Abstract
Opioids have multiple effects on the immune system (IS). Experimentally, the effects of opioid administration range from a severe inhibition to strong activation of immune responses, depending on the compound, schedule administration, experimental model, or clinical condition. On the other hand, endogenous opioids play a central role in the complex circuitry that mediates the IS and nervous system (NS) communication, tuning the intensity of reactions such as inflammation and pain or the mechanisms for sensing tissue damage and triggering a stress response. This chapter reviews studies showing increased susceptibility to infections and altered immune parameters produced by opioids and some mechanisms involved in direct and indirect actions of opioids on innate and adaptive immunity, the influence on genetic factors and aging on opioid effects, and pathologies where opioids exert immunomodulatory actions, including current information about COVID-19.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
To understand the effects of opioids on immune responses, it is necessary to consider the complexity of the two highly organized systems involved: (1) the opioidergic system with all its ligands, receptors, signaling cascades, and final effects on distinct cell types (Chaps. 8 and 9) and (2) the immune system (IS) with its primary and secondary lymphoid organs, particular responding cells and active molecules produced against damage.
The major design principles of the IS are:
-
Layering: new processes are built on top of initial, more general processes.
-
Scaffolding: early steps in the immune system provide the conditions needed for the later steps.
-
Parallel processing: several events occur at the same time, not always synchronized, in distinct parts of the body.
-
Dynamic engagement: cells act briefly and are then replaced by other cells.
-
Variable network connectivity: mediators and cells acting in one immune process can be incorporated into another ongoing response, thus altering the outcome [1].
Opioids can modify the intensity of processes involved in all the organizational levels of the IS, both directly and indirectly, altering the direction and final consequences of a given immune response.
Classical μ-, δ-, and κ-opioid receptors (ORs), as well as non-classical ORs expressed in immune cells, mediate the direct actions of opioids on the IS. Indirect actions are mainly related to opioid effects on the hypothalamus-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS), as explained in the following sections. Also, conditions involving changes in the IS functioning, such as aging, modify opioid effects. Altogether, evidence shows that opioids alter the individual response to infection and tissue damage, as supported by numerous studies documenting increased susceptibility of opioid users to infections and chronic diseases.
2 Altered Immune Parameters Associated with Opioid Administration
Initial observations of immunosuppressive opioid actions in humans showed that people dependent on heroin or morphine and methadone-treated patients had a high incidence of infectious diseases of viral, bacterial, and fungal etiology [2, 3]. It was initially thought that such infections resulted from transmission of microorganisms through fomites (passive vectors), including the drug paraphernalia used for injection, shared hypodermic needles, or contaminated syringes. It was believed that non-diagnosed acquired immunodeficiency syndrome (AIDS) and lifestyle factors such as malnutrition influenced opioid users’ immunosuppression. However, further controlled studies clearly showed an altered IS function in opioid users [4, 5], and comparisons between healthy and non-dependent subjects who received opioids further supported the notion that these drugs might have immunosuppressive or immunomodulatory effects on their own [6].
Opioids appear to be immunosuppressive regardless of the reasons for use (pain relief, anesthetic, psychoactive effects) (Table 12.1). Recent studies show that prescribed opioids increase the incidence of Gram-positive infections and death [7]. Opioid use also contributes to human immunodeficiency virus (HIV) neuropathogenesis by acting on immune cells such as monocytes, macrophages (MΦs), microglia, and T cells [11, 19, 20]. In addition, patients with rheumatoid arthritis or burn injuries (conditions that occur with local and systemic inflammation) have a higher infection risk after opioid treatment [18, 29]. Activation of μ-OR affects cell differentiation, migration, and cytokine synthesis in the human epidermis, creating a niche that favors microbial survival in patients with burns [33].
High doses of opioid analgesics in trauma patients are also associated with infectious complications related to pneumonia, bacteremia, urinary tract infection, and wound infection [23]. Other studies have shown a higher incidence of diseases associated with repeated parenteral opioid administration, such as viral infections and infective endocarditis (Table 12.1) [34].
Clinical use of opioids has been related to poor surgical outcomes and the appearance of other diseases, such as infections and cancer [35]. In humans, immunosuppression after surgery has traditionally been associated with factors such as hypothermia, poor lung ventilation, or pre-existing health conditions [36], but evidence indicates that fentanyl, remifentanil, and morphine, can produce deleterious effects because of their immunosuppressive actions [37,38,39].
Fukada et al., in 2016, showed that paradoxical effects of opioids on the IS can be related to different administration schedules because the timing of administration can determine whether morphine exacerbates or inhibits an infection. For example, morphine treatment before a lipopolysaccharide (LPS) challenge (which mimics an infection with Gram-negative bacteria) suppresses lethal endotoxic shock in mice, but exacerbates it when administered after LPS [40].
Morphine and other μ-OR agonists do not produce similar immunosuppressive effects. Recent clinical trials suggest that there are two distinct groups of opioids: those with significant immunosuppressive effects (i.e., codeine, dihydrocodeine, methadone, morphine, or fentanyl) and those with less immunosuppressive effects (i.e., buprenorphine, hydromorphone, oxycodone, or tramadol) [41]. Moreover, the administration scheme and individuals’ condition and health status modify the final effect of a specific opioid on selected immune responses.
The mechanisms proposed for opioid-induced immunosuppression are diverse. Some involve direct activation of ORs on immune cells; others require stress response activation. A few mechanisms are related to alterations in gut microbiota (dysbiosis), which compromise the intestinal barrier and allow changes in gut permeability, leading to systemic deleterious inflammation. The following section presents some general aspects of the IS function to understand the consequences of opioid actions on immune responses.
3 Overview of the Immune System
The immune system is organized based on the type and time course of reactions elicited by infection or tissue damage.
The innate IS (also called the innate immunity responses) constitutes the first line of defense against germs or damage in the body. In general, it is nonspecific, acts rapidly, and has limited efficiency. It comprises physical barriers for the entrance of external substances and specialized secretions (such as tears, mucus, and saliva) and involves the activation of tissue-resident mast cells (MCs), macrophages (MΦs), and natural killer (NK) cells, among others.
The adaptive immune system orchestrates adaptive immunity responses, activating T and B lymphocytes which proliferate and produce cytokines or differentiate and produce antibodies, respectively. Adaptive immunity responses via T cells initiate after antigen presentation by dendritic cells to T-cell receptor (TCR), whereas B cell antibody production starts after antigen recognition via the B-cell receptor (BCR). Alteration of the early and efficient innate immune response against pathogenic insults promotes an impaired adaptive response that results in uncontrolled inflammation and host tissue damage (Fig. 12.1).
Features of innate and adaptive immunity. Innate immunity constitutes the early line of defense that is in place even before infection. It is composed by various cell types, such as mast cells (MCs), monocytes, macrophages (MΦ), dendritic cells (DCs), and natural killer cells (NKs), and active molecules, such as proteins of the complement system, cytokines, and enzymes. Structures that are common to groups of related microbes or are released by damaged cells activate innate immune responses, which shape adaptive immunity trough the interaction of phagocytic cells with T cells (among other mechanisms). Those events result in the production of antigen-specific T and B lymphocytes. Adaptive immunity is based on lymphocytes properties; they can respond selectively to a huge number of different antigens, leading to specific memory and efficient effector response. Created with BioRender.com
Effective connection between innate and adaptive immune responses is mainly orchestrated by cytokines. Cytokines are low molecular weight, signaling proteins that are released in response to diverse stimuli. They have specific effects on the activation, proliferation, and differentiation of immune cells. Chemokines (chemotactic cytokines) are also proteins produced by immune cells, but their main action is to induce the chemotaxis needed for the recruitment of cells to sites of infection and/or tissue damage. By doing this, cytokine and chemokine production modifies the interaction between the cellular elements of a given immune response.
Pro-inflammatory cytokines alert the IS against invading pathogens. Some pro-inflammatory cytokines are interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-2, IL-6, and chemokines such as IL-8, CCL2, and CCL5 (also known as RANTES). Anti-inflammatory cytokines limit the actions of pro-inflammatory ones. Major anti-inflammatory cytokines include IL-10, IL-4, transforming growth factor (TGF)-β, and soluble cytokines receptors [42].
Opioids can alter the activity of distinct immune cells and modify the course of an immune reaction by direct interaction with immune cells or by activation of neuroendocrine mechanisms that modulate global immune responses. The following sections present current knowledge on that matter.
4 Direct Effects of Opioids on the IS
4.1 Direct Actions of Opioids on Innate Immune Cells: Focus on Inflammation
Immune cells express all described ORs. As mentioned in Chap. 9, ORs are G protein-coupled receptors (GPCR). To date, five ORs have been identified; three classical, μ-, κ-, and δ-OR; and two non-classical receptors, the nociceptin/orphanin FQ (ORL-1 or NOP) receptor and the novel atypical Mas-related G protein-coupled receptor X2 (MRGPRX2), expressed in MCs, basophils, eosinophils, and other immune cells [43,44,45].
Opioid receptors activate similar signal transduction cascades in neurons and immune cells, but with some relevant particularities, since the latter ones are not considered excitable cells. As mentioned in Chap. 9, activation of classical ORs and NOP promotes Gi/o dissociation into Gαi/o and Gβγ subunits. Gαi/o inhibits adenylyl cyclase and PKA activity, modulating ion channels activated by capsaicin (TRVP1) and voltage-gated sodium channels. Meanwhile, the Gβγ complex blocks Ca2+ channels (Cav2+), increases K+ channels conductance (GIRK and KATP), and activates PKC and mitogen-activated protein kinases (MAPKs) [46]. ORs are desensitized by phosphorylation and then internalized by clathrin-dependent pathways, a mechanism initially controlled by GPCR kinases (GRKs) and followed by β-arrestin binding [47].
The second messengers evoked through opioid stimulation of MRGPRX2 are not completely identified. Activation of these receptors induces Ca2+ influx and MAPKs activation, which suggests that MRGPRX2 receptors could couple to a Gαq protein [48]. It remains unclear whether opioids have the same effects on Ca2+ and K+ channels in immune cells and neurons.
The inflammatory response is the most important innate immune process (Fig. 12.2). It is classically triggered by the activation of pattern recognition receptors (PRRs), e.g., Toll-like receptors (TLRs) expressed by immune tissue-resident cells, such as MΦs, MCs, and others.
