Abstract
Orbital and periorbital malignancies account for a significant proportion of cancers in ophthalmic oncology, encompass a wide variety of histologies, and are more frequently encountered than intraocular tumors. In this chapter, we focus on the major types of orbital and periorbital cancers: lymphoma, melanoma, squamous cell carcinoma, basal cell carcinoma, and lacrimal gland tumors. Treatments are often multidisciplinary and aim to eliminate cancer with the least ocular morbidity possible. Surgery remains the mainstay treatment; however, newer medical treatments such as immunotherapy and targeted biologic treatments have been a welcome addition for locally advanced and metastatic disease. For low-grade ocular adnexal lymphoma, significantly lower doses of radiation compared with “standard dose” have demonstrated good efficacy, lowering the morbidity and cost associated with radiotherapy. Finally, perspectives on sentinel node biopsy for detection of early microscopic disease associated with eyelid cancers and multidisciplinary eye-sparing treatments for lacrimal gland carcinomas will be highlighted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Orbital tumors
- Basal cell carcinoma
- Squamous cell carcinoma
- Ocular adnexal lymphoma
- Lacrimal gland tumors
- Eyelid and periocular melanoma
- Immunotherapy
- Targeted therapy
1 Eyelid and Periocular Melanoma
Melanoma of the periocular region is rare, comprising less than 2% of all eyelid lesions [1, 2]. It may arise on eyelid skin, conjunctiva, or both. Eyelid skin melanoma behaves similarly to cutaneous melanoma in other anatomic sites and thus histologic features such as tumor thickness (Breslow thickness), presence of ulceration (microscopic, histologic ulceration), and mitotic figures are correlated with the risk of nodal and distant metastases and disease-free survival [3, 4]. The status of sentinel lymph node (SLN) is viewed as the single most important prognostic factor for patients with early-stage cutