Abstract
Stress-response kinases, the mitogen-activated protein kinases (MAPKs), are activated in response to the challenge of a myriad of stressors. c-jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and MAPK p38 are the important members of the MAPK family in the heart. Extensive studies have revealed critical roles of activated MAPKs in the processes of cardiac injury, cardiac arrhythmias, heart failure, and other cardiovascular diseases. Advancing our understanding regarding the functional impacts of MAPKs in the development of heart diseases could shed new light on developing novel therapeutic approaches to improve cardiac function and prevent arrhythmia development in patients. This chapter summarizes relevant current knowledge on the pivotal roles of MAPKs in physiopathological and molecular remodeling in cardiac myocytes during the disease development and for the therapeutic potentials of developing MAPK inhibitors and/or activators.

Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mitogen-activated protein kinases
- Stress
- Arrhythmia
- Heart failure
- Calcium homeostasis
- Cardiovascular diseases
Introduction
The heart exhibits enhanced cellular stressors given its high level of energy consumption and output (i.e., oxidative stress, inflammatory stress, senescence stress) and with increasing age and cardiovascular diseases (CVDs), which leads to a higher susceptibility to additional extrinsic stress stimuli (i.e., ischemia, inflammation, excessive alcohol exposure, obesity) [1,2,3,4,5,6,7,8,9]. The mitogen-activated protein kinases (MAPKs) are stress kinases that are activated in response to both intrinsic and extrinsic stress challenges and critically regulate cell survival and growth. Over the years, MAPK activation has been found to be critical in the development of various diseases such as diabetes, cancer, Alzheimer’s disease, as well as various cardiac diseases such as cardiac hypertrophy, heart failure, and atrial fibrillation [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Among ~500 recognized protein kinase genes in the human genome, the MAPK family is composed of three major members, c-jun NH2-terminal kinases (JNK), p38 mitogen-activated protein (MAP) kinase (p38), and extracellular signal-regulated kinases (ERK1/2). These three subfamilies have been the focus of extensive studies to uncover their contributions to pathological cardiac remodeling and disease development [10, 14,15,16,17,18,19,20,21,22,23,24,25,26,27].
Stress Kinase MAPK Signaling in the Heart
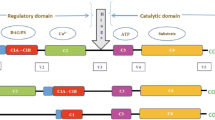
MAPKs are serine/threonine kinases that phosphorylate serine or threonine residues in a consensus sequence of Pro-X-Thr/Ser-Pro on the target protein [28]. The canonical pathways leading to MAPK activation require a kinase cascade in which MAP kinase kinases phosphorylate MAPKs that in turn activate MAPKs. The MAPK cascade controls the activity of downstream target proteins including numerous transcription factors, through the regulation of binding partners, protein conformational changes, protein stability, and subcellular localization [29].
JNK
JNK is an important member of the MAPK family that is activated in response to various stress challenges and regulates cell proliferation, differentiation, apoptosis, autophagy, cell survival, cell mobility, and cytokine production [10, 21, 28, 30,31,32]. The JNK kinase family was discovered in the early 1990s by Kyriakis and Avruch and reported as a novel protein named as pp54 MAP-2 kinase, which is activated by phosphorylation of the amino acid residues of threonine-183 and tyrosine-185 [33, 34]. Next, two isoforms were identified with molecular weights of 46 and 56 kDa and were named JNK1 and JNK2, respectively [35]. Later, it was revealed that these JNK kinases could be activated by various extracellular stimuli. Given that these JNKs contained a threonine-proline-tyrosine phosphorylation motif (TPY), they were characterized as a member of the MAPK family. Finally, JNK3 was discovered in 1995 as the last member of this MAPK subfamily and is mainly expressed in neurons [10, 22, 36, 37]. In the heart, JNK1 and JNK2 are the major isoforms, while JNK3 is expressed at a much lower level [24, 38].
JNKs function within a protein kinase cascade and are activated by dual phosphorylation of on the specific threonine-X-tyrosine motif by the upstream kinases MKK4 and MKK7 in response to stress challenges [21, 28, 31, 39]. JNK itself is a Ser/Thr kinase that phosphorylates its substrates on serine or threonine residues in a consensus sequence of Pro-X-Thr/Ser-Pro [28]. Numerous JNK substrates have been identified and include but are not limited to Ca2+/calmodulin-dependent protein kinase II δ (CaMKIIδ), c-jun, Jun-B, ATF2, c-Myc, and p53 [28, 40]. JNK activation has been observed with aging, excessive binge alcohol-triggered “holiday heart syndrome,” and with CVDs such as myocardial ischemic injury and heart failure. Accumulating evidence suggests that JNK signaling is critically involved in the development of diabetes, atrial fibrillation, and other CVDs such as heart failure (HF), ischemic myocardial infarction (MI), and atherosclerosis [9, 10, 20,21,22, 30, 41,42,43,44].
P38
p38 MAP kinase, also referred to as RK, p40, or CSBP2 (cytokinin-specific-binding protein 2), is also a member of the MAPK family and is ubiquitously expressed in all somatic cell types [45]. Although p38 participates in signaling cascades controlling cellular responses to cytokines and other stress stimuli, the function of the p38 kinases appears to be both protective and deleterious in the stressed heart [46]. The p38 was initially found as an unidentified 38-kDa protein, which exhibited increased phosphorylation of tyrosine residues by lipopolysaccharide [47]. Later, it was discovered that there are four identified genes of the p38 MAPK: p38α, p38β, p38γ, and p38δ. P38α shares sequence homology with p38β (~75%), p38γ (~62%), and p38δ (~61%), while p38γ and p38δ are ~70% identical [48, 49]. While the heart expresses all the sub-isoforms, p38α is the most abundant, followed by p38γ [50,51,52]. The canonical activation of p38 MAPK was found to be achieved through dual phosphorylation of the threonine (Thr)-glycine (Gly)-tyrosine(Tyr) motif in its activation loop [53]. The phosphorylation of this threonine-X-tyrosine motif of p38 in vivo is predominantly mediated by upstream MAPK kinases, MKK3 and MKK6, which are activated by MAPK kinase kinases such as TAK1, ASK1, DLK, and mitogen-kinase protein kinase kinase kinase 4 (MEKK4) [54,55,56,57]. This cascade can be instigated by one of multiple MKK-activating MAP3Ks in a stimulus-/stress-dependent manner relevant for specific cell type. Studies suggest that the different isoforms require differential activation of MAPK kinases for full activation, one such example being p38α which requires both MKK6 and MKK3 activation to be phosphorylated in response to cytokines, while p38δ is activated by MKK6, but negatively regulated by MKK3 [49]. In addition, low-molecular-weight GTP-binding proteins in the Rho family (i.e., Rac1, Cdc42, RhoA, and RhoF (Rif)) and heterotrimeric G-protein-coupled receptors could activate p38 [58,59,60]. Furthermore, the activity of p38 can also be modulated by dual-specificity phosphatases, such as PAC-1 and MKP-1 [53]. Finally, the autoactivation of p38, as a noncanonical mode of p38 activation, was observed through the activation of scaffolding proteins such as TAB1 along with the “priming” phosphorylation of Tyr-323 by a tyrosine kinase of the SYK family [61,62,63].
ERK
The ERK family, another MAPK member group, can be divided into five subfamilies named ERK1–ERK5. ERK1 and ERK2 share 90% homology and thus are usually referred to as ERK1/2 [64, 65]. And ERK1 and ERK2 (44kDa and 42kDa, respectively [66, 67]) are the most thoroughly investigated isoforms in the ERK family. The basic ERK signal transduction cascade has been shown to follow the typical MAPK cascade reaction (Ras-Raf-MEK-ERK pathway). ERK1/2 is activated by MEK1/2-mediated phosphorylation at Thr-183 and Tyr-185 [68]. This dual-site phosphorylation enhances the ERK1/2 activity by >1000 folds [68, 69]. ERK can also be activated by tyrosine kinase receptors and Gi/o-, Gq-, and Gs-coupled receptors via a range of different signaling pathways [70,71,72,73,74,75]. One of the best characterized MKKKs to activate ERK is Raf-1, a Ser/Thr protein kinase [76], which binds directly to activated GTP-bound Ras leading to a full activation of MKKK [77]. Once it is fully activated, Raf-1 phosphorylates and activates MKK1 or MKK2. MKK1/2, which in turn phosphorylates ERK1 or ERK2 (ERK1/2) on the Thr-X-Tyr motif in its activation loop, leads to ERK activation. Like JNK and p38, ERK is a Thr/serine (Ser) kinase that is normally located in the cytoplasm and translocates into the nucleus when it is activated [29, 78]. Fully activated ERKs phosphorylate a wide spectrum of substrates with a general amino acid consensus sequence of proline (Pro)-X-Ser/Thr-Pro that can be localized at the plasma membrane, in the cytosol and the nucleus that regulate important aspects of cell physiology including cell proliferation, differentiation, adhesion, migration, and survival [79,80,81,82,83].
MAPKs and Calcium Homeostasis in Myocytes
One of the hallmarks of a diseased heart is an altered protein phosphorylation state that critically contributes to ion transporter and channel dysfunctions leading to the disruption of Ca2+ homeostasis in response to both intrinsic and extrinsic stress stimuli in the heart [10, 20,21,, 21, 22, 28, 31, 131, 132]. Calcium (Ca2+) is an important cation in the conversion of an electrical signal to mechanical function (termed as excitation-contraction coupling) during each heartbeat to maintain normal cardiac function and is also important in the cellular signal transduction pathways that control myocyte survival and growth [84,85,86,87,88,89,90,91]. Excitation-contraction coupling (ECC) is an essential link between myocyte excitation (membrane depolarization of the action potential) and Ca2+ release from the sarcoplasmic reticulum (SR) for myocyte contraction, and this series of events is critical in the beat-to-beat cardiac muscle contraction and relaxation.
Normal and Abnormal Calcium (Ca2+) Signaling in Myocytes
The cardiac action potential occurs when myocyte membrane potential is depolarized upon the initiation of a rapid sodium influx (INa) followed by Ca2+ influx (ICa), while repolarization occurs by the outward potassium current (Ik). During systole in normal cardiomyocytes, the Ca2+ entry via L-type Ca2+ channels (LTCCs) along with a much smaller amount of Ca2+ influx via the Na+ /Ca2+ exchanger (NCX) activates ryanodine receptor channels (RyR2) on the SR membrane to release a large amount of SR stored Ca2+. The LTCCs located in the plasma membrane are activated by the rapid sodium influx (INa) and depolarization of the myocyte membrane [92,93,94,95,96,97]. A small amount of inward Ca2+ flux (ICa) through activated LTCCs triggers large quantities of Ca2+ to be released from the SR via cardiac ryanodine receptor type 2 (RyR2; also called Ca2+-triggered SR Ca2+ release channels) to produce a large intracellular Ca2+ ([Ca2+]i) transient, driving myocyte contraction [89, 98,99,100]. This Ca2+-induced Ca2+ release (CICR) event occurs locally within the clusters of RyR2 channels on the SR membrane that are in close proximity to LTCCs located on the plasma membrane [101, 102]. CICR is further facilitated by dyads, which are the structures consisting of terminal cisternae of SR, composed of clusters of RyR2 channels, paired with transverse tubules (T-tubules), and LTCCs [103]. Upon action potential arrival at the T-tubule, Ca2+ influx via LTCCs activates RyR2 channels on the cytosolic side of SR allowing for the occurrence of CICR, which activates neighboring RyR2 channels, resulting in a rapidly increased cytosolic Ca2+ [104, 105]. CICR is also the trigger for Ca2+-troponin C binding, leading to myofilament activation and cardiac muscle contraction [106, 107]. During cardiac muscle relaxation, LTCCs close and terminate the influx of Ca2+, and RyR2 channels usually are also closed. Meanwhile, the excess amount of cytosolic Ca2+ is removed mainly through cardiac sarcoplasmic reticulum Ca2+-ATPase 2 (SERCA2) Ca2+ uptake back to the SR and Ca2+ extrusion from the myocyte to the extracellular space through NCX, while another small portion of Ca2+ is taken up by mitochondria via mitochondrial Ca2+ uniporters as well as a small Ca2+ efflux via the plasma membrane Ca2+-ATPase (also known as plasma membrane calcium-/calmodulin-dependent ATPase or PMCA) [93, 98, 108,109,110]. Normal contraction of the heart requires high Ca2+ levels in systole and low levels in diastole [111, 112]. Therefore, SR Ca2+ release via RyR2 channels and reuptake via the predominating Ca2+ pump SERCA2a isoform (SERCA2a) and, to a much lesser extent SERCA2b isoform, critically mediate the cytoplasmic Ca2+ concentration, which is essential in cardiac contraction and relaxation of each heartbeat [87, 90].
Given the tightly regulated role of Ca2+ in ECC, even a small amount of aberrant Ca2+ release resulting from slowly developed pathological changes of the intracellular Ca2+ homeostasis can potentially have escalating negative consequences for the myocyte and ultimately the entire heart. With increasing age and abnormal stressed conditions (e.g., heart failure (HF), ischemia-reperfusion (I/R) injury, myocardial infarction (MI), post-MI, and excessive alcohol exposure), impaired Ca2+ homeostasis causes myocardial molecular remodeling, including aberrant gene expression, myocyte death, electrical and mechanical dysfunctions, contractile dysfunction, and triggered arrhythmic activities [24, 25, 113,114,115,116,117,118,119,120,121,122]. Abnormal Ca2+ dynamics such as reduced SR Ca2+ content via decreased uptake by SERCA2 and increased diastolic SR calcium leak via RyR2 channels are involved in the development of maladaptive cardiac remodeling. The abnormal diastolic SR Ca2+ leak via RyR2 openings may produce large/frequent Ca2+ sparks that may trigger propagating diastolic Ca2+ waves [113, 121, 123]. These aberrant Ca2+ waves result in an excess outward NCX current, which is electrogenic (3 Na+ in, 1 Ca2+ out), and, thus, may produce triggered arrhythmic activities such as delayed afterdepolarization (DAD) that may initiate cardiac arrhythmias [98, 123]. Under certain pathological conditions such as HF, decreased SR Ca2+ refill during the SR Ca2+ cycling in myocytes due to reduced Ca2+ uptake by SERCA2a leads to a reduced Ca2+ transient amplitude and consequently decreased cardiac contractility as seen in the failing heart [124,125,126,127]. In the normal diastolic phase, CICR-mediated SR Ca2+ release shuts off almost completely (∼99%). However, increased diastolic RyR2 channel activity leads to increased diastolic SR Ca2+ leak and further reduced SR Ca content, which results in a reduced systolic fractional Ca2+ release for a given ICa as the release trigger [123, 128, 129]. Meanwhile, this increased diastolic SR Ca2+ leakage along with an impaired SR Ca2+ uptake in HF slows down the intracellular Ca2+ decline and then elevates the amount of diastolic intracellular Ca2+ concentration, which leads to increased Na+ influx via NCX for removing the elevated intracellular Ca2+ outside of the cell membrane. As a result, increased diastolic SR Ca2+ leak promotes aberrant Ca2+ events (Ca2+ sparks and waves), and the inward NCX current produces abnormal triggered activities, DADs, to initiate atrial arrhythmias such as atrial fibrillation (AF) and ventricular arrhythmias including ventricular tachycardia and ventricular fibrillation; fatal types of cardiac arrhythmias [94, 98, 99, 123, 130].
MAPKs in Stress-Evoked Ca2+ Mishandling in Myocytes
JNK is a key member of the MAPK family, which plays a critical role in maladaptive cardiac remodeling [10, 20,21,, 21, 30, 31, 43, 133,134,135]. Although the contributions of JNK1 in cellular apoptosis and proliferation as well as cardiac contractile function have been well studied, the function of JNK2, one of the two major cardiac isoforms, has received significantly less attention [10, 20, 133]. Recently, a causal role of JNK2 in abnormal Ca2+ handling was discovered for the first time in animal models and humans with both binge alcohol exposure and increasing age with preserved cardiac function and no history of cardiac arrhythmias or any major CVDs [20, 24,25,26]. JNK2, but not JNK1, drives a significant diastolic SR Ca2+ leak and a higher SR Ca2+ load at the same time in the stressed myocytes. This JNK2-enhanced SR Ca2+ uptake partially compensates for the greater diastolic SR Ca2+ leak and maintains a normal level of Ca2+ transients and normal cardiac function, while the greater JNK2-driven diastolic SR Ca2+ leak acts as a key contributor to enhanced atrial arrhythmic Ca2+ events and arrhythmia susceptibility [24, 25].
Very recently, JNK2 was identified as a previously unrecognized enhancer of SERCA2 function via the elevation of the maximal rate (Vmax) of SERCA2 activity by phosphorylating SERCA2 protein [24]. The SERCA2 pump activity is known to be regulated by phospholamban (PLB), sarcolipin, myoregulin, striated muscle enriched protein kinase (SPEG) and DWORF (dwarf open reading frame) micropeptide [136,137,138,139]. All those regulators that are known to increase SERCA2 Ca2+ affinity (Km) do not change the Vmax, whereas, JNK2 significantly enhances the Vmax of SERCA2-ATPase activity but not the Km [24, 136,137,138,139]. Intriguingly, JNK2-enhanced SR Ca2+([Ca2+]SR) load by itself (in the absence of CaMKII-dependent RyR2 channel sensitization) may not be sufficient to cause significant diastolic leak, a combined higher load and CaMKII-sensitized RyR2 channels may promote the diastolic leak as previously demonstrated [24, 113, 120, 122, 123]. Thus, JNK2 is an important stress-induced regulator driving to maintain a high SR Ca2+ uptake and load in order to preserve cardiac function, while JNK2 also drives a greater CaMKII-dependent SR Ca2+ leak to promote abnormal triggered activities (discussed in detail below), as seen in both humans and animal models.