The inflammatory response. (1) After a harmful stimulus or infection, PAMPs or DAMPs bind to PRRs and activate tissue-resident MCs and MΦs. For example, triggering of TLRs promotes the release of pro-inflammatory mediators (e.g., TNF-α, IL-1β, and IL-12, among others), favoring neutrophil migration toward the inflamed tissue. Nod-like receptor (NLRP) activation leads to the formation of inflammasomes that results in IL-1β secretion. (2) Neutrophils travel from the circulation to the damaged tissues through blood vessels with the participation of adhesion molecules highly expressed in endothelial cells. (3) Neutrophils secrete mediators to kill pathogens or dead cells into the compromised tissue and phagocyte them. (4) In addition, neutrophils release chemical mediators and extracellular DNA traps by the induction of programmed cell death (NETosis and apoptosis) capturing and killing pathogens to prevent them from spreading. (5) In parallel, non-inflammatory monocytes arrive and eliminate the pro-inflammatory molecules, pathogens, and cellular debris, promoting its reprogramming to mature MΦs. Both leukocytes, neutrophils and monocytes, release opioid peptides into the inflamed tissue supporting the beginning of the resolution steps of the inflammatory response. (6) Reprogrammed macrophages release anti-inflammatory cytokines to stop leukocyte infiltration and attract immune cells that will support the tissue remodeling and homeostasis recovery. Created with BioRender.com
Pathogen-associated molecular patterns (PAMPs), like bacterial lipopolysaccharide (LPS), and damage-associated molecular patterns (DAMPs), like heat-shock proteins or intracellular proteins, activate PRRs, causing the release of pro-inflammatory mediators, such as biogenic amines (histamine, serotonin), cytokines (TNF-α, IL-6, IL-1β), chemokines (CCL2, CCL5), and active lipids that depend on cyclooxygenase 2 (COX2) activity (leukotrienes, prostaglandins, and tromboxanes). Also, PRR activation leads to the synthesis of proteins like the inducible nitric oxide synthase (iNOS), which increases the concentration of nitric oxide and promotes oxidative stress. These mediators and enzymes translate damage sensing into a coordinated response of endothelial, neuronal, and immune cells directed to remove or contain the pathogen or tissue damage and to resolve inflammation and restore the tissue homeostasis [49, 50].
The resolution phase of inflammation involves the limitation of leukocyte infiltration, the induction of cell apoptosis and their phagocytic removal, clearance of pro-inflammatory dead cells and cytokines, and finally, the beginning of the healing processes, culminating in the reconstruction of vascular, lymphatic, and nerve networks, which regenerate a functional tissue (Fig. 12.2) [50].
Inflammation can be acute or chronic, depending on the time that it lasts and the mechanisms involved in its initiation and resolution. Acute inflammation develops in minutes to hours and lasts for days, whereas chronic inflammation remains active for as long as infection or tissue damage persists. Chronic inflammation is associated with the development of multiple degenerative diseases.
Opioids alter various steps of the inflammatory response through multiple mechanisms triggered by their binding to classical or non-classical ORs expressed on immune cells. Later, in the course of physiological inflammation, granulocytes, monocytes, MΦs, and lymphocytes synthesize endogenous opioid peptides, which infiltrate the site of injury in high quantities [51,52,53,54,55]. Adhesion molecules and chemokines control the accumulation of these opioid peptide-containing cells into the extravascular inflamed tissue [56, 57].
Stressors and local-inflammatory factors trigger the release of endogenous opioid peptides that activate ORs on peripheral terminals of sensory neurons, evoking analgesia and anti-inflammatory effects [53]. Continuous production and release of opioid peptides from immune cells into injured tissue are active processes in the resolution phase of inflammation. Due to those actions, opioids can suppress this first defense mechanism, promoting infection or damage progression (Fig. 12.2).
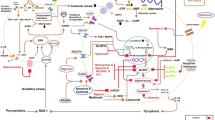
Studies on the molecular mechanism of opioids’ inhibitory actions on inflammation triggered by PRRs can help identify molecules that could be targets to prevent opioid-induced immunosuppression. For example, the TLR4 signaling system involves activation of intracellular kinases, ubiquitin ligases, and the NF-κB transcription factor (Box 12.1) implicated in pro-inflammatory cytokine synthesis.
Box 12.1 TLR4 Signaling Pathways

PAMPs (e.g., LPS) and DAMPs (e.g., HMGB proteins) activate the TLR4 receptor complex, composed by the TLR4/MD-2 dimer [58]. Signaling pathways include those coordinated by MyD88 or TRIF adapter proteins. In the MyD88-dependent pathway, after activation of several kinases, AP-1 and NF-κB transcription factors are translocated to the nucleus to initiate de novo mRNA synthesis for distinct pro-inflammatory cytokines. In the TRIF-dependent pathway, TRIF adapter binds to the ubiquitin ligase TRAF3, promoting the activation of the interferon regulatory factor 3 (IRF3) and stimulating the transcription of type I interferon genes (IFNs) [42, 59]
PAMPs pathogen-associated molecular patterns, LPS lipopolysaccharide, DAMPs damage-associated molecular patterns, HMGB high mobility group box 1 proteins, MyD88 myeloid differentiation primary response gene 88), IRAK interleukin-1 receptor-associated kinases, TRAF6 TNF receptor-associated factor 6, TAK1 TGF-β-activated kinase 1, MAPKs mitogen-activated protein kinases, IKK the IκB kinase complex, AP-1 activating protein 1, NF-κB nuclear factor κ-light chain enhancer of activated B cells. Created with BioRender.com
Opioids can inhibit several steps in the TLR4 signaling cascades (Fig. 12.3). For example, in tissue-resident MCs, μ-OR activation by morphine and fentanyl and δ-OR activation by morphine prevent the early secretion of TNF-α induced by TLR4 stimulation through a process that involves the formation of a β-arrestin 2 and TRAF6 complex [59,60,62]. TNF-α is an inflammatory cytokine responsible for the activation of endothelial cells and leukocytes and induction of acute-phase response, among others roles. Also, μ-OR and δ-OR activation in MΦs inhibits TNF-α and IL-1β, IL-6, and IL-12 production after TLR4 triggering via NF-κB inhibition. In addition, opioids reduce the levels of nitric oxide (NO), iNOS, and COX-2 promoted by LPS [62,63,64,66], bioactive molecules that, as mentioned, play an important role in vascular function and host defense. Furthermore, activation of ORs in MΦs also impacts the phagocytic activity of those cells [66,67,69]. For example, morphine enhances MΦs’ phagocytic capacity induced by TLR4 activation [70, 71]. However, bacteria clearance is unsuccessful, because morphine suppresses phagosome maturation, which is critical for destroying pathogens [71]. Figure 12.3 summarizes the intracellular mechanisms involved in the impaired response of innate cells against injury stimulus due to OR activation.
Molecular opioid-controlled check points on TLR4 receptor signaling cascade. Opioids modulate innate immune response to pathogens through the interaction with ORs, such as μ-, δ-, and κ, and non-ORs, including MRGPRX2 and TLR4. Activation of ORs by their ligands (e.g., morphine and fentanyl) activates Gαi/o protein-dependent pathways, but also modifies the activity of several molecules involved in the signaling pathways triggered by TLR4, affecting the effector function of immune cells involved in innate and adaptive immunity. TLR4 signal cascade includes the activation of TRAF6, MAPKs, IKK, and the translocation of NF-κB (see Box 12.1 for more details). Main molecular changes induced by the interaction of ORs and TLR4 signaling cascades are the following: (1) β-arrestin 2 forms a complex with TRAF6, inhibiting the secretion of TNF-α; (2) NF-κB activation is blocked, affecting the production of pro-inflammatory mediators; (3) NLPR3 inflammasome formation and oligomerization is enhanced, inducing the maturation and secretion of IL-1β; (4) although opioids improve phagocytic activity, they limit deubiquitination of p62 protein, leading to a deficient pathogen clearance. Mentioned effects are prevented by (−) opioid antagonist, e.g., naloxone and naltrexone. Opioid ligands also bind non-ORs, activating signaling cascades downstream of those receptors. MRGPRX2 can be activated by morphine and codeine, supporting the influx of Ca2+ and the activation of p38 MAPK and IKK and favoring the production of inflammatory mediators. TLR4 is activated by morphine and its metabolite through the interaction with MD-2, promoting a pro-inflammatory signaling pathway that requires the activation of MAPK and NF-κB and leads to the production of pro-inflammatory mediators and theFig. 12.3 (continued) formation of the NLRP3 inflammasome. Opioid antagonists, both (+ and -) isomers equally, binds to MD-2 as opioid agonists do and inhibit the signaling pathway that depend on TRIF/IRF3, suppressing the production of IFNs and other pro-inflammatory mediators. Ligands are indicated by colors, e.g., green = opioid agonists; opioid-stimulated receptor are illustrated by figures, e.g., circles = ORs; and effect are shown by different line head, e.g., → = activation. Therefore, opioid agonists activate ORs promoting activation. Created with BioRender.com
Additionally, morphine, fentanyl, and remifentanil decrease neutrophil and monocyte transmigration across endothelial cells via μ-ORs, increasing susceptibility to infections [69, 71,72,74]. Moreover, opioid agonists acting at μ-ORs in endothelial cells attenuate cell activation during inflammation [72] and suppress the expression of intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 required for cell migration, through a mechanism dependent on NF-κB [73,74,75,77].
After a noxious stimulus, μ-ORs also modulate innate immune cells activation in the central nervous system (CNS), including glial cells, microglia, and astrocytes. In most cases, opioid effects are pro-inflammatory, which are opposite to the effects observed on peripheral immune cells. For example, in microglia, morphine enhances the production of TNF-α, IL-1β, IL-6, and NO induced by LPS, by increasing TRAF6 [78], MAPKs [78, 79], and IKK [80] activation (see Box 12.1) promoted by TLR4. These effects are mediated by classical ORs because they can be blocked by naloxone [79]. Also, in combination with the HIV protein Tat or the bacteria Streptococcus pneumoniae, morphine improves the expression of cytokines and chemokines, such as TNF-α, IL-6, CCL2, and RANTES favoring the trafficking of T cells and monocytes into the CNS [81, 82].
Morphine and fentanyl activate the NOD-like receptor protein 3 (NLRP3) inflammasome in microglia, favoring caspase 1 activation and IL-1β production [83, 84]. Furthermore, activation of μ-ORs induces DAMP HSP70 (heat shock protein 70) [85] and HMGB1 (high mobility group box 1) production [86], which, in turn, activates TLR4, causing TNF-α and IL-1β production through NF-κB and the formation of NLRP3 inflammasome [86]. Taken together, these data indicate that μ-ORs mediate a sensitization stage of microglia that enhances its activation by harmful stimuli, favoring the development of neuroinflammation that could result in neuronal damage and even cell death.
Paradoxical effects of opioids seem to be related to their binding to non-classical ORs and non-ORs. For example, morphine activates both MRGPRX2 and TLR4. Similarly, codeine, a low affinity μ-OR agonist, activates MRGPRX2 expressed in MCs, favoring their degranulation and the production of histamine, TNF-α, CCL2, RANTES, and IL-8, by a Ca2+-dependent mechanism and activation of MAPKs and IkB kinase (IKK) [86,87,89].
Morphine, like LPS, binds to MD-2 protein, promotes TLR4 oligomerization, and activates TLR4’s signaling pathway (see Box 12.1) [90, 91]. Moreover, morphine increases TLR4, TAK1, and NLRP3 expression, and these effects do not occur in cells lacking TLR4 [92].
Opioids interact differently with classical ORs and TLR4. Some main differences are:
-
Both OR agonists and OR antagonists bind to and activate TLR4.
-
Morphine-3-glucuronide (M3G), morphine’s inactive metabolite (as analgesic), triggers TLR4 signaling and induces IL-1β production.
-
Both dextro- (+) and levo (−) isomers of naloxone and naltrexone block TLR4 [93], but only (−)-naloxone and (−)-naltrexone block ORs.
