Abstract
While the human central nervous system (CNS) has historically been believed to be devoid of a lymphatic drainage system, recent immunological and physiological studies have provided evidence in support of a CNS lymphatic drainage system in vertebrate animals [1–4]. More specifically, accumulating evidence has suggested that communication exists between brain parenchyma (e.g., tissue and blood microvasculature), perivascular and interstitial spaces, astrocyte activity, and draining lymphatic vessels and cervical lymph nodes (Fig. 59.1). This glymphatic system is believed to operate to clear interstitial solutes and in coordination with meningeal lymphatic vessels to clear fluid and metabolic waste products toward cervical lymph nodes. This recently proposed pathway has gained much attention in light of its potential role in diseases of the circulation and clearance deficiencies [5–7], including Αβ accumulation present in Alzheimer’s disease and aging [8], neuroinflammatory disorders [9], and sleep disorders [10].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
While the human central nervous system (CNS) has historically been believed to be devoid of a lymphatic drainage system, recent immunological and physiological studies have provided evidence in support of a CNS lymphatic drainage system in vertebrate animals [1,2,3,4]. More specifically, accumulating evidence has suggested that communication exists between brain parenchyma (e.g., tissue and blood microvasculature), perivascular and interstitial spaces, astrocyte activity, and draining lymphatic vessels and cervical lymph nodes (Fig. 59.1). This glymphatic system is believed to operate to clear interstitial solutes and in coordination with meningeal lymphatic vessels to clear fluid and metabolic waste products toward cervical lymph nodes. This recently proposed pathway has gained much attention in light of its potential role in diseases of the circulation and clearance deficiencies [5,6,7], including Αβ accumulation present in Alzheimer’s disease and aging [8], neuroinflammatory disorders [9], and sleep disorders [10].
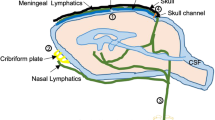
Components of the proposed human central nervous system lymphatic drainage system. (a) Sagittal T1-weighted MRI showing the traditional cerebrospinal fluid (CSF) flow pathway consisting of (1) choroid plexus, which produces CSF; (2) cerebral aqueduct through which CSF flows from third to fourth ventricles, after which CSF circulates through arachnoid space where it can be (3) removed through arachnoid projections into the venous system. This system has been adapted to include (b) a glymphatic pathway, in which CSF influx occurs along periarterial spaces. Aquaporin-4 (AQP4) channels (purple) mediate the flow of fluid from the periarterial space to interstitial space. Convection currents from astrocytes allow for net fluid flow through interstitial space and via AQP4 channels fluid flows into venous perivascular space. Net fluid flow reaches the cervical lymph nodes (a; 4) through small CNS lymphatic channels
Due to the relative novel discovery of this system, gaps remain in our knowledge regarding the precise mechanisms underlying lymphatic-based waste and pathogen clearance in the CNS, as well as how possible glymphatic dysfunction may be implicated in disease. Here, we briefly review what is known regarding the peripheral lymphatic system; we subsequently summarize conventional viewpoints of CSF circulation and CNS fluid clearance in humans and how this view is being modified as a result of recent data pertaining to a possible CNS lymphatic pathway. We also include discussions on emerging, early work outlining how glymphatic dysfunction may be implicated in multiple CNS clearance disorders with largely unknown pathophysiology, report on the challenges from a radiological perspective on glymphatic measurement tools, and present this body of work in the context of current limitations and future directions for this field.
Anatomy and Function of the Peripheral Lymphatic System
Before summarizing the components of the proposed glymphatic system, it is useful to review the anatomy and function of the peripheral lymphatic system to understand similarities as well as differences between these two systems. Briefly, the peripheral lymphatic system has been known to be a central component of the vascular system since the seventeenth century, and consists of a network of lymphatic vessels and organs including lymph nodes, spleen, tonsils and thymus. In healthy adults, approximately 20 L of fluid per day is processed through capillary filtration, with the majority of this fluid (approximately 17 L) returning directly to the blood vasculature and the balance removed to interstitial spaces. The lymphatic system operates to process and clear this interstitial fluid, and ultimately returns this fluid to the blood vasculature following processing in lymphoid tissues.
Unlike the blood circulation, the lymphatic system is unidirectional and open-ended, in which lymphatic fluid moves from the blood circulation into interstitial spaces as a result of ultrafiltration from blood capillaries, interstitial fluid, and lymphatic capillaries [11,12,13,14]. Entry of lymph, protein-enriched fluid, into the lymphatic system occurs at the level of the lymphatic capillaries, which communicate closely with blood capillaries in the interstitium; these lymphatic capillaries absorb macromolecules (10–100 nm in diameter) through open junctions [15, 16]. Open junctions permit fluid entrance into the lymphatic capillaries when the total interstitial pressure, including hydrostatic and colloid osmotic pressures, is less than intravascular blood capillary pressure. Semielastic fibers, or anchoring filaments, which connect the lymphatic capillaries to the surrounding connective tissue, respond to increases in interstitial pressure pulling open the overlapping junctions of lymphatic capillaries unidirectionally to permit fluid containing macromolecules to enter the lymphatic capillaries [17]. The volume of lymphatic fluid entering the lymphatic capillaries is referred to as the lymphatic load. Small lymphatic precollectors join the lymphatic capillaries which then transport the lymphatic load to larger lymphatic vessels or collectors (0.1–0.6 mm in diameter). Fluid is carried to lymph nodes via these lymphatic collectors through forces supplied by smooth muscle contractions at a comparatively slow velocity in relation to other fluid transport within the body, generally at a mean velocity much less than 1 cm/s. The lymphatic collectors contain valves that ensure the unidirectional flow of fluid, as well as lymphangions which contract (autonomic contraction referred to as lymphangiomotoricity; 10–12 contractions per minute) to assist with lymphatic flow. The lymphatic collectors forward lymphatic fluid to the larger lymphatic trunks and lymph nodes.
