Abstract
Immune checkpoint inhibitors (ICI) have improved prognosis in advanced malignancies but may be associated with extensive ocular immune-related adverse events (irAEs) that may be sight-threatening. Although ocular side effects account for 1% of total adverse events, they are becoming more commonplace as more people undergo treatment with ICI. This chapter aims to identify the presentation, characteristics, and management of ocular irAEs. Prompt recognition and management of ocular irAEs can improve patient care and outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ocular oncology
- Checkpoint immunotherapy
- Immune-related adverse events
- Toxicity
- PD1
- PDL-1
- CTLA-4 inhibitors
1 Introduction
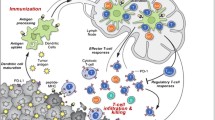
The advent of immune checkpoint inhibitors (ICI) has revolutionized the field of oncology. Immunotherapy harnesses the patient’s own immune system to overcome cancer [1]. Many malignancies have had minimal response to traditional therapies such as radiation and chemotherapy [2]. However, these tumors have shown remarkable response to ICI, leading to improved prognosis in advanced or metastatic cancers [2].
The origins of modern immunotherapy date back to the efforts of William Coley in the nineteenth century [3]. Known as the father of immunotherapy, Coley described several cases where cancer patients went into spontaneous remission after developing acute bacterial infections. Summoning the immune system appeared to halt cancer growth and progression. He went on to inoculate his cancer patients with strains of Streptococcus and Serratia and successfully achieved remission in several malignancies including sarcoma [3]. Years of subsequent research have increased our understanding of the immune system and its relationship to tumor growth. Tumor cells activate immune checkpoint pathways that inhibit the immune system [4]. In 2018, James Allison and Tasuku Honjo were awarded the Nobel Prize in Physiology or Medicine for their discovery of specific proteins involved in suppressing immune checkpoint regulation [5]. The primary targets of checkpoint inhibition are cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), program cell death receptor 1 (PD-1), and programmed cell death ligand 1 (PD-L1) [6]. These ICI interrupt the activation of checkpoint pathways and promote immune-mediated elimination of tumor cells [4]. Thus, ICI enhances the immune system and has significantly improved the prognosis for patients with advanced malignancies [7].
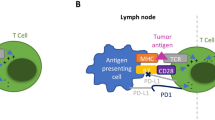
T cell activation requires at least two signals [8]. The first signal comes from an antigen-presenting cell (APC) and occurs when its major histocompatibility complex II (MHC II) interacts with a T cell receptor (TCR) on the surface of a T cell [8]. To complete activation, CD28 on a T cell then binds to a B7 complex on an APC. However, initiation of the first signal also upregulates CTLA-4 [8]. This protein then competes with the stimulatory CD28 binding to the B7 complex and provides a signal to inhibit the T cell’s activation. Ipilimumab, a CTLA-4 antibody, was the first ICI approved by the Food and Drug Administration (FDA) in 2010 [9]. Blockade of CTLA-4 results in enhanced T cell response.
PD-1 is commonly found on T cells and normally prevents T cells from attacking other cells in the body when bound to PD-L1 [6]. Tumor cells can evade an immune attack by secreting large amounts of PD-L1 [6]. Monoclonal antibodies that target PD-1 or PD-L1 can block this binding and increase the T cell response [6]. Interestingly, PD-1 and PD-L1 play a role in ocular immune privilege. PD-1 is constitutively expressed in the eye by retinal pigment epithelial cells [4, 5]. When blocked this can trigger an immune response against intraocular tissues [5]. In a mouse model that underwent corneal transplantation, the transplants developed rejection when PD-1 or PD-L1 was blocked [5]. While CTLA-4 inhibition occurs at the time of T cell initiation, PD-1 inhibition occurs downstream to promote T cell function. PD-1 inhibitors include nivolumab, pembrolizumab, and cemiplimab. FDA-approved drugs that target PD-L1 include atezolizumab, avelumab, and durvalumab.
Ipilimumab was approved by the FDA for the management of metastatic melanoma [10]. Nivolumab and pembrolizumab were approved for advanced melanoma and pretreated non-small cell lung cancer (NSCLC) [10]. Atezolizumab may be used for pretreated NSCLC and advanced urothelial carcinoma [10]. Cemiplimab was authorized by the FDA to treat metastatic or unresectable squamous cell cancer [2]. Other indications for ICI are renal cell carcinoma, head and neck squamous cell carcinoma, Hodgkin lymphoma, and Merkel cell carcinoma [7].
2 General Principles
Despite their success, ICI have been associated with a broad spectrum of side effects. These have been coined immune-related adverse events (irAEs) [7]. IrAEs are thought to arise from the enhancement of the immune system [7]. ICI not only allows the T cells to attack tumor cells but also allows them to respond against normal healthy tissue [5]. Common adverse events include rash, diarrhea, and fatigue. Ocular irAEs are quite rare, occurring in approximately 1% of treated patients [12]. Most reports of ocular irAEs are limited to single-digit case series and case reports [12]. Nevertheless, as use of ICI increases, ocular adverse events are becoming more relevant. Ocular toxicities may range from transient blurred vision and ocular discomfort to uveitis and optic neuropathy. Ocular toxicity may be sight-threatening and has a significant impact on a patient’s quality of life. Specialized and multidisciplinary teams including ophthalmologists, oncologists, internal medicine, etc. are critical to prevent further aggravation of irAEs.
The National Cancer Institute has created Common Terminology Criteria for Adverse Events (CTCAE), which provides descriptive terminology to report grading (severity) of medication adverse events (toxicities) [11]. The CTCAE includes grading tables for the severity of ocular pathology (Table 1). These include blurred vision, cataract formation, conjunctivitis, flashing lights, floaters, dry eye, extraocular muscle paresis, retinopathy, and uveitis among others [11].
The grading system is divided into five categories: grades 1–5 [11]. Grade 1 with mild to no symptoms is discovered only by observation and requires no intervention. Grade 2 is defined as mildly limiting age-appropriate activities of daily living (ADL) with minimal, local, noninvasive intervention indicated. Visual acuity is 20/40 or better (or loss of 3 lines or fewer from baseline). Grade 3 occurs when there is a severe or medically significant adverse effect; however, it is not immediately life-threatening. Hospitalization may be indicated due to disabling or limited ability to perform ADLs. Vision is worse than 20/40 with more than 3 lines decreased from baseline but better than 20/200. For grade 4 toxicities that are life-threatening, urgent intervention is required. Visual acuity is worse than 20/200 and qualifies as blindness. Grade 5 involves death that is related to the adverse event [11].
In general, patients with mild (i.e., grade 1) toxicity can be monitored closely for the progression of adverse events. Patients who develop moderate to severe toxicity (i.e., grade 2 or above) should consider reducing the dose or withholding treatment. Grade 3 and above toxicity should prompt consideration of holding or permanently discontinuing treatment. Rapid and aggressive use of systemic corticosteroids may be required followed by a slow taper. Other treatments such as plasmapheresis, intravenous immunoglobulin, and other immunosuppressants may be used [7].