Both naloxone’s and naltrexone’s isomers inhibit the production of NO, ROS, TNF-α, and phagocytosis induced by TLR4 signaling in microglia and macrophage cell cultures [90, 94]. Naloxone and naltrexone do not inhibit MAPKs and NF-κB activation but do suppress IFN regulatory factor 3 (IRF3) activation promoted by TLR4 stimulation [94]. These studies open new avenues for studying the cellular and molecular changes that opioid ligands can induce in cells of the IS.
4.2 Direct Actions of Opioids on Lymphocytes
Cells of the lymphocyte lineage express ORs [95]. Morphine and endogenous opioid peptides, including β endorphin, modulate B and T lymphocytes’ function. Several researchers have proposed that opioids act like cytokines, orchestrating complex responses where distinct cell types participate. For example, the mixed agonist-antagonist buprenorphine (Chap. 8) suppresses splenic NK cell activity, lymphocyte proliferation, and IFN-γ production in rats [96]. Opioids also suppress the movement and the number of circulating white blood cells [97, 98] and play a role in suppressing a variety of immunological endpoints such as cell proliferation and cytokine synthesis [99].
Studies have evolved from anecdotic reports of immunosuppressive actions of opioids to well-designed and controlled studies in humans. For example, a randomized pilot study in gynecological laparotomy patients evaluated the effect of morphine and oxycodone on immune responses [100]. Patients were randomized to receive morphine, oxycodone, or nonopioid analgesia during and after surgery. Using different molecular techniques, the researchers analyzed gene expression, NK cell activation, and serum cytokine concentration at different times after opioid treatment. The results showed that morphine, but not oxycodone or epidural analgesia, produced immunosuppression 2 hours post incision [100].
Similarly, morphine showed a higher immunosuppressive effect than oxycodone in patients who suffered a radical resection of rectal cancer [101]. Recent studies have shown that morphine antagonizes the chemotaxis induced by TNF-α and IL-1β in human leucocytes, decreases IL-2 and IFN-γ levels, and increases IL-4 and IL-5 plasma concentration, suggesting that morphine can block an effective immune response against pathogens or tissue damage. In addition, long-term use of opiates produces atrophy of lymphoid organs, decreases lymphoid content, alters antigen-specific antibody production, causes loss of T helper (Th) cells, and decreases T cell reactivity [102, 103].
5 Indirect Mechanisms of Action of Opioids on the IS
5.1 Opioid Actions Through HPA Axis Activation
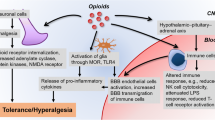
The hypothalamus-pituitary-adrenal axis (HPA) is the neuroendocrine system involved in mediating the stress response. It starts with the release of corticotropin-releasing hormone (CRH), which is produced in neurons in the hypothalamic paraventricular nucleus (PVN) and travels through the portal vasculature to stimulate the release of adrenocorticotropic hormone (ACTH) from corticotropes of the anterior lobe of the pituitary gland. ACTH acts in the adrenal cortex, promoting glucocorticoid biosynthesis and release, mediating stress responses, and regulating the axis through negative feedback [104]. Acute stress benefits survival of the individual, but chronic stress induces HPA axis dysregulation, leading to the development of various pathologies, including general immunosuppression. The neuroendocrine response of HPA axis activation mainly inhibits pro-inflammatory cytokine production induced by the innate immune response [105]. The main mechanism involved is mediated by glucocorticoids which, by activating their receptors in the cytoplasm of immune cells, act directly in inhibiting the activation of NF-κB or indirectly increasing the transcription and translation of the NF-κB inhibitory protein (IκB) (Fig. 12.4).
Indirect actions of opioids on the immune system. Opioid receptors lead to activation of the hypothalamus-pituitary-adrenal (HPA) axis, the sympathetic (SNS), and the parasympathetic nervous system (PNS). Vagus nerve innervates lymphoid organs modulating immune cells’ differentiation. Activation of adrenergic, acetylcholine, and glucocorticoid receptors in immune cells inhibits pro-inflammatory cytokine synthesis by different intracellular pathways. Glucocorticoid receptors promote the synthesis of NF-κB inhibitor (IkB) protein [1]. β-Adrenergic receptor activation increases cAMP levels and PKA activation [2]. Nicotinic acetylcholine receptor rises intracellular Ca2+ concentration, leading to protein kinase C (PKC) activation, and produces Hem Oxygenase 1 [3]. Alternate pathways depending on nicotinic receptors interfere with NFκB signaling [4]. CRH: corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone, nuclear factor erythroid 2-related factor 2 (Nrf2). Created with BioRender.com
The acute immunosuppressive effects of μ-OR agonists (e.g., morphine, fentanyl) mediated by the HPA axis have been studied using animal models or in vitro cell systems [99, 106, 107]. These studies indicate that opioid effects depend on the type of opioid administered, treatment time, and experimental conditions.
In clinical trials, patients treated for at least 6 months with oxycodone, morphine, fentanyl, or buprenorphine had a suboptimal initial cortisol response after stimulating the HPA axis with exogenous ACTH [108]. However, heroin-dependent individuals had elevated ACTH and cortisol levels that returned to basal levels after a single dose of opioids [109]. Few clinical trials have addressed the relationship of the immunosuppressive effects of opioids with cortisol levels in prescription opioid users [110]. Opioid anesthetic and analgesic drugs acutely stimulate the HPA axis during the perioperative period, producing immunosuppression, but not all OR agonists have the same effects. For example, tramadol induces very little immunosuppression compared to remifentanil, fentanyl, or morphine in both animal models and clinical trials [111].
Notably, the immunosuppressive effects of chronic opioids can be dissociated from their analgesic effects even when acting through the same μ-ORs [60]. Thus, it is likely that opioids that produce more significant immunosuppression show a distinctive pattern of HPA axis activation than those with mild effects and that the final outcome depends both on the pharmacological properties of each opioid and the treatment time.
The contribution of the HPA axis to the immunosuppressive effects of prescription opioids remains unclear because studies addressing this mechanism are scarce, and there are confounding factors, such as the stress prior to surgery and other clinical conditions that produce stress mediators in patients. For this reason, it is important to continue the study of opioid effects on the immune system in animal models and clinical trials, especially in conditions where continuous monitoring and pain control is needed.
5.2 Actions of Opioids on the IS Via the Sympathetic Nervous System (SNS) and Vagus Nerve
Opioids can also modulate immune responses via the sympathetic nervous system (SNS) (Fig. 12.4). Systemic morphine administration increases acetylcholine (ACh) release in the rat spinal dorsal horn, suggesting that opioids activate the SNS [112]. Most of the evidence that suggests SNS participation in immunosuppressive opioid effects comes from in vitro and animal studies [113, 114]. ACh binds to nicotinic receptors in postganglionic neurons, promoting the release of norepinephrine and adrenaline from the adrenal medulla. Norepinephrine binds to α- and β-adrenergic receptors located in immune cells. β-Adrenergic agonists suppress lymphocyte and MΦs functions by elevating intracellular cAMP and, consequently, inhibiting pro-inflammatory cytokine synthesis [105, 113].
Another mechanism involved in opioids’ immunosuppressive actions is parasympathetic nervous system (PNS) activation, which, through the stimulation of the vagus nerve, initiates a response known as the cholinergic anti-inflammatory reflex. Diverse in vitro studies show that inhibition of TNF-α secretion occurs through nicotinic cholinergic receptors located in murine and human MΦs [114,115,117]. ACh inhibits immune cell function by binding to ionotropic α7 nicotinic receptors that promote Ca2+ influx, activate several signaling pathways, and cause the synthesis of anti-inflammatory mediators [118]. In MCs, nicotinic receptors block the LPS-induced production of TNF by inhibiting the phosphorylation of the MAPK ERK1/2, which phosphorylates and activates the metalloproteinase responsible for TNF-α processing in the plasma membrane [119]. Other inhibitory mechanisms of ACh involve a direct interaction of ACh receptors with G protein activation of Janus kinase 2 (JAK2)-dependent signaling, which elicits downstream cascades dependent or independent of Ca2+ influx, leading to NFκB inhibition [118] (Fig. 12.4).
6 Conditions that Modulate Opioid Actions on the Immune System: Genetic Polymorphisms and Aging
6.1 Genetic Polymorphisms
Some genetic variants alter HPA axis reactivity, resulting in variations in opioid actions on immune cells. These gene variants exist for GABAA receptors, μ-ORs, serotonin transporters, the monoamine oxidase enzyme, MAO-A, adrenergic receptors, brain-derived neurotrophic factor (BDNF), and others [120]. In particular, the μ-OR variant containing a single-nucleotide polymorphism (SNP; see Chap. 9) in the extracellular domain (A118G) leads to the change of an amino acid from asparagine to an aspartate during protein translation (Asn40Asp). In vitro studies have found that this variant increases threefold the affinity of μ-OR for endorphins [121]. Thus, carrier individuals of this polymorphism experience less analgesic opioid effects in both postoperative pain and cancer-associated pain and have altered HPA axis activation.
Studies carried out in healthy people with different genotypes (AA, AG, and GG) in the same position of the gene have shown that the produced variants are not associated with changes on baseline cortisol levels but respond to a naloxone challenge with a higher concentration of cortisol [122, 123]. It is possible that individuals carrying this polymorphism, mainly found in European American individuals [123], present a higher inhibitory tone on CRH neurons expressing μ-opioid receptors, and their response to different psychological and physical stressors could be lower when compared to that of individuals with other genetic sequences [124, 125].
Because corticosterone exerts a significant inhibition on the immune system and μ-OR Asp40 polymorphism results in higher inhibition of the HPA axis, it is likely that opioid users with such genetic variation would have low cortisol levels. Hence, it would be interesting to study key immune parameters in patients with this polymorphism and analyze if opioids produce immunosuppression.
6.2 Influence of Aging
Cellular stress related to age and senescence induce an immune condition called “inflammaging.” This condition refers to a low-grade inflammatory process accompanied by aging, mainly characterized by an increase in pro-inflammatory cytokine levels [126]. During this immune process, cells such as myeloid-derived suppressor cells and other immunosuppressive cell populations become active to counteract the inflammatory response and promote immune system remodeling, in a phenomenon called “immunosenescence” [127].
At this point, we do not know whether immunosenescence is a mechanism that counteracts chronic inflammation or the consequence of the low-grade inflammatory process associated with aging. Several causes and risk factors that lead to immunosenescence include inadequate micronutrient consumption, the decrease in the length of cell telomeres, reactivity to self-antigens, reactive oxygen species accumulation, lifelong stress, and the increase in HPA function along aging [128, 129].
Studies about the effects of aging on the opioid system and opioid actions on immune responses are scarce. What is known is that aging affects the response of the IS, decreasing defenses against pathogens [129,130,132], lowering vaccination efficacy [133], and impairing anticancer immunity [134]. There is evidence that both endogenous opioid peptides and ORs levels in the brain of rodents decrease with aging [135]. In the elderly, an increased pro-inflammatory general state seems to be related to exacerbated actions of β-endorphin on immune cells, which is evidenced by augmented NK activity, neutrophil adherence, and histamine adherence after opioid administration [135, 136] (Fig. 12.5).
