describe the regular switching between long-term and locomotory adhesion (Kang et al., 2020). By analogy to ciliary gliding, food collection using muco-ciliary systems is also classified as transitory adhesion. Considering both locomotion and feeding, transitory adhesion is represented in 13 phyla (Fig. 16.2). Temporary adhesion, on the other hand, is used by organisms such as sea stars and sea urchins (Echinodermata) that are able to adhere firmly yet temporarily to the substratum, allowing them to repeatedly attach and detach. This type of adhesion is also frequently found in small invertebrates that inhabit the interstitial environment, such as various species of Platyhelminthes, Gastrotricha, Nematoda and Annelida (Tyler, 1988; Lengerer & Ladurner, 2018). These animals use bioadhesives to temporarily secure themselves to the sand grains of marine or freshwater beaches to avoid dislodgement. Some echinoderms and mollusks also rely on temporary adhesion to capture their food and release it into the mouth. Overall, this type of adhesion occurs in 11 phyla (Fig. 16.2). Instantaneous adhesion, finally, describes a type of adhesion whereby the adhesive is rapidly discharged from single-use adhesive organs or glands and is immediately sticky. In aquatic animals, this type of adhesion is only seen in ctenophores during prey capture and in sea cucumbers through the release of Cuvierian tubules as a defense mechanism. Many bioadhesives produced by terrestrial animals for defense or prey capture satisfy the definition of instantaneous adhesives, even though they are not released by single-use organs or cells as seen in sea cucumbers. Examples of terrestrial species that use this type of adhesive can be found in four phyla (Fig. 16.2): Craniata (frogs and salamanders), Mollusca (slugs), Arthropoda (insects, centipedes, spiders), and Onychophora (velvet worms).
The fundamental design elements of a biological suction attachment system are a circular or elliptical attachment disc, a sealing rim to prevent leakage, and a mechanism to enable the lowering of the internal pressure. Using these parameters to identify suction organs it is clear that suction attachment has evolved independently in highly disparate branches of the tree of life (Fig. 16.2). It is important to bear in mind, however, that while morphological similarities are useful for an initial assessment of whether an attachment organ may be a suction organ, mechanistic studies are required to confirm that reduced pressure gives rise to the attachment force. It will become apparent that of the multiple species mentioned in this chapter as possessing suction attachment organs, only a few have fully satisfied this requirement, which highlights significant opportunities for future research. Examples of suction organs can be found in numerous species across five phyla (Annelida, Arthropoda, Craniata, Mollusca, and Platyhelminthes; Fig. 16.2 and Supplementary Table 16.1).
16.3 An Organ-Level Approach to Attachment Mechanisms in Aquatic Organisms (Macroscopic)
At the organ level, animals with single-unit attachment organs can be easily distinguished from those with multi-unit organs, irrespective of whether the organs rely on bioadhesives or suction attachment (Fig. 16.3). For example, whereas limpets and barnacles have evolved a single attachment pad, sea urchins, sea stars and mussels rely on multiple-point attachments. One might think these two distinct structural strategies are function-related (Nachtigall, 1974) but this is unlikely because both single- and multi-unit attachments may be used exclusively for anchoring (e.g., barnacle and mussel permanent adhesion) or cumulatively for anchoring, locomotion and feeding (e.g., limpet transitory adhesion and sea urchin/sea star temporary adhesion) (Fig. 16.3).
Diversity of attachment organs in aquatic organisms. These organs may be distinguished based on the mechanism of attachment (columns: adhesive secretion versus suction) and the number of attachment points (rows: single versus multiple), as exemplified by four generalized organisms: (a) limpets, (b) fishes, (c) sea urchins, and (d) octopuses. In each case, a lateral view (left) and a ventral/oral view (right) are represented with the zone(s) of contact with the substratum highlighted in red
Limpets (Mollusca) are intertidal inhabitants that attach to the surface of rocks using a muscular pedal sole (Fig. 16.3a). The exact mechanism of attachment appears to differ between members of the families Patellidae (true limpets) and Lottiidae. Several studies have demonstrated that lottiid limpets alternate between suction attachment at high tide (when they are actively moving around to feed) and adhesive mucus secretion at low tide (when they are exposed to the environment and to predators and require more powerful, long-term attachment) (Smith, 1991a, 1992; Smith et al., 1993, 1999). Although patellid limpets also inhabit the intertidal zone and respond to the tide, they primarily rely on adhesive mucus secretions for attachment (Kang et al., 2020). Single attachment organs are also found in other common inhabitants of the intertidal zone, such as acorn and stalked barnacles (Arthropoda), which live their adult life permanently anchored to the substratum, and sea anemones (Cnidaria), some of which use transitory adhesion (Cowles, 1977; Young et al., 1988; Clarke et al., 2020).
Sea urchins and sea stars (Echinodermata), meanwhile, attach using multiple specialized adhesive organs called tube feet (Figs. 16.1 and 16.3b) (Nichols, 1966). Tube foot attachment is temporary, allowing strong attachment to the substratum and easy detachment before the initiation of another attachment–detachment cycle (Thomas & Hermans, 1985; Flammang, 1996; Flammang et al., 2016; Federle & Labonte, 2019). Most tube feet consist of a basal hollow cylinder (the stem) and an enlarged and flattened apical extremity (the disc) that work together to make tube feet efficient and versatile, allowing echinoderms to resist hydrodynamic forces and to perform tasks such as climbing, righting, covering their bodies with objects, or opening mollusk shells (Lawrence, 1987; Flammang et al., 2016). Another example of multi-point attachment occurs in mussels (Mollusca) that permanently anchor to rocks using multiple thread-like tethers, collectively called byssus. Each thread contains three parts: a spatulate adhesive plaque, a stiff distal portion and a compliant proximal portion (Waite, 2017).
Within the organisms that possess multiple adhesive organs there is high variability in the number of organs and the adhesive contact areas of each organ. Even within the same taxonomic group (e.g., Echinoidea, the sea urchins) there are species that can increase their maximum adhesive surface area by increasing the number of adhesive organs (e.g., 0.8 tube feet/mm2 of test area in Colobocentrotus atratus versus 0.2 tube feet/mm2 in Arbacia lixula) or by increasing the contact area of each adhesive organ (e.g., 1.16 mm2 tube foot disc area in A. lixula versus 0.81 mm2 tube foot disc area in C. atratus) (Santos & Flammang, 2006, 2007, 2008). Moreover, unlike single adhesive organs, the strength of multi-component adhesive organs is the product of the number and mechanical properties of the individual tethers. This allows animals using multiple attachment points to adjust the number of tethers they use according to the environmental conditions. Sea urchins, for example, appear to respond to increased wave height by dedicating more tube feet to attachment, thereby increasing the overall attachment force (Santos & Flammang, 2007).
Bioadhesive-producing organs usually contain (or are associated with) connective tissues or other types of load-bearing support structures and muscles that facilitate locomotion or mechanical detachment. For example, support structures, such as ossicles and a circular plate of connective tissue within the adhesive discs of echinoderm tube feet, help to withstand the tensile forces that result from external loading. This connective tissue plate, at its proximal end, is continuous with the connective tissue sheath of the stem, and at its distal end divides into numerous branching connective tissue septa that attach apically to the support cells of the epidermis (Flammang et al., 2016). Additionally, these organs possess retractor muscles that might facilitate detachment, thereby complementing the action of a de-adhesive secretion (discussed in Sect. 16.4) (Lengerer & Ladurner, 2018). Because a chain is only as strong as its weakest link, there should be a good balance between the adhesive strength developed by the secretion and the mechanical properties of the load-bearing parts of the adhesive organ. However, other factors may play a role. In sea urchins, the force needed to break the stem (the proximal part of the tube foot linked to the animal) is greater than that needed to detach the distal disc (the distal part attached to the substratum). This can be explained by the fact that if the disc detaches from the substratum it can easily re-attach as re-attachment requires only a fresh adhesive secretion; if the stem breaks, however, the tube foot must be completely regenerated (Santos & Flammang, 2005, 2006, 2008). In mussels, byssal threads converge to a structure, also called the stem, which is contiguous with the byssal retractor muscles (within the body of the mussel) used to control thread tension (Waite, 1992; Sagert et al., 2006). The weakest link of the byssus is typically the proximal region of the thread (the part linked to the animal) or the adhesive plaque (the part attached to the substrate) (Bell & Gosline, 1996; Carrington et al., 2015). Therefore, multi-component adhesive organs or structures, although clearly not homologous, might be similarly designed so as to balance energy costs against over-engineered material properties.
Muscles and structural parts can also play a significant role in single adhesive organs, regardless of the type of adhesion involved. In barnacles (Arthropoda, Crustacea) the retractor muscle pulls the peripheral shell plate downward at the time the permanent cement is secreted, thereby improving adhesion (Kamino, 2016). In limpets, the contraction of the powerful foot muscle clamps the shell against the substratum, playing an important role in the adhesion mechanism because friction generated by this behavior resists dislodgement by shear forces (Ellem et al., 2002). Meanwhile, in reversibly attaching animals lacking a duo-gland system, detachment is mostly achieved through mechanical forces (Lengerer & Ladurner, 2018) (see also Sect. 16.4). In Hydra and sea anemones (Cnidaria) release is induced by muscular contractions in the basal disc (Rodrigues et al., 2016a). Some cephalopods (Mollusca), such as Idiosepius, Euprymna and Sepia, seem to detach as a result of dermal muscle contraction (von Byern & Klepal, 2006).
