Abstract
Diffusion MRI has many potential advantages over conventional structural MRI for detecting and characterizing both acute seizures and epilepsy. This chapter reviews how diffusion tensor imaging and tractography have provided novel insights into epilepsy-induced changes to the functional and structural organization of the hippocampus, cortex, and underlying white matter for patients with various epilepsy disorders. In temporal lobe epilepsy (TLE) patients, diffusion tensor imaging has demonstrated that (1) there are extensive, often bilateral white matter and extra-hippocampal changes; (2) significant white matter structural differences are evident in patients with right versus left temporal lobe epilepsy; (3) seizure propagation and white matter reorganization may be more widespread when seizures are generated in the dominant cerebral hemisphere due to greater pre-existing connectivity; (4) the extent of white matter and hippocampal diffusion changes may correlate with the duration or age of onset of seizures, supporting the hypothesis that seizures damage the brain in a dose-dependent fashion; and (5) changes in connectivity may help predict positive surgical outcomes. White matter assessment with diffusion tractography in temporal lobe epilepsy patients prior to surgical resection can predict visual field defects based on the extent of resection and also provide objective surrogate markers for language lateralization, verbal and memory function. In malformations of cortical development or tuberous sclerosis, diffusion MRI also demonstrates clinically relevant abnormalities of structural organization both in focal lesions and in nearby or remote normal-appearing white matter. Diffusion tensor imaging continues to expand our knowledge of functional and structural changes associated with various seizure disorders and with further investigation is likely to have a positive impact on the clinical management for individual epilepsy patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Seizures represent abnormal, unregulated discharges of neurons in the brain that disrupt normal function (5% of the population experience at least one seizure before age 80). Epilepsy refers to a chronic condition where seizures repeatedly occur in the absence of a reversible systemic cause (~1% of the population suffers from epilepsy). The International League Against Epilepsy (ILAE) criteria for epilepsy include: At least two unprovoked seizures >24 h apart, one unprovoked seizure and a probability of future seizures similar to the general recurrence risk after two unprovoked seizures or diagnosis of an epilepsy syndrome [1,2,3] (Fig. 43.1). The morbidity and mortality of epilepsy are high. Epilepsy patients have an increased risk of sudden death [4] and epilepsy ranks second only to stroke when comparing years of life lost [5].
Current classification of epilepsies as defined by the International League Against Epilepsy. Adapted with permission from Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. doi: https://doi.org/10.1111/epi.13709
MRI plays an important role in the clinical evaluation of epilepsy patients [6]. However, conventional anatomic MRI lacks sensitivity for detecting epileptogenic lesions in a substantial portion of patients (“MRI-negative” epilepsy) and after 40 years of use may be less likely to produce novel insights into disease detection or pathophysiology. Diffusion MRI can be sensitive to both acute nervous tissue water changes associated with excess neuronal activation and to chronic alterations to the functional organization of the tissue from axonal and synaptic loss—these latter changes can lead to further seizure initiation, propagation and synchronization. Diffusion MRI is a sensitive structural imaging method to assess the impact of epilepsy on the in vivo functional organization and connectivity of the hippocampus, cortex, and white matter.
This chapter reviews how diffusion MRI has been applied to different epilepsy disorders. We first provide an overview of the key technical concepts for diffusion MRI and the white matter pathways that are most commonly involved in epilepsy. We review diffusion MRI changes in the peri-ictal state. The bulk of the chapter addresses various applications of diffusion MRI to temporal lobe epilepsy, the most common adult form of epilepsy. The use of diffusion MRI in malformations of cortical development is then considered briefly. Finally, we suggest potential future directions for research of diffusion imaging in epilepsy disorders.
Fundamental Concepts in Diffusion MRI
In its most simplified form, the addition of two balanced spatially encoding gradients across a spin echo creates an MRI sequence that is sensitive to tissue water diffusion. We review the key concepts for understanding water diffusion observable by MRI in nervous tissue—the more technical details are well described by several reviews for the interested reader [7]. Water molecules in a glass of water will diffuse in a random Brownian motion. In healthy nervous tissue, however, mean diffusivity will appear reduced (relative to “free water” or CSF) as water movement is altered when it encounters and interacts with large proteins, organelles and semi-permeable cellular membranes. This “sampling” of the local tissue structure on the mesoscopic length scale by MRI-observable water is the reason diffusion MRI has become so fundamental to clinical neuroradiology. The sensitivity of a diffusion MRI sequence to water diffusion changes is generally determined by the maximum b-value (a scalar value determined by the size, duration and separation of the diffusion gradients; a typical clinical value is 1000 s/mm2). In acute pathology, like ischemic stroke, mean diffusivity is reduced—the exact biophysical basis for this is not understood, but attributed to simultaneous dynamic changes in the restrictive properties of cellular membranes, altered tortuosity from macromolecules, and changes associated with water re-distribution between the intra- and extracellular compartments [8]. The use of diffusion MRI to detect reduced diffusivity in ischemic stroke remains its most important clinical application [9]. In chronic pathology (like hippocampal sclerosis), mean diffusivity is increased due to neuron loss, gliosis, extracellular space expansion and a loss of normal tissue organization. Unlike acute changes, chronic diffusion MRI changes correlate better with changes observed by histology. Recent analytical methods using diffusion MRI acquisitions with higher b-values (e.g., 2000 s/mm2) also can characterize the non-Gaussian diffusion behavior of water molecules in tissue—this diffusion kurtosis may be more sensitive than diffusivity for subtle changes to nervous tissue complexity in chronic conditions [10].
Myelin provides a formidable barrier to water movement such that diffusivity will appear much lower transverse to membranes, particularly myelinated axons—this orientation-dependence of water diffusivity is defined as anisotropy. Interpretation of diffusion MRI in nervous tissue, particularly in white matter, can be confounded by anisotropy. The diffusion MRI sequence can be made sensitive to water diffusion anisotropy by increasing the number and varying the spatial orientation of the diffusion gradients relative to the brain (increasing the “angular resolution”). The orientation-dependence of observed water diffusion within an image voxel in clinical data is most commonly described using a tensor representation (diffusion tensor imaging; DTI) that provides measures of mean diffusivity and fractional anisotropy (the latter using a scale between 0 and 1, where that glass of water has a value close to “0” and the densely myelinated corpus callosum approaches “0.8”). A dominant vector orientation for diffusion anisotropy in a voxel is also determined. Algorithms can be created to follow the vector orientations voxel-by-voxel through a 3D image dataset under certain user-defined conditions (e.g., fractional anisotropy remains above 0.3) to create diffusion tractograms that capture large portions of known brain white matter pathways (diffusion tractography) [11]. Table 43.1 provides an overview of white matter pathways possibly involved by seizures and epilepsy. Improvements in computational analyses of DTI allows for studies of structural connectivity or “connectomics,” and combining DTI with functional imaging has the potential to expand our understanding of large scale networks and connectivity in patients with epilepsy [12, 13] (Fig. 43.2). Over the past 20 years, diffusion MRI acquisition strategies and the analytical representations of the data have become increasing complex with multiple different models of the underlying nervous tissue microstructure that estimate selective properties for the intra- and extracellular volume fractions. The specific models, their assumptions and limitations, and the data supporting their validation are beyond the scope of this review. It should be noted that these models thus far have largely focused on white matter, which is likely easier to model than gray matter structures.
Flowchart that illustrates the steps of connectivity analysis. MP-RAGE sequence is segmented into ROIs, which are then parcellated into cortical and subcortical ROIs. Whole-brain white matter tractography is performed after voxel-wise tensor calculation, and the density of fibers that connect each pair of cortical ROIs is used to calculate structural connectivity. Reproduced with permission from DeSalvo MN, Douw L, Tanaka N, Reinsberger C, Stufflebeam SM. Altered Structural Connectome in Temporal Lobe Epilepsy. Radiology. 2014;270(3):842–848. doi: https://doi.org/10.1148/radiol.13131044
The signal-to-noise is limited in clinical MRI compared to other imaging techniques. In diffusion-weighted MRI, the signal is reduced ~90% between images obtained with b-value of 0 and 1000 s/mm2. Because of these limitations, clinicians and researchers must choose compromises between the strength of diffusion weighting (resolution in “q-space”), degree of angular resolution and the spatial resolution of the image—this is a key challenge when using diffusion MRI to investigate epilepsy. Further, image discrimination of small temporal lobe structures, the most common lobar substrate for seizures, are particularly confounded by partial volume averaging of the diffusion signal from brain tissue with adjacent isotropic diffusion signal from surrounding cerebrospinal fluid. The fornix, a major output of the hippocampus, is poorly resolved even in normal patients with current advanced diffusion MRI resolutions (2-mm isotropic resolution). The fornix is reduced in size ipsilateral to fronto-temporal seizures [14] making partial volume confounds to diffusion MRI data even worse. Some groups have advocated the use of CSF suppression techniques in diffusion MRI [15], but this approach further reduces SNR by up to 30% [16]. A recent study used relatively low diffusion-weighting and limited coverage to obtain 1-mm isotropic diffusion parameter maps of medial temporal lobe structures in controls [17]—presumably such an approach also will be applied to patients with epilepsy in the near future. Simultaneous multislice acquisitions [18] compressed sensing image reconstructions [19] and recent novel image denoising methods [20] should improve our ability to push spatial, angular or q-space resolution for epilepsy investigations in the near future.
Eddy current, patient motion, geometric distortion and low signal-to-noise (SNR) inherent to commonly used echoplanar imaging sequences [7] also create challenges for diffusion MRI characterization of certain parts of the brain frequently involved in seizure disorders. The medial temporal lobes are especially vulnerable to susceptibility-induced blurring and geometric distortion due to air-filled structures within the underlying temporal bones. These artifacts, which partially depend on the number of phase encoding steps and readout duration, can be mitigated to some degree with improved gradient coil performance, partial Fourier acquisition and parallel imaging [21]. Multishot echoplanar imaging (EPI) [22,23,24] also can significantly reduce distortion at the expense of increased imaging time. 3D and ultrahigh field acquisition methods also have the potential to improve SNR [25]. Periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) is a multishot technique that can be used to decrease susceptibility artifacts [26] with the added benefit of reduced sensitivity to rigid-body patient motion that may help to improve visualization of medial temporal lobe structures [27]. Finally, when whole-brain imaging is not required, reduced field-of-view diffusion (rFOV) imaging can be used to image clinically important medial temporal lobe structures with high spatial resolution [15].
