Abstract
This chapter is a comprehensive review of the basics of ultrasound imaging. It contains general principles of the physics and main ultrasonographic techniques that are useful to practice dermatologic ultrasound. There is a glossary of common ultrasound terms and information on the new developments in the field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Ultrasound is a unique medical imaging tool in the investigation of dermatological diseases that provides anatomical and functional data in real time with high-resolution grayscale images. It has no ionizing radiation. Grayscale images show lesion morphology; echogenicity; inner texture such as solid, cystic, or mixed; homogeneous or inhomogeneous; foci of calcifications; location and depth of penetration; and lesion size and volume. Doppler ultrasound provides blood flow information. Color and spectral Doppler ultrasound is able to determine vascularity in real time, showing flow pattern, flow direction, and velocity. Furthermore, ultrasound enables safe guidance of interventional procedures such as needle localization of lesions, biopsy of suspected malignancies, drainage of collections, and foreign-body localization and removal. Sonography provides detailed anatomic information that is useful for surgical planning.
To produce high-quality diagnostic images, it is essential to understand the physics of interactions between transmitted acoustic energy and reflected echoes from interfaces within tissues. Optimizing the techniques used to produce the ultrasound display is essential. Successful diagnosis requires knowledge of the clinical setting, operator skills, and knowledge of the physics and instrumentation used to optimize the imaging information. If the operator is facile with ultrasound technique, then pitfalls from misinterpretation of artifacts can be avoided. In comparison to other imaging examinations, ultrasound is a safe and effective noninvasive, low-cost, and widely available test that is useful for diagnosis and follow-up.
Basic Acoustics and Instrumentation
Grayscale Ultrasound Imaging
The mainstay of ultrasound imaging is provided by B-mode, gray scale, and real-time display [1]. B-mode display refers to the brightness level of the echoes and is related to the strength of the reflected signal. Reflected signals of different amplitudes are displayed in different intensities or levels of brightness. Gray scale depicts the signal intensity from white to black through different shades of gray. Signals of greatest intensity are displayed as white, those with intermediate intensity as shades of gray, and absence of signals as black. These are referred to as hyperechoic (white), hypoechoic (gray), and echo-free or anechoic (black) (Fig. 4.1). Real-time ultrasound is the dynamic display of organs achieved by generating 2D images at a rate of 15–60 frames per second.
B-mode grayscale image. Transverse (axial) image of a pediatric liver, showing a hyperechoic convex line (large arrow) representing the diaphragm, a highly reflective interface between the liver and the aerated lung; anechoic structures (short arrow) that are the inferior vena cava and the hepatic veins; and the hypoechoic liver parenchyma (asterisk)
Doppler Sonography
The ability of sonography to show and characterize subtle nuances in tissue composition has been enhanced by the development of Doppler sonography [2]. The reflected sound from a stationary target has the same frequency as the transmitted sound, whereas the reflected sound from a moving target has a different and changing frequency from the transmitted sound. The Doppler effect reflects this change in frequency and is directly proportional to the velocity of the moving target. Doppler measurements have to be made at an angle of insonation of 60 degrees or less. No Doppler shift is detected at 90 degrees, because there is no relative movement of the target towards or away from the transducer i.e.- the cosine of 90 degrees is zero). Accurate estimation of target velocity requires precise measurement of the Doppler frequency shift at a correct angle of insonation of the moving target. Doppler frequency shifts fall in the audible range. The received signal may also provide useful information about flow characteristics.
Both color and power Doppler display the Doppler shifts in color superimposed on the grayscale image in real time (Fig. 4.2). Color Doppler mode displays flowing blood in a color map information on flow direction. Usually red means towards the transducer and blue away from the transducer; however, the color map can be changed in the configuration of the machine. Relative velocity information is signified by lighter tints corresponding to higher velocities (Fig. 4.3). Power Doppler mode uses a single-color map to display the power or amplitude of the Doppler signals. Power mode has increased sensitivity for flow detection, but lacks information about flow direction and flow velocity. Low-velocity flow and poorly vascularized lesions are better displayed by power Doppler. Color Doppler provides more information in higher flow velocity hypervascular lesions because it demonstrates both flow direction and relative velocities. On color Doppler mode, color artifacts may be reduced to a minimum by optimizing the color Doppler amplification; i.e. - degrading it until only pulsating color pixels are left. Lack of intralesional vessel detection may be due to low Doppler amplification, which can be corrected by increasing amplification just until colored artifacts are seen in or around the lesion.