If we shift our focus to biological suction attachments, it is also evident that suction organs have evolved multiple times. This speaks to the utility of the organ for carrying out a variety of biological functions, from maintaining a position against strong hydrodynamic forces to facilitating locomotion, feeding, and reproduction. Whenever animals with suction organs are discussed, perhaps the most recognizable, and one of the most well-studied examples is the octopus (Fig. 16.3d). Octopuses use numerous suckers on their arms to catch prey, manipulate objects, locomote, and maintain position. (Although octopod suckers also serve as mechano- and chemo-sensors, we here focus on their role in attachment.) Decapods, such as squids and cuttlefish, are related to octopuses and they also possess suckers that serve similar functions as octopod suckers; hence, we refer to them collectively as coleoid suckers. Suckers present on the arms of octopods and decapods are superficially similar, whereas some tentacular suckers of decapods may also possess large hooks and spines for piercing prey (Nixon & Dilly, 1977). In general, coleoid suckers are circular in ventral view, with a rim for sealing, a central opening, and musculature that helps lower the pressure within the cavity enclosed between the sucker and the substrate (Kier & Smith, 1990, 2002; Smith, 1991b). (Microstructures present on the sucker surface are explored in Sect. 16.4.)
Among annelids, leeches (e.g., Placobdella parasitica and Hirudo verbana) use suction organs (one at the anterior and another at the posterior end) for locomotion and maintenance of position. Leech suction organs have muscles for raising the central region of the attachment disc and both in vivo pressure recordings and attachment performance measurements have confirmed that both reduced pressures and proper sealing are important for attachment (Gradwell, 1972a; Kampowski et al., 2016). In arthropods, suction organs are found in two disparate families: net-winged midges (Blephariceridae) and diving beetles (Dytiscidae). Blepharicerid larvae are found in fast-flowing alpine streams and each larva uses six specialized suction organs to attach to rock surfaces (Rietschel, 1961; Kang et al., 2019, 2021). These suction organs bear a striking resemblance to coleoid suckers, with a circular attachment disc, a sealing rim, and a central piston controlled by muscles that lower the pressure upon retraction. In dytiscid beetles the males alone carry numerous suckers on their prolegs and these are primarily used for holding onto females during courtship and copulation (Aiken and Khan 1992; Karlsson et al., 2013; Chen et al., 2014). There are no muscles within the individual suckers and it is thought that suction attachment is afforded passively through a combination of stored elastic energy and larger movements of the leg and body.
An impressive variety of suction organs has evolved in the Craniata. Many fishes and amphibian larvae use suction attachment for locomotion and maintenance of position. In fish, ventral suckers have been developed through modifications of the pelvic fins and pelvic girdle, pectoral fins and pectoral girdle, or the periphery of the mouth (Arita, 1967; Lujan & Conway, 2015). These analogous structures thus appear to be derived from different organs illustrating multiple evolutionary convergences. Clingfishes (Gobiesocidae), lumpsuckers (Cyclopteridae; Fig. 16.3c), and snailfishes (Liparidae) use their suckers to maintain position, either against strong currents or crashing tidal waves (Arita, 1967; Budney & Hall, 2010). Clingfishes are one of the model species for the study of biological suction attachment, and several detailed investigations of their attachment performance to various substrates are available (Wainwright et al., 2013; Ditsche et al., 2014, 2017). Gobies (Gobiidae) have independently evolved ventral suction organs that are also derived from their pelvic fins (Budney & Hall, 2010). Some gobies use suction attachment (employing both oral and posterior suction organs) to climb waterfalls, which is a well-documented behavior that clearly demonstrates the adhesive power that can be generated by suckers (Schoenfuss & Blob, 2003; Maie et al., 2012). The remoras (Echeneidae) are another well-studied group of fishes that have a single large elliptical suction pad derived from a highly modified dorsal fin. The remoras use their suction pad to attach to many different hosts, including turtles, sharks, dolphins, whales, and other fishes. The morphology and function of these organs are explored in more detail in Sect. 16.4.
Although suction feeding is a common feeding strategy in fishes, many species living in fast-flowing waterways have modified the periphery of their mouthparts to facilitate suction attachment (Lujan & Conway, 2015). Species of the genus Garra (Cyprinidae), for example, inhabit sub-Himalayan mountain streams and use their suction organs for maintaining position (Das & Nag, 2006, 2009). Their suction organs are derived from modified lips and encircle the mouth, the lower lip being further modified into a structure called the callous pad, which appears to have retractor muscles that can reduce the pressure during attachments (Saxena & Chandy, 1966). It would be remiss to mention oral suckers without acknowledging lampreys (Petromyzontidae). Although there are no detailed studies of the mechanism of attachment, lampreys are capable of generating significantly reduced pressures within the oral hood (Gradwell, 1972b). While it is unclear whether lampreys possess a specialized sealing rim, the margin of their mouth is free of teeth and could function as a soft sealing rim. In addition, their numerous teeth may provide additional friction by piercing the skin of the host and anchoring the lamprey.
Before proceeding to the next phylum, there is one more aquatic taxon in the Craniata that uses suction attachment organs: frogs. The larvae of several families of frogs (Bufonidae, Ranidae, and Hylidae) possess oral and abdominal suction organs and are collectively referred to as gastromyzophorous tadpoles. While some species inhabit fast-flowing streams (e.g., Rhinella quechua, Huia cavitympanum, Atelopus sp.), hyliid tadpoles (e.g., Phyllodytes gyrinaethes) develop in bromeliads (Kaplan, 1997; Aguayo et al., 2009; Haad et al., 2014; Gan et al., 2016; Vera Candioti et al., 2017). The musculature beneath abdominal suckers suggests that the suckers can actively reduce the internal pressure, although further functional studies are required for verification. Species of Atelopus also possess protuberances on the posterior part of the abdominal suckers that may increase friction during attachment (Kaplan, 1997). Several other anuran genera have enlarged oral suckers (e.g., Litoria, Mixophyes, Ascaphus) that resemble the specialised oral suckers of fishes and can actively reduce the pressure during attachments (Gradwell, 1971, 1975).
In Mollusca, besides coleoids, lottiid limpets are capable of reducing the pressure beneath their muscular feet during attachment (Smith, 1991a). In contrast, patellid limpets do not produce as low sub-pedal pressures as lottiid limpets (approximately −0.6 kPa compared to −20 kPa, relative to ambient) (Jones & Trueman, 1970; Smith, 1991a; Kang et al., 2020). As mentioned in the introductory text, it can be difficult to clearly delineate between attachments that rely on suction and adhesive secretions, and thorough investigations using pressure recordings and molecular biological techniques are necessary for a more complete understanding of the underlying mechanism(s).
Although numerous tapeworms (Platyhelminthes) possess circular attachment structures that resemble suckers, additional studies are needed to verify whether they are able to reduce the pressure within the cavity. The anterior part of the tapeworm, the scolex, bears remarkably diverse structures ranging from suckers and hooks to hair-like structures called microtriches (de Chambrier & Scholz, 2008). Some authors refer to sucker-like structures as bothridia, but the distinction between suckers and bothridia is unclear. Suckers have longitudinal and radial muscles (Pospekhova & Bondarenko, 2014), and bothridia contain radial muscles and a single retractor muscle (Jones, 2000), but these differences in musculature do not appear to be used for categorisation. While both structures have been imaged with plugs of tissue within their cavities, it is currently unknown if the organs act as mechanical clamps or as suction organs by contracting their muscles to create pressure difference-based attachment (Andersen & Lysfjord, 1982; Borucinska & Caira, 1993; Ibraheem, 1998). Since we only have morphological data relating to tapeworm attachment, further work is needed to verify that tapeworm “suckers” or bothridia can indeed function as suction organs.
It is interesting to note that, like adhesive secretion organs, suction organs can be found as a single relatively large attachment unit (e.g., in remora fish, lottiid limpets, gastromyzophorous tadpoles, lampreys, and clingfish) or as a group of many relatively small suckers (e.g., in coleoids, blepharicerid larvae, diving beetles, and tapeworms). There are advantages and disadvantages for both strategies: in terms of benefits, having a single large attachment organ means that the same suction attachment force can be generated with a lower internal pressure, which demands less work from the muscles. On the other hand, a larger contact area increases the probability of encountering a random topography that interrupts the seal, thereby weakening the suction attachment or causing failure. In contrast, an attachment organ comprising many smaller suckers has the advantage that each unit is less likely to come into contact with a challenging surface feature, and even if one fails, there are numerous others to provide attachment. The disadvantage of multiple suckers is that if the total contact area is less than that of a single large organ (e.g., if the boundary is constrained to a circle, even the most optimal packing arrangement of smaller circles will result in ~20% loss of area), then each sucker must work harder to attain the same amount of total attachment force. Based on our current understanding, there does not appear to be a strong determinant for whether an organism uses a single or multiple suction attachments.
16.4 A Cell/Microstructure-Level Approach to Attachment Mechanisms in Aquatic Organisms (Microscopic)
At the microscopic level, similar designs and organizations appear to have emerged independently in different phylogenetic lineages for both adhesive and suction organs.
The biosynthesis, packaging and release of adhesive secretions take place at the level of specialized secretory cells. In some rare cases (e.g., the cement glands of barnacles; Liang et al., 2019), these secretory cells are associated with collecting ducts to form complex glands. In most cases, however, each secretory cell delivers its products directly at the epithelial surface of the body area where adhesion takes place. These secretory cells can, however, be aggregated to form large secretory structures which are also often named glands in the literature—this is the case, for example, for the cement glands of annelid tubeworms (Becker et al., 2012) or the byssal glands of mussels (Waite, 2017). Alternatively, secretory cells may be homogeneously distributed among other cell types, as in the pedal sole epidermis of gastropod molluscs such as limpets (Grenon & Walker, 1978; Kang et al., 2020). Independent of the taxon and type of adhesion, there are animals in which the adhesive material is produced by a single type of secretory cell (e.g., barnacle cement cells (Liang et al., 2019), platyhelminth rhabdite-secreting cells (Martin, 1978c), sea urchin tube feet adhesive cells (Flammang et al., 2016), and ctenophore collocytes (von Byern et al., 2010) for permanent, transitory, temporary and instantaneous adhesion, respectively) and others in which this material is made up by the blending of molecules produced by two or more secretory cell types (e.g., polychaete tubeworm cement cells (Becker et al., 2012), limpet pedal glands (Kang et al., 2020), and sea star tube foot adhesive cells (Flammang et al., 2016) for permanent, transitory and temporary adhesion, respectively).