Peri-ictal Diffusion MRI of Seizures
The ionic changes, increased energy demands and increased presence of extracellular glutamate associated with sustained, repetitive electrical activity may lead to the development of cytotoxic edema within nervous tissue in seizures [28, 29] that can be detected as reduced water diffusion using MRI. Diffusion MRI studies of patients suffering from status epilepticus and children with complex febrile seizures (e.g., sustained seizures) have shown reduced diffusivity in a number of brain structures [29,30,31]. Peri-ictal studies, obtained shortly after a more discrete seizure, present a less consistent pattern, especially in patients with longstanding epilepsy [32,33,34,35]. Detection of acute seizure-induced water diffusion changes appears to depend both on the seizure duration and rapid MRI follow-up [32, 36, 37]. Mean diffusivity changes can resolve even within 1–2 h [32, 36]. In patients with unilateral localization-related epilepsy, peri-ictal reduced diffusion may be concordant with epileptogenic lesion localization [30, 35]; however, other studies have suggested limited concordant diffusion and EEG seizure localization [34]. Poor diffusion MRI localization of the seizure focus may be attributed to rapid, widespread involvement of neuronal networks by seizures (similar to what is often observed with EEG). It is impractical to image patients immediately following a seizure; therefore, typical seizure episodes may not be detected with peri-ictal diffusion MRI if they are associated with only transient diffusion changes. With even longer initial follow-up intervals, it appears that most acute seizure-induced diffusion changes have resolved [30, 31, 38]. Less often, changes in water diffusion may progress or be associated with permanent MRI signal changes, particularly if there was excitotoxic injury, such as following prolonged status epilepticus [29]. Peri-ictal reductions in water diffusivity seem to be associated with more severe seizures and suggest increased risk for atrophy on subsequent follow-up imaging [31, 39]. This supports the hypothesis that individual seizure episodes can cause long-term structural damage to the brain.
Diffusion MRI may add to our understanding of the association between complex febrile seizures in children and the subsequent development of temporal lobe epilepsy, the most common adult seizure disorder. A complex seizure lasts greater than 15 min and/or was associated with focal seizure onset or recurrent seizures within 24 h. Between 2% and 8% of children with complex febrile seizures develop epilepsy and 30%–50% of temporal lobe epilepsy patients had complex febrile seizures as children (versus only 6% of the normal population) [40]. Complex febrile seizures can give rise to transient peri-ictal reductions in hippocampal diffusion that progress to atrophy on follow-up imaging months later [31, 39, 41]. Complex febrile seizure patients also do not demonstrate normal age-dependent decreases in hippocampal diffusivity compared to controls at later time points [42]. These data suggest hippocampal damage induced during febrile seizures may progress to hippocampal sclerosis [40] or increase a patient’s susceptibility to developing epilepsy by altering normal neuropil and axonal organization in the hippocampus during development [40]. DTI of children following prolonged febrile seizures at 1 and 6 months demonstrate diffusely reduced FA at 1 and 6 months, which normalized at 12 months post seizure [43]. Diffusion MRI 8 years postfebrile seizures found increases in FA in early maturing white matter tracts and increased AD and MD in peripheral white matter tracts that mature later in life [44]. These studies also suggest febrile seizures may affect white matter maturation. Some studies of patients with perinatal injury or familial predilection for febrile seizures suggest that complex febrile seizures occur because the hippocampus was already abnormal [40]—if true, diffusion may be able to characterize these differences.
Temporal Lobe Epilepsy
Temporal lobe epilepsy (TLE) is the most common adult seizure disorder that is often associated with epigastric auras, behavioral arrest, altered consciousness, amnesia and behavioral automatisms [42]. Many TLE patients do not achieve seizure control with medical therapy, but can achieve seizure-free outcomes, or at least significant reduction in seizure frequency and secondary generalization, by unilateral temporal lobectomy [45]. Conventional MRI plays a major role in the identification of mesial temporal sclerosis (MTS) in patients with longstanding TLE. Architectural distortion, T2 prolongation and atrophy on MRI in MTS correlate with gliosis and neuronal loss in several cell layers, granule cell dispersion and mossy fiber sprouting observed on postresection surgical pathology [46]. Medically refractory TLE patients with concordant lateralization by EEG and hippocampal sclerosis on MRI have the highest postresection seizure-free success rates. Preoperative MRI also can sometimes detect “dual pathology,” whereby epileptogenic lesions are present beyond the mesial temporal structures in 15% of patients—failure to resect this second lesion diminishes postsurgical seizure freedom or control. Patients with a clinical diagnosis of TLE, but without MTS (or other structural MRI abnormalities) are described as “MRI negative” and have less favorable seizure-control following temporal lobectomy. Applications of diffusion tensor imaging and tractography in the temporal lobe epilepsy patient population have focused on improved characterization (or detection) of the mesial temporal lobe pathology, understanding the impact of TLE on white matter connectivity, and prediction of surgical outcome utilizing these techniques.
Hippocampal Pathology
One of the first applications of diffusion MRI to epilepsy was to characterize hippocampal disease in patients with known temporal lobe epilepsy [47, 48]. Conventional MRI findings in hippocampal sclerosis correlate to relatively macroscopic histological features. T2 prolongation is attributed to gliosis and extracellular space expansion, while hippocampal atrophy and loss of normal architecture on MRI correlates with neuronal loss. Water diffusion and fractional anisotropy measurements in the normal hippocampus and its layers depend on specific cytoarchitectural features of pyramidal and granule cell neurons, their surrounding neuropil, and local axonal connectivity (Fig. 43.3) [49, 50]. These features reflect the specific functional organization of the hippocampus so it is reasonable to hypothesize that diffusion MRI may be more sensitive to early or subtle changes associated with seizures (e.g., granule cell dispersion, mossy fiber sprouting and synaptic loss). Diffusion MRI then might have clinical value for accurate seizure lateralization and localization, as well as correlating with specific clinical features, functional impairments and prognosis.
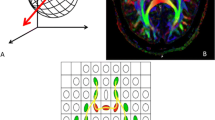
Color fiber orientation map demonstrates the functional cytoarchitectural organization of the ex vivo human hippocampus (1 = hilus, 2 = molecular layer, 3 = mossy fibers, 4 = fimbria, 5 = alveus, 6 = stratum lacunosum-moleculare, 7 = stratum radiatum and pyramidale, 8 = stratum oriens, 9 = prosubiculum, 10 = subiculum, 11 = angular bundle). Developing similar in vivo high-resolution DTI of the hippocampus may increase its specificity and sensitivity for changes related to hippocampal sclerosis and temporal lobe epilepsy. Note, color intensity depends on fractional anisotropy and the color scheme differs from the clinical standard (here, left-right = blue, anterior-posterior = red, and cranio-caudal = green). Reproduced with permission from Shepherd TM, Ozarslan E, Yachnis AT, King MA, Blackband SJ. Diffusion tensor microscopy indicates the cytoarchitectural basis for diffusion anisotropy in the human hippocampus. AJNR Am J Neuroradiol 2007 May;28(5):958–964
In unilateral temporal lobe epilepsy, interictal MRI studies have reported 10%–25% elevations in mean diffusivity in hippocampal sclerosis compared with controls or the contralateral hippocampus [31, 48, 51,52,53,54,55,56,57]. Ipsilateral hippocampus FA also is reduced compared to controls or the contralateral hippocampus in unilateral temporal lobe epilepsy patients [36, 52, 57,58,59]. Reductions in FA in the ipsilateral posterior cingulum and fornix have been identified in patients with MTS [60], as well as the parahippocampal gyrus, bilateral thalami and posterior callosum with connections to the temporal lobe [61]. Increased diffusion and reduced FA within the ipsilateral hippocampus of TLE patients may reflect loss of synaptic complexity and neuronal connectivity, but early studies with low spatial resolution also may have observed such changes just from increase intravoxel contributions from CSF due to hippocampal volume loss [51]. The head, body and tail of the hippocampus have all demonstrated elevated diffusion in unilateral TLE [62], with the anterior portion or hippocampal head demonstrating more substantial changes to diffusion [56] and fractional anisotropy [36]. The amygdala does not demonstrate associated diffusion changes in most TLE patients [56, 63]. Evaluating the dentate gyrus or CA1 region, which are selectively vulnerable to seizure pathology [64], may increase the specificity and/or sensitivity of diffusion MRI for temporal lobe epilepsy with or without hippocampal sclerosis. Ex-vivo ultra-high field imaging and DTI have elucidated hippocampal organization [65] and demonstrated disruptions in hippocampal connectivity [66] in TLE. A high-resolution 7-T diffusion MRI study also found layer specific changes in MRI negative TLE patients [67] (see further discussion below).
Ipsilateral diffusion changes in hippocampal sclerosis suggest diffusion MRI has the potential to independently lateralize temporal lobe epilepsy. Visual recognition by neuroradiologists for intensity changes or asymmetries in diffusion-weighted images, though, may not be as sensitive as absolute quantification for detecting mean diffusivity abnormalities in temporal lobe epilepsy patients [51, 56]. Our experience also is that B1 heterogeneity, poor fat saturation and skull base artifacts from 3-T MRI can make the medial temporal lobe structures in normal individuals appear asymmetrically bright on both FLAIR and diffusion trace images. Several quantitative studies have demonstrated asymmetric unilateral elevations of hippocampal diffusion above study-specific thresholds can have high sensitivity and specificity (>80%) for correct lateralization in TLE based on semiology and EEG as reference standards [56, 68,69,70]. Combining machine learning in combination with graph theoretical analysis of diffusion MRI also has recently demonstrated >70% accuracy for correct lateralization of right and left TLE [71]. Diffusion kurtosis imaging, which may be more sensitive to subtle epilepsy-induced reductions to nervous tissue complexity, detected additional differences in TLE patients not captured by conventional DTI [72, 73] (Fig. 43.4). When combined with support vector machine (SVM), diffusion kurtosis outperformed diffusivity and FA when classifying TLE [74]. One would predict that deep learning methods, potentially using diffusion MRI data, also will perform well classifying TLE.