Influence of aging on the opioid system and the effects of opioids on immune system. A comparison of studies carried out in animal models and clinical trials is observed. The senescent condition column shows main results in studies performed in whole healthy elderly individuals or cells isolated from them. The opioid-treated column shows results obtained from studies involving individuals under acute or chronic treatment with exogenous opioids and also studies where ex vivo cells were analyzed (for a more detailed description of these studies, see the text). ↑ = increase, ↓ = decrease. Created with BioRender.com
A comparative study of the effects of various prescription opioids in young versus old rats found that systemic fentanyl injection interfered with cellular immunity, by decreasing the lytic activity of NK cells before and after surgery in old rats. In addition, fentanyl administration after surgery did not change the number of lung metastases of chemically induced mammary adenocarcinoma in young animals, but reduced their occurrence in old animals [137]. These results indicate a dual effect of fentanyl on older individuals since, on the one hand, it impairs the function of the cellular immune response, but on the other hand, it protects from cancer metastasis development.
Endogenous opioid peptides appear to enhance cellular immunity in humans (Fig. 12.5). However, endorphin levels in humans and μ-ORs density and affinity increase, not decrease, with age [138, 139]. Moreover, the NK activity and lymphocyte numbers induced by β-endorphins are higher in healthy elderly volunteers (65–89 years) than in young subjects [140].
Oral administration of a sustained-release morphine formulation decreases antibody production but not peripheral mononuclear proliferation in patients (mean age of 50 years old) with chronic pain as compared to healthy controls [141]. However, prolonged treatment (lasting more than 6 months) with morphine, oxycodone, methadone, or buprenorphine does not affect NK cells [142]. In older patients (median age of 77 years old), the susceptibility to respiratory tract infections and complications such as pneumonia occurs mainly during the first 2 weeks of opioid treatment [32]. These results indicate that further studies are needed to address opioid effects on the IS in older individuals, attending variables that frequently occur during this stage of life, such as the presence of comorbidities and polymedication.
7 Selected Examples of Pathology-Related Actions of Opioids on the IS
7.1 Opioids in the Relationship Between IS and Tumor Growth
Opioids have been used for a long time to manage the perioperative pain associated with tumor ablation or control cancer-induced pain (see Chaps. 10 and 11). Nowadays, the opioid approach to cancer therapy focuses not only on controlling pain but also on fighting tumor growth and metastases. Discrepancies exist among the results of studies analyzing opioid effects on malignant tumor growth (Table 12.2). While some studies indicate that opioids increase tumor mass, others suggest that opioids help block cancer development (Fig. 12.6). Results seem to depend on the type of tumor studied, opioid dose, and administration schedule, as well as the OR subtype activated.
Actions of opioids on specific pathologies. Left panel: Opioids inhibit cytokines related to tumor growth that diminish angiogenesis and tumor mass. Activation of ORs generates damage of mitochondria and DNA, to conduce secretion of apoptosis-related molecules (like Bcl-2) and caspase activation. Caspase 1 activates gasdermin D (GSDMD) to form pores in the cell membrane. On the other hand, opioids inhibit transcription factor HIF-1α, which is related to tumor growth. All mentioned effects promote apoptosis of cancer cells. Negative effects of opioids on cancer are related to the induction of pro-inflammatory and pro-angiogenic cytokines that promote tumor growth. Central panel. Bacteria composing gut microbiota metabolize morphine to morphine-6-glucuronide, which alters permeability of the intestinal barrier and promotes systemic inflammation. Opioid induces permeability of the intestine allowing the passage of the bacteria. Right panel: In intubated patients, treatment with opioids has been associated with higher probability of survival associated with a blockage of the cytokine storm (see text for details). Created with BioRender.com
As to positive effects on cancer, evidence indicates that opioids restrict tumor growth by the following mechanisms: (1) inhibition of cytokine synthesis and release; (2) blockage of tumor angiogenesis; and (3) promotion of cancer cell death.
The tumor microenvironment comprises different cells with non-malignant phenotypes, such as tumor-associated MΦs, endothelial cells, and fibroblasts. These cells release vascular endothelial growth factor (VEGF)-A, which increases vascular permeability and promotes endothelial cell survival and proliferation, cell migration, and blood vessel formation. Blood supply is essential for tumors to grow. Morphine decreases the release of VEGF by tumor-associated MΦs when they are co-cultured with 4 T1 murine mammary breast carcinoma cells, but not when cultured alone. This effect is not dependent on μ-OR activation, because (−)naloxone pretreatment does not prevent it [157]. Interestingly, morphine treatment inhibits activation of MΦs in co-culture with breast carcinoma, an effect that does depend on μ-OR activation [158]. These results indicate that distinct receptors can mediate opioid effects on tumors (Table 12.2), but the molecular mechanisms involved are still unknown.
The hypoxia-inducible transcription factor 1α (HIF-1α) controls the expression of genes involved in angiogenesis, glucose transport, and tumor metabolism after its nuclear translocation. VEGF is one of the genes induced by HIF-1α. Morphine, through a μ-OR-dependent mechanism, inhibits HIF-1α activity, lowering VEGF production in Lewis lung carcinoma cells, and decreases angiogenesis and tumor growth in mice [159]. Also, morphine reduces tumor infiltration of neutrophils and MΦs due to diminished angiogenesis, which contribute to the decrease in lung tumor size [150]. Besides inhibiting VEGF, morphine decreases the production of other pro-inflammatory mediators that contribute to tumor growth. For example, matrix metalloproteinase proteins (MMP) are a family of proteins involved in the breakdown of extracellular matrix in normal physiological processes. However, they also participate in tumor growth and metastasis. Morphine inhibits the number of metastatic foci of breast cancer and decreases the level of circulating proteases in mice. Furthermore, opioids influence the expression of adhesion molecules in cancer cells. For example, colon cancer cells pretreated with morphine have a lower expression of type IV collagen, which is necessary for tumor growth [160].
In humans, the endogenous opioid peptide [Met5]-enkephalin is a negative growth regulator identified as such with the additional name of opioid growth factor [OGF]. This peptide interacts with the growth factor receptor (GFR), inhibiting DNA synthesis and interfering with the cell cycle. A study in adult patients with unresectable pancreatic adenocarcinoma, who did not benefit from chemotherapy, showed that treatment with OGF (250 μg/kg) administered each week for 8 weeks led to a significant reduction in tumor size in 62% of patients [161]. Based on these results, it appears that the study of opioid effects on cancer progression is a promising field to find new therapeutic strategies against tumors.
Opioids also favor the apoptotic processes (programmed cell death) of malignant cells. Morphine induces pro-apoptotic effect through the activation of caspases, release of cytochrome C, reactive oxygen species, and DNA damage [155, 160]. Methadone, when used in combination with the chemotherapeutic compound doxorubicin, induces cell death in chemo- and radioresistant glioblastoma human cells [149]. Methadone also increases the expression and activity of apoptotic mediators, such as caspase 3, caspase 8, caspase 9, and HSP70 protein in different leukemic cells, ultimately leading to leukemia cell death [162]. Moreover, in leukemia cell lines, methadone decreases the percentage of cell viability to less than 50% in 24 h [155]. These studies have led to propose that methadone could be a good therapeutic agent against leukemia.
On the other hand, several lines of evidence suggest that the OR antagonist naltrexone inhibits blood vessel formation. The mechanism behind this effect seems to be related to the inactivation of signaling pathways leading to angiogenesis [143].
Paradoxically, under certain circumstances, opioids can contribute to increase the tumor mass. Main mechanisms proposed behind these effects are (1) increased angiogenesis and (2) inhibition of active and protective immune cells. For example, DAMGO, a highly specific synthetic μ-OR-specific agonist, induces proliferation and cell migration in human H358 non-small cell lung cancer. Morphine and fentanyl drive epithelial-mesenchymal transition, a phenomenon associated with increased tumor metastatic capacity [152]. Repeated morphine treatment leads to significant increase in tumor burden, breast carcinoma, and the formation of new lymphatic vessels (lymphangiogenesis) in mice [151]. Also, morphine induces cell recruitment, release of cytokines, and degranulation of MCs inside breast tumors [151]. A proposed mechanism for the increase of malignant tumor size is that morphine inhibits NK cells, which are one of the first lines of defense against transformed cells [163]. To date, evidence indicates that some opioids (like methadone) could be useful for the treatment of certain types of cancers, but methadone effects seem not to be positive in all the types of tumors [144].
Because patients with morphine treatment have elevated levels of morphine’s metabolite M3G, some studies have analyzed the effect of this metabolite in cancer. M3G binds to and activates TLR4, which is highly expressed in tumors and is associated with tumor malignancy. In human lung cancer cells, M3G, in a dose-dependent manner and through TLR4 binding, increases the expression of programmed death-ligand 1 (PD-L1), a molecule that transmits an inhibitory signal to the cytotoxic T lymphocytes (CTL) and promotes the inhibition of the protective actions of IS against the tumor. Even opioid metabolites, such as M3G, seem to exert inhibitory actions on immune cells and modulate their interaction with transformed cancer cells, which indicates that opioids alter the participation of the IS on the recognition of transformed cells and the initiation, progress, and success of immune response against tumors, which makes it difficult to predict the final outcome of opioid administration on cancer development.
7.2 Opioids, the IS, and Tolerance to Microbiota
Gut microbiota include all microorganisms within the gastrointestinal (GI) tract, including fungi, viruses, bacteria, archaea, and eukaryotes [164]. The microbiota constitutes a complex system that interacts with the host through the secretion of different metabolites, vitamins, and other mediators able to modify metabolic pathways in different organs, such as the brain and the IS [165]. Physiological homeostasis exists when there is an equilibrium between commensal microbiota growth and a low (tolerant) immune response [166]. Intense research in the last years indicates that gut microbiota modulates the development of immune reactions, neural functioning, and the onset of distinct pathologies [167], although the involved mechanisms are not well understood. Alterations in microbiota composition or metabolism, called dysbiosis, are associated with many pathologies, such as autoimmune diseases, neurological disorders, obesity, diabetes, allergies, and other diseases [164].
Opioids modulate GI function. For example, morphine inhibits the intestinal epithelium’s protective mucus and bicarbonate secretion [168]. Morphine also attenuates epithelial immune function, inhibiting cytokine secretion in an in vitro model of inflammation [169]. In general, opioid administration causes constipation, leaky intestinal barrier function, nausea, and vomiting [170]. In addition, morphine increases bacterial overgrowth and the PAMP N-formyl-methionyl-leucyl-phenylalanine (FMLP) production, which induces mucosal permeability on the intestine of rats [170]. MCs appear to be the cellular target of FMLP and morphine effects on the rat ileum, since changes in permeability caused by FMLP and their blockage with morphine do not occur in ilea from mice treated with doxantrazole (a stabilizer of MCs) or in ilea from MC-deficient mice [170].
