Abstract
Microvascular decompression (MVD) is a neurosurgical technique for the treatment of vasculature related compression of various cranial nerves, mainly CN V, VII, and IX/X, that cause trigeminal neuralgia, hemifacial spasm, and glossopharyngeal neuralgia (GPN), respectively. Trigeminal neuralgia causes unilateral recurrent episodes of sharp pain. Failing medical treatments require vascular decompression. Complications of the surgery include local ischemia and damage or irritation of the cranial nerve. Neurophysiological intraoperative monitoring (IOM) can be used to identify functional changes that can result in minimizing such complications. Changes of IOM signals can alert the surgeon to reverse course in order to preserve function. IOM changes can be of technical, physiological, pharmacological, positional, or surgical origin. Proper and timely management of such changes may prevent postoperative neurological deficits. Hemifacial spasm (HFS) is characterized by unilateral spasms of the facial musculature beginning with the orbicularis oculi muscle and later spreading to other muscles of facial expression. While the disease is not life-threatening, it can profoundly reduce quality of life (Heuser et al., Eur J Neurol 14:335–340, 2007; Samii et al., Neurosurgery 50:712–718, 2002; Sekula et al., Muscle Nerve 48:770–776, 2013). One proposed etiology of HFS is vascular compression of the centrally myelinated portion of the facial nerve. In this respect, MVD is the only treatment of HFS that directly addresses etiology of the disease and achieves success in nearly 90% of initial operations (Miller and Miller, Br J Neurosurg 26:438–444, 2012). The role of electrophysiology and magnetic resonance imaging (MRI) in these patients has become an indispensable tool in identifying surgical candidates. Glossopharyngeal neuralgia (GPN) is an uncommon cause of facial pain and it is characterized by intermittent, lancinating pain involving the posterior tongue and pharynx, often with radiation to deep ear structures (Resnick et al., Neurosurg 36:64–68, 1995). MVD is indicated in drug intolerant and medically refractory cases of GPN and has improved success rates in the last few decades. IOM using brainstem auditory-evoked potentials and cranial nerve VII, IX, X electromyography (EMG) during MVD reduces postoperative incidence of hearing loss, facial paresis, and dysphonia/dysphagia (Habeych et al., J Clin Neurophysiol 31:337–343, 2014).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Trigeminal neuralgia
- Hemifacial spasms
- Glossopharyngeal neuralgia
- Microvascular decompression
- Brainstem auditory evoked potentials
- Neuromonitoring
- Cranial nerve monitoring
- Motor evoked potentials
- Sensory evoked potentials
-
Trigeminal neuralgia (TN) causes unilateral, recurrent episodes of severe pain that can be triggered by minor stimulation.
-
TN is more common in females, on the right side and the distribution of V2 and V3.
-
Microvascular decompression is effective management when medical treatments fail.
-
Surgery may be associated with complications related to position, retractors, ischemia to the brainstem, eighth cranial nerve, and other cranial nerves.
-
Bradycardia, asystole, and trigeminal cardiac reflex can be associated with surgery.
-
The causes of evoked potential changes can be related to technical, positional, pharmacological, physiological, or surgical factors.
-
HFS consists of unilateral facial nerve dysfunction resulting in spasms of the ipsilateral facial muscles. MVD is a surgical procedure that addresses the suggested etiologic causes of HFS, vascular compression causing facial nerve and facial nerve nuclei hyperexcitability, and is successful in the majority of well-selected patients.
-
Electromyography revealing characteristic, spontaneous activity, and an abnormal motor response of the facial musculature and MRI confirming vascular compression of the facial nerve are important tools for distinguishing the disease and determining the role of operative intervention.
-
Compression of the facial nerve along any portion of the centrally myelinated nerve root is the target for decompression during MVD and is achieved by use of shredded Teflon implants or, in some instances, “slinging” of the artery away from the nerve.
-
Considerations for anesthesia specific to MVD for HFS include optimal positioning and avoidance of neuromuscular blockade during the decompression phase of the surgery.
-
Intraoperative use of abnormal motor response monitoring can be used as a guide for adequate facial nerve decompression and has been shown to correlate with increased odds of symptom resolution postoperatively.
-
Due to the proximity of the vestibulocochlear nerve to the facial nerve, hearing loss following MVD for HFS is a possible complication of MVD for HFS, and the use of brainstem auditory-evoked responses to mitigate the risk of hearing loss should be considered.
-
Glossopharyngeal neuralgia (GPN) is an uncommon cause of facial pain and it is characterized by intermittent, lancinating pain involving the posterior tongue and pharynx, often with radiation to deep ear structures.
-
MVD for GPN is an effective treatment and it is indicated in drug intolerance and medically refractory cases. IOM using brainstem auditory-evoked potentials and cranial nerve VII, IX, X EMG during MVD reduces postoperative incidence of hearing loss, facial paresis, and dysphonia/dysphagia.
Introduction
Microvascular decompression (MVD) is a neurosurgical technique for the treatment of vasculature related compression of various cranial nerves, mainly CN V, VII, and IX/X, that cause trigeminal neuralgia, hemifacial spasm, and glossopharyngeal neuralgia, respectively. MVD includes separation of various offending arteries from the affected cranial nerves by placing an inert material (e.g., Teflon felt) between them.
Cranial Nerve Disorders from Vascular Compression
Trigeminal Neuralgia
Trigeminal neuralgia (TN) is described as sudden, severe, unilateral, brief, recurrent episodes of sharp shooting pain in the distribution of one or more of the trigeminal branches. It is more common in females, the right side of the face, and in the maxillary and mandibular branches of the trigeminal nerve, V2 and V3 [1]. The incidence is five in 100,000. Pain can be a sequel to normal stimuli such as eating or shaving. TN has been classified into three categories: classical, idiopathic, and secondary TN. TN is idiopathic when no diagnostic tests can confirm a lesion or disease to explain TN. In classical TN, there is a vascular compression of the trigeminal nerve root showing morphological changes on imaging or during surgery. However, in secondary TN, there is a known underlying disease causing TN, for example, tumor in the cerebellopontine angle, arteriovenous malformation, and multiple sclerosis [2]. There are some proposed mechanisms for idiopathic TN such as Na-voltage gated channel gain of function mutations, neural inflammation, and nondemyelinating brainstem lesions [3,4,5,6]. In this chapter, we will focus on classical TN where MVD has a great role in its management.
Pathophysiology of Classical TN
Classical TN occurs from nerve compression at the entry zone by an artery or vein [2]. At the entry zone of the trigeminal nerve, the myelination from peripheral Schwann cells transitions to central Oligodendroglia myelination, and this area is susceptible to pressure [2]. Vascular compression at this area causes focal dysmyelination, demyelination, and direct apposition of demyelinated axons with absence of intervening glial processes [7, 8]. These pathological changes support the theories of pain production in TN that include ectopic electrogenesis, and cross-talk between denuded axons [8].
Multiple vascular contacts including veins are common and not identifying all the contacts can be the reason for surgical treatment failure. The entry zone was first observed by Dandy in 1929. Later, Dodd proposed demyelination at the entry zone as the cause of pain that Gardner described as a short circuit of afferent stimuli. In addition, King suggested a central mechanism for pain in the trigeminal distribution. Jannetta reviewed the above information and promoted microvascular decompression (MVD) as an effective treatment [9]. Left untreated, the patient with trigeminal neuralgia proceeds to have shorter intervals free of pain and may progress to have another more complicated type 2 pain syndrome. Patients who fail medical management either because of inadequate pain control or excessive side effects are candidates for surgical treatment. An extensive radiological examination may include reliable three-dimensional (3D) high-resolution magnetic resonance imaging (MRI) with constructive interference in steady state (CISS), or fast imaging employing steady state acquisition sequence (FIESTA) that can identify the location and degree of compression at the entry zone [10]. The clinical diagnosis combined with this expanded radiological examination and the intraoperative use of indocyanine (IC) green will lead to better preoperative planning and an improved surgical outcome [11,12,13].
Early surgical strategies were performed in the prone position with the goal of partially or completely cutting the trigeminal nerve. Since then, the surgery is commonly performed in the lateral position with the goal of decompressing the nerve through a small suboccipital, retromastoid craniectomy utilizing the microscope and more recently using an endoscopic technique [9, 14,15,16]. The use of endoscopy offers several advantages: a smaller incision, less tissue manipulation, excellent visualization, fewer complications, less postoperative pain, and a shorter hospital stay [17]. Other treatment modalities have been added to the management of trigeminal neuralgia such as stereotactic radiosurgery, percutaneous balloon compression, glycerol rhizolysis, percutaneous radiofrequency lesioning and the newly, albeit experimental, and gasserian ganglion neuromodulation [2]. In this chapter, we discuss a MVD done in the lateral position. However, there are complications that include incisional infection (1.3%), hearing loss (1.9%), brainstem and cerebral infarction, cerebrospinal fluid (CSF) leak (1.6%), facial nerve palsy (2.9%), facial numbness (9.1%) and to lesser degree diplopia, ataxia, meningitis, and hydrocephalus [1, 14]. The observed complications after MVD of the cranial nerves directly involved in the surgical approach, and specifically the cochlear cranial nerve, gave rise to cranial nerve monitoring during such surgical procedures [18, 19]. Monitoring select portions of the nervous system to minimize such complications is the essence of this book.
Presentation of Clinical Scenarios
Surgical success starts before incision. Proper positioning will make it easier to perform surgery and improper positioning may compromise the results. The patient is usually placed in the lateral position after induction of anesthesia and needed lines are secured. Pressure points should be padded, a pillow should be placed between the legs, and an axillary roll should be utilized. The patient position is secured using a bean bag, bolsters, or another mechanism. The head should be positioned and stabilized using a three-pin holding device, and rotated 10° away from the surgical side so that the plane of the surgical field is kept parallel to the floor. The neck is flexed so as to preserve at least a 2-cm distance between the mandible and sternum. Positioning is a shared responsibility between the anesthesiologist and surgeon to ensure adequate access into the superior posterior cranial fossa and to maintain a line of sight to the fifth cranial nerve while not compromising intracranial arterial blood flow, venous drainage, or airway patency.
Attention must be paid to the positioning of both upper extremities. The dependent arm is at risk of vascular compromise and as a result an axillary roll must be carefully placed so that the pulse oximeter can be placed on this arm to detect vascular compromise. The nondependent arm is actually at a higher risk of nerve injury due to positioning. In order to provide more working room, the shoulder of the ipsilateral arm should be gently and carefully pulled inferiorly so as to avoid any excess traction on the upper brachial plexus. Monitoring during MVD will include all American Society of Anesthesiologists (ASA) standard monitors in addition to the neurophysiologic monitoring. Neurophysiologic monitoring should be directed to the structures at risk. The most commonly used monitoring modality is the auditory brainstem response (ABR), which is used to detect hearing loss. Hearing loss can occur in up to 5.58% of cases after MVD in TN [20]. Thirumala et al. found that patients with hearing loss after MVD usually have higher probability of showing intraoperative loss of ABR [21]. (For more information about ABRs, see Chap. 3, “Auditory-Evoked Potentials”). A recent review of the literature of MVD operations found the occurrence of facial nerve palsy to be 2.9% (0.5–6.2), facial numbness to be 9.1% (1.3–19.6), and postoperative mortality to be 0.1% (0.02–0.2). As a result, in addition to the use of ABRs during MVD procedures, such complications support the use of somatosensory-evoked potentials (SSEPs) and/or motor-evoked potentials (MEPs) and cranial nerve monitoring as well [1]. Monitoring using these modalities allows for the detection of compromised circulation to the brainstem as well as functional changes due to positioning. Laser-evoked potentials and trigeminal-evoked potentials have been used as research tools but are not routinely used [22, 23]. More recently, blink reflex monitoring has been shown to be a promising tool to monitor trigeminal sensory function and can predict postoperative facial hypoesthesia [24, 25]. The rest of this chapter includes six cases: five MVD cases monitored with only ABRs, and one case (#2) monitored with ABRs and MEPs.