3 Immunotherapy Considerations
Adverse events are most common with the use of nivolumab compared to any other ICI [13]. The reported ocular toxicity rate of ipilimumab is 1.3% including anterior uveitis, optic neuropathy, and thyroid orbitopathy [14]. Atezolizumab carries the highest association with ocular inflammation, while ipilimumab has the greatest association with uveitis [15]. Adverse events with CTLA-4 inhibitors appear to be dose dependent, but this may not be necessarily true for PD-1 and PD-L1 inhibitor monotherapy [13]. Additionally, the combination of ipilimumab with nivolumab is associated with greater toxicity than either ICI alone. Several studies have also shown that patients who had systemic manifestations such as colitis were also more likely to have uveitis or episcleritis [14].
4 Pathogenesis of ICI
The pathogenesis of ICI leading to ocular toxicity is not well understood. One study proposed that CTLA-4 inhibitors impair the survival and function of T cells which yields to the formation of autoimmune inflammatory disorders [16]. Conversely, PD-1 and PD-L1 inhibitors not only affect T cell function but also produce pathological autoantibodies [16]. This may be why PD-1 inhibitors are more likely to result in ocular myasthenia [16]. Further studies need to be conducted to elucidate the mechanism of adverse events.
5 Clinical Manifestations of ICI Toxicities
5.1 Eyelids, Eyebrows, and Eyelashes
5.1.1 Blepharitis and Meibomian Gland Dysfunction (MGD)
Blepharitis is a chronic inflammatory condition of the eyelids [17]. Meibomian glands in the eyelids secrete the oily layer of the tear film. This prevents tear evaporation, and thus, plays an important role in maintaining normal lubrication of the eye. Patients may present with itching, blurry vision improved with blinking, a foreign body sensation, and dry eye symptoms. Examination may reveal crusting of the eyelids and margin inflammation with inspissated oil glands [17]. It is hypothesized that treatment with checkpoint immunotherapy may trigger a T cell-mediated autoimmune response in the eyelids leading to ocular surface disease [18]. Ipilimumab has less than 1% incidence of blepharitis [17].
Management of symptoms includes daily eyelid scrubs and the use of therapeutic medications such as antibiotics and combination steroids to reduce infection and inflammation [17, 18]. Warm compresses may also be helpful as well as omega-3 fatty acids. There have been no case reports thus far of discontinuation of ICI due to blepharitis or MGD.
5.1.2 Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN)
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) belong to a spectrum of rare but potentially life-threatening complications affecting the skin and mucous membranes [19]. This dermatological emergency manifests as patches of the skin and mucous membrane inflammation that rapidly progress to epidermal necrosis and sloughing. SJS typically involves less than 10% of the total body surface area, while TEN accounts for greater than 30% of total body surface area. Medications are the causative factor in 80% of cases [19]. Patients treated with nivolumab have been reported to develop SJS [2, 20, 21]. Additionally, TEN was reported with both pembrolizumab and atezolizumab [22].
A high level of suspicion and close monitoring is recommended as SJS and TEN are potentially fatal [2]. The duration from onset of drug exposure to the development of eruption is variable, and there may be delay of several months or several cycles of treatment. SJS is automatically classified as grade 3 or higher toxicity on the CTAE scale, while TEN is grade 4 or higher [11]. Thus, discontinuation of ICI is necessary. Treatment is largely supportive, but medical therapy may include corticosteroid, intravenous immunoglobulin, cyclosporine, and TNF-α inhibitors [2].
5.2 Conjunctiva and Sclera
5.2.1 Conjunctivitis
Conjunctivitis is the inflammation of the transparent conjunctiva and typically presents as a pink hue over the white part of the eye [17]. This can be associated with swelling or chemosis, increased tearing, and discharge. It is generally not accompanied by eye pain or vision changes. Although conjunctivitis may be induced from any of the ICI, it can also be caused by infectious and allergic etiologies [23]. Therefore, patient history is important [17]. Conjunctivitis was reported in two cases with irritation and injection after nivolumab [24].
Most cases of conjunctivitis are self-limited [17]. Symptoms may last 4–6 weeks. If there is suspicion for bacterial infectious etiology, a short course of antibiotic ointment may be prescribed. Allergic conjunctivitis may be treated with over-the-counter antihistamines and mast cell inhibitors [17]. There have been no reported cases requiring cessation of ICI due to conjunctivitis [24]. One case was treated with topical corticosteroids [24].
5.2.2 Sclera
Scleral disease has been reported in all the ICI; however, they are rare presentations. Together scleritis and episcleritis make up 1% of adverse events with ICI [25].
5.2.3 Episcleritis
Episcleritis is the inflammation of the tissue between the conjunctiva and sclera [17]. Patients will report a sudden onset redness, irritation, and watering of the eye. There is minimal pain and vision is preserved. Examination may reveal vasodilation of the superficial episcleral vessels without swelling or thinning of the sclera. Cases of episcleritis have been reported with ipilimumab [8, 26]. Episcleritis can be managed with topical lubricants, oral nonsteroidal anti-inflammatory drugs (NSAIDs), or topical corticosteroid [17].
5.2.4 Scleritis
Scleritis involves the deeper connective tissue and appears darker and more violaceous compared to episcleritis [25]. This condition can be localized, nodular, or diffuse with vasodilation. Pain ranges from mild to out of proportion to examination findings based on the severity of disease. Rarely, necrosis may be seen in the most severe type. However, necrotizing scleritis has not been reported as an adverse event with ICI. Diagnosis of scleritis is based on location, redness, pain, and tenderness to touch. B-scan ultrasonography may help to diagnose posterior scleritis when a classic T-sign is observed due to the presence of fluid in Tenon’s space [27]. Nivolumab had one case report of scleritis treated with oral corticosteroids [27]. Scleritis is generally managed with NSAIDs and topical corticosteroid [25]. Severe cases may require oral corticosteroids and cessation of the ICI if poorly controlled [27].
5.3 Cornea and Tear Film
ICI can affect the cornea leading to dry eye, corneal ulcers, and deposits. The most common adverse event is dry eye. Clinical trial reports of CTLA4 and PD-1-targeting antibodies showed that the incidence of dry eye is 1.2–24.2% [10]. Another study suggested that dry eye occurred in one in four treated patients [12]. The most common ICI associated with dry eye are ipilimumab, nivolumab, and pembrolizumab [13]. Dry eyes are often not reported in clinical trials and are often underdiagnosed even in clinical practice [12].
5.3.1 Dry Eye Syndrome
Dry eye syndrome results in burning, gritty, sandy, or foreign body sensation [25]. Some may experience increased redness, sensitivity to light, and blurred vision. Corneal defects associated with dry eyes are variable. Blurry vision often improves with blinking. Diagnosis is achieved primarily from the patient’s history and symptoms. Staining with fluorescein, rose bengal, or lissamine green can reveal epithelial defects. Additionally, dry eye can be documented with rapid tear breakup time (TBUT) of <10 s (normal is >10 s) and reduced tear secretion using the Schirmer tests.