Color and power Doppler sonography. Flow direction is displayed in colors toward or away from the transducer. Transverse (axial) image of ventral wrist. (a) Radial artery appears red (arrow) and radial veins appear blue, on both sides of the artery on color Doppler. (b) Same vessels displayed on power Doppler. Power flow uses a single-color map to display the amplitude of the Doppler signals. Power Doppler increases sensitivity for flow detection at lower velocities, although it lacks information on flow direction and flow velocity
Color Doppler sonography. Dermatofibrosarcoma at the mid-thigh. (a) On grayscale image, the tumor is very hypoechoic (arrow), surrounded by edematous hyperechoic subcutaneous fat. (b) Color Doppler shows the rich vascularity of the lesion. Red color represents flow towards the transducer and blue away from the transducer
The Doppler frequency spectrum (pulsed Doppler; spectral curve analysis) displays Doppler shifts in graphic form. The Doppler frequency spectrum shows flow velocity and direction by vertical deflections of the waveform above and below the baseline. Doppler parameters such as maximum systolic velocity, end diastolic velocity, and mean blood flow velocity may be measured. Pulsatility and resistive indices may be calculated. Spectral Doppler display allows differentiation between venous and arterial flow by showing flow characteristics and velocity measurements (Fig. 4.4). By combining grayscale ultrasound with Doppler ultrasound, the accurate definition of lesion features may be obtained, including internal echogenicity, size, shape, margins, deep-layer involvement, and blood flow. Doppler sonography sometimes may be useful to differentiate between vascular lesions, inflammation, and tumors; however, hypervascularity is nonspecific because it is found both in inflammatory and malignant lesions.
Color and spectral Doppler sonography. A hypervascular lesion at the dorsal foot is shown. (a) Color Doppler display of vessels and flow direction displayed in a color map, superimposed on the grayscale image in real time. Red towards the transducer and blue away from the transducer. Lighter hues correspond to higher velocity. The color bar is shown on the right side of the image. (b) Spectral Doppler display of arterial flow. A cursor (two parallel lines) is placed into the vessel to display flow. Peak systolic velocity (PSV), end diastolic velocity (EDV), mean diastolic velocity (MDV), and resistive index (RI) are automatically calculated and displayed during one cardiac cycle, enclosed between the vertical lines on the spectral display at the bottom of the image. (c) Spectral Doppler display of venous flow. Spectral Doppler allows differentiation between arterial and venous flow based on flow characteristics. Note that in this vein, flow direction is towards the transducer, which is displayed in red and is located above the baseline
Modern high-resolution equipment using high-frequency probes and a very sensitive power Doppler technique allows a clear definition of superficial structures, grayscale characteristics, and vascularity. Color Doppler sonography can be used to increase the specificity of ultrasonography in the evaluation of focal lesions. Benign tumors may be associated with hyperemia and ectasia of vessels but not with any appreciable formation of new vessels. Conversely, angiogenesis is seen in many malignant tumors where it can be an important indicator of prognosis. The detection of Doppler signals is a simple, noninvasive method of analyzing vascularity. Intratumoral vascularity can influence treatment planning and selection as well as extent of excision. Extensive vascularity in a primary tumor may indicate a less favorable prognosis due to the propensity for early spread. However, vascularity and tissue perfusion may be favorable for penetration by chemotherapy and other forms of treatment. In summary, Doppler sonography allows blood flow detection and definition of flow direction, characteristics, and velocity.
