Abstract
This chapter covers the clinical and radiological diagnosis of placenta accreta spectrum and the ability of sonographic and MRI findings to predict definitive diagnosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
The Importance of Antenatal Diagnosis of Placenta Accreta Spectrum
Placenta accreta spectrum (PAS) is a morbid complication of pregnancy that results from abnormal placentation. In normal pregnancies, the placenta implants in the decidua functionalis and does not adhere to deeper tissue. It is suggested that the partial or complete lack of decidua basalis results in deeper penetration of the placenta into myometrium. Placental growth may be limited to the endometrium (accreta), to myometrium (increta), or through the uterine serosa (percreta) with possible involvement of the adjacent organs. As the cesarean delivery rate increases, there has been a significant increase in the incidence of PAS. In the United States, the incidence increased from 1 in 30,000 pregnancies in 1960s to 1 in 2500 pregnancies in the 1990s [1] and further increased to 1 in 533 pregnancies in the early 2000s [2]; however, the true incidence of PAS is unknown as the reported rates vary when outside of research protocols [3].
Clinically, the major risk factors for PAS are history of prior cesarean delivery and presence of placenta previa in the current pregnancy. The risk significantly increases with history of multiple cesarean deliveries [4,5,6]. Other risk factors include prior uterine surgery other than cesarean [7,8,9], assisted reproduction [10, 11], advanced maternal age [12], and multiple gestation [13, 14].
The predominant morbidity associated with PAS is obstetric hemorrhage, often requiring multiple units of blood products and possible complications with massive transfusion [15,16,17,18]. Due to the complexity of surgery at the time of delivery, often accompanied with hysterectomy, surgical complications are common with PAS including bladder and ureteral injury, or other adjacent organs in the pelvis.
Maternal outcomes are improved when there is antenatal diagnosis prior to delivery, allowing for advanced planning and multidisciplinary management of PAS to achieve favorable outcomes [19, 20]. This reflects the importance of accurate diagnostic tools particularly in the high-risk population to avoid poor outcomes. PAS evaluation should be considered in any pregnancy with placenta previa and history of cesarean delivery. Antepartum diagnosis helps with planning the delivery under optimized conditions in experienced centers.
Antenatal Diagnosis: Role of Different Imaging Modalities
Definitive diagnosis is by histopathologic evaluation of the placenta and the uterus. However, imaging techniques are available that can predict PAS with good accuracy. The sensitivity of ultrasound in the diagnosis of PAS is reported to be 90.7% (95% CI, 87.2–93.6) with specificity of 96.9% (95% CI, 96.3–97.5%) [21]. However, the experience of the operator and clinician reading the ultrasound images may affect the accuracy. In addition, recent study suggests intraoperative clinical diagnosis correlate well with pathologic diagnosis [22].
In 1982, the first documented antenatal diagnosis of PAS was reported [23]. Since then several markers are introduced to improve the accuracy of PAS diagnosis on imaging. Advances in technology also have helped for better visualization and description of various markers and findings. Ultrasound is the modality of choice for the evaluation of the placenta. It is safe during pregnancy, it provides easy availability with real-time assessment, and it is the cheapest diagnostic imaging available compared to other tools such as MRI. There have been recent efforts to establish a standardized protocol for ultrasound evaluation of PAS in relation to clinical and pathological findings. There are several sonographic markers associated with PAS, and some can be seen as early as the first trimester pregnancy. For all pregnancies, standard of care is to evaluate placental location and implantation universally during the midtrimester anatomy scan. Magnetic resonance imaging (MRI) can also help when placenta cannot clearly be visualized on ultrasound or the findings are not conclusive. There are scoring systems introduced for antenatal assessment of PAS involving both clinical risk factors and US markers [24,25,26]. The presence of placenta previa in patients with history of prior cesarean delivery has the most influence in these scoring systems suggested.
First Trimester Imaging
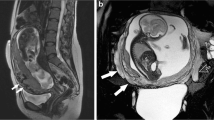
Early in pregnancy, a gestational sac implanted in the lower uterine segment in close proximity to the previous uterine scar increases the risk of PAS [27, 28]. It has been reported that 28% of patients with PAS have low implantation of the gestational sac on the first trimester ultrasound [28]. Cesarean section scar pregnancy is a marker for PAS, and all the sonographic PAS markers described on a second or third trimester ultrasound can also be seen on a first trimester scan [29], including as anechoic placental areas and an irregular uteroplacental interface (Fig. 6.1) [30]. Particularly concerning for early PAS is when the residual myometrial thickness is less than 5 mm at the implantation site within the previous cesarean scar; this finding increases the risk for need for hysterectomy if the pregnancy continues [28]. In case of cesarean scar pregnancy, there are studies reporting a new sonographic sign (the crossover sign or COS) that can predict the severity of subsequent PAS and possibility of a successful pregnancy [31]. The COS is a measure of the relationship between the gestational sac, anterior uterine wall, and cesarean scar.