A retrospective review of the FDA Adverse Event Reporting System (FAERS) database from 2015 to 2020 demonstrated 75 reports of dry eye syndrome secondary to PD1/PD-1 inhibition [28]. Nivolumab represented 51 cases (68%) followed by pembrolizumab with 22 cases (29%). There was only one documented incidence each of dry eye with atezolizumab (1%) and durvalumab (1%) [28].
Lubrication with artificial tears is the first line of treatment as they are simple to administer and available over the counter [25]. Lid hygiene and warm compresses also play an important role in tear quality. Patients may benefit from punctal occlusion if they have reflex excess tearing caused by dry eyes. Severe cases may be improved with topical corticosteroids or ophthalmic cyclosporine [25]. Autologous serum tears have also been used with success.
5.3.2 Corneal Epithelial Defects, Keratitis, and Corneal Melts
Persistent corneal epithelial defects are often secondary to poor healing of the epithelial layer of the cornea [25]. Symptoms associated with corneal epithelial defects are similar to dry eye but more severe. Patients may experience blurred vision, burning, and eye pain. Measurement of visual acuity is critical. Nonhealing epithelial defects are at increased risk of developing infectious keratitis and corneal ulcers [25]. However, not all corneal ulcers are related to exposure. Parker et al. described a peripheral corneal ulcer secondary to nivolumab use which was resolved after discontinuation of nivolumab [23]. Corneal ulcers or infiltrates can appear as white-tan-colored lesions in the clear cornea. These conditions are sight-threatening and require immediate ophthalmologic attention [23].
Aggressive lubrication with preservative-free artificial tears or ointments is helpful. However, patients with corneal epithelial defects are often not improved on topical lubrication alone [25]. Patients generally are treated with topical antibiotics to prevent superinfection. Some cases may benefit from a bandage contact lens or temporary tarsorrhaphy although care must be taken to ensure there is no infection present [25]. Nivolumab-induced ulcerative keratitis was treated successfully with topical corticosteroids alone [29]. Another patient on atezolizumab developed autoimmune keratitis that was treated with both topical and oral corticosteroids [30].
Corneal thinning and melt are severe epithelial defects [29, 30]. They are often difficult to diagnose unless viewed through a slit lamp with a thin beam transecting the cornea. These are vision-threatening conditions that may lead to corneal perforation if left untreated. There has been one case report of corneal perforation following the use of nivolumab that did not improve with corticosteroids (both systemic and subconjunctival) [31].
5.4 Cataracts
Cataracts are opacities of the lens of the eye and are generally associated with age. Cataracts are classified as nuclear sclerotic, cortical, or posterior subcapsular based on the location in the lens where the opacification occurs. Patients will typically complain of painless, progressive vision loss. There may be symptoms of glare or halos around lights at night. Cataract formation from ICI is rare and typically found as a sequela of chronic or severe uveitis [12, 32, 33]. Posterior subcapsular cataracts are most reported in pembrolizumab, ipilimumab, and nivolumab [32, 33]. There were two cases of cataract formation with the use of nivolumab [34]. There have been no reports of cataracts with the use of atezolizumab, avelumab, or durvalumab to date. Treatment is surgical excision of the cataract and placement of an intraocular lens.
5.5 Uveitis
The uvea is composed of the iris, ciliary body, and choroid. Uveitis can be divided into four broad categories, anterior, intermediate, posterior, and panuveitis, depending on the location of the inflammation. Presentation typically occurs within 2 months after starting ICI therapy; however, there has been a case report of a patient with anterior uveitis presenting 2 years after treatment with nivolumab [12, 35]. Inflammation of the uveal tract can produce variable ophthalmic complaints depending on the location of involvement including vision loss, floaters, pain, redness, and photophobia [17]. The incidence of uveitis ranges from 0.3 to 6% [10]. Conrady et al. performed a literature review of 33 cases of uveitis secondary to ICI [36]. One-third of patients experienced anterior uveitis alone; one-third demonstrated anterior uveitis with posterior segment changes such as macular edema, retinitis, or papillitis; and one-third had panuveitis [36]. The odds ratio for uveitis ranged from 4.6 to 10.8 [12]. The risk of uveitis is greatest with the use of ipilimumab, nivolumab, pembrolizumab, and atezolizumab [13].
5.5.1 Anterior Uveitis
Anterior uveitis is characterized by white cells in the anterior chamber of the eye [17]. When it affects the iris, it is frequently referred to as iritis. When the ciliary body is also involved, it is known as iridocyclitis. Symptoms of anterior uveitis are pain, photophobia, and redness. Examination often reveals limbal flush (inflammation around the cornea and sclera). The cornea may demonstrate keratic precipitates. The key finding is white cells in the anterior chamber. The severity of anterior uveitis is determined by the number of cells seen in high-power magnification on a slit lamp. In addition, there may be a haze or flare noted which represents protein in the aqueous humor caused by disruption of the blood-aqueous barrier [17]. In severe inflammation, posterior synechiae or scarring of the iris to the lens may occur resulting in an abnormal pupil shape on attempted dilation [25].
5.5.2 Intermediate Uveitis
Leukocytes in the vitreous or inflammation of the ciliary body is referred to as intermediate uveitis [17]. Unlike anterior uveitis, intermediate uveitis is typically painless; however, vision changes such as vision loss and floaters are more prevalent [17].
5.5.3 Posterior Uveitis and Panuveitis
Posterior uveitis involves the choroid [17]. Severe choroiditis can affect the retina and optic nerve. Vision loss can be profound without pain [17]. Patients may present with floaters, photopsias, and blind spots [12]. Fluorescein angiography (FA) and indocyanine green angiography (ICG) play an important role in the diagnosis of posterior uveitis [25]. Panuveitis involves all three compartments of the eye and may present with all symptoms from eye pain to vision loss [17].
Prompt referral to an ophthalmologist is critical for the proper management of uveitis [17]. Treatment involves the initiation of topical cycloplegics and corticosteroids to prevent scarring and reduce ciliary spasms of the eye which cause pain [35]. Sometimes periocular and intraocular steroids are necessary in cases with involvement of the macula. Systemic corticosteroids are used to treat posterior or panuveitis. If systemic steroids were used, the American Society of Clinical Oncology (ASCO) recommended suspending the ICI until the patient is either off all steroids or is receiving a daily dose of 10 mg of oral prednisone or less [12]. Grade 2–4 drug toxicities may require discontinuation of ICI. In fact, most patients with uveitis in a case series (five out of seven patients) ultimately have had their treatment discontinued [12]. Patients may see a reversal of vision loss after treatment; however, a small minority may have permanent vision loss. A rechallenge can be considered in patients with mild irAE once symptoms resolve [14].