Case 1
A 60-year-old, 80-kg woman with left-sided trigeminal neuralgia refractory to medical management was admitted for MVD. She was brought to the operating room, moved onto the surgical table, and ASA standard monitors were applied. Induction of anesthesia included lidocaine (100 mg); propofol, 1.5 mg/kg; and rocuronium, 0.7 mg/kg, with simultaneous initiation of infusions of remifentanil, 0.1 μg/kg/min, and propofol, 25 μg/kg/min. Direct laryngoscopy and endotracheal intubation were performed with a 7.5-mm endotracheal tube secured to the right side of the mouth. A soft bite block was inserted between molars on the left side. A radial arterial line, an additional intravenous line, and a Foley catheter were placed after induction of anesthesia. The patient was securely placed in the right lateral position. Monitoring included electrocardiography (ECG), both arterial and noninvasive blood pressure monitoring, end-expiratory CO2, oxygen saturation, temperature, and compressed EEG using a bispectral index (BIS) monitor, respiratory rate, tidal volume, peak airway pressure, and urine output. Neurophysiologic monitoring was performed using brainstem auditory-evoked potentials (ABRs). (For more details, see Chap. 3). The pulse oximetry probe was placed on the dependent arm to monitor blood flow to that arm. A BIS monitor was used to guide the depth of anesthesia and to adjust the infusion of propofol. The mean arterial blood pressure was maintained within 20% of baseline values. Manipulation of the blood pressure was accomplished by adjusting the dose of remifentanil or the administration of phenylephrine. Maintenance of anesthesia consisted of infusions of remifentanil (0.1–0.5 μg/kg/min) and propofol (25–150 μg/kg/min) and ≤0.5 MAC of the inhalation agent desflurane. After positioning the patient and obtaining an ABR baseline, a second set of responses were obtained while the surgeon was draping the surgical field. This second set revealed a complete loss of ABR responses to left ear stimulation (Fig. 23.1).
What Was the Cause of This Change? Was It Surgical, Pharmacologic, Physiologic, Positional, or Technical?
A surgical cause could be easily eliminated since the change occurred prior to surgical incision. Changes related to anesthetic agents are usually bilateral. However, because the ABR changes were unilateral (the responses to right ear stimulation were normal), an anesthetic-related cause was unlikely. In addition, short latency ABRs (those measured early in the first 10 ms after stimulation) are generally resilient to anesthetic changes. When changes do occur in the presence of inhalation anesthetic agents, they are limited to small increases in latency that do not exceed 0.75 ms. Nitrous oxide has no effect on ABRs, and narcotics, propofol, and barbiturates minimally affect ABRs [26]. Midlatency ABRs (≥50 ms) are affected by anesthetics, but these were not monitored in this case. The anesthetic agents used for this particular patient had very little effect and could be excluded as the cause of the change.
Physiologic changes related to hypothermia may occur while surgery is in progress—when the brain is exposed to a relatively cool ambient temperature and cold solutions are used for irrigation. The effect of hypothermia has been reported to either increase or decrease the amplitude of the ABR. Some reports state that in the face of hypothermia, latency increases of about 7% for each 1 °C will affect wave I of the ABR, and below 26 °C this effect will double [26]. This did not occur in this case. Other physiologic factors such as hypotension, hypoxemia, and low arterial CO2 concentration can cause bilateral changes. In this case, there were no such changes in any of these physiologic parameters. In rare situations when a major pathological condition is present in one ear, a physiologic change may lead to bilateral signal changes, with the changes more pronounced on the side with pathology.
We are left with the possibility of either a positional or technical etiology for the encountered change. Because the normal first tracing was done after the patient was positioned with the head fixed without any head or position readjustment, positioning as the cause of the ABR change could be excluded as well.
Technical reasons for ABR changes may include the failure to generate or deliver proper stimulation or the inability to collect and analyze the signals [26]. Disconnected and broken wires and operator errors account for some of the technical changes. Fluid in the ear canal, kinking of the silicone extension tube, or complete or partial dislodgement of an earpiece can all interfere with stimulation intensity and result in decreased amplitude or completely obliterated responses. Placing cotton and wax or ointment over the earpiece protects against such a problem. The inability to properly collect signals may be related to high impedance, broken wires, noise artifacts, and the use of electrical cautery. The use of an ultrasound aspirator or monopolar cautery can saturate the amplifier and lead to poor signal acquisition. In addition, the use of an integer number for the stimulation rate can cause the acquisition system to lock onto rather than to cancel out 60/50 Hz interference commonly associated with the frequency at which power is being delivered and thus interfere with signal acquisition. For more information about technical problems, see Chap. 16, “Wiring and Electrical Interference in Intraoperative Monitoring.”
In this case, a review of the potential technical causes for loss of the ABR response revealed that a near complete kink of the silicone extension tube following draping was the cause. The signals recovered immediately after releasing the kink, allowing the appropriate intensity sound stimulus to be applied.
Case 2
In another scenario similar to case 1, a 58-year-old man with left-sided disease was placed in the lateral position. The surgeon requested IOM monitoring, to include ABR, cranial nerves 5 and 7 in addition to MEP. Baseline responses revealed normal ABRs on both sides. The MEP baseline responses revealed an absent left hand, but present left foot responses and the presence of both hand and foot responses from the right side (Fig. 23.2).
What Could Be the Cause?
In this case, we have normal ABRs with absent left-hand MEP responses. As in the first case, the surgical procedure had not yet begun, so a surgical cause for the absence of the response was excluded. Anesthesia could be ruled out as a cause since there were no changes in the anesthetic delivery or depth of anesthesia. In addition, changes due to anesthetics are usually bilateral. Physiologic changes such as those due to hypotension, hypothermia, hypocarbia, and hypoxemia are usually bilateral as well. Because there were no changes in any of these parameters, physiologic causes for the absent left-hand MEP response were eliminated. However, localized ischemia in the left arm due to a tourniquet is possible, but none had been used. The possibility of a technical cause for the absent response exists. A technical cause would involve either stimulation or recording. Two needles were used for MEP stimulation and because responses were present from the right side and the left lower extremity, stimulation can be excluded as a cause for the absent response. The other possibility to explain this absence is the recording needles in the left hand. These were examined and found to be fine. Low-intensity stimulation is not a possible cause since there were small responses from the other side indicating that the stimulation intensity was strong enough to elicit responses from both sides. In addition, if responses were absent, it would usually be the foot rather than the hand response if the stimulation intensity was too weak. We are left with positioning as the cause of the absent response. Could this be caused by head position and ischemia-related changes? This is unlikely since the ABRs were normal bilaterally and the right side and left foot MEP responses were normal as well. A very localized ischemic event or a stroke is possible but these cannot be diagnosed based on a clinical examination only. A close examination of the left arm revealed that the arm had been stretched backward by the tape used to keep the arm away from the surgical field. Releasing the tape and moving the arm slightly forward resulted in an immediate recovery of the MEP signals. Their absence may have been due to stretching of nerves or ischemia produced by the abnormal position. A pulse oximeter had been placed on the fingers of the right rather than the left hand to monitor for possible ischemia resulting from the lateral positioning, and therefore we were not able to confirm ischemia as the cause of the absent signals. The fast signal recovery points to ischemia as the cause of the absent responses rather than stretching. Left uncorrected, the positioning may have resulted in nerve injury.
Changes in the ABR due to extreme head positions have been reported by Grundy et al. [27]. Severe flexion and twisting of the head resulting from a patient being positioned in the lateral position may alter the relationship of intracranial structures, increase intracranial pressure, decrease cerebral blood flow, and disturb blood flow to the eighth cranial nerve and other areas of the brain, which can result in changes of the ABRs and MEPs. Such changes are generally corrected with readjustment of the head position and restoration of normal blood flow. Inspection of the head position in this case indicated a slight distortion of the head position but major distortion of the arm. After repositioning of the arm to a more neutral position, the signals readily recovered (Fig. 23.3). This patient may also have had abnormal anatomy or some pathology accentuated by the extreme position that led to changes in the MEPs. If the ABR, SSEP, and MEP baselines are obtained after a patient’s positioning is completed and if the signals are severely abnormal, it may be necessary to readjust the position of the head and extremities.
Case 3
This case is similar to the first case. Baseline values were obtained and the signals were stable before and after positioning of the patient. The surgical procedure began, the dura was opened, and the surgical microscope was used. The surgeon placed and then adjusted a self-retaining surgical retractor several times in order to facilitate surgical exposure. During this period, the technologist observed gradual changes of the ABR signals, which included a 5–15% increase in wave V latency and a 40% decrease in wave V amplitude from the ipsilateral ear (Fig. 23.4).
Is This a Significant Change?
Polo et al. [28] designated a 0.4-ms (7%) change in latency of wave V as an early warning sign, a 0.6-ms (10%) change in latency as the time to alert the surgeon, and a 1-ms (17%) latency change as serious enough that it needed to be addressed—especially if it was associated with a change in amplitude [28]. In contrast, in 2006, Ramnarayan and Mackenzie [29] found a 0.9-ms latency increase and 50% amplitude drop to be significant and associated with postoperative hearing loss. Our in-house criteria for alerting the surgeon of a potentially significant change are a progressive 5, 10, and 15% increase in latency. Some authors depend on latency alone, but Hatayama and Moller [30] believe changes in wave V amplitude are important and should be used as warning criteria when they decrease by 40%. The changes observed in this case did reach a significant level that required the surgeon’s attention.
What Is the Cause of This Change?
Technical and positional causes were excluded, as the patient remained in a stable position and proper functioning of the monitoring system was verified. Both the ambient temperature and the patient’s measured temperature were unchanged, no cold irrigation had been used, and the vital signs were stable. Physiologic causes were therefore unlikely to be the cause of the observed ABR changes. Since the changes were unilateral, pharmacologic- and anesthetic-related causes were doubtful, so surgical factors remained as the likely potential etiology.
Surgical causes of ABR changes can be divided into three categories [31]. The first group includes gradual changes in ABR signals from stimulation of the ear on the operative side that recover with surgical maneuvers such as repositioning of the retractor. These are usually not associated with hearing loss. The second group includes ABR changes where there is an abrupt loss of all waves except wave I on the operative side that do not recover with surgical maneuvers. Such changes are usually associated with postoperative hearing loss. The third group of changes consists of a loss of all ABR signals after wave I on the contralateral side of surgery. This change is usually associated with brainstem dysfunction and may result in severe postoperative neurological deficits beyond just hearing loss.