Voxel-wise maps of white matter abnormalities in patients with TLE compared with controls. Statistical maps are overlaid on an average of probabilistic white matter maps from all subjects. The scale bars represent absolute values of t scores. The first and second rows demonstrate areas with an increase in MD and radial diffusion. The third row demonstrates areas with reduced FA in patients, while the fourth, fifth, and sixth rows demonstrate areas of reduced mean kurtosis, axial kurtosis, and radial kurtosis. Reproduced with permission from Bonilha L, Lee CY, Jensen JH, et al. Altered Microstructure in Temporal Lobe Epilepsy: A Diffusional Kurtosis Imaging Study. American Journal of Neuroradiology. 2015;36(4):719–724. doi: https://doi.org/10.3174/ajnr.A4185
Could diffusion MRI be more sensitive than conventional MRI for structural medial temporal lobe abnormalities underlying temporal lobe epilepsy, particularly in MRI-negative patients? In one study, diffusion was increased and FA reduced compared to controls in one-third of MRI-negative focal epilepsy patients [75]. Similarly, observations of increased ADC without other MRI abnormalities were attributed to early tissue changes in a subset of five TLE patients [76]. More recently, diffusion tensor imaging applied to TLE patients with HS and MRI negative TLE patients have detected decreased FA in the ipsilateral cingulate gyrus and fornix for both groups, with decreased FA in the hippocampus in the MRI negative group [59]. Increased MD also was found in the ipsilateral hippocampus in both groups; however, this was more significantly increased in patients with HS [59]. In contrast, several early studies of MRI-negative TLE patients did not detect significant underlying water diffusion changes [48, 54, 62]. This may have been due to technical and study design limitations at the time; however, publication bias may limit the number of reports that have failed to observe diffusion changes in MRI negative patients and a priori placement of ROIs in known TLE patients limits the external validity of the positive studies. Assessing the sensitivity of diffusion contrast in MRI-negative patients is challenging, since most studies are unable to obtain pathological confirmation of the non-MRI localized epileptogenic lesion. One study reported MRI-negative, diffusion-negative resected hippocampi had gliosis without associated neuron loss [62]—this suggests more substantive tissue changes may be required for detection with diffusion MRI. Recently, high-resolution 7T tractography in patients with MRI-negative TLE demonstrated decreased connectivity in the contralateral CA1 region and subiculum with increased connectivity of ipsilateral subfields such as the subiculum, presubiculum and parasubiculum when compared to controls [67]. Increasing the spatial resolution of diffusion MRI and/or combining it with accurate volumetry (and other quantitative MRI methods) may increase correct localization of MRI-negative TLE patients [77].
Water diffusion changes indicate altered functional organization of the hippocampus and may correlate with certain clinical or prognostic features of TLE. Ipsilateral diffusivity negatively correlated with performance on cognitive tests of recognition and delayed recall [55]. Increased hippocampal diffusivity and reduced FA correlates with increased seizure duration and earlier age at seizure onset [54, 61]. Another interesting result was that TLE patients with the highest ipsilateral hippocampal ADC more frequently reported epigastric sensation auras [57]. Two studies with 40 and 55 patients contradict one another whether increased hippocampal ADC correlated with better temporal lobectomy outcomes [63, 68]. Clinical correlations with diffusion changes in the ipsilateral hippocampus or thalamus may relate to connected neural networks that are involved in seizure epileptogenesis or propagation. In the future, structural connectome studies may help to clarify these seizure networks.
Diffusion changes in the hippocampus also can be associated with increased diffusion in the ipsilateral thalamus [78, 79], parahippocampal gyrus [55, 78], or other temporal lobe structures [57, 78]. Diffusion changes in the contralateral hippocampus of patients with unilateral TLE have been reported as unchanged [52, 78], elevated [51, 62], or decreased [57, 61, 80]. FA also can be reduced in the contralateral hippocampus compared with controls [58]. The counterintuitive reductions in water diffusion reported in the contralateral hippocampus by one group reversed 8 months following resection of the sclerotic, opposite hippocampus, and were attributed to swelling due to neuronal dysfunction without actual cell loss [57, 80]. Other studies have reported bilateral diffusion changes to the adjacent fornix in unilateral TLE patients that did not reverse with surgery [81, 82]. It has been shown that the contralateral hippocampus can demonstrate delayed decline in FA and increase in MD several years after epilepsy surgery, although the clinical significance of this is not yet determined [83]. As diffusion MRI techniques become more sensitive, more changes are observed in more structures—this may reflect the widespread pathology associated with TLE.
White Matter Pathology
Diffusion tensor imaging, diffusion kurtosis and diffusion MRI-based graph theoretical analysis demonstrate widespread white matter abnormalities in TLE patients that are difficult to appreciate using conventional MRI contrast mechanisms (see Table 43.2 for key references). A meta-analysis of diffusion imaging of white matter abnormalities in epilepsy demonstrated that there are greater white matter differences in focal epilepsies than generalized epilepsy and that changes were age- and duration-dependent [89]. Both right and left TLE are associated with abnormal white matter diffusion in the corpus callosum [90,91,92,93], ipsilateral temporal lobe white matter [68, 94,95,85], and external capsule [90, 91]. Statistical parametric mapping demonstrated shared diffusion abnormalities in the ipsilateral limbic system and inferior frontal gyrus for either right or left TLE [78]. DTI tractography studies have demonstrated diffusion changes to the uncinate fasciculus [96] and cingulum for both right and left TLE patients [82, 95, 85]. Further, FA is reduced in the ipsilateral inferior longitudinal fasciculus (ILF), inferior fronto-occipital fasciculus (IFOF), and arcuate fasciculus for both right and left TLE patients [85]. A study using voxel-based and track-based postprocessing of diffusion images demonstrated changes in the superior longitudinal fasciculus (SLF), ILF, IFOF, and uncinate fasciculi, with differences mainly in fractional anisotropy and radial diffusivity, suggesting that multiple parameters should be used to evaluate for white matter changes in epilepsy [97]. The fornix may [82, 98] or may not [99] be affected in all patients with TLE. In histopathologic correlation studies, FA reductions in the ipsilateral fornix in TLE patients undergoing temporal lobectomy correlated with myelin sheath irregularities, increased extracellular space, more variable axonal size [100] (Fig. 43.5) and reduced axonal density [101]. Comparison of postoperative patients with seizure-free outcome to patients with persistent seizures demonstrated significant diffusivity differences in the dorsal ipsilateral fornix and contralateral parahippocampal white matter, and were associated with smaller resections of the uncinate fasciculus [102]. Increased ADC, reduced FA, and/or reduced fiber tract density in the white matter of TLE patients have been attributed variously to edema, gliosis, occult cortical dysplasias, demyelination, and/or neuronal loss.
Correlation of fractional anisotropy (a, d) and deterministic DTI tractography findings (b, e) from the fimbria-fornix pathway with postsurgical electron microscopy of the more rostral fimbria (c, f) for temporal lobe epilepsy (TLE) patients with and without mesial temporal sclerosis (MTS) (HC = hippocampal commissure). TLE patients with MTS had decreased axonal density, membrane circumference, and myelin fractions compared to patients without MTS (P ≤ 0.05). These white matter microscopy changes also showed some statistic correlations with fractional anisotropy in the fimbria-fornix DTI tracts. Reproduced with permission from Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J Neurosci 2010 January 20;30(3):996–1002
Using graph theoretical analysis to evaluate topological and complex networks in TLE has demonstrated overall increased path length and decreased global efficiency, indicating disrupted normal white matter networks [103]. Additionally, graph diffusion based models have been used to predict areas of propagation of neuronal degeneration in TLE with and without MTS [104]. Differences in white matter alteration also are present between patients with clinically established TLE with or without mesial temporal sclerosis. Those with MTS demonstrated more extensive white matter changes with decreased FA in the cingulum, external capsule, internal capsule and uncinate fasciculus that were not seen in patients without MTS [105] Furthermore, studies demonstrated reduced FA in ten major WM tracks in patients with MTS as compared to only changes in the parahippocampal cingulum and tapetum in patients without MTS [106]. Restriction Spectrum Imaging (RSI) studies of patients with temporal lobe epilepsy have shown decreased neurite density in similar locations to changes in FA; however, with greater reductions of neurite density (ND) in the ipsilateral hemisphere, as well as correlation with disease duration [107].
DTI studies also have emphasized the bilateral extent of white matter pathology in unilateral TLE. Both right and left TLE demonstrate increased diffusion in the bilateral temporal lobe white matter [78] and uncinate fasciculus [96]. The bilateral cingulum and fornix demonstrated reduced FA in right or left TLE patients [82]. In patients with unilateral hippocampal sclerosis, there is reduction of cingulum fibers connecting the cingulate gyrus and parahippocampal gyrus [108]. A separate study in adults found bilateral cingulum DTI changes only for left, but not right TLE patients [85]. Children with TLE, but normal conventional MRI, also demonstrate bilateral DTI changes in the uncinate fasciculus, arcuate fasciculus, ILF and corticospinal tracts [109]. Some bilateral white matter diffusion abnormalities in unilateral TLE persist after resective surgery [81, 110], refuting the early hypothesis that extensive white matter changes are only due to neuron dysfunction and edema. In a few studies, white matter diffusion abnormalities did not correlate with differences to postsurgical seizure-free outcomes [81, 91]; however, in more recent studies, patients with poor postsurgical outcomes had more changes in the ipsilateral fornix and contralateral parahippocampal white matter [111]. Widespread white matter changes, often remote from structural abnormalities, suggest widespread involvement of neural networks during seizure propagation. It also has been speculated that bilateral white matter disease detected by DTI represents abnormal connectivity that may precede clinical seizures and predisposes patients to epilepsy [40, 81].
Many DTI studies have highlighted specific connectivity differences between right and left TLE patients. Left TLE patients demonstrate additional FA reductions in the ipsilateral IFOF, arcuate fasciculus, fornix and anterior thalamic radiation, and contralateral changes to the cingulum, anterior thalamic radiation and IFOF [85]. Similar extensive white matter changes were present in a pediatric cohort of TLE patients [109]. Although contralateral white matter changes are not always reported for left TLE [112], several DTI studies have also detected more extensive changes with left compared to right TLE [78, 113,114,115,116]. One study demonstrated more significant changes in the hippocampus and cingulum with right TLE, but more prominent remote changes in left TLE [117]. The more extensive white matter changes associated with left TLE may be the result of more widespread seizure propagation in the dominant left hemisphere due to its greater connectivity [78]. A study comparing DTI and PET activity in preoperative TLE-HS patients demonstrated abnormalities on both modalities involving similar regions [118]. Patients with anterior temporal hypometabolism had abnormalities in anterior association and commissural tracts, while those with posterior temporal hypometabolism had differences in anterior and posterior tracts. DTI probabilistic tractography in left TLE demonstrates loss of asymmetry in the arcuate fasciculus associated with increased bilateral connections compared to controls. In patients after anterior temporal lobectomy, those with left TLE demonstrated normalization in the SLF and uncinate fasciculus FA compared to right TLE, indicating that there is potential for reorganization [119]. These changes reflect side-specific reorganization of language networks underlying verb generation and reading comprehension as demonstrated by functional MRI, whereas right temporal lobe epilepsy patients were similar to controls [120]. An additional study using DSI to evaluate whole brain connectivity demonstrated different structural alterations in right and left TLE [121].