Shahzad et al. developed an algorithm based on their review of literature and international guidelines on the management of uveitis [12]. It is designed for multidisciplinary teams for use in clinical settings [12]. For patients with mild to moderate symptoms, ICI may be continued but requires a referral to ophthalmology in 1–2 weeks. For patients with moderate to severe symptoms or neurological symptoms such as diplopia, color vision, or visual field deficits, ICI should be paused, and urgent referral should be made to an ophthalmologist. Ophthalmologists can diagnose uveitis and begin management with corticosteroids and escalate therapy as needed. Additional testing such as fluorescein angiography, autofluorescence, and optical coherence tomography (OCT) may be performed. After monitoring treatment response, the ophthalmologist and oncologist can then create a multidisciplinary assessment and tailor ICI therapy [12].
Poorly controlled uveitis may lead to cataract formation, glaucoma, or choroidal neovascularization [12, 33]. Conversely, treatment of uveitis with glucocorticoids may result in elevated intraocular pressures and subsequent optic nerve damage leading to glaucoma and irreversible blindness. Thus, patients with uveitis should be followed closely [17].
5.5.4 Vogt-Koyanagi-Harada (VKH)-Like Syndrome
A recent review of 126 cases of ICI-associated uveitis found that 35% of panuveitis cases were part of Vogt-Koyanagi-Harada (VKH)-like syndrome [12]. VKH is an inflammatory disease process against melanocytes characterized by chronic diffuse granulomatous panuveitis [25, 35]. VKH is mediated by T lymphocytes similar to the mechanism of action of ICI, with presumed mechanisms to make ICI have a high incidence of VKH. CTLA-4 and PD1 and PD-L1 antibodies may disrupt the immune tolerance to melanocytes and trigger VKH [25]. VKH-like syndrome has been reported in patients receiving combination therapy ipilimumab and pembrolizumab as well as nivolumab [37, 38].
Classically, VKH presents as a bilateral panuveitis with exudative retinal detachments. Vision loss can be profound in both eyes. Associated symptoms include cutaneous changes—vitiligo, poliosis, alopecia, and neurological deficits—tinnitus, hearing loss, meningismus, and headache. Cerebrospinal fluid analysis may reveal pleocytosis. However, VKH-like syndrome may have some distinct features. For example, VKH-like syndrome did not feature pleocytosis or meningismus symptoms [39]. The diagnosis of VKH should be in the absence of prior ocular trauma or surgery and any other ocular disease based on clinical or laboratory evidence [35].
VKH has four stages with different clinical features [35, 39]. Stage I is the prodromal stage in which the patient may suffer nonspecific viral illness with nausea, vomiting, and headache for up to a week. Stage 2 presents with ophthalmic symptoms and signs of uveitis. This may lead to serous retinal detachments and subsequent vision loss. During stage 3, convalescence, choroidal depigmentation results in a sunset glow fundus. At this point, the patient generally develops vitiligo and poliosis due to an autoimmune attack on melanocytes. Stage 4 is the chronic recurrent stage in which recurrent uveitis is predominant. Patients may develop complications from uveitis as described above. Ipilimumab and nivolumab have been implicated in a presentation very similar to classic VKH [35, 40]. The time to onset after immunotherapy ranges from 2 weeks to 13 months [17].
Treatment is the same as the management of uveitis with topical and systemic corticosteroids [17]. Most cases of drug-induced VKH require stopping ICI. In one case report, treatment with ICI was continued with the use of subconjunctival steroids, and no recurrence of symptoms has occurred to date [37].
5.5.5 Uveal Effusion
Uveal effusion is a rare adverse event with the use of ICI, particularly with PD-1 and PD-LI inhibitors atezolizumab, nivolumab, or pembrolizumab [41]. Only three patients have been reported to develop large uveal effusions. These first appeared between 1 and 3 months after two infusions of ICI [41]. Two out of three patients discontinued therapy with resolution of uveal effusion after several weeks. The last patient succumbed to melanoma [41]. Similarly, Telfah described two patients on pembrolizumab who presented with vision loss bilaterally and were found to have choroidal effusions leading to exudative retinal detachments [42]. The first patient developed irAE 1 year after initiating treatment, while the second suffered vision loss more acutely within weeks. Once the ICI was stopped, the vision returned to baseline in both patients. One of the patients resumed ICI after 4 months; however, his symptoms recurred, and ICI was permanently discontinued [42].
5.6 Retina and Vitreous
ICI can produce variable toxicity to the retina. The retinal vasculature may be at risk in developing intraretinal hemorrhages, cotton wool spots, and choroidal neovascularization. Damage to the retinal pigment epithelium may lead to pigmentary retinopathy or intraretinal fluid known as macular edema. Involvement of photoreceptors may lead to flashes or night blindness.
5.6.1 Retinal Tears and Detachments
According to the retrospective review of the FAERS study from 2015 to 2020, there were 49 reported cases of retinal detachments secondary to PD-1 and PD-L1 inhibition [28]. Nivolumab had 30 associated cases (61%), while pembrolizumab had 11 reported cases (22%). Eight cases were reported with the use of atezolizumab [28]. Treatment of retinal detachments includes scleral buckle, pars plana vitrectomy, or cryotherapy.
5.6.2 Macular Edema
Cystoid macular edema is defined as multiple cyst-like fluid spaces in the central retina resulting in swelling or edema. Patients present with painless blurry vision or metamorphopsia, or the perception that straight lines appear wavy. Most cases of macular edema occur in conjunction with uveitis or optic neuropathy.
5.6.3 Choroidal Neovascularization
Only one case report has documented choroidal neovascularization with ipilimumab treatment thus far [43]. The patient presented with blurred vision 1 year after starting ICI therapy. Macular edema and choroidal neovascularization were confirmed on OCT. The patient was treated with intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy with resolution of symptoms 2 years later without discontinuation of ipilimumab [43].
5.6.4 Other
Grade 3 chorioretinitis was seen in one patient treated with ipilimumab which led to treatment discontinuation [44]. There has been one case report of a patient treated with pembrolizumab who developed a white dot syndrome known as birdshot-like chorioretinopathy 2 years after treatment [45]. Combined FA/ICG showed hypocyanescent lesions without retinal vasculitis in both eyes. The patient underwent testing for HLA-A29 which generally is positive in 95% of patients with birdshot chorioretinopathy; however, this patient was negative. The patient was treated with triamcinolone injections in both eyes with partial resolution [45]. There has been one case report of retinal vasculitis which developed after pembrolizumab treatment which improved after vitrectomy and external beam radiotherapy for vitreous metastases [46]. Another case report details the development of melanoma-associated retinopathy (MAR) with atypical chorioretinal lesions after treatment with pembrolizumab [47]. On the other hand, another study reported photoreceptor toxicity secondary to nivolumab [48].
5.7 Neuro-ophthalmology
Neuro-ophthalmic adverse events are extremely rare but visually significant. Ocular myasthenia risk was only associated with nivolumab and pembrolizumab [13]. The reported cumulative incidence of severe neurotoxicities related to ICI is estimated at less than 1% [49]. A review of ocular irAE found that neurological-related events occurred at a median of 35 days (5 weeks) after initiating ICI therapy [13, 49]. This included cases of optic neuritis and myasthenia gravis [13, 48].