Surgical causes for ABR changes may be due to mechanical or thermal irritation or injury to the eighth cranial nerve or its blood supply, which result in abrupt loss of the signals [26]. Coagulation of small blood vessels that provide blood flow to the cochlea or of veins that drain from the brainstem may result in such changes. Slower changes in the signals without complete loss may be a consequence of direct compression or traction on the nerve or indirect traction on the nerve through cerebellar retraction and tension on the bridging arachnoid bands. Indirect interference with blood supply to the cochlear nerve caused by compression, coagulation, spasm, or intentional occlusion can lead to varying degrees of ABR changes. Retraction of the cerebellum to improve surgical exposure may lead to stretching and potential damage of the eighth cranial nerve, and be associated with an alteration in ABR signals. Such damage can be more critical in MVD than in cases of tumor resection. During cases of tumor resection, distortion of the nerve by stretch or compression may be better tolerated having occurred over time, whereas during MVD, this anatomical stretch represents an acute insult. Changes related to traction of the cerebellum recover soon, often within minutes after retractor adjustment. If abnormal signals persist, the retractor should be completely withdrawn, and the eighth cranial nerve checked for any evidence of compression including from the Teflon pledgets utilized by the surgeon to isolate the trigeminal nerve from surrounding structures impinging on it.
A clinical situation in which there is wave I delay or loss, with wave V delayed but present, may be the result of mechanical or thermal damage to the cochlea. The presence of wave I associated with equal changes in waves III and V is related to a more proximal injury to the eighth cranial nerve and could be attributed to causes such as cerebellar retraction, nerve compression, or small vessel vasospasm. Preserved waves I and III with delay or loss of wave V may be caused by mechanical, thermal, or vascular injury in the mid to upper pons. Injury to the auditory pathways cephalic to the lower mesencephalon may be associated with normal ABRs. A vascular injury in the posterior fossa that spares the auditory pathway can be associated with normal ABRs.
Of note, an interesting phenomenon is vasospasm, which may be seen in the vascular loop surrounding the cranial nerves during MVD. Successful treatment of spasm of the loop by the application of topical papaverine either directly or via a papaverine-soaked pledget yields immediate improvement of blood flow in the loop but may be associated with decreased blood flow to the cochlea and a loss of the ABR signals within a few minutes. The cause of this change is not clear, although the low pH of papaverine has been suspected [32]. In this case, the changes were gradual, ipsilateral, and involved waves I, III, and V. Delayed latency changes were equal for waves III and V, which pointed to an issue with the eighth cranial nerve proximal to the cochlea and related to the retraction on the cerebellum. The changes in wave I could be related to a compromise in blood flow to the internal auditory artery, either by partial obstruction or spasm. The surgeon inspected the operative site and decided to reposition the retractor. Soon after repositioning, the signals started to recover back to baseline. The most likely cause of such changes was retractor pressure on the cerebellum, which decreased blood flow to the eighth cranial nerve. Strategies to improve the signals include relaxing the retractor, dividing the arachnoid tension bands between the eighth cranial nerve and the cerebellum, and pharmacologically raising the blood pressure. Raising the blood pressure could help by increasing collateral blood flow to the area, especially if retractor repositioning alone did not provide recovery of the signals.
Case 4
In this case, a healthy 45-year-old woman who failed medical management for trigeminal neuralgia was scheduled for MVD. Anesthesia and surgery proceeded in similar fashion to the previous cases. The patient was quite stable, signals were normal and consistent, and the surgery began and progressed without incident. The surgeon was far into the dissection around the fifth cranial nerve to isolate it from the artery when adhesions were noted between the compressing artery and the sensory portion of the fifth cranial nerve. The anesthesiologist noticed an increase in blood pressure and heart rate and thus administered a bolus dose of 0.5 μg/kg of remifentanil and increased the remifentanil infusion rate to 0.2 μg/kg/min. Vital signs stabilized over the next 10 min followed by a period of fluctuating blood pressure during which the pulse varied 20–30% from baseline. The BIS monitor was stable and the surgical procedure proceeded at a slow pace. Suddenly, the heart rate dropped to 30 beats per minute followed by 8 s of asystole with a precipitous drop in systemic blood pressure (Fig. 23.5).
What Happened? Is This Related to the Remifentanil Infusion, a Cardiac Incident, Brainstem Manipulation, or Something Else?
Remifentanil is associated with production of a sympathetic blockade that may lead to indirect bradycardia and hypotension. In rare situations, the change in vital signs may resemble those in the case at hand. Such changes in pulse and blood pressure usually follow a bolus injection and recover spontaneously over a short period of time. Cardiac incidents may also occur in patients with coronary artery disease or other comorbidities. Direct brainstem manipulation can be associated with abrupt fluctuations in pulse and blood pressure that may exceed a 20% change from baseline values. This etiology should be considered and reported to the surgeon who may adjust the amount of direct contact with the brainstem. The clinical picture could also be the result of the trigeminal cardiac reflex. It is a well-described phenomenon that may be triggered during surgical procedures at the cerebellopontine angle area or skull base, an MVD, or a sudden and significant manipulation of the dura. In addition, the trigeminal cardiac reflex may also be elicited during ophthalmologic surgery, surgery of the maxilla, and other areas innervated by the trigeminal nerve [33]. Release of surgical traction or cessation of the trigger stimulation will allow the reflex to cease and the vital signs to return to normal without further intervention. However, if the stimulation resumes, the reflex may reoccur. On occasion, the reflex may produce such a profound change in heart rate and blood pressure that the clinical picture progresses to cardiac arrest. The mechanism for this reflex was described in The Journal of Neurosurgery in 1999 [34]. In summary, stimulation of the trigeminal nerve sends an afferent signal to the sensory nucleus at the brainstem, which then crosses to the motor nucleus of the vagus nerve. The signal then travels through the efferent arm to the heart, lungs, and stomach to cause bradycardia, apnea, and gastric hypersecretion. This reflex may be a cerebral oxygen conserving reflex [35].
In this patient, the trigeminal cardiac reflex was suspected and the surgeon was alerted to stop any manipulations. The surgeon was not in direct contact with the brainstem but was manipulating a blood vessel adhering to the trigeminal nerve near the brainstem. Blood pressure and heart rate returned to normal soon after the surgeon stopped the manipulation.
Case 5
The following case depicts a rather rare scenario for MVD in an otherwise healthy patient refractory to medical management. The anesthesia, positioning, and surgical course followed the same regimen as the previous cases. The surgical course proceeded without incident during which the MVD was completed. The ABR remained stable throughout the procedure and the surgeon started to close. Upon closure of the dura, the ABR signals started to deteriorate and were completely lost with the closure of the fascia.
What Is the Problem and What Should Be Done?
Unilateral ABR changes at this stage of the operation are not characteristic of those caused by anesthetic depth, physiologic causes, or patient positioning. Occasionally, irrigation with cold saline in the surgical field just before dural closure can cause a decline in signals, but these occur immediately. In this case, the changes occurred after the dura was closed. The two most likely causes for this change were technical or surgical. In this case, no technical cause could be identified. Since the dura was closed, neither direct manipulation of the eighth cranial nerve or low blood flow from retractor pressure could be implicated. Indirect factors related to intradural anatomical changes that could decrease blood flow to the eighth cranial nerve at different locations remained a possibility. Bleeding, hematoma, and clot formation could affect the integrity of the nerve. Changes and loss of ABRs, caused by these factors, after the closure of the dura have been reported previously [18, 36,37,38].
Bleeding and cerebral edema may be the result of movement caused by light anesthesia, high blood pressure, or inadequate hemostasis by the conclusion of surgery. In some patients, coagulation and cutting of a petrosal vein larger than 2 mm in caliber may lead to obstruction of drainage from parts of the brainstem. In turn, this can result in venous congestion, edema, and an increased intracranial pressure (ICP) and may be the cause of the ABR changes. Contralateral hearing loss has been reported due to vascular congestion causing brainstem shift, edema, and high ICP, and has been associated with ABR changes [39]. Before disruption of such a vein, an occlusion test with a temporary aneurysm clip placed on the vein in conjunction with ABR monitoring may be helpful to prevent such a complication [40].
In this patient, the decision was made to reopen the surgical field and explore the operative site. The surgeon looked for a possible source of bleeding either in the CP angle or within the cerebellar parenchyma, some limitation of blood flow related to vascular kinking, or malpositioning secondary to shift of the Teflon felt. During this time, the anesthesiologist raised the blood pressure to increase collateral circulation. The surgical exploration revealed a small blood collection engulfing the eighth cranial nerve. This was related to a bleeding stump of a branch of the petrosal vein, which had been sectioned on the approach. Once the blood was evacuated, ABR signals started to recover.
Case 6
A 46-year-old 85-kg woman is having right-sided MVD surgery for trigeminal neuralgia. As in previous cases, the patient was positioned in the lateral position and the surgery progressed very well without any noticeable changes in the ABRs. After the main procedure was completed and while securing hemostasis, the technologist noticed significant changes of the right ABR at 10:35 and complete loss at 10:39 (Fig. 23.6). No changes in the left ABR were observed.
What happened? What is the cause? If we go through the differential diagnosis and review all five causes of EP changes—technical, physiological, pharmacological, surgical, or positional—we should get the cause. Changes on one side are unlikely to be due to anesthetic agents or physiological alterations. Special physiological conditions such as regional hypothermia, while possible, are unlikely to be a cause, since only warm irrigations were used for the hemostasis. Severe regional ischemia that affects the distribution of the eighth cranial nerve is possible, but why? No clips or retractors were used at that time. Technical causes should always be considered when localized changes or losses occur. A survey of all technical possibilities did not reveal any cause. Changes due to positioning are unlikely to have occurred since no such changes occurred since the start of the case. At the time when the changes occurred, the surgeon was only performing hemostasis; there were no retractors, no major manipulations, and the dura was not closed with overinfusion of saline [41]. It seems that none of the five causes of EP changes can be easily identified. What is the cause? In this case, the author was walking into the room when he heard the neurosurgical chief resident announcing the use of papaverine to reverse spasm. The surgical team was alerted to its potential effects and stopped any further usage. While the anesthesiologist was talking to the surgeon, the technologist announced that signal changes were occurring. The chief resident was instructed to flush the field with warm saline to minimize the effects of the papaverine. The signals gradually recovered and were back to normal by 10:51. The possible cause of this effect is the low pH of papaverine, which can affect the eighth cranial nerve [32]. If papaverine is needed, its use should be limited to a precise location.
In these six cases we reviewed different clinical situations in which changes of ABRs and MEP signals occurred during MVD surgery. In each case, we followed an algorithm with a stepwise approach to identify the etiology and management. It is critical for both the anesthesiologist and surgeon to work in concert to identify changes, analyze their cause, start treatments, and to perform surgical maneuvers that may reverse changes in a timely fashion in order to optimize the surgical outcome.
Hemifacial Spasm
Hemifacial spasm (HFS), a syndrome of unilateral facial nerve hyperactive dysfunction, is a severe and disabling condition that causes impairments in a patient’s quality of life [42,43,44,45]. Spasms begin insidiously in the orbicularis oculi muscle and spread over time to the muscles of the face with variable involvement of the frontalis and platysma muscles. Ultimately, the patient may develop prolonged contractions of all the involved muscles, causing severe, disfiguring grimacing with partial closure of the eye and drawing up of the corner of the mouth, the so-called tonus phenomenon [46]. The majority of patients also exhibit the reverse Babinski sign, which is appreciated as paradoxical raising of the eyebrow during closing of the eye [47, 48]. The best available data suggest that the prevalence rate of HFS is ten patients per 100,000 in the population of the United States and Norway [49, 50].