Humans have on average 600 to 700 lymph nodes, with the majority located in head and neck (cervical and supraclavicular), intestinal (inguinal) and underarm (axilla) regions. Lymph nodes vary in size (typically with a maximum diameter of 5–30 mm) in adults and are present at intervals along lymphatic collectors in clusters or chain form. Afferent lymphatic collectors transport lymphatic fluid to the lymph node cortex, where it is filtered, whereas efferent lymphatic collectors transport fluid from the lymph node hilum either to lymphatic trunks or additional lymph nodes. Lymphatic fluid is ultimately returned to the blood circulation through the venous system. Approximately 75% of lymphatic fluid is returned via the thoracic duct into the left venous angle (left internal jugular and left subclavian vein), whereas approximately 25% of lymphatic fluid is returned via the right lymphatic duct into the right venous angle (right internal jugular and subclavian veins) [18,19,20].
The lymphatic system plays a crucial role in the immune system for host defense, in nutrient absorption function, and in maintaining osmolyte homeostasis for fluid balance. Lymphatic organs and the vasculature are both the source and transport pathway of the innate and adaptive immune system. Lymphatic endothelial cells are capable of recognizing invasive pathogens, and signaling for immune cell responses, whereas lymphatic organs store immune cells such as lymphocytes, T cells, and B cells. The role of lymphatics in assisting with metabolite transport and metabolic regulation is increasingly recognized. The lymphatic vessels from the intestines absorb chyle (fatty acids), transport lipoproteins [21], and participate in fatty acid oxidation [22]. In addition, the lymphatic system is critical for maintaining the homeostasis of body fluid and thereby tissue volume, as it processes and returns capillary ultrafiltrate and plasma proteins back to the blood circulation. In most tissues except intestinal mucosa and kidneys, the lymphatic capillaries reabsorb the arterial capillary filtrate that would otherwise accumulate in the interstitial space as depicted through the revised Starling–glycocalyx model [23]. This revised Starling model takes into account the contributions the semipermeable endothelial glycocalyx layer has on opposing the filtration rate and amends the role of the interstitial fluid pressures where slight filtration remains in the microvasculature [23, 24]. The capillary ultrafiltrate is drained by lymphatic vessels, processed by lymph nodes, and returned to the blood circulation. When lymphatic load exceeds the lymphatic transport capacity (i.e., highest possible lymphatic flow per unit time), fluid balance is disrupted, protein-rich lymph fluid accumulates in the interstitium, and macroscopic swelling, or lymphedema, results. Without effective lymphatic function, protein-rich filtrate will progressively accumulate within the subcutaneous and deep tissue, setting the stage for permanent lymphatic vascular impairment and compromised immune function. A damaged lymphatic system is prime for chronic inflammation, lymphedema, tissue fibrosis within the tissue, and risk for recurrent infections.
Peripheral lymphatic disorders present when the functioning of the lymphatic system is insufficient such that the lymphatic transport capacity cannot compensate for the lymphatic load. Lymphedema is classified as either primary lymphedema (developmental abnormality) or secondary lymphedema (lymphatic mechanical impairment following medical procedures or trauma). Primary lymphedema can present due to (1) hypoplasia (most common, malformation of the lymphatic vessels, and lymphatic vessels are reduced in number and/or smaller than normal), (2) hyperplasia (macrolymphatics or lymphangiectasia where lymph vessels are larger than normal and oftentimes valvular insufficiencies), (3) aplasia (congenital absence or defective development of lymphatic vessels, nodes or capillaries), or (4) primary lymph node fibrosis (fibrosis of the lymph node capsule and trabecular area impacting lymph flow in afferent lymph collectors) [25]. The lymphatic anomalies reduce the lymphatic transport capacity and are further traditionally defined by the time of development, though may better be classified by phenotype–genotype correlations [26]. Congenital lymphedema is present at birth (Milroy’s disease), Lymphedema praecox (Meige’s disease) develops after birth and before 35 years of age, and Lymphedema Tarda is primary lymphedema that develops after 35 years of age [16]. Congenital lymphedema is an inherited (familial) condition whereas the other forms of primary lymphedema are considered sporadic (nonfamilial) conditions.
Secondary lymphedema is the development of lymphedema due to an identified cause such as cancer (malignant lymphedema), surgery, radiation therapy, trauma, infection, and/or chronic venous insufficiencies. Filariasis is the most common reason for secondary lymphedema, though in western countries, secondary lymphedema is most frequently a complication of cancer treatment, which often involves radiation and/or surgical resection of lymph nodes [26], and is a chronic and lifelong condition. More than 250,000 new cases of invasive breast cancer are diagnosed per year in the United States and associated breast cancer treatment-related lymphedema (BCRL) has a chronic and lifelong condition with a high mean 2-year incidence of 30% in cancer survivors treated with lymph node removal [27]. Additionally, secondary lymphedema from other cancer treatments which have an overall incidence similar to BCRL are lower extremity melanoma (28%), gynecological cancers (20%), and sarcoma (30%) [28]. The underlying mechanism of cancer related lymphedema is well-characterized: when lymphatic transport capacity exceeds lymphatic load, protein-rich fluid accumulates in the interstitium, and macroscopic swelling, or lymphedema, results. However, there is more uncertainty regarding subclinical mechanisms: why only some patients develop lymphedema, whether surgical practices can be adapted to reduce incidence, and how emerging pharmacological or surgical interventions impact lymphatics [29, 30]. Chronic venous insufficiencies may lead to lymphatic disease when a failing venous transport capacity eventually results in extravascular fluid accumulation that is compensated for by lymphatic uptake. After chronic and progressive demands on the lymphatic system, chronic venous insufficiency initially develops into dynamic lymphatic insufficiency (phlebo-lymphodynamic insufficiency) and progresses into functional and morphological alterations of the lymph vessels and blood capillaries.