In general, evidence in humans and mouse models indicates that opioid administration leads to increased intestinal barrier permeability, bacterial translocation, increased risk of enteric infection, and life-threatening conditions, such as gut-derived sepsis [171, 172]. The mechanisms through which opioids cause GI dysfunction are not clear. However, some studies have demonstrated that morphine disrupts intestinal barrier function and damages the organization of the tight junction proteins. These events activate TLR, whose signaling cascade leads to the phosphorylation of the myosin light-chain kinase [173] and alters the cell cytoskeleton. Research on the effects of opioids on GI tract has led to propose the use of naltrexone as a therapeutic strategy for active Crohn’s disease [174].
Opioids induce gut microbial dysbiosis and lead to sustained systemic inflammation by disrupting the pathway of bile acid metabolism [175]. Opioid metabolism affects microbiota and, in consequence, modifies distinct immune responses. For example, morphine metabolites M6G and M3G are hydrolyzed by beta-glucuronidase in intestinal mucosal cells and gut bacteria, [176, 177]. Anaerobic bacteria such as Bacteroides and Bifidobacteria are major sources of beta-glucuronidase [177]. In consequence, a gut microbioma enriched with these bacteria could promote an increase degradation of morphine metabolites and limit opioid actions on IS.
Also, morphine can cause lethal gut-derived sepsis in mice [172, 178], presumably by disrupting the gut-barrier function, increasing bacteria translocation and bacterial virulence expression [179].
Finally, in humans, it has been observed that opioid-dependent alteration of GI tract modifies the time course of bacterial and viral infections, such as HIV [173]. An analysis of a cohort of 2933 patients with functional GI disorders found that opioid use was associated with increased vomiting, constipation, and the severity of GI disease [180].
8 Opioids and Immune Responses Against COVID-19
The recent global health burden due to SARS-CoV-2 virus infection has led to a worldwide effort toward understanding COVID-19 and the search for therapeutic strategies targeting key aspects of this potentially lethal syndrome. From the beginning of the pandemics, it has been clear that hyper-inflammation relates to symptom severity and mortality. Severely affected COVID-19 patients have GI, respiratory, neuronal, renal, and cardiac symptoms. In addition, several critical immunological parameters are affected, such as the number of leukocytes (especially NK cells) and markers of inflammation [181, 182]. Furthermore, these patients suffer from a significant systemic inflammation that leads to increased blood levels of cytokines and chemokines, something referred to as a “cytokine storm.” Elevated cytokines comprise IL1-β, IL1RA, IL7, IL8, IL9, IL-10, basic FGF2, GCSF, GM-CSF, IFNγ, IP10, CCL-2, MIP1α, MIP1β, PDGF-B, TNF-α, and VEGF [183]. Specific therapeutic approaches include immunoglobulins, recombinant human IL-6 receptor monoclonal antibody (tocilizumab), chloroquine, hydroxychloroquine, JAK inhibitors (Ruxolitinib®, Jakotinib®), convalescent plasma, and glucocorticoids, among others [184]. However, when severe inflammation exists, the nonspecific, anti-inflammatory properties of opioids have been used to control damage induced by the over-activation of IS [185].
Different calculations have reported that around 40% of patients with severe COVID-19 require mechanical ventilation, 15% develop acute respiratory distress syndrome (ARDS), and 6% develop septic shock [186]. Sedation is necessary to manage acute agitation, pain treatment, and facilitation of mechanical ventilation. Normally, morphine and fentanyl help patients reach light sedation levels needed for intensive care unit manipulations. In those conditions, the immunosuppressive actions of opioids can help to diminish the cytokine storm [185]. The proposed mechanism for opioid actions includes HPA axis activating because morphine significantly increases plasma corticosterone levels, contributing to damp hyper-inflammation. From this point of view, immunosuppressive actions of opioids make them a valuable tool as a multitarget therapeutic strategy for acute hyper-inflammatory conditions, such as COVID-19.
Like other treatments, the use of opioids in the management of COVID-19 is not free of controversy. For example, opioid-induced inhibition of an initially protective anti-inflammatory response in the lungs can be a risk for COVID-19 complications, and prolonged opioid use has been related to pneumonia development [35]. Due to these effects, caution is recommended for the use of opiates in distinct phases of the COVID-19 disease [187].
9 Perspectives
Due to the diverse effects of opioids in IS function, broad terms such as “immunosuppression“or “immunoactivation” referring to the actions of any particular opioid should be used with care, considering the context of the specific immune reaction studied before reaching conclusions on long-term responses. To date, evidence indicates that the opioid system connects and affects immune responses modifying the intensity of critical processes involved in several of the design principles of the IS and, in consequence, opioids alter the direction of a given immune response. This idea is supported by basic studies on the presence of opioid ligands and receptors in distinct groups of organisms, which strongly suggest that opioids constitute a unique evolution-conserved mechanism of cross-regulation between the immune and the nervous systems in metazoans [188]. Thus, alterations on any branch of this communication network, which also participate in the activity of the HPA axis, modify the final immune response against pathogens, tumor recognition, initiation, and resolution of inflammation and affect immunosurveillance or antibody production, besides pain control.
Knowledge of the main effects of opioids on the IS has started to be used to control not only pain but also several pathologic conditions. The potential role of the opioid system on the resolution of inflammatory responses is a promising area for the therapeutics of distinct acute and chronic diseases.
References
Orosz CG. An introduction to immuno-ecology and immuno-informatics. In: Segel LA, Cohen IR, editors. Design principles for the immune system and other distributed autonomous systems; 2001. p. 125–49.
Lazzarin A, Mella L, Trombini M, Uberti-Foppa C, Franzetti F, Mazzoni G, et al. Immunological status in heroin addicts: effects of methadone maintenance treatment. Drug Alcohol Depend. 1984;13(2):117–23.
Mclachlan C, Crofts N, Wodak A, Crowe S. The effects of methadone on immune function among injecting drug users: a review. Addiction. 1993;88(2):257–63.
Tubaro E, Avico U, Zuccaro P, Cavallo G, Pac R, Croce C, et al. Morphine and methadone impact on human phagocytic physiology. Int J Immunopharmacol. 1985;7(6):865–74.
Delafuente JC, Lindsay DC. Immunologic effects of cocaine and related alkaloids. Immunopharmacol Immunotoxicol. 1991;13(1–2):11–23.
Peterson PK, Gekker G, Brummitt C, Pentel P, Bullock M, Simpson M, et al. Suppression of human peripheral blood mononuclear cell function by methadone and morphine. J Infect Dis. 1989;159(3):480–7.
Zhang R, Meng J, Lian Q, Chen X, Bauman B, Chu H, et al. Prescription opioids are associated with higher mortality in patients diagnosed with sepsis: a retrospective cohort study using electronic health records. PLoS One. 2018;(1):1–8.
Bauchat JR. Neuraxial morphine and oral herpes reactivation in the obstetric population, Anesthesia and analgesia, vol. 111. Lippincott Williams and Wilkins; 2010. p. 1238–41.
Østerdal OB, Salminen PR, Jordal S, Sjursen H, Wendelbo O, Haaverstad R. Cardiac surgery for infective endocarditis in patients with intravenous drug use. Interact Cardiovasc Thorac Surg. 2016;22(5):633–40.
Barocas JA, Morgan JR, Wang J, McLoone D, Wurcel A, Stein MD. Outcomes associated with medications for opioid use disorder among persons hospitalized for infective endocarditis. Clin Infect Dis. 2021;72(3):472–8.
Zhu JW, Liu FL, Mu D, Deng DY, Zheng YT. Heroin use is associated with lower levels of restriction factors and type I interferon expression and facilitates HIV-1 replication. Microbes Infect. 2017;19(4–5):288–94.
Do Q-CN, Wallace MS, Ashar N, Mathews C. Long-term methadone treatment: effect on CD4+ lymphocyte counts and HIV-1 plasma RNA level in patients with HIV infection. Eur J Pain. 2001;5(4):415–20.
Wang X, Ye L, Zhou Y, Liu MQ, Zhou DJ, Ho WZ. Inhibition of anti-HIV microRNA expression: a mechanism for opioid-mediated enhancement of HIV infection of monocytes. Am J Pathol. 2011;178(1):41–7.
Ovalle F, Dembinski D, Yalamanchili S, Minkara A, Stern PJ. Hand and upper extremity infections in intravenous drug users: epidemiology and predictors of outcomes. J Hand Surg Am. 2020;45(6):503–11.
Lewer D, Harris M, Hope V. Opiate injection–associated skin, soft tissue, and vascular infections, England, UK, 1997–2016. Emerg Infect Dis. 2017;23(8):1400–3.
Suzuki J, Park EM. Buprenorphine/naloxone and dental caries: a case report. Am J Addict. 2012;21:494–5.
Farr A, Kiss H, Hagmann M, Holzer I, Kueronya V, Husslein PW, et al. Evaluation of the vaginal flora in pregnant women receiving opioid maintenance therapy: a matched case-control study. BMC Pregnancy Childbirth [Internet]. 2016;16(1):1–8. Available from: https://doi.org/10.1186/s12884-016-1003-z
Wiese AD, Griffin MR, Schaffner W, Stein CM, Greevy RA, Mitchel EF, et al. Long-acting opioid use and the risk of serious infections: a retrospective cohort study. Clin Infect Dis. 2019;68(11):1862–9.
Liao Y, Jiang J, Liang B, Wei F, Huang J, Pan P, et al. Opiate use inhibits TLR9 signaling pathway in vivo: possible role in pathogenesis of HIV-1 infection. Sci Rep. 2017;7(1)
Masvekar RR, El-Hage N, Hauser KF, Knapp PE. GSK3β-activation is a point of convergence for HIV-1 and opiate-mediated interactive neurotoxicity. Mol Cell Neurosci [Internet]. 2015;65:11–20 Available from: https://doi.org/10.1016/j.mcn.2015.01.001
Zou S, Fitting S, Hahn YK, Welch SP, El-Hage N, Hauser KF, et al. Morphine potentiates neurodegenerative effects of HIV-1 Tat through actions at μ-opioid receptor-expressing glia. Brain. 2011;134(12):3613–28.
Edelman EJ, Gordon KS, Crothers K, Akgün K, Bryant KJ, Becker WC, et al. Association of prescribed opioids with increased risk of community-acquired pneumonia among patients with and without HIV. JAMA Intern Med. 2019;179(3):297–304.
Oppeltz RF, Holloway TL, Covington CJ, Schwacha MG. The contribution of opiate analgesics to the development of infectious complications in trauma patients [Internet]. Int J Burn Trauma. 2015;5(2):56–65. Available from: www.IJBT.org.
Schwacha MG, McGwin G, Hutchinson CB, Cross JM, MacLennan PA, Rue LW. The contribution of opiate analgesics to the development of infectious complications in burn patients. Am J Surg. 2006;192(1):82–6.
Bonilla-García JSL, Cortiñas-Sáenz M, Del Pozo-Gavilán E. Opioids and immunosupression in oncological postoperative patients. Rev Assoc Med Bras. 2017;63(9):753–63.
Shao Y-J, Liu W-S, Guan B-Q, Hao J-L, Ji K, Cheng X-J, et al. Contribution of opiate analgesics to the development of infections in advanced cancer patients. Clin J Pain. 2017;33(4):295–9.