Electrophysiology and Pathophysiology of HFS
The diagnosis of HFS can largely be made clinically, but electromyography (EMG) and magnetic resonance imaging (MRI) may help in distinguishing the disorder from other abnormal facial movement disorders, such as blepharospasm, tics, partial motor seizures, synkinesis, craniocervical dystonia, neuromyotonia, and facial myokymia [51, 52]. The electrophysiologic hallmarks of HFS consist of spontaneous, high-frequency (as many as 150 impulses per second), synchronized firing of EMG activity. In addition, an abnormal motor response (AMR), also known as the “lateral spread response,” can be elicited by electrically stimulating one branch of the facial nerve and recording triggered electromyographic (t-EMG) responses from muscles innervated by other branches of the facial nerve. Specifically, electrical stimulation of the zygomatic branch of the facial nerve will result in an AMR recording from the mentalis muscles when the t-EMG response should be limited to only the orbicularis oculi muscle.
There remains no consensus regarding the etiopathogenesis of HFS. One hypothesis asserts that symptoms are caused by ephaptic transmission at the location of the vascular contact along the facial nerve [53, 54]. Ephaptic transmission refers to the “crosstalk” between axon fibers as a result of axon demyelination. Alternatively, it has been submitted that the symptoms are secondary to hyperactivity of the facial nucleus [55]. While the hypothesis of ephaptic transmission was popularized for many years, the hypothesis of facial motor nucleus hyperactivity has been supported by intraoperative electrophysiological recordings made during MVD [56, 57], clinical studies [58, 59], and the results of intraoperative blink reflex testing [60]. The blink reflex is elicited by stimulation of the supraorbital nerve and consists of afferent and efferent pathways along the trigeminal and facial nerves, respectively. In anesthetized patients without HFS, the blink reflex is typically suppressed if a single stimulus over the supraorbital nerve is applied. However, it was described by Deletis et al. [61] that elicitability of blink reflex after delivering a short train of stimuli on the supraorbital nerve is feasible and reliable even under general anesthesia. In HFS patients, due to the hyperexcitability of the facial nerve nucleus, the blink reflex might be elicitable after just one single stimulus in the anesthetized conditions [59]. After successful MVD, changes in excitability could be reflected in additional difficulty to obtain blink reflex. It is of interest to evaluate bilateral blink reflex in order to confirm changes due to decompression ipsilateral to the HFS.
A third proposed contributor is excitation of terminal facial nerve branches near the supraorbital nerve stimulation point during blink reflex. Excitation of these terminal zygomatic facial nerve branches would carry the signal antidromically to the site of presumed demyelination (ostensibly at the area of vascular compression) and induce an ephaptic response through axono-axonal activation in the lower facial muscles [62]. The term “lateral spread” is often used interchangeably with the term “AMR.” The term “lateral spread,” however, should be reserved for describing the AMR in relationship to the theory of ephaptic transmission, as the term “lateral spread” neglects the theory of motor nucleus hyperexcitability.
It is certainly possible that all three mechanisms contribute in part to the generation of abnormal reflex responses. As the AMR often disappears with vascular decompression of the centrally myelinated facial nerve, ephaptic transmission is thought to be a more likely, or more common, cause of the AMR. Isolated facial motor nucleus hyperactivity may account for a minority of HFS cases refractory to MVD or, alternatively, to a continuum of disease severity (i.e., motor nucleus hyperexcitability follows ephaptic transmission at the site of vascular compression).
Anatomy of the Facial Nerve and Etiology of HFS
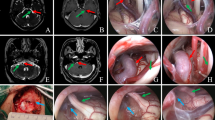
A discussion of anatomy requires a consistent vocabulary. The anatomical terms, hereto used, were first proposed by Tomii et al. [63] and subsequently expanded by Campos-Benitez and Kaufmann [64]. The facial nerve emerges from the brainstem at the nerve’s root exit point from the pontomedullary sulcus. Along its course, it adheres (i.e., the attached segment of the facial nerve) to the pons for 8–10 mm. The nerve then separates from the pons at the so-called root detachment point. The next segment of the nerve is termed the transition zone or the Obersteiner-Redlich zone where the oligodendrocyte-derived central myelin transitions to the peripheral Schwann cells. A study by Tomii et al. [63] of this transition zone revealed that (1) the maximum length from the root detachment point to the most proximal portion of the transition zone is 1.4 mm and (2) the maximum length from the most proximal to the most distal portion of the transition zone is 2.1 mm. In practice, our group uses 4 mm as the maximum distance from the root detachment point to the end of the transition zone (Fig. 23.7). Beyond the transition zone, the seventh nerve lies adjacent to the anterior rostral border of the CN VIII complex and continues into the acoustic meatus. Rostral and anterior to the VII/VIII complex, the trigeminal root is visible as it emerges from the pons, eventually traveling laterally toward the petrous apex to Meckel’s cave. Caudal to the VII/VIII complex, emerging from the lateral surface of the medulla, are the rootlets of the glossopharyngeal and vagus nerves. Our experience with HFS suggests that vascular compression along any portion of the centrally myelinated facial nerve may contribute to symptoms. This portion extends from the root exit point to the distal transition zone. As of note, our group hesitates to use the term “root entry zone” or “root exit zone” as (1) it is imprecisely defined but approximately corresponds to the root detachment point and the transition zone, and (2) does not include the root exit point or the attached segment, which is the most common site of nerve compression [65, 66]. The most common offending vessels are the anterior inferior cerebellar artery (AICA) and the posterior inferior cerebellar artery (PICA), which occur in the setting of a tortuous vertebrobasilar tree in half of affected patients [67].
Imaging in HFS
Imaging of patients with HFS who are considered for MVD is intended to delineate the facial nerve and adjacent vessels. Studies should include thin-section multiplanar steady-state free precession (SSFP) MRI sequences, which are heavily T2 weighted and provide excellent contrast between CSF and adjacent tissue [68]. The role of SSFP imaging in the evaluation of vascular compression syndromes has been described [69,70,71,72] and recently optimized for HFS by our group [65, 66]. Studies are performed on either 1.5 or 3 T MRI scanners (Optima and Discovery; GE Healthcare, Milwaukee, WI) and include whole-brain sagittal T1, axial fluid-attenuated inversion recovery (FLAIR), and diffusion weighted imaging (DWI) sequences. Thin-section axial, coronal, and sagittal SSFP images through the brainstem are obtained. It is important to note that the role of imaging in HFS is supportive and not diagnostic. As reported by our group, imaging has a sensitivity of 75–92.9% and a specificity of 28.6–75% [73]. The high sensitivity warrants appropriate counseling in patients deemed clinically favorable for surgery but have no vascular compression on thin-slice T2-weighted MRI. Furthermore, the low specificity does not warrant justification of surgery for positive imaging findings in clinically unfavorable surgical candidates.
Operative Technique of Microvascular Decompression of the Facial Nerve
Medications have been shown to be largely ineffective in treatment of HFS [74,75,76,77]. Serial botulinum toxin injections of the facial musculature may provide a temporary respite but are not curative. In addition, prolonged use of botulinum toxin may result in persistent facial nerve paresis or palsy. For this reason, MVD, the only etiological therapy for HFS, is the preferred treatment [76, 78].
Microvascular decompression is performed under general anesthesia with the patient in the contralateral decubitus position (Fig. 23.8, utilizing auditory brainstem-evoked potentials [ABRs]), facial EMG, and monitoring of the AMR in a previously described manner [78]. A retromastoid incision is made behind the hairline, and a small craniectomy below the asterion is performed. The edge of the sigmoid sinus is identified, and the dura mater is opened. After appropriate brain relaxation is achieved with cerebrospinal fluid (CSF) drainage, the facial nerve is exposed from the root exit point to the transition zone and examined for vascular contact. In concert with our neuromonitoring team, arteries are decompressed from the facial nerve using shredded Teflon® implants. Occasionally, our surgeon (RS) transposes or “slings” the artery away from the facial nerve. ABRs and facial EMG are used to monitor the patients in all cases. Direct monopolar facial nerve stimulation is used in selected cases (Videos 23.1 and 23.2).
Photograph of patient in the lateral decubitus position. Patient’s head is placed at the foot of the operating table to allow more leg room for the surgeon during the microsurgical portion of the procedure. The head is secured with three-point fixation and the patient is turned in the lateral decubitus position. The head is rotated slightly away from the affected side and flexed to allow approximately two fingerbreadths from the sternum
Anesthetic Considerations During MVD for HFS
Induction of general anesthesia may begin with propofol or etomidate. A depolarizing muscle relaxant is often used for the intubation. It has been our experience that even a small defasciculating dose of nondepolarizing muscle relaxant used during the intubation period is enough to obscure the detection of the AMR at the beginning of the case. Maintenance of general anesthesia can be achieved with a variety of techniques as long as the patient remains motionless without the use of muscle relaxants. Some patients may require only an inhalational agent while others may require inhalation as well as an infusion of either propofol or narcotic such as remifentanil. Meticulous attention to proper positioning takes into consideration avoidance of extreme head and neck flexion or rotation, proper placement of an axillary roll, as well as proper padding of both upper and lower extremities to prevent peripheral nerve injury. There should always be a final confirmation of bilateral breath sounds to confirm the absence of endotracheal tube migration.
Principles of Intraoperative Neuromonitoring for HFS
The AMR can be elicited in nearly every patient with HFS. Nondepolarizing muscle relaxants can obscure results. Bipolar subdermal needle recording electrodes are placed in the orbicularis oculi and mentalis muscles. Paired electrodes are placed approximately 0.5–1 cm apart. Bipolar stimulating electrodes were inserted subdermally over the zygomatic branch of the facial nerve midway between the outer canthus and tragus and over the marginal mandibular branch on mandibular bone. Monophasic pulses were delivered at an intensity of 1–20 mA, a frequency of 4.0 Hz, and a pulse width of 0.2 ms. Final positioning of the stimulating electrodes is based on the location that maximizes the orbicularis oculi response and the AMR recorded from the mentalis muscle group after the stimulation of zygomatic branch and/or the location that maximizes the mentalis muscle response and the AMR recorded from orbicularis oculi after the stimulation of mandibular branch. Once identified, suprathreshold stimulation intensity is utilized throughout the procedure. Electrodes are then affixed to the skin in their optimal position with tape.
To avoid nerve fatigue, the AMR is evoked at approximately 5-min intervals until dural opening. Once the dura is opened, the AMR should be recorded continuously throughout the dissection and decompression and then again periodically during closure to detect the potential reappearance of the AMR. The zygomatic and mandibular branches are alternatively stimulated. Occasionally, the AMR disappears as CSF is drained, ostensibly as a result of relaxation of vascular compression of the facial nerve. Of note, our group has not used brain retraction in the past 5 years [79]. An attempt is made to restore the AMR by increasing the current intensity (up to 20 mA), increasing the stimulation frequency, and finally by increasing pulse widths in increments of 50 μs. After decompression of suspected arteries and veins from the facial nerve and disappearance of the AMR, a further attempt is made to “drive” or stimulate the AMR by increasing the frequency to 30 Hz. If the AMR cannot be elicited, we consider the AMR to have completely resolved.