Furthermore, disruption of lymphatic function is implicated in many healthcare challenges of the twenty-first century, including infection and cellulitis [19, 31], human immunodeficiency virus (HIV) reservoirs [32], and cancer metastasis [33]. Until recently, it was largely believed that the CNS was devoid of a similar lymphatic waste and pathogen clearance system; however, this assumption has been called into question in light of several recent developments. It is also not unreasonable that the CNS, which is central to the coordination of bodily functioning at multiple levels, requires a sophisticated waste and pathogen identification and clearance system. As will be described further, many CNS conditions with unknown etiology exhibit phenotypical signatures of fluid transport dysfunction, which could manifest as edema or mis-compartmentalization of molecules, implicating lymphatic disruption and leading either directly or indirectly through various cascades to cellular toxicities.
Unmet Needs: Chronic Neurodegenerative Disorders
Part of the motivation for considering a human CNS lymphatic system comes from a general lack of understanding of many chronic neurodegenerative disorders, which have been implicated to have central features related to waste clearance deficiency. Specifically, our collective understanding of the pathophysiology of chronic neurologic disease has lagged behind that of acute neurologic injury. In the setting of traumatic injury to the brain, dramatic improvements in morbidity and mortality have been achieved with timely surgical intervention. The foundation of these advances is our comprehension of the underlying pathophysiology in these processes. For example, subarachnoid hemorrhage leading to hydrocephalus allows us to know that many patients with subarachnoid hemorrhage will benefit from ventricular drain placement. Epidural hemorrhage which often precedes severe mass effect on the brain is very frequently a clear indication for acute neurosurgical decompression. In contrast, despite our longstanding recognition of many chronic neurological disorders these diseases are often poorly understood, and the most effective treatments are consequently precluded.
Over the past decade, imaging characterization of Alzheimer’s disease has substantially progressed, particularly with the advent of volumetric MRI and fluorodeoxyglucose (FDG) PET and amyloid PET imaging using a growing number of available radiotracers [34]. Disproportionate atrophy in the medial temporal lobe and entorhinal cortex on MRI and decreased glucose metabolism in the parietotemporal association cortices, posterior cingulate, and precuneus regions are important features that can aid in earlier disease recognition [35]. Furthermore, association with beta amyloid (Αβ) plaques and intracellular accumulation of neurofibrillary tangles has provided important insight into the mechanisms of Alzheimer’s disease pathophysiology. However, despite these advancements, the fundamental causative mechanisms of Alzheimer’s disease remain opaque and consequently prevention and treatment remain profoundly limited.
In addition to Alzheimer’s disease there is a broad collection of chronic neurologic diseases that disproportionally affect the elderly patient. One such entity is Normal Pressure Hydrocephalus (NPH) which is commonly as associated with the clinical triad of gait apraxia, urinary incontinence and dementia. Imaging features such as disproportionate enlargement of the ventricular system and sparing of sulcal prominence at the vertex can provide important clues that complement clinical diagnosis. Despite these helpful signs and symptoms, the underlying mechanism of disease is very poorly understood. Shunting of CSF away from the ventricles does provide some symptomatic relief in well-selected patients [36]. Unfortunately, this disease is often not substantially treatable with shunting at the time of diagnosis and clinical options are substantially limited. It remains possible that more robust understanding of the pathophysiology of this disease would allow for earlier detection and more effective therapies.
Compared to the previously mentioned chronic neurologic diseases, relatively more progress has been made unraveling the pathophysiology of multiple sclerosis, with treatment options now available that can profoundly alter disease course [37]. An autoimmune mechanism of disease characterized by activated T cells leading to destruction of myelinated axons is now recognized as central in multiple sclerosis [38]. This insight in turn has provided important targets for pharmaceutical intervention. Furthermore, MRI combined with clinical examination and laboratory analysis provides important information regarding disease activity and need for management changes [39]. Despite this substantial progress, the primary causative agent and/or process responsible for this disease remain unclear.
Finally, while overt stroke is described by a severe disruption of blood and related nutrient supply to the brain, and has well-characterized etiology most often in terms of arterial steno-occlusion and associated atherosclerosis in most western countries, the etiology of small vessel disease is much less well characterized. Small vessel disease describes a spectrum of neuropathological processes that are generally age-dependent and affect arterioles, capillaries, and venules and can lead to gray and white matter damage visible on anatomical imaging. Such damage can manifest as white matter lesions, lacunes, enlarged perivascular spaces, and/or cerebral microbleeds. While the etiology of small vessel disease and its multiple variants is not well characterized, small vessel disease is known to be linked with stroke risk as well as cognitive decline. Traditional ischemic risk factors such as hypoperfusion, severely stenosed or occluded supplying arteries (e.g., macrovascular arterial steno-occlusion), and atherothromboembolic disease also cannot fully explain small vessel disease, as these conditions are only inconsistently present in patients with small vessel disease. Therefore, it is logical that small vessel disease has origins in dysfunctional microvascular processes that are not well understood.