Duo-gland adhesive systems involved in temporary adhesion present an additional level of complexity as, in addition to adhesive secretory cells, they also incorporate de-adhesive secretory cells, hence their name (Fig. 16.4). De-adhesive cells release a second type of secretion, poorly characterized to date, that allows the detachment of the adhesive organ from the substratum (Lengerer & Ladurner, 2018). Indeed, temporary adhesion can be defined as a reversible attachment process in which strong adhesion is followed, after a certain interval, by voluntary detachment leading to a loss of contact between the adhesive organ and the surface (Lengerer & Ladurner, 2018). Duo-gland adhesive structures are found in many unrelated taxa. They were originally described for small invertebrates inhabiting the interstitial environment (Boaden, 1968; Tyler, 1976). In these meiofaunal organisms, belonging to the phyla Platyhelminthes (Tyler, 1976; Lengerer et al., 2014), Gastrotricha (Tyler & Rieger, 1980), Nematoda (Adams & Tyler, 1980), and Annelida (Martin, 1978a), they are involved in maintaining position. Duo-gland adhesive systems have also been described for echinoderm tube feet (Santos et al., 2009b; Flammang et al., 2016). Tube feet can be involved in position maintenance and locomotion (sea stars, sea urchins, and sea cucumbers), feeding (sea cucumbers, brittle stars, and feather stars), or shelter building (burrowing sea urchins). A duo-gland adhesive system has also been suggested to be present in the captacula (i.e., the food-collecting tentacles) of scaphopod molluscs (Shimek, 1988; Byrum & Ruppert, 1994), further widening the distribution range of this adhesive system in aquatic invertebrates.
Convergent cellular organisation of duo-gland secretory complexes in different metazoans. Transmission electron microscopic images of transverse sections through the adhesive epidermis of (a) a turbellarian flatworm body wall (adapted from Tyler (1976) with permission from Springer Nature), (b) a polychaete worm pygidium (adapted from Martin (1978a) with permission from Springer Nature), (c) a brittle star tube foot (original), and (d) a cuttlefish ventral mantle (adapted from von Byern et al. (2011) with permission from Wiley). The center of the figure shows a generalized drawing of a longitudinal section through such a secretory complex with the horizontal line showing the plane of section for images a to d (original drawing). Adhesive gland cells are indicated in red and de-adhesive gland cells in green. Scale bars: 1 μm. M microtubule, R releasing (de-adhesive) granule, rg releasing (de-adhesive) gland, V viscid (adhesive) granule, vg viscid (adhesive) gland
Despite the more important morphological and functional complexity of duo-gland adhesive systems, strikingly similar cellular organizations have been reported for distantly related animals. In every species studied, the adhesive structures contain two types of closely associated secretory cells (Fig. 16.4). Adhesive cells are specialized epidermal cells, morphologically similar to the secretory cells involved in the other types of adhesion. They are filled with secretory granules which can vary greatly in shape, size, and contents. De-adhesive cells are thought to be derived from nerve cells in different taxa (Tyler, 1976; Flammang, 1996). They generally enclose small spherical, electron-dense secretory granules. The simplest organization of a duo-gland adhesive system consists of one adhesive cell with one de-adhesive cell, as seen in the flatworm Macrostomum lignano and in the sea urchin Echinocardium cordatum (Flammang et al., 1991; Lengerer et al., 2014). In the former, these two secretory cells are associated with one epidermal anchor cell, and the set of three cells has been named the duo-gland adhesive organ. In the latter, the adhesive and de-adhesive cells are associated with two sensory cells and the resulting structures have been called sensory-secretory complexes. There are also slightly more complex systems made up of the association of two adhesive cells flanking one de-adhesive cell (Fig. 16.4). This organization has been described for groups as diverse as flatworms (Tyler, 1976), annelids (Martin, 1978a), brittle stars (Flammang, 1996), and cuttlefishes (von Byern et al., 2011). For this last-mentioned cephalopod, Sepia tuberculata, it was proposed that detachment results from muscular contraction (von Byern et al., 2011). However, the close morphological convergence with other duo-gland adhesive systems suggests that de-adhesive secretions could help mechanical detachment.
As emphasized by the mechanism of detachment in Sepia, duo-gland adhesive systems do not seem to be the only adhesive systems involved in temporary adhesion. In a few taxa, structures possessing only one type of secretory cell attach and detach quickly. Such adhesive systems occur in some turbellarians (Tyler, 1976), gastrotrichs (Tyler & Rieger, 1980), and nematodes (Lippens, 1974). These structures were also described for cnidarians: the medusae of several species of hydrozoan possess adhesive tentacles that can attach and detach repeatedly (Honegger, 1984). Finally, barnacle larvae also fit into this category (Raine et al., 2020). In all these organisms the detachment process is purely mechanical (Lengerer & Ladurner, 2018).
In artificial suction cups (e.g., rubber suction cups used to attach mobile devices to glass), the disc wall—the side that attaches to the surface—is smooth. This is rarely the case in biological suction organs, where regions of the organ that contacts the attachment surface are highly textured. This texturing may arise from stiff microstructures (e.g., remora suction pads and net-winged midge larvae suction discs), dense arrays of cilia or microvilli (e.g., clingfish, lumpfish, limpets), or networks of channels and polygonal microstructures (e.g., coleoid suckers and clingfish). We provide an overview of the morphology and function of stiff microstructures below.
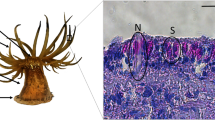
Spine-like microstructures called spinules are found within the suction pad of remoras (Fig. 16.5a–c). Spinules are mineralized projections that are approximately 500 μm in length and are found on top of lamellae (Fulcher & Motta, 2006; Beckert et al., 2015; Wang et al., 2017). The lamellae can be erected so that the spinules come into contact with the host surface. Several studies have demonstrated that the stiff spinules enhance friction on rough surfaces, thereby increasing drag resistance underwater (Beckert et al., 2015; Wang et al., 2017; Gamel et al., 2019). It is important that the spinules are sufficiently stiff and strong so that they retain their structural integrity when in contact with rough surfaces—otherwise they might either buckle or break under high loads. Interestingly, the orientation of the spinules with respect to drag forces may facilitate passive engagement. The spinules are posterior-facing, the drag force on hitch-hiking remoras (which act in the anterior-to-posterior direction) automatically promotes interlocking against surface asperities (Fulcher & Motta, 2006; Beckert et al., 2015). In addition, the soft fleshy rim around the suction disc also plays an important role during attachment as it creates a seal and contributes to friction on smooth surfaces (Fulcher & Motta, 2006; Wang et al., 2017).
Stiff microstructures in suction organs of disparate metazoans. Unlike synthetic suction cups, the surfaces of suction organs that contact the substrate are often highly textured. Stiff microstructures found on the suction discs of remora fish (spinules, a–c) and blepharicerid larvae (microtrichia, d–f) interlock with surface asperities to increase friction and help resist strong drag forces. Keratinized protuberances on the suction organ of cyprinid fish (e.g., in Garra gotyla gotyla, g–i) and cuticular pegs found on coleoid suckers (e.g., Spirula spirula, j–l) are also thought to increase friction during suction attachment
Surprisingly, spine-like microstructures similar to remora spinules are found on suction organs of a family of insects (Blephariceridae; Fig. 16.5d–f). As mentioned previously, blepharicerid larvae are found in fast-flowing alpine water systems, where they use their suction organs to attach to and move on rocks. The spine-like microstructures on their suction organs are called microtrichia, and evidence suggests that they are stiff cuticular structures capable of interlocking with surface asperities (Rietschel, 1961; Kang et al., 2019, 2021). Since microtrichia tips are oriented towards the center of the suction organ, inward sliding of the organ likely results in passive engagement with the surface to increase friction, in a similar fashion to the posterior-facing spinules in remoras (Kang et al., 2019).
Many fish species that live in fast-flowing waters (rheophilic fish) have microstructures called unculi within their suction organs (note that unculi can also be present on other regions of their bodies) (Roberts, 1982). Unculi are keratinized outgrowths of single epithelial cells of approximately 10 to 20 μm in length, and in Garra sp. they are found atop tubercles within the oral sucker (Fig. 16.5g–i) (Saxena, 1959; Roberts, 1982; Teimori et al., 2011; Hussain & Bordoloi, 2018). It is possible that some fishes use their unculi to help scrape food from the substrate; however, unculi are also present on the frictional pads of the pectoral fins of non-suctorial fishes (Conway et al., 2012; De Meyer & Geerinckx, 2014), which suggests a role in friction enhancement. Indeed, a functional study of attachment performance in Hypostomus sp. demonstrated that both oral suction organs and frictional pads contribute towards improved resistance against high flow rates (Gerstner, 2007).
Lastly, returning to the group of animals that symbolises biological suction attachment, many coleoid species possess microstructures called cuticular pegs on their suckers (Fig. 16.5j–l) (Nixon & Dilly, 1977; Salcedo-Vargas, 1995; Schmidtberg, 1999; Minnocci et al., 2015). Cuticular pegs, similarly to the arthropod cuticle, are made of chitin-protein complexes and may also be stiff structures (Hunt & Nixon, 1981; Accogli et al., 2017). Morphological studies have shown that cuticular pegs are found on a region of the suction organ called the infundibulum, which is firmly pressed against the surface during attachment (Nixon & Dilly, 1977; Kier & Smith, 1990; Salcedo-Vargas, 1995). Despite coleoid suckers being one of the most studied biological suction systems, not much is known about the function of these cuticular pegs. Researchers have hypothesized that they may increase friction when in contact with the surface and could also help to maximize attachment strength by transmitting low internal pressures throughout the disc (Kier & Smith, 1990). High mechanical wear from contacting and increasing friction may be why the lining of the infundibulum is periodically shed (Kier & Smith, 1990; Minnocci et al., 2015). Further research is needed to reveal the material properties of these cuticular pegs and how they generate additional friction during coleoid suction attachment.