Monitoring for Complications
Cranial nerve injuries during MVD may result in facial weakness, hearing impairment, vestibular dysfunction, and dysphagia and/or hoarseness, which can affect satisfaction with MVD despite the absence of HFS postoperatively. ABRs are used to monitor cochlear nerve function throughout the procedure. In addition, continuous monitoring of the AMR (Fig. 23.9) and occasional monopolar facial EMG are performed during the procedure. Indeed, neuromonitoring in MVD surgery has been shown not only to improve patient outcome in terms of symptom resolution, but also to decrease the occurrence of hearing loss and facial nerve weakness after surgery [80,81,82]. Monitoring of somatosensory-evoked potentials (SSEPs) used to detect brainstem stroke secondary to vascular manipulation may be considered but is not routinely performed by our group. Monitoring of the ninth and tenth cranial nerves is not performed.
In the following examples, we review cases from our experience over the past 30+ years of MVD for HFS.
Case Illustrations
Case 1: Understanding the AMR During MVD of the Facial Nerve
A 42-year-old woman with right-sided HFS underwent an MVD after serial botulinum toxin injections resulted in unsatisfactory results. During the operation, the AICA and PICA were noted to be tightly compressing the right facial nerve. During decompression of the PICA, the AMR resolved and could not be elicited (Fig. 23.10). The patient awoke from the operation with much reduced spasms, which gradually decreased and resolved entirely 4 months after the operation.
Team Notes
Even after appropriate decompression of the facial nerve, as many as half of the patients have persistent spasms, which resolve over the course of a few weeks to 23 months [78]. Since the AMR often disappears when the offending vessel is lifted off of the facial nerve in a microvascular decompression procedure, its use has been suggested as an intraoperative guide to success [59, 83,84,85,86]. Because HFS disappears or gradually resolves over time in many patients in whom the AMR persists intraoperatively, many authors have questioned the utility of intraoperative EMG [87,88,89,90]. In a large study evaluating 300 patients, Kong et al. [86] found a statistical difference at 1-year follow-up in the outcomes between two groups based on whether the AMR resolved or persisted [86]. Their report is the only one to show a statistically different outcome in cases where the AMR did not resolve. A meta-analysis by Sekula et al. [78] of the data concerning the relationship between resolution or persistence of the AMR after MVD and the resolution or persistence of HFS showed that the chance of a cure if the AMR was abolished after MVD was 4.2 times greater than when the AMR persisted. Thirumala et al. [91], reporting on 259 patients undergoing MVD with intraoperative monitoring of the lateral spread response (LSR), found that abolishment of the LSR during surgery was associated with statistically significant rates of spasm relief immediately postoperatively and at discharge. This increased rate of spasm relief was not observed at later follow-up between the two groups [91]. A similar finding was reported by Nugroho et al. in their meta-analysis that at long term follow up of ≥1 year, intra-operative resolution of AMR failed to predict spasm relief, whereas, it correlated well with spasm relief in short term follow up (≤1 week) [92]. Furthermore, reoperation for persistent symptoms and botulinum toxin treatments prior to surgery does not affect rates of intraoperative abolishment of the LSR [93, 94]. Based on these results, we support that AMR should be monitored routinely in the operating room, and surgical decision-making in the operating room should be guided by the absence or presence of the AMR.
Case 2: “Frozen Shoulder” or Adhesive Capsulitis of the Glenohumeral Joint After MVD in the Contralateral Decubitus Position
A 52-year-old woman with right-sided HFS underwent an MVD after serial botulinum toxin injections resulted in unsatisfactory results. During the operation, the PICA was noted to be tightly compressing the right facial nerve. During decompression of the PICA, the AMR resolved and could not be elicited. No change in SSEPs was noted. The patient awoke without spasms but complained of pain, stiffness, and limited range of movement of her left shoulder.
The surgeon recommended observation and increasing use of the left shoulder for the first 6 weeks following the operation. During those 6 weeks, the patient’s shoulder pain increased to the point that she did not use the left shoulder and upper extremity and required assistance with dressing. The surgeon referred the patient for physical therapy, which was ineffective. The patient was then referred to an orthopedic surgeon who diagnosed her with a “frozen shoulder” or adhesive capsulitis of the glenohumeral joint. MRI of the left shoulder confirmed adhesive capsulitis. The surgeon performed “manipulation” of the left shoulder under a general anesthetic in the operating room, and the patient awoke with reduced pain and improved range of motion. Over the next few weeks, the pain resolved entirely and range of motion was restored.
Team Notes
The pathophysiology of adhesive capsulitis is elusive. Although this complication is rare with the contralateral decubitus position, it should be considered in the differential diagnosis of those patients complaining of pain and reduced range of motion of either shoulder. Additionally, extreme care should be taken when retracting the nondependent shoulder inferiorly due to the potential for stretching of the brachial plexus. This is true when shoulders are taped and pulled away from the operative field (see Fig. 23.8) whether in the supine (such as in an anterior cervical discectomy and fusion), prone (such as in a suboccipital decompression), or in lateral position, as in this case, a retromastoid approach for microvascular decompression.
Case 3: Hearing Loss as a Result of MVD for HFS
A 67-year-old woman with right-sided HFS underwent an MVD after serial botulinum toxin injections resulted in permanent facial weakness (House-Brackmann grade II/VI) [95]. After dural opening, a brain retractor was used to elevate the cerebellum away from the brainstem for exposure of the facial nerve. During this maneuver, the neuromonitoring technician advised the surgeon that waves III and V of the right ABRs had increased in latency by 0.8 ms. Within a few minutes, the neuromonitoring technician advised that the amplitude of the ABRs had decreased by 50%.
After notification of a reduction in amplitude of the ABRs, the surgeon removed the retractor and stopped dissection. The anesthesiologist increased the mean arterial blood pressure 10 mm Hg to a MAP of 80 mm Hg. Within 2 min, the latency improved and the amplitude increased. Within 5 min, the ABRs had returned to baseline, and the surgeon resumed exposure of the facial nerve.
Team Notes
Hearing loss remains a significant risk with MVD of the facial nerve for HFS, with partial hearing loss ranging from 0.5 to 9.5% and complete hearing loss ranging from 0.7 to 7.6% [28, 43, 76, 96]. Polo et al. [28] have provided data concerning a stepwise reduction in hearing with progressive latency increases of Peak V of the ABR during microvascular decompression. In their study of 84 consecutive patients undergoing MVD for HFS, they report that in the group with more than a 20-dB loss in pure tone audiogram, delays in the latency of Peak V were on average 1 ms. To this end, a study by our group showed that intraoperative loss of wave V resulted in significantly increased odds of hearing loss [81]. Similarly, Park et al. showed that loss of wave V or decrease in wave V amplitude by ≥50% with ≥1 ms latency prolongation is highly predictive of postoperative hearing loss [97]. Our group attempted to address this with avoidance of a fixed, self-retained cerebellar retractor in favor of dynamic retraction and subsequently demonstrated a reduction in ipsilateral high-frequency hearing loss (HFHL) from 50 to 7.4% [79].
Case 4: Anesthesia and the AMR
A 54-year-old woman with a 10-year history of right-sided HFS is brought to the operating room for MVD of the facial nerve. After positioning the patient in the contralateral decubitus position, the neuromonitoring technician reports an inability to obtain an AMR of the right facial nerve.
After confirming that the AMR was documented in the Cranial Nerve and Brainstem Disorders Clinic, discussion among the team revealed that a nondepolarizing muscle relaxant had been given during induction for intubation. The team waited 30 min before beginning the procedure, allowing the agent to wear off.
Team Notes
Although the evidence is anecdotal, we believe that there is no role for the use of nondepolarizing muscle relaxants in cases involving microvascular decompression for HFS. It has been our experience that a small defasciculating dose of a nondepolarizing muscle relaxant used during the intubation period is enough to obscure the detection of the abnormal spread at the start of the case. The return of the AMR after the use of a nondepolarizing agent becomes unpredictable.
Case 5: Stroke During MVD for HFS
A 35-year-old woman with right-sided HFS underwent MVD of the right facial nerve. After exposing the facial nerve, the anterior inferior cerebellar arteries as well as multiple perforators of that vessel were noted to be tightly compressing the facial nerve. A preoperative MRI had revealed right-sided dolichoectasia of the vertebrobasilar system with clear compression of the right facial nerve. During the operation, the AICA with perforators and the vertebral artery were decompressed without incident. The AMR was abolished. SSEPs were not measured during the procedure.
Upon awakening, the patient was free of spasms, but, within an hour, she noted contralateral trunk and extremity hypalgesia and thermoanesthesia, ipsilateral Horner syndrome, ipsilateral hypohidrosis, and gait ataxia consistent with a Wallenberg syndrome [98, 99]. Stroke involving the PICA, vertebral artery, or their respective perforators was suspected [100], and a postoperative MRI of the brain confirmed a small infarct involving the lower lateral medulla and posterior cervical spinal cord. Because intraoperative videos did not indicate an avulsion of a perforator or major vessel during decompression of the vertebral and posterior inferior cerebellar arteries (including a complicated tangle of arterioles compressing the facial nerve likely representing PICA medullary perforators), occult compression or vasospasm of perforators was suspected. Although some authors have suggested the use of papaverine to prevent vasospasm-related ischemia [87], we do not routinely use papaverine during MVD due to concerns of vestibulocochlear toxicity [32]. The patient was discharged from acute rehabilitation on postoperative day 13 with improved and independent ambulation. At 10.3-month follow-up, ipsilateral hypohidrosis and gait ataxia had improved significantly with moderate improvement of contralateral extremity thermoanesthesia. The patient notes some incoordination with jogging and persistent difficulty with high humidity due to hypohidrosis.
Team Notes
Because of the low incidence of stroke with MVD of the facial nerve (i.e., <0.2%), we have not utilized SSEPs for MVD for any cranial neuralgia in recent years. It is likely, however, that a change in the SSEPs would be noted, at least in a delayed fashion, in the event of an ischemic event involving the brainstem. If so, application of papaverine to the affected blood arterial vessels (see above explanation), repositioning of Teflon pledgets, and increasing the mean arterial blood pressure (particularly with hypertonic saline) may have been valuable in this patient.
Case 6: Facial Weakness and MVD for HFS
A 42-year-old man with right-sided HFS underwent MVD of the facial nerve at another institution. Although the operative record noted a significant latency increase of the ABR, specific details were not provided. The patient awoke with persistent spasms and a deaf right ear. Because as many as half of patients awake from MVD for HFS with persistent but reduced spasms and proceed to a spasm-free state over the course of a few weeks to as long as 24 months, the patient was observed by the surgeon for 1 year. At 1 year, the patient was referred to our center with no improvement in spasms.
A repeat MVD was offered. During the MVD, a vein tightly compressing the facial nerve was noted. Prior to and after coagulation of the vein with low-power bipolar cautery, the facial nerve was stimulated at 0.2 mA. Prior to coagulation, the nerve responded robustly. After coagulation, the nerve did not respond at 0.2 mA but did respond at 0.5 mA. The patient awoke with weakness (House-Brackmann, Grade V/VI), which improved completely within 9 months [95].