The recent discovery of the so-called glymphatic system offers a new perspective of how fluid circulation and thus waste clearance occurs within the CNS. It is worthwhile to note that a new perspective on fluid circulation and clearance is not only relevant to CSF disorders, but potentially to multiple other conditions where reduced CSF flow may contribute to accumulating toxic substrates, inefficient circulation of blood products, and even miscompartmentalization of immune cells as described below. Given this new insight, it is important to consider how this newly illuminated system may relate to chronic neurologic disease. Numerous chronic neurologic diseases are long recognized though poorly understood, are associated with substantial morbidity in large portions and are not adequately mitigated by robust prevention and treatment therapies. Perhaps our limited understanding of the underlying pathophysiologies of these respective diseases is related to a fundamental misunderstanding of how waste and fluid clearance is accomplished within the CNS.
Overview of the Human Glymphatic System
Traditional Model of CSF Flow and Clearance
The classical model of CSF production and clearance dictates that fluid is produced by the choroid which is located throughout the ventricular system, though is predominantly located in the atria of the lateral ventricles. Net fluid flow subsequently occurs through the foramen of Monro into the third ventricle, then progresses through the cerebral aqueduct and fourth ventricle. From this location CSF then traverses the foramen of Magendie and Luschka and subsequently extends throughout the subarachnoid space to bathe the spinal cord and remainder of the brain surfaces. Resorption of CSF is traditionally thought to occur at the level of the arachnoid granulations, which then allows fluid from the CSF to be resorbed into the venous circulation.
Expansion of CSF Flow and Clearance in the Glymphatic Pathway
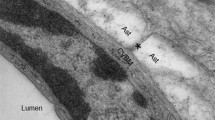
The above model of CSF circulation is being adapted in light of recent immunological and physiological studies, primarily in animals, that have provided evidence in support of a CNS lymphatic drainage system [1,2,3]. This system has been proposed to exist throughout the brain and suggests a pathway along perivascular spaces whereby CSF exchanges with interstitial fluid within the brain parenchyma itself (Table 59.1). Specifically, as shown in Fig. 59.1, it has been suggested that CSF can also circulate into brain parenchyma along perivascular spaces surrounding penetrating arteries. The perivascular space is flanked by blood vasculature and astrocytic endfeet. Fluid entry into the interstitial space, from the perivascular space, is believed to be facilitated by aquaporin-4 (AQP4) water channels, which are localized to the astrocytic endfeet. Fluid transported through these AQP4 channels then moves through the interstitial space by a motion believed to be driven by convection as a result of astrocyte and respiratory activity, and fluid can exit the interstitial space again via AQP4 channels on astrocytic endfeet that line perivenous spaces. Once the fluid exits into the perivenous channel, it is believed that this fluid can exit the CNS either by being absorbed into the venous vasculature via arachnoid granular projections, or, via small meningeal lymphatic vessels that communicate with the perivenous spaces and ultimately drain to cervical lymph nodes. The pathway and mechanism of efflux of perivenous fluid is currently the least well developed component of this newly proposed pathway. Importantly, compared with the peripheral lymphatic circulation described previously, the mechanism of fluid uptake and most likely clearance in this glymphatic system, as currently proposed, differs substantially. As such, the peripheral and CNS lymphatic systems have little overlap in terms of the actual mechanism of waste and pathogen clearance; however, the implications of dysfunction of both systems may have severe and pathological consequences in terms of edema, inflammation, and toxicity.
The above pathways have been proposed primarily as a result of ex vivo and in vivo animal studies (e.g., Table 59.2). In 2012, observation of CSF flow patterns following the administration of a fluoroscopic tracer injection directly into the cistern a magna of mice provided the first clues of CSF and interstitial fluid exchange [40]. This foundational work emphasized the particular significance of the AQP4 channel as an important gatekeeper located at the interface of the perivascular space and the interstitial space. Interest in this system was substantially accelerated following an important discovery that this exchange of fluid is tightly associated with the sleep wake cycle and anesthesia in mice [41]. Importantly, both of these studies focused particular attention to the efflux of radioactive Αβ protein which appeared to closely parallel the flow of fluid to and from the CSF to the interstitial fluid, characterized as glymphatic flow. Due largely to a lack of available methods with sensitivity to perivascular flow, evidence in favor of a glymphatic pathway is primarily based on animal models used in conjunction with immunohistochemistry techniques and/or tracer-based approaches that utilize exogenous CSF or blood tracers [7, 40, 42, 43].
However, in addition to compelling animal data in support of a glymphatic circulation, there are now a few promising human experiments that also demonstrate robust evidence of a human glymphatic circuit. In the CNS, these experiments primarily focus on development of new MRI tools, and recent technical development work quantifying basic MRI relaxation times of lymphatic fluid [44] and lymph nodes [45] are also helping to improve the quantitative accuracy of these emerging tools. In 2017, Taoka and colleagues introduced a method called “Diffusion Tensor Image Analysis Along the Perivascular Space (DTI-ALPS)” which seeks to measure fluid movement associated with glymphatic flow using diffusion tensor imaging [46]. This novel technique measures relative excess flow of fluid in specific areas of the cerebral hemispheres that are parallel to the perivascular space and orthogonal to the projection and association tracts. These regions occur at the axial level of the lateral ventricles and in the region of the lateral projections of the medullary veins Fig. 59.2. Advantages of this technique are that it is noninvasive and that data acquired from most routine DTI MRI acquisitions with sufficiently high b-values (e.g., b > 1000 s/mm2), to reduce sensitivity to venous flow, can be used for this analysis. The authors of this study demonstrated promising results indicating that water diffusion, thought to be reflective of glymphatic flow, correlated significantly with measures of cognitive performance in older adults with Alzheimer’s disease.