16.5 A Molecule-Level Approach to Attachment Mechanisms in Aquatic Organisms (Nanoscopic)
In aquatic animals, the biochemical composition of adhesive secretions varies greatly from one taxonomic group to another (Tyler, 1988; Whittington & Cribb, 2001; Flammang et al., 2005, 2016). As a general rule, permanent adhesives consist almost exclusively of proteins. On the other hand, non-permanent adhesives (transitory as well as temporary) are made up of an association of proteins and carbohydrates, the latter being represented mostly in the form of acidic and sulfated glycans conjugated or associated to proteins (Hennebert et al., 2018). There is typically more protein than carbohydrate, usually in a ratio of approximately 2:1 (Flammang et al., 2016), but there may be substantial variation on this. The composition of instantaneous adhesives has only been investigated for sea cucumber Cuvierian tubules. Their adhesive is reminiscent of non-permanent adhesives through its constitution of proteins and carbohydrate in a 3:2 ratio (De Moor et al., 2003). However, it differs from them in that the carbohydrate fraction is in the form of neutral rather than acidic sugars. In all aquatic metazoans, therefore, adhesive secretions are predominantly made up of proteins (Hennebert et al., 2015). It is now well-established that the common properties of aquatic bioadhesives (e.g., the ability to displace water from the substratum, to spread and rapidly form strong adhesive bonds with the surface, and to cure) are related to the physico-chemical characteristics of their constituent proteins, including their post-translational modifications such as hydroxylation, phosphorylation and glycosylation (Stewart et al., 2011; Petrone, 2013; Davey et al., 2021). Thus far, hydroxylation and phosphorylation are the most thoroughly investigated modifications (Davey et al., 2021). Studies on mussel and tubeworm adhesive composition have revealed a high content of 3,4-dihydroxy-L-phenylalanine (DOPA), which is formed by post-translational hydroxylation of tyrosine, and of phosphoserine, which results from the phosphorylation of serine residues. These modified amino acids play important interfacial and cross-linking roles in aquatic adhesive secretions (Sagert et al., 2006), and in the case of mussel and tubeworm permanent adhesives, they are thought to be the result of convergent evolution (Kamino, 2010).
As far as the amino acid composition of the protein fraction is concerned, aquatic adhesives also vary considerably from one species to another. We used the method of Rocha et al. (2019) to quantify the level of relatedness among proteins. We performed a principal component analysis (PCA) of the amino acid compositions of bulk adhesive secretions that are usually mixtures of different proteins. The PCA, based on a variance-covariance matrix, was performed using the PAST 4.02 software (Hammer et al., 2001) on the relative amino acid content of whole adhesive secretions from 34 species belonging to seven phyla, including some terrestrial glues. An average protein (based on UniProtKB/SwissProt databases) and an average human secreted protein amino acid compositions were included for comparison (see Supplementary Table 16.2). Some post-translationally modified amino acids (i.e., half-cystine and DOPA) were included in the analysis because they are important constituents of some aquatic adhesives (Kamino, 2010; Hennebert et al., 2015; Davey et al., 2021), but phosphoserine residues were not considered as they are dephosphorylated into serine residues during the acid hydrolysis step of the amino acid analysis (Stewart et al., 2004). Similarly, aspartic acid and asparagine, and glutamic acid and glutamine were grouped as Asx and Glx respectively since the acid hydrolysis induces a deamidation of Asn and Gln. Two principal factors extracted from the PCA, PC1 and PC2, accounted for 64.2% of the cumulative variance. Figure 16.6 shows that the adhesives of phylogenetically related species using the same type of adhesion generally cluster together, which suggests they are homologous. For example, the permanent cements of both acorn and goose barnacles, the transitory adhesives of limpets, and the instantaneous adhesives of sea cucumber Cuvierian tubules form tight, taxon-specific clusters. For some other taxa, however, the species are more distantly spaced but are still clustered together: for example, the temporary adhesives of echinoderm tube feet, the permanent adhesives of mussels and of tubeworms, and the slimes of velvet worms. It should be noted, however, that some of these taxa are represented by only two species. A notable exception is the loose cluster comprising the temporary adhesives of monogenean flatworms, for which divergence between species is more pronounced. Terrestrial glues are intermixed with aquatic adhesives, although they tend to cluster in the lower right part of the PCA plot.
Comparative amino acid composition of bioadhesives (PCA). Scatter plot of principal component axis 1 (PC1) and axis 2 (PC2) based on the relative amino acid composition of the secreted adhesives from various animal species, where the two first principal components (1 × 2) account for 64.2% of the cumulative variance. (a) Projection of metazoan bioadhesives (each dot represents one species; n = 34) on the factor plane showing clusters based on phylogenetic and functional aspects. (b) Projection of amino acid (n = 18) levels on the factor plane, showing amino acids that contribute the most to the characterisation of each group of bioadhesives. Ar Asterias rubens, Bc Balanus crenatus, Be Balanus eburneus, Bech Brachycentrus echo, Bh Balanus hameri, Bs Bohadschia subrubra, Df Dosima fascicularis, Dm Dermacentor marginatus, Ek Euperipatoides kanangrensis, Es Entobdella soleae, Ga Gasterosteus aculeatus, Gd Geukensia demissa, Hf Holothuria forskali, Hl Holothuria leucospilota, La Lepas anatifera, Ll Lottia limatula, Ma Merizocotyle australensis, Me Mytilus edulis, Mi Merizocotyle icopae, Mh Monocotyle helicophallus, Mr Megabalanus rosa, Ms Monocotyle spiremae, Nb Notaden bennetti, Nr Neoheterocotyle rhinobatidis, Pc Phragmatopoma californica, Pg Pearsonothuria graeffei, Pl Paracentrotus lividus, Plap Phragmatopoma lapidosa, Pm Peripatopsis moseleyi, Pmoe Phragmatopoma moerchi, Pv Patella vulgata, Sf Sabellaria floridensis, Sk Sabellaria kaiparaensis, Tr Troglocephalus rhinobatidis. The average amino acid composition of proteins from the UniProtKB/SwissProt database and of human secreted proteins are also included as comparison points (black dots). Amino acid compositions and references can be found in Supplementary Table 16.2
Glycine and serine are over-represented in the adhesives of almost half of the species included in the analysis, and the first component of the PCA separates adhesives with a bias towards these amino acids (right part of Fig. 16.6b) from adhesives with a more average composition (left part of Fig. 16.6b). In most cases, there is little overlap between taxonomic groups, but several non-permanent adhesives from a number of species are grouped together even though they belong to disparate phyla (i.e., platyhelminths (Hamwood et al., 2002), mollusks (Grenon & Walker, 1980; Smith et al., 1999), and echinoderms (Flammang et al., 1998; Santos et al., 2009a) (Fig. 16.6a, grey dotted frame). This relationship might indicate convergence in amino acid composition driven by shared function and selective pressures. A similarity between transitory and temporary adhesives was already evident in terms of glycan composition (Hennebert et al., 2018; Kang et al., 2020) (see also above). In contrast, no such compositional convergence is observed for adhesives from sessile species using permanent adhesion. Indeed, the adhesives of mussels, tubeworms and barnacles differ greatly from each other (Fig. 16.6). In their composition, the protein fractions of mussel byssal plaques and polychaete cement have the presence of DOPA in common in their composition (Benedict & Waite, 1986; Jensen & Morse, 1988; Waite et al., 1989). However, the tubeworm adhesives are separated by their high content of phosphoserine (Mitterer, 1971; Stewart et al., 2004), which is a characteristic they share with the adhesive silk of caddisfly larvae (Stewart & Wang, 2010), a permanent adhesive used in building shelters. Barnacle cements, on the other hand, contain neither DOPA nor phosphoserine, and seem to have more in common with non-permanent adhesives, in which disulfide bonds serve an important function (Fig. 16.6b) (Walker, 1972; Kamino et al., 1996; Naldrett & Kaplan, 1997; Engel et al., 2021). As for the instantaneous adhesives of holothuroid Cuvierian tubules, they differ from all other aquatic bioadhesives because they are particularly rich in glycine (De Moor et al., 2003; Flammang et al., 2005), and instead share resemblance to the defensive onychophoran slimes (Röper, 1977; Benkendorff et al., 1999).
For all investigated species, adhesive secretions consist of at least two or more proteins. According to their sequence and structure, these proteins may achieve various sub-functions within the secreted adhesive (e.g., interfacial adhesive or bulk cohesive interactions). This means that bioadhesives are usually composed of a variety of different proteins. Thus, although the amino acid composition of barnacle cement resembles that of an average secreted mammalian protein (Fig. 16.6), these cements are in fact made up of several proteins of very different compositions and sequences (Rocha et al., 2019) (Fig. 16.7).