Team Notes
Facial weakness following MVD of the facial nerve for HFS may occur immediately or be delayed. Facial weakness detected immediately following MVD indicates dysfunction occurring at operation (e.g., mechanical dislocation/pressure, thermal trauma or ischemic injury by vascular occlusion, vascular compression, or vasospasm), whereas facial weakness, which develops in a delayed manner, is less well understood. The incidence of delayed facial palsy has been reported to be between 2.8 and 14.5% [44, 101,102,103,104]. Although the phenomenon of delayed facial weakness following MVD for HFS is well known, no explanation is entirely satisfactory, and the evidence is incomplete. Some have suggested reactivation of a dormant virus or delayed facial nerve edema [105, 106]. Other authors have suggested delayed cranial nerve VII injury from manipulation of the nerve near the transition zone during the surgery as a probable mechanism for delayed facial weakness after MVD [104]. Fortunately, resolution of delayed facial weakness following MVD for HFS is almost uniformly complete [101,102,103, 105]. The prognosis of immediate facial weakness is less auspicious. In their series of 1524 operations, Huh et al. [96] reported that 11.4 and 1% of patients undergoing MVD for HFS developed immediate transient and permanent facial weakness, respectively. It should also be noted that many patients who present for surgical evaluation of HFS have peripheral weakness caused by botulinum toxin injections, and many others (those with tonus phenomenon, in particular) have mild to peripheral weakness.
Over the years, we have learned that the removal of veins near the facial nerve is better achieved with purposeful avulsion using a microhook rather than bipolar electrocautery. Facial nerve EMG can be used to thoroughly identify the centrally myelinated portion of the facial nerve before and after decompression of arteries or removal of veins in selected cases. In our experience, an intact facial nerve will respond at 0.2 mA.
Case 7: Vestibular Nerve Dysfunction (After MVD for HFS)
A 68-year-old woman underwent MVD for HFS. During the MVD, dissection rostral to the superior vestibular portion of the vestibulocochlear nerve was required. During exposure of the rostral portion of the facial nerve, the latency of the ABR increased by 1.2 ms. After the decompression, the ABR slowly returned to baseline over 10 min. Upon awakening, the patient was free of spasms with preserved hearing, but complained of vertigo and disequilibrium requiring assistance with ambulation. The patient’s condition improved slowly over a few months.
Team Notes
Data regarding vestibular nerve dysfunction following MVD for HFS are scarce. Because the centrally myelinated portion of the facial nerve lies in proximity to the vestibulocochlear nerve, complete decompression of the facial nerve may result in transient or permanent injury to the vestibular portion of the vestibulocochlear nerve. Samii et al. [43] reported transient and permanent vestibular nerve dysfunction following MVD of the facial nerve for HFS in 9.6 and 2.7% of patients, respectively, and the authors hypothesized that the dysfunction “was probably attributable to direct mechanical trauma or temporary reduction of the blood supply to the vestibular nerves” [43]. Our own group reports 2.7% occurrence of transient vestibular dysfunction in the elderly with no permanent symptoms and 3.7 and 1.9% occurrence of transient and permanent vestibular dysfunction in nonelderly patients, respectively [44]. Because the vestibulocochlear nerve is intimately associated with the facial nerve at the brainstem, vestibular nerve dysfunction can occur particularly with HFS where rostral compression is the norm [107, 108]. In our experience, risk of vestibular nerve dysfunction following MVD for HFS is increased when decompression is rostral to the facial nerve, adjacent to the vestibular portion of the vestibulocochlear nerve, and dissection of adhesions about the vestibulocochlear nerve in reoperations is required. The ABR can be used as an indirect guide to potential injury of the vestibular portion of the vestibulocochlear nerve. When the latency of the ABR increases (particularly beyond 0.5 ms), the team should consider potential causes such as excessive retraction, systemic hypotension, compression of the cochlear portion of the vestibulocochlear nerve by Teflon, and others. As of note, in the past 5 years, our surgeon (RS) has abandoned decompression rostral to the vestibulocochlear nerve with no diminution in positive outcome.
Case 8: Dysphagia/Hoarseness Following MVD for HFS
An 82-year-old woman with right-sided HFS underwent MVD of the facial nerve. During the operation, a dolichoectatic vertebrobasilar system had shifted into the lower cranial nerves including the facial, vestibulocochlear, glossopharyngeal, and vagus nerves. After decompression of the vertebrobasilar system away from the lower cranial nerves, the patient awoke spasm free but with obvious hoarseness and inability to swallow on the right side. Physical examination showed left uvular deviation and minimal movement of the left side of the pharynx. Flexible laryngoscopy revealed good mobility of the vocal cords. After a few days of intravenous fluids and oral restriction, the patient’s swallowing improved to the point that she could safely resume oral intake.
Team Notes
Infrequently, patients awaken with hoarseness and/or dysphagia after MVD of the facial nerve due to manipulation and/or stretching of the glossopharyngeal and vagus nerves. Neurogenic dysphagia can involve dysfunction of any of the three phases of deglutition [109]. In the past, we have noticed that some patients, particularly elderly patients, experience significant transient dysphagia and/or hoarseness following microvascular decompression of the facial nerve. Because the facial nerve lies in proximity to the glossopharyngeal and vagus nerves, complete decompression of the facial nerve may result in transient or permanent injury to those nerves.
A study previously published by our group of 131 HFS patients undergoing MVD showed that 14.8% of elderly patients experienced transient dysphagia and/or hoarseness with no patients experiencing permanent symptoms. No elderly patients required a feeding tube or medialization of the vocal cords; 2.9 and 1.9% of young patients experienced transient and permanent dysphagia and/or hoarseness, respectively. One young patient required medialization of a vocal cord. Resolution of transient dysphagia and/or hoarseness may be protracted, and in this series of patients, resolution of transient dysphagia and/or hoarseness ranged from 1 to 10 months in the elderly cohort and 2 weeks to 6 months in the young cohort [44]. It has been recently described the methodology to elicit the laryngeal adductor reflex under general anesthesia [110]. This new technique could be of help detecting lesions to the afferent and efferent portions of the vagal nerve in neurosurgery before they are irreversible. However, a meta-analysis by Sindou et al. reported 0.5–1% risk for lower cranial nerve dysfunction after MVD for HFS [111].
Glossopharyngeal Neuralgia
Glossopharyngeal neuralgia (GPN) is an uncommon cause of facial pain with incidence of 0.2–0.7/100,000 people per year and accounting for 0.2–1.3% of facial pain syndromes and it is characterized by intermittent, lancinating pain involving the posterior tongue and pharynx, often with radiation to deep ear structures [112]. The most common offending vessel implicated to cause GPN is the posterior inferior cerebellar artery, followed by the vertebral artery, and anterior inferior cerebellar artery [113]. MVD is indicated in drug intolerance and medically refractory cases of GPN and in the last few decades it has improved success rates in pain relief (from 79 to >90%) without increase in complication rates [114, 115]. MVD is an effective treatment for GPN and pain relief can persist beyond 10 years after surgery [116, 117]. Even in pain recurrence including post-rhizotomy cases, MVD remains effective [118]. MVD has better success rates (>90%) in postoperative pain relief in GPN compared to other techniques such as rhizotomy (55%) [114, 119]. Also, MVD alone has been found to be efficacious in treating GPN compared to combined rhizotomy and MVD procedures which showed no increased efficacy in treating GPN and caused increased adverse effects [120, 121]. In a large meta-analysis, complications after MVD in GPN were reported in 21% (92/436) cases. Of these 92 cases, 65 (71%) had lower cranial nerve palsy and 66% of them were transient. CSF leak was present in 15% (14/92) cases. Mortality occurred in 1% (4/436) cases but these were reported in studies published before 2003 [115, 116, 122]. Dry cough, dysphonia/dysphagia, and brain swelling can also occur transiently after MVD [123].
IOM using ABR and cranial nerve VII, IX, X EMG during MVD reduces the postoperative incidence of hearing loss, facial paresis, and dysphonia/dysphagia [124]. Habeych et al. reported that addition of cranial nerve V and VI monitoring does not improve the efficacy of IOM of MVD for GPN [124]. Motoyama et al. have also reported the use of transcranial motor-evoked potentials (TcMEP) recorded from muscles innervated by CNs VII, IX, and X during MVD for GPN [125]. These authors found that one of three patients who did not undergo CN IX or X TcMEP monitoring experienced transient mild dysphagia. In the other two patients, one of them had a decrease in CN IX (pharyngeal) TcMEP response amplitude by >50% from baseline. This prompted the surgeon to release the self-retaining retractor on cerebellum and subsequently, the TcMEP response improved back to baseline and the rest of the procedure was completed within normal range of TcMEP responses. The second of these two patients did not sustain any lower cranial nerve TcMEP changes intraoperatively. These two patients showed no lower cranial nerve deficits (dysphagia or dysphonia) postoperatively [125].
References
Xia L, Zhong J, Zhu J, et al. Effectiveness and safety of microvascular decompression surgery for treatment of trigeminal neuralgia: a systematic review. J Craniofac Surg. 2014;25(4):1413–7.
Bendtsen L, Zakrzewska JM, Heinskou TB, et al. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. 2020;19(9):784–96.
Arrese I, Lagares A, Alday R, Ramos A, Rivas JJ, Lobato RD. Typical trigeminal neuralgia associated with brainstem white matter lesions on MRI in patients without criteria of multiple sclerosis. Acta Neurochir. 2008;150(11):1157–61.
Ericson H, Abu Hamdeh S, Freyhult E, et al. Cerebrospinal fluid biomarkers of inflammation in trigeminal neuralgia patients operated with microvascular decompression. Pain. 2019;160(11):2603–11.
Tanaka BS, Zhao P, Dib-Hajj FB, et al. A gain-of-function mutation in Nav1.6 in a case of trigeminal neuralgia. Mol Med. 2016;22(1):338–48.
Siqueira SRDT, Alves B, Malpartida HMG, Teixeira MJ, Siqueira JTT. Abnormal expression of voltage-gated sodium channels Nav1.7, Nav1.3 and Nav1.8 in trigeminal neuralgia. Neuroscience. 2009;164(2):573–7.
Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001;124(12):2347–60.
Harry Rappaport Z, Govrin-Lippmann R, Devor M. An electron-microscopic analysis of biopsy samples of the trigeminal root taken during microvascular decompressive surgery. Stereotact Funct Neurosurg. 1997;68(1–4):182–6.
Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(1):159–62.
Li GW, Zhang WC, Min Y, Ma QF, Zhong WX. Surgical skills of adhesions and transposition of trigeminal nerve for primary trigeminal neuralgia. J Craniofac Surg. 2014;25(4):1296–8.
Zacest AC, Magill ST, Miller J, Burchiel KJ. Preoperative magnetic resonance imaging in Type 2 trigeminal neuralgia. J Neurosurg. 2010;113(3):511–5.
Leal PRL, Hermier M, Froment JC, Souza MA, Cristino-Filho G, Sindou M. Preoperative demonstration of the neurovascular compression characteristics with special emphasis on the degree of compression, using high-resolution magnetic resonance imaging: a prospective study, with comparison to surgical findings, in 100 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Acta Neurochir. 2010;152(5):814–25.
Ferroli P, Acerbi F, Broggi M, Broggi G. Arteriovenous micromalformation of the trigeminal root: intraoperative diagnosis with indocyanine green videoangiography: case report. Neurosurgery. 2010;67(3 Suppl Operative):onsE309–10.
McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg. 1999;90(1):1–8.