The atrial action potential morphology differs from that of the ventricle, where the atrial action potential is generally shorter with a triangular shape due to a smaller Ca2+ influx and a more gradual repolarization period [140, 141]. Ca2+ handling in atrial myocytes while similar to that of ventricular myocytes has some important structural and molecular differences. Atrial myocytes are thinner and longer and exhibit a longer lag time between APs and Ca2+ transients at the center of the cell. This property of the atrial cell contributes to a higher instability of Ca2+ propagation, which is pro-arrhythmogenic [142]. Notably, atrial Ca2+ transient amplitude is smaller, and the rate of intracellular Ca2+ decay is higher than in ventricular myocytes due to a greater SERCA2 uptake and a stronger NCX removal of cytosolic Ca2+ during the diastolic phase [142, 143]. The increased SERCA2-dependent intracellular Ca2+ removal is attributed to a greater amount of SERCA2 protein and lower level of SERCA2 inhibitory protein PLB [142, 143]. This stronger cytosolic Ca2+ removal machinery in the atria leads to a higher SR Ca2+ content than that of ventricular myocytes, which makes atrial myocytes prone to diastolic SR Ca2+ leak when RyR2 is pathologically sensitized and SR Ca2+ overload is increased due to the dual functional impact of stress-activated JNK2 [24, 120, 123, 142, 144,145,146]. Thus, markedly increased JNK2 in atria exposed to stress stimuli (i.e., aging, binge alcohol) is a major contributor to continued pathological remodeling and enhanced AF risk [20, 24,25,26, 120]. T-tubules are a subcellular network involved in SR Ca2+ dynamics by the coupling of LTCCs to RyR2 channels on the SR membrane to allow a rapid intracellular Ca2+-triggered SR Ca2+ release in response to electrical excitation [147,148,149,150]. Previously it was believed that atrial T-tubules were virtually absent in isolated myocytes from small rodents; [151, 152] however, accumulating evidence suggests that the T-tubule network is present in intact atrial myocytes and plays a functional role in atrial myocytes from both large mammalian species and small rodents (including humans, sheep, dogs, cows, and horses, rats, mice) [153,154,155,156,157]. These inconsistent research findings may likely be due to the nature of fast T-tubule structural deformation during myocyte isolation and preparation. However, atrial T-tubular networks are clearly less abundant and less well-organized compared to ventricular. In fact, rapid pacing-induced HF dogs showed reduced atrial T-tubular abundance that was linked to altered subcellular Ca2+ dynamics and AF development [122, 154, 155]. However, the involvement of the stress-response MAPKs in T-tubular remodeling remains unknown to date.
Like JNK, other MAPK members ERK and p38 are also involved in various types of stress-caused cardiac pathogenesis [10, 21, 22, 131, 132, 158]. Although enhanced activity of ERK or p38 alone may or may not be required or sufficient for facilitating cardiac hypertrophy, both ERK and p38 were found to be involved in pathological remodeling and AF development in the failing heart [159,160,161,162,163,164,165]. Hypertrophic stimuli lead to an increase in ICa and downregulation of SERCA2 expression via activated ERK [17,18,19]. While Ras, a GTPase, is able to activate ERK through a Ras-Raf-MEK cascade [166], Ras signaling-activated ERK was found to contribute to downregulation of L-type Ca2+ channels and reduced channel activity along with reduced SERCA2 protein expression in cultured myocytes [15, 16]. Thus, Ras-ERK-modulated molecular remodeling led to decreased intracellular Ca2+ transients and impaired SR Ca2+ uptake, which could lead to enhanced arrhythmogenicity [167]. In both isolated cardiomyocytes and Langendorff-perfused intact hearts, activation of p38 MAPK signaling was found to induce SR Ca2+ overload through enhanced SERCA2 activity and increased SR Ca2+ uptake during cardiac I/R injury, which in turn prompted myocardial apoptosis [168]. Overall, the stress-response MAPK signaling cascades are critically involved in cardiac Ca2+ handling and stress-caused maladaptive cardiac remodeling. However, many questions remain unanswered such as the extent and detailed mechanisms of how the three MAPK members interact and functionally overlap with regards to Ca2+ handling in cardiac myocytes under physiological and stressed conditions.
MAPKs and Ca Handling Proteins in Myocytes
JNK2 was recently recognized as a critical activator and transcriptional regulator of CaMKIIδ, a highly validated pro-arrhythmic signal [25, 40, 120]. CaMKIIδ is a well-recognized regulator of Ca2+ dysregulation in cardiomyocytes through its critical contribution in phosphorylation of the Ca2+ handling proteins RyR2-Ser2815 (sensitizing RyR2 channels to increase diastolic SR Ca2+ leak) and PLB-Thr17 (elevating the SERCA activity to enhance the SR Ca2+ uptake), which results in triggered activities and arrhythmia pathogenesis in humans and animal models [25, 145, 146, 169,170,171]. In addition, CaMKIIδ also regulates other ion channels such as Ca2+, Na+, K+ channels as well as NCX and myofilament proteins including troponin T (TnT) and myosin-binding protein C (MyBP-C) via phosphorylation [172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191]. Thus, hyper-activated CaMKIIδ drives RyR2 channel-mediated diastolic Ca2+ dysfunction that causes and triggers arrhythmic activities but also contributes to cardiac contractile function. Intriguingly, recent studies revealed that JNK2 has specific actions in regulating both expression and activation of CaMKIIδ, which consequently drives CaMKIIδ-dependent SR Ca2+ mishandling in the stressed heart. Specifically, JNK2 and CaMKIIδ were found to be tethered with each other, and JNK2 increases phosphorylation of CaMKIIδ at the autophosphorylation site Thr286 to activate CaMKIIδ in a JNK2 dose-dependent manner [25]. Protein phosphatase 1 (PP1) is known to target this specific Thr286 site to dephosphorylate CaMKIIδ [192]. Thus, a possible interrelationship between PP1 and JNK2 might exist in regulating the CaMKIIδ activity, and this is worthy of further investigation.
Although CaMKIIδ is essential in regulating a large number of cellular substrates including ion channels, pumps, transporters, and transcription factors [25, 120, 172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191, 193], exactly how the CaMKIIδ gene and protein expression is controlled remains surprisingly understudied. A recent study reported for the first time that JNK2 downstream transcription factors c-jun and activating transcription factor 2 (ATF2) both bind to the CaMKIIδ gene promoter and regulate CaMKIIδ expression [40]. Surprisingly, c-jun was found to be a key transcription factor for the basal level expression of CaMKIIδ mRNAs and proteins. This was evidenced by the suppression of CaMKIIδ promoter baseline activity when c-jun was knocked out in the cells or the binding consensus sequence for c-jun was mutated to alter binding. Moreover, robustly activated JNK2, mimicking a stressed condition, significantly increases the binding of c-jun, but did not change the binding of ATF2, to the CaMKIIδ promoter, while JNK2 inhibition alleviated this enhanced c-jun binding activity. In addition, the JNK2 specific action in c-jun-regulated CaMKIIδ promoter activity was strongly supported by the suppressed CaMKIIδ promoter activity from JNK2 knockdown or suppressed activity [40]. These findings take on special translational importance given that the development of drugs that target CaMKIIδ activity has been considered as an appealing anti-arrhythmic intervention point; however, specificity issues driving off-target effects greatly hinder the clinical applications. Additionally, upstream or downstream components of the CaMKII signaling cascades are then considered as new potential therapeutic targets. Stress-driven JNK2 activation and the JNK2-CaMKII cross talk are likely critical mechanisms that couple stressors and maladaptive cardiac responses. Therefore, modulating JNK2 activity could be a likely therapeutic approach to prevent and treat cardiac arrhythmias.
An extensive number of studies have demonstrated that JNK1 activation is critically involved in preservation of cardiac function and promoting apoptosis after myocardial I/R, MI, and HF via the regulation of signaling pathways that modulate gene expression [10, 21, 165, 194,195,196,197,198,199,200]. Also, emerging evidence suggests that p38 regulates SERCA2 mRNA and protein expression via the transcription factors Egr-1 and SP1 [14]. However, the functional role of all three MAPK members and sub-isoforms of each kinase in Ca2+ handling proteins in cardiomyocytes under physiological conditions or stress challenges requires further investigation. Our advancement of understanding the functional role of MAPKs will likely aid the effort of developing novel preventive and therapeutic strategies for CVDs.
MAPKs and Molecular Remodeling in Myocytes
The MAPK signaling pathway transduces and integrates diverse stress stimuli into complex cytoplasmatic and nuclear processes and finally leads to altered cellular function including proliferation, gene expression, differentiation, and apoptosis. MAPKs regulate cellular processes via direct phosphorylation of downstream targets and/or indirectly regulate gene expression in maintaining normal cell function and cellular responses under stress stimuli challenges. Generally, the JNKs and p38 kinases regulate the stress or injury responses, while the ERKs are more specialized for mitogenic and growth factor stimulations [201, 202].
MAPKs and Gene Regulation in Myocytes
Gene regulation is one of the important functions of the MAPK family via the downstream transcription factors such as c-jun, ATF2, JunD, c-Fos, SRF, AP-1, c-Myc, MEF2, GATA, SMAD, STAT-1, and NFκB. The JNKs directly phosphorylate a number of their downstream transcription factors such as c-jun, ATF2, AP-1, JunD, Sp1, Elk1, and c-Myc [26, 40, 203,204,205,206]. The AP-1 complex is composed of homodimers of c-jun or heterodimers of c-jun/ATF2 or other combinations of transcription factors, which induce target gene expression by binding the AP-1 consensus site(s) in the promoter region of the gene or dissociating from the promoter region to upregulate or suppress the specific gene expression [40]. These activated transcription factors critically contribute to the transcriptional regulation of proteins that are involved in I/R caused myocardial injury and ATP depletion. JNK1 is also known to regulate several important genes (i.e., Notch1, SOD-3) in response to inflammation, oxidative stress, and heat stress through phosphorylation of several transcription factors (i.e., c-jun, Sp1, DAF-16) [207,208,209]. Under long-term stress challenges (i.e., aging, repeated binge alcohol exposure), JNK2, but not JNK1, is activated, which leads to reduced AP-1 activity but increased binding of c-jun and unchanged binding of ATF2 to the promoters of JNK2 target genes such as the “cell-to-cell communicator” gap junction protein connexin43(Cx43), “pro-arrhythmic kinase” CaMKIIδ, and “epigenetic regulatory molecule” DNA methyltransferase1 (DNMT1) to either suppress or enhance their transcriptional activities and gene expression. Consequently, this JNK2-mediated gene regulation leads to impaired cellular function including intercellular uncoupling between cardiac myocytes along with the slowing of electrical conduction or enhanced cardiac Ca2+-mediated arrhythmic triggered activities or increases DNA methylation [20, 24,25,26, 40, 210].
Like the JNKs, p38 also directly phosphorylates transcription factors including ATF1/2/6, c-Myc, c-Fos, GATA4, MEF2A/C, SRF, STAT-1, and CHOP [53, 211,212,213]. Upon activation, p38 translocates into the nucleus and reenters the cytosol when inactivated which is essential for its function across all cell types [58]. The phosphorylation status and the interaction of p38 with other proteins determine its subcellular location and activities of its downstream targets [214]. For instance, activated p38/MAPKAP kinase-2 (MK2) can form a complex with activated p38 in the nucleus leading to the export of MK2 from the nucleus to phosphorylate its cytosolic substrates [215, 216]. The mitogen and stress-activated protein kinase 1/2 (MSK1/2) is a downstream target of p38 but also regulates the subcellular location of p38 and the p38/MSK complex in regulating the transcriptional activity of CREB, STAT3, and NFκB [217,218,219,220,221]. Moreover, p38-regulated transcription factors contribute to the upregulation of several important stress-response genes including phosphatase and tensin homolog on chromosome 10 (PTEN) that acts to limit the phosphorylation and activation of Akt as well as the transforming growth factor beta (TGF-β) signaling pathway in regulating cell growth/differentiation/apoptosis and other cellular functions [222, 223].
Activated ERK1/2, another MAPK family member, has been found to undergo nucleus-translocation and directly phosphorylate transcription factors and bind to chromatin inside the nuclei [224,225,226]. A recent discovery shows that autophosphorylation of ERK2 at the site of Thr188 (Thr208 in ERK1) promotes the nucleotide binding but attenuates ERK kinase activities, while inhibiting the upstream regulator MEK1/2 also abolishes the nucleotide binding and reduces the activity of ERK [227, 228]. ERK has diverse cytoplasmic targets such as the p90 ribosomal S6 (p90RSK) family with isoforms 1–4, p70 ribosomal S6 (p70RSK) [229], MNK [230], and glycogen synthase kinase-3 (GSK-3) [231], which consequently phosphorylate a wide range of substrates involved in gene transcription, translation, cell cycle regulation, and cell survival [232, 233]. Moreover, ERK can phosphorylate a complex family of transcription factors, the ternary complex factors (TCFs; SAP-1, Elk-1, Net, etc.), which are vertebrate ETS-domain proteins that link transcription to MAPK signaling in order to regulate the expression of c-Fos, c-Myc, and c-jun and in turn contribute to transcriptional regulation of various late-response genes that promote cell survival, cell division, and mobility, which are opposite to the regulatory role of JNK and p38 on those cellular responses as discussed above [158, 234,235,236,237]. In addition, several key transcription factors including FOXO, BETA2/NeurD1 (the basic helix-loop-helix protein partner), E4, and PDX-1 were found to be related to glucose regulation and insulin gene transcription, which also underlie the anti-apoptosis effect in myocytes and cardiac protective effect of ERK1/2 [238,239,240,241].
In summary, MAPK signaling pathways are key mediators of cell transcriptional responses to stress-induced extracellular signals. These pathways critically control gene expression in a number of ways including the phosphorylation of cytosolic proteins and regulation of transcription factors and co-regulatory proteins.
MAPKs and Apoptotic Signaling Pathways in Stress-Exposed Myocytes
Apoptosis is a highly regulated process in the cell composed of a balance between pro-death and pro-survival cell signals, and apoptotic cell death plays a pivotal role in myocyte survival in response to stress stimuli such as ischemic cardiac injury and heart failure. Apoptosis can be roughly divided into extrinsic apoptosis, meaning the apoptosis signaling coming from the environment, and intrinsic apoptosis, meaning the apoptosis signaling coming from the cell itself. The main route of apoptosis in the heart is intrinsic apoptosis, and the key initiator of intrinsic apoptosis is the mitochondrial release of cytochrome c [242]. Cytochrome c released from mitochondria forms a complex from pro-caspase 9 and its cofactor APAF-1 and eventually leads to the activation of caspase 9 and apoptosome formation in the cytosol followed by the activation of caspase 3 leading to apoptosis [242]. Various adverse conditions can lead to intrinsic apoptosis in cardiac myocytes including but not limited to redox stress, energy deprivation, and activation of Gαq signaling [243,244,245]. On the other hand, activation of the extrinsic apoptotic signaling pathway is a common phenomenon in stressed hearts such as seen with myocardial ischemic I/R, postinfarction remodeling, end-stage heart failure, and diabetes [246,247,248,249]. Two families of independent receptors that mediate the extrinsic apoptosis signaling are mainly regulated by the Fas receptor (receptor for Fas ligand, FasL) and TNFα receptor 1 (TNFα-R1). Activation of Fas receptor and TNFα-R1 by their specific ligands leads to cleavage of pro-caspase 8 into caspase 8 which further activates caspase 3 to promote apoptosis. In both extrinsic and intrinsic apoptosis, the Bcl-2 family of proteins with Bcl-homology domains plays important roles. To date, this Bcl-2 family is known to contain two subgroups, anti-apoptotic proteins including Bcl-2, Bcl-X(L), Bcl-W, Bfl-1, and Mcf-1 and pro-apoptotic proteins including Bad, Bac, Bak, Bix, Box, Bid, Bim, Bnip3, and Nix [250, 251]. Phosphorylation of Bcl-2 family members plays a key role in regulating mitochondrial membrane integrity, and MAPK has been shown to regulate the Bcl-2 family by phosphorylation [250, 251]. While anti-apoptotic Bcl-2 proteins bind to the proteins forming mitochondrial pores, controlling their opening and closure, some pro-apoptotic Bcl-2 proteins including Bax and Bak can insert into mitochondrial outer membrane upon activation via phosphorylation and form pores into the mitochondria [252,253,254,255]. The Bcl-2 family achieve the anti-apoptotic effect through the inhibition of pro-apoptotic proteins (i.e., Bad, Bax, and Bim) [256,257,258,259,260].