Second or Third Trimester Ultrasound Evaluation
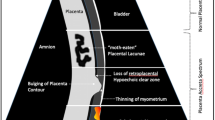
There are multiple ultrasound markers associated with PAS in the second or third trimester [26, 32]. Most of these markers are present at the time of midtrimester anatomy scan. Transvaginal sonography with a partially full urinary bladder is in particular recommended for evaluation of the lower uterine segment and vesicouterine interface [33]. Color Doppler is the other imaging tool that can be helpful and should be used to visualize PAS-associated vascular abnormalities.
These markers include (Fig. 6.2) the following:
-
1.
Placenta lacunae: hypoechoic spaces with irregular margins within the placental tissue visible on gray scale; color Doppler often reveals a swirling of venous flow. The following criteria are associated with high-risk PAS:
-
2.
Irregular uteroplacental interface includes the loss of the retroplacental hypoechoic zone between the placenta and myometrium as well as thinning of the retroplacental myometrium (<1 mm):
-
This marker should be evaluated particularly along the posterior bladder wall; a partial or complete interruption of the uterovesical interface can often be seen.
-
It is important to ensure the correct angle of insonation and avoid undue pressure on the abdomen or with the transvaginal ultrasound probe as this has been shown to obscure imaging accuracy [36].
-
-
3.
Uterine wall bulging as a result of placental tissue distorting uterine contour.
-
4.
Placental extrusion beyond the uterine wall:
-
This marker can represent uterine wall dehiscence as well.
-
-
5.
Bridging vessels:
-
Placental vasculature crossing from the placenta into the myometrium (and sometimes beyond).
-
Neovascularization at the placental implantation/invasion site is often represented as vessels crossing the uterine serosa into uterovesical space or other adjacent anatomic planes [37].
-
Attention is required to distinguish lower uterine segment hypervascularity often associated with placenta previa from bridging vessels.
-
Ultrasound markers of placenta accreta spectrum on images of lower uterine segment; (a) sagittal view with placenta lacunae present; (b) transverse view with placenta lacunae present; (c) loss of the retroplacental hypoechoic zone between the placenta and myometrium; (d, e). sagittal view with irregular uteroplacental interface and bulging of the placenta toward the bladder with loss of the retroplacental hypoechoic zone between the placenta and myometrium; (f) sagittal view of placental extrusion beyond the uterine wall into the bladder; (g, h) sagittal view of hypervascularity of the placenta myometrium interface with bridging vessels
If PAS is suspected, the extent of placental involvement, focal or global, and the depth of placental invasion, confined to the uterus or extending to the adjacent organs such as the bladder or parametrium, should also be evaluated.
There are other techniques that can help to improve diagnosis but are not well described yet. Measurement of the peak systolic velocity of placental vasculature has been shown to have a direct correlation with PAS; however, it lacks sensitivity with a low negative predictive value [38]. Three-dimensional (3D) US in combination with power Doppler may also help to better evaluate the placental-myometrial interface looking for hypervascularity, and tortuous vascularity with chaotic branching, and also help to better evaluate for involvement of adjacent organs [39]. Detection of hypervascularity of the uterine-bladder interface by 3D ultrasound is reported to have superior diagnostic sensitivity and specificity over 2D imaging with 100% positive predictive value [40]. However, 3D imaging remains a complex technique requiring operator expertise and may not be an available option in some centers.
It should be taken into account that the presence of these markers in the low-risk population without placenta previa or history of previous cesarean delivery may be hard to interpret and often does not convey an increased risk for PAS [41].
MRI Evaluation
Additional MRI can provide similar diagnostic accuracy when compared to ultrasound. Due to the higher cost of MRI, it is recommended to consider MRI when ultrasound evaluation of the placenta is technically difficult such as in posterior placentation, morbid obesity, or multiple gestation [42]. Other limiting factors for US evaluation are operator dependency, quality of equipment used, and acoustic effects such as fetal position, prior scars, uterine contraction, myomas, and insufficient or excessive maternal urinary bladder filling. Overall, MRI provides similar detection rate compared to US for PAS diagnosis [42, 43]; however, it can provide more details regarding the depth of invasion and adjacent organ involvement [26, 44].