Sekula RF, Frederickson AM, Jannetta PJ, Bhatia S, Quigley MR. Microvascular decompression after failed Gamma Knife surgery for trigeminal neuralgia: a safe and effective rescue therapy? J Neurosurg. 2010;113(1):45–52.
Vaz-Guimaraes F, Gardner PA, Fernandez-Miranda JC. Fully endoscopic retrosigmoid approach for posterior petrous meningioma and trigeminal microvascular decompression. Acta Neurochir. 2015;157(4):611–5.
Artz GJ, Hux FJ, LaRouere MJ, Bojrab DI, Babu S, Pieper DR. Endoscopic vascular decompression. Otol Neurotol. 2008;29(7):995–1000.
Moller AR, Moller MB. Does intraoperative monitoring of auditory evoked potentials reduce incidence of hearing loss as a complication of microvascular decompression of cranial nerves? Neurosurgery. 1989;24(2):257–63.
Brock S, Scaioli V, Ferroli P, Broggi G. Neurovascular decompression in trigeminal neuralgia: role of intraoperative neurophysiological monitoring in the learning period. Stereotact Funct Neurosurg. 2004;82(5–6):199–206.
Bartindale M, Kircher M, Adams W, et al. Hearing loss following posterior fossa microvascular decompression: a systematic review. Otolaryngol Head Neck Surgery. 2018;158(1):62–75.
Thirumala PD, Carnovale G, Loke Y, et al. Brainstem auditory evoked potentials’ diagnostic accuracy for hearing loss: systematic review and meta-analysis. J Neurol Surgery B Skull Base. 2017;78(1):43–51.
Truini A, Cruccu G. Laser evoked potentials in patients with trigeminal disease: the absence of Adelta potentials does not unmask C-fibre potentials. Clin Neurophysiol. 2008;119(8):1905–8.
Dong CCJ, MacDonald DB, Akagami R, et al. Intraoperative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base surgery. Clin Neurophysiol. 2005;116(3):588–96.
Simioni V, Capone JG, Sette E, et al. Intraoperative monitoring of sensory part of the trigeminal nerve using blink reflex during microvascular decompression for trigeminal neuralgia. Acta Neurochir. 2018;160(1):165–9.
Ying T, Bao B, Yuan Y, et al. Blink reflex monitoring in microvascular decompression for trigeminal neuralgia. Neurol Res. 2021;43(7):591–4.
Legatt AD. Mechanisms of intraoperative brainstem auditory evoked potential changes. J Clin Neurophysiol. 2002;19(5):396–408.
Grundy BL, Procopio PT, Jannetta PJ, Lina A, Doyle E. Evoked potential changes produced by positioning for retromastoid craniectomy. Neurosurgery. 1982;10(6 Pt 1):766–70.
Polo G, Fischer C, Sindou MP, et al. Brainstem auditory evoked potential monitoring during microvascular decompression for hemifacial spasm: intraoperative brainstem auditory evoked potential changes and warning values to prevent hearing loss—prospective study in a consecutive series of 84 patients. Neurosurgery. 2004;54(1):97–106.
Ramnarayan R, Mackenzie I. Brain-stem auditory evoked responses during microvascular decompression for trigeminal neuralgia: predicting post-operative hearing loss. Neurol India. 2006;54(3):250–4.
Hatayama T, Møller AR. Correlation between latency and amplitude of peak V in the brainstem auditory evoked potentials: intraoperative recordings in microvascular decompression operations. Acta Neurochir. 1998;140(7):681–7.
Raudzens PA, Shetter AG. Intraoperative monitoring of brain-stem auditory evoked potentials. J Neurosurg. 1982;57(3):341–8.
Chadwick GM, Asher AL, van der Veer CA, Pollard RJ. Adverse effects of topical papaverine on auditory nerve function. Acta Neurochir. 2008;150(9):901–9.
Schaller B. Trigemino-cardiac reflex during microvascular trigeminal decompression in cases of trigeminal neuralgia. J Neurosurg Anesthesiol. 2005;17(1):45–8.
Schaller B, Probst R, Strebel S, Gratzl O. Trigeminocardiac reflex during surgery in the cerebellopontine angle. J Neurosurg. 1999;90(2):215–20.
Sandu N, Spiriev T, Lemaitre F, Filis A, Schaller B. New molecular knowledge towards the trigemino-cardiac reflex as a cerebral oxygen-conserving reflex. Sci World J. 2010;10:811–7.
Wahlig J, Kaufmann A, Balzer JR, Lovely T, Jannetta P. Intraoperative loss of auditory function relieved by microvascular decompression of the cochlear nerve. Can J Neurol Sci. 1999;26(1):44–7.
Neu M, Strauss C, Romstöck J, Bischoff B, Fahlbusch R. The prognostic value of intraoperative BAEP patterns in acoustic neurinoma surgery. Clin Neurophysiol. 1999;110(11):1935–41.
Grundy BL, Jannetta PJ, Procopio PT, Lina A, Boston JR, Doyle E. Intraoperative monitoring of brain-stem auditory evoked potentials. J Neurosurg. 1982;57(5):674–81.
Strauss C, Naraghi R, Bischoff B, Huk WJ, Romstock J. Contralateral hearing loss as an effect of venous congestion at the ipsilateral inferior colliculus after microvascular decompression: report of a case. J Neurol Neurosurg Psychiatry. 2000;69(5):679–82.
Zhong J, Li ST, Xu SQ, Wan L, Wang X. Management of petrosal veins during microvascular decompression for trigeminal neuralgia. Neurol Res. 2008;30(7):697–700.
Jo KW, Kong DS, Park K. Microvascular decompression for hemifacial spasm: long-term outcome and prognostic factors, with emphasis on delayed cure. Neurosurg Rev. 2013;36(2):297–302.
Heuser K, Kerty E, Eide PK, Cvancarova M, Dietrichs E. Microvascular decompression for hemifacial spasm: postoperative neurologic follow-up and evaluation of life quality. Eur J Neurol. 2007;14(3):335–40.
Samii M, Günther T, Iaconetta G, et al. Microvascular decompression to treat hemifacial spasm: long-term results for a consecutive series of 143 patients. Neurosurgery. 2002;50(4):712–9.
Sekula RF, Frederickson AM, Arnone GD, Quigley MR, Hallett M. Microvascular decompression for hemifacial spasm in patients >65 years of age: an analysis of outcomes and complications. Muscle Nerve. 2013;48(5):770–6.
Miller LE, Miller VM. Safety and effectiveness of microvascular decompression for treatment of hemifacial spasm: a systematic review. Br J Neurosurg. 2012;26(4):438–44.
Jannetta P, Samii M. The Cranial nerves. Berlin: Springer Berlin Heidelberg; 1981.
Babinski J. Hemipasme facial peripherique. 1905.
Stamey W, Jankovic J. The other Babinski sign in hemifacial spasm. Neurology. 2007;69(4):402–4.
Auger RG, Whisnant JP. Hemifacial spasm in Rochester and Olmsted County, Minnesota, 1960 to 1984. Arch Neurol. 1990;47(11):1233–4.
Nilsen B, Le KD, Dietrichs E. Prevalence of hemifacial spasm in Oslo, Norway. Neurology. 2004;63(8):1532–3.
Martí-Fàbregas J, Montero J, López-Villegas D, Quer M. Post-irradiation neuromyotonia in bilateral facial and trigeminal nerve distribution. Neurology. 1997;48(4):1107–9.
Valls-Solé J. Facial palsy, postparalytic facial syndrome, and hemifacial spasm. Movement disorders 2002;17(SUPPL. 2).
Gardner WJ. Cross talk—the paradoxical transmission of a nerve impulse. Arch Neurol. 1966;14(2):149–56.
Nielsen VK. Pathophysiology of hemifacial spasm: II. Lateral spread of the supraorbital nerve reflex. Neurology. 1984;34(4):427–31.
Ferguson JH. Hemifacial spasm and the facial nucleus. Ann Neurol. 1978;4(2):97–103.
Møller AR. Hemifacial spasm: ephaptic transmission or hyperexcitability of the facial motor nucleus? Exp Neurol. 1987;98(1):110–9.
Moller A. Cranial nerve dysfunction syndromes: pathophysiology of microvascular compression. In: Barrow D, editor. Neurosurgical Topics Book 13: surgery of cranial nerves of the posterior fossa. Park Ridge, IL: American Association of Neurologic Surgeons; 1993.
Esteban A, Molina-Negro P. Primary hemifacial spasm: a neurophysiological study. J Neurol Neurosurg Psychiatry. 1986;49(1):58–63.
Møller AR, Jannetta PJ. Physiological abnormalities in hemifacial spasm studied during microvascular decompression operations. Exp Neurol. 1986;93(3):584–600.
Fernández-Conejero I, Ulkatan S, Sen C, Deletis V. Intra-operative neurophysiology during microvascular decompression for hemifacial spasm. Clin Neurophysiol. 2012;123(1):78–83.
Deletis V, Urriza J, Ulkatan S, Fernandez-Conejero I, Lesser J, Misita D. The feasibility of recording blink reflexes under general anesthesia. Muscle Nerve. 2009;39(5):642–6.
Montero J, Junyent J, Calopa M, Povedano M, Valls-Sole J. Electrophysiological study of ephaptic axono-axonal responses in hemifacial spasm. Muscle Nerve. 2007;35(2):184–8.
Tomii M, Onoue H, Yasue M, Tokudome S, Abe T. Microscopic measurement of the facial nerve root exit zone from central glial myelin to peripheral Schwann cell myelin. J Neurosurg. 2003;99(1):121–4.
Campos-Benitez M, Kaufmann AM. Neurovascular compression findings in hemifacial spasm. J Neurosurg. 2008;109(3):416–20.
Hughes MA, Branstetter BF, Taylor CT, et al. MRI findings in patients with a history of failed prior microvascular decompression for hemifacial spasm: how to image and where to look. AJNR Am J Neuroradiol. 2015;36(4):768–73.
Hughes MA, Branstetter BF, Frederickson AM, Oskin JE, Yankevich U, Sekula RF. Imaging hemifacial spasm. Neurographics. 2015;5(1):2–8.
Nagatani T, Inao S, Suzuki Y, Yoshida J. Perforating branches from offending arteries in hemifacial spasm: anatomical correlation with vertebrobasilar configuration. J Neurol Neurosurg Psychiatry. 1999;67(1):73–7.
Sheth S, Branstetter BF, Escott EJ. Appearance of normal cranial nerves on steady-state free precession MR images. Radiographics. 2009;29(4):1045–55.
Borges A, Casselman J. Imaging the trigeminal nerve. Eur J Radiol. 2010;74(2):323–40.
Zeng Q, Zhou Q, Liu Z, Li C, Ni S, Xue F. Preoperative detection of the neurovascular relationship in trigeminal neuralgia using three-dimensional fast imaging employing steady-state acquisition (FIESTA) and magnetic resonance angiography (MRA). J Clin Neurosci. 2013;20(1):107–11.
Jo KW, Kong DS, Hong KS, Lee JA, Park K. Long-term prognostic factors for microvascular decompression for trigeminal neuralgia. J Clin Neurosci. 2013;20(3):440–5.
Garcia M, Naraghi R, Zumbrunn T, Rösch J, Hastreiter P, Dörfler A. High-resolution 3D-constructive interference in steady-state MR imaging and 3D time-of-flight MR angiography in neurovascular compression: a comparison between 3T and 1.5T. AJNR Am J Neuroradiol. 2012;33(7):1251–6.