Ultrasound Transducers
The ultrasound transducer, (also known as the probe), is the handheld part of the ultrasound machine that produces transmitted sound and detects reflected ultrasound waves. Convex array transducers have a lower frequency (1–6 MHz), a greater tissue penetration, but a lower resolution and are selected to image deeper structures like abdominal organs (Fig. 4.5). Linear array transducers have a higher frequency (6–18 MHz), lesser penetration, and greater spatial resolution than convex array transducers (Fig. 4.6). High-resolution linear transducers are used for superficial applications like thyroid, breast, testis, superficial vascular, musculoskeletal, and skin. Modern ultrasound systems employ transducers with a broad high variable-frequency ultrasound range or broad “bandwidth” (HVFUS) [1]. High-frequency linear transducers that are currently used in skin imaging range from 6 to 18 MHz for grayscale imaging and from 7 to 14 MHz for Doppler frequency ranges. This wide range of frequency availability and adjustable focal length allow echo sources at different depths to be accurately pinpointed. The small “hockey stick”-shape footprint compact lightweight 7–15 MHz linear array probe allows complete contact with the skin surface. This contact reduces scattering artifacts and improves access to mobile structures such as the tongue, or small appendages such as children’s fingers (Fig. 4.7). “Hockey stick”-shape transducers make a good choice for superficial and vascular imaging. High-resolution linear transducers with combinations of variable (tunable) frequencies (6–18 MHz) can define lesions in the submillimeter range down to 0.1 mm and may reach tissue depths of 60 mm. Transducers using frequencies of 15 MHz or above can clearly define skin layer morphology including changes in epidermal thickness. Variable (tunable)-frequency ultrasound (HVFUS) transducers allow a complete view of the skin and the deeper structures (muscles, tendons, and bone margins) with minimal dispersion of the sound energy waves by selection of the transmission frequency and adjustment of focal length at a selected depth (Fig. 4.8). Frequencies in the upper range (14–15 MHz) are used to demonstrate the superficial and dermal layers, while frequencies in the lower range (7–13 MHz) are for imaging the deeper tissues. The resulting real-time composite imaging integrates the effects of frequency-specific tissue reflectance and full-field automatic scanning. The images produced are sharp. By applying Doppler ultrasound, estimation of blood flow is provided [3,4,5,6,7]. Fixed (not variable) high-frequency ultrasound transducers are activated at a single high operating frequency (20–100 MHz) that determines the resolution and depth of penetration: 6–7 mm at 20 MHz and 3 mm at 75 MHz. Most lesions examined in skin ultrasound involve subepidermal structures, which would be out of reach for devices of low penetrating power that cannot show deep subcutaneous tissues. Images produced by fixed high-frequency ultrasound transducers are static and lack blood flow and vascular pattern information [6]. The spatial resolution of Magnetic resonance imaging (MRI) is dependent on matrix size, field of view, and slice thickness, whereas the spatial resolution of positron emission/computed tomography (PET-CT) generally is limited in comparison to small-parts US imaging. Both MRI and PET-CT typically use an intravenously administered contrast material that renders them more expensive [8]. Thus, among all the available imaging techniques, variable-frequency ultrasound (HVFUS) has favorable spatial resolution, ideal focal depth, and lesser cost, thereby empowering high-quality images of the skin layers, subcutaneous fat, tendons, muscles, and bone (Fig. 4.9). Skin ultrasound images are cross-sectional views of the scanned areas in grayscale images that show the location, morphology, size, and echogenicity of normal and pathological structures.
“Hockey stick” transducer. Versatile multifrequency (up to 13 MHz) linear compact, hockey stick-shape transducer, with a high Doppler and color flow sensitivity. The “hockey stick” shape allows complete contact with the skin surface, reducing scattering artifacts, making it a good choice for small superficial structures, including vascular imaging
Gain, depth, and focal length adjustments on grayscale image. Axial scan at the level of the wrist. (a) Focal length is adjusted at the area of interest: vertical line on the left side of the image, a correct depth (2.5 cm penetration in this scan), and gain are selected, for imaging of the skin and deeper structures. (b) Incorrect depth selection, with depth. (c) Too much depth. (d) Incorrect gain selection, with too little gain. (e) Too much gain
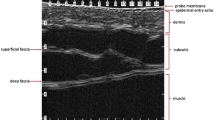
High-resolution image of skin and deeper layers. Focus is adjusted at the superficial level (vertical line on the left side of the image) to allow better definition of the skin. A generous amount of gel (G) over the skin is recommended in order to avoid near-field artifacts. A clear separation of skin layers (S) is shown. The epidermis is seen as a thin hyperechoic line and the dermis as a hyperechoic band of variable thickness (thin in the forearm and thicker in the lumbar region, due to a high collagen content). The subcutaneous tissue (SC) is hypoechoic, generated by fat lobules, which are surrounded by hyperechoic fibrous septa. Muscles (M) are hypoechogenic, with parallel echogenic lines. The bone margin (B) appears as a distinct hyperechoic line
Software Developments
Modern ultrasound equipment includes capabilities for harmonic imaging, compound imaging, extended field of view (EFOV), elastography, 3D ultrasound, contrast-enhanced ultrasound (CEUS), microvascularity, and fusion imaging.
Tissue Harmonic Imaging
Harmonics are multiples of the fundamental transmitted frequency. Transmitting a band of frequencies centered at 3 MHz will result in the production of harmonic frequency bands centered at 6, 9, 12 MHz, etc. The generated images exhibit reduced noise and improved spatial resolution [9, 10] (Fig. 4.10). The second harmonic is being used to produce the image because the subsequent harmonics are of are insufficient to generate a proper image. Harmonics reduce imaging artifacts caused by the interaction of the ultrasound beam with superficial structures or due to aberrations at the edges of the beam profile. The artifact-producing signals are of low energy and insufficient to generate harmonic frequencies.