Acharya C, Betrapally NS, Gillevet PM, Sterling RK, Akbarali H, White MB, et al. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45(2):319–31.
Lee AA, Baker JR, Wamsteker EJ, Saad R, DiMagno MJ. Small intestinal bacterial overgrowth is common in chronic pancreatitis and associates with diabetes, chronic pancreatitis severity, low zinc levels, and opiate use. Am J Gastroenterol. 2019;114(7):1163–71.
Wiese AD, Griffin MR, Stein CM, Mitchel EF, Grijalva CG. Opioid analgesics and the risk of serious infections among patients with rheumatoid arthritis: a self-controlled case series study. Arthritis Rheumatol. 2016;68(2):323–31.
Wiese AD, Griffin MR, Schaffner W, Michael Stein C, Greevy RA, Mitchel EF, et al. Opioid analgesic use and risk for invasive pneumococcal diseases a nested case-control study. Ann Intern Med. 2018;168(6):396–404.
Hamina A, Taipale H, Karttunen N, Tanskanen A, Tiihonen J, Tolppanen AM, et al. Hospital-treated pneumonia associated with opioid use among community dwellers with Alzheimer’s disease. J Alzheimers Dis. 2019;69(3):807–16.
Dublin S, Walker RL, Jackson ML, Nelson JC, Weiss NS, Von Korff M, et al. Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case-control study. J Am Geriatr Soc. 2011;59(10):1899–907.
Bigliardi-Qi M, Bigliardi P. The roles of opioid receptors in cutaneous wound healing. In: Handbook of experimental pharmacology. Springer New York LLC; 2018. p. 335–45.
Franchi S, Moschetti G, Amodeo G, Sacerdote P. Do all opioid drugs share the same immunomodulatory properties? A review from animal and human studies. Front Immunol. 2019;10:1–11.
Sacerdote P, Franchi S, E. Panerai A. Non-analgesic effects of opioids: mechanisms and potential clinical relevance of opioid-induced Immunodepression. Curr Pharm Des. 2012;18(37)
Cata JP, Bauer M, Sokari T, Ramirez MF, Mason D, Plautz G, et al. Effects of surgery, general anesthesia, and perioperative epidural analgesia on the immune function of patients with non-small cell lung cancer. J Clin Anesth. 2013;25(4)
Sacerdote P, Bianchi M, Gaspani L, Manfredi B, Maucione A, Terno G, et al. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesth Analg. 2000;90(6):1411–4.
Beilin B, Shavit Y, Hart J, Mordashov B, Cohn S, Notti I, et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesth Analg. 1996;82(3):492–7.
Inagi T, Suzuki M, Osumi M, Bito H. Remifentanil-based anaesthesia increases the incidence of postoperative surgical site infection. J Hosp Infect. 2015;89(1):61–8.
Fukada T, Kato H, Ozaki M, Yagi J. Impact of the timing of morphine administration on lipopolysaccharide-mediated lethal endotoxic shock in mice. Shock. 2016;45(5):564–9.
Khosrow-Khavar F, Kurteva S, Cui Y, Filion KB, Douros A. Opioids and the risk of infection: a critical appraisal of the pharmacologic and clinical evidence, Expert opinion on drug metabolism and toxicology, vol. 15. Taylor and Francis Ltd; 2019. p. 565–75.
Abbas A, Lichtman A, Pilla S. Cellular and molecular immunology. 9th ed. Elsevier; 2018.
Shang Y, Filizola M. Opioid receptors: Structural and mechanistic insights into pharmacology and signaling. Eur J Pharmacol. 2015;763:206–13.
Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, Kroeze WK, et al. In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol. 2017;13(5):529–36.
Akuzawa N, Obinata H, Izumi T, Takeda S. Morphine is an exogenous ligand for MrgX2, a G protein-coupled receptor for cortistatin. J Cell Anim Biol. 2007;2(1):004–9.
Stein C. Opioid receptors. Annu Rev Med. 2016;67:433–51.
Darcq E, Kieffer BL. Opioid receptors: drivers to addiction? Nat Rev Neurosci. 2018;19:499–514.
Wu H, Zeng M, Cho EYP, Jiang W, Sha O. The origin, expression, function and future research focus of a G protein-coupled receptor, Mas-related Gene X2 (MrgX2). Prog Histochem Cytochem. 2015;50:11–7.
Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35.
Sugimoto MA, Sousa LP, Pinho V, Perretti M, Teixeira MM. Resolution of inflammation: what controls its onset? Front Immunol [Internet]. 2016;7:160. Available from: www.frontiersin.org.
Rittner HL, Hackel D, Voigt P, Mousa S, Stolz A. Mycobacteria attenuate nociceptive responses by formyl peptide receptor triggered opioid peptide release from neutrophils. PLoS Pathog [Internet]. 2009;5(4):1000362. Available from: www.dfg.de.
Rittner HL, Labuz D, Richter JF, Brack A, Schäfer M, Stein C, et al. CXCR1/2 ligands induce p38 MAPK-dependent translocation and release of opioid peptides from primary granules in vitro and in vivo. Brain Behav Immun. 2007;21(8):1021–32.
Malafoglia V, Ilari S, Vitiello L, Tenti M, Balzani E, Muscoli C, et al. The interplay between chronic pain, opioids, and the immune system. Neurosci [Internet]. 2021;107385842110304. Available from: http://journals.sagepub.com/doi/10.1177/10738584211030493.
Pannell M, Labuz D, Celik M, Keye J, Batra A, Siegmund B, et al. Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J Neuroinflammation. 2016;13(1):262.
Labuz D, Celik M, Seitz V, Machelska H. Interleukin-4 induces the release of opioid peptides from M1 macrophages in pathological pain. J Neurosci. 2021;41(13):2870–82.
Brack A, Rittner HL, Machelska H, Beschmann K, Sitte N, Schäfer M, et al. Mobilization of opioid-containing polymorphonuclear cells by hematopoietic growth factors and influence on inflammatoty pain. Anesthesiology. 2004;100(1):149–57.
Machelska H, Mousa SA, Brack A, Schopohl JK, Rittner HL, Schäfer M, et al. Opioid control of inflammatory pain regulated by intercellular adhesion molecule-1. J Neurosci. 2002;22(13)
Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. 2020;180(6):1044–66.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124, 4:783–801.
Molina-Martínez LM, González-Espinosa C, Cruz SL. Dissociation of immunosuppressive and nociceptive effects of fentanyl, but not morphine, after repeated administration in mice: fentanyl-induced sensitization to LPS. Brain Behav Immun. 2014;1(42):60–4.
Madera-Salcedo IK, Cruz SL, Gonzalez-Espinosa C. Morphine decreases early peritoneal innate immunity responses in Swiss-Webster and C57BL6/J mice through the inhibition of mast cell TNF-α release. J Neuroimmunol. 2011;232(1–2):101–7.
Madera-Salcedo IK, Cruz SL, Gonzalez-Espinosa C. Morphine prevents lipopolysaccharide-induced TNF secretion in mast cells blocking IκB kinase activation and SNAP-23 phosphorylation: correlation with the formation of a β-Arrestin/TRAF6 complex. J Immunol. 2013;191(6):3400–9.
Martucci C, Franchi S, Lattuada D, Panerai AE, Sacerdote P. Differential involvement of RelB in morphine-induced modulation of chemotaxis, NO, and cytokine production in murine macrophages and lymphocytes. J Leukoc Biol. 2007;81(1):344–54.
Roy S, Cain KJ, Chapin RB, Charboneau RG, Barke RA. Morphine modulates NFκB activation in macrophages. Biochem Biophys Res Commun. 1998;245(2):392–6.
Zeng S, Zhong Y, Xiao J, Ji J, Xi J, Wei X, et al. Kappa opioid receptor on pulmonary macrophages and immune function. Transl Perioper Pain Med. 2020;7(3):225–33.
Luan G, Pan F, Bu L, Wu K, Wang A, Xu X. Butorphanol promotes macrophage phenotypic transition to inhibit inflammatory lung injury via κ receptors. Front Immunol. 2021;12:692286.
Tubaro E, Avico U, Santiangeli C, Zuccaro P, Cavallo G, Pacifici R, et al. Morphine and methadone impact on human phagocytic physiology. Int J Immunopharmacol. 1985;7(6):865–74.
Rojavin M, Szabo I, Bussiere JL, Rogers TJ, Adler MW, Eisenstein TK. Morphine treatment in vitro or in vivo decreases phagocytic functions of murine macrophages. Life Sci. 1993;53(12):997–1006.
Wang J, Barke RA, Charboneau R, Roy S. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174(1):426–34.
Jana N, Vidhu A, Raini D, Zhang L, Saluja A, Meng J, et al. Differential effects of gram-positive and gram-negative bacterial products on morphine induced inhibition of phagocytosis. Sci Rep. 2016;6:21094.
Wan J, Ma J, Anand V, Ramakrishnan S, Roy S. Morphine potentiates LPS-induced autophagy initiation but inhibits autophagosomal maturation through distinct TLR4 dependent and independent pathways HHS Public Access. Acta Physiol. 2015;214(2):189–99.
Wang TL, Chang H, Hung CR, Tseng YZ. Attenuation of neutrophil and endothelial activation by intravenous morphine in patients with acute myocardial infarction. Am J Cardiol. 1997;80(12):1532–5.
Koodie L, Yuan H, Pumper JA, Yu H, Charboneau R, Ramkrishnan S, et al. Morphine inhibits migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice. Am J Pathol [Internet]. 2014;184(4):1073–84. Available from: https://doi.org/10.1016/j.ajpath.2013.12.019.
Hofbauer R, Frass M, Gmeiner B, Sandor N, Schumann R, Wagner O, et al. Effects of remifentanil on neutrophil adhesion, transmigration, and intercellular adhesion molecule expression. Acta Anaesthesiol Scand. 2000;44(10):1232–7.
Min TJ, Park S-H, Ji Y-H, Lee Y-S, Kim TW, Kim JH, et al. Morphine attenuates endothelial cell adhesion molecules induced by the supernatant of LPS-stimulated colon cancer cells. J Korean Med Sci [Internet]. 2011;26:747–52. Available from: http://jkms.org
Zhang J-N, Ma Y, Wei X-Y, Liu K-Y, Wang H, Han H, et al. Remifentanil protects against lipopolysaccharide-induced inflammation through PARP-1/NF-κB signaling pathway. Mediators Inflamm. 2019;2019:3013716. Available from: https://doi.org/10.1155/2019/3013716.
Andrews EJ, Wang JH, Winter DC, Laug WE, Redmond HP. Tumor cell adhesion to endothelial cells is increased by endotoxin via an upregulation of β-1 integrin expression. J Surg Res. 2001;97(1):14–9.
Wang L, Yin C, Xu X, Liu T, Wang B, Abdul M, et al. Pellino1 contributes to morphine tolerance by microglia activation via MAPK signaling in the spinal cord of mice. Cell Mol Neurobiol. 2020;40(7)
Merighi S, Gessi S, Varani K, Fazzi D, Stefanelli A, Borea PA. Morphine mediates a proinflammatory phenotype via m-opioid receptor-PKCε-Akt-ERK1/2 signaling pathway in activated microglial cells. Biochem Pharmacol. 2013;86(4):487–96.