Several studies have tried to localize (or lateralize) TLE based on white matter DTI differences, many of which also combine clinical data and multimodality imaging with PET or MEG. An early report found concordant increased diffusion and reduced anisotropy in the ipsilateral temporal lobe white matter [75]. However, another study demonstrated that increased diffusion in the ipsilateral temporal stem white matter was less accurate than hippocampal diffusivity measurements for lateralization of epilepsy concordant with semiology, EEG, and conventional MRI findings [68]. Utilizing discriminate function analysis of DTI tractography lateralized TLE patients concordant to video EEG with 90% accuracy. Further, this study was 100% accurate for right TLE patients and for a small subset of five patients that were MRI-negative for hippocampal sclerosis [85]. Combination of MEG with diffusion imaging may help as a means of noninvasive lateralization [122]. In MRI-negative children with TLE, extensive white matter diffusion abnormalities were present in the bilateral uncinate fasciculus, arcuate fasciculus, ILF and corticospinal tracts [110]. Another study of adult TLE patients without hippocampal sclerosis demonstrated reduced FA in the fronto-temporal components of the corpus callosum and external capsule, plus reduced diffusion in the cingulum compared with controls. More extensive white matter abnormalities were present in patients with hippocampal sclerosis, but these patients also had longer durations of epilepsy [120]. Multiple studies to date suggest that DTI can detect white matter structural and functional connectivity abnormalities without detectable structural lesions by conventional MRI contrast mechanisms. Additional studies are necessary to determine the clinical relevance of these differences between the various imaging manifestations of temporal lobe epilepsy, which include patients with or without hippocampal sclerosis, cortical dysplasias, dual pathologies, and/or bilateral disease.
Similar to diffusion changes in the hippocampus of TLE patients, some studies of white matter diffusion abnormalities have shown correlations with age of onset or duration of seizure disorder. Higher magnitude reduced anisotropy and increased diffusivity in the posterior corpus callosum correlated with early seizure onset [123]. Similarly, FA decreases in the uncinate fasciculus correlate with the age of onset [112]. Early onset of TLE also is associated with a larger volume of bilateral arcuate fasciculus connections, which may reflect altered language development (discussed later) [124]. A recent study evaluating thalamic connectivity in TLE patients using diffusion imaging demonstrated differences in MD and FA in patients with <15 years of seizures compared to those with >15 years of seizures. Differences in MD and FA also were found in those with better Engel classification following surgery [125]. In a separate study, increased diffusivity in both the uncinate fasciculus and arcuate fasciculus positively correlated with duration of seizures in children with left TLE [110]. Duration of epilepsy has also been correlated to increased mean diffusivity within the left hippocampus in left TLE [117]. One group has suggested unlike diffusivity, FA changes may only occur in the temporal lobe and cingulum white matter after longer durations of disease [85]. Further, patients with longer durations of clinical epilepsy (27 versus 18 years) had reduced anisotropy in the bilateral fornix and cingulum, although this result may be confounded by the presence of hippocampal sclerosis [120]. DTI changes from age of onset and duration of TLE can be hard to disentangle from each other in small patient cohorts, but both factors may relate to a dose-related progressive damage from repetitive seizures [126]. These chronological correlations also could reflect seizure-induced derangement of normal white matter development in children or young adults [123]. Similar to hippocampal diffusivity clinical correlations, findings have not been consistent and there may be a publication bias in the literature, especially since small patient populations limit the statistical power to disprove a small or weak correlation. Unlike hippocampus diffusion changes, white matter connectivity changes may be more amenable to systems-based clinical correlations with specific cognitive abilities (e.g., language and memory). DTI studies of white matter changes also can be combined with functional MRI methods to simultaneously examine concordant activation and structural connectivity changes in TLE [94, 120, 127]. In a study analyzing 24 patients with left TLE in comparison to controls, decreased distant connectivity was found in the medial orbitofrontal cortex, temporal cortex, posterior cingulate, and precuneus, whereas increased local connectivity was found in the medial and lateral frontal cortices, insular cortex, posterior cingulate cortex, precuneus, and occipital cortex [13].
DTI studies also have begun to correlate specific changes to verbal memory and naming ability to the white matter changes observed in TLE. In TLE patients, ADC or FA of the left parahippocampal gyrus white matter correlated with verbal memory [99, 100]. The parahippocampal gyrus white matter may contain several fiber tracts associated with the hippocampus or limbic system, including the uncinate and arcuate fasciculi. The uncinate fasciculus and arcuate fasciculus demonstrate increased diffusion and reduced anisotropy in both adults [112] and children [109] with TLE. Further, TLE is associated with a loss of the normal left-right asymmetry in the diffusion properties of the arcuate fasciculus [109, 128] and uncinate fasciculus [96, 129]. Poor verbal memory performance correlated with increased diffusion in the left uncinate fasciculus for patients with either left or right TLE, but also correlated with increased diffusion in the left IFOF and bilateral arcuate fasciculus [99]. An additional study in patients with L TLE have found alterations in the left arcuate fasciculus in patients during cognitive processes, including decreased connectivity in the left fronto-temporal components of the arcuate fasciculus and increased parietal connectivity [130]. Alternatively, the ILF also has been proposed as the strongest predictor of verbal memory [87]. Additionally, combining hippocampal volume and MD of the ILF may also model prediction of verbal memory impairment [87]. Decreased FA within the left UF in patients with TLE [131] and DTI microstructural change in the thalamocortical pathways [132] was associated with poor executive function on multiple clinical tasks. Alternatively, uncinate fasciculus diffusion changes correlated with Wechsler Memory Scale scores in left, but not right TLE patients [96]. This was further validated in another study demonstrating that patients with left TLE had positive correlations between WM diffusivity characteristics and cognitive performance and that patients with significant diffusion abnormalities had decreased cognitive performance [133]. Poor naming ability correlated with increased diffusivity and decreased anisotropy in the both uncinate and arcuate fasciculus [99]. In patients with left TLE, overall poor executive function correlated with decreased FA of the uncinate fasciculus [131]. Another study suggested verbal naming ability in TLE patients might correlate better with more lateralized arcuate fasciculus asymmetry [124]. In contrast, there is conflicting data whether correlations exist between nonverbal memory and white matter diffusion changes. In TLE patients, volume and fractional anisotropy of the right parahippocampal gyrus white matter correlated with nonverbal memory [134], but another study found no correlations between nonverbal memory or fluency and different white matter tracts [99].
It is helpful clinically to understand the impact of TLE on patient’s cognitive abilities when determining treatment options. In patients with medically refractory seizures, surgical resection of the ipsilateral temporal lobe structures (hippocampus, amygdala, parahippocampal gyrus, and/or entire temporal pole) can eliminate or significantly decrease the frequency of seizures [45]. There are a number of studies evaluating white matter changes postsurgery, with the goal of being able to predict patients that will be seizure-free postoperatively. Both right and left TLE patients free of seizures after resection of hippocampal sclerosis demonstrate reduced fractional anisotropy in the corpus callosum, ipsilateral arcuate fasciculus, cingulum, uncinate fasciculus, inferior frontal-occipital fasciculus, and corticospinal tracts compared with controls [112]. In one published longitudinal follow-up after surgical resection of hippocampal sclerosis, DTI changes to the corpus callosum, external capsule, contralateral fornix, and cingulum persisted despite 1-year seizure-free outcomes, suggesting a persistent and/or predisposing developmentally abnormal network for seizure propagation in these patients [81]. Continued work is needed to better understand how epileptogenic networks are affected by surgical resection of presumed epileptogenic foci in pediatric and adult patients, especially in the patient population that demonstrates seizure recurrence at more chronic follow-up time points.
Surgical Planning
Presurgical planning for TLE patients involves a multimodality, interdisciplinary assessment of the patient to establish medically refractory disease, localize the epileptogenic focus, and accurately predict risk for complications from resection. Complications result from injury to eloquent, still-functional temporal lobe structures or pathways from the surgical dissection required to reach and resect the hippocampus and other medial temporal lobe structures.
Previous studies evaluating hippocampal and white matter changes in pre- and postsurgical cases of temporal lobe epilepsy were limited. In one study, bilateral white matter changes to the cingulum and fornix in preoperative DTI did not portend poor postsurgical prognosis [81, 91], in contrast to the known poor prognosis associated with resective surgery in patients with bilateral hippocampal sclerosis. With regard to the hippocampus, one study has reported a positive correlation between increased hippocampal ADC and positive surgical outcomes in 55 patients [63] yet another study of 40 patients failed to detect a similar relationship [68].
More recently, multiple studies have focused on predictive features of TLE with positive postsurgical outcomes. Using functional resting state and DTI imaging, a connectivity model was used to predict outcomes postepilepsy surgery on a small sample size [135]. Although larger studies are necessary, patients with DTI images prior to and postsurgery, with connectivity aberrations outside of the surgical resection, demonstrate poorer prognosis postsurgically [136]. Combining DTI data with clinical data has shown up to 88% accuracy in predicting patients with a positive postsurgical outcome [136]. Computational models based upon DTI imaging have been proposed to improve surgery success rates in TLE [88]. Machine learning algorithms in temporal lobe epilepsy have reported seizure-free outcome with 79% accuracy and 65% sensitivity in a small retrospective study [137] with predictive models identifying important features involving ipsilateral and contralateral structures [138]. Patients with altered DTI involving bilateral thalamotemporal connections are not as likely to have a seizure-free outcome in the postsurgical state [139]. Comparison of postoperative patients with seizure-free outcomes and those with continued seizures demonstrated significant diffusivity differences in the dorsal ipsilateral fornix and contralateral parahippocampal white matter, as well as smaller resections of the uncinate fasciculus [111].
An additional topic that requires future research involves determining structural changes associated with different surgical approaches. Previously reported, resecting the anterior two-thirds of the temporal pole causes further reduction in fractional anisotropy of the inferior longitudinal fasciculus compared with selective amygdala-hippocampectomy [112]. Yet, another small study did not detect a significant relationship between type of surgery and white matter changes, particularly in the limbic system [81]. Many patients experience decline in naming ability following anterior temporal lobectomy. Initial studies suggest that the greatest decline in naming ability occurs for individual temporal lobe epilepsy patients that undergo temporal lobectomy in the dominant hemisphere as assigned by differences in fMRI activation and DTI assessment of the arcuate fasciculus (Fig. 43.5) [124]. Direct correlation between surgical disruptions of the arcuate fasciculus (or other language-related pathways) detected by DTI with the severity of postoperative naming difficulties has not yet been reported.
Patients undergoing temporal lobectomy also develop visual field deficits due to surgical disruption of Meyer’s loop. Ironically, driving privileges that patients hoped would be restored with seizure-free outcomes then may be denied due to surgery-induced visual field defects. Surgeries for temporal lobe epilepsy often resect the most anterior portions of this fiber bundle leading to contralateral superior homonymous quadrantanopsia that can spread from the medial to lateral sector depending on the extent of injury [140]. DTI-based tractography studies demarcate the anterior extent of Meyer’s loop between 32 and 37 mm posterior to the temporal pole tip, but there is significant individual patient variation [140,141,142]. Also, the left Meyer’s loop may extend slightly more anterior compared to the right, consistent with more visual field defects reported following left anterior temporal lobectomies [142]. Comparison between pre- and intraoperative DTI demonstrated that the position of Meyer’s loop also shifts several millimeters cranially and medially during the majority of anterior temporal lobectomies [141]. Postoperative DTI has demonstrated decreased fractional anisotropy in the ipsilateral sagittal stratum correlated with the severity of visual field defects after anterior temporal lobectomy [142]. The severity of visual field defects following surgery correlated both with the anterior extent of Meyer’s loop [86] and the distance the resection extends into an individual’s Meyer’s loop as determined by DTI tractography [142]. These results suggest preoperative DTI may have an important role in the preoperative assessment of individual risk for visual impairment following anterior temporal lobectomy.