T2-weighted imaging is the modality for placental evaluation. The MRI markers for PAS include (Fig. 6.3):
-
1.
Dark intraplacental bands.
-
2.
Heterogenous signal intensity in the placenta.
-
3.
Focal areas of uterine bulging.
-
4.
Loss of the interface with adjacent organs.
Of the above markers, the first two are the most sensitive and the latter two are the most specific findings for PAS [45].
Other MRI markers include thick T2-hypointense septa within the myometrium likely due to myometrial invasion, tenting of the urinary bladder, and abnormal vascular formation in the placenta as tortuous and enlarged spaces on T2-weighted sequence [45].
It is recommended that at least two of the above findings should be present to raise concern for PAS [46, 47]. MRI has sensitivity and specificity increases after 24 weeks of gestation, up to 79% and 94%, respectively [48]. Although MRI is overall safe in pregnancy, use of gadolinium-based intravenous contrast is not recommended for possible fetal side effects [49, 50].
Special Considerations
-
Despite ongoing efforts to improve antenatal diagnosis of PAS, PAS is often not diagnosed until the time of delivery. Under such circumstances, when appropriate resources for managing such patients are not available, temporary closure of the abdomen and rapid transfer to a tertiary center with higher level of care can be considered as long as the patient is hemodynamically stable.
-
False-positive diagnosis of PAS may introduce unnecessary perinatal morbidity. However, a recent study investigating the outcomes in such patients revealed acceptable outcomes if managed in a referral center with expertise in managing PAS. The incidence of unnecessary hysterectomy was reported to be 2% or less [51].
References
Miller DA, Vhollet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol. 1997;177:210–4.
Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005;192:1458–61.
Bailit JL, Grobman WA, Rice MM, Reddy UM, Wapner RJ, Varner MW, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal Fetal Medicine Units (MFMU) Network. Morbidly adherent placenta treatments and outcomes. Obstet Gynecol. 2015;125(3):683–9.
Clark SL, Koonings PP, Phelan JP. Placenta previa/accreta and prior cesarean section. Obstet Gynecol. 1985;66:89–92.
Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107:1226–32.
Erfani H, Fox KA, Clark SL, Rac M, Rocky Hui SK, Rezaei A, Aalipour S, Shamshirsaz AA, Nassr AA, Salmanian B, Stewart KA, Kravitz ES, Eppes C, Coburn M, Espinoza J, Teruya J, Belfort MA, Shamshirsaz AA. Maternal outcomes in unexpected placenta accreta spectrum disorders: single-center experience with a multidisciplinary team. Am J Obstet Gynecol. 2019;221(4):337.e1–5.
Gyamfi-Bannerman C, Gilbert S, Landon MB, Spong CY, Rouse DJ, Varner MW, et al. Risk of uterine rupture and placenta accreta with prior uterine surgery outside of the lower segment. Obstet Gynecol. 2012;120:1332–7.
Kohn JR, Shamshirsaz AA, Popek E, Guan X, Belfort MA, Fox KA. Pregnancy after endometrial ablation: a systematic review. BJOG. 2018;125(1):43–53.
Carusi DA, Fox KA, Lyell DJ, Perlman NC, Aalipour S, Einerson BD, Belfort MA, Silver RM, Shamshirsaz AA. Placenta accreta spectrum without placenta previa. Obstet Gynecol. 2020;136(3):458–65.
Salmanian B, Fox KA, Arian SE, Erfani H, Clark SL, Aagaard KM, Detlefs SE, Aalipour S, Espinoza J, Nassr AA, Gibbons WE, Shamshirsaz AA, Belfort MA, Shamshirsaz AA. In vitro fertilization as an independent risk factor for placenta accreta spectrum. Am J Obstet Gynecol. 2020;223(4):568.e1–5.
Modest AM, Toth TL, Johnson KM, Shainker SA. Placenta accreta spectrum: in vitro fertilization and non-in vitro fertilization and placenta Accreta Spectrum in a Massachusetts Cohort. Am J Perinatol. 2021;38(14):1533–9.
Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa and placenta accreta. Am J Obstet Gynecol. 1997;177(1):210–4.