Gessi S, Borea PA, Bencivenni S, Fazzi D, Varani K, Merighi S. The activation of μ-opioid receptor potentiates LPS-induced NF-kB promoting an inflammatory phenotype in microglia. FEBS Lett. 2016;590(17):2813–26.
Dutta R, Krishnan A, Meng J, Das S, Ma J, Banerjee S, et al. Morphine modulation of toll-like receptors in microglial cells potentiates neuropathogenesis in a HIV-1 model of coinfection with pneumococcal pneumoniae. J Neurosci. 2012;32(29):9917–30.
Dutta R, Roy S. Chronic morphine and HIV-1 Tat promote differential central nervous system trafficking of CD3+ and Ly6C+ immune cells in a murine Streptococcus pneumoniae infection model. J Neuroinflammation. 2015;12:120.
Cai Y, Kong H, Pan YB, Jiang L, Pan XX, Hu L, et al. Procyanidins alleviates morphine tolerance by inhibiting activation of NLRP3 inflammasome in microglia. J Neuroinflammation. 2016;13(1):53.
Carranza-Aguilar CJ, Hernández-Mendoza A, Mejias-Aponte C, Rice KC, Morales M, González-Espinosa C, et al. Morphine and fentanyl repeated administration induces different levels of NLRP3-dependent pyroptosis in the dorsal raphe nucleus of male rats via cell-specific activation of TLR4 and opioid receptors. Cell Mol Neurobiol. 2020;42(3):677–94.
Qian J, Zhu Y, Bai L, Gao Y, Jiang M, Xing F, et al. Chronic morphine-mediated upregulation of high mobility group box 1 in the spinal cord contributes to analgesic tolerance and hyperalgesia in rats. Neurotherapeutics. 2020;17(2):722–42. Available from: https://doi.org/10.1007/s13311-019-00800-w.
Qu J, Tao X-Y, Teng P, Zhang Y, Guo C-L, Hu L, et al. Blocking ATP-sensitive potassium channel alleviates morphine tolerance by inhibiting HSP70-TLR4-NLRP3-mediated neuroinflammation. Journal of Neuroinflammation. 2017;14:228.
Babina M, Wang Z, Roy S, Guhl S, Franke K, Artuc M, et al. MRGPRX2 is the codeine receptor of human skin mast cells: desensitization through β-Arrestin and lack of correlation with the FcεRI pathway. J Invest Dermatol. 2021;141(5):1286–96.
Lazki-Hagenbach P, Ali H, Sagi-Eisenberg R. Authentic and ectopically expressed MRGPRX2 elicit similar mechanisms to stimulate degranulation of mast cells. Cells. 2021;10(2):376. Available from: https://doi.org/10.3390/cells10020376.
Sheen CH, Schleimer RP, Kulka M. Codeine induces human mast cell chemokine and cytokine production: involvement of G-protein activation. Allergy. 2007;62(5):532–8.
Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24(1):83–95.
Wang X, Loram LC, Ramos K, De Jesus AJ, Thomas J, Cheng K, et al. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A. 2012;109(16):6325–30.
Wang H, Huang M, Wang W, Zhang Y, Ma X, Luo L, et al. Microglial TLR4-induced TAK1 phosphorylation and NLRP3 activation mediates neuroinflammation and contributes to chronic morphine-induced antinociceptive tolerance. Pharmacol Res. 2021;165:6325–30.
Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, et al. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1β. Neuroscience. 2010;165(2)
Wang X, Zhang Y, Peng Y, Hutchinson MR, Rice KC, Yin H, et al. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br J Pharmacol [Internet]. 2016;173:856–69. Available from: www.brjpharmacol.org.
Sibinga NES, Goldstein A. Opioid peptides and opioid receptors in cells of the immune system. Annu Rev Immunol. 1988;6:219–49.
Bidlack JM, Khimich M, Parkhill AL, Sumagin S, Sun B, Tipton CM. Opioid receptors and signaling on cells from the immune system. J Neuroimmune Pharmacol. 2006;
Miyagi T, Chuang LF, Lam KM, Kung HF, Wang JM, Osburn BI, et al. Opioids suppress chemokine-mediated migration of monkey neutrophils and monocytes – an instant response. Immunopharmacology. 2000;47(1):53–62.
Pérez-Castrillón JL, Pérez-Arellano JL, García-Palomo JD, Jiménez-López A, De CS. Opioids depress in vitro human monocyte chemotaxis. Immunopharmacology. 1992;23(1)
Vallejo R, de Leon-Casasola O, Benyamin R. Opioid therapy and immunosuppression: a review. Am J Ther. 2004;11(5):354–65.
Wodehouse T, Demopoulos M, Petty R, Miraki-Moud F, Belhaj A, Husband M, et al. A randomized pilot study to investigate the effect of opioids on immunomarkers using gene expression profiling during surgery. Pain. 2019;160(12):2691–8.
Cui JH, Jiang WW, Liao YJ, Wang QH, Xu M, Li Y. Effects of oxycodone on immune function in patients undergoing radical resection of rectal cancer under general anesthesia. Medicine (Baltimore). 2017;96(31):e7519.
McDonough RJ, Madden JJ, Falek A, Shafer DA, Pline M, Gordon D, et al. Alteration of T and null lymphocyte frequencies in the peripheral blood of human opiate addicts: in vivo evidence for opiate receptor sites on T lymphocytes. J Immunol. 1980;125(6):2539–43.
Donahoe RM, Bueso-Ramos C, Donahoe F, Madden JJ, Falek A, Nicholson JKA, et al. Mechanistic implications of the findings that opiates and other drugs of abuse moderate T-cell surface receptors and antigenic markers. Ann N Y Acad Sci. 1987;496(1):711–21.
Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–80.
Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–28.
Mellon RD, Bayer BM. Evidence for central opioid receptors in the immunomodulatory effects of morphine: review of potential mechanism(s) of action. J Neuroimmunol. 1998;83:19–28.
Zubelewicz B, Muc-Wierzgoń M, Harbuz MS, Brodziak A. Central single and chronic administration of morphine stimulates corticosterone and interleukin (IL)-6 in adjuvant-induced arthritis. J Physiol Pharmacol. 2000;51(4):897–906.
Gibb FW, Stewart A, Walker BR, Strachan MWJ. Adrenal insufficiency in patients on long-term opioid analgesia. Clin Endocrinol. 2016;85(6):831–5.
Walter M, Gerber H, Kuhl HC, Schmid O, Joechle W, Lanz C, et al. Acute effects of intravenous heroin on the hypothalamic-pituitary-adrenal axis response: a controlled trial. J Clin Psychopharmacol. 2013;33(2):193–8.
Bakr MAEM, Amr SAER, Mohamed SA, Hamed HB, EL-Rahman AMA, Mostafa MAM, et al. Comparison between the effects of intravenous morphine, tramadol, and ketorolac on stress and immune responses in patients undergoing modified radical mastectomy. Clin J Pain. 2016;32(10):889–97.
Moyano J, Aguirre L. Opioids in the immune system: from experimental studies to clinical practice. Rev Assoc Med Bras. 2019;65:262–9.
Nallu R, Radhakrishnan R. Spinal release of acetylcholine in response to morphine. J Pain [Internet]. 2007;8(4):S19. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1526590007004543.
Hall DM, Suo J-L, Weber RJ. Opioid mediated effects on the immune system: sympathetic nervous system involvement. J Neuroimmunol. 1998;83:29–35.
Fecho K, Dykstra LA, Lysle DT. Evidence for beta adrenergic receptor involvement in the immunomodulatory effects of morphine. J Pharmacol Exp Ther. 1993;265:1079–87.
Wang H, Yu M, Ochani M, Amelia CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384–8.
Bernik TR, Friedman SG, Ochani M, Diraimo R, Ulloa L, Yang H, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway [Internet]. J Exp Med. The Rockefeller University Press; 2002 195:781–8. Available from: http://www.jem.org/cgi/content/full/195/6/781.
Parrish WR, Rosas-Ballina M, Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, et al. Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol Med. 2008;14(9–10):567–74.
Fujii T, Mashimo M, Moriwaki Y, Misawa H, Ono S, Horiguchi K, et al. Expression and function of the cholinergic system in immune cells. Front Immunol. Frontiers Media S.A.;. 2017;8:1085.
Guzmán-Mejía F, López-Rubalcava C, González-Espinosa C. Stimulation of nAchRα7 receptor inhibits TNF synthesis and secretion in response to LPS treatment of mast cells by targeting ERK1/2 and TACE activation. J Neuroimmune Pharmacol. 2018;13(1):39–52.
Derijk RH. Single nucleotide polymorphisms related to HPA axis reactivity. Neuroimmunomodulation. 2009;16:340–52.
Bond C, Laforge KS, Tian M, Melia D, Zhang S, Borg L, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters-endorphin binding and activity: possible implications for opiate addiction [Internet]. Proc Natl Acad Sci U S A. 1998;95(16):9608–13. Available from: www.pnas.org.
Wand GS, Mccaul M, Yang X, Reynolds J, Gotjen D, Lee S, et al. The Mu-opioid receptor gene polymorphism (A118G) alters HPA Axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26(1):106–14.
Hernandez-Avila CA, Covault J, Wand G, Zhang H, Gelernter J, Kranzler HR. Population-specific effects of the Asn40Asp polymorphism at the l-opioid receptor gene (OPRM1) on HPA-axis activation [Internet]. Vol. 17, Pharmacogenetics and Genomics. Wolters Kluwer Health | Lippincott Williams & Wilkins; 2007. Available from: http://www.ncbi.nlm.nih.gov/SNP/.
Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The mu-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31(1):204–11.
Ducat E, Ray B, Bart G, Umemura Y, Varon J, Ho A, et al. Mu-opioid receptor A118G polymorphism in healthy volunteers affects hypothalamic-pituitary-adrenal axis adrenocorticotropic hormone stress response to metyrapone. Addict Biol. 2013;18(2):325–31.
Franceschi C, Campisi J. Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. J Gerontol Ser A Biol Sci Med Sci. Oxford University Press;. 2014;69:S4–9.
Salminen A, Kaarniranta K, Kauppinen A. Immunosenescence: the potential role of myeloid-derived suppressor cells (MDSC) in age-related immune deficiency. Cell Mol Life Sci. Birkhauser Verlag AG;. 2019;76:1901–18.
Bauer ME. Stress, glucocorticoids and ageing of the immune system. Stress. 2005;8:69–83.
Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014;30:16–22.
Murciano C, Villamón E, Yáñez A, O’Connor JE, Gozalbo D, Gil ML. Impaired immune response to Candida albicans in aged mice. J Med Microbiol. 2006;55(12):1649–56.
Goldmann O, Lehne S, Medina E. Age-related susceptibility to Streptococcus pyogenes infection in mice: underlying immune dysfunction and strategy to enhance immunity. J Pathol. 2010;220(5):521–9.
Inomata M, Xu S, Chandra P, Meydani SN, Takemura G, Philips JA, et al. Macrophage LC3-associated phagocytosis is an immune defense against Streptococcus pneumoniae that diminishes with host aging. Proc Natl Acad Sci U S A. 2020;117(52):33561–9. Available from: www.pnas.org/cgi/doi/10.1073/pnas.2015368117.