Malformations of Cortical Development
Malformations of cortical development represent a heterogeneous spectrum of disorders with abnormal cerebral cortex laminar or columnar structure due to deranged neuronal proliferation, migration or cortical network organization [143]. The underlying etiology may be perinatal ischemia or infection, versus germline or somatic mosaic gene mutations. The MRI-visible spectrum of abnormalities includes lissencephaly, focal cortical dysplasia, subependymal, subcortical or band gray matter heterotopias. Individual patients have variable clinical presentations with partial and/or generalized seizures [144]. Focal cortical dysplasia can represent dual pathology in a subset of temporal lobe epilepsy patients that reduces seizure-free prognosis following temporal lobe resection [145]. Conventional MRI of cortical dysplasia may demonstrate visible focal cortical thickening, gray-white junction blurring and T2 prolongation in the underlying white matter radiating from the lateral ventricle to the cortical surface concordant to localization by EEG. Overall, advanced diffusion MRI investigations of cortical malformations have been more limited than TLE, but have demonstrated increased diffusivity and reduced fractional anisotropy within lesions visible on conventional MRI when compared to controls or contralateral homologous brain regions [75, 146,147,148]. Limited data suggested band heterotopias may be the only visible cortical malformation that do not have these diffusion abnormalities [146]. Water diffusion changes are consistent with decreased neuronal density, the presence of abnormal cells and loss of diffusion anisotropy due to abnormal functional organization.
Similar to temporal lobe epilepsy, conventional MRI fails to detect all epileptogenic cortical abnormalities [144], or underestimates the full extent of abnormal nervous tissue. This latter problem could affect seizure-free outcomes if surgical resection fails to remove the full margins of epileptogenic tissue. Subtle structural abnormalities not visible with conventional MRI may demonstrate DTI abnormalities due to the associated abnormal functional organization of the tissue. Diffusion or fractional anisotropy changes in normal-appearing brain tissue were concordant with EEG localization in 7 of 30 MRI-negative patients with partial seizures. However, in this study, three additional diffusion abnormalities were discordant with EEG localization [75]. Voxel-based analysis demonstrated diffusion and fractional anisotropy changes in refractory epilepsy patients without structural abnormalities, but only 50% of those findings were concordant with EEG [79]. Changes to mean diffusivity may be more sensitive than altered anisotropy in MRI-negative patients with partial seizures [75], perhaps due to the low intrinsic fractional anisotropy in normal cortex. DTI studies of extratemporal epilepsy also have suggested involvement of the thalamocortical network [149]. Overall these results suggest DTI may increase sensitivity for epileptogenic cortical malformations in MRI-negative patients, but has limited specificity.
Several groups have reported white matter subjacent or surrounding malformations of cortical development has increased diffusivity and/or decreased anisotropy [146, 150, 151]. All but one study have reported diffusion abnormalities extended beyond white matter with abnormal T2 prolongation. Further, white matter diffusion abnormalities may extend several centimeters remote to the conventional MRI lesion [152], involve deep white matter projecting from the cortical lesion [151], or affect adjacent large fiber bundles such as the posterior corona radiata [152], uncinate fasciculus, SLF, and IFOF [153]. White matter subjacent to seizure activity on MEG (beyond the conventional MRI lesion) also has increased diffusion and reduced anisotropy [150]. Diffusion MRI at 7T demonstrated decreased u-fibers in patients with nonlesional focal epilepsy [154]. Abnormal white matter diffusion in patients with cortical malformations may have multiple etiologies including the presence of dysplastic neurons, decreased axonal density and increased extracellular space due to failed neurogenesis, abnormal neuronal migration and/or subsequent cell loss from chronic repetitive seizures. Specific changes to diffusion perpendicular to the principal eigenvector suggests these normal-appearing white matter lesions also may reflect abnormal myelination [151]. Neurite orientation dispersion and density imaging (NODDI) suggested decreased intracellular volume fraction in cortical malformations with normal conventional MRI [155, 156].
Similar to temporal lobe epilepsy, DTI findings also suggest malformations of cortical development may have more widespread and potentially bilateral white matter abnormalities. One study examined 23 patients with histopathologically confirmed FCD and determined that there was decreased FA involving the corpus callosum, bilateral IFO, ILF, and SLF compared to controls with a greater decrease in the side of the SLF corresponding to the FCD. In a connectivity meta-analysis, patients with focal epilepsy were determined to have reduced integration and increased segregation on network organization [157]. It remains to be determined whether a component of the cortical or white matter lesions detected by DTI that extends beyond the conventional MRI lesion is epileptogenic or just represents a pathway of preferred seizure propagation. Currently, EEG, magnetoencephalography (MEG), and/or electocorticography are used to guide the surgical margins for curative resection of epileptogenic focal cortical malformations. The role for DTI in such preoperative assessment has not been established.
Diffusion MRI also has been applied to tuberous sclerosis (TSC) patients. Tubers demonstrate increased mean diffusivity compared with either contralateral cortical regions in patients [158, 159] or control subjects [160]—these diffusion changes may not be appreciated on visual inspection [158]. Further, several studies have suggested the tuber or perilesional white matter with highest diffusivity corresponded to the epileptogenic lesion on EEG [158, 161, 162], although to our knowledge this has not been implemented clinically as a strong selection factor for tuber resection. The supratentorial white matter in TSC patients has increased mean diffusivity [163, 164] and reduced fractional anisotropy [165] well away from conventional MRI-detected lesions when compared to age-matched control subjects. Structural whole-brain connectivity in 20 patients with tuberous sclerosis demonstrated increased diffusivity throughout the brain that correlated with tuber load. Additionally, TSC patients with developmental delay or autism have higher diffusivity for the interhemispheric connections [166]. Further studies are warranted to determine whether differences in tuber or white matter connectivity by diffusion tractography correlate to seizure origin, propagation, network and prognosis.
Conclusion: Future Directions
Diffusion tensor imaging and tractography have provided many novel insights into epilepsy-induced changes to the functional and structural organization of the hippocampus, cortex and underlying white matter. These changes appear more widespread with diffusion MRI techniques compared to conventional MRI. Diffusion may be more sensitive at detecting seizure-induced cytotoxic edema in epileptogenic regions during the peri-ictal period and detecting subtle chronic structural alterations to brain tissue not visible with conventional MRI contrast mechanisms. DTI has demonstrated extensive, often bilateral white matter and extrahippocampal changes in temporal lobe epilepsy. Further, right and left temporal lobe epilepsy have significant white matter structural differences. Seizure propagation and white matter reorganization appears more widespread when seizures are generated in the dominant cerebral hemisphere due to greater pre-existing connectivity. White matter changes can correlate with the duration or age of onset of seizures, supporting the hypothesis that seizures may damage the brain in a dose-dependent fashion. Normal-appearing white matter in malformations of cortical development or tuberous sclerosis remote from visible structural lesions also can be abnormal. White matter assessment in temporal lobe epilepsy patients prior to temporal lobe resection can predict visual field defects based on the extent of resection and provide objective surrogate findings for language lateralization, verbal, and memory function. Additionally, better understanding of white matter changes in the preoperative period may aid in determination of which patients will benefit from surgical resection.
The most advanced DTI and tractography methods have been applied to cross-sectional studies of adult patients with unilateral temporal lobe epilepsy well localized by semiology, EEG, and sometimes conventional MRI, but one of the challenges to research and clinical imaging of epilepsy is the heterogeneous nature of the patient population. Thus, significant further insight into temporal lobe epilepsy may be gained by applying advanced diffusion methods and analysis to more longitudinal studies and to larger cohorts of specific patient populations including MRI-negative patients, patients with bilateral disease, or dual pathology. It would be interesting to compare white matter involvement in medically refractory temporal lobe epilepsy patients to those with successful pharmacologic management. More diffusion studies of children with complex seizures or families with increased incidence of temporal lobe epilepsy may give insight into predisposing connectivity differences or early pathological changes. Over the last several years, mathematical models have used diffusion MRI probabilistic tractography data to assess connectivity of whole brain neural networks by analyzing its topological properties with graphical theoretical approaches. Future studies with large cohorts and evaluation of subtypes of epilepsy will help to elucidate clinical prognosis and treatment strategies.
References
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE Official Report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82.
Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–30.
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–21.
Jones LA, Thomas RH. Sudden death in epilepsy: insights from the last 25 years. Seizure. 2017;44:232–6.
Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia. 2014;55(10):1479–85.
Kuzniecky RI. Neuroimaging of epilepsy: therapeutic implications. NeuroRx. 2005;2(2):384–93.
Mukherjee P, Chung SW, Berman JI, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: technical considerations. Am J Neuroradiol. 2008;29(5):843–52.
Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain injury - a review. NMR Biomed. 2002;15(7–8):561–9.
Fung SH, Roccatagliata L, Gonzalez RG, Schaefer PW. MR diffusion imaging in ischemic stroke. Neuroimaging Clin N Am. 2011;21(2):345–77.
Marrale M, Collura G, Brai M, Toschi N, Midiri F, La Tona G, et al. Physics, techniques and review of neuroradiological applications of diffusion kurtosis imaging (DKI). Clin Neuroradiol. 2015;26(4):391–403.
Jeurissen B, Descoteaux M, Mori S, Leemans A. Diffusion MRI fiber tractography of the brain. NMR Biomed. 2019;32(4):e3785.
Munsell BC, Wee C-Y, Keller SS, Weber B, Elger C, da Silva LAT, et al. Evaluation of machine learning algorithms for treatment outcome prediction in patients with epilepsy based on structural connectome data. NeuroImage. 2015;118:219–30.
DeSalvo MN, Douw L, Tanaka N, Reinsberger C, Stufflebeam SM. Altered structural connectome in temporal lobe epilepsy. Radiology. 2014;270(3):842–8.
Ozturk A, Yousem DM, Mahmood A, El Sayed S. Prevalence of asymmetry of mamillary body and fornix size on MR imaging. AJNR Am J Neuroradiol. 2008;29(2):384–7.
Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. Am J Neuroradiol. 2005;26(9):2267–74.
Ma X, Kadah YM, LaConte SM, Hu X. Enhancing measured diffusion anisotropy in gray matter by eliminating CSF contamination with FLAIR. Magn Reson Med. 2004;51(2):423–7.
Treit S, Steve T, Gross DW, Beaulieu C. High resolution in-vivo diffusion imaging of the human hippocampus. NeuroImage. 2018;182:479–87.
Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210–24.