Cluster analysis of adhesive protein sequences and identification of shared protein motifs. (a) CLANS analysis of selected adhesive proteins using an E-value threshold of 1E-10. (In BLAST analyses, the E-value is defined as the probability, due to chance, that there is another alignment with a similarity greater than the obtained score). Only proteins presenting a similarity above the threshold are connected by lines. The lines are color-coded according to their E-values. (b) Alpha-macroglobulin domains observed in barnacle settlement-inducing protein complex and echinoderm adhesive proteins. (c) Lectin domains observed in echinoderm (continued overleaf) and Hydra adhesive proteins. (d) VWD domains observed in echinoderm and limpet adhesive proteins. (e) EGF domains found in echinoderm and mussel adhesive proteins. The list of the adhesive proteins used in the CLANS analysis can be found in Supplementary Table 16.3
The evolutionary origins of metazoan adhesive proteins remain largely enigmatic. While some authors have proposed a complete independent evolution of bioadhesive proteins (Kamino, 2010), more recent works—driven by omics approaches—suggest some evolutionary-related sequence similarities and, more specifically, the presence of common protein domains between different bioadhesive proteins (Davey et al., 2021). Indeed, although some adhesive protein sequences are short and intrinsically disordered, others are long or very long, comprising multiple domains involved in various subtasks important for their adhesion and/or cohesive functions. Protein domains are “high-level parts of proteins that either occur alone or together with partner domains on the same protein chain” (Forslund & Sonnhammer, 2012). Many protein domains can perform a particular function or contribute in a specific way to the function of the overall protein. Most domains correspond to tertiary structural elements and are able to fold independently.
We also investigated adhesive proteins secreted by a wide variety of aquatic animals (i.e., Cnidaria, Annelida, Mollusca, Platyhelminthes, Echinodermata, Craniata) using a sequence similarity-based clustering analysis to highlight potential similarities between these bioadhesives. Sequence similarity searches (often performed using BLAST) can identify “homologous” proteins by detecting excess similarity corresponding to the statistically significant similarity that reflects common ancestry (Pearson, 2013). Adhesive protein sequences were retrieved from publicly-accessible databases or from previous studies (Rodrigues et al., 2016b). The sequence similarity-based clustering was performed using CLANS (Frickey & Lupas, 2004). An all-against-all BLASTp was conducted using the scoring matrix BLOSUM62 and linkage clustering was performed with an E-value of 1E-10 to identify coherent clusters. The clustering was first performed in 3-dimensions and then collapsed into 2D in order to generate the plot shown in Fig. 16.7a (see Supplementary Table 16.3 for the list of adhesive proteins). The connections between the dots indicate clear similarity and highlight potential homology between the proteins. Our analyses only included protein sequences that have been confirmed to be part of bioadhesive secretions. Many candidates that did not meet our rigorous criteria could be included in the future as new evidence becomes available.
While many proteins appear to be specific to the investigated organisms (represented as isolated dots or clusters of dots of the same color in our analysis; Fig. 16.7a), some exhibit clear sequence homologies between phylogenetically distant organisms (shown as connections between dots of different colors). At least four clusters of adhesive proteins from phylogenetically distant organisms have been identified. Our protein domain analyses showed that the similarity between all of these adhesive proteins is specifically associated with similar (and likely homologous) protein domains: lectin domains, epidermal growth factor-like (EGF) domains, alpha-2-macroglobulin-like (A2M) domains, and von Willebrand factor type D (VWD) domains. These domains are known to bind to other proteins and sugar groups, forming oligomers and adsorbing onto substrates—functions that are particularly relevant for adhesive proteins (Davey et al., 2021). A2M domains are specifically shared by two echinoderm proteins (found in the sea star Asterias rubens and in the sea urchin Paracentrotus lividus) and one barnacle protein (Settlement Inducing Protein Complex or SIPC of Amphibalanus amphitrite) (Fig. 16.7a). Comparison of the protein domains highlighted a general similarity of the two proteins that share a rather long alpha-2-macroglobulin-like multi-subdomain set of around 800 amino acids (Fig. 16.7b). Galactose/rhamnose binding lectin domains are observed in multiple adhesive proteins of Hydra (Rodrigues et al., 2016b) and are also present in various echinoderm adhesive proteins (i.e., from both sea stars and sea urchins) (Fig. 16.7c). VWD domains have also been found in various adhesive proteins from fish (Gasterosteus), flatworms, limpets, and echinoderms. As illustrated in the protein domain prediction, the Sea star Footprint Protein 1 of Asterias rubens contains numerous domains including three VWD domains. This domain is also found in one of the adhesive proteins isolated from the limpet Patella vulgata (P-vulgata_4), although only in one “copy”. EGF domains have been detected in various adhesive proteins, including proteins from mussels and echinoderms (Fig. 16.7d). This domain also occurs in adhesive proteins from limpets and flatworms, but it appears that, with our stringent threshold, connections between these proteins and those of mussels and echinoderms are not visible on the CLANS analyses (Fig. 16.7a). It is noteworthy, however, that in most of the cases EGF domains are present in multiple copies in adhesive proteins (Fig. 16.7d).
Proteins evolve not only by point mutations but also by modular rearrangements generally occurring at the level of domains (Weiner et al., 2006). It is generally accepted that the vast majority of proteins have domain architectures that emerged through evolutionary descent rather than due to functional necessity and convergence (Gough, 2005). Many biological processes involved in the evolutionary emergence of domain architectures have been studied to date, including: gene fusion by a mobile element (such as a retrotransposon), gene fusion by loss of a stop signal or deletion of much of the intergenic region, domain insertion through recombination, gene fission by the introduction of transcription stop and start codons, and domain loss by the introduction of a stop codon with subsequent degeneration of the now untranslated domain (Björklund et al., 2005; Weiner et al., 2006; Chothia & Gough, 2009). Because protein domains exhibit evolutionary conservation, adhesive proteins from phylogenetically distant organisms undoubtedly share related features. However, it is highly probable that these domains, which are also present in a variety of non-adhesive proteins, were convergently acquired from ancestral proteins with unrelated general functions (even though the general domain subfunctions could be similar or identical). Thus, it seems that there is no common ancestral bioadhesive protein; instead, evolutionarily related protein domains were likely repurposed to achieve similar functions in different bioadhesives.
16.6 Conclusion and Outlook
Investigating how multiple evolutionary scenarios converge on functionally similar traits is important for understanding the evolution of complex biological processes. Many aquatic animals, whether they are sessile or mobile, marine or freshwater, require strategies to allow them to attach to substrates in wet environments. We have explored the metazoan phylogeny and identified the two main mechanisms of aquatic attachment: bioadhesive secretions and suction attachment. Based on our survey, most of the recognized extant metazoan phyla contain at least one species that uses bioadhesives or suction organs, and numerous cases of convergent evolution can be identified that span the length-scales from molecules to organisms. We have shown that attachment systems are complex traits with similar functions that have emerged repeatedly during evolution. From the molecular point of view, it is likely that homologous features (i.e., protein domains) were independently requisitioned in different lineages. There remain, however, many gaps in our knowledge of biological attachment strategies and their evolution. For instance, although a growing number of studies have isolated and characterized proteins and sugars from adhesive secretions, functional studies of the individual components are scarce. Likewise, while it is relatively easy to classify an organ as a suction attachment organ, it is much more challenging to convincingly prove that the animal indeed generates pressure differences for attachment. Future studies that successfully explore these aspects in detail will be of great value to the bioadhesive community.
Our review demonstrates the utility of a multi-level approach in exploring the evolution of biological attachment strategies in aquatic metazoans. We show that convergence can be identified at many different organizational levels, which means that studies focusing solely on one level (e.g., adhesive proteins) can miss insights into other important components of adhesive systems (e.g., the glandular system that delivers the proteins to the substrate). Due to a combination of the breadth of our taxonomic coverage and the lack of studies that quantify convergence of specific traits of adhesive systems, our work is light on detailed discussions. We believe that there are ample opportunities for both continuing to explore the tree of life for strategies of adhesion as well as delving deeper into identified species to better understand the mechanism of action. Furthermore, if our multi-level approach is adopted in future studies, we expect a more holistic understanding of attachment strategies within and across different species to emerge. Such endeavors will undoubtedly uncover new and exciting examples of adhesion and will help to enrich our understanding of the role of convergent evolution in the development of complex biological traits.
References
Accogli, G., Scillitani, G., Mentino, D., & De Santis, S. (2017). Characterization of the skin mucus in the common octopus Octopus vulgaris (Cuvier). Reared paralarvae. European Journal of Histochemistry, 61, 2815.
Adams, P. J., & Tyler, S. (1980). Hopping locomotion in a nematode: Functional anatomy of the caudal gland apparatus of Theristus caudasaliens sp. n. Journal of Morphology, 164, 265–285.
Aguayo, R., Lavilla, E. O., Vera Candioti, M. F., & Camacho, T. (2009). Living in fast-flowing water: Morphology of the gastromyzophorous tadpole of the bufonid Rhinella quechua (R. veraguensis group). Journal of Morphology, 270, 1431–1442.
Aiken, R. B., & Khan, A. (1992). The adhesive strength of the palettes of males of a boreal water beetle, Dytiscus alaskanus J. Balfour Browne (Coleoptera: Dytiscidae). Canadian Journal of Zoology, 70, 1321–1324.
Andersen, K. I., & Lysfjord, S. (1982). The functional morphology of the scolex of two Tetrabothrius Rudolphi 1819 species (Cestoda; Tetrabothriidae) from penguins. Parasitology Research, 67, 299–307.
Arita, G. S. (1967). A comparative study of the structure and function of the adhesive apparatus of the Cyclopteridae and Gobiesocidae. Doctoral dissertation, The University of British Columbia.
Becker, P. T., Lambert, A., Lejeune, A., Lanterbecq, D., & Flammang, P. (2012). Identification, characterization, and expression levels of putative adhesive proteins from the tube-dwelling polychaete Sabellaria alveolata. The Biological Bulletin, 223, 217–225.
Beckert, M., Flammang, B. E., & Nadler, J. H. (2015). Remora fish suction pad attachment is enhanced by spinule friction. Journal of Experimental Biology, 218, 3551–3558.
Bell, E., & Gosline, J. (1996). Mechanical design of mussel byssus: Material yield enhances attachment strength. Journal of Experimental Biology, 199, 1005–1017.
Benedict, C. V., & Waite, J. H. (1986). Ultrastructure and composition of the byssus of Mytilus edulis L. Journal of Morphology, 189, 261–270.