Pinti M, Appay V, Campisi J, Frasca D, Fülöp T, Sauce D, et al. Aging of the immune system: focus on inflammation and vaccination. Eur J Immunol. Wiley-VCH Verlag;. 2016;46:2286–301.
Zhang X, Meng X, Chen Y, Leng SX, Zhang H. The biology of aging and cancer frailty, inflammation, and immunity the biology of aging [internet]. Cancer J. 2017;23(4):201–5. Available from: www.mmaapf.org.
Morley JE, Flood JF, Silver AJ. Opioid peptides and aging. Ann N Y Acad Sci. 1990;579(1):123–32.
SG B, Kimura T, Stefano JM III, Patrick Finn J, Leung MK, Smith E, et al. Autoimmunomodulation: age-related opioid differences in vertebrate and invertebrate immune systems. Ann N Y Acad Sci. 1992;663(1):396–402.
Forget P, Collet V, Lavand’homme P, De Kock M. Does analgesia and condition influence immunity after surgery? Effects of fentanyl, ketamine and clonidine on natural killer activity at different ages. Eur J Anaesthesiol. 2010 Mar;27(3):233–40.
Gross-Isseroff R, Dillon KA, Israeli M, Biegon A. Regionally selective increases in opioid receptor density in the brains of suicide victims. Brain Res. 1990;530(2):312–6.
González-Maeso J, Torre I, Rodríguez-Puertas R, García-Sevilla JA, Guimón J, Meana JJ. Effects of age, postmortem delay and storage time on receptor-mediated activation of G-proteins in human brain [Internet]. Neuropsychopharmacology. 2002;26:468–78. Available from: www.acnp.org/citations/Npp.
Solomon GF, Fiatarone MA, Benton D, Morley JE, Bloom E, Makinodan T. Psychoimmunologic and endorphin function in the aged. Ann N Y Acad Sci. 1988;521(1):43–58.
Palm S, Lehzen S, Mignat C, Steinmann J, Leimenstoll G, Maier C. Does prolonged oral treatment with sustained-release Morphine tablets influence immune function? Anesth Analg. 1998;86(1):166–72.
Tabellini G, Borsani E, Benassi M, Patrizi O, Ricotta D, Caimi L, et al. Effects of opioid therapy on human natural killer cells. Int Immunopharmacol. 2014;18(1):169–74.
Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62(15):4491–8.
Shavit Y, Ben-Eliyahu S, Zeidel A, Beilin B. Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats: dose and timing study. Neuroimmunomodulation. 2004;11(4):255–60.
Koodie L, Ramakrishnan S, Roy S. Morphine suppresses tumor angiogenesis through a HIF-1α/p38MAPK pathway. Am J Pathol [Internet]. 2010;177(2):984–97. Available from: https://doi.org/10.2353/ajpath.2010.090621.
Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, et al. The novel role of the mu opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg. 2011;112(3):558–67.
Singh A, Jayanthan A, Farran A, Elwi AN, Kim SW, Farran P, et al. Induction of apoptosis in pediatric acute lymphoblastic leukemia (ALL) cells by the therapeutic opioid methadone and effective synergy with Bcl-2 inhibition. Leuk Res [Internet]. 2011;35(12):1649–57. Available from: https://doi.org/10.1016/j.leukres.2011.06.035.
Afsharimani B, Baran J, Watanabe S, Lindner D, Cabot PJ, Parat MO. Morphine and breast tumor metastasis: the role of matrix-degrading enzymes. Clin Exp Metastasis. 2014;31(2):149–58.
Friesen C, Hormann I, Roscher M, Fichtner I, Alt A, Hilger R, et al. Opioid receptor activation triggering downregulation of cAMP improves effectiveness of anti-cancer drugs in treatment of glioblastoma. Cell Cycle. 2014;13(10):1560–70.
Koodie L, Yuan H, Pumper JA, Yu H, Charboneau R, Ramkrishnan S, et al. Morphine inhibits migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice. Am J Pathol. 2014;184(4):1073–84.
Nguyen J, Luk K, Vang D, Soto W, Vincent L, Robiner S, et al. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth. 2014;113(1):4–13.
Lennon FE, Mirzapoiazova T, Mambetsariev B, Poroyko VA, Salgia R, Moss J, et al. The Mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and Epithelial Mesenchymal Transition (EMT) in human lung cancer. PLoS One. 2014;9(3):1–13.
Khabbazi S, Goumon Y, Parat MO. Morphine modulates interleukin-4- or breast cancer cell-induced pro-metastatic activation of macrophages. Sci Rep [Internet]. 2015;5:1–12. Available from: https://doi.org/10.1038/srep11389.
Khabbazi S, Nassar ZD, Goumon Y, Parat MO. Morphine decreases the pro-angiogenic interaction between breast cancer cells and macrophages in vitro. Sci Rep [Internet]. 2016;6:1–10. Available from: https://doi.org/10.1038/srep31572.
Kua VMD, Rasul A, Sreenivasan S, Rasool B, Younis T, Lai NS. Methadone hydrochloride and leukemia cells: effects on cell viability, DNA fragmentation and apoptotic proteins expression level. Pak J Pharm Sci. 2019;32(4):1797–803.
Wang K, Wang J, Liu T, Yu W, Dong N, Zhang C, et al. Morphine-3-glucuronide upregulates PD-L1 expression via TLR4 and promotes the immune escape of non-small cell lung cancer. Cancer Biol Med. 2021;18(1):155–71.
Khabbazi S, Nassar ZD, Goumon Y, Parat MO. Morphine decreases the pro-angiogenic interaction between breast cancer cells and macrophages in vitro. Sci Rep. 2016;6:1–10.
Khabbazi S, Goumon Y, Parat MO. Morphine modulates interleukin-4- or breast cancer cell-induced pro-metastatic activation of macrophages. Sci Rep. 2015;5(June):1–12.
Koodie L, Ramakrishnan S, Roy S. Morphine suppresses tumor angiogenesis through a HIF-1α/p38MAPK pathway. Am J Pathol. 2010;177(2):984–97.
Afsharimani B, Cabot P, Parat MO. Morphine and tumor growth and metastasis. Cancer Metastasis Rev. 2011;30(2):225–38.
Smith JP, Bingaman SI, Mauger DT, Harvey HH, Laurence M, Zagon IS. Patients with advanced pancreatic. Cancer. 2010;2010(2):37–48.
Singh A, Jayanthan A, Farran A, Elwi AN, Kim SW, Farran P, et al. Induction of apoptosis in pediatric acute lymphoblastic leukemia (ALL) cells by the therapeutic opioid methadone and effective synergy with Bcl-2 inhibition. Leuk Res. 2011;35(12):1649–57.
Riou B, Lennon FE, Moss J, Singleton PA. Clinical concepts and commentary the-opioid receptor in cancer progression is there a direct effect? Anesthesiology. 2012;116:940–5.
Gordon JI. Honor thy gut symbionts redux. Science. 2012;336:1251–3.
Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36.
Brown EM, Sadarangani M, Finlay BB. The role of the immune system in governing host-microbe interactions in the intestine. Nat Immunol. 2013;14:660–7.
Sommer F, Bäckhed F. The gut microbiota-masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–38.
Rogers DF, Barnes PJ. Opioid inhibition of neurally mediated mucus secretion in human bronchi. Lancet. 1989;333(8644):930–2.
Brosnahan AJ, Jones BJ, Dvorak CM, Brown DR. Morphine attenuates apically-directed cytokine secretion from intestinal epithelial cells in response to enteric pathogens. Pathogens. 2014;3(2)
Harari Y, Weisbrodt NW, Moody FG. The effect of morphine on mast cell-mediated mucosal permeability. Surgery. 2006;139(1)
Mora AL, Salazar M, Pablo-Caeiro J, Frost CP, Yadav Y, Dupont HL, et al. Moderate to high use of opioid analgesics are associated with an increased risk of Clostridium difficile infection. Am J Med Sci. 2012;343(4)
Babrowski T, Holbrook C, Moss J, Gottlieb L, Valuckaite V, Zaborin A, et al. Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Ann Surg. 2012;255(2)
Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, et al. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One. 2013;8(1)
Smith JP, Bingaman SI, Ruggiero F, Mauger DT, Mukherjee A, McGovern CO, et al. Therapy with the opioid antagonist naltrexone promotes mucosal healing in active Crohn’s disease: a randomized placebo-controlled trial. Dig Dis Sci. 2011;56(7)
Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang L, et al. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol. 2016;9(6)
Hawksworth G, Drasar BS, Hill MJ. Intestinal bacteria and the hydrolysis of glycosidic bonds. J Med Microbiol. 1971;4(4)
Walsh CT, Levine RR. Studies of the enterohepatic circulation of morphine in the rat. J Pharmacol Exp Ther. 1975;195(2)
Hilburger ME, Adler MW, Truant AL, Meissler JJ, Satishchandran V, Rogers TJ, et al. Morphine induces sepsis in mice. J Infect Dis. 1997;176(1)
Banerjee S, Meng J, Das S, Krishnan A, Haworth J, Charboneau R, et al. Morphine induced exacerbation of sepsis is mediated by tempering endotoxin tolerance through modulation of miR-146a. Sci Rep. 2013;3:1977. Available from: www.nature.com/scientificreports,
Melchior C, Desprez C, Wuestenberghs F, Leroi AM, Lemaire A, Goucerol G. Impact of opioid consumption in patients with functional gastrointestinal disorders. Front Pharmacol. 2020;11:596467.
Del Rio C, Malani PN. Novel coronavirus – important information for clinicians. JAMA. 2019, 2020;323(11):1039–40.
Henry BM, Vikse J. Clinical characteristics of Covid-19 in China. N Engl J Med. 2020;382(19)
Ye Q, Wang B, Mao J. The pathogenesis and treatment of the Cytokine Storm in COVID-19. J Infect. 2020;80(6):607–13.
Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1039–40.
Hudzik B, Nowak J, Zubelewicz-Szkodzinska B. Consideration of immunomodulatory actions of morphine in COVID-19 – short report. Eur Rev Med Pharmacol Sci. 2021;25(24):13062–4.
Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. JAMA. 2020;323:1839–41.
Ataei M, Shirazi FM, Lamarine RJ, Nakhaee S, Mehrpour O. A double-edged sword of using opioids and COVID-19: a toxicological view. Subst Abuse Treat Prev Policy. 2020;15:1–4.
Esch T, Kream RM, Stefano GB. Emerging regulatory roles of opioid peptides, endogenous morphine, and opioid receptor subtypes in immunomodulatory processes: metabolic, behavioral, and evolutionary perspectives. Immunol Lett. Elsevier B.V;. 2020;227:28–33.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gonzalez-Espinosa, C., Madera-Salcedo, I.K., Molina-Martínez, L.M., Martínez-Cuevas, F.L. (2022). Opioids and the Immune System. In: Cruz, S.L. (eds) Opioids. Springer, Cham. https://doi.org/10.1007/978-3-031-09936-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-09936-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-09935-9
Online ISBN: 978-3-031-09936-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)