Shi X, Ma X, Wu W, Huang F, Yuan C, Guo H. Parallel imaging and compressed sensing combined framework for accelerating high-resolution diffusion tensor imaging using inter-image correlation. Magn Reson Med. 2014;73(5):1775–85.
Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. NeuroImage. 2016;142:394–406.
Jaermann T, Crelier G, Pruessmann KP, Golay X, Netsch T, van Muiswinkel AMC, et al. SENSE-DTI at 3 T. Magn Reson Med. 2004;51(2):230–6.
Skare S, Newbould RD, Clayton DB, Albers GW, Nagle S, Bammer R. Clinical multishot DW-EPI through parallel imaging with considerations of susceptibility, motion, and noise. Magn Reson Med. 2007;57(5):881–90.
Holdsworth SJ, Skare S, Newbould RD, Bammer R. Robust GRAPPA-accelerated diffusion-weighted readout-segmented (RS)-EPI. Magn Reson Med. 2009;62(6):1629–40.
Yeom KW, Holdsworth SJ, Van AT, Iv M, Skare S, Lober RM, et al. Comparison of readout-segmented echo-planar imaging (EPI) and single-shot EPI in clinical application of diffusion-weighted imaging of the pediatric brain. Am J Roentgenol. 2013;200(5):W437–43.
Wu W, Miller KL. Image formation in diffusion MRI: a review of recent technical developments. J Magn Reson Imaging. 2017;46(3):646–62.
Wang F-N, Huang T-Y, Lin F-H, Chuang T-C, Chen N-K, Chung H-W, et al. PROPELLER EPI: an MRI technique suitable for diffusion tensor imaging at high field strength with reduced geometric distortions. Magn Reson Med. 2005;54(5):1232–40.
Eriksson SH, Thom M, Bartlett PA, Symms MR, McEvoy AW, Sisodiya SM, et al. PROPELLER MRI visualizes detailed pathology of hippocampal sclerosis. Epilepsia. 2008 Jan;49(1):33–9.
Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349(13):1257–66.
Kim JA, Chung JI, Yoon PH, Kim DI, Chung TS, Kim EJ, et al. Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. Am J Neuroradiol. 2001;22(6):1149–60.
Di Bonaventura C, Bonini F, Fattouch J, Mari F, Petrucci S, Carnì M, et al. Diffusion-weighted magnetic resonance imaging in patients with partial status epilepticus. Epilepsia. 2009;50(Suppl 4):45–52.
Farina L, Bergqvist C, Zimmerman RA, Haselgrove J, Hunter JV, Bilaniuk LT. Acute diffusion abnormalities in the hippocampus of children with new-onset seizures: the development of mesial temporal sclerosis. Neuroradiology. 2004;46(4):251–7.
Hufnagel AEA. Brain diffusion after single seizures. Epilepsia. 2003;44:54–63.
Oh J-B, Lee SK, Kim K-K, Song IC, Chang K-H. Role of immediate postictal diffusion-weighted MRI in localizing epileptogenic foci of mesial temporal lobe epilepsy and non-lesional neocortical epilepsy. Seizure. 2004;13(7):509–16.
Salmenpera TM, Symms MR, Boulby PA, Barker GJ, Duncan JS. Postictal diffusion weighted imaging. Epilepsy Res. 2006;70(2–3):133–43.
Williams JA, Bede P, Doherty CP. An exploration of the spectrum of peri -ictal MRI change; a comprehensive literature review. Seizure. 2017;50:19–32.
Salmenpera TM, Simister RJ, Bartlett P, Symms MR, Boulby PA, Free SL, et al. High-resolution diffusion tensor imaging of the hippocampus in temporal lobe epilepsy. Epilepsy Res. 2006;71(2–3):102–6.
Jabeen SA, Cherukuri P, Mridula R, Harshavardhana KR, Gaddamanugu P, Sarva S, et al. A prospective study of diffusion weighted magnetic resonance imaging abnormalities in patients with cluster of seizures and status epilepticus. Clin Neurol Neurosurg. 2017;155:70–4.
Hübers A, Thoma K, Schocke M, Fauser S, Ludolph AC, Kassubek J, et al. Acute DWI reductions in patients after single epileptic seizures - more common than assumed. Front Neurol. 2018;9:303–7.
Natsume J, Bernasconi N, Miyauchi M, Naiki M, Yokotsuka T, Sofue A, et al. Hippocampal volumes and diffusion-weighted image findings in children with prolonged febrile seizures. Acta Neurol Scand. 2007 Apr;115(4 Suppl):25–8.
Cendes F. Febrile seizures and mesial temporal sclerosis. Curr Opin Neurol. 2004;17(2):161–4.
Scott RC, King MD, Gadian DG, Neville BGR, Connelly A. Prolonged febrile seizures are associated with hippocampal vasogenic edema and developmental changes. Epilepsia. 2006;47(9):1493–8.
Adams RD, Victor M, AH R. Principles of neurology. 6th ed. San Francisco: McGraw-Hill; 1997. 30 p.
Yoong M, Seunarine K, Martinos M, Chin RF, Clark CA, Scott RC. Prolonged febrile seizures cause reversible reductions in white matter integrity. Neuroimage Clin. 2010;3:515–21.
Pujar SS, Seunarine KK, Martinos MM, Neville BGR, Scott RC, Chin RFM, et al. Long-term white matter tract reorganization following prolonged febrile seizures. Epilepsia. 2017;58(5):772–80.
Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–8.
Van Paesschen W. Qualitative and quantitative imaging of the hippocampus in mesial temporal lobe epilepsy with hippocampal sclerosis. Neuroimaging Clin N Am. 2004;14(3):373–400.
Hugg JW, Butterworth EJ, Kuzniecky RI. Diffusion mapping applied to mesial temporal lobe epilepsy: preliminary observations. Neurology. 1999;53(1):173–6.
Wieshmann UC, Clark CA, Symms MR, Barker GJ, Birnie KD, Shorvon SD. Water diffusion in the human hippocampus in epilepsy. Magn Reson Imaging. 1999;17(1):29–36.
Shepherd TM, Ozarslan E, Yachnis AT, King MA, Blackband SJ. Diffusion tensor microscopy indicates the cytoarchitectural basis for diffusion anisotropy in the human hippocampus. Am J Neuroradiol. 2007;28(5):958–64.
Beaujoin J. Post-mortem inference of the human hippocampal connectivity and microstructure using ultra-high field diffusion MRI at 11.7 T. Brain Struct Funct. 2018;223(5):2157–79.
Yoo SY, Chang K-H, Song IC, Han MH, Kwon BJ, Lee SH, et al. Apparent diffusion coefficient value of the hippocampus in patients with hippocampal sclerosis and in healthy volunteers. Am J Neuroradiol. 2002;23(5):809–12.
Assaf BA, Mohamed FB, Abou-Khaled KJ, Williams JM, Yazeji MS, Haselgrove J, et al. Diffusion tensor imaging of the hippocampal formation in temporal lobe epilepsy. Am J Neuroradiol. 2003;24(9):1857–62.
Lee JH, Chung C-K, Song IC, Chang KH, Kim HJ. Limited utility of interictal apparent diffusion coefficient in the evaluation of hippocampal sclerosis. Acta Neurol Scand. 2004;110(1):53–8.
Düzel E, Kaufmann J, Guderian S, Szentkuti A, Schott B, Bodammer N, et al. Measures of hippocampal volumes, diffusion and 1H MRS metabolic abnormalities in temporal lobe epilepsy provide partially complementary information. Eur J Neurol. 2004;11(3):195–205.
Lui YW, Nusbaum AO, Barr WB, Johnson G, Babb JS, Orbach D, et al. Correlation of apparent diffusion coefficient with neuropsychological testing in temporal lobe epilepsy. Am J Neuroradiol. 2005;26(7):1832–9.
Hakyemez B, Erdogan C, Yildiz H, Ercan I, Parlak M. Apparent diffusion coefficient measurements in the hippocampus and amygdala of patients with temporal lobe seizures and in healthy volunteers. Epilepsy Behav. 2005;6(2):250–6.
Thivard L, Lehéricy S, Krainik A, Adam C, Dormont D, Chiras J, et al. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. NeuroImage. 2005;28(3):682–90.
Kimiwada T, Juhász C, Makki M, Muzik O, Chugani DC, Asano E, et al. Hippocampal and thalamic diffusion abnormalities in children with temporal lobe epilepsy. Epilepsia. 2006;47(1):167–75.
Bao Y, He R, Zeng Q, Zhu P, Zheng R, Xu H. Investigation of microstructural abnormalities in white and gray matter around hippocampus with diffusion tensor imaging (DTI) in temporal lobe epilepsy (TLE). Epilepsy Behav. 2018;83:44–9.
Nazem-Zadeh M-R, Schwalb JM, Elisevich KV, Bagher-Ebadian H, Hamidian H, Akhondi-Asl A-R, et al. Lateralization of temporal lobe epilepsy using a novel uncertainty analysis of MR diffusion in hippocampus, cingulum, and fornix, and hippocampal volume and FLAIR intensity. J Neurol Sci. 2014;342(1–2):152–61.
Keller SS, Schoene-Bake J-C, Gerdes JS, Weber B, Deppe M. Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: cross-sectional evidence for progressive neurologic injury. Zhan W, editor. PLoS One. 2012;7(10):e46791.
Wehner T, Lapresto E, Tkach J, Liu P, Bingaman W, Prayson RA, et al. The value of interictal diffusion-weighted imaging in lateralizing temporal lobe epilepsy. Neurology. 2007;68(2):122–7.
Gonçalves Pereira PM, Oliveira E, Rosado P. Apparent diffusion coefficient mapping of the hippocampus and the amygdala in pharmaco-resistant temporal lobe epilepsy. Am J Neuroradiol. 2006;27(3):671–83.
Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26(3):321–30.
Colon-Perez LM, King M, Parekh M, Boutzoukas A, Carmona E, Couret M, et al. High-field magnetic resonance imaging of the human temporal lobe. Neuroimage Clin. 2015;9:58–68.
Modo M, Hitchens TK, Liu JR, Richardson RM. Detection of aberrant hippocampal mossy fiber connections: ex vivo mesoscale diffusion MRI and microtractography with histological validation in a patient with uncontrolled temporal lobe epilepsy. Hum Brain Mapp. 2015;37(2):780–95.
Rutland JW, Feldman RE, Delman BN, Panov F, Fields MC, Marcuse LV, et al. Subfield-specific tractography of the hippocampus in epilepsy patients at 7 tesla. Seizure. 2018;62:3–10.
Kantarci K, Shin C, Britton JW, So EL, Cascino GD, Jack CR. Comparative diagnostic utility of 1H MRS and DWI in evaluation of temporal lobe epilepsy. Neurology. 2002;58(12):1745–53.
Focke NK, Yogarajah M, Symms MR, Gruber O, Paulus W, Duncan JS. Automated MR image classification in temporal lobe epilepsy. NeuroImage. 2012;59(1):356–62.