Benkendorff, K., Beardmore, K., Gooley, A. A., Packer, N. H., & Tait, N. N. (1999). Characterisation of the slime gland secretion from the peripatus, Euperipatoides kanangrensis (Onychophora: Peripatopsidae). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 124, 457–465.
Björklund, A. K., Ekman, D., Light, S., Frey-Skött, J., & Elofsson, A. (2005). Domain rearrangements in protein evolution. Journal of Molecular Biology, 353, 911–923.
Boaden, P. J. S. (1968). Water movement—a dominant factor in interstitial ecology. Sarsia, 34, 125–136.
Borucinska, J., & Caira, J. N. (1993). A comparison of mode of attachment and histopathogenicity of four tapeworm species representing two orders infecting the spiral intestine of the nurse shark, Ginglymostoma cirratum. The Journal of Parasitology, 79, 238–246.
Budney, L. A., & Hall, B. K. (2010). Comparative morphology and osteology of pelvic fin-derived midline suckers in lumpfishes, snailfishes and gobies. Journal of Applied Ichthyology, 26, 167–175.
von Byern, J., & Klepal, W. (2006). Adhesive mechanisms in cephalopods: a review. Biofouling, 22, 329–338.
von Byern, J., Mills, C. E., & Flammang, P. (2010). Bonding tactics in ctenophores — morphology and function of the colloblast system. In J. von Byern & I. Grunwald (Eds.), Biological adhesive systems (pp. 29–40). Springer Vienna.
von Byern, J., Müller, C., Voigtländer, K., Dorrer, V., Marchetti-Deschmann, M., Flammang, P., & Mayer, G. (2017). Examples of bioadhesives for defence and predation. In S. N. Gorb & E. V. Gorb (Eds.), Functional surfaces in biology III, biologically-inspired systems (pp. 141–191). Springer International Publishing.
von Byern, J., Scott, R., Griffiths, C., Micossi, A., Grunwald, I., & Cyran, N. (2011). Characterization of the adhesive areas in Sepia tuberculata (Mollusca, Cephalopoda). Journal of Morphology, 272, 1245–1258.
Byrum, C. A., & Ruppert, E. E. (1994). The ultrastructure and functional morphology of a captaculum in Graptacme calamus (Mollusca, Scaphopoda). Acta Zoologica, 75, 37–46.
Carrington, E., Waite, J. H., Sarà, G., & Sebens, K. P. (2015). Mussels as a model system for integrative ecomechanics. Annual Review of Marine Science, 7, 443–469.
de Chambrier, A., & Scholz, T. (2008). Tapeworms (Cestoda: Proteocephalidea) of firewood catfish Sorubimichthys planiceps (Siluriformes: Pimelodidae) from the Amazon River. Folia Parasitologica, 55, 17–28.
Chen, Y., Shih, M.-C., Wu, M.-H., Yang, E.-C., & Chi, K.-J. (2014). Underwater attachment using hairs: The functioning of spatula and sucker setae from male diving beetles. Journal of the Royal Society, Interface, 11, 20140273.
Chothia, C., & Gough, J. (2009). Genomic and structural aspects of protein evolution. The Biochemical Journal, 419, 15–28.
Clarke, J. L., Davey, P. A., & Aldred, N. (2020). Sea anemones (Exaiptasia pallida) use a secreted adhesive and complex pedal disc morphology for surface attachment. BMC Zoology, 5, 5.
Conway, K. W., Lujan, N. K., Lundberg, J. G., Mayden, R. L., & Siegel, D. S. (2012). Microanatomy of the paired-fin pads of ostariophysan fishes (Teleostei: Ostariophysi). Journal of Morphology, 273, 1127–1149.
Cowles, D. (1977). Locomotion by Epiactis prolifera (Coelenterata: Actiniaria). Marine Biology, 39, 67–70.
Das, D., & Nag, T. C. (2006). Fine structure of the organ of attachment of the teleost, Garra gotyla gotyla (Ham). Zoology, 109, 300–309.
Das, D., & Nag, T. C. (2009). Organs of adhesion in some mountain-stream teleosts of India: Structure-function relationship. In S. N. Gorb (Ed.), Functional surfaces in biology (pp. 105–122). Springer.
Davey, P. A., Power, A. M., Santos, R., Bertemes, P., Ladurner, P., Palmowski, P., et al. (2021). Omics-based molecular analyses of adhesion by aquatic invertebrates. Biological Reviews, 96, 1051–1075.
De Meyer, J., & Geerinckx, T. (2014). Using the whole body as a sucker: Combining respiration and feeding with an attached lifestyle in hill stream loaches (Balitoridae, Cypriniformes). Journal of Morphology, 275, 1066–1079.
De Moor, S., Waite, J. H., Jangoux, M., & Flammang, P. (2003). Characterization of the adhesive from Cuvierian tubules of the sea cucumber Holothuria forskali (Echinodermata, Holothuroidea). Marine Biotechnology, 5, 45–57.
Ditsche, P., Hicks, M., Truong, L., Linkem, C., & Summers, A. (2017). From smooth to rough, from water to air: The intertidal habitat of Northern clingfish (Gobiesox maeandricus). Naturwissenschaften, 104, 33.
Ditsche, P., & Summers, A. P. (2014). Aquatic versus terrestrial attachment: Water makes a difference. Beilstein Journal of Nanotechnology, 5, 2424–2439.
Ditsche, P., Wainwright, D. K., & Summers, A. P. (2014). Attachment to challenging substrates--fouling, roughness and limits of adhesion in the northern clingfish (Gobiesox maeandricus). Journal of Experimental Biology, 217, 2548–2554.
Edmunds, M., Potts, G. W., Swinfen, R. C., & Waters, V. L. (1976). Defensive behaviour of sea anemones in response to predation by the opisthobranch mollusc Aeolidia papillosa (L.). Journal of the Marine Biological Association of the United Kingdom, 56, 65–83.
Ellem, G. K., Furst, J. E., & Zimmerman, K. D. (2002). Shell clamping behaviour in the limpet Cellana tramoserica. Journal of Experimental Biology, 205, 539–547.
Engel, B., Suppan, J., Nürnberger, S., Power, A. M., & Marchetti-Deschmann, M. (2021). Revisiting amino acid analyses for bioadhesives including a direct comparison of tick attachment cement (Dermacentor marginatus) and barnacle cement (Lepas anatifera). International Journal of Adhesion and Adhesives, 105, 102798.
Federle, W., & Labonte, D. (2019). Dynamic biological adhesion: Mechanisms for controlling attachment during locomotion. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 374, 20190199.
Feigenbaum, D. L. (1976). Development of the adhesive organ in Spadella schizoptera (Chaetognatha) with comments on growth and pigmentation. Bulletin of Marine Science, 26, 600–603.
Flammang, P. (1996). Adhesion in echinoderms. In Echinoderm Studies (Vol. 5, pp. 1–60). Balkema.
Flammang, P., Demeuldre, M., Hennebert, E., & Santos, R. (2016). Adhesive secretions in echinoderms: A review. In A. M. Smith (Ed.), Biological adhesives (pp. 193–222). Springer International Publishing.
Flammang, P., De Ridder, C., & Jangoux, M. (1991). Ultrastructure of the penicillate podia of the spatangoid echinoid Echinocardium cordatum (Echinodermata) with special emphasis on the epidermal sensory-secretory complexes. Acta Zoologica, 72, 151–158.
Flammang, P., Michel, A., Cauwenberge, A. V., Alexandre, H., & Jangoux, M. (1998). A study of the temporary adhesion of the podia in the sea star Asterias rubens (Echinodermata, Asteroidea) through their footprints. Journal of Experimental Biology, 201(Pt 16), 2383–2395.
Flammang, P., Santos, R., & Haesaerts, D. (2005). Echinoderm adhesive secretions: From experimental characterization to biotechnological applications. Progress in Molecular and Subcellular Biology, 39, 201–220.
Forslund, K., & Sonnhammer, E. L. L. (2012). Evolution of protein domain architectures. Methods in Molecular Biology, 856, 187–216.
Frickey, T., & Lupas, A. (2004). CLANS: a Java application for visualizing protein families based on pairwise similarity. Bioinformatics, 20, 3702–3704.
Fulcher, B. A., & Motta, P. J. (2006). Suction disk performance of echeneid fishes. Canadian Journal of Zoology, 84, 42–50.
Gamel, K. M., Garner, A. M., & Flammang, B. E. (2019). Bioinspired remora adhesive disc offers insight into evolution. Bioinspiration & Biomimetics, 14, 056014.
Gan, L. L., Hertwig, S. T., Das, I., & Haas, A. (2016). The anatomy and structural connectivity of the abdominal sucker in the tadpoles of Huia cavitympanum, with comparisons to Meristogenys jerboa (Lissamphibia: Anura: Ranidae). Journal of Zoological Systematics and Evolutionary Research, 54, 46–59.
Gerstner, C. L. (2007). Effect of oral suction and other friction-enhancing behaviors on the station-holding performance of suckermouth catfish (Hypostomus spp.). Canadian Journal of Zoology, 85, 133–140.
Giribet, G., & Edgecombe, G. D. (2020). The invertebrate tree of life. Princeton University Press.
Gorb, S. N. (2008). Biological attachment devices: Exploring nature’s diversity for biomimetics. Philosophical Transactions Series A, Mathematical, Physical, and Engineering Sciences, 366, 1557–1574.
Gough, J. (2005). Convergent evolution of domain architectures (is rare). Bioinformatics, 21, 1464–1471.
Gradwell, N. (1971). Ascaphus tadpole: Experiments on the suction and gill irrigation mechanisms. Canadian Journal of Zoology, 49, 307–332.
Gradwell, N. (1972a). Behaviors of the leech, Placobdella, and transducer recordings of suctorial pressures. Canadian Journal of Zoology, 50, 1325–1332.