5.7.1 Optic Nerve Edema and Neuropathy
Optic neuropathy may present as optic nerve edema, optic atrophy, idiopathic intracranial hypertension, or extraocular motility deficits. It has a variable presentation from asymptomatic to enlarged blind spot or constriction of visual fields. Two cases of optic neuropathy have been associated with ipilimumab [51]. The patients presented with visual disturbances such as scotomas or visual field deficits [51]. Another case report documented a patient with previously 20/20 vision who developed vision loss down to 20/200 within days after his fourth infusion of ipilimumab [52]. Examination demonstrated optic nerve edema with correlating visual field defects. OCT imaging confirmed moderate optic nerve atrophy [52]. Six months after treatment with topical steroids, the patient’s vision returned to baseline; however, the visual field defects persisted [52].
5.7.2 Optic Neuritis
Optic neuritis is a rare presentation with ICI. Patients will complain of blurred vision, color vision loss, and pain with eye movements. One patient with nivolumab-induced optic neuritis complained of photopsias only [53]. Examination may reveal afferent pupillary defect if there is asymmetry of optic nerve involvement. Fundus exam may show optic nerve edema if the anterior optic nerve is involved or a normal fundus in which case may represent retrobulbar optic neuritis. MRI orbits will reveal optic nerve enhancement. Duong reported a patient who developed demyelinating disease secondary to combination ipilimumab with nivolumab therapy [49]. MRI revealed bilateral optic nerve enhancement. Cerebrospinal fluid (CSF) studies were negative for infection and neuromyelitis optica (NMO) and paraneoplastic (PNP) antibodies. The patient was treated with intravenous steroids for 5 days followed by intravenous immunoglobulin (IVIG) for 2 days and then transitioned to oral prednisone [49]. Later she relapsed and was treated with one dose of infliximab with some improvement overall in vision [49]. Another case report detailed a pediatric patient who was treated with nivolumab monotherapy and developed bilateral optic neuritis after the second infusion [54]. A large case series of 18 patients found that combination therapy created a higher short-term risk for optic neuritis, while protracted use of a single-agent ICI was associated with a delayed risk [50].
5.8 Orbital Inflammation
ICI have been reported to develop inflammation of the orbit, particularly with ipilimumab [14]. Symptoms associated with orbital inflammation are pain with eye movement, orbital congestion, proptosis, subsequent exposure keratopathy, ophthalmoplegia, diplopia, and blindness if severe. Examination should include visual acuity, pupil assessment to rule out an afferent pupillary defect, color plates to look for desaturation, extraocular motility testing in addition to slit lamp, and dilated fundus examination. Imaging with CT scan and MRI of the orbits helps elucidate extraocular muscle enlargement and involvement of muscle tendons [17].
5.8.1 Thyroid Eye Disease
Thyroid eye disease (TED) is also known as Graves’ disease. It occurs when there is an abnormal expression of thyrotropin receptor antibody and T cell response. The T cell response seen in TED is similar to the mechanism of action in CTLA-4 and PD1/PD-L1 antibodies [55]. The result is inflammation of the orbit and extraocular muscles sparing the tendons [55]. Other associated symptoms include eye pain, redness, diplopia, and blurred vision from compressive ophthalmopathy [25]. Signs of TED may be eyelid retraction, and lid lag in downgaze with proptosis, and restrictive strabismus. Symptoms can present after the first dose of ICI but may be delayed even 2 years later [56]. Hertel measurements can be used to monitor for progression [57]. Enlargement of extraocular muscles and fat on imaging may lead to compressive optic neuropathy [25]. Laboratory workup for thyroid function may assist with diagnosis; however, normal test results do not necessarily exclude disease if clinical suspicion is high [56]. Treatment may range from lubrication and NSAIDs for redness and pain to corticosteroids and even decompressive surgery if vision is threatened by compressive orbitopathy [25]. Fresnel prisms can be used to control diplopia [57].
A case report described a patient who developed severe Graves’ disease with proptosis and diplopia after two doses of ipilimumab [55, 58]. The patient’s blood work returned elevated antithyroglobulin and thyroid-stimulating hormone receptor antibodies, while TSH, T3, and T4 were within normal limits [55, 58]. Imaging revealed diffuse enlargement of all extraocular muscles consistent with TED [55]. The patient required oral corticosteroids and orbital decompression with resolution of symptoms. Similarly, pembrolizumab has also been linked to new-onset Graves’ ophthalmopathy [57]. Thyroid function studies may be unremarkable. Imaging revealed asymmetric proptosis consistent with TED. Treatment is the cessation of ICI and initiation of corticosteroid.
5.8.2 Orbital Myositis
Orbital myositis has been reported in patients treated with ICI. A patient treated with ipilimumab developed ophthalmoplegia and proptosis after the third infusion [59]. Imaging with MRI revealed diffuse EOM enlargement that involved the tendons. Typically, the tendons are spared in thyroid ophthalmopathy. Ultrasound demonstrated hypo-reflectivity of the extraocular muscles which was more consistent with myositis or orbital inflammatory syndrome [59]. Ipilimumab was discontinued and the patient required prolonged steroid taper over 5 months [59]. Another case was diagnosed with orbital inflammatory syndrome and hypophysitis, secondary to ipilimumab [60]. The patient similarly presented with ophthalmoplegia. Treatment with ICI was stopped, and the patient underwent oral corticosteroid treatment with mild residual abduction deficit remaining [60].
5.8.3 Myasthenia Gravis
Myasthenia gravis (MG) is a neuromuscular disorder of the postsynaptic neuromuscular junction that causes variable muscle weakness involving the eyes, mouth, limbs, and respiration. Nivolumab had the highest association with an OR of 22.82 followed by pembrolizumab with an OR of 20.17 [13, 15]. Ipilimumab has also been implicated in the development of MG [61]. Patients with ocular MG may present with variable ptosis and diplopia [62]. Kamo described two cases of patients taking pembrolizumab who developed autoimmune myositis resembling MG with the involvement of the levator palpebrae superioris and extraocular muscles [63]. However, MG-related antibodies were negative [63]. Diagnosis is clinical as autoantibodies to acetylcholine receptors, binding, blocking, and modulating may be negative. The patients may be managed with discontinuation of ICI and the start of steroids or intravenous immunoglobulin may be considered [15]. Additionally, patients can be treated with plasmapheresis and pyridostigmine [51].
5.8.4 Giant Cell Arteritis
Giant cell arteritis (GCA) or temporal arteritis is inflammation of the large vessels of the body. GCA has been reported more frequently for ipilimumab (n = 10) than for nivolumab (n = 3) or pembrolizumab (n = 4) [64]. Vision can be affected profoundly. Two patients presented with blurred vision, headache, jaw claudication, and myalgias after treatment with ipilimumab [51, 65]. Both patients were treated with high-dose systemic corticosteroids [51, 65]. The third patient presented with scalp necrosis 6 weeks after initiation of nivolumab [64]. She did not complain of any visual changes at the time. Temporal artery biopsy confirmed GCA [64].