Tissue harmonic imaging at the wrist. (a) Fundamental scan, without applying any software development. Radial vessels (straight arrows) and median nerve (oblique arrow). (b) Harmonic imaging reduces near-field artifacts caused by the interaction of the ultrasound beam with superficial structures. The generated images exhibit reduced noise and improved spatial resolution. Sharper imaging of radial vessels (straight arrows) and median nerve (oblique arrow)
Spatial Compounding Imaging
Spatial compounding obtains information from several different angles of insonation, combining them into a single image. Compound imaging generates higher quality images by improving border definition and increasing signal-to-noise ratio by reducing artifacts generated by the scattering of ultrasound from small tissue reflectors (speckle) [11] (Fig. 4.11).
Spatial compounding imaging. Compound imaging of the wrist. Compounding significantly improves contrast-to-noise ratio by reducing artifacts generated by the scattering of ultrasound from small tissue reflectors (speckle). Improved imaging of radial vessels (wide straight arrows) and median nerve (thin oblique arrow), as compared to fundamental image in Fig. 4.10a at same level
Extended Field of View (EFOV)
EFOV allows acquisition and display of a panoramic images offering the possibility of viewing topographic anatomic structures with no loss in resolution [12]. EFOV applies a sophisticated computer processing algorithm that analyzes multiple real-time images to produce a static image of considerable length (Figs. 4.12 and 4.13).
Extended field of view (EFOV). An oval lesion in the subcutaneous tissue, almost 8 cm long (cursors), is compatible with a subcutaneous lipoma. EFOV or panoramic view allows acquisition and display of large structures and the topographic anatomic relationships with the surrounding tissues with no loss in resolution
Three-Dimensional (3D) Imaging
3D imaging permits volume data to be displayed in multiple planes and allows accurate measurement of lesion volume. 3D imaging when combined with power Doppler or contrast-enhanced imaging improves anatomic and vascular visualization [13] (Fig. 4.14).
Three-dimensional (3D) imaging. Infantile hemangioma of the lower lip. 3D reconstruction of a power Doppler ultrasound highlighting the rich vascularity of the lesion. Images may also be displayed in separate planes. 3D imaging combined with either B-mode, power Doppler mode, or nonlinear contrast imaging allows for quantification of volume and vascularity within a defined anatomical structure (courtesy of Dr. Ximena Wortsman)
Elastography
Elastography maps the elastic properties and stiffness of the tissue that can indicate the presence or status of disease. Malignant tumors will often be stiffer than the surrounding tissue, as are diseased organs compared to healthy ones. Fibrosis is another example of stiff tissues. Ultrasound elastography produces a stiffness map with an anatomical image for comparison [14, 15] (Fig. 4.15).
Contrast-Enhanced Ultrasound
The introduction of microbubble contrast injection for ultrasound (CEUS) redefined the role of ultrasound in resolving tissue vascularity [16,17,18,19,20] (Fig. 4.16). CEUS can help delineate small vascular structures (0.1–0.3 mm in diameter) and enhance Doppler signals from small volumes with low-velocity blood flow. Ultrasound contrast media aids in characterizing the vascularity of benign and malignant tumors. After performing a conventional grayscale and Doppler scan, the mode is changed to contrast-enhanced imaging with intravenous injection of a signal-enhancing agent. One of the major features of malignancy is the degree of intratumoral vascularity as measured by the percentage vessel area (p.v.a.) of the lesions. The p.v.a. is defined as the ratio of intratumoral vessel area compared to the “gray” parts of the tumor. A significant difference in vascularity was found between malignant and benign tumors (p = 0.01). The mean p.v.a. of malignant tumors was 9.6% before and 18.8% on CEUS. The mean p.v.a. of benign tumors was 1.1% and 3.3%, respectively. Analysis of tumor vascularity using the p.v.a. after injection of the microbubble agent was superior to B-mode, spectral Doppler ultrasound, and flow indices. When combined with 3D US, nonlinear contrast agent imaging is a valuable tool for relative vascularity and perfusion quantification [18]. Contrast-enhanced ultrasound is regarded as safe. At the tiome of this writing, Ultrasound contrast agents have safety comparable the safety MRI contrast agents with a better safety profile than typical iodinated contrast agents that are used in CT scans.
Contrast enhancement ultrasound imaging (CEUS). Hemangioma in a child at the parietal region. A bolus injection (4 mL) of a microbubble contrast agent was injected into a peripheral vein, followed by a “flush” of 10 mL of saline. CEUS helps delineate small vascular structures (0.1–0.3 mm in diameter) and enhance signals from low-velocity small volumes of blood (courtesy of Dr. Marcio Bouer)
Microvascularity
Microvascular software such as superb microvascular imaging (SMI) is a recently developed ultrasound imaging application that aims to visualize low-velocity and small-diameter blood vessel flow [21]. SMI achieves a greater sensitivity than color and power Doppler by suppressing noise caused by motion artifacts without removing the weak signals from low-velocity flow (Fig. 4.17).