JNK and Apoptotic Signaling Pathways in Myocytes
In the heart, JNK is activated in response to various stimuli signals including mechanical stretch [261, 262], pressure overload [263,264,265], I/R [266, 267], and catecholamine stimulation [263], which are known to activate apoptotic signaling pathways [262, 268, 269]. Apoptosis signal-regulating kinases (ASK, including ASK1 and ASK2) promote the activation of MKK4 and MKK7, which are the upstream activators of JNK [21]. However, mixed findings regarding the role of JNK in apoptosis have been reported, suggesting complicated stress responses of JNK signaling in different cell types and potentially different isoform activation at different time frames following various stress challenges. In ROS-challenged myocyte models (H2O2-treated or norepinephrine-treated adult rat ventricular myocytes), JNK activation is involved in ROS-induced apoptosis, evidenced by alleviated ROS production and reduced apoptosis through JNK-specific inhibiton [270, 271]. In addition, JNK has been localized to the mitochondria in cardiac myocytes and is known to promote the mitochondrial cytochrome c release in a capase-9-dependent but capase-8-independent manner, suggesting a direct functional role of JNK in mitochondria-associated appotosis [272]. This pro-apoptotic effect of JNK was also found in noncardiac mammalian cells via enhanced phosphorylation and degradation of anti-apoptotic proteins Bcl-2 and Bcl-X(L), which removes the inhibitory regulation of Bcl-2 and consequently activates the pro-apoptotic proteins Bad, Bax, and Bim [256,257,258,259,260]. Furthermore, suppressing JNK1 or the MKK4 pathway from overexpression of dominant negative proteins enhances apoptosis during NO treatment and I/R injuries, further supporting the anti-apoptotic effect of cardiac JNK in response to ischemic stress [273,274,275]. While JNK inhibition reduces apoptosis during numerous stress conditions including transient energy deprivation (glucose deprivation and mitochondria inhibition), I/R, and hyperglycemia in cultured H9c2 cells and myocytes, JNK-deficiency in fibroblasts activates mitochondrial apoptotic signaling [276,277,278,279,280]. Thus, the functional role of JNKs in cellular apoptotic pathways of the heart may vary depending on the cell type and context in response to different types of stress stimuli. While the findings discussed above are predominantly regarding the contributions of JNK1 in cellular apoptosis and proliferation that have been well studied [10, 20, 133], the functional roles of cardiac JNK2 and JNK3 (with less expression in the heart) in the apoptotic pathway under different stressed conditions remain largely unknown. Further investigations are clearly needed to enhance our understanding regarding the functional role of JNK in myocardial remodeling and maladaptive development.
p38 and Apoptotic Signaling Pathways in Myocytes
p38 is critically involved in cardiac apoptosis in various animal models of cardiac disease such as cardiac injury and HF [281,282,283,284,285]. p38 was found to activate p53 and promote apoptosis by enhancing the expression and translocation of Bax in mitochondria [286]. p38 can also phosphorylate Bcl-2 via translocation into mitochondria to suppress the anti-apoptotic effect of cytosolic Bcl-2 [287]. In platelet-activating factor (PAF)-treated H2c2 cardiac myocytes with elevated cytosolic Ca2+, caspase 3 activity and mitochondria release of cytochrome c increase in a p38-dependent manner, further supporting the pivotal role of p38 in promoting myocyte apoptosis [288]. Also, a reduced level of Bcl-2 and enhanced apoptosis was found in cardiac myocytes with overexpression of wild-type p38 but not in the myocytes with overexpression of an inactive dominant negative form of p38α [283]. This pro-apoptotic function of p38α was further demonstrated in a mouse myocardial infarction model where overexpression of p38α alleviates the inhibition of apoptosis guard Bcl-X(L) and Bcl-2 reduces the Bcl-X(L) deamination consequently activating the pro-apoptotic signaling cascades [282]. The I/R injury response is crucially determined by mitochondrial function and activity and p38 inhibition during I/R decreases mitochondrial swelling and ultrastructural alterations and mitigates mitochondrial membrane depolarization [289]. There is also evidence that p38 activation during I/R contributes to cardiac damage by triggering intracellular Ca2+ overload [290]. While the pro-apoptotic role of p38α in myocytes has been well-recognized, the pro-apoptotic role of p38δ in deteriorating cardiac function was recently reported with chemotherapeutic doxorubicin-induced cardiomyopathy [291]. Moreover, p38 is a key player in α1-adrenoreceptor blocker doxazosin-induced apoptosis in cardiac myocytes that increases the risk of HF development [292, 293]. Although the overwhelming evidence suggests a pro-apoptotic role of p38, an anti-apoptosis role of p38 has also been described under certain stressed conditions. For instance, with specific β-adrenergic receptor signaling mediated through Gi-dependent receptors, p38 could exert anti-apoptotic roles, while osmotic stress also showed anti-apoptotic function of p38 via phosphorylation of small heat shock protein αB-crystallin [294,295,296]. Therefore, p38 may have a differential functional impact on myocyte apoptotic signaling pathways in response to different stress stimuli. In addition, the distinct functional roles of the different sub-isoforms of p38 under different stress stimuli require further investigation.
Other than a direct regulation of p38 in the apoptotic signaling cascade proteins, p38 can also promote apoptosis in cardiac myocytes by regulating apoptotic genes via a wide panel of transcription factors including but not limited to ATF2, AP-1, GATA, SMAD, STAT-1, and NFκB [297]. For instance, in I/R injury and hypoxia animal and cell models, p38-dependent phosphorylation of ATF2 leads to enhanced expression of phosphatase PTEN which limits the phosphorylation and activation of Akt, a powerful survival pathway regulator [222]. Suppressing p38 by overexpressing dominant negative p38 or PTEN both attenuated myocyte death, cardiac injury, and functional loss after the I/R injury [222]. Enhanced expression of FasL is a well-established route toward DNA fragmentation and apoptosis [298, 299]. p38 has been shown to upregulate Fas/FasL expression via increased phosphorylation of the downstream transcription factor STAT-1 at the Ser727 site to enhance its transcriptional activity in myocytes treated with angiotensin II, norepinephrine, and hypoxia [300, 301]. Moreover, p38-dependent AP-1 and GATA transcriptional regulation were also found to upregulate TGF-β expression, which triggers the activation of transcription factor SMAD-mediated apoptosis in angiotensin II-treated myocytes [223]. GADD153 (growth-arrest-and-DNA-damage-inducible protein 153 also known as C/EBP homologous protein (CHOP)) is one of the pro-apoptotic transcription factors engaged in response to enhanced ER stress. p38-dependent activation of GADD153 has been shown to promote the expression of downstream-of-CHOP gene 1(DOC-1), which encodes for a stress-induced form of carbonic anhydrase VI that catalyzes the formation of H2CO3 to increase cellular stress-induced apoptosis [302, 303]. In addition, p38-activated GADD153 enhances NFκB phosphorylation and nuclear translocation, which was found in doxorubicin-induced inflammation and apoptosis in cardiac myocytes [304]. Furthermore, inhibition of p38/NFκB signaling alleviates isoproterenol-induced cardiac dysfunction in a rat model [297]. Thus far, the evidence supports a key role for p38 in myocyte apoptosis and the development of cardiac maladaptive function.
ERK and Apoptotic Signaling Pathways in Myocytes
ERK activation is largely anti-apoptotic in the heart under various stressed conditions such as I/R injury, oxidative stress, hypoxia stimulation, and β-adrenergic stimulation [305, 306]. ERK inhibition enhanced the oxidative stress-induced cell injury and apoptosis, while also pharmacologically potentiated ERK activation showed a protective effect against apoptosis induced with a chemotherapy reagent doxorubicin in cardiac myocytes [307,308,309]. In contrast, activated ERK1/2 attenuates I/R injury in both cell and animal models [268, 310,311,312,313]. Another interesting finding is that the cell survival signaling through β2-adrenergic receptor activation has been shown to be regulated through activated ERK [314].
The cardiac protective roles of ERK1/2 during a stress challenge are multidimensional. ERK substrates include nuclear substrates (transcription factors) and cytosolic substrates promote apoptosis through activity and transcriptional regulation of certain key apoptosis-related proteins in the cytosol or nucleus. For example, activated cardiac ERK1/2 can phosphorylate cytosolic proteins such as phospholipase A2 and transcription-regulating kinases p90SRK, GSK3, which are critically involved in cellular apoptosis under stressed conditions [315,316,317,318,319,320,321,322]. ERK1/2 also inhibits a key pro-apoptotic protein Bad by facilitating the protein kinase Cε (PKCε)-mediated phosphorylation of Bad in mitochondria and downregulates the expression of Bax, another pro-apoptotic protein in the Bcl-2 family, resulting in the inhibition of cytochrome c release from mitochondria in cardiac myocytes [312, 316,317,318]. Moreover, ERK-GATA4 signaling was shown to be involved in the anti-apoptosis function of ERK by upregulated expression of anti-apoptotic protein Bcl-X(L) via enhanced phosphorylation of the transcription factor GATA4 at the Ser105 site to enhance the promoter activity of Bcl-X(L) [312, 323]. It is known that activating ERK promotes the activation and nuclear translocation of transcription factor Nrf2 which promotes the expression of genes that potentiate the glutathione antioxidant response, while ERK also upregulates COX-2 via enhanced AP-1 and NFκB-2 transcriptional activity to sustain the cardiac myocyte survival and metabolism in response to an oxidative stress challenge [213, 324]. This convincing evidence suggests that ERK1/2 fulfills an anti-apoptotic role to promote cell survival and growth in response to stress stimuli to protect cardiac function. However, ERK was also found to be pro-apoptotic in certain disease models. For instance, in a diabetic rat model with upregulated HMGB1 (high mobility group box 1 protein), ERK was found to promote apoptosis via the activation of Ets-1, which eventually leads to enhanced Bax protein and caspase 3 activation [325].
Taken together, the three MAPK members fulfill unique but overlapping intracellular signaling mechanisms in responding to a myriad of mitogens and stressors mediating the signaling networks of cell survival and death as well as cardiac metabolism and pathogenesis in a cell type and context dependent manner.
Dynamic Relationships of MAPKs in Pathological Cardiac Remodeling in Stressed Hearts
Accumulating evidence suggests that the three MAPK family members play important roles in the development of pathological remodeling and maladaptive cardiac function during disease progression in a cellular context- and time-dependent manner [10, 21, 22, 131, 132, 326]. Cardiac remodeling encompasses the molecular and structural changes accompanying the electrical physiological and pathological changes in stress-exposed hearts. Those changes are manifested clinically in the progression of HF including increased heart size, deteriorated cardiac contractile function, and reduced cardiac output along with a series of clinical symptoms of HF. Cardiomyocytes are the major cardiac cells involved in the remodeling process, although other components are also involved including the interstitium, fibroblasts, and coronary vasculature. Being terminally differentiated cells, cardiac myocytes respond to stress stimuli by adaptive growth, also known as hypertrophy, as the adaptive response in stress-exposed hearts [327]. Cardiac hypertrophy can be roughly divided into physiological (or adaptive) hypertrophy and pathological (or maladaptive) hypertrophy [328]. Physiological hypertrophy is reversible and occurs in response to continuously increased demand for cardiac function, which bears the main characteristics of increased cell size, increased fatty acid oxidation and protein synthesis, sarcomeric reorganization, and increased gene transcription related to cell growth [329, 330]. Pathological hypertrophy may also occur after myocardial ischemic injury, inflammatory heart muscle diseases (i.e., myocarditis), idiopathic dilated cardiomyopathy, or unfavorable cardiac volume loading such as hypertension, aortic stenosis, or valvular regurgitation. Pathological hypertrophy is usually accompanied by enhanced inflammation signaling, fetal gene expression, interstitial fibrosis, and risks of decompensation and progression toward to HF [329,330,331,332].
Regardless of cardiomyocyte MAPK activation under different stress conditions, a different temporal response to various stress stimuli clearly plays a role [161, 333,334,335,336]. Cardiac ERK1/2 plays an essential role in promoting myocyte survival and growth during the progression of adaptive hypertrophy and pathological maladaptive remodeling [337,338,339]. In response to stress stimuli, ERK1/2 can be activated by either G protein-coupled receptor (altered angiotensin II, endothelin-1, phenylephrine, catecholamines, etc. ) [331, 332, 340,341,342,343], receptor tyrosine kinases (altered fibroblast growth factor, TGF-β1, growth differentiation factor 15, etc. ) [344,345,346], or mechanical stretch [347]. Activated ERK drives a wide network of intricate pathways which activate secondary signaling to regulate cardiac myocyte cytosolic activity and gene regulation leading to cardiac hypertrophy without premature death or impaired cardiac function [84, 348,349,350]. While suppression of ERK1/2 signaling by inhibition of ERK1/2 or MEK1/2 with pharmacological agents and dominant negative protein overexpression abolishes hypertrophic response in myocytes during pathological insults (e.g., pressure overload, I/R injury, oxidative stress), this ERK inhibition however leads to aggravated cardiac function and exacerbated myocyte death [161, 324, 351,352,353,354]. Thus, ERK-mediated hypertrophic remodeling appears to be a beneficial early response to maintain a normal cardiac function under stress challenges.
A dynamic relationship between the three MAPKs was found in various cardiac disease models. While some studies observed only transient ERK activation in a pressure-overload mouse model induced with transverse aortic constriction (TAC), other reports suggest that ERK remained activated for 2- to 4-week post-TAC [333, 335, 336]. However, JNK and p38 were found to be constantly activated over the course of pressure overload [161, 333,334,335,336]. On the other hand, severity of the pressure overload and HF status likely plays a key role in the differential activation status of the three MAPK members [333, 335, 336]. For instance, JNK and p38 activation in the heart and in white blood cells were in positive correlation with the severity of pressure overload measured with trans-stenotic systolic pressure gradient and the severity of hypertrophy (left ventricular weight/body weight ratio) [355]. This was supported by another study in animals showing that JNK is significantly increased with a mild to severe pressure overload (35–85% aortic constriction), while p38 is activated in HF with a more severe pressure overload (85% aortic constriction), but ERK was only transiently activated [356]. In human hypertensive patients with uncontrolled blood pressure, p38 and JNK activation is significantly higher in white blood cells compared to patients with controlled blood pressure [355]. Myocardial I/R is another common and complicated pathophysiological stressor that can lead to myocardial stunning, arrhythmias, and eventually HF [357]. Different time scales of MAPK activation have been shown in the infarct or infarct border zone of post-MI mouse, and p38 phosphorylation is increased initially, while JNK phosphorylation increased approximately 2 weeks after MI; on the other hand, ERK phosphorylation increased after about 4 weeks after MI, likely contributing to the post-MI myocardial remodeling [358]. In a rat model of I/R injury, increased p38 and unchanged ERK occurred during the acute ischemia phase, while JNK was only increased during the reperfusion phase [359]. Similarly, in a long-term ischemia porcine model of coronary embolism, JNK, p38, and ERK activations were all enhanced, contributing to the postischemic remodeling from the microinfarction and apoptosis [360]. The enhanced phosphorylation of p38 and JNK after I/R injury further decreased the cell viability and promoted cardiac cell apoptosis [361] via decreased anti-apoptotic Bcl-2 and increased pro-apoptotic Bax [280]. Moreover, acute MI (6 hours after LAD ligation) could activate both JNK and p38, yet ERK was decreased, accompanied by increased activity of pro-apoptotic protein caspase-3 [362,363,364]. ROS has been shown to be critical in activating MAPKs in response to I/R injury. Suppression of ROS production alleviates MAPK activation and attenuates the loss of cardiac function and decreased infarct size [365]. This is supported by the findings of ROS-dependent JNK and p38 activation in post-MI ventricular tissues [366]. In animal models of diabetes and post-MI diabetes, increased ROS was found to be responsible for intensified myocardial injury and loss of cardiac function along with increased activation of JNK and p38, and the contribution of ROS-activated JNK and p38 was confirmed by the striking rescue effect of ROS suppression and stress kinase inhibiton [367, 368]. Moreover, antioxidant/anti-inflammatory agents also prove to enhance ERK1/2 activation and preserve the cardiac function along with suppressed activities of JNK and p38 [369, 370].
Overall, dynamic changes of the three MAPKs appear to occur at different time points during the pathological cardiac remodeling process in response to a myriad of mitogens and stressors. In general, the end result of this temporally dynamic activation of p38 and JNK leads to the aggravation of cardiac injury, promotes maladaptive cardiac remodeling, and impairs cardiac function, while the activation of ERK promotes cell survival and preserves cardiac function. To date, the MAPK family has received extensive interest due to the far-reaching implications its members manifest in signaling and cross talk with other signaling networks. Further understanding regarding the dynamic relationship between the three MAPK members under different stress conditions will be required for the development of effective therapeutic strategies and the discovery of novel therapeutic targets for early intervention and treatment of cardiac diseases.
MAPKs and Therapeutic Potentials
MAPKs are critically involved in cardiac pathological remodeling, and disease development (i.e., hypertrophy, ischemic injury, HF, and cardiac arrhythmias) positioning the modulation of MAPK activity as a novel point for therapeutic intervention. Pharmacological inhibition and genetic deletion of the three MAPK kinases has proven to change the course of stress-induced cardiac adaptive and maladaptive remodeling [333, 371]. Inhibition of JNK and p38 activities has been shown to reduce cardiac injury and preserve cardiac function in the stressed heart, and this was supported by manipulating upstream regulators of JNK and/or p38 to suppress their activities. The knockdown of Grb2 adaptor protein suppressed JNK and p38 activities (no effect on ERK), which effectively alleviated cardiac hypertrophy and apoptosis in pressure-overload hearts [334, 372]. Another example is that deletion/suppression of multiple upstream regulatory genes (i.e., BAMBI, NECTIN2, DKK3, and the 14-3-3 family) inhibits JNK and p38 activity along with exacerbated apoptosis, fibrosis, and cardiac function loss [373,374,375,376].