O’Brien TJ, David EP, Kilpatrick CJ, Desmond P, Tress B. Contrast-enhanced perfusion and diffusion MRI accurately lateralize temporal lobe epilepsy: a pilot study. J Clin Neurosci. 2007;14(9):841–9.
Kamiya K, Amemiya S, Suzuki Y, Kunii N, Kawai K, Mori H, et al. Machine learning of DTI structural brain connectomes for lateralization of temporal lobe epilepsy. MRMS. 2016;15(1):121–9.
Zhang Y, Gao Y, Zhou M, Wu J, Zee C, Wang D. A diffusional kurtosis imaging study of idiopathic generalized epilepsy with unilateral interictal epileptiform discharges in children. J Neuroradiol. 2016;43(5):339–45.
Bonilha L, Lee CY, Jensen JH, Tabesh A, Spampinato MV, Edwards JC, et al. Altered microstructure in temporal lobe epilepsy: a diffusional kurtosis imaging study. Am J Neuroradiol. 2015;36(4):719–24.
Del Gaizo J, Mofrad N, Jensen JH, Clark D, Glenn R, Helpern J, et al. Using machine learning to classify temporal lobe epilepsy based on diffusion MRI. Brain Behav. 2017;7(10):e00801.
Rugg-Gunn FJ, Eriksson SH, Symms MR, Barker GJ, Duncan JS. Diffusion tensor imaging of cryptogenic and acquired partial epilepsies. Brain. 2001;124(Pt 3):627–36.
Londoño A, Castillo M, Lee YZ, Smith JK. Apparent diffusion coefficient measurements in the hippocampi in patients with temporal lobe seizures. Am J Neuroradiol. 2003;24(8):1582–6.
Ercan K, Gunbey HP, Bilir E, Zan E, Arslan H. Research article comparative lateralizing ability of multimodality MRI in temporal lobe epilepsy. Dis Markers. 2016;2016:5923243.
Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. NeuroImage. 2008;40(2):728–37.
Chen Q, Lui S, Li C-X, Jiang L-J, Ou-Yang L, Tang H-H, et al. MRI-negative refractory partial epilepsy: role for diffusion tensor imaging in high field MRI. Epilepsy Res. 2008;80(1):83–9.
Thivard L, Tanguy M-L, Adam C, Clémenceau S, Dezamis E, Lehéricy S, et al. Postoperative recovery of hippocampal contralateral diffusivity in medial temporal lobe epilepsy. Epilepsia. 2007;48(3):599–604.
Concha L, Beaulieu C, Wheatley BM, Gross DW. Bilateral white matter diffusion changes persist after epilepsy surgery. Epilepsia. 2007;48(5):931–40.
Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol. 2005;57(2):188–96.
Elliott CA, Gross DW, Wheatley BM, Beaulieu C, Sankar T. Longitudinal hippocampal and extra-hippocampal microstructural and macrostructural changes following temporal lobe epilepsy surgery. Epilepsy Res. 2018;140:128–37.
Catani M, de Schotten MT. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105–32.
Ahmadi ME, Hagler DJ, McDonald CR, Tecoma ES, Iragui VJ, Dale AM, et al. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR Am J Neuroradiol. 2009;30(9):1740–7.
Yogarajah M, Focke NK, Bonelli S, Cercignani M, Acheson J, Parker GJM, et al. Defining Meyer’s loop-temporal lobe resections, visual field deficits and diffusion tensor tractography. Brain. 2009 Jun;132(Pt 6):1656–68.
McDonald CR, Leyden KM, Hagler DJ, Kucukboyaci NE, Kemmotsu N, Tecoma ES, et al. White matter microstructure complements morphometry for predicting verbal memory in epilepsy. Cortex. 2014;58:139–50.
Hutchings F, Han CE, Keller SS, Weber B, Taylor PN, Kaiser M. Predicting surgery targets in temporal lobe epilepsy through structural connectome based simulations. Sporns O, editor. PLoS Comput Biol. 2015;11(12):e1004642.
Slinger G, Sinke MRT, Braun KPJ, Otte WM. White matter abnormalities at a regional and voxel level in focal and generalized epilepsy: a systematic review and meta-analysis. Neuroimage Clin. 2016;12:902–9.
Arfanakis K, Hermann BP, Rogers BP, Carew JD, Seidenberg M, Meyerand ME. Diffusion tensor MRI in temporal lobe epilepsy. Magn Reson Imaging. 2002;20(7):511–9.
Gross DW. Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia. 2011;52(Pt 3):32–4.
Kim H, Piao Z, Liu P, Bingaman W, Diehl B. Secondary white matter degeneration of the corpus callosum in patients with intractable temporal lobe epilepsy: a diffusion tensor imaging study. Epilepsy Res. 2008;81(2–3):136–42.
Lyra KP, Chaim KT, Leite CC, Park EJ, Andrade CS, Passarelli V, et al. Corpus callosum diffusion abnormalities in refractory epilepsy associated with hippocampal sclerosis. Epilepsy Res. 2017;137:112–8.
Briellmann RS, Mitchell LA, Waites AB, Abbott DF, Pell GS, Saling MM, et al. Correlation between language organization and diffusion tensor abnormalities in refractory partial epilepsy. Epilepsia. 2003;44(12):1541–5.
Nilsson D, Go C, Rutka JT, Rydenhag B, Mabbott DJ, Snead OC III, et al. Bilateral diffusion tensor abnormalities of temporal lobe and cingulate gyrus white matter in children with temporal lobe epilepsy. Epilepsy Res. 2008;81(2–3):128–35.
Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Lüders HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49(8):1409–18.
Sanches P, Fujisao EK, Braga AMS, Cristaldo NR, Reis dos R, Yamashita S, et al. Voxel-based analysis of diffusion tensor imaging in patients with mesial temporal lobe epilepsy. Epilepsy Res. 2017;132:100–8.
Imamura H, Matsumoto R, Takaya S, Nakagawa T, Shimotake A, Kikuchi T, et al. Network specific change in white matter integrity in mesial temporal lobe epilepsy. Epilepsy Res. 2016;120:65–72.
McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, et al. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71(23):1869–76.
Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J Neurosci. 2010;30(3):996–1002.
Rodríguez-Cruces R, Concha L. White matter in temporal lobe epilepsy: clinico-pathological correlates of water diffusion abnormalities. Quant Imaging Med Surg. 2015;5(2):264–78.
Keller SS, Roberts N, Baker G, Sluming V, Cezayirli E, Mayes A, et al. A voxel-based asymmetry study of the relationship between hemispheric asymmetry and language dominance in Wada tested patients. Hum Brain Mapp. 2018;39(7):3032–45.
Xu Y, Qiu S, Wang J, Liu Z, Zhang R, Li S, et al. Disrupted topological properties of brain white matter networks in left temporal lobe epilepsy: a diffusion tensor imaging study. Neuroscience. 2014;279(C):155–67.
Abdelnour F, Raj A, Devinsky O, Thesen T. Network analysis on predicting mean diffusivity change at group level in temporal lobe epilepsy. Brain Connect. 2016;6(8):607–20.
Scanlon C, Mueller SG, Cheong I, Hartig M, Weiner MW, Laxer KD. Grey and white matter abnormalities in temporal lobe epilepsy with and without mesial temporal sclerosis. J Neurol. 2013;260(9):2320–9.
Liu M, Concha L, Lebel C, Beaulieu C, Gross DW. Mesial temporal sclerosis is linked with more widespread white matter changes in temporal lobe epilepsy. Neuroimage Clin. 2012;1(1):99–105.
Loi RQ, Leyden KM, Balachandra A, Uttarwar V, Hagler DJ Jr, Paul BM, et al. Restriction spectrum imaging reveals decreased neurite density in patients with temporal lobe epilepsy. Epilepsia. 2016;57(11):1897–906.
Urbach H, Egger K, Rutkowski K, Nakagawa JM, Schmeiser B, Reisert M, et al. Bilateral cingulum fiber reductions in temporal lobe epilepsy with unilateral hippocampal sclerosis. Eur J Radiol. 2017;94:53–7.
Govindan RM, Makki MI, Sundaram SK, Juhász C, Chugani HT. Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Res. 2008;80(1):30–41.
Schoene-Bake J-C, Faber J, Trautner P, Kaaden S, Tittgemeyer M, Elger CE, et al. Widespread affections of large fiber tracts in postoperative temporal lobe epilepsy. Hum Brain Mapp J. 2009;46(3):569–76.
Keller SS, Glenn GR, Weber B, Kreilkamp BAK, Jensen JH, Helpern JA, et al. Preoperative automated fibre quantification predicts postoperative seizure outcome in temporal lobe epilepsy. Brain. 2016;140(1):68–82.
Lin JJ, Riley JD, Juranek J, Cramer SC. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Res. 2008;82(2–3):162–70.
Yogarajah M, Powell HWR, Parker GJM, Alexander DC, Thompson PJ, Symms MR, et al. Tractography of the parahippocampal gyrus and material specific memory impairment in unilateral temporal lobe epilepsy. Hum Brain Mapp J. 2008;40(4):1755–64.
Kreilkamp BAK, Weber B, Richardson MP, Keller SS. Automated tractography in patients with temporal lobe epilepsy using TRActs Constrained by UnderLying Anatomy (TRACULA). Neuroimage Clin. 2017;14(C):67–76.
Kemmotsu N, Girard HM, Bernhardt BC, Bonilha L, Lin JJ, Tecoma ES, et al. MRI analysis in temporal lobe epilepsy: cortical thinning and white matter disruptions are related to side of seizure onset. Epilepsia. 2011;52(12):2257–66.
Pustina D, Doucet G, Sperling M, Sharan A, Tracy J. Increased microstructural white matter correlations in left, but not right, temporal lobe epilepsy. Hum Brain Mapp. 2014;36(1):85–98.
Chiang S, Levin HS, Wilde E, Haneef Z. White matter structural connectivity changes correlate with epilepsy duration in temporal lobe epilepsy. Epilepsy Res. 2016;120:37–46.
Aparicio J, Carreño M, Bargalló N, Setoain X, Rubí S, Rumià J, et al. Combined 18F-FDG-PET and diffusion tensor imaging in mesial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage Clin. 2016;12(C):976–89.
Pustina D, Doucet G, Evans J, Sharan A, Sperling M, Skidmore C, et al. Distinct types of white matter changes are observed after anterior temporal lobectomy in epilepsy. Draganski B, editor. PLoS One. 2014;9(8):e104211.
Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(3):312–9.
Lemkaddem A, Daducci A, Kunz N, Lazeyras F, Seeck M, Thiran J-P, et al. Connectivity and tissue microstructural alterations in right and left temporal lobe epilepsy revealed by diffusion spectrum imaging. Neuroimage Clin. 2014;5(C):349–58.