Fusion Imaging
Performing fusion of real-time ultrasound with reference series of CT, MRI, or PET examinations using simultaneous live navigation enables direct anatomic and pathologic correlation between both modalities [22,23,24,25]. Fusion imaging is useful for the interpretation of complex cases and for ultrasound guidance of procedures (Fig. 4.18).
Fusion imaging. Co-registration of aortic aneurysm between ultrasound and computerized tomography angiography. Ultrasound (left plot) registered simultaneously with computed tomography (right plot). (a) Aneurysm (arrows) on axial plane. (b) Aneurysm (arrows) on sagittal plane. A better estimation of a fusiform aneurysm may be achieved on fusion imaging, hence opening a possibility for surveillance by ultrasound examinations, reducing repeated exposure to radiation and contrast material
Operator Performance
US examination is largely operator dependent. The quality of the obtained information depends mostly on the skill and experience of the examiner. Optimal adjustment of the technical settings such as depth, focal length, and gain is needed for every ultrasound scan (Fig. 4.8). Color Doppler and spectral Doppler settings have to be adjusted according to flow velocity (Figs. 4.3 and 4.4). A generous amount of gel on the skin is recommended to avoid near-field artifacts (Fig. 4.9). The operator should avoid compressing vigorously with the transducer, which may result in disappearing or false thinning of lesions. Ultrasound examinations should be recorded and reported, preferably using a standardized ultrasound report template [26]. The EFSUMB (European Federation of Societies for Ultrasound in Medicine and Biology) Steering Committee for Dermatologic Ultrasound has developed a series of consensus position statements regarding technical and practice-training requirements [27]. To arrive at the correct ultrasound diagnosis, the operator should have information on the clinical setting and the clinical question. Besides high-quality equipment, knowledge of basic ultrasound physics, facility with ultrasound technology, and the operator’s familiarity with the patient’s clinical picture essential to produce a high-quality exploration and a correct diagnosis. Ultrasound can be used to guide the performance of procedures like tissue biopsy, drainage of fluid collections, foreign-body extractions, and demonstrations of biopsy needle positions [28,29,30] (Figs. 4.19 and 4.20). Success of US-guided procedures is widely dependent on the clinical expertise and training of the operator. Standardization of ultrasound imaging technique is advisable to produce a correct and reproducible examination [31].
US-guided biopsy. Ultrasound-guided biopsy of a slightly enlarged cervical lymph node at level IV—lower internal jugular (deep cervical) chain. The needle shaft (wide arrow) and needle tip (thin arrow) are perfectly delineated as a highly echogenic line into the target. Note the proximity of the tip to the common carotid artery (at the left) and the subclavian vein (posteriorly)
Future Directions
The increasingly wide usage of ultrasound contrast material will advance the increasingly important role of ultrasound for imaging diagnosis and interventions. The implementation of ultrasound imaging contrast materials to not only reveal tumors but also deliver a payload of therapeutic agents enables a theranostic approach. Thus, ultrasound can not only “find it,” but also “fix it” [32]. Artificial intelligence with machine learning using big data, fast processing, and smart algorithms offers segmentation, feature analysis, and classification of findings that is becoming automated such that unsupervised computerized deep learning is now a reality. No doubt this will soon become mainstream, including for ultrasonography [33]. The increasing use of telesonography, especially during the current Coronavirus pandemic, will likely also persist long after the infection is controlled, with further potential as a mechanism for remote transmission and interpretation of ultrasound images for urgent and emergent indications, but potentially also for enhanced home care [34].
Tips and Teaching Points
-
1.
Ultrasound examinations are operator dependent. Optimal performance of an US scan requires live adjustment of B-mode and Doppler technical settings.
-
2.
A large amount of gel on the skin should be spread. It is advisable to avoid over-compression with the transducer. Knowledge of the clinical setting helps to produce a correct diagnosis.
-
3.
High variable-frequency ultrasound transducers (HVFUS) with Doppler capability allow excellent resolution (as low as 0.1 mm), although with low penetration, clearly defining skin layers and deeper structures, and showing vascularization patterns and flow velocity.
-
4.
Power Doppler is more sensitive for low-velocity flow and thus better for poorly vascularized or low-velocity flow lesions, but lacks information on flow direction or flow velocity.
-
5.