5.9 Glaucoma
Glaucoma is a disease of the optic nerve that can lead to irreversible vision loss [66]. Characteristic features of glaucoma include cupping of the optic disc and corresponding visual field deficits due to retinal ganglion cell loss. Glaucoma is thought to arise from increased resistance to aqueous outflow which may be associated with elevated intraocular pressures [66]. The mechanism is not well understood, but studies have shown that lowering intraocular pressure is effective at preventing progression of glaucoma [66]. ICI have indirectly been linked to the development of glaucoma such as a complication of poorly controlled uveitis or from autoimmune thyroid disease [12, 33]. To date there have been no reports of direct formation of glaucoma from ICI.
5.9.1 Hypotony
Hypotony is the presence of low intraocular pressure defined as below 6 mmHg [67]. Ocular hypotony has been documented in a patient on treatment with pembrolizumab. Nguyen described sequential vision loss down to 20/200 after mild blunt trauma [67]. Examination revealed a pressure of 0 mmHg with minimal inflammation. Intraoperatively, the patient had profound ciliary body atrophy. The patient underwent multiple tests and procedures to reverse the hypotony including high-dose systemic steroids, but the response was limited [67].
6 Patient Education and Multidisciplinary Approach
Early recognition and treatment initiation is essential to the management of irAEs. Communication between ophthalmologists, primary care physicians, and oncologists can help guide diagnosis and management for patients with ocular irAE related to checkpoint immunotherapy [68]. Patient education also provides awareness and promotes discussion on what to expect during cancer treatment [69, 70]. With improved education, patients are more likely to adhere to treatment regimens and have a better overall prognosis. Oncological societies such as the American Cancer Society, cancer centers, and pharmaceutical companies are launching patient education programs to share important signs and symptoms that may herald an adverse event [69, 70]. A multidisciplinary approach will minimize the impact of ocular toxicity.
7 Conclusion
ICI has provided new hope in the treatment of advanced malignancies. Despite their success, ICI have been associated with a broad spectrum of adverse events. Although ocular side effects account for 1% of total adverse events, they are becoming more commonplace as more people undergo treatment with ICI. Recognizing ocular adverse events early is important as they can impact a patient’s quality of life. Most irAEs are mild and do not require discontinuation of ICI. Treatment with corticosteroids assists with more moderate cases. Patients with severe and sight-threatening adverse events should have their immunotherapy discontinued. This may come at a cost as tumor progression can be noted after cessation of ICI. Management should involve ophthalmologists and oncologists. Risks and benefits should be addressed with patients. With improved education, patients are more likely to adhere to treatment regimens and have a better overall prognosis of advanced malignancies. In conclusion patients who present with ocular complaints should be referred to an ophthalmologist for early identification and intervention.
References
Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist. 2015;20(7):812–22. https://doi.org/10.1634/theoncologist.2014-0422. Epub 2015 Jun 11.
Tattersall IW, Leventhal JS. Cutaneous toxicities of immune checkpoint inhibitors: the role of the dermatologist. Yale J Biol Med. 2020;93(1):123–32.
Dobosz P, Dzieciątkowski T. The intriguing history of cancer immunotherapy. Front Immunol. 2019;10:2965. https://doi.org/10.3389/fimmu.2019.02965.
Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11. https://doi.org/10.1038/s12276-018-0191-1.
Wierenga APA, Cao J, Luyten GPM, Jager MJ. Immune checkpoint inhibitors in uveal and conjunctival melanoma. Int Ophthalmol Clin. 2019;59(2):53–63. https://doi.org/10.1097/IIO.0000000000000263.
Immune checkpoint inhibitors and their side effects. In: Immunotherapy, American Cancer Society; 2019.
Postow M, et al. Toxicities associated with checkpoint inhibitor immunotherapy. UpToDate, Wolters Kluwer; 2021.
Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Restifo NP, Haworth LR, Levy C, Mavroukakis SA, Nichol G, Yellin MJ, Rosenberg SA. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–53. https://doi.org/10.1200/JCO.2005.06.205. Epub 2005 Aug 8.
Ito F, Ernstoff MS. Immune checkpoint inhibitors in cancer. Elsevier; 2019.
Abdel-Rahman O, Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, Schöb O, Giryes A. Immune-related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev Anticancer Ther. 2017;17(4):387–94. https://doi.org/10.1080/14737140.2017.1296765. Epub 2017 Feb 24.
Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. National Institutes of Health, U.S. Department of Health and Human Services; 2017.
Shahzad O, Thompson N, Clare G, Welsh S, Damato E, Corrie P. Ocular adverse events associated with immune checkpoint inhibitors: a novel multidisciplinary management algorithm. Ther Adv Med Oncol. 2021;13:1758835921992989. https://doi.org/10.1177/1758835921992989.
Fang T, Maberley DA, Etminan M. Ocular adverse events with immune checkpoint inhibitors. J Curr Ophthalmol. 2019;31(3):319–22. https://doi.org/10.1016/j.joco.2019.05.002.
Papavasileiou E, Prasad S, Freitag SK, Sobrin L, Lobo AM. Ipilimumab-induced ocular and orbital inflammation—a case series and review of the literature. Ocul Immunol Inflamm. 2016;24(2):140–6. https://doi.org/10.3109/09273948.2014.1001858. Epub 2015 Mar 11.
Bindiganavile S, Bhat N, Lee A, Gombos S, Al-Zubidi N. Targeted cancer therapy and its ophthalmic side effects: a review. J Immunother Precis Oncol. 2021;4(1):6–15.
Inno A, Metro G, Bironzo P, Grimaldi AM, Grego E, Di Nunno V, Picasso V, Massari F, Gori S. Pathogenesis, clinical manifestations and management of immune checkpoint inhibitors toxicity. Tumori. 2017;103(5):405–21. https://doi.org/10.5301/tj.5000625. Epub 2017 May 10.
Liu C, et al. Ocular side effects of systemically administered chemotherapy. UpToDate, Wolters Kulwer; 2021.
Brouwer NJ, Haanen JBAG, Jager MJ. Development of ocular rosacea following combined ipilimumab and nivolumab treatment for metastatic malignant skin melanoma. Ocul Oncol Pathol. 2017;3(3):188–92. https://doi.org/10.1159/000455150. Epub 2017 Feb 2.
Oakley AM, Krishnamurthy K. Stevens Johnson syndrome. [Updated 2020 Nov 20]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK459323/.
Keerty D, Koverzhenko V, Belinc D, LaPorta K, Haynes E. Immune-mediated toxic epidermal necrolysis. Cureus. 2020;12(8):e9587. https://doi.org/10.7759/cureus.9587.
Haratake N, Tagawa T, Hirai F, Toyokawa G, Miyazaki R, Maehara Y. Stevens-Johnson syndrome induced by pembrolizumab in a lung cancer patient. J Thorac Oncol. 2018;13(11):1798–9. https://doi.org/10.1016/j.jtho.2018.05.031. Epub 2018 Jun 6.
Hsu TJ, Yeh HH, Lee CH, Liu KL. Stevens-Johnson syndrome and toxic epidermal necrolysis in a referral center in Taiwan. Int J Dermatol. 2021;60(8):964–72. https://doi.org/10.1111/ijd.15586.