Gradwell, N. (1972b). Hydrostatic pressures and movements of the lamprey, Petromyzon, during suction, olfaction, and gill ventilation. Canadian Journal of Zoology, 50, 1215–1223.
Gradwell, N. (1975). Experiments on oral suction and gill breathing in five species of Australian tadpole (Anura: Hylidae and Leptodactylidae). Journal of Zoology, 177, 81–98.
Grenon, J. F., & Walker, G. (1978). The histology and histochemistry of the pedal glandular system of two limpets, Patella vulgata and Acmaea tessulata (Gastropoda: Prosobranchia). Journal of the Marine Biological Association of the UK, 58, 803–816.
Grenon, J. F., & Walker, G. (1980). Biochemical and rheological properties of the pedal mucus of the limpet, Patella vulgata L. Comparative Biochemistry and Physiology, 66B, 451–458.
Haad, M. B., Candioti, F. V., & Baldo, D. (2014). The stream tadpoles of Rhinella rumbolli (Anura: Bufonidae). Herpetologica, 70, 184–197.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontological Association, 4, 1–9.
Hamwood, T. E., Cribb, B. W., Halliday, J. A., Kearn, G. C., & Whittington, I. D. (2002). Preliminary characterisation and extraction of anterior adhesive secretion in monogenean (platyhelminth) parasites. Folia Parasitologica, 49, 39–49.
Hennebert, E., Gregorowicz, E., & Flammang, P. (2018). Involvement of sulfated biopolymers in adhesive secretions produced by marine invertebrates. Biology Open, 7.
Hennebert, E., Maldonado, B., Ladurner, P., Flammang, P., & Santos, R. (2015). Experimental strategies for the identification and characterization of adhesive proteins in animals: A review. Interface Focus, 5, 20140064.
Hennebert, E., Santos, R., & Flammang, P. (2012). Echinoderms don’t suck: Evidence against the involvement of suction in tube foot attachment. Zoosymposia, 7, 25–32.
Honegger, T. G. (1984). Ultrastructure of the adhesive tentacles of the limnomedusa Vallentinia gabriella (Hydrozoa, Olindiadidae). Zoomorphology, 104, 26–32.
Hunt, S., & Nixon, M. (1981). A comparative study of protein composition in the chitin-protein complexes of the beak, pen, sucker disc, radula and oesophageal cuticle of cephalopods. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 68, 535–546.
Hussain, J. F., & Bordoloi, S. (2018). Adaptive modifications in four fish species of the genus Garra (Teleostei; Cyprinidae) in Basistha river, Assam, India. Microscopy and Microanalysis, 24, 310–317.
Ibraheem, M. H. (1998). The mode of attachment of two cestode species infesting the Nile electric catfish Malapterurus electricus (Froskal), with special reference to the functional morphology of the scolex and histopathology. Arab Gulf Journal of Scientific Research, 16, 159–172.
Jensen, R. A., & Morse, D. E. (1988). The bioadhesive of Phragmatopoma californica tubes: A silk-like cement containing L-DOPA. Journal of Comparative Physiology B, 158, 317–324.
John, C. C. (1933). Habits, structure, and development of Spadella cephaloptera. Journal of Cell Science, 2, 625–696.
Jones, H. D., & Trueman, E. R. (1970). Locomotion of the limpet, Patella vulgata L. Journal of Experimental Biology, 52, 201–216.
Jones, M. K. (2000). Ultrastructure of the scolex, rhyncheal system and bothridial pits of Otobothrium mugilis (Cestoda: Trypanorhyncha). Folia Parasitologica, 47, 29–38.
Kamino, K. (2010). Molecular design of barnacle cement in comparison with those of mussel and tubeworm. The Journal of Adhesion, 86, 96–110.
Kamino, K. (2016). Barnacle underwater attachment. In A. M. Smith (Ed.), Biological adhesives (pp. 153–176). Springer International Publishing.
Kamino, K., Odo, S., & Maruyama, T. (1996). Cement proteins of the acorn barnacle, Megabalanus rosa. The Biological Bulletin, 190, 403–409.
Kampowski, T., Eberhard, L., Gallenmüller, F., Speck, T., & Poppinga, S. (2016). Functional morphology of suction discs and attachment performance of the Mediterranean medicinal leech (Hirudo verbana Carena). Journal of the Royal Society, Interface, 13.
Kang, V., Johnston, R., van de Kamp, T., Faragó, T., & Federle, W. (2019). Morphology of powerful suction organs from blepharicerid larvae living in raging torrents. BMC Zoology, 4, 10.
Kang, V., Lengerer, B., Wattiez, R., & Flammang, P. (2020). Molecular insights into the powerful mucus-based adhesion of limpets (Patella vulgata L.). Open Biology, 10, 200019.
Kang, V., White, R. T., Chen, S., & Federle, W. (2021). Extreme suction attachment performance from specialised insects living in mountain streams (Diptera: Blephariceridae). eLife, 10, e63250.
Kaplan, M. (1997). Internal and external anatomy of the abdominal disc of Atelopus (Bufonidae) larvae. Caldasia, 19, 61–69.
Karlsson Green, K., Kovalev, A., Svensson, E. I., & Gorb, S. N. (2013). Male clasping ability, female polymorphism and sexual conflict: fine-scale elytral morphology as a sexually antagonistic adaptation in female diving beetles. Journal of the Royal Society, Interface, 10, 20130409.
Kier, W. M., & Smith, A. M. (1990). The morphology and mechanics of octopus suckers. The Biological Bulletin, 178, 126–136.
Kier, W. M., & Smith, A. M. (2002). The structure and adhesive mechanism of octopus suckers. Integrative and Comparative Biology, 42, 1146–1153.
Lawrence, J. M. (1987). A functional biology of echinoderms. Croom Helm.
Lengerer, B., & Ladurner, P. (2018). Properties of temporary adhesion systems of marine and freshwater organisms. Journal of Experimental Biology, 221, jeb182717.
Lengerer, B., Pjeta, R., Wunderer, J., Rodrigues, M., Arbore, R., Schärer, L., Berezikov, E., Hess, M. W., Pfaller, K., Egger, B., Obwegeser, S., Salvenmoser, W., & Ladurner, P. (2014). Biological adhesion of the flatworm Macrostomum lignano relies on a duo-gland system and is mediated by a cell type-specific intermediate filament protein. Frontiers in Zoology, 11, 12.
Liang, C., Strickland, J., Ye, Z., Wu, W., Hu, B., & Rittschof, D. (2019). Biochemistry of barnacle adhesion: An updated review. Frontiers in Marine Science, 6.
Lippens, P. L. (1974). Ultrastructure of a marine nematode, Chromadorina germanica (Buetschli, 1874). Zoomorphology, 78, 181–192.
Lujan, N. K., & Conway, K. W. (2015). Life in the fast lane: A review of rheophily in freshwater fishes. In R. Riesch, M. Tobler, & M. Plath (Eds.), Extremophile fishes (pp. 107–136). Springer International Publishing.
Maie, T., Schoenfuss, H. L., & Blob, R. W. (2012). Performance and scaling of a novel locomotor structure: Adhesive capacity of climbing gobiid fishes. Journal of Experimental Biology, 215, 3925–3936.
Martin, G. G. (1978a). The duo-gland adhesive system of the archiannelids Protodrilus and Saccocirrus and the turbellarian Monocelis. Zoomorphology, 91, 63–75.
Martin, G. G. (1978b). Ciliary gliding in lower invertebrates. Zoomorphology, 91, 249–261.
Martin, G. G. (1978c). A new function of rhabdites: Mucus production for ciliary gliding. Zoomorphology, 91, 235–248.
Minnocci, A., Cianchetti, M., Mazzolai, B., Sebastiani, L., & Laschi, C. (2015). Cryo-scanning electron microscopy investigation of the Octopus vulgaris arm structures for the design of an octopus-like arm artefact. Microscopy Research and Technique, 78, 1133–1145.
Mitterer, R. M. (1971). Comparative amino acid composition of calcified and non-calcified polychaete worm tubes. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 38, 405–409.
Nachtigall, W. (1974). Biological mechanisms of attachment: The comparative morphology and bioengineering of organs for linkage, suction, and adhesion. Springer.
Naldrett, M. J., & Kaplan, D. L. (1997). Characterization of barnacle (Balanus eburneus and B. cenatus) adhesive proteins. Marine Biology, 127, 629–635.
Nichols, D. (1966). Functional morphology of the water-vascular system. Physiology of Echinodermata, 219, 240.
Nixon, M., & Dilly, P. (1977). Sucker surfaces and prey capture. Symposia of the Zoological Society of London, 38, 447–511.
Pearson, W. R. (2013). An introduction to sequence similarity (“homology”) searching. Current Protocols in Bioinformatics, Chapter 3, Unit3.1.
Petrone, L. (2013). Molecular surface chemistry in marine bioadhesion. Advances in Colloid and Interface Science, 195–196, 1–18.
Pospekhova, N. A., & Bondarenko, S. K. (2014). Morpho-functional characteristics of the scolex of Wardium chaunense (Cestoda: Aploparaksidae). Penetrated into host intestine. Parasitology Research, 113, 131–137.
Raine, J. J., Aldred, N., & Clare, A. S. (2020). Anatomy and ultrastructure of the cyprid temporary adhesive system in two species of acorn barnacle. Journal of Marine Science and Engineering, 8, 968.
Rietschel, P. (1961). Bau, Funktion und Entwicklung der Haftorgane der Blepharoceridae. Zeitschrift für Morphologie und Ökologie der Tiere, 50, 239–265.
Roberts, T. R. (1982). Unculi (horny projections arising from single cells), an adaptive feature of the epidermis of ostariophysan fishes. Zoologica Scripta, 11, 55–76.