US-guided procedures are widely used for biopsies, drainages, foreign-body extractions, and needle insertions.
Useful Glossary of Ultrasound
Transducer: Device that converts one form of energy into another. In ultrasound, the transducer converts electric energy provided by the transmitter to mechanical energy (acoustic pulses) and vice versa, as reflected echoes are converted to electric signals. Transducers are made of thin piezoelectric crystals that expand and contract to generate acoustic vibrations (frequencies).
Resolution: The ability to separate two points along the path of the ultrasound beam, which are in close proximity (spatial resolution) or have similar echogenicity (contrast resolution). Axial resolution is the smallest thickness, while lateral resolution is the smallest width that can be resolved. Higher ultrasound frequencies raise the spatial resolution, at the cost of lowering the depth of penetration into the tissues.
Artifacts: Imaging pitfalls that may interfere with or completely obscure visibility of the intended target or suggest the presence of false structures that are not actually present. Examples of artifacts suggesting structures that are not actually present are reverberation, refraction, and side lobe artifacts.
-
(a)
Reverberation artifacts are due to repeated reflection of the ultrasound signal between highly reflective interfaces that are usually located near the transducer.
-
(b)
Refraction artifacts are due to bending of the sound beam causing targets that are not situated along the axis of the transducer to appear in a misleading location.
-
(c)
Side lobe artifacts are due to a strong out-of-plane reflector, generating confusing echoes.
-
(d)
Posterior acoustic shadowing artifact is due to total reflection of the US waves by a strong reflector that causes loss of information from the tissues deep to the reflecting structure. It is usually seen in the calcified or osseous structures.
-
(e)
Posterior acoustic enhancement or reinforcement artifact is due to more rapid passage of the sound waves through a fluid-filled structure. In the screen, it appears as a white band underneath the lesion and is commonly seen in cysts.
-
(f)
Snowstorm artifact is a diffuse reverberation artifact usually generated by the presence of silicone oil (synthetic filler).
-
(g)
Mini-comet tail artifact is a focal reverberation artifact commonly produced by the presence of polymethylmethacrylate (synthetic filler).
Grayscale Image: Referred to as B (brightness)-mode, in which tissues of interest are depicted in the scale of grays of variable brightness, from black (anechoic) and different grades of gray (hypoechoic) to white (hyperechoic). The formation of a B-mode image relies on the pulse-echo principle (i.e., assuming that the speed of sound remains constant, the position of a target of interest may be inferred by the time taken from emission to its return to the transducer).
Echogenicity: It is related to the echo amplitude and brightness of the image compared to the surrounding tissue. Isoechoic: Reflected signals (echoes) of the same intensity or amplitude as the surrounding tissue. The lesion is detected only if it has defined margins.
-
(a)
Hypoechoic: Reflected signals (echoes) of intermediate intensity or amplitude, generating images of low brightness in different shades of gray. Examples of hypoechoic tissues are liver, spleen, renal parenchyma, and muscles.
-
(b)
Hyperechoic: Reflected signals (echoes) of high intensity or amplitude, generating images of high brightness (white). Examples of hyperechoic tissues are fat, fibrous tissues and bones. Examples of hyperechoic structures are foreign bodies and calcified stones.
-
(c)
Anechoic: Echo-free or sonolucent: Absence of reflected signals (echoes), generating images in black. Examples of anechoic targets are normal vessels, gallbladder, urine in the bladder, and cysts.
Real Time: A moving cross-sectional image generated by sequential summation of a multitude of neighboring scan lines, generating 2D images at a rate of 15–60 frames per second.
Focus: Beam focusing refers to creating a narrow point in the cross section of the ultrasound beam called the focal point. It is at the focal point that the lateral resolution of the beam is the greatest. Focus places the ultrasound beam on the area of interest.
Doppler Effect: Change in frequency of the sound when scattered by a moving target. The Doppler frequency shift is described by the Doppler equation:
ΔF: Doppler frequency shift.FR: frequency of sound reflected from the moving target.FT: frequency of sound emitted from the transducer.V: target velocity.c: sound velocity in the medium.θ: angle between the flow axis and the incident ultrasound beam (angle of insonation). This angle must be kept at 60° or less.
Pulsatility Index (PI): Tissue resistance to flow at each complete cardiac cycle. It is calculated as PI = PSV-EDV/mean velocity.
Resistive Index (RI): Tissue resistance to blood flow at systole and diastole. It is calculated as RI = PSV–EDV/PSV.
Harmonics: Multiples or harmonic echoes of the transmitted fundamental frequency generated by the acoustical pulse as it travels within tissues.