Parker JS, Feagin W, Wang C, Heersink M, Parker JS. Corneal ulceration associated with Nivolumab use. Am J Ophthalmol Case Rep. 2019;14:26–7. https://doi.org/10.1016/j.ajoc.2019.01.013.
Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I, Schmidgen MI, Gutzmer R, Utikal JS, Göppner D, Hassel JC, Meier F, Tietze JK, Forschner A, Weishaupt C, Leverkus M, Wahl R, Dietrich U, Garbe C, Kirchberger MC, Eigentler T, Berking C, Gesierich A, Krackhardt AM, Schadendorf D, Schuler G, Dummer R, Heinzerling LM. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–25. https://doi.org/10.1016/j.ejca.2016.02.024. Epub 2016 Apr 13.
Liu X, Wang Z, Zhao C, Wang H, Guo X, Zhou J, Duan L, Si X, Zhang L, Li Y, Wang M, Zhang M, Zhang L. Clinical diagnosis and treatment recommendations for ocular toxicities of targeted therapy and immune checkpoint inhibitor therapy. Thorac Cancer. 2020;11(3):810–8. https://doi.org/10.1111/1759-7714.13327. Epub 2020 Feb 4.
Khoja L, Atenafu EG, Ye Q, et al. Real-world efficacy, toxicity and clinical management of ipilimumab treatment in metastatic melanoma. Oncol Lett. 2016;11(2):1581–5. https://doi.org/10.3892/ol.2015.4069.
Gonzales JA, Shantha J, Acharya NR. Combination nivolumab- and cabiralizumab-associated acute bilateral anterior and posterior scleritis and anterior uveitis. Am J Ophthalmol Case Rep. 2018;10:117–8. https://doi.org/10.1016/j.ajoc.2018.02.005.
Sahu Y, Ensor J, Burns E, et al. Ocular adverse events due to PD-1 and PD-L1 checkpoint inhibitors: a retrospective review of FDA adverse events reporting system (FAERS). In: ESMO immuno-oncology virtual congress; 2020.
Losonczy G, Gijs M, Nuijts RMMA. Nivolumab-induced ulcerative keratitis-a case report. Cornea. 2021;40(5):656–8. https://doi.org/10.1097/ICO.0000000000002460.
Oh J. Autoimmune keratitis after atezolizumab treatment. N Engl J Med. 2020;383:1468. https://doi.org/10.1056/NEJMicm1910925.
Le Fournis S, Gohier P, Urban T, Jeanfaivre T, Hureaux J. Corneal graft rejection in a patient treated with nivolumab for primary lung cancer. Lung Cancer. 2016;102:28–9. https://doi.org/10.1016/j.lungcan.2016.10.008. Epub 2016 Oct 22.
Lee JC, Al-Humimat G, Kooner KS. Acute bilateral uveitis, hypotony, and cataracts associated with ipilimumab and nivolumab therapy: optical coherence tomography angiography findings. Case Rep Ophthalmol. 2020;11(3):606–11. https://doi.org/10.1159/000509629.
Basilious A, Lloyd JC. Posterior subcapsular cataracts and hypotony secondary to severe pembrolizumab induced uveitis: case report. Can J Ophthalmol. 2016;51(1):e4–6. https://doi.org/10.1016/j.jcjo.2015.09.008.
Maruyama D, Hatake K, Kinoshita T, Fukuhara N, Choi I, Taniwaki M, Ando K, Terui Y, Higuchi Y, Onishi Y, Abe Y, Kobayashi T, Shirasugi Y, Tobinai K. Multicenter phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma. Cancer Sci. 2017;108(5):1007–12. https://doi.org/10.1111/cas.13230. Epub 2017 May 19.
Witmer MT. Treatment of ipilimumab-induced Vogt-Koyanagi-Harada syndrome with oral dexamethasone. Ophthalmic Surg Lasers Imaging Retina. 2017;48(11):928–31. https://doi.org/10.3928/23258160-20171030-09.
Conrady CD, Larochelle M, Pecen P, Palestine A, Shakoor A, Singh A. Checkpoint inhibitor-induced uveitis: a case series. Graefes Arch Clin Exp Ophthalmol. 2018;256(1):187–91. https://doi.org/10.1007/s00417-017-3835-2. Epub 2017 Oct 27.
Yoshida S, Shiraishi K, Mito T, Sayama K. Vogt-Koyanagi-Harada-like syndrome induced by immune checkpoint inhibitors in a patient with melanoma. Clin Exp Dermatol. 2020;45(7):908–11. https://doi.org/10.1111/ced.14282. Epub 2020 Jul 5.
Gambichler T, Seifert C, Lehmann M, Lukas C, Scheel C, Susok L. Concurrent Vogt-Koyanagi-Harada disease and impressive response to immune checkpoint blockade in metastatic melanoma. Immunotherapy. 2020;12(7):439–44. https://doi.org/10.2217/imt-2019-0206. Epub 2020 Apr 19.
Kikuchi R, Kawagoe T, Hotta K. Vogt-Koyanagi-Harada disease-like uveitis following nivolumab administration treated with steroid pulse therapy: a case report. BMC Ophthalmol. 2020;20(1):252. https://doi.org/10.1186/s12886-020-01519-5.
Obata S, Saishin Y, Teramura K, Ohji M. Vogt-Koyanagi-Harada disease-Like Uveitis during Nivolumab (Anti-PD-1 Antibody) treatment for metastatic cutaneous malignant melanoma. Case Rep Ophthalmol. 2019;10(1):67–74. https://doi.org/10.1159/000496682.
Thomas M, Armenti ST, Ayres MB, Demirci H. Uveal effusion after immune checkpoint inhibitor therapy. JAMA Ophthalmol. 2018;136(5):553–6. https://doi.org/10.1001/jamaophthalmol.2018.0920.
Telfah M, Whittaker TJ, Doolittle C, G. Vision loss with pembrolizumab treatment: a report of two cases. J Oncol Pharm Pract. 2019;25(6):1540–6. https://doi.org/10.1177/1078155219841683. Epub 2019 Apr 18.
Modjtahedi BS, Maibach H, Park S. Multifocal bilateral choroidal neovascularization in a patient on ipilimumab for metastatic melanoma. Cutan Ocul Toxicol. 2013;32(4):341–3. https://doi.org/10.3109/15569527.2013.781618. Epub 2013 May 28.
Jacobsoone-Ulrich A, Jamme P, Alkeraye S, Dzwiniel V, Faure E, Templier C, Mortier L. Ipilimumab in anti-PD1 refractory metastatic melanoma: a report of eight cases. Melanoma Res. 2016;26(2):153–6. https://doi.org/10.1097/CMR.0000000000000221.
Acaba-Berrocal LA, Lucio-Alvarez JA, Mashayekhi A, Ho AC, Dunn JP, Shields CL. Birdshot-like chorioretinopathy associated with pembrolizumab treatment. JAMA Ophthalmol. 2018;136(10):1205–7. https://doi.org/10.1001/jamaophthalmol.2018.1851.