Evaluating surrogates of perivascular flow in humans. (a) Minimum intensity projection (10 mm slab thickness) and matching slice of a (b) magnitude SWI, (c) T1-weighted, and (d) primary eigenvector diffusion tensor imaging (DTI) map. The colors correspond to the primary diffusion directions outlined in (e). In (d), three 4 mm isotropic regions of interest (white circles) are placed corresponding to the projection, association and subcortical fibers in the axial plane at the level of the lateral ventricles where the medullary veins course orthogonal to the projection and association fiber tracts. (f) Diffusivity (eigenvalue) values for each axis in each of the three regions for this subject. Note that the x-component of the diffusion direction (Dxx; black arrow) in the projection region may have perivascular flow relevance, given the expected direction of perivascular flow along medullary veins and projection and association fibers running orthogonal to the medullary veins at this location
It is also possible to interrogate the structure and function of the CNS lymphatic system thought to potentially be related to the glymphatic system using intravenous contrast agents of different size. This strategy has been used to visualize potential meningeal lymphatic vessels directly in vivo in both humans and nonhuman primates [6]. Here, T2-weighted FLAIR MRI images were acquired before and after injection of either gadobutrol, a gadolinium-based contrast agent capable of traversing the semipermeable capillary barrier, or larger gadofosveset (molecular weight of approximately 67 kDa), which is not capable of traversing this barrier and remains exclusively intravascular. In both nonhuman primates (marmoset monkeys) and humans, conspicuous hyperintense signal surrounding dural venous sinuses was observed following gadobutrol but not gadofosveset injection, suggestive of vessels distinct from the blood pool. This hyperintense signal demonstrated a curvilinear orientation typical of vessels. On T2-weighted FLAIR imaging following the administration of gadobutrol, the venous sinuses themselves and other known blood vascular structures did not enhance, allowing for selective imaging characterization of these distinct vessels. Additionally, immunohistochemistry was performed on the apparent nonhuman primate lymphatic vessels, which demonstrated that these vessels displayed a similar panel of endothelial markers as what is present in peripheral lymphatic vessels.
While intrathecal gadolinium contrast injection is not approved by the Food and Drug Administration in the United States, gadolinium contrast is occasionally injected into the intrathecal space in challenging cases where a CSF leak is suspected. This clinical scenario has allowed for an imaging description of normal CSF flow in this pseudo control population. These studies have demonstrated that fluid injected into the intrathecal space in the lumbar region progressively ascends through the subarachnoid space of the spine and enters the basal cisterns of the intracranial compartment [47]. Importantly, contrast is then noted to extend along the surfaces of the brain with particular concentration along the course of the anterior and middle cerebral arteries. The contrast is noted to then progressively spread from the extra-axial space to the surface of the brain. Contrast then subsequently extends to the deeper brain parenchyma. These findings are consistent with earlier animal studies documenting glymphatic flow and are highly supportive of a functional glymphatic system in humans.
Potential Relevance of Glymphatic Dysfunction in Disease
Sleep
Shortly after animal studies demonstrated that subarachnoid fluid communicates with interstitial fluid within the brain parenchyma, Xie and colleagues demonstrated that glymphatic flow in mice was highly correlated with sleeping and the anesthetized state [41]. Glymphatic flow in mice was essentially nonfunctional in the awake state. The authors of this study demonstrated that the extracellular space of the brain parenchyma was markedly increased during sleep and the anesthetized state as compared the awake state. In contrast, intracellular volume was decreased during sleep and the anesthetized state.
Another important observation from this study was that the clearance of Αβ protein was highly correlated with the sleep-wake cycle and the anesthetized state, with clearance occurring essentially exclusively in the sleeping or the anesthetized state. Taken together with previous observations that Αβ protein is often associated with Alzheimer’s disease and that Αβ protein is found within the perivascular space and glymphatic pathway, this study provided compelling evidence that impaired waste clearance in the setting of a dysfunctional glymphatic pathway could contribute to Alzheimer’s pathophysiology.
Normal Pressure Hydrocephalus
Normal Pressure Hydrocephalus (NPH) remains a relatively poorly understood disease where there is disproportionate dilatation of the ventricular system relative to the prominence of the subarachnoid space. These patients are classically characterized clinically with the triad of gait apraxia, urinary incontinence and dementia. The presentation however is inconsistent though with many patients not exhibiting all three characteristics. The patients by definition do not have elevated intracranial pressure measurements. In some carefully selected patients, CSF diversion via shunting can significantly reduce symptoms in patients with this condition. MRI has been helpful in the selection of patients for shunting, as higher stroke volumes at the level of the cerebral aqueduct on MRI CSF velocity studies have been shown to have higher rates of clinical improvement following shunting [48].