Compounding: Image resulting from summing ultrasound images obtained from different scanning angles.
Extended Field of View (EFOV): Panoramic image generated by manual movement of a real-time probe in the direction of the transducer array. Imaging-processing technology estimates translation and rotation of the probe by comparing successive images. The EFOV image buffer combines the images to produce a panoramic image.
3D Ultrasound: Tissue volume acquired by dedicated 3D transducers employing hardware-based image registration, high-density 2D arrays, or software registration of scan planes.
Elastography: Modern method that uses sound waves for assessing stiffness and elasticity of tissues in response to mechanical pressure. It allows detection of different pathologies by using differences in mechanical properties of tissues. Elastography techniques include shear wave or transient elastography and strain elastography, also called static or compression elastography.
Contrast-Enhanced Ultrasound Agents: Intravascular blood pool agents comprised of encapsulated microbubbles of gas, smaller than red blood cells, capable of circulating freely (“blood pool” agents). CEUS seeks to enhance the echo amplitude by increasing the backscatter from moving red cells while increasing attenuation from the static tissue.
Superb Microvascularity Imaging (SMI): A novel technique to demonstrate flow in small vessels. SMI achieves a greater sensitivity than color and power Doppler by suppressing noise caused by motion artifacts without removing the weak signals arising from blood flow into small vessels.
Fusion Imaging: Co-registered display of live ultrasound with a reference series from another modality, such as CT, MRI, or PET. In a simultaneous live navigation, reformatted planes from the reference series are generated, matching the ultrasound imaging planes. Images are displayed either as an overlay or side by side.
Conclusion
Grayscale sonography with color and spectral Doppler capabilities is a real-time noninvasive imaging technique used as a first-line diagnostic tool in the clinical evaluation of many conditions. Sonography does not require administration of intravenous contrast media and has no ionizing radiation. It is noninvasive, widely available, and of low cost. It provides valuable information about lesion characteristics, extent of disease, and vascularity patterns. Measurements in US images have a good correlation with pathology. US-guided procedures are widely used for biopsies, drainages, foreign-body extractions, and biopsy needle insertions. Preoperative imaging aids in surgical planning by identifying the anatomical location and extent of a lesion and the presence of subclinical satellite lesions. Follow-up ultrasound examinations allow monitoring of surgical results, especially for suspected recurrent tumor or in cases with persistent pain. Sonographic monitoring of lesions following medical treatment allows an objective verification of results and adjustment of treatment if necessary.
There are few limitations of the ultrasound technique that mostly relate to its lack of sensitivity to detect extremely thin (<0.1 mm) lesions. The technique is operator dependent (training is needed). Optimal adjustment of B-mode and Doppler technical settings must be made while performing ultrasound examinations.
References
Merrit CRB. Physics of ultrasound. In: Rumack CM, editor. Diagnostic ultrasound. 5th ed. Philadelphia, PA: Elsevier; 2018. p. 1–33.
Kremkau FW. Doppler principles. Semin Roentgenol. 1992;27(1):6–16.
Wortsman X, Wortsman J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J Am Acad Dermatol. 2010;62:247–56.
Lassau N, Spatz A, Avril MF, et al. Value of high-frequency US for preoperative assessment of skin tumors. Radiographics. 1997;17:1559–651.
Cammarota T, Pinto F, Magliaro A, Sarno A. Current uses of diagnostic high-frequency US in dermatology. Eur J Radiol. 1998;27:215–23.
Szymanska E, Nowicki A, Mlosek K, et al. Skin imaging with high frequency ultrasound: preliminary results. Eur J Ultrasound. 2000;12:9–16.
Shung KK. High frequency ultrasonic imaging. J Med Ultrasound. 2009;17:25–30.
Antoch G, Vogt FM, Freudenberg LS, Nazaradeh F, Goehde SC, Barkhausen J, et al. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA. 2003;290:3199–206.
Hedrick WR, Metzger L. Tissue harmonic imaging: a review. J Diagn Med Sonogr. 2005;21:183–9.
Choudhry S, Gorman B, Charboneau JW, et-al. Comparison of tissue harmonic imaging with conventional US in abdominal disease. Radiographics. 2000;20(4):1127–35.
Wortsman XC, Holm EA, Wulf HC, Jemec GBE. Real-time spatial compound ultrasound imaging of skin. Skin Res Technol. 2004;10:23–31.
Weng L, Tirumalai AP, Lowery CM, et al. US extended-field-of-view imaging technology. Radiology. 1997;203:877–80.
Prager RW, Ijaz UZ, Gee AH, Treece GM. Three-dimensional ultrasound imaging. Proc Inst Mech Eng H. 2010;224(2):193–223.