Manusow JS, Khoja L, Pesin N, et al. Retinal vasculitis and ocular vitreous metastasis following complete response to PD-1 inhibition in a patient with metastatic cutaneous melanoma. J Immunother Cancer. 2014;2:41.
Roberts P, Fishman GA, Joshi K, Jampol LM. Chorioretinal lesions in a case of melanoma-associated retinopathy treated with pembrolizumab. JAMA Ophthalmol. 2016;134(10):1184–8. https://doi.org/10.1001/jamaophthalmol.2016.2944.
Reddy M, Chen JJ, Kalevar A, Terribilini R, Agarwal A. Immune retinopathy associated with nivolumab administration for metastatic non-small cell lung cancer. Retin Cases Brief Rep. 2020;14(2):120–6. https://doi.org/10.1097/ICB.0000000000000675.
Duong SL, Barbiero FJ, Nowak RJ, Baehring JM. Neurotoxicities associated with immune checkpoint inhibitor therapy. J Neuro-Oncol. 2021;152(2):265–77. https://doi.org/10.1007/s11060-021-03695-w. Epub 2021 Jan 17.
Francis JH, Jaben K, Santomasso BD, Canestraro J, Abramson DH, Chapman PB, Berkenstock M, Aronow ME. Immune checkpoint inhibitor-associated optic neuritis. Ophthalmology. 2020;127(11):1585–9. https://doi.org/10.1016/j.ophtha.2020.05.003. Epub 2020 May 8.
Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina. 2018;38(6):1063–78. https://doi.org/10.1097/IAE.0000000000002181.
Yeh OL, Francis CE. Ipilimumab-associated bilateral optic neuropathy. J Neuroophthalmol. 2015;35(2):144–7. https://doi.org/10.1097/WNO.0000000000000217.
Ahluwalia A, Kohli AA. Photopsias in the setting of nivolumab therapy. J Neuroophthalmol. 2021;41(1):e25–6. https://doi.org/10.1097/WNO.0000000000000909.
Kartal Ö, Ataş E. Bilateral Optic neuritis secondary to nivolumab therapy: a case report. Medicina (Kaunas). 2018;54(5):82. doi:https://doi.org/10.3390/medicina54050082.
Borodic G, Hinkle DM, Cia Y. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthalmic Plast Reconstr Surg. 2011;27(4):e87–8. https://doi.org/10.1097/IOP.0b013e3181ef72a1.
McElnea E, Ní Mhéalóid A, Moran S, Kelly R, Fulcher T. Thyroid-like ophthalmopathy in a euthyroid patient receiving Ipilimumab. Orbit. 2014;33(6):424–7. https://doi.org/10.3109/01676830.2014.949792. Epub 2014 Sep 10.
Park ESY, Rabinowits G, Hamnvik OR, Dagi LR. A case of Graves’ ophthalmopathy associated with pembrolizumab (Keytruda) therapy. J AAPOS. 2018;22(4):310–2. https://doi.org/10.1016/j.jaapos.2018.01.006. Epub 2018 Apr 4.
Renouf DJ, Velazquez-Martin JP, Simpson R, Siu LL, Bedard PL. Ocular toxicity of targeted therapies. J Clin Oncol. 2012;30(26):3277–86. https://doi.org/10.1200/JCO.2011.41.5851. Epub 2012 May 29.
Sheldon CA, Kharlip J, Tamhankar MA. Inflammatory orbitopathy associated with ipilimumab. ophthalmic. Plast Reconstr Surg. 2017;33(3S Suppl 1):S155–8. https://doi.org/10.1097/IOP.0000000000000509.
Henderson AD, Thomas DA. A case report of orbital inflammatory syndrome secondary to ipilimumab. Ophthalmic Plast Reconstr Surg. 2015;31(3):e68–70. https://doi.org/10.1097/IOP.0000000000000081.
Johnson DB, Saranga-Perry V, Lavin PJ, et al. Myasthenia gravis induced by ipilimumab in patients with metastatic melanoma. J Clin Oncol. 2015;33(33):e122–4. https://doi.org/10.1200/JCO.2013.51.1683.
Onda A, Miyagawa S, Takahashi N, Gochi M, Takagi M, Nishino I, Suzuki S, Oishi C, Yaguchi H. Pembrolizumab-induced ocular myasthenia gravis with anti-titin antibody and necrotizing myopathy. Intern Med. 2019;58(11):1635–8. https://doi.org/10.2169/internalmedicine.1956-18. Epub 2019 Feb 1.
Kamo H, Hatano T, Kanai K, Aoki N, Kamiyama D, Yokoyama K, Takanashi M, Yamashita Y, Shimo Y, Hattori N. Pembrolizumab-related systemic myositis involving ocular and hindneck muscles resembling myasthenic gravis: a case report. BMC Neurol. 2019;19(1):184. https://doi.org/10.1186/s12883-019-1416-1.
Kreuter A, Koushk-Jalali B, Cusenza A, Oellig F, Tigges C. Nivolumab-associated giant cell arteritis with scalp necrosis. JAMA Dermatol. 2019;155(9):1086–7. https://doi.org/10.1001/jamadermatol.2019.1411.
Goldstein BL, Gedmintas L, Todd DJ. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis Rheumatol. 2014;66(3):768–9. https://doi.org/10.1002/art.38282.
Anderson DR, Normal Tension Glaucoma Study. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003;14(2):86–90. https://doi.org/10.1097/00055735-200304000-00006.
Nguyen M, Islam MR, Lim SW, Sahu A, Tamjid B. Pembrolizumab induced ocular hypotony with near complete vision loss, interstitial pulmonary fibrosis and arthritis. Front Oncol. 2019;9:944. https://doi.org/10.3389/fonc.2019.00944.
Antoun J, Titah C, Cochereau I. Ocular and orbital side-effects of checkpoint inhibitors: a review article. Curr Opin Oncol. 2016;28(4):288–94. https://doi.org/10.1097/CCO.0000000000000296.
Naing A, Hajjar J, Gulley JL, Atkins MB, Ciliberto G, Meric-Bernstam F, Hwu P. Strategies for improving the management of immune-related adverse events. J Immunother Cancer. 2020;8(2):e001754. https://doi.org/10.1136/jitc-2020-001754.
Reynolds KL, Cohen JV, Zubiri L, Stern TA, editors. Facing immunotherapy. Boston: Octal Productions, LLC; 2020.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mazharuddin, A., Gombos, D.S., Al-Zubidi, N. (2022). The Rise in Immunotherapy and Associated Ocular Toxicities. In: Chawla, B.V., Aronow, M.E. (eds) Global Perspectives in Ocular Oncology. Springer, Cham. https://doi.org/10.1007/978-3-031-08250-4_33
Download citation
DOI: https://doi.org/10.1007/978-3-031-08250-4_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-08249-8
Online ISBN: 978-3-031-08250-4
eBook Packages: MedicineMedicine (R0)