Shamshirsaz AA, Carusi D, Shainker SA, Einerson B, Khandelwal M, Erfani H, Shamshirsaz AA, Modest AM, Aalipour S, Fox KA, Lyell DJ, Belfort MA, Silver RM. Characteristics and outcomes of placenta accreta spectrum in twins versus singletons: a study from the pan American Society for Placenta Accreta Spectrum (PAS(2)). Am J Obstet Gynecol. 2020;222(6):624–5.
Miller HE, Leonard SA, Fox KA, Carusi DA, Lyell DJ. Placenta accreta spectrum among women with twin gestations. Obstet Gynecol. 2020:3. Online ahead of print
Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. The management and outcomes of placenta accreta, increta, and percreta in the UK: a population-based descriptive study. BJOG. 2014;121(1):62–70.
Wright JD, Pri-Paz S, Herzog TJ, Shah M, Bonanno C, Lewin SN, et al. Predictors of massive blood loss in women with placenta accreta. Am J Obstet Gynecol. 2011;205(38):e1–6.
Eller AG, Porter TF, Soisson P, Silver RM. Optimal management strategies for placenta accreta. BJOG. 2009;116:648–54.
Warshak CR, Ramos GA, Eskander R, Benirschke K, Saenz CC, Kelly TF, et al. Effect of predelivery diagnosis in 99 consecutive cases of placenta accreta. Obstet Gynecol. 2010;115:65–9.
Shamshiraz AA, Fox KA, Salmanian B, Diaz-Arrastia CR, Lee W, Baker BW, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212(218):e1–9.
Shamshirsaz AA, Fox KA, Erfani H, et al. Multidisciplinary team learning in the management of the morbidly adherent placenta: outcome improvements over time. Am J Obstet Gynecol. 2017;216(612):e1–5.
D'Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2013;42(5):509–17.
Salmanian B, Shainker SA, Hecht JL, Modest AM, Castro EC, Seaman RD, Meshinchiasl N, Hessami K, Brown A, Tounsi S, Shamshirsaz AA, Fox KA, Clark SL, Belfort MA, Shamshirsaz AA. The Society for Pediatric Pathology Task Force grading system for placenta accreta spectrum and its correlation with clinical outcomes. Am J Obstet Gynecol. 2022; S0002-9378(22)00100-4
Tabsh KM, Brinkman CR 3rd, King W. Ultrasound diagnosis of placenta increta. J Clin Ultrasound. 1982;10(6):288–90.
Jauniaux E, Kingdom JC, Silver RM. A comparison of recent guidelines in the diagnosis and management of placenta accreta spectrum disorders. Best Pract Res Clin Obstet Gynaecol. 2020;
Jauniaux E, Bhide A. Prenatal ultrasound diagnosis and outcome of placenta previa accreta after cesarean delivery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217(1):27–36.
Pagani G, Cali G, Acharya G, et al. Diagnostic accuracy of ultrasound in detecting the severity of abnormally invasive placentation: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97(1):25–37.
Calì G, Timor-Trisch IE, Palacios-Jaraquemada J, et al. Changes in ultrasonography indicators of abnormally invasive placenta during pregnancy. Int J Gynaecol Obstet. 2018;140:319–25.
Calì G, Timor-Tritsch IE, Palacios-Jaraquemada J, et al. Outcome of cesarean scar pregnancy managed expectantly: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51:169–75.
Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound Obstet Gynecol. 2014;43:383–95.
Ballas J, Pretorius D, Hull AD, Resnik R, Ramos GA. Identifying sonographic markers for placenta accreta in the first trimester. J Ultrasound Med. 2012;31(11):1835–41.
Cali G, Forlani F, Timor-Tritsch IE, et al. Natural history of Cesarean scar pregnancy on prenatal ultrasound: the crossover sign. Ultrasound Obstet Gynecol. 2017;50(1):100–4.
Shainker SA, Coleman B, Timor IE, Bhide A, Bromley B, Cahill AG, Gandhi M, Hecht JL, Johnson KM, Levine D, Mastrobattista J, Philips J, Platt LJ, Shamshirsaz AA, Shipp TD, Silver RM, Simpson LL, Copel JA, Abuhamad A. Society for Maternal-Fetal Medicine. Electronic address: pubs@smfm.org. Special Report of the Society for Maternal-Fetal Medicine Placenta Accreta Spectrum Ultrasound Marker Task Force: consensus on definition of markers and approach to the ultrasound examination in pregnancies at risk for placenta accreta spectrum. Am J Obstet Gynecol. 2021;224(1):B2–B14.