However, selective inhibition of each MAPK often offers different outcomes, which is in line with the evidence of differential involvement of the three MAPKs and their downstream signaling pathways at different stages during the progression of cardiac remodeling and disease development under different stress conditions. While some studies have shown a beneficial effect of competitive inhibition of the p38 signaling alone by overexpression of dominant negative p38 or pharmacological inhibition in promoting cardiac hypertrophy by preventing apoptosis and fibrosis and preserving cardiac function in response to pressure overload and I/R injury [281, 283, 372, 375, 377,378,379], other studies suggest that inhibiting p38 alone promotes hypertrophy but aggravates the deterioration of cardiac function in both pressure overload and I/R injury models and even abolishing the protective effects of ischemia preconditioning [380,381,382,383]. There are current clinical trials testing the safety and efficacy of p38 inhibition in myocardial infarction [384, 385]. The p38 inhibitor SB-681323 decreased the circulating inflammatory marker high-sensitivity C-reactive protein (hs-CRP) in statin-receiving patients undergoing elective percutaneous coronary intervention [386]. A phase 2 clinical trial suggests treating patients with non-ST-segment elevation myocardial infarction with the p38 inhibitor losmapimod (GW856553X) decreased circulating levels of inflammatory markers including IL-6 and hs-CRP short term (72 hour); however, no differences in inflammatory markers or risk of cardiovascular events were shown in a longer term trial (12 weeks) [384, 387, 388]. Similarly, JNK inhibition without the influence in ERK and p38 led to increased myocyte size; upregulated activation of pro-apoptotic proteins including p53, Bad, and Bax; and exacerbated deterioration of cardiac function in different disease models [389,390,391]. Thus, therapeutic potentials of selective inhibition of either JNK or p38 for cardiac function protection under certain pathological conditions need to be further evaluated. Recently, JNK2, an understudied JNK isoform, showed therapeutical potentials in anti-arrhythmogenesis [20, 24,25,26, 392]. While accumulating evidence suggests that pro-arrhythmic molecule CaMKII may represent a target for therapy and provoked the development of CAMKII inhibitors [113, 393, 394], CAMKII inhibitors have off-target effects that hinder their clinical application [395]. With the discovery of a novel form of pathogenic kinase-on-kinase cross talk, JNK2 directly regulates CAMKII activity, and the development of compounds directed at JNK2 may represent an alternative drug target. More research is warranted to explore JNK2 as an anti-arrhythmic drug target in patients.
Considering the protective role of ERK and detrimental role of JNK and p38 in cardiac injury and pathological remodeling, developing specific strategies for use of selective MAPK inhibitors has a high clinical potential in preventing and treating cardiac injury and pathological remodeling and ultimately preserving or improving cardiac function. In fact, activation of the ERK1/2 pathway has been identified as a central component of the so-called Reperfusion Injury Salvage Kinase (RISK) pathway [396]. ERK1/2-specific in vitro kinase activity in adult hearts subjected to 20 minutes of ischemia followed by 15 minutes of reperfusion was doubled [362]. In addition to this study, others support a protective role for the MEK1-ERK2-signaling pathway against I/R injury [268, 397, 398]. A thorough experimental dissection of the RISK pathway revealed a combination of two parallel cascades, PI3K-Akt and MEK1-ERK1/2, that produced a protective effect when blocked with the co-administration of both PI3K and ERK inhibitors at different time points [399]. Thus, broadly the RISK pathway refers to a group of pro-survival protein kinases, which confer cardioprotection when activated specifically at the time of reperfusion [399, 400]. The RISK pathway has recently been seen as a universal signaling cascade for cardioprotection and is likely recruited not only by ischemic conditioning but also by other pharmacological agents such insulin, bradykinin adenosine, or statins [401] shared by most cardioprotective therapies [402]. Therefore, the cardiac protective effect of activated ERK shed new light on drug development studies. Intriguingly, pharmacological reagents that are suppressive to JNK and p38 activities while augmenting the activation of ERK lead to attenuated scar formation and improved cardiac outcome, underscoring the functional cross talk between the MAPKs [282, 362,363,364]. As it has been discussed in this chapter, differential changes of the three MAPKs occur during the development of adaptive and maladaptive cardiac remodeling and cardiac disease progression in cellular context and time-dependent manners. Further investigation is urgently needed to enhance our understanding of the dynamic relationships between the three MAPK members under different stress conditions, which is essential for the development of effective therapeutic strategies and the discovery of novel therapeutic targets for early intervention and treatment of cardiac diseases.
Abbreviations
- ASK1-2 :
-
Apoptosis signal-regulating kinases-1/2
- AF :
-
Atrial fibrillation
- BAMBI:
-
BMP and activin membrane-bound inhibitor
- Ca2+-ATPase:
-
Plasma membrane calcium/calmodulin-dependent ATPase or PMCA
- CICR :
-
Ca2+ induced Ca2+ release
- ICa :
-
Ca2+ influx
- CaMKIIδ :
-
Calcium calmodulin kinase IIδ
- Ca2+ :
-
Calcium
- CVDs:
-
Cardiovascular diseases
- CHOP:
-
C/EBP homologous protein
- JNK :
-
c-jun N-terminal kinase
- Cx43 :
-
Connexin43
- CSBP :
-
Cytokinin-specific binding protein
- DAD:
-
Delayed afterdepolarization
- DNMT1 :
-
DNA methyltransferase-1
- DOC-1 :
-
Downstream-of-CHOP gene1
- DWORF :
-
Dwarf open reading frame
- ECC:
-
Excitation-contraction coupling
- ERK:
-
Extracellular signal-regulated kinase
- GSK-3:
-
Glycogen synthase kinase-3
- HF :
-
Heart failure
- HMGB1 :
-
High mobility group box 1 protein
- CRP:
-
C-reactive protein
- [Ca2+]i :
-
Intracellular Ca2+
- I/R :
-
Ischemia-reperfusion
- LTCCs :
-
L-type Ca2+ channels
- MK2 :
-
MAPKAP kinase-2
- Vmax :
-
Maximal rate
- MAPKs :
-
Mitogen-activated protein kinases
- MNK :
-
Mitogen-activated protein kinase interacting protein kinase
- MSK1/2 :
-
Mitogen and stress-activated protein kinase1/2
- MEKK4:
-
Mitogen-kinase protein kinase kinase kinase-4
- MI :
-
Myocardial infarction
- MyBP-C :
-
Myosin-binding protein C
- NCX :
-
Na+ /Ca2+ exchanger
- Ik :
-
Outward potassium current
- PKCε:
-
Protein kinase Cε
- PP1:
-
Protein phosphatase 1
- PLB:
-
Phospholamban
- PAF :
-
Platelet-activating factor
- p70RSK:
-
p70 ribosomal S6
- p90RSK:
-
p90 ribosomal S6
- INa :
-
Rapid sodium influx
- RISK :
-
Reperfusion injury salvage kinase
- RyR2 :
-
Ryanodine receptor channel-2
- SR:
-
Sarcoplasmic reticulum
- SERCA2a:
-
Sarcoplasmic reticulum Ca2+-ATPase 2a
- SPEG :
-
Striated muscle enriched protein kinase
- PTEN:
-
Tensin homolog on chromosome 10
- TNFα-R1:
-
Tumor necrosis factor alpha receptor1
- TnT:
-
Troponin T
- TGF-β:
-
Transforming growth factor beta
- TAC :
-
Transverse aortic constriction
- T-tubules :
-
Transverse tubules
- TPY :
-
Threonine-proline-tyrosine phosphorylation motif
References
Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–81.
Neuman RB, et al. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007;53:1652–7. https://doi.org/10.1373/clinchem.2006.083923. clinchem.2006.083923 [pii]
Juhaszova M, Rabuel C, Zorov DB, Lakatta EG, Sollott SJ. Protection in the aged heart: preventing the heart-break of old age? Cardiovasc Res. 2005;66:233–44. https://doi.org/10.1016/j.cardiores.2004.12.020. S0008-6363(04)00592-9 [pii].
He BJ, et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17:1610–8. https://doi.org/10.1038/nm.2506.
Belmin J, et al. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268:H2288–93.
Ismahil MA, et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–82. https://doi.org/10.1161/CIRCRESAHA.113.301720.
Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol. 2007;292:C1983–92. https://doi.org/10.1152/ajpcell.00285.2006. 00285.2006 [pii]
Yang Z, Shen W, Rottman JN, Wikswo JP, Murray KT. Rapid stimulation causes electrical remodeling in cultured atrial myocytes. J Mol Cell Cardiol. 2005;38:299–308. https://doi.org/10.1016/j.yjmcc.2004.11.015.
Li SY, et al. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell. 2005;4:57–64. https://doi.org/10.1111/j.1474-9728.2005.00146.x. ACE146 [pii].
Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–46. https://doi.org/10.1152/physrev.00054.2009. 90/4/1507 [pii].
Yarza R, Vela S, Solas M, Ramirez MJ. c-Jun N-terminal Kinase (JNK) signaling as a therapeutic target for Alzheimer's disease. Front Pharmacol. 2015;6:321. https://doi.org/10.3389/fphar.2015.00321.
Xiao B, et al. Extracellular translationally controlled tumor protein promotes colorectal cancer invasion and metastasis through Cdc42/JNK/ MMP9 signaling. Oncotarget. 2016; https://doi.org/10.18632/oncotarget.10315. 10315 [pii].
Brozzi F, Eizirik DL. ER stress and the decline and fall of pancreatic beta cells in type 1 diabetes. Ups J Med Sci. 2016;121:133–9. https://doi.org/10.3109/03009734.2015.1135217.
Scharf M, et al. Mitogen-activated protein kinase-activated protein kinases 2 and 3 regulate SERCA2a expression and fiber type composition to modulate skeletal muscle and cardiomyocyte function. Mol Cell Biol. 2013;33:2586–602. https://doi.org/10.1128/MCB.01692-12.
Ho PD, et al. Ras reduces L-type calcium channel current in cardiac myocytes. Corrective effects of L-channels and SERCA2 on [Ca(2+)](i) regulation and cell morphology. Circ Res. 2001;88:63–9.
Ho PD, et al. The Raf-MEK-ERK cascade represents a common pathway for alteration of intracellular calcium by Ras and protein kinase C in cardiac myocytes. J Biol Chem. 1998;273:21730–5.
Huang H, Joseph LC, Gurin MI, Thorp EB, Morrow JP. Extracellular signal-regulated kinase activation during cardiac hypertrophy reduces sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) transcription. J Mol Cell Cardiol. 2014;75:58–63. https://doi.org/10.1016/j.yjmcc.2014.06.018.
Hagiwara Y, et al. SHP2-mediated signaling cascade through gp130 is essential for LIF-dependent I CaL, [Ca2+]i transient, and APD increase in cardiomyocytes. J Mol Cell Cardiol. 2007;43:710–6. https://doi.org/10.1016/j.yjmcc.2007.09.004.
Takahashi E, et al. Leukemia inhibitory factor activates cardiac L-Type Ca2+ channels via phosphorylation of serine 1829 in the rabbit Cav1.2 subunit. Circ Res. 2004;94:1242–8. https://doi.org/10.1161/01.RES.0000126405.38858.BC.
Yan J, et al. c-Jun N-terminal kinase activation contributes to reduced connexin43 and development of atrial arrhythmias. Cardiovasc Res. 2013;97:589–97. https://doi.org/10.1093/cvr/cvs366.
Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52.
Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–95. https://doi.org/10.1080/15216540500097111. H527470040088211 [pii]
Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–19. https://doi.org/10.1016/j.biocel.2008.04.009.
Yan J, et al. JNK2, a newly-identified SERCA2 enhancer, augments an arrhythmic [Ca(2+)]SR leak-load relationship. Circ Res. 2021;128:455–70. https://doi.org/10.1161/CIRCRESAHA.120.318409.
Yan J, et al. Role of stress Kinase JNK in binge alcohol-evoked atrial arrhythmia. J Am Coll Cardiol. 2018;71:1459–70. https://doi.org/10.1016/j.jacc.2018.01.060. S0735-1097(18)30435-2 [pii].
Yan J, et al. The stress kinase JNK regulates gap junction Cx43 gene expression and promotes atrial fibrillation in the aged heart. J Mol Cell Cardiol. 2017;114:105–15. https://doi.org/10.1016/j.yjmcc.2017.11.006.
Ai L, et al. Inhibition of Abeta Proteotoxicity by Paeoniflorin in Caenorhabditis elegans through regulation of oxidative and heat shock stress responses. Rejuvenation Res. 2018;21:304–12. https://doi.org/10.1089/rej.2017.1966.
Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–95. https://doi.org/10.1128/MMBR.00025-06. 70/4/1061 [pii]
Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79:143–80.
Karin M. Inflammation-activated protein kinases as targets for drug development. Proc Am Thorac Soc. 2005;2:386–390; discussion 394-385. https://doi.org/10.1513/pats.200504-034SR. 2/4/386 [pii]
Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–12. https://doi.org/10.1038/sj.onc.1210392.
Shimizu S, et al. Involvement of JNK in the regulation of autophagic cell death. Oncogene. 2010;29:2070–82. https://doi.org/10.1038/onc.2009.487. onc2009487 [pii].
Kyriakis JM, Avruch J. pp54 microtubule-associated protein 2 kinase. A novel serine/threonine protein kinase regulated by phosphorylation and stimulated by poly-L-lysine. J Biol Chem. 1990;265:17355–63.
Kyriakis JM, Brautigan DL, Ingebritsen TS, Avruch J. pp54 microtubule-associated protein-2 kinase requires both tyrosine and serine/threonine phosphorylation for activity. J Biol Chem. 1991;266:10043–6.
Hu MC, Qiu WR, Wang YP. JNK1, JNK2 and JNK3 are p53 N-terminal serine 34 kinases. Oncogene. 1997;15:2277–87. https://doi.org/10.1038/sj.onc.1201401.
Mohit AA, Martin JH, Miller CA. p493F12 kinase: a novel MAP kinase expressed in a subset of neurons in the human nervous system. Neuron. 1995;14:67–78. https://doi.org/10.1016/0896-6273(95)90241-4.
Nakano R, Nakayama T, Sugiya H. Biological properties of JNK3 and its function in neurons, astrocytes, pancreatic β-cells and cardiovascular cells. Cells. 2020;9 https://doi.org/10.3390/cells9081802.
Kuan CY, et al. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–76., S0896-6273(00)80727-8 [pii].
Moriguchi T, et al. A novel SAPK/JNK kinase, MKK7, stimulated by TNFalpha and cellular stresses. EMBO J. 1997;16:7045–53. https://doi.org/10.1093/emboj/16.23.7045.
Gao X, et al. Transcriptional regulation of stress kinase JNK2 in pro-arrhythmic CaMKIIdelta expression in the aged atrium. Cardiovasc Res. 2018;114:737–46. https://doi.org/10.1093/cvr/cvy011. 4817461 [pii].
Izumi Y, Kim S, Murakami T, Yamanaka S, Iwao H. Cardiac mitogen-activated protein kinase activities are chronically increased in stroke-prone hypertensive rats. Hypertension. 1998;31:50–6.
Peart JN, Gross ER, Headrick JP, Gross GJ. Impaired p38 MAPK/HSP27 signaling underlies aging-related failure in opioid-mediated cardioprotection. J Mol Cell Cardiol. 2007;42:972–80. https://doi.org/10.1016/j.yjmcc.2007.02.011. S0022-2828(07)00050-8 [pii].
Rich MW. Epidemiology of atrial fibrillation. J Interv Card Electrophysiol. 2009;25:3–8. https://doi.org/10.1007/s10840-008-9337-8.
Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–6., S153458070300323X [pii].
Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. https://doi.org/10.1016/s0898-6568(99)00071-6. S0898-6568(99)00071-6 [pii].
Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–11. https://doi.org/10.1126/science.7914033.
Han J, Lee JD, Tobias PS, Ulevitch RJ. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268:25009–14.
Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–75. https://doi.org/10.1016/j.bbamcr.2007.03.010.
Remy G, et al. Differential activation of p38MAPK isoforms by MKK6 and MKK3. Cell Signal. 2010;22:660–7. https://doi.org/10.1016/j.cellsig.2009.11.020.
Seta K, Sadoshima J. What is the unique function of SAPK3/p38gamma in cardiac myocytes? J Mol Cell Cardiol. 2002;34:597–600. https://doi.org/10.1006/jmcc.2002.2000.
Court NW, dos Remedios CG, Cordell J, Bogoyevitch MA. Cardiac expression and subcellular localization of the p38 mitogen-activated protein kinase member, stress-activated protein kinase-3 (SAPK3). J Mol Cell Cardiol. 2002;34:413–26. https://doi.org/10.1006/jmcc.2001.1523.
Dingar D, et al. Effect of pressure overload-induced hypertrophy on the expression and localization of p38 MAP kinase isoforms in the mouse heart. Cell Signal. 2010;22:1634–44. https://doi.org/10.1016/j.cellsig.2010.06.002. S0898-6568(10)00167-1 [pii].
Raingeaud J, et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–6. https://doi.org/10.1074/jbc.270.13.7420. S0021-9258(18)71780-8 [pii].
Moriguchi T, et al. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996;271:13675–9. https://doi.org/10.1074/jbc.271.23.13675.
Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–55. https://doi.org/10.1128/MCB.16.3.1247.
Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. https://doi.org/10.1038/35065000. 35065000 [pii].
Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–17. https://doi.org/10.1042/BJ20100323. BJ20100323 [pii].
Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–8. https://doi.org/10.1038/sj.cr.7290257.
Marinissen MJ, Chiariello M, Pallante M, Gutkind JS. A network of mitogen-activated protein kinases links G protein-coupled receptors to the c-jun promoter: a role for c-Jun NH2-terminal kinase, p38s, and extracellular signal-regulated kinase 5. Mol Cell Biol. 1999;19:4289–301.
Zhang S, et al. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–6.
Ge B, et al. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–4. https://doi.org/10.1126/science.1067289.
Salvador JM, et al. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat Immunol. 2005;6:390–5. https://doi.org/10.1038/ni1177.
Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. https://doi.org/10.1038/nri2765.
Boulton TG, Cobb MH. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991;2:357–71. https://doi.org/10.1091/mbc.2.5.357.
Boulton TG, et al. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–75. https://doi.org/10.1016/0092-8674(91)90098-j. 0092-8674(91)90098-J [pii].
Sturgill TW, Ray LB, Erikson E, Maller JL. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988;334:715–8. https://doi.org/10.1038/334715a0.