Rocha, M., Antas, P., Castro, L. F. C., Campos, A., Vasconcelos, V., Pereira, F., & Cunha, I. (2019). Comparative analysis of the adhesive proteins of the adult stalked goose barnacle Pollicipes pollicipes (Cirripedia: Pedunculata). Marine Biotechnology, 21, 38–51.
Rodrigues, M., Leclère, P., Flammang, P., Hess, M. W., Salvenmoser, W., Hobmayer, B., & Ladurner, P. (2016a). The cellular basis of bioadhesion of the freshwater polyp Hydra. BMC Zoology, 1.
Rodrigues, M., Ostermann, T., Kremeser, L., Lindner, H., Beisel, C., Berezikov, E., Hobmayer, B., & Ladurner, P. (2016b). Profiling of adhesive-related genes in the freshwater cnidarian Hydra magnipapillata by transcriptomics and proteomics. Biofouling, 32, 1115–1129.
Röper, H. (1977). Analytische Untersuchungen des Wehrsekretes von Peripatopsis moseleyi (Onychophora)/Analytical Investigations of the Defensive Secretion from Peripatopsis moseleyi (Onychophora). Zeitschrift für Naturforschung C, 32, 57-b.
Sagert, J., Sun, C., & Waite, J. H. (2006). Chemical subtleties of mussel and polychaete holdfasts. In A. M. Smith & J. A. Callow (Eds.), Biological adhesives (pp. 125–143). Springer.
Salcedo-Vargas, M. A. (1995). Systematic value of the ultrastructure of the sucker surface in the squid family Mastigoteuthidae (Mollusca: Cephalopoda). Contributions to Zoology, 65, 65–77.
Santos, R., da Costa, G., Franco, C., Gomes-Alves, P., Flammang, P., & Coelho, A. V. (2009a). First insights into the biochemistry of tube foot adhesive from the sea urchin Paracentrotus lividus (Echinoidea, Echinodermata). Marine Biotechnology, 11, 686–698.
Santos, R., & Flammang, P. (2005). Morphometry and mechanical design of tube foot stems in sea urchins: A comparative study. Journal of Experimental Marine Biology and Ecology, 315, 211–223.
Santos, R., & Flammang, P. (2006). Morphology and tenacity of the tube foot disc of three common European sea urchin species: a comparative study. Biofouling, 22, 187–200.
Santos, R., & Flammang, P. (2007). Intra- and interspecific variation of attachment strength in sea urchins. Marine Ecology Progress Series, 332, 129–142.
Santos, R., & Flammang, P. (2008). Estimation of the attachment strength of the shingle sea urchin, Colobocentrotus atratus, and comparison with three sympatric echinoids. Marine Biology, 154, 37–49.
Santos, R., Hennebert, E., Coelho, A. V., & Flammang, P. (2009b). The echinoderm tube foot and its role in temporary underwater adhesion. In S. N. Gorb (Ed.), Functional Surfaces in Biology (pp. 9–41). Springer.
Saxena, S. C., & Chandy, M. (1966). Adhesive apparatus in certain Indian hill stream fishes. Journal of Zoology, 148, 315–340.
Saxena, S. C. (1959). Adhesive apparatus of a hill stream cyprinid fish Garra mullya (Sykes). Proceedings of the National Institute of Sciences of India, 25, 205–214.
Schmidtberg, H. (1999). Ultrastructural studies of the suckers of newly hatched Eledone moschata and Octopus vulgaris (Mollusca; Cephalopoda). In F. Olóriz & F. J. Rodríguez-Tovar (Eds.), Advancing research on living and fossil cephalopods (pp. 203–221). Springer US.
Schoenfuss, H. L., & Blob, R. W. (2003). Kinematics of waterfall climbing in Hawaiian freshwater fishes (Gobiidae): Vertical propulsion at the aquatic–terrestrial interface. Journal of Zoology, 261, 191–205.
Shimek, R. L. (1988). The functional morphology of scaphopod captacula. The Veliger, 30, 213–221.
Smith, A. M. (1991a). The role of suction in the adhesion of limpets. Journal of Experimental Biology, 161, 151–169.
Smith, A. M. (1991b). Negative pressure generated by octopus suckers: A study of the tensile strength of water in nature. Journal of Experimental Biology, 157, 257–271.
Smith, A. M. (1992). Alternation between attachment mechanisms by limpets in the field. Journal of Experimental Marine Biology and Ecology, 160, 205–220.
Smith, A. M., Kier, W. M., & Johnsen, S. (1993). The effect of depth on the attachment force of limpets. The Biological Bulletin, 184, 338–341.
Smith, A. M., Quick, T. J., & St Peter, R. L. (1999). Differences in the composition of adhesive and non-adhesive mucus from the limpet Lottia limatula. The Biological Bulletin, 196, 34–44.
Stewart, R. J., Ransom, T. C., & Hlady, V. (2011). Natural underwater adhesives. Journal of Polymer Science Part B, Polymer Physics, 49, 757–771.
Stewart, R. J., & Wang, C. S. (2010). Adaptation of caddisfly larval silks to aquatic habitats by phosphorylation of h-fibroin serines. Biomacromolecules, 11, 969–974.
Stewart, R. J., Weaver, J. C., Morse, D. E., & Waite, J. H. (2004). The tube cement of Phragmatopoma californica: A solid foam. Journal of Experimental Biology, 207, 4727–4734.
Teimori, A., Esmaeili, H., & Ansari, M. (2011). Micro-structure consideration of the adhesive organ in doctor fish, Garra rufa (Teleostei; Cyprinidae) from the Persian Gulf Basin. Turkish Journal of Fisheries and Aquatic Sciences, 11, 407–411.
Thomas, L. A., & Hermans, C. O. (1985). Adhesive interactions between the tube feet of a starfish, Leptasterias hexactis, and substrata. The Biological Bulletin, 169, 675–688.
Tyler, S. (1976). Comparative ultrastructure of adhesive systems in the Turbellaria. Zoomorphology, 84, 1–76.
Tyler, S. (1988). The role of function in determination of homology and convergence - Examples from invertebrates adhesive organs. Fortschritte der Zoologie, 36, 331–347.
Tyler, S., & Rieger, G. E. (1980). Adhesive organs of the Gastrotricha. Zoomorphology, 95, 1–15.
Vera Candioti, F., Haas, A., Altig, R., & Peixoto, O. (2017). Cranial anatomy of the amazing bromeliad tadpoles of Phyllodytes gyrinaethes (Hylidae: Lophyohylini), with comments about other gastromyzophorous larvae. Zoomorphology, 136, 61–73.
Wainwright, D. K., Kleinteich, T., Kleinteich, A., Gorb, S. N., & Summers, A. P. (2013). Stick tight: suction adhesion on irregular surfaces in the northern clingfish. Biology Letters, 9, 20130234.
Waite, J. H. (1992). The formation of mussel byssus: anatomy of a natural manufacturing process. Results and Problems in Cell Differentiation, 19, 27–54.
Waite, J. H. (2017). Mussel adhesion - Essential footwork. Journal of Experimental Biology, 220, 517–530.
Waite, J. H., Hansen, D. C., & Little, K. T. (1989). The glue protein of ribbed mussels (Geukensia demissa): a natural adhesive with some features of collagen. Journal of Comparative Physiology B, 159, 517–525.
Walker, G. (1972). The biochemical composition of the cement of two barnacle species, Balanus hameri and Balanus crenatus. Journal of the Marine Biological Association of the United Kingdom, 52, 429–435.
Wang, Y., Yang, X., Chen, Y., Wainwright, D. K., Kenaley, C. P., Gong, Z., Liu, Z., Liu, H., Guan, J., Want, T., Weaver, J. C., Wood, R. J., & Wen, L. (2017). A biorobotic adhesive disc for underwater hitchhiking inspired by the remora suckerfish. Science Robotics, 2, eaan8072.
Weiner, J., III, Beaussart, F., & Bornberg-Bauer, E. (2006). Domain deletions and substitutions in the modular protein evolution. The FEBS Journal, 273, 2037–2047.
Whittington, I. D., & Cribb, B. W. (2001). Adhesive secretions in the Platyhelminthes. Advances in Parasitology, 48, 101–224.
Young, G. A., Yule, A. B., & Walker, G. (1988). Adhesion in the sea anemones Actinia equina L. and Metridium senile (L.). Biofouling, 1, 137–146.
Acknowledgments
This work was supported by the ARC project “PROTEST” (Production and testing of recombinant sea star adhesive proteins; “Communauté française de Belgique—Actions de Recherche Concertées”, ARC-17/21 UMONS 3), by the Fund for Scientific Research of Belgium (F.R.S.-FNRS) under grant “Projet de Recherche” n° T.0088.20, by the Fundação para a Ciência e Tecnologia through Project grants (IF/00006/2015/CP1276/CT0001; UIDP/04292/2020), and by the European Cooperation in Science and Technology (COST) Action CA15216. JD and PF are respectively Postdoctoral Researcher and Research Director of the F.R.S.-FNRS. This paper is a contribution to the Centre Interuniversitaire de Biologie Marine (CIBIM).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
16.1 Electronic Supplementary Material
Supplementary Table 16.1
List of metazoans using either bioadhesive secretions or suction attachment and for which some information is available in the literature (XLSX 102 kb)
Supplementary Table 16.2
Amino acid compositions (values in mol%) of bioadhesive secretions from various species of metazoans as well as of average proteins (see main text for details) (XLSX 12 kb)
Supplementary Table 16.3
List of adhesive proteins used in the CLANS analysis (XLSX 16 kb)
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Delroisse, J., Kang, V., Gouveneaux, A., Santos, R., Flammang, P. (2023). Convergent Evolution of Attachment Mechanisms in Aquatic Animals. In: Bels, V.L., Russell, A.P. (eds) Convergent Evolution. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-031-11441-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-11441-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-11440-3
Online ISBN: 978-3-031-11441-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)