Maynard H, Zamudio S, Jauniaux E, Collins SL. The importance of bladder volume in the ultrasound diagnosis of placenta accreta spectrum disorders. Int J Gynecol Obstet. 2018;140(3):332–7.
Bhide A, Sebire N, Abuhamad A, Acharya G, Silver R. Morbidly adherent placenta: the need for standardization. Ultrasound Obstet Gynecol. 2017;49:559–63.
Finberg HJ, Williams JW. Placenta accreta: prospective sonographic diagnosis in patients with placenta previa and prior cesarean section. J Ultrasound Med. 1992;11:333–43.
Collins SL, Ashcroft A, Braun T, et al. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol. 2016;47:271–5.
Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218:75–87.
Zhang J, Li H, Wang F, Qin H, Qin Q. Prenatal diagnosis of abnormal invasive placenta by ultrasound: measurement of highest peak systolic velocity of subplacental blood flow. Ultrasound Med Biol. 2018;44(8):1672–8.
Shih JC, Palacios Jaraquemada JM, Su YN, et al. Role of three-dimensional power doppler in the antenatal diagnosis of placenta accreta: comparison with gray-scale and color doppler techniques. Ultrasound Obstet Gynecol. 2009;33(2):193–203.
Cali G, Giambanco L, Puccio G, Forlani F. Morbidly adherent placenta: evaluation of ultrasound diagnostic criteria and differentiation of placenta accreta from percreta. Ultrasound Obstet Gynecol. 2013;41(4):406–12.
Philips J, Gurganus M, DeShields S, et al. Prevalence of sonographic markers of placenta accreta spectrum in low-risk pregnancies. Am J Perinatol. 2019;36(8):733–80.
D’Antonio F, Iacovella C, Palacios-Jarquemada J, Bruno CH, Manzoli L, Bhide A. Prenatal identification of invasive placentation using magnetic resonance imaging :systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2014;44:8–16.
Meng X, Xie L, Song W. Comparing the diagnostic value of ultrasound and magnetic resonance imaging for placenta accreta: a systematic review and meta-analysis. Ultrasound Med Biol. 2013;39(11):1958–65.
Palacios-Jaraquemada JM, Bruno CH, Martin E. MRI in the diagnosis and surgical management of abnormal placentation. Acta Obstet Gynecol Scand. 2013;92(4):392–7.
Dighe M. MR imaging of abnormal placentation. Magn Reson Imaging Clin N Am. 2017;25(3):601–10.
Maurea S, Romeo V, Mainenti PP, et al. Diagnostic accuracy of magnetic resonance imaging in assessing placental adhesion disorder in patients with placenta previa: correlation with histological findings. Eur J Radiol. 2018;106:77–84.
Chu C, Zhao S, Ding M, et al. Combining clinical characteristics and specific magnetic resonance imaging features to predict placenta accreta. J Comput Assist Tomogr. 2019;43(5):775–9.
Horowitz JM, Berggruen S, McCarthy RJ, et al. When timing is everything: are placental MRI examinations performed before 24 weeks' gestational age reliable? AJR Am J Roentgenol. 2015;205(3):685–92.
Patenaude Y, Pugash D, Lim K, et al. The use of magnetic resonance imaging in the obstetric patient. J Obstet Gynaecol Can. 2014;36(4):349–63.
Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37(3):501–30.
Salmanian B, Shamshirsaz A, Fox K, Meshinchi Asl N, Erfani H, Detlefs S, Coburn M, Espinoza J, Nassr A, Belfort M, Clark SL, Shamshirsaz AA. Clinical outcomes of a false positive antenatal diagnosis of placenta accreta spectrum. Am J Perinatol. 2021; Epub ahead of print
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Salmanian, B., Shainker, S.A., Shamshirsaz, A.A., Nassr, A.A. (2023). Diagnosis of Placenta Accreta Spectrum: Clinical and Radiological Diagnosis of Placenta Accreta Spectrum and the Ability of Sonographic and MRI Findings to Predict Definitive Diagnosis. In: Shazly, S.A., Nassr, A.A. (eds) Placenta Accreta Spectrum. Springer, Cham. https://doi.org/10.1007/978-3-031-10347-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-10347-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10346-9
Online ISBN: 978-3-031-10347-6
eBook Packages: MedicineMedicine (R0)