Nazem-Zadeh M-R, Bowyer SM, Moran JE, Davoodi-Bojd E, Zillgitt A, Weiland BJ, et al. MEG coherence and DTI connectivity in mTLE. Brain Topogr. 2016;29(4):598–622.
Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47(8):1360–3.
Powell HWR, Parker GJM, Alexander DC, Symms MR, Boulby PA, Barker GJ, et al. Imaging language pathways predicts postoperative naming deficits. J Neurol Neurosurg Psychiatry. 2008;79(3):327–30.
Santyr BG, Lau JC, Mirsattari SM, Burneo JG, de Ribaupierre S, Steven DA, et al. Novel connectivity map normalization procedure for improved quantitative investigation of structural thalamic connectivity in temporal lobe epilepsy patients. J Magn Reson Imaging. 2018;23(85):31–19.
Sutula TP, Hagen J, Pitkänen A. Do epileptic seizures damage the brain? Curr Opin Neurol. 2003;16(2):189–95.
Ellmore TM, Beauchamp MS, Breier JI, Slater JD, Kalamangalam GP, O’Neill TJ, et al. Temporal lobe white matter asymmetry and language laterality in epilepsy patients. NeuroImage. 2010;49(3):2033–44.
Matsumoto R, Okada T, Mikuni N, Mitsueda-Ono T, Taki J, Sawamoto N, et al. Hemispheric asymmetry of the arcuate fasciculus: a preliminary diffusion tensor tractography study in patients with unilateral language dominance defined by Wada test. J Neurol. 2008;255(11):1703–11.
Rodrigo S, Oppenheim C, Chassoux F, Golestani N, Cointepas Y, Poupon C, et al. Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. Eur Radiol. 2007;17(7):1663–8.
Takaya S, Liu H, Greve DN, Tanaka N, Leveroni C, Cole AJ, et al. Altered anterior-posterior connectivity through the arcuate fasciculus in temporal lobe epilepsy. Hum Brain Mapp. 2016;37(12):4425–38.
Diao L, Yu H, Zheng J, Chen Z, Huang D, Yu L. Abnormalities of the uncinate fasciculus correlate with executive dysfunction in patients with left temporal lobe epilepsy. Magn Reson Imaging. 2015;33(5):544–50.
Law N, Smith ML, Widjaja E. Thalamocortical connections and executive function in pediatric temporal and frontal lobe epilepsy. AJNR Am J Neuroradiol. 2018;39(8):1523–9.
Rodríguez-Cruces R, Velázquez-Pérez L, Rodríguez-Leyva I, Velasco AL, Trejo-Martínez D, Barragán-Campos HM, et al. Association of white matter diffusion characteristics and cognitive deficits in temporal lobe epilepsy. Epilepsy Behav. 2018;79(C):138–45.
Yogarajah M, Powell HWR, Parker GJM, Alexander DC, Thompson PJ, Symms MR, et al. Tractography of the parahippocampal gyrus and material specific memory impairment in unilateral temporal lobe epilepsy. Human Brain Mapp J. 2008;40(4):1755–64.
Morgan VL, Englot DJ, Rogers BP, Landman BA, Cakir A, Abou-Khalil BW, et al. Magnetic resonance imaging connectivity for the prediction of seizure outcome in temporal lobe epilepsy. Epilepsia. 2017;58(7):1251–60.
Bonilha L, Keller SS. Quantitative MRI in refractory temporal lobe epilepsy: relationship with surgical outcomes. Quant Imaging Med Surg. 2015;5(2):204–24.
Taylor PN, Sinha N, Wang Y, Vos SB, de Tisi J, Miserocchi A, et al. The impact of epilepsy surgery on the structural connectome and its relation to outcome. Neuroimage Clin. 2018;18:202–14.
Gleichgerrcht E, Munsell B, Bhatia S, Vandergrift WA, Rorden C, McDonald C, et al. Deep learning applied to whole-brain connectome to determine seizure control after epilepsy surgery. Epilepsia. 2018;59(9):1643–54.
Keller SS, Richardson MP, Schoene-Bake J-C, O’Muircheartaigh J, Elkommos S, Kreilkamp B, et al. Thalamotemporal alteration and postoperative seizures in temporal lobe epilepsy. Ann Neurol. 2015;77(5):760–74.
Taoka T, Sakamoto M, Nakagawa H, Nakase H, Iwasaki S, Takayama K, et al. Diffusion tensor tractography of the Meyer loop in cases of temporal lobe resection for temporal lobe epilepsy: correlation between postsurgical visual field defect and anterior limit of Meyer loop on tractography. Am J Neuroradiol. 2008;29(7):1329–34.
Chen X, Weigel D, Ganslandt O, Buchfelder M, Nimsky C. Prediction of visual field deficits by diffusion tensor imaging in temporal lobe epilepsy surgery. NeuroImage. 2009;45(2):286–97.
Taoka T, Sakamoto M, Iwasaki S, Nakagawa H, Fukusumi A, Hirohashi S, et al. Diffusion tensor imaging in cases with visual field defect after anterior temporal lobectomy. Am J Neuroradiol. 2005;26(4):797–803.
Barkovich AJ, Guerrini R, Kuzniecky RI, Jackson GD, Dobyns WB. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135(Pt 5):1348–69.
Colombo N, Salamon N, Raybaud C, Ozkara C, Barkovich AJ. Imaging of malformations of cortical development. Epileptic Disord. 2009;11(3):194–205.
Engel J. Update on surgical treatment of the epilepsies. Clin Exp Neurol. 1992;29:32–48.
Eriksson SH, Rugg-Gunn FJ, Symms MR, Barker GJ, Duncan JS. Diffusion tensor imaging in patients with epilepsy and malformations of cortical development. Brain. 2001;124(Pt 3):617–26.
Trivedi R, Gupta RK, Hasan KM, Hou P, Prasad KN, Narayana PA. Diffusion tensor imaging in polymicrogyria: a report of three cases. Neuroradiology. 2006;48(6):422–7.
Deleo F, Thom M, Concha L, Bernasconi A, Bernhardt BC, Bernasconi N. Histological and MRI markers of white matter damage in focal epilepsy. Epilepsy Res. 2018;140:29–38.
Peng S-J, Hsin Y-L. Altered structural and functional thalamocortical networks in secondarily generalized extratemporal lobe seizures. Neuroimage Clin. 2017;13(C):55–61.
Widjaja E, Zarei Mahmoodabadi S, Otsubo H, Snead OC, Holowka S, Bells S, et al. Subcortical alterations in tissue microstructure adjacent to focal cortical dysplasia: detection at diffusion-tensor MR imaging by using magnetoencephalographic dipole cluster localization. Radiology. 2009;251(1):206–15.
Widjaja E, Blaser S, Miller E, Kassner A, Shannon P, Chuang SH, et al. Evaluation of subcortical white matter and deep white matter tracts in malformations of cortical development. Epilepsia. 2007;48(8):1460–9.
Lee S-K, Kim DI, Mori S, Kim J, Kim HD, Heo K, et al. Diffusion tensor MRI visualizes decreased subcortical fiber connectivity in focal cortical dysplasia. NeuroImage. 2004;22(4):1826–9.
Cendes F. White matter abnormalities in patients with focal cortical dysplasia revealed by diffusion tensor imaging analysis in a voxelwise approach. Front Neurol. 2012;3:121.
O’Halloran R, Feldman R, Marcuse L, Fields M, Delman B, Frangou S, et al. A method for u-fiber quantification from 7 T diffusion-weighted MRI data tested in patients with nonlesional focal epilepsy. Neuroreport. 2017;28(8):457–61.
Winston GP, Micallef C, Symms MR, Alexander DC, Duncan JS, Zhang H. Advanced diffusion imaging sequences could aid assessing patients with focal cortical dysplasia and epilepsy. Epilepsy Res. 2014;108(2):336–9.
Adler S, Lorio S, Jacques TS, Benova B, Gunny R, Cross JH, et al. Towards in vivo focal cortical dysplasia phenotyping using quantitative MRI. Neuroimage Clin. 2017;15:95–105.
van Diessen E, Zweiphenning WJEM, Jansen FE, Stam CJ, Braun KPJ, Otte WM. Brain network organization in focal epilepsy: a systematic review and meta-analysis. Doesburg S, editor. PLoS One. 2014;9(12):e114606.
Jansen FE, Braun KPJ, van Nieuwenhuizen O, Huiskamp G, Vincken KL, van Huffelen AC, et al. Diffusion-weighted magnetic resonance imaging and identification of the epileptogenic tuber in patients with tuberous sclerosis. Arch Neurol. 2003;60(11):1580–4.
Piao C, Yu A, Li K, Wang Y, Qin W, Xue S. Cerebral diffusion tensor imaging in tuberous sclerosis. Eur J Radiol. 2009;71(2):249–52.
Karadag D, Mentzel H-J, Güllmar D, Rating T, Löbel U, Brandl U, et al. Diffusion tensor imaging in children and adolescents with tuberous sclerosis. Pediatr Radiol. 2005;35(10):980–3.
Chandra PS, Salamon N, Huang J, Wu JY, Koh S, Vinters HV, et al. FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia. 2006;47(9):1543–9.
Yogi A, Hirata Y, Karavaeva E, Harris RJ, Wu JY, Yudovin SL, et al. DTI of tuber and perituberal tissue can predict epileptogenicity in tuberous sclerosis complex. Neurology. 2015;85(23):2011–5.
Garaci FG, Floris R, Bozzao A, Manenti G, Simonetti A, Lupattelli T, et al. Increased brain apparent diffusion coefficient in tuberous sclerosis. Radiology. 2004;232(2):461–5.
Arulrajah S, Ertan G, Jordan L, Tekes A, Khaykin E, Izbudak I, et al. Magnetic resonance imaging and diffusion-weighted imaging of normal-appearing white matter in children and young adults with tuberous sclerosis complex. Neuroradiology. 2009;51(11):781–6.
Makki MI, Chugani DC, Janisse J, Chugani HT. Characteristics of abnormal diffusivity in normal-appearing white matter investigated with diffusion tensor MR imaging in tuberous sclerosis complex. Am J Neuroradiol. 2007;28(9):1662–7.
Im K, Ahtam B, Haehn D, Peters JM, Warfield SK, Sahin M, et al. Altered structural brain networks in tuberous sclerosis complex. Cereb Cortex. 2016;26(5):2046–58.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rispoli, J.M., Hess, C.P., Shepherd, T.M. (2023). Clinical Applications of Diffusion MRI in Epilepsy. In: Faro, S.H., Mohamed, F.B. (eds) Functional Neuroradiology. Springer, Cham. https://doi.org/10.1007/978-3-031-10909-6_43
Download citation
DOI: https://doi.org/10.1007/978-3-031-10909-6_43
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10908-9
Online ISBN: 978-3-031-10909-6
eBook Packages: MedicineMedicine (R0)