Despite the presence of a treatment that prompts improved symptomatology in some patients, the pathophysiology of NPH is poorly understood. It has been speculated that NPH is the end result of long term dysfunction of the glymphatic system in those individuals where the traditional route of CSF absorption by the arachnoid villa is compromised [49]. More recently, studies documenting the glymphatic circulation of contrast on MRI following intrathecal gadolinium contrast injection have prompted a more detailed understanding of compromised CSF flow in this population. Abnormal reflux of CSF was demonstrated to fill the enlarged ventricular system following lumbar intrathecal injection of contrast [47]. This is a known feature observed on nuclear medicine cisternography which is often employed in the NPH workup. The major novel finding from this study was that compared to a pseudo control population, NPH patients demonstrated delayed perivascular contrast opacification at the brain surface and delay in subsequent enhancement of the deeper brain parenchyma. This flow of fluid from the subarachnoid space to the periarterial space and then to the brain parenchyma is precisely that of the inflow into the glymphatic system observed in animal studies. Thus, this delayed flow in NPH patients, seen directly in human MR imaging, is highly supportive on an impaired glymphatic system in the NPH population.
Cerebrovascular Disease
Several studies have provided relevance of the glymphatic system in cerebrovascular disease. In a nonhuman primate model, the introduction of subarachnoid hemorrhage was demonstrated to result in the occupation of the perivascular space of the remote brain with blood product. Furthermore, introduction of subarachnoid hemorrhage profoundly limited glymphatic flow of fluid, as seen following the injection of gadolinium contrast [50]. It has been hypothesized that blockage of perivascular spaces by blood products is possible, and this hypothesis has been supported by studies that have shown that intraventricular injection of tissue plasminogen activator (tPA) improves CSF flow [50, 51]. Studies such as these suggest that poor fluid exchange between the CSF and interstitial fluid may be implicated in diverse types of CNS dysfunction.
Additionally, glymphatic dysfunction may predispose patients with subarachnoid hemorrhage to more serious complications such as vasospasm, which is itself incompletely characterized. While calcium-dependent mechanisms are established as a contributing factor to vasospasm, the development of therapeutic interventions is substantially hindered by a poor understanding of the overall underlying pathophysiology [52]. As seen in the animal studies referenced above, subarachnoid hemorrhage preferentially and progressively concentrates along the glymphatic inflow circuit in the arterial perivascular space. This arterial perivascular space surrounds the vessels which are most prone to vasospasm. Furthermore, the time required for blood to travel from the site of hemorrhage in the subarachnoid space to perivascular space mimics the well-established delay that is seen between hemorrhage and initiation of vasospasm. Thus, investigation into potential glymphatic compromise as a predisposing factor underlying the development of vasospasm is merited.
In ischemic stroke, glymphatic clearance has also been implicated. Using gadolinium injection into the cisterna magna of rodents following middle cerebral artery occlusion, it was shown that subarachnoid CSF flow was reduced in the acute window postocclusion (approximately 3 h) but recovered after arterial recanalization [51]. It has also been demonstrated that following middle cerebral artery occlusion in mice, fluorescent tracers present in the infarct core were found to be transported through the glial scar and out of the brain along perivascular spaces [53]. These studies provide evidence that CSF clearance dysfunction and potential glymphatic flow may be relevant for ischemic stroke outcomes; however, much more work is required to further identify these pathways and if present, understand how they can be leveraged to triage patients for acute stroke therapies.
While there is limited convincing data linking cerebral small vessel disease to potential CSF and glymphatic clearance dysfunction, there are multiple avenues in which this link could exist and several studies have provided evidence in support of this possibility, albeit indirectly. White matter lesion burden is largely associated with cognitive performance in older adults. In rats, amount of extravascular imaging tracers, indicative of blood–brain barrier dysfunction, has been shown to be associated with cognitive performance in models of vascular dementia and diabetes [54, 55]. It is logical that reduced interstitial fluid flow and associated solute uptake in the interstitial space could have toxic implications, including development of white matter lesions, lacunes, and other types of small vessel disease. Currently this link remains largely speculative; however, given the poorly characterized etiology of many variants of small vessel disease and their known link with cognitive dysfunction, it is reasonable that glymphatic pathways may have relevance in this condition as well.
Multiple Sclerosis
Glymphatic dysfunction has also been implicated in neuroinflammatory disorders. Multiple sclerosis is the most extensively characterized of these conditions and can be characterized by oligodendrocyte demyelination as a result of autoimmune dysfunction. As such, immune cell access to their cellular targets, which is generally prevented by the blood–brain barrier, is fundamental to disease onset and likely progression. Perivascular astroglial compartmentalization may be compromised in patients with multiple sclerosis, and evidence has been provided for this possibility through rodent models of autoimmune encephalomyelitis in which disease progression was found to be associated with poor compartmentalization of immune cells [56]. In this same model, reduced AQP4 localization is observed, which could be consistent with inadequate localization of immune cells to intended compartments and possible routes for these immune cells to their pathological cellular targets. Finally, the demyelinating disorder neuromyelitis optica shares many phenotypical hallmarks as multiple sclerosis, but neuromyelitis optica patients also test positive for AQP4 auto-antibodies. The logical extension of this finding is that if AQP4 function is disrupted in such patients, then it follows that glymphatic flow and CSF clearance dynamics should be altered as well. Though by slightly different mechanisms, the sequela of these neuroinflammatory disorders is that neurotoxic solutes may accumulate in the parenchyma and/or interstitial spaces, and such accumulation may lead to neurodegeneration.