Kohno M, Pouyssegur J. Alpha-thrombin-induced tyrosine phosphorylation of 43,000- and 41,000-Mr proteins is independent of cytoplasmic alkalinization in quiescent fibroblasts. Biochem J. 1986;238:451–7.
Prowse CN, Deal MS, Lew J. The complete pathway for catalytic activation of the mitogen-activated protein kinase, ERK2. J Biol Chem. 2001;276:40817–23. https://doi.org/10.1074/jbc.M105860200. S0021-9258(20)77927-5 [pii].
Ahn NG, et al. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991;266:4220–7., S0021-9258(20)64310-1 [pii].
Lowes VL, Ip NY, Wong YH. Integration of signals from receptor tyrosine kinases and g protein-coupled receptors. Neurosignals. 2002;11:5–19., 57317 [pii] 57317.
Wang LP, et al. Erythropoietin decreases the occurrence of myocardial fibrosis by inhibiting the NADPH-ERK-NF-x03BA;B pathway. Cardiology. 2016;133:97–108. https://doi.org/10.1159/000440995. 000440995 [pii].
Chen LJ, et al. Angiotensin-converting enzyme 2 ameliorates renal fibrosis by blocking the activation of mTOR/ERK signaling in apolipoprotein E-deficient mice. Peptides. 2016;79:49–57. https://doi.org/10.1016/j.peptides.2016.03.008. S0196-9781(16)30043-2 [pii].
Hao G, et al. Ets-1 upregulation mediates angiotensin II-related cardiac fibrosis. Int J Clin Exp Pathol. 2015;8:10216–27.
Peng K, et al. Novel EGFR inhibitors attenuate cardiac hypertrophy induced by angiotensin II. J Cell Mol Med. 2016;20:482–94. https://doi.org/10.1111/jcmm.12763.
Li Q, Liu X, Wei J. Ageing related periostin expression increase from cardiac fibroblasts promotes cardiomyocytes senescent. Biochem Biophys Res Commun. 2014;452:497–502. https://doi.org/10.1016/j.bbrc.2014.08.109. S0006-291X(14)01541-1 [pii].
Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–85. https://doi.org/10.1038/nrm1498. nrm1498 [pii].
Muslin AJ. Role of raf proteins in cardiac hypertrophy and cardiomyocyte survival. Trends Cardiovasc Med. 2005;15:225–9. https://doi.org/10.1016/j.tcm.2005.06.008. S1050-1738(05)00089-7 [pii].
Chen Z, et al. MAP kinases. Chem Rev. 2001;101:2449–76., cr000241p [pii].
Hatano N, et al. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells. 2003;8:847–56., 680 [pii].
Saba-El-Leil MK, et al. An essential function of the mitogen-activated protein kinase Erk2 in mouse trophoblast development. EMBO Rep. 2003;4:964–8. https://doi.org/10.1038/sj.embor.embor939. embor939 [pii].
Blasco RB, et al. c-Raf, but not B-Raf, is essential for development of K-Ras oncogene-driven non-small cell lung carcinoma. Cancer Cell. 2011;19:652–63. https://doi.org/10.1016/j.ccr.2011.04.002. S1535-6108(11)00147-4 [pii].
Yao Y, et al. Extracellular signal-regulated kinase 2 is necessary for mesoderm differentiation. Proc Natl Acad Sci U S A. 2003;100:12759–64. https://doi.org/10.1073/pnas.2134254100. 2134254100 [pii].
Alvarez E, et al. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991;266:15277–85., S0021-9258(18)98613-8 [pii].
Bueno OF, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–50. https://doi.org/10.1093/emboj/19.23.6341.
Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985;85:247–89.
Rougier O, Vassort G, Garnier D, Gargouil YM, Coraboeuf E. Existence and role of a slow inward current during the frog atrial action potential. Pflugers Arch. 1969;308:91–110.
Afzal N, Dhalla NS. Differential changes in left and right ventricular SR calcium transport in congestive heart failure. Am J Physiol. 1992;262:H868–74. https://doi.org/10.1152/ajpheart.1992.262.3.H868.
Makarewich CA, et al. The DWORF micropeptide enhances contractility and prevents heart failure in a mouse model of dilated cardiomyopathy. Elife. 2018;7 https://doi.org/10.7554/eLife.38319.
Minamisawa S, Sato Y, Cho MC. Calcium cycling proteins in heart failure, cardiomyopathy and arrhythmias. Exp Mol Med. 2004;36:193–203. https://doi.org/10.1038/emm.2004.27.
Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015;112:11389–94. https://doi.org/10.1073/pnas.1513047112.
Smith GL, Eisner DA. Calcium Buffering in the Heart in Health and Disease. Circulation. 2019;139:2358–71. https://doi.org/10.1161/circulationaha.118.039329.
Fernández-Miranda G, Romero-Garcia T, Barrera-Lechuga TP, Mercado-Morales M, Rueda A. Impaired activity of Ryanodine receptors contributes to calcium mishandling in Cardiomyocytes of metabolic syndrome rats. Front Physiol. 2019;10:520. https://doi.org/10.3389/fphys.2019.00520.
Gorski PA, Ceholski DK, Hajjar RJ. Altered myocardial calcium cycling and energetics in heart failure--a rational approach for disease treatment. Cell Metab. 2015;21:183–94. https://doi.org/10.1016/j.cmet.2015.01.005.
Lindner M, Böhle T, Beuckelmann DJ. Ca2+-handling in heart failure--a review focusing on Ca2+ sparks. Basic Res Cardiol. 2002;97(Suppl 1):I79–82. https://doi.org/10.1007/s003950200034.
Santulli G, Nakashima R, Yuan Q, Marks AR. Intracellular calcium release channels: an update. J Physiol. 2017;595:3041–51. https://doi.org/10.1113/jp272781.
Shiferaw Y, Watanabe MA, Garfinkel A, Weiss JN, Karma A. Model of intracellular calcium cycling in ventricular myocytes. Biophys J. 2003;85:3666–86. https://doi.org/10.1016/s0006-3495(03)74784-5.
Tamayo M, et al. Intracellular calcium mishandling leads to cardiac dysfunction and ventricular arrhythmias in a mouse model of propionic acidemia. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165586. https://doi.org/10.1016/j.bbadis.2019.165586.
Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87:275–81.
Ather S, Respress JL, Li N, Wehrens XH. Alterations in ryanodine receptors and related proteins in heart failure. Biochim Biophys Acta. 1832;2425-2431:2013. https://doi.org/10.1016/j.bbadis.2013.06.008.
Lugenbiel P, et al. Atrial fibrillation complicated by heart failure induces distinct remodeling of calcium cycling proteins. Plos One. 2015;10:e0116395. https://doi.org/10.1371/journal.pone.0116395.
Hu ST, et al. Altered intracellular Ca2+ regulation in chronic rat heart failure. J Physiol Sci. 2010;60:85–94. https://doi.org/10.1007/s12576-009-0070-6.
Lehnart SE, et al. Sarcoplasmic reticulum proteins in heart failure. Ann N Y Acad Sci. 1998;853:220–30. https://doi.org/10.1111/j.1749-6632.1998.tb08270.x.
Wescott AP, Jafri MS, Lederer WJ, Williams GS. Ryanodine receptor sensitivity governs the stability and synchrony of local calcium release during cardiac excitation-contraction coupling. J Mol Cell Cardiol. 2016;92:82–92. https://doi.org/10.1016/j.yjmcc.2016.01.024.
Gambardella J, Trimarco B, Iaccarino G, Santulli G. New insights in cardiac calcium handling and excitation-contraction coupling. Adv Exp Med Biol. 2018;1067:373–85. https://doi.org/10.1007/5584_2017_106.
Landstrom AP, Dobrev D, Wehrens XHT. Calcium signaling and cardiac arrhythmias. Circ Res. 2017;120:1969–93. https://doi.org/10.1161/circresaha.117.310083.
Maier LS. CaMKIIdelta overexpression in hypertrophy and heart failure: cellular consequences for excitation-contraction coupling. Brazil J Med Biol Res = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al]. 2005;38:1293–302. https://doi.org/10.1590/s0100-879x2005000900002.
Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest. 2013;123:46–52. https://doi.org/10.1172/jci62834.
Balke CW, Shorofsky SR. Alterations in calcium handling in cardiac hypertrophy and heart failure. Cardiovasc Res. 1998;37:290–9. https://doi.org/10.1016/s0008-6363(97)00272-1.
Dobrev D, Wehrens XHT. Calcium-mediated cellular triggered activity in atrial fibrillation. J Physiol. 2017;595:4001–8. https://doi.org/10.1113/jp273048.
Ottolia M, Torres N, Bridge JH, Philipson KD, Goldhaber JI. Na/Ca exchange and contraction of the heart. J Mol Cell Cardiol. 2013;61:28–33. https://doi.org/10.1016/j.yjmcc.2013.06.001.
Eisner DA, Caldwell JL, Kistamás K, Trafford AW. Calcium and excitation-contraction coupling in the heart. Circ Res. 2017;121:181–95. https://doi.org/10.1161/circresaha.117.310230.
Kistamás K, et al. Calcium handling defects and cardiac arrhythmia syndromes. Front Pharmacol. 2020;11:72. https://doi.org/10.3389/fphar.2020.00072.
Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–22. https://doi.org/10.1161/01.RES.0000194329.41863.89.
Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. https://doi.org/10.1146/annurev.physiol.70.113006.100455.
Bovo E, Mazurek SR, Zima AV. Oxidation of ryanodine receptor after ischemia-reperfusion increases propensity of Ca(2+) waves during beta-adrenergic receptor stimulation. Am J Physiol Heart Circ Physiol. 2018;315:H1032–40. https://doi.org/10.1152/ajpheart.00334.2018.
Breckenridge R. Heart failure and mouse models. Dis Model Mech. 2010;3:138–43. https://doi.org/10.1242/dmm.005017.
Denham NC, et al. Calcium in the pathophysiology of atrial fibrillation and heart failure. Front Physiol. 2018;9:1380. https://doi.org/10.3389/fphys.2018.01380.
Kashimura T, et al. In the RyR2(R4496C) mouse model of CPVT, beta-adrenergic stimulation induces Ca waves by increasing SR Ca content and not by decreasing the threshold for Ca waves. Circ Res. 2010;107:1483–9. https://doi.org/10.1161/CIRCRESAHA.110.227744.
Liu B, et al. Ablation of HRC alleviates cardiac arrhythmia and improves abnormal Ca handling in CASQ2 knockout mice prone to CPVT. Cardiovasc Res. 2015;108:299–311. https://doi.org/10.1093/cvr/cvv222. cvv222 [pii].
Yan J, et al. Stress signaling JNK2 crosstalk with CaMKII underlies enhanced atrial Arrhythmogenesis. Circ Res. 2018; https://doi.org/10.1161/CIRCRESAHA.117.312536.
Respress JL, et al. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110:1474–83. https://doi.org/10.1161/circresaha.112.268094.
Yeh YH, et al. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol. 2008;1:93–102. https://doi.org/10.1161/CIRCEP.107.754788.
Bers DM. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu Rev Physiol. 2014;76:107–27. https://doi.org/10.1146/annurev-physiol-020911-153308.
Houser SR, Piacentino V 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–607. https://doi.org/10.1006/jmcc.2000.1206.
Louch WE, et al. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res. 2004;62:63–73. https://doi.org/10.1016/j.cardiores.2003.12.031.
Sjaastad I, Wasserstrom JA, Sejersted OM. Heart failure -- a challenge to our current concepts of excitation-contraction coupling. J Physiol. 2003;546:33–47. https://doi.org/10.1113/jphysiol.2002.034728.
Xie LH, Sato D, Garfinkel A, Qu Z, Weiss JN. Intracellular Ca alternans: coordinated regulation by sarcoplasmic reticulum release, uptake, and leak. Biophys J. 2008;95:3100–10. https://doi.org/10.1529/biophysj.108.130955.
Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268:C1313–9.
Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys J. 2000;78:334–43. https://doi.org/10.1016/S0006-3495(00)76596-9.
Desantiago J, et al. Arrhythmogenic effects of beta2-adrenergic stimulation in the failing heart are attributable to enhanced sarcoplasmic reticulum Ca load. Circ Res. 2008;102:1389–97. https://doi.org/10.1161/CIRCRESAHA.107.169011. CIRCRESAHA.107.169011 [pii].
Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–69.
Kyoi S, et al. Opposing effect of p38 MAP kinase and JNK inhibitors on the development of heart failure in the cardiomyopathic hamster. Cardiovasc Res. 2006;69:888–98. https://doi.org/10.1016/j.cardiores.2005.11.015. S0008-6363(05)00527-4 [pii].
Bogoyevitch MA. The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): differences revealed by gene targeting. Bioessays. 2006;28:923–34. https://doi.org/10.1002/bies.20458.
Go AS, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5., jcc10004 [pii].
Benjamin EJ, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–4.
Chen Z, Stokes DL, Rice WJ, Jones LR. Spatial and dynamic interactions between phospholamban and the canine cardiac Ca2+ pump revealed with use of heterobifunctional cross-linking agents. J Biol Chem. 2003;278:48348–56. https://doi.org/10.1074/jbc.M309545200.
Nelson BR, et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351:271–5. https://doi.org/10.1126/science.aad4076.
Bluhm WF, Kranias EG, Dillmann WH, Meyer M. Phospholamban: a major determinant of the cardiac force-frequency relationship. Am J Physiol Heart Circ Physiol. 2000;278:H249–55.
Quan C, et al. SPEG controls calcium reuptake into the sarcoplasmic reticulum through regulating SERCA2a by its second Kinase-Domain. Circ Res. 2019;124:712–26. https://doi.org/10.1161/CIRCRESAHA.118.313916.
Hume JR, Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J Physiol. 1985;368:525–44. https://doi.org/10.1113/jphysiol.1985.sp015874.
Amos GJ, et al. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J Physiol. 1996;491(Pt 1):31–50. https://doi.org/10.1113/jphysiol.1996.sp021194.
Walden AP, Dibb KM, Trafford AW. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol. 2009;46:463–73. https://doi.org/10.1016/j.yjmcc.2008.11.003.
Freestone NS, et al. Differential lusitropic responsiveness to beta-adrenergic stimulation in rat atrial and ventricular cardiac myocytes. Pflugers Arch. 2000;441:78–87.
Venetucci LA, Trafford AW, O'Neill SC, Eisner DA. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res. 2008;77:285–92. https://doi.org/10.1093/cvr/cvm009.
Chelu MG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–51.
Neef S, et al. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–44. https://doi.org/10.1161/CIRCRESAHA.109.203836. CIRCRESAHA.109.203836 [pii].
Franzini-Armstrong C, Protasi F, Tijskens P. The assembly of calcium release units in cardiac muscle. Ann N Y Acad Sci. 2005;1047:76–85. https://doi.org/10.1196/annals.1341.007.
Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–6. https://doi.org/10.1038/35069083.
Ibrahim M, et al. Prolonged mechanical unloading affects cardiomyocyte excitation-contraction coupling, transverse-tubule structure, and the cell surface. FASEB J. 2010;24:3321–9. https://doi.org/10.1096/fj.10-156638.
Brette F, Orchard C. T-tubule function in mammalian cardiac myocytes. Circ Res. 2003;92:1182–92. https://doi.org/10.1161/01.RES.0000074908.17214.FD.
Berlin JR. Spatiotemporal changes of Ca2+ during electrically evoked contractions in atrial and ventricular cells. Am J Physiol. 1995;269:H1165–70.
Forbes MS, Van Niel EE, Purdy-Ramos SI. The atrial myocardial cells of mouse heart: a structural and stereological study. J Struct Biol. 1990;103:266–79.
Richards MA, et al. Transverse tubules are a common feature in large mammalian atrial myocytes including human. Am J Physiol Heart Circ Physiol. 2011;301:H1996–2005. https://doi.org/10.1152/ajpheart.00284.2011.
Lenaerts I, et al. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ Res. 2009;105:876–85. https://doi.org/10.1161/CIRCRESAHA.109.206276.
Dibb KM, et al. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ Heart Fail. 2009;2:482–9. https://doi.org/10.1161/CIRCHEARTFAILURE.109.852228.
Wakili R, et al. Multiple potential molecular contributors to atrial hypocontractility caused by atrial tachycardia remodeling in dogs. Circ Arrhythm Electrophysiol. 2010;3:530–41. https://doi.org/10.1161/CIRCEP.109.933036.
Frisk M, et al. Variable t-tubule organization and Ca2+ homeostasis across the atria. Am J Physiol Heart Circ Physiol. 2014;307:H609–20. https://doi.org/10.1152/ajpheart.00295.2014.
Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. https://doi.org/10.1080/02699050500284218.
Li D, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001;104:2608–14.
Nishida K, et al. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24:10611–20. https://doi.org/10.1128/MCB.24.24.10611-10620.2004.
Purcell NH, et al. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–9. https://doi.org/10.1073/pnas.0610906104.
Wang Y, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–8.
Zechner D, Thuerauf DJ, Hanford DS, McDonough PM, Glembotski CC. A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J Cell Biol. 1997;139:115–27.
Li M, et al. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation. 2005;111:2494–502. https://doi.org/10.1161/01.CIR.0000165117.71483.0C.
Cardin S, et al. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res. 2003;60:315–25.
Avruch J, et al. Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog Horm Res. 2001;56:127–55.
Zheng M, et al. Sarcoplasmic reticulum calcium defect in Ras-induced hypertrophic cardiomyopathy heart. Am J Physiol Heart Circ Physiol. 2004;286:H424–33. https://doi.org/10.1152/ajpheart.00110.2003.