Sekula RF, Frederickson AM, Branstetter BF, et al. Thin-slice T2 MRI imaging predicts vascular pathology in hemifacial spasm: a case-control study. Mov Disord. 2014;29(10):1299–303.
Savino PJ, Sergott RC, Bosley TM, Schatz NJ. Hemifacial spasm treated with botulinum A toxin injection. Arch Ophthalmol (Chicago, Ill: 1960). 1985;103(9):1305–6.
Kraft SP, Lang AE. Cranial dystonia, blepharospasm and hemifacial spasm: clinical features and treatment, including the use of botulinum toxin. Can Med Assoc J. 1988;139(9):837–44.
Barker FG, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD. Microvascular decompression for hemifacial spasm. J Neurosurg. 1995;82(2):201–10.
Wang A, Jankovic J. Hemifacial spasm: clinical findings and treatment. Muscle Nerve. 1998;21(12):1740–7.
Sekula RF, Bhatia S, Frederickson AM, et al. Utility of intraoperative electromyography in microvascular decompression for hemifacial spasm: a meta-analysis. Neurosurg Focus. 2009;27(4):E10.
Thirumala P, Frederickson AM, Balzer J, et al. Reduction in high-frequency hearing loss following technical modifications to microvascular decompression for hemifacial spasm. J Neurosurg. 2015;123(4):1059–64.
Thirumala PD, Carnovale G, Habeych ME, Crammond DJ, Balzer JR. Diagnostic accuracy of brainstem auditory evoked potentials during microvascular decompression. Neurology. 2014;83(19):1747–52.
Thirumala PD, Krishnaiah B, Habeych ME, Balzer JR, Crammond DJ. Hearing outcomes after loss of brainstem auditory evoked potentials during microvascular decompression. J Clin Neurosci. 2015;22(4):659–63.
Thirumala PD, Wang X, Shah A, et al. Clinical impact of residual lateral spread response after adequate microvascular decompression for hemifacial spasm: a retrospective analysis. Br J Neurosurg. 2015;29(6):818–22.
Moller AR, Jannetta PJ. On the origin of synkinesis in hemifacial spasm: results of intracranial recordings. J Neurosurg. 1984;61(3):569–76.
Møller AR, Jannetta PJ. Hemifacial spasm: results of electrophysiologic recording during microvascular decompression operations. Neurology. 1985;35(7):969–74.
Yamashita S, Kawaguchi T, Fukuda M, Watanabe M, Tanaka R, Kameyama S. Abnormal muscle response monitoring during microvascular decompression for hemifacial spasm. Acta Neurochir. 2005;147(9):933–8.
Kong DS, Park K, Shin BG, Jeong AL, Eum DO. Prognostic value of the lateral spread response for intraoperative electromyography monitoring of the facial musculature during microvascular decompression for hemifacial spasm. J Neurosurg. 2007;106(3):384–7.
Sindou MP. Microvascular decompression for primary hemifacial spasm. Importance of intraoperative neurophysiological monitoring. Acta Neurochir. 2005;147(10):1019–26.
Hatem J, Sindou M, Vial C. Intraoperative monitoring of facial EMG responses during microvascular decompression for hemifacial spasm. Prognostic value for long-term outcome: a study in a 33-patient series. Br J Neurosurg. 2001;15(6):496–9.
Kiya N, Bannur U, Yamauchi A, Yoshida K, Kato Y, Kanno T. Monitoring of facial evoked EMG for hemifacial spasm: a critical analysis of its prognostic value. Acta Neurochir. 2001;143(4):365–8.
Joo W, Lee KJ, Park HK, Chough CK, Rha HK. Prognostic value of intra-operative lateral spread response monitoring during microvascular decompression in patients with hemifacial spasm. J Clin Neurosci. 2008;15(12):1335–9.
Thirumala PD, Shah AC, Nikonow TN, et al. Microvascular decompression for hemifacial spasm: evaluating outcome prognosticators including the value of intraoperative lateral spread response monitoring and clinical characteristics in 293 patients. J Clin Neurophysiol. 2011;28(1):56–66.
Nugroho SW, Perkasa SAH, Gunawan K, Manuhutu YN, Rahman MA, Rizky A. Predicting outcome of hemifacial spasm after microvascular decompression with intraoperative monitoring: a systematic review. Heliyon. 2021;7(2):e06115.
Wang X, Thirumala PD, Shah A, et al. Microvascular decompression for hemifacial spasm: focus on late reoperation. Neurosurg Rev. 2013;36(4):637–44.
Wang X, Thirumala PD, Shah A, et al. Effect of previous botulinum neurotoxin treatment on microvascular decompression for hemifacial spasm. Neurosurg Focus. 2013;34(3):E3.
House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surgery. 1985;93(2):146–7.
Huh R, Han IB, Moon JY, Chang JW, Chung SS. Microvascular decompression for hemifacial spasm: analyses of operative complications in 1582 consecutive patients. Surg Neurol. 2008;69(2):153–7.
Park SK, Joo BE, Lee S, et al. The critical warning sign of real-time brainstem auditory evoked potentials during microvascular decompression for hemifacial spasm. Clin Neurophysiol. 2018;129(5):1097–102.
Currier R, Dejong R. The lateral medullary (Wallenberg’s) syndrome. Med Bull (Ann Arbor). 1962;28:106–13.
Currier RD, Giles CL, DeJong RN. Some comments on Wallenberg’s lateral medullary syndrome. Neurology. 1961;11(9):778–91.
Lister JR, Rhoton AL, Matsushima T, Peace DA. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982;10(2):170–99.
Kim B-T, Hwang S-C, Chang J-C, Shin W-H, Choi S-K, Byun B-J. Delayed facial palsy following microvascular decompression in hemifacial spasm patients. J Korean Neurosurg Soc. 1999;28(9):1332–6.
Kuroki A, Itagaki S, Nagai O. Delayed facial palsy after microvascular decompression for hemifacial spasm. Facial Nerve Res. 1991;11(11):147–50.
Lovely TJ, Getch CC, Jannetta PJ. Delayed facial weakness after microvascular decompression of cranial nerve VII. Surg Neurol. 1998;50(5):449–52.
Lee JM, Park HR, Choi YD, et al. Delayed facial palsy after microvascular decompression for hemifacial spasm: friend or foe? J Neurosurg. 2018;129(2):299–307.
Rhee DJ, Kong DS, Park K, Lee JA. Frequency and prognosis of delayed facial palsy after microvascular decompression for hemifacial spasm. Acta Neurochir. 2006;148(8):839–43.
Furukawa K, Sakoh M, Kumon Y, et al. Delayed facial palsy after microvascular decompression for hemifacial spasm due to reactivation of varicella-zoster virus. No Shinkei Geka. 2003;31(8):899–902.
Ryu H, Yamamoto S, Miyamoto T. Atypical hemifacial spasm. Acta Neurochir. 1998;140(11):1173–6.
Jannetta PJ. Surgical treatment of cranial rhizopathies. In: Congress of neurological surgeons. Montreal, QC, Canada: Williams & Wilkins; 1996. p. 181–95.
Pollack IF, Pang D, Kocoshis S, Putnam P. Neurogenic dysphagia resulting from chiari malformations. Neurosurgery. 1992;30(5):709–19.
Sinclair CF, Téllez MJ, Tapia OR, Ulkatan S. Contralateral R1 and R2 components of the laryngeal adductor reflex in humans under general anesthesia. Laryngoscope. 2017;127(12):E443–8.
Sindou M, Mercier P. Microvascular decompression for hemifacial spasm: outcome on spasm and complications. A review. Neuro-Chirurgie. 2018;64(2):106–16.
Resnick DK, Jannetta PJ, Bissonnette D, Jho HD, Lanzino G. Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery. 1995;36(1):64–9.
Hiwatashi A, Matsushima T, Yoshiura T, et al. MRI of glossopharyngeal neuralgia caused by neurovascular compression. AJR Am J Roentgenol. 2008;191(2):578–81.
Kandan SR, Khan S, Jeyaretna DS, Lhatoo S, Patel NK, Coakham HB. Neuralgia of the glossopharyngeal and vagal nerves: long-term outcome following surgical treatment and literature review. Br J Neurosurg. 2010;24(4):441–6.
Patel A, Kassam A, Horowitz M, et al. Microvascular decompression in the management of glossopharyngeal neuralgia: analysis of 217 cases. Neurosurgery. 2002;50(4):705–11.
Kondo A. Follow-up results of using microvascular decompression for treatment of glossopharyngeal neuralgia. J Neurosurg. 1998;88(2):221–5.
Sampson JH, Grossi PM, Asaoka K, et al. Microvascular decompression for glossopharyngeal neuralgia: long-term effectiveness and complication avoidance. Neurosurgery. 2004;54(4):884–90.
Owler BK, Johnston I, Kennedy M. Late recurrence of glossopharyngeal neuralgia after IXth and partial Xth nerve rhizotomy: treatment by microvascular decompression. J Neurol Neurosurg Psychiatry. 2000;69(6):834–5.
Kano H, Urgosik D, Liscak R, et al. Stereotactic radiosurgery for idiopathic glossopharyngeal neuralgia: an international multicenter study. J Neurosurg. 2016;125(Suppl 1):147–53.
Zheng X, Wei XY, Zhu J, Yuan Y, Ying TT, Li ST. Microvascular decompression alone without rhizotomy is an effective way of treating glossopharyngeal neuralgia: clinical analysis of 46 cases. Stereotact Funct Neurosurg. 2020;98(2):129–35.
Wang J, Yu R, Qu C, et al. Does glossopharyngeal neuralgia need rhizotomy in neurovascular decompression surgery? J Craniofac Surg. 2018;29(8):2192–4.
Lu VM, Goyal A, Graffeo CS, Perry A, Jonker BP, Link MJ. Glossopharyngeal neuralgia treatment outcomes after nerve section, microvascular decompression, or stereotactic radiosurgery: a systematic review and meta-analysis. World Neurosurg. 2018;120:572–582.e7.
Kim MK, Park JS, Ahn YH. Microvascular decompression for glossopharyngeal neuralgia: clinical analyses of 30 cases. J Korean Neurosurg Soc. 2017;60(6):738–48.
Habeych ME, Crammond DJ, Gardner P, Thirumala PD, Horowitz MB, Balzer JR. Intraoperative neurophysiological monitoring of microvascular decompression for glossopharyngeal neuralgia. J Clin Neurophysiol. 2014;31(4):337–43.
Motoyama Y, Nakagawa I, Takatani T, et al. Microvascular decompression for glossopharyngeal neuralgia using intraoperative neurophysiological monitoring: technical case report. Surg Neurol Int. 2016;7(Suppl 2):S28–35.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Electronic Supplementary Material
Positioning and marking for MVD (MOV 223316 kb)
Placement of a Teflon sponge under the offending artery (MP4 37554 kb)
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shandal, V. et al. (2023). Microvascular Decompression for Cranial Nerve Disorders. In: Seubert, C.N., Balzer, J.R. (eds) Koht, Sloan, Toleikis's Monitoring the Nervous System for Anesthesiologists and Other Health Care Professionals. Springer, Cham. https://doi.org/10.1007/978-3-031-09719-5_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-09719-5_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-09718-8
Online ISBN: 978-3-031-09719-5
eBook Packages: MedicineMedicine (R0)