Alzheimer’s Disease
Alzheimer’s disease is known to be closely associated with extracellular deposition of Αβ-containing plaques [57], and progressive cognitive impairment. While the precise role of Αβ as an initiating factor in AD pathogenesis is debated [58], Αβ brain burden measured with PET is a well-known hallmark of Alzheimer’s disease pathology, and the origins of Αβ clearance deficiencies have been broadly association with vascular risk factors [59]. However, it remains unknown as to why Αβ accumulation begins in subjects in early clinical Alzheimer’s disease stages, typically referred to as mild cognitive impairment (MCI). A logical explanation is that Αβ accumulation is partly associated with abnormal glymphatic function [8], and furthermore, Αβ production rate has been shown not to be different in Alzheimer’s disease and cognitively intact controls, yet the rate of Aβ clearance has been observed to be reduced in Alzheimer’s disease patients [57]. Aβ clearance is known to be partially attributable to proteolytic degradation, efflux across the blood–brain barrier, phagocytosis by microglia, and clearance along cerebrovascular spaces [8, 57, 60,61,62]; however, pathology of these systems has not been shown to be uniquely present in subjects progressing to Alzheimer’s disease.
Aβ accumulation in MCI patients is of particular interest in clinical research, as the stage represents a potential pathophysiological intervention point. The aging process alone results in Aβ accumulation, e.g., older adults (55+ years) without MCI have been demonstrated to have 17% more Aβ accumulation (per PET) than younger adults [63]. However, older adults (55+ years) with amnestic MCI (aMCI) have been demonstrated to have 22% more Aβ accumulation (per PET) than their non-MCI peers [63]. The Aβ accumulation (per PET) does not seem limited to aMCI patients, with findings suggesting mixed-dysexecutive MCI older adults have the highest frequency (73%) of reaching amyloid positivity per PET compared to aMCI (63%) or non-MCI controls (34%) [64]. In preliminary data, evidence for reduced glymphatic flow in humans measured using the above DTI-ALPS methods has been suggested. In this work [46], it was shown in elderly adults with cognitive complaints that the degree of cognitive impairment as quantified by a basic mini-mental state exam was inversely correlated with the extent of glymphatic flow as quantified by the DTI-ALPS measure.
Conclusion
For approximately three centuries, a peripheral lymphatic clearance system has been known to be present and to be central to waste clearance, fluid balance, and host defense. During this period, it was largely believed that the CNS was devoid of a lymphatic system. Over the past decade, it has been suggested that a so-called glymphatic system may be operative in the CNS, which is believed to function to clear waste products via an alternative pathway of CSF, interstitial fluid, and possibly meningeal lymphatic vessels. Central to this system is movement of CSF along periarterial spaces, into the interstitial space via transport through AQP4 channels located on the astrocytic endfeet, and out of the CNS via perivenous spaces. Importantly, while this system is referred to frequently as a CNS lymphatic clearance system, as proposed it has only very limited overlap with the conventional model of capillary filtrate and interstitial fluid transport via lymphatic capillaries and collectors that occur in the rest of the body. However, operative glymphatic clearance dysfunction may have central relevance to many CNS disorders with unknown etiology, including Alzheimer’s disease and neuroinflammatory conditions. The development of new, sensitive imaging measures capable of interrogating the central processes of the glymphatic system will be imperative to understand the relevance of this system in health and disease.
References
Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34(49):16180–93.
Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke. 2013;44(6 Suppl 1):S93–5.
Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. 2017;127(9):3210–9.
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–9.
Sun BL, Wang LH, Yang T, Sun JY, Mao LL, Yang MF, et al. Lymphatic drainage system of the brain: a novel target for intervention of neurological diseases. Prog Neurobiol. 2017;163:118–43.
Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017;6:e29738.
Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–24.
Boespflug EL, Iliff JJ. The emerging relationship between interstitial fluid–cerebrospinal fluid exchange, amyloid-beta, and sleep. Biol Psychiatry. 2018;83(4):328–36.
Simon MJ, Iliff JJ. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta. 2016;1862(3):442–51.
Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, et al. Beta-amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018;115(17):4483–8.
Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. J Clin Invest. 2014;124(3):953–9.
Stranford S, Ruddle NH. Follicular dendritic cells, conduits, lymphatic vessels, and high endothelial venules in tertiary lymphoid organs: parallels with lymph node stroma. Front Immunol. 2012;3:350.
Loukas M, Abel N, Shane Tubbs R, Grabska J, Birungi J, Anderson RH. The cardiac lymphatic system. Clin Anat. 2011;24(6):684–91.
Miller MJ, McDole JR, Newberry RD. Microanatomy of the intestinal lymphatic system. Ann N Y Acad Sci. 2010;1207(Suppl.1):1–11.
Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1(3):219–27.
Choi I, Lee S, Hong YK. The new era of the lymphatic system: no longer secondary to the blood vascular system. Cold Spring Harb Perspect Med. 2012;2(4):a006445.
Leak LV, Burke JF. Fine structure of the lymphatic capillary and the adjoining connective tissue area. Am J Anat. 1966;118(3):785–809.
Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73(1):1–78.
Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. J Clin Invest. 2014;124(3):915–21.
Swartz MA. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50(1–2):3–20.
Randolph GJ, Miller NE. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest. 2014;124(3):929–35.
Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, et al. The role of fatty acid beta-oxidation in lymphangiogenesis. Nature. 2017;542(7639):49–54.
Levick JR, Michel CC. Microvascular fluid exchange and the revised starling principle. Cardiovasc Res. 2010;87(2):198–210.
Woodcock TE, Woodcock TM. Revised starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108(3):384–94.
Kinmonth JB, Wolfe JH. Fibrosis in the lymph nodes in primary lymphoedema. Histological and clinical studies in 74 patients with lower-limb oedema. Ann R Coll Surg Engl. 1980;62(5):344–54.
Grada AA, Phillips TJ. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77(6):1009–20.
DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–15.
Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer. 2010;116(22):5138–49.
Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17(11):1371–80.
Donahue PM, Crescenzi R, Scott AO, Braxton V, Desai A, Smith SA, et al. Bilateral changes in deep tissue environment after manual lymphatic drainage in patients with breast cancer treatment-related lymphedema. Lymphat Res Biol. 2017;15(1):45–56.
Pallin DJ, Camargo CA Jr, Schuur JD. Skin infections and antibiotic stewardship: analysis of emergency department prescribing practices, 2007–2010. West J Emerg Med. 2014;15(3):282–9.
Tawakol A, Ishai A, Li D, Takx RA, Hur S, Kaiser Y, et al. Association of arterial and lymph node inflammation with distinct inflammatory pathways in human immunodeficiency virus infection. JAMA Cardiol. 2017;2(2):163–71.
Goldblatt SA, Nadel EM. Cancer cells in the circulating blood: a critical review II. Acta Cytol. 1965;9:6–20.
Mosconi L, Berti V, Glodzik L, Pupi A, De Santi S, de Leon MJ. Pre-clinical detection of Alzheimer’s disease using FDG-PET, with or without amyloid imaging. J Alzheimer’s Dis. 2010;20(3):843–54.
Frisoni GB, Fox NC, Jack CR Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6(2):67–77.
Shprecher D, Schwalb J, Kurlan R. Normal pressure hydrocephalus: diagnosis and treatment. Curr Neurol Neurosci Rep. 2008;8(5):371–6.
Torkildsen O, Myhr KM, Bo L. Disease-modifying treatments for multiple sclerosis—a review of approved medications. Eur J Neurol. 2016;23(Suppl 1):18–27.
Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162(1):1–11.
Bakshi R, Thompson AJ, Rocca MA, Pelletier D, Dousset V, Barkhof F, et al. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008;7(7):615–25.
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra11.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–7.
Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76(6):845–61.
Pizzo ME, Wolak DJ, Kumar NN, Brunette E, Brunnquell CL, Hannocks MJ, et al. Intrathecal antibody distribution in the rat brain: surface diffusion, perivascular transport and osmotic enhancement of delivery. J Physiol. 2018;596(3):445–75.
Rane S, Donahue PM, Towse T, Ridner S, Chappell M, Jordi J, et al. Clinical feasibility of noninvasive visualization of lymphatic flow with principles of spin labeling MRI imaging: implications for lymphedema assessment. Radiology. 2013;269(3):893–902.
Crescenzi R, Donahue PM, Braxton VG, Scott AO, Mahany HB, Lants SK, et al. 3.0 T relaxation time measurements of human lymph nodes in adults with and without lymphatic insufficiency: implications for magnetic resonance lymphatic imaging. NMR Biomed. 2018;31:e4009.
Taoka T, Masutani Y, Kawai H, Nakane T, Matsuoka K, Yasuno F, et al. Evaluation of glymphatic system activity with the diffusion MRI technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol. 2017;35(4):172–8.
Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain J Neurol. 2017;140(10):2691–705.
Bradley WG Jr, Scalzo D, Queralt J, Nitz WN, Atkinson DJ, Wong P. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MRI imaging. Radiology. 1996;198(2):523–9.
Bradley WG Jr. CSF flow in the brain in the context of normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2015;36(5):831–8.
Goulay R, Flament J, Gauberti M, Naveau M, Pasquet N, Gakuba C, et al. Subarachnoid hemorrhage severely impairs brain parenchymal cerebrospinal fluid circulation in nonhuman primate. Stroke. 2017;48(8):2301–5.
Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke. 2014;45(10):3092–6.
Kolias AG, Sen J, Belli A. Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: putative mechanisms and novel approaches. J Neurosci Res. 2009;87(1):1–11.
Zbesko JC, Nguyen TV, Yang T, Frye JB, Hussain O, Hayes M, et al. Glial scars are permeable to the neurotoxic environment of chronic stroke infarcts. Neurobiol Dis. 2018;112:63–78.
Venkat P, Chopp M, Zacharek A, Cui C, Zhang L, Li Q, et al. White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies. Neurobiol Aging. 2017;50:96–106.
Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2017;37(4):1326–37.
Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462(7269):94–8.
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774.
Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron. 2011;69(2):203–13.
Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363(9415):1139–46.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–41.
Masseguin C, LePanse S, Corman B, Verbavatz JM, Gabrion J. Aging affects choroidal proteins involved in CSF production in Sprague–Dawley rats. Neurobiol Aging. 2005;26(6):917–27.
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain—implications for Alzheimer disease. Nat Rev Neurol. 2016;12(4):248.
Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, et al. 18f-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68(3):319–29.
Bangen KJ, Clark AL, Werhane M, Edmonds EC, Nation DA, Evangelista N, et al. Cortical amyloid burden differences across empirically-derived mild cognitive impairment subtypes and interaction with APOE varepsilon4 genotype. J Alzheimer’s Dis. 2016;52(3):849–61.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Donahue, M.J., Donahue, P.M.C., Crescenzi, R., McKnight, C.D. (2023). Anatomical and Functional Features of the Central Nervous System Lymphatic System. In: Faro, S.H., Mohamed, F.B. (eds) Functional Neuroradiology. Springer, Cham. https://doi.org/10.1007/978-3-031-10909-6_59
Download citation
DOI: https://doi.org/10.1007/978-3-031-10909-6_59
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10908-9
Online ISBN: 978-3-031-10909-6
eBook Packages: MedicineMedicine (R0)