Sarvazyan A, Hall TJ, Urban MW, Fatemi M, Aglyamov SR, Garra BS. Overview of elastography–an emerging branch of medical imaging. Curr Med Imaging Rev. 2011;7:255–82.
Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. 2013;94(5):487–95.
Burns PN. Contrast agents for ultrasound. In: Rumack CM, editor. Diagnostic ultrasound. 5th ed. Philadelphia, PA: Elsevier; 2018. p. 53–73.
Wilson Deham SL, Alexander LF, Robbin ML. Contrast- enhanced ultrasound: practical review for the assessment of hepatic and renal lesions. Ultrasound Q. 2016;32:116–25.
Schroder RJ, Maurer J, Zlowodski M, et al. Vascularization of malignant and benign skin tumors measured by D-galactose-based signal enhanced colour Doppler sonography. Acta Radiol. 2001;42:294–301.
Hoogi A, Adam D, Hoffman A, Kerner H, Reisner S, Gaitini D. Carotid plaque vulnerability: quantification of neovascularization on contrast-enhanced ultrasound with histopathologic correlation. Am J Roentgenol. 2011;196:431–6.
Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (long version). Ultraschall Med. 2018;39:e2–e44.
Zhan J, Diao XH, Jin JM, et al. Superb microvascular imaging- a new vascular detecting ultrasonographic technique for avascular breast masses. A preliminary study. Eur J Radiol. 2016;85:915–21.
Stoll J. Ultrasound fusion imaging. Perspect Med. 2012;1:80–1.
Ahn SJ, Lee JM, Lee DH, et al. Real time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2017;66:347–54.
Bercovich E, Leiderman M, Beck-Razi N, Gaitini D, Javitt M. Ultrasound–unenhanced CT fusion for detection and localization of ureteral stones. Am J Roentgenol. 2018;210:W8–W11.
Zur G, Andraous M, Bercovich E, Litvin M, Ofer A, Gaitini D, Javitt M. CT ultrasound fusion for abdominal aortic aneurysm measurement. Am J Roentgenol. 2020;214:472–6.
Wortsman X, Alfageme F, Roustan G, Arias-Santiago S, Martorell A, Catalano C, Scotto di Santolo M, Zarchi K, Bouer M, Gonzalez C, Bard R, Mandava A, Gaitini D. Guidelines for performing dermatologic ultrasound examinations by the DERMUS group. J Ultrasound Med. 2006;35:577–80.
Alfageme F, Wortsman X, Catalano O, et al. European federation of societies for ultrasound in medicine and biology (EFSUMB) position statement on dermatologic ultrasound. Ultraschall Med. 2020 7. Online ahead of print. https://doi.org/10.1055/a-1161-8872.
Dogra VS, Saad WEA. Ultrasound-guided procedures. New York: Thieme; 2009.
AIUM Practice parameters for the performance of selected ultrasound-guided procedures. 2014. https://www.aium.org/resources/guidelines/usGuidedProcedures.pdf
Barr L, Hatch N, Roque PJ, Wu TS. Basic ultrasound-guided procedures. Crit Care Clin. 2014;30:275–304.
European Society of Radiology (ESR), Clevert DA, Nyhsen C, et al. Position statement and best practice recommendations on the imaging use of ultrasound from the European Society of Radiology ultrasound subcommittee. Insights Imaging. 2020;11:115.
Zullino S, Argenziano M, Stura I, Guiot C, Cavalli R. From micro- to nano-multifunctional theranostic platform: effective ultrasound imaging is not just a matter of scale. Mol Imaging. 2018;17 https://doi.org/10.1177/1536012118778216.
U.S. Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)-based software as a medical device (SaMD). Discussion paper and request for feedback. https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device. Content current as of: 01/12/2021. Accessed 8 Mar 2021.
Pian, L., Gillman, L. M., McBeth, P. B., Xiao, Z., Ball, C. G., Blaivas, M., Hamilton, D. R., & Kirkpatrick, A. W. Potential use of remote telesonography as a transformational technology in underresourced and/or remote settings. Emerg Med Int. 2013;2013, 986160. https://doi.org/10.1155/2013/986160. Accessed 8 Mar 2021.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gaitini, D., Ullmann, Y., Javitt, M. (2022). Ultrasound Imaging: Basic Principles and Terminology. In: Wortsman, X. (eds) Textbook of Dermatologic Ultrasound. Springer, Cham. https://doi.org/10.1007/978-3-031-08736-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-08736-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-08735-6
Online ISBN: 978-3-031-08736-3
eBook Packages: MedicineMedicine (R0)