Zhu S, et al. Luteolin enhances sarcoplasmic reticulum Ca2+-ATPase activity through p38 MAPK signaling thus improving Rat cardiac function after ischemia/reperfusion. Cell Physiol Biochem. 2017;41:999–1010. https://doi.org/10.1159/000460837. 000460837 [pii].
Greiser M, et al. Distinct contractile and molecular differences between two goat models of atrial dysfunction: AV block-induced atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2009;46:385–94. https://doi.org/10.1016/j.yjmcc.2008.11.012.
Chiang DY, et al. Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release. Circ Arrhythm Electrophysiol. 2014;7:1214–22. https://doi.org/10.1161/CIRCEP.114.001973.
Voigt N, et al. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–56. https://doi.org/10.1161/CIRCULATIONAHA.113.006641.
Mustroph J, Maier LS, Wagner S. CaMKII regulation of cardiac K channels. Front Pharmacol. 2014;5:20. https://doi.org/10.3389/fphar.2014.00020.
Nerbonne JM. Repolarizing cardiac potassium channels: multiple sites and mechanisms for CaMKII-mediated regulation. Heart Rhythm. 2011;8:938–41. https://doi.org/10.1016/j.hrthm.2011.01.018.
Hegyi B, et al. Enhanced depolarization drive in failing Rabbit ventricular myocytes: calcium-dependent and β-adrenergic effects on late sodium, L-type calcium, and sodium-calcium exchange currents. Circ Arrhythm Electrophysiol. 2019;12:e007061. https://doi.org/10.1161/circep.118.007061.
Wagner S, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–38. https://doi.org/10.1172/JCI26620.
Yoon JY, Ho WK, Kim ST, Cho H. Constitutive CaMKII activity regulates Na+ channel in rat ventricular myocytes. J Mol Cell Cardiol. 2009;47:475–84. https://doi.org/10.1016/j.yjmcc.2009.06.020.
Xu L, et al. Analysis of Na(+)/Ca (2+) exchanger (NCX) function and current in murine cardiac myocytes during heart failure. Mol Biol Rep. 2012;39:3847–52. https://doi.org/10.1007/s11033-011-1163-x.
Yang Y, et al. Xanthine oxidase inhibitor allopurinol improves atrial electrical remodeling in diabetic rats by inhibiting CaMKII/NCX signaling. Life Sci. 2020;259:118290. https://doi.org/10.1016/j.lfs.2020.118290.
Ronkainen JJ, et al. Ca2+-calmodulin-dependent protein kinase II represses cardiac transcription of the L-type calcium channel alpha(1C)-subunit gene (Cacna1c) by DREAM translocation. J Physiol. 2011;589:2669–86. https://doi.org/10.1113/jphysiol.2010.201400.
Wang Y, et al. Ca2+/calmodulin-dependent protein kinase II-dependent remodeling of Ca2+ current in pressure overload heart failure. J Biol Chem. 2008;283:25524–32. https://doi.org/10.1074/jbc.M803043200.
Van Wagoner DR, et al. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–36.
Christ T, et al. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–7. https://doi.org/10.1161/01.CIR.0000145659.80212.6A. 01.CIR.0000145659.80212.6A [pii].
Zhang R, et al. A dynamic alpha-beta inter-subunit agonist signaling complex is a novel feedback mechanism for regulating L-type Ca2+ channel opening. FASEB J. 2005;19:1573–5. https://doi.org/10.1096/fj.04-3283fje.
Greer-Short A, et al. Calmodulin kinase II regulates atrial myocyte late sodium current, calcium handling, and atrial arrhythmia. Heart Rhythm. 2020;17:503–11. https://doi.org/10.1016/j.hrthm.2019.10.016.
McCauley MD, et al. Ion channel and structural remodeling in obesity-mediated atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13:e008296. https://doi.org/10.1161/CIRCEP.120.008296.
Yoo S, et al. Oxidative stress creates a unique, CaMKII-mediated substrate for atrial fibrillation in heart failure. JCI Insight. 2018;3 https://doi.org/10.1172/jci.insight.120728.
Syeda F, et al. PITX2 modulates atrial membrane potential and the antiarrhythmic effects of sodium-channel blockers. J Am Coll Cardiol. 2016;68:1881–94. https://doi.org/10.1016/j.jacc.2016.07.766.
Justo F, et al. Inhibition of the cardiac late sodium current with eleclazine protects against ischemia-induced vulnerability to atrial fibrillation and reduces atrial and ventricular repolarization abnormalities in the absence and presence of concurrent adrenergic stimulation. Heart Rhythm. 2016;13:1860–7. https://doi.org/10.1016/j.hrthm.2016.06.020.
Henry BL, et al. Relaxin suppresses atrial fibrillation in aged rats by reversing fibrosis and upregulating Na+ channels. Heart Rhythm. 2016;13:983–91. https://doi.org/10.1016/j.hrthm.2015.12.030.
Jaquet K, Fukunaga K, Miyamoto E, Meyer HE. A site phosphorylated in bovine cardiac troponin T by cardiac CaM kinase II. Biochim Biophys Acta. 1995;1248:193–5. https://doi.org/10.1016/0167-4838(95)00028-s.
Hartzell HC, Glass DB. Phosphorylation of purified cardiac muscle C-protein by purified cAMP-dependent and endogenous Ca2+-calmodulin-dependent protein kinases. J Biol Chem. 1984;259:15587–96.
Hidalgo CG, et al. The multifunctional Ca(2+)/calmodulin-dependent protein kinase II delta (CaMKIIdelta) phosphorylates cardiac titin's spring elements. J Mol Cell Cardiol. 2013;54:90–7. https://doi.org/10.1016/j.yjmcc.2012.11.012.
Rokita AG, Anderson ME. New therapeutic targets in cardiology: arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII). Circulation. 2012;126:2125–39. https://doi.org/10.1161/CIRCULATIONAHA.112.124990.
Dhanasekaran DN, Reddy EP. JNK-signaling: a multiplexing hub in programmed cell death. Genes Cancer. 2017;8:682–94. https://doi.org/10.18632/genesandcancer.155.
Knight RJ, Buxton DB. Stimulation of c-Jun kinase and mitogen-activated protein kinase by ischemia and reperfusion in the perfused rat heart. Biochem Biophys Res Commun. 1996;218:83–8. https://doi.org/10.1006/bbrc.1996.0016.
Mohammad J, et al. JNK inhibition blocks piperlongumine-induced cell death and transcriptional activation of heme oxygenase-1 in pancreatic cancer cells. Apoptosis. 2019;24:730–44. https://doi.org/10.1007/s10495-019-01553-9.
Omura T, et al. Activation of mitogen-activated protein kinases in in vivo ischemia/reperfused myocardium in rats. J Mol Cell Cardiol. 1999;31:1269–79. https://doi.org/10.1006/jmcc.1999.0959.
Pombo CM, et al. The stress-activated protein kinases are major c-Jun amino-terminal kinases activated by ischemia and reperfusion. J Biol Chem. 1994;269:26546–51.
Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–44. https://doi.org/10.1128/mcb.19.4.2435.
Zhang W, et al. USP49 inhibits ischemia-reperfusion-induced cell viability suppression and apoptosis in human AC16 cardiomyocytes through DUSP1-JNK1/2 signaling. J Cell Physiol. 2019;234:6529–38. https://doi.org/10.1002/jcp.27390.
Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–8. https://doi.org/10.1016/s0955-0674(99)80028-3.
Kehat I, Molkentin JD. Extracellular signal-regulated kinase 1/2 (ERK1/2) signaling in cardiac hypertrophy. Ann N Y Acad Sci. 2010;1188:96–102. https://doi.org/10.1111/j.1749-6632.2009.05088.x. NYAS5088 [pii].
Kaoud TS, et al. From in Silico discovery to intra-cellular activity: targeting JNK-protein interactions with small molecules. ACS Med Chem Lett. 2012;3:721–5. https://doi.org/10.1021/ml300129b.
Morooka H, Bonventre JV, Pombo CM, Kyriakis JM, Force T. Ischemia and reperfusion enhance ATF-2 and c-Jun binding to cAMP response elements and to an AP-1 binding site from the c-jun promoter. J Biol Chem. 1995;270:30084–92. https://doi.org/10.1074/jbc.270.50.30084.
Sugden PH, Clerk A. “Stress-responsive” mitogen-activated protein kinases (c-Jun N-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ Res. 1998;83:345–52. https://doi.org/10.1161/01.res.83.4.345.
Zeke A, Misheva M, Reményi A, Bogoyevitch MA. JNK signaling: regulation and functions based on complex protein-protein partnerships. Microbiol Mol Biol Rev. 2016;80:793–835. https://doi.org/10.1128/mmbr.00043-14.
Oh SW, et al. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–9. https://doi.org/10.1073/pnas.0500749102. 0500749102 [pii].
Zhao L, et al. The transcription factor DAF-16 is essential for increased longevity in C. elegans exposed to Bifidobacterium longum BB68. Sci Rep. 2017;7:7408. https://doi.org/10.1038/s41598-017-07974-3. 10.1038/s41598-017-07974-3 [pii].
Pan M, et al. JNK1 induces Notch1 expression to regulate genes governing photoreceptor production. Cell. 2019;8 https://doi.org/10.3390/cells8090970. E970 [pii]. cells8090970 [pii].
Yang M, et al. Causal roles of stress kinase JNK2 in DNA methylation and binge alcohol withdrawal-evoked behavioral deficits. Pharmacol Res. 2021;164:105375. https://doi.org/10.1016/j.phrs.2020.105375.
Vlahopoulos SA, et al. The role of ATF-2 in oncogenesis. Bioessays. 2008;30:314–27. https://doi.org/10.1002/bies.20734.
Braz JC, et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111:1475–86. https://doi.org/10.1172/JCI17295.
Chiu PY, Chen N, Leong PK, Leung HY, Ko KM. Schisandrin B elicits a glutathione antioxidant response and protects against apoptosis via the redox-sensitive ERK/Nrf2 pathway in H9c2 cells. Mol Cell Biochem. 2011;350:237–50. https://doi.org/10.1007/s11010-010-0703-3.
Wood CD, Thornton TM, Sabio G, Davis RA, Rincon M. Nuclear localization of p38 MAPK in response to DNA damage. Int J Biol Sci. 2009;5:428–37. https://doi.org/10.7150/ijbs.5.428.
Ben-Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol. 1998;8:1049–57. https://doi.org/10.1016/s0960-9822(98)70442-7. S0960-9822(98)70442-7 [pii]
White A, Pargellis CA, Studts JM, Werneburg BG, Farmer BT 2nd. Molecular basis of MAPK-activated protein kinase 2:p38 assembly. Proc Natl Acad Sci U S A. 2007;104:6353–8. https://doi.org/10.1073/pnas.07016791040701679104 [pii].
Drobic B, Perez-Cadahia B, Yu J, Kung SK, Davie JR. Promoter chromatin remodeling of immediate-early genes is mediated through H3 phosphorylation at either serine 28 or 10 by the MSK1 multi-protein complex. Nucleic Acids Res. 2010;38:3196–208. https://doi.org/10.1093/nar/gkq030. gkq030 [pii].
Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 2003;22:1313–24. https://doi.org/10.1093/emboj/cdg139.
Wierenga AT, Vogelzang I, Eggen BJ, Vellenga E. Erythropoietin-induced serine 727 phosphorylation of STAT3 in erythroid cells is mediated by a MEK-, ERK-, and MSK1-dependent pathway. Exp Hematol. 2003;31:398–405. https://doi.org/10.1016/s0301-472x(03)00045-6. S0301472X03000456 [pii].
Pierrat B, Correia JS, Mary JL, Tomas-Zuber M, Lesslauer W. RSK-B, a novel ribosomal S6 kinase family member, is a CREB kinase under dominant control of p38alpha mitogen-activated protein kinase (p38alphaMAPK). J Biol Chem. 1998;273:29661–71. https://doi.org/10.1074/jbc.273.45.29661. S0021-9258(19)59366-8 [pii].
Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75:50–83. https://doi.org/10.1128/MMBR.00031-10. 75/1/50 [pii].
Qian J, et al. Regulation of phosphatase and tensin homolog on chromosome 10 in response to hypoxia. Am J Physiol Heart Circ Physiol. 2012;302:H1806–17. https://doi.org/10.1152/ajpheart.00929.2011. ajpheart.00929.2011 [pii].
Schroder D, Heger J, Piper HM, Euler G. Angiotensin II stimulates apoptosis via TGF-beta1 signaling in ventricular cardiomyocytes of rat. J Mol Med (Berl). 2006;84:975–83. https://doi.org/10.1007/s00109-006-0090-0.
Lenormand P, et al. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–88. https://doi.org/10.1083/jcb.122.5.1079.
Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med (Berl). 1996;74:589–607. https://doi.org/10.1007/s001090050063.
Winston LA, Hunter T. Intracellular signalling: putting JAKs on the kinase MAP. Curr Biol. 1996;6:668–71. https://doi.org/10.1016/s0960-9822(09)00445-x. S0960-9822(09)00445-X [pii].
Lawrence MC, et al. Chromatin-bound mitogen-activated protein kinases transmit dynamic signals in transcription complexes in beta-cells. Proc Natl Acad Sci U S A. 2008;105:13315–20. https://doi.org/10.1073/pnas.0806465105. 0806465105 [pii].
McReynolds AC, et al. Phosphorylation or mutation of the ERK2 activation loop alters oligonucleotide binding. Biochemistry. 2016;55:1909–17. https://doi.org/10.1021/acs.biochem.6b00096.
Toda T, Shimanuki M, Yanagida M. Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev. 1991;5:60–73. https://doi.org/10.1101/gad.5.1.60.
Joshi S, Platanias LC. Mnk kinase pathway: cellular functions and biological outcomes. World J Biol Chem. 2014;5:321–33. https://doi.org/10.4331/wjbc.v5.i3.321.
McCubrey JA, et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget. 2014;5:2881–911. https://doi.org/10.18632/oncotarget.2037. 2037 [pii].
Casalvieri KA, Matheson CJ, Backos DS, Reigan P. Selective targeting of RSK isoforms in cancer. Trends Cancer. 2017;3:302–12. https://doi.org/10.1016/j.trecan.2017.03.004. S2405-8033(17)30059-6 [pii]
Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–58. https://doi.org/10.1038/nrm2509. nrm2509 [pii].
Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. https://doi.org/10.1038/sj.onc.1210421. 1210421 [pii].
Hollenhorst PC, McIntosh LP, Graves BJ. Genomic and biochemical insights into the specificity of ETS transcription factors. Annu Rev Biochem. 2011;80:437–71. https://doi.org/10.1146/annurev.biochem.79.081507.103945.
Xu Z, et al. CCL19 suppresses angiogenesis through promoting miR-206 and inhibiting Met/ERK/Elk-1/HIF-1alpha/VEGF-A pathway in colorectal cancer. Cell Death Dis. 2018;9:974. https://doi.org/10.1038/s41419-018-1010-2. 10.1038/s41419-018-1010-2 [pii].
Yang R, et al. EGFR activates GDH1 transcription to promote glutamine metabolism through MEK/ERK/ELK1 pathway in glioblastoma. Oncogene. 2020;39:2975–86. https://doi.org/10.1038/s41388-020-1199-2. 10.1038/s41388-020-1199-2 [pii].
Khoo S, et al. Regulation of insulin gene transcription by ERK1 and ERK2 in pancreatic beta cells. J Biol Chem. 2003;278:32969–77. https://doi.org/10.1074/jbc.M301198200. S0021-9258(20)83856-3 [pii].
Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25. https://doi.org/10.1038/sj.onc.1209086. 1209086 [pii].
Farhan M, et al. FOXO signaling pathways as therapeutic targets in cancer. Int J Biol Sci. 2017;13:815–27. https://doi.org/10.7150/ijbs.20052. ijbsv13p0815 [pii].
Wang X, Chen WR, Xing D. A pathway from JNK through decreased ERK and Akt activities for FOXO3a nuclear translocation in response to UV irradiation. J Cell Physiol. 2012;227:1168–78. https://doi.org/10.1002/jcp.22839.
Bishopric NH, Andreka P, Slepak T, Webster KA. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr Opin Pharmacol. 2001;1:141–50. https://doi.org/10.1016/s1471-4892(01)00032-7S1471-4892(01)00032-7 [pii].
Stefanelli C, et al. Polyamines directly induce release of cytochrome c from heart mitochondria. Biochem J. 2000;347(Pt 3):875–80.
Bialik S, et al. The mitochondrial apoptotic pathway is activated by serum and glucose deprivation in cardiac myocytes. Circ Res. 1999;85:403–14. https://doi.org/10.1161/01.res.85.5.403.
Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN. Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev. 2019;99:1765–817. https://doi.org/10.1152/physrev.00022.2018.
Freude B, et al. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol. 2000;32:197–208. https://doi.org/10.1006/jmcc.1999.1066. S0022-2828(99)91066-0 [pii].
Guerra S, et al. Myocyte death in the failing human heart is gender dependent. Circ Res. 1999;85:856–66. https://doi.org/10.1161/01.res.85.9.856.
Sam F, et al. Progressive left ventricular remodeling and apoptosis late after myocardial infarction in mouse heart. Am J Physiol Heart Circ Physiol. 2000;279:H422–8. https://doi.org/10.1152/ajpheart.2000.279.1.H422.
Zhou YT, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9. https://doi.org/10.1073/pnas.97.4.178497/4/1784 [pii].
Singh V, et al. Phosphorylation: implications in cancer. Protein J. 2017;36:1–6. https://doi.org/10.1007/s10930-017-9696-z. 10.1007/s10930-017-9696-z [pii].
Wang XX, Zhang B, Xia R, Jia QY. Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci. 2020;24:9601–14. https://doi.org/10.26355/eurrev_202009_23048. 23048 [pii].
Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol Med. 2003;9:196–205. https://doi.org/10.1016/s1471-4914(03)00046-7. S1471491403000467 [pii]
Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. https://doi.org/10.1101/gad.13.15.1899.
Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–607. https://doi.org/10.1038/sj.onc.1207102. 1207102 [pii].
Chittenden T. BH3 domains: intracellular death-ligands critical for initiating apoptosis. Cancer Cell. 2002;2:165–6. https://doi.org/10.1016/s1535-6108(02)00128-9. S1535610802001289 [pii].
Fan M, et al. Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J Biol Chem. 2000;275:29980–5. https://doi.org/10.1074/jbc.M003776200. S0021-9258(18)44324-4 [pii].
Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–78. https://doi.org/10.1128/MCB.19.12.8469.
Donovan N, Becker EB, Konishi Y, Bonni A. JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J Biol Chem. 2002;277:40944–9. https://doi.org/10.1074/jbc.M206113200. S0021-9258(19)72207-8 [pii].
Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. https://doi.org/10.1016/s0092-8674(03)00757-8. S0092867403007578 [pii].
Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–7. https://doi.org/10.1073/pnas.0438011100. 0438011100 [pii].
Yano M, Kim S, Izumi Y, Yamanaka S, Iwao H. Differential activation of cardiac c-jun amino-terminal kinase and extracellular signal-regulated kinase in angiotensin II-mediated hypertension. Circ Res. 1998;83:752–60. https://doi.org/10.1161/01.res.83.7.752.
Seko Y, Takahashi N, Tobe K, Kadowaki T, Yazaki Y. Pulsatile stretch activates mitogen-activated protein kinase (MAPK) family members and focal adhesion kinase (p125(FAK)) in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1999;259:8–14. https://doi.org/10.1006/bbrc.1999.0720. S0006-291X(99)90720-9 [pii].
Ramirez MT, et al. The MEKK-JNK pathway is stimulated by alpha1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. J Biol Chem. 1997;272:14057–61. https://doi.org/10.1074/jbc.272.22.14057. S0021-9258(19)62512-3 [pii].
He H, Li HL, Lin A, Gottlieb RA. Activation of the JNK pathway is important for cardiomyocyte death in response to simulated ischemia. Cell Death Differ. 1999;6:987–91. https://doi.org/10.1038/sj.cdd.4400572.
Choukroun G, et al. Regulation of cardiac hypertrophy in vivo by the stress-activated protein kinases/c-Jun NH(2)-terminal kinases. J Clin Invest. 1999;104:391–8. https://doi.org/10.1172/JCI6350.
Shvedova M, Anfinogenova Y, Atochina-Vasserman EN, Schepetkin IA, Atochin DN. c-Jun N-Terminal Kinases (JNKs) in Myocardial and Cerebral Ischemia/Reperfusion Injury. Front Pharmacol. 2018;9:715. https://doi.org/10.3389/fphar.2018.00715.
Duplain H. Salvage of ischemic myocardium: a focus on JNK. Adv Exp Med Biol. 2006;588:157–64. https://doi.org/10.1007/978-0-387-34817-9_14.
Yue TL, et al. Inhibition of extracellular signal-regulated kinase enhances Ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000;86:692–9. https://doi.org/10.1161/01.res.86.6.692.
Laderoute KR, Webster KA. Hypoxia/reoxygenation stimulates Jun kinase activity through redox signaling in cardiac myocytes. Circ Res. 1997;80:336–44. https://doi.org/10.1161/01.res.80.3.336.
Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS. H(2)O(2) regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol. 2003;35:615–21. https://doi.org/10.1016/s0022-2828(03)00084-1. S0022282803000841 [pii].
Remondino A, et al. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–8. https://doi.org/10.1161/01.res.0000054624.03539.b4.
Aoki H, et al. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–50. https://doi.org/10.1074/jbc.M112355200. S0021-9258(19)36158-7 [pii].
Andreka P, et al. Cytoprotection by Jun kinase during nitric oxide-induced cardiac myocyte apoptosis. Circ Res. 2001;88:305–12.
Dougherty CJ, et al. Activation of c-Jun N-terminal kinase promotes survival of cardiac myocytes after oxidative stress. Biochem J. 2002;362:561–71.
Hreniuk D, et al. Inhibition of c-Jun N-terminal kinase 1, but not c-Jun N-terminal kinase 2, suppresses apoptosis induced by ischemia/reoxygenation in rat cardiac myocytes. Mol Pharmacol. 2001;59:867–74.
Tournier C, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–4. https://doi.org/10.1126/science.288.5467.870. 8440 [pii].
Gabai VL, et al. Suppression of stress kinase JNK is involved in HSP72-mediated protection of myogenic cells from transient energy deprivation. HSP72 alleviates the stewss-induced inhibition of JNK dephosphorylation. J Biol Chem. 2000;275:38088–94. https://doi.org/10.1074/jbc.M006632200. S0021-9258(20)88528-7 [pii].
Ma L, et al. Ginsenoside Rb3 protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of JNK-mediated NF-kappaB pathway: a mouse cardiomyocyte model. Plos One. 2014;9:e103628. https://doi.org/10.1371/journal.pone.0103628. PONE-D-14-00008 [pii].
Pan Y, et al. Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes. 2014;63:3497–511. https://doi.org/10.2337/db13-1577. db13-1577 [pii].
Li C, et al. Quercetin attenuates cardiomyocyte apoptosis via inhibition of JNK and p38 mitogen-activated protein kinase signaling pathways. Gene. 2016;577:275–80. https://doi.org/10.1016/j.gene.2015.12.012. S0378-1119(15)01483-3 [pii].
Ma XL, et al. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–91. https://doi.org/10.1161/01.cir.99.13.1685.
Ren J, Zhang S, Kovacs A, Wang Y, Muslin AJ. Role of p38alpha MAPK in cardiac apoptosis and remodeling after myocardial infarction. J Mol Cell Cardiol. 2005;38:617–23. https://doi.org/10.1016/j.yjmcc.2005.01.012. S0022-2828(05)00033-7 [pii].
Kaiser RA, et al. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J Biol Chem. 2004;279:15524–30. https://doi.org/10.1074/jbc.M313717200. S0021-9258(19)63956-6 [pii].
Zhang L, Guo Z, Wang Y, Geng J, Han S. The protective effect of kaempferol on heart via the regulation of Nrf2, NF-kappabeta, and PI3K/Akt/GSK-3beta signaling pathways in isoproterenol-induced heart failure in diabetic rats. Drug Dev Res. 2019;80:294–309. https://doi.org/10.1002/ddr.21495.
Clark JE, Sarafraz N, Marber MS. Potential of p38-MAPK inhibitors in the treatment of ischaemic heart disease. Pharmacol Ther. 2007;116:192–206. https://doi.org/10.1016/j.pharmthera.2007.06.013. S0163-7258(07)00150-7 [pii].
Kim SJ, Hwang SG, Shin DY, Kang SS, Chun JS. p38 kinase regulates nitric oxide-induced apoptosis of articular chondrocytes by accumulating p53 via NFkappa B-dependent transcription and stabilization by serine 15 phosphorylation. J Biol Chem. 2002;277:33501–8. https://doi.org/10.1074/jbc.M202862200. S0021-9258(20)74511-4 [pii].
Torcia M, et al. Nerve growth factor inhibits apoptosis in memory B lymphocytes via inactivation of p38 MAPK, prevention of Bcl-2 phosphorylation, and cytochrome c release. J Biol Chem. 2001;276:39027–36. https://doi.org/10.1074/jbc.M102970200. S0021-9258(20)74167-0 [pii].
Zhao D, et al. PAF exerts a direct apoptotic effect on the rat H9c2 cardiomyocytes in Ca2+-dependent manner. Int J Cardiol. 2010;143:86–93. https://doi.org/10.1016/j.ijcard.2009.01.068. S0167-5273(09)00128-4 [pii].
Kumphune S, Surinkaew S, Chattipakorn SC, Chattipakorn N. Inhibition of p38 MAPK activation protects cardiac mitochondria from ischemia/reperfusion injury. Pharm Biol. 2015;53:1831–41. https://doi.org/10.3109/13880209.2015.1014569.
D'Oria R, et al. The role of oxidative stress in cardiac disease: from physiological response to injury factor. Oxid Med Cell Longev. 2020;2020:5732956. https://doi.org/10.1155/2020/5732956.
George SA, et al. p38delta genetic ablation protects female mice from anthracycline cardiotoxicity. Am J Physiol Heart Circ Physiol. 2020;319:H775–86. https://doi.org/10.1152/ajpheart.00415.2020.
Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. https://doi.org/10.1038/sj.cdd.4401373. 4401373 [pii].
Eiras S, et al. Doxazosin induces activation of GADD153 and cleavage of focal adhesion kinase in cardiomyocytes en route to apoptosis. Cardiovasc Res. 2006;71:118–28. https://doi.org/10.1016/j.cardiores.2006.03.014. S0008-6363(06)00127-1 [pii]
Hoover HE, Thuerauf DJ, Martindale JJ, Glembotski C. C. alpha B-crystallin gene induction and phosphorylation by MKK6-activated p38. A potential role for alpha B-crystallin as a target of the p38 branch of the cardiac stress response. J Biol Chem. 2000;275:23825–33. https://doi.org/10.1074/jbc.M003864200. S0021-9258(19)66047-3 [pii].
Communal C, Colucci WS, Singh K. p38 mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against beta -adrenergic receptor-stimulated apoptosis. Evidence for Gi-dependent activation. J Biol Chem. 2000;275:19395–400. https://doi.org/10.1074/jbc.M910471199.
Communal C, Colucci WS. The control of cardiomyocyte apoptosis via the beta-adrenergic signaling pathways. Arch Mal Coeur Vaiss. 2005;98:236–41.
Li L, Hao J, Jiang X, Li P, Sen H. Cardioprotective effects of ulinastatin against isoproterenol-induced chronic heart failure through the PI3KAkt, p38 MAPK and NF-kappaB pathways. Mol Med Rep. 2018;17:1354–60. https://doi.org/10.3892/mmr.2017.7934.
Hsu SC, Gavrilin MA, Tsai MH, Han J, Lai MZ. p38 mitogen-activated protein kinase is involved in Fas ligand expression. J Biol Chem. 1999;274:25769–76. https://doi.org/10.1074/jbc.274.36.25769. S0021-9258(19)55339-X [pii].
Kawahara A, Enari M, Talanian RV, Wong WW, Nagata S. Fas-induced DNA fragmentation and proteolysis of nuclear proteins. Genes Cells. 1998;3:297–306. https://doi.org/10.1046/j.1365-2443.1998.00189.x.
Stephanou A, et al. Induction of apoptosis and Fas receptor/Fas ligand expression by ischemia/reperfusion in cardiac myocytes requires serine 727 of the STAT-1 transcription factor but not tyrosine 701. J Biol Chem. 2001;276:28340–7. https://doi.org/10.1074/jbc.M101177200. S0021-9258(19)31644-8 [pii].
Sharov VG, et al. Hypoxia, angiotensin-II, and norepinephrine mediated apoptosis is stimulus specific in canine failed cardiomyocytes: a role for p38 MAPK, Fas-L and cyclin D1. Eur J Heart Fail. 2003;5:121–9. https://doi.org/10.1016/s1388-9842(02)00254-4. S1388984202002544 [pii]
Wang XZ, et al. Identification of novel stress-induced genes downstream of chop. EMBO J. 1998;17:3619–30. https://doi.org/10.1093/emboj/17.13.3619.
Sok J, et al. CHOP-Dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol Cell Biol. 1999;19:495–504. https://doi.org/10.1128/MCB.19.1.495.
Guo RM, et al. Activation of the p38 MAPK/NF-kappaB pathway contributes to doxorubicin-induced inflammation and cytotoxicity in H9c2 cardiac cells. Mol Med Rep. 2013;8:603–8. https://doi.org/10.3892/mmr.2013.1554.
Sheng Z, et al. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway. Divergence from downstream CT-1 signals for myocardial cell hypertrophy. J Biol Chem. 1997;272:5783–91. https://doi.org/10.1074/jbc.272.9.5783. S0021-9258(18)41273-2 [pii].
Parrizas M, LeRoith D. Insulin-like growth factor-1 inhibition of apoptosis is associated with increased expression of the bcl-xL gene product. Endocrinology. 1997;138:1355–8. https://doi.org/10.1210/endo.138.3.5103.
Chen YL, Loh SH, Chen JJ, Tsai CS. Urotensin II prevents cardiomyocyte apoptosis induced by doxorubicin via Akt and ERK. Eur J Pharmacol. 2012;680:88–94. https://doi.org/10.1016/j.ejphar.2012.01.034. S0014-2999(12)00104-5 [pii].
Su HF, et al. Oleylethanolamide activates Ras-Erk pathway and improves myocardial function in doxorubicin-induced heart failure. Endocrinology. 2006;147:827–34. https://doi.org/10.1210/en.2005-1098. en.2005-1098 [pii].
Montgomery MD, et al. An Alpha-1A Adrenergic receptor agonist prevents acute doxorubicin cardiomyopathy in male mice. Plos One. 2017;12:e0168409. https://doi.org/10.1371/journal.pone.0168409. PONE-D-16-31909 [pii].
Lips DJ, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–41. https://doi.org/10.1161/01.CIR.0000127126.73759.23. 01.CIR.0000127126.73759.23 [pii].
Zhu W, et al. MAPK superfamily plays an important role in daunomycin-induced apoptosis of cardiac myocytes. Circulation. 1999;100:2100–7. https://doi.org/10.1161/01.cir.100.20.2100.
Nebigil CG, Etienne N, Messaddeq N, Maroteaux L. Serotonin is a novel survival factor of cardiomyocytes: mitochondria as a target of 5-HT2B receptor signaling. FASEB J. 2003;17:1373–5. https://doi.org/10.1096/fj.02-1122fje. 02-1122fje [pii].
Iwai-Kanai E, et al. Basic fibroblast growth factor protects cardiac myocytes from iNOS-mediated apoptosis. J Cell Physiol. 2002;190:54–62. https://doi.org/10.1002/jcp.10036. 10.1002/jcp.10036 [pii].
Shizukuda Y, Buttrick PM. Subtype specific roles of beta-adrenergic receptors in apoptosis of adult rat ventricular myocytes. J Mol Cell Cardiol. 2002;34:823–31. https://doi.org/10.1006/jmcc.2002.2020. S0022282802920201 [pii].
Erikson RL. Structure, expression, and regulation of protein kinases involved in the phosphorylation of ribosomal protein S6. J Biol Chem. 1991;266:6007–10., S0021-9258(18)38072-4 [pii].
Baines CP, et al. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–7. https://doi.org/10.1161/01.res.0000012702.90501.8d.
Smith JA, Poteet-Smith CE, Malarkey K, Sturgill TW. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J Biol Chem. 1999;274:2893–8. https://doi.org/10.1074/jbc.274.5.2893. S0021-9258(19)88070-5 [pii].
Valks DM, et al. Phenylephrine promotes phosphorylation of Bad in cardiac myocytes through the extracellular signal-regulated kinases 1/2 and protein kinase A. J Mol Cell Cardiol. 2002;34:749–63. https://doi.org/10.1006/jmcc.2002.2014. S0022282802920146 [pii].
Cho J, Rameshwar P, Sadoshima J. Distinct roles of glycogen synthase kinase (GSK)-3alpha and GSK-3beta in mediating cardiomyocyte differentiation in murine bone marrow-derived mesenchymal stem cells. J Biol Chem. 2009;284:36647–58. https://doi.org/10.1074/jbc.M109.019109. S0021-9258(20)37474-3 [pii].
Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298:H16–23. https://doi.org/10.1152/ajpheart.00588.2009. 00588.2009 [pii].
Pabbidi MR, et al. Inhibition of cAMP-dependent PKA activates beta2-Adrenergic receptor stimulation of cytosolic phospholipase A2 via Raf-1/MEK/ERK and IP3-dependent Ca2+ signaling in atrial myocytes. PLoS One. 2016;11:e0168505. https://doi.org/10.1371/journal.pone.0168505. PONE-D-16-28898 [pii].
Micova P, et al. Chronic intermittent hypoxia affects the cytosolic phospholipase A2alpha/cyclooxygenase 2 pathway via beta2-adrenoceptor-mediated ERK/p38 stimulation. Mol Cell Biochem. 2016;423:151–63. https://doi.org/10.1007/s11010-016-2833-8. 10.1007/s11010-016-2833-8 [pii].
Kitta K, et al. Hepatocyte growth factor induces GATA-4 phosphorylation and cell survival in cardiac muscle cells. J Biol Chem. 2003;278:4705–12. https://doi.org/10.1074/jbc.M211616200. S0021-9258(19)32767-X [pii].
Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274:5038–46. https://doi.org/10.1074/jbc.274.8.5038. S0021-9258(19)87829-8 [pii].
Wang WK, et al. HMGB1 mediates hyperglycaemia-induced cardiomyocyte apoptosis via ERK/Ets-1 signalling pathway. J Cell Mol Med. 2014;18:2311–20. https://doi.org/10.1111/jcmm.12399.
Maruyama J, Naguro I, Takeda K, Ichijo H. Stress-activated MAP kinase cascades in cellular senescence. Curr Med Chem. 2009;16:1229–35.
Chien KR, Knowlton KU, Zhu H, Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 1991;5:3037–46. https://doi.org/10.1096/fasebj.5.15.1835945.
Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res. 2010;61:269–80. https://doi.org/10.1016/j.phrs.2009.11.012. S1043-6618(09)00281-3 [pii].
Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res. 2003;92:139–50. https://doi.org/10.1161/01.res.0000053618.86362.df.
Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. https://doi.org/10.1016/j.pharmthera.2010.04.005. S0163-7258(10)00079-3 [pii].
Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest. 1983;72:732–8. https://doi.org/10.1172/JCI111023.
Knowlton KU, et al. The alpha 1A-adrenergic receptor subtype mediates biochemical, molecular, and morphologic features of cultured myocardial cell hypertrophy. J Biol Chem. 1993;268:15374–80., S0021-9258(18)82267-0 [pii].
Ai F, et al. Schisandrin B attenuates pressure overload-induced cardiac remodeling in mice by inhibiting the MAPK signaling pathway. Exp Ther Med. 2019;18:4645–52. https://doi.org/10.3892/etm.2019.8154. ETM-0-0-8154 [pii].
Esposito G, et al. Cardiac overexpression of a G(q) inhibitor blocks induction of extracellular signal-regulated kinase and c-Jun NH(2)-terminal kinase activity in in vivo pressure overload. Circulation. 2001;103:1453–8.
You J, et al. Differential cardiac hypertrophy and signaling pathways in pressure versus volume overload. Am J Physiol Heart Circ Physiol. 2018;314:H552–62. https://doi.org/10.1152/ajpheart.00212.2017ajpheart.00212.2017 [pii].
Liu Y, et al. Cardiac-specific PID1 overexpression enhances pressure overload-induced cardiac hypertrophy in mice. Cell Physiol Biochem. 2015;35:1975–85. https://doi.org/10.1159/000374005. 000374005 [pii].
Lavoie H, Gagnon J, Therrien M. ERK signalling: a master regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol. 2020;21:607–32. https://doi.org/10.1038/s41580-020-0255-7. 10.1038/s41580-020-0255-7 [pii].
Bodart JF, Chopra A, Liang X, Duesbery N. Anthrax, MEK and cancer. Cell Cycle. 2002;1:10–5., 11020115 [pii].
McCubrey JA, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84. https://doi.org/10.1016/j.bbamcr.2006.10.001. S0167-4889(06)00315-6 [pii].
Clerk A, Bogoyevitch MA, Anderson MB, Sugden PH. Differential activation of protein kinase C isoforms by endothelin-1 and phenylephrine and subsequent stimulation of p42 and p44 mitogen-activated protein kinases in ventricular myocytes cultured from neonatal rat hearts. J Biol Chem. 1994;269:32848–57., S0021-9258(20)30069-7 [pii].
Xiao L, et al. MEK1/2-ERK1/2 mediates alpha1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol. 2001;33:779–87. https://doi.org/10.1006/jmcc.2001.1348. S0022-2828(01)91348-3 [pii].
Yue TL, et al. Extracellular signal-regulated kinase plays an essential role in hypertrophic agonists, endothelin-1 and phenylephrine-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:37895–901. https://doi.org/10.1074/jbc.M007037200. S0021-9258(20)88503-2 [pii].
Cipolletta E, et al. Targeting the CaMKII/ERK Interaction in the Heart Prevents Cardiac Hypertrophy. PLoS One. 2015;10:e0130477. https://doi.org/10.1371/journal.pone.0130477. PONE-D-14-54390 [pii].
Heger J, et al. Growth differentiation factor 15 acts anti-apoptotic and pro-hypertrophic in adult cardiomyocytes. J Cell Physiol. 2010;224:120–6. https://doi.org/10.1002/jcp.22102.
House SL, et al. Fibroblast growth factor 2 mediates isoproterenol-induced cardiac hypertrophy through activation of the extracellular regulated Kinase. Mol Cell Pharmacol. 2010;2:143–54. https://doi.org/10.4255/mcpharmacol.10.20.
Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–6. https://doi.org/10.1016/j.yjmcc.2010.10.033S0022-2828(10)00431-1 [pii].
Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res. 2000;47:23–37. https://doi.org/10.1016/s0008-6363(00)00076-6. S0008-6363(00)00076-6 [pii].
Molkentin JD, Robbins J. With great power comes great responsibility: using mouse genetics to study cardiac hypertrophy and failure. J Mol Cell Cardiol. 2009;46:130–6. https://doi.org/10.1016/j.yjmcc.2008.09.002. S0022-2828(08)00584-1 [pii].
Ruppert C, et al. Interference with ERK(Thr188) phosphorylation impairs pathological but not physiological cardiac hypertrophy. Proc Natl Acad Sci U S A. 2013;110:7440–5. https://doi.org/10.1073/pnas.12219991101221999110 [pii].
Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15:75–83. https://doi.org/10.1038/nm.1893. nm.1893 [pii].
Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: targeting Raf/MEK/ERK1/2-signaling. Int J Biochem Cell Biol. 2009;41:2351–5. https://doi.org/10.1016/j.biocel.2009.08.002. S1357-2725(09)00225-8 [pii].
van Berlo JH, Elrod JW, Aronow BJ, Pu WT, Molkentin JD. Serine 105 phosphorylation of transcription factor GATA4 is necessary for stress-induced cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2011;108:12331–6. https://doi.org/10.1073/pnas.1104499108. 1104499108 [pii].
Harris IS, et al. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–23. https://doi.org/10.1161/01.CIR.0000138190.50127.6A. 01.CIR.0000138190.50127.6A [pii].
Punn A, Mockridge JW, Farooqui S, Marber MS, Heads RJ. Sustained activation of p42/p44 mitogen-activated protein kinase during recovery from simulated ischaemia mediates adaptive cytoprotection in cardiomyocytes. Biochem J. 2000;350(Pt 3):891–9.
Esposito G, et al. Induction of mitogen-activated protein kinases is proportional to the amount of pressure overload. Hypertension. 2010;55:137–43. https://doi.org/10.1161/HYPERTENSIONAHA.109.135467. HYPERTENSIONAHA.109.135467 [pii].
Sopontammarak S, et al. Mitogen-activated protein kinases (p38 and c-Jun NH2-terminal kinase) are differentially regulated during cardiac volume and pressure overload hypertrophy. Cell Biochem Biophys. 2005;43:61–76. https://doi.org/10.1385/CBB:43:1:061. CBB:43:1:061 [pii].
Rentrop KP, Feit F. Reperfusion therapy for acute myocardial infarction: concepts and controversies from inception to acceptance. Am Heart J. 2015;170:971–80. https://doi.org/10.1016/j.ahj.2015.08.005. S0002-8703(15)00515-3 [pii].
Yeh CC, et al. Distinctive ERK and p38 signaling in remote and infarcted myocardium during post-MI remodeling in the mouse. J Cell Biochem. 2010;109:1185–91. https://doi.org/10.1002/jcb.22498.
Bogoyevitch MA, et al. Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res. 1996;79:162–73.
Liu T, et al. Coronary Microembolization induces Cardiomyocyte apoptosis in swine by activating the LOX-1-dependent mitochondrial pathway and Caspase-8-dependent pathway. J Cardiovasc Pharmacol Ther. 2016;21:209–18. https://doi.org/10.1177/1074248415599265. 1074248415599265 [pii].
Song CL, et al. Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1. Oncotarget. 2016; https://doi.org/10.18632/oncotarget.9240. 9240 [pii].
Milano G, et al. A peptide inhibitor of c-Jun NH2-terminal kinase reduces myocardial ischemia-reperfusion injury and infarct size in vivo. Am J Physiol Heart Circ Physiol. 2007;292:H1828–35. https://doi.org/10.1152/ajpheart.01117.2006. 01117.2006 [pii].
Jeong CW, et al. Curcumin protects against regional myocardial ischemia/reperfusion injury through activation of RISK/GSK-3beta and inhibition of p38 MAPK and JNK. J Cardiovasc Pharmacol Ther. 2012;17:387–94. https://doi.org/10.1177/1074248412438102. 1074248412438102 [pii].
Liu X, Gu J, Fan Y, Shi H, Jiang M. Baicalin attenuates acute myocardial infarction of rats via mediating the mitogen-activated protein kinase pathway. Biol Pharm Bull. 2013;36:988–94., DN/JST.JSTAGE/bpb/b13-00021 [pii].
Zhu Z, et al. All-trans retinoic acid ameliorates myocardial ischemia/reperfusion injury by reducing cardiomyocyte apoptosis. Plos One. 2015;10:e0133414. https://doi.org/10.1371/journal.pone.0133414PONE-D-15-03759 [pii].
Yang R, et al. Sodium tanshinone IIA sulfonate protects cardiomyocytes against oxidative stress-mediated apoptosis through inhibiting JNK activation. J Cardiovasc Pharmacol. 2008;51:396–401. https://doi.org/10.1097/FJC.0b013e3181671439. 00005344-200804000-00008 [pii].
Samuel SM, et al. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 2010;121:1244–55. https://doi.org/10.1161/CIRCULATIONAHA.109.872481. CIRCULATIONAHA.109.872481 [pii].
Liu J, et al. Palmitate promotes autophagy and apoptosis through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun. 2015;463:262–7. https://doi.org/10.1016/j.bbrc.2015.05.042. S0006-291X(15)00957-2 [pii].
Suchal K, et al. Kaempferol attenuates myocardial ischemic injury via inhibition of MAPK signaling pathway in experimental model of myocardial ischemia-reperfusion injury. Oxid Med Cell Longev. 2016;2016:7580731. https://doi.org/10.1155/2016/7580731.
Zhang N, et al. Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: induced diabetic cardiomyopathy. Mol Cell Biochem. 2016;417:87–96. https://doi.org/10.1007/s11010-016-2716-z. 10.1007/s11010-016-2716-z [pii].
Rapacciuolo A, et al. Important role of endogenous norepinephrine and epinephrine in the development of in vivo pressure-overload cardiac hypertrophy. J Am Coll Cardiol. 2001;38:876–82. https://doi.org/10.1016/s0735-1097(01)01433-4. S0735-1097(01)01433-4 [pii].
Zhang S, et al. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111:833–41. https://doi.org/10.1172/JCI16290.
Villar AV, et al. BAMBI (BMP and activin membrane-bound inhibitor) protects the murine heart from pressure-overload biomechanical stress by restraining TGF-beta signaling. Biochim Biophys Acta. 2013;1832:323–35. https://doi.org/10.1016/j.bbadis.2012.11.007. S0925-4439(12)00258-X [pii].
Satomi-Kobayashi S, et al. Deficiency of nectin-2 leads to cardiac fibrosis and dysfunction under chronic pressure overload. Hypertension. 2009;54:825–31. https://doi.org/10.1161/HYPERTENSIONAHA.109.130443. HYPERTENSIONAHA.109.130443 [pii].
Zhang S, et al. Role of 14-3-3-mediated p38 mitogen-activated protein kinase inhibition in cardiac myocyte survival. Circ Res. 2003;93:1026–8. https://doi.org/10.1161/01.RES.0000104084.88317.91. 01.RES.0000104084.88317.91 [pii].
Zhang Y, et al. Dickkopf-3 attenuates pressure overload-induced cardiac remodelling. Cardiovasc Res. 2014;102:35–45. https://doi.org/10.1093/cvr/cvu004. cvu004 [pii].
Sari FR, et al. Attenuation of CHOP-mediated myocardial apoptosis in pressure-overloaded dominant negative p38alpha mitogen-activated protein kinase mice. Cell Physiol Biochem. 2011;27:487–96. https://doi.org/10.1159/000329970. 000329970 [pii].
van Eickels M, et al. 17beta-estradiol attenuates the development of pressure-overload hypertrophy. Circulation. 2001;104:1419–23. https://doi.org/10.1161/hc3601.095577.
Wu L, et al. Loss of TRADD attenuates pressure overload-induced cardiac hypertrophy through regulating TAK1/P38 MAPK signalling in mice. Biochem Biophys Res Commun. 2017;483:810–5. https://doi.org/10.1016/j.bbrc.2016.12.104. S0006-291X(16)32164-7 [pii].
Liu J, et al. Pressure overload induces greater hypertrophy and mortality in female mice with p38alpha MAPK inhibition. J Mol Cell Cardiol. 2006;41:680–8. https://doi.org/10.1016/j.yjmcc.2006.07.007. S0022-2828(06)00720-6 [pii].
Gorog DA, et al. Inhibition of p38 MAPK activity fails to attenuate contractile dysfunction in a mouse model of low-flow ischemia. Cardiovasc Res. 2004;61:123–31. https://doi.org/10.1016/j.cardiores.2003.09.034. S0008636303006655 [pii].
Mocanu MM, Baxter GF, Yue Y, Critz SD, Yellon DM. The p38 MAPK inhibitor, SB203580, abrogates ischaemic preconditioning in rat heart but timing of administration is critical. Basic Res Cardiol. 2000;95:472–8. https://doi.org/10.1007/s003950070023.
Nakano A, Cohen MV, Critz S, Downey JM. SB 203580, an inhibitor of p38 MAPK, abolishes infarct-limiting effect of ischemic preconditioning in isolated rabbit hearts. Basic Res Cardiol. 2000;95:466–71. https://doi.org/10.1007/s003950070022.
Melloni C, et al. The study of LoSmapimod treatment on inflammation and InfarCtSizE (SOLSTICE): design and rationale. Am Heart J. 2012;164:646–653 e643. https://doi.org/10.1016/j.ahj.2012.07.030. S0002-8703(12)00581-9 [pii].
O'Donoghue ML, et al. Rationale and design of the LosmApimod To Inhibit p38 MAP kinase as a TherapeUtic target and moDify outcomes after an acute coronary syndromE trial. Am Heart J. 2015;169:622–630 e626. https://doi.org/10.1016/j.ahj.2015.02.012. S0002-8703(15)00115-5 [pii].
Sarov-Blat L, et al. Inhibition of p38 mitogen-activated protein kinase reduces inflammation after coronary vascular injury in humans. Arterioscler Thromb Vasc Biol. 2010;30:2256–63. https://doi.org/10.1161/ATVBAHA.110.209205. ATVBAHA.110.209205 [pii].
Newby LK, et al. Losmapimod, a novel p38 mitogen-activated protein kinase inhibitor, in non-ST-segment elevation myocardial infarction: a randomised phase 2 trial. Lancet. 2014;384:1187–95. https://doi.org/10.1016/S0140-6736(14)60417-7. S0140-6736(14)60417-7 [pii].
O'Donoghue ML, et al. Effect of Losmapimod on cardiovascular outcomes in patients hospitalized with acute myocardial infarction: a randomized clinical trial. JAMA. 2016;315:1591–9. https://doi.org/10.1001/jama.2016.3609. 2511223 [pii].
Liu W, et al. Deprivation of MKK7 in cardiomyocytes provokes heart failure in mice when exposed to pressure overload. J Mol Cell Cardiol. 2011;50:702–11. https://doi.org/10.1016/j.yjmcc.2011.01.013. S0022-2828(11)00053-8 [pii].
Liu W, et al. Cardiac-specific deletion of mkk4 reveals its role in pathological hypertrophic remodeling but not in physiological cardiac growth. Circ Res. 2009;104:905–14. https://doi.org/10.1161/CIRCRESAHA.108.188292. CIRCRESAHA.108.188292 [pii].
Calamaras TD, et al. Mixed lineage kinase-3 prevents cardiac dysfunction and structural remodeling with pressure overload. Am J Physiol Heart Circ Physiol. 2019;316:H145–59. https://doi.org/10.1152/ajpheart.00029.2018.
Yan J, et al. Stress signaling JNK2 crosstalk with CaMKII underlies enhanced atrial Arrhythmogenesis. Circ Res. 2018;122:821–35. https://doi.org/10.1161/CIRCRESAHA.117.312536. CIRCRESAHA.117.312536 [pii].
Gao X, et al. Transcriptional regulation of stress Kinase JNK2 in pro-arrhythmic CaMKIIdelta expression in the aged atrium. Cardiovasc Res. 2018; https://doi.org/10.1093/cvr/cvy011.
Zhang R, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–17.
Anderson ME. Calmodulin kinase and L-type calcium channels; a recipe for arrhythmias? Trends Cardiovasc Med. 2004;14:152–61. https://doi.org/10.1016/j.tcm.2004.02.005. S1050173804000283 [pii].
Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–60. https://doi.org/10.1016/j.cardiores.2003.09.024. S0008636303006540 [pii].
Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 2009;296:H1236–43. https://doi.org/10.1152/ajpheart.00100.2009. 00100.2009 [pii].
Yang X, et al. Cardioprotection by mild hypothermia during ischemia involves preservation of ERK activity. Basic Res Cardiol. 2011;106:421–30. https://doi.org/10.1007/s00395-011-0165-0.
Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–6. https://doi.org/10.1152/ajpheart.00374.2004. 00374.2004 [pii].
Schulman D, Latchman DS, Yellon DM. Urocortin protects the heart from reperfusion injury via upregulation of p42/p44 MAPK signaling pathway. Am J Physiol Heart Circ Physiol. 2002;283:H1481–8. https://doi.org/10.1152/ajpheart.01089.2001. 01089.2001 [pii].
Rossello X, Yellon DM. A critical review on the translational journey of cardioprotective therapies! Int J Cardiol. 2016;220:176–84. https://doi.org/10.1016/j.ijcard.2016.06.131. S0167-5273(16)31141-X [pii].
Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005;15:69–75, S1050-1738(05)00025-3 [pii]. https://doi.org/10.1016/j.tcm.2005.03.001.
Declarations
This work was supported by National Institutes of Health [P01-HL06426, R01-AA024769, R01-HL113640, R01-HL146744 to XA].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ai, X., Yan, J., Bare, D. (2022). Stress Kinase Signaling in Cardiac Myocytes. In: Parinandi, N.L., Hund, T.J. (eds) Cardiovascular Signaling in Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-031-08309-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-08309-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-08308-2
Online ISBN: 978-3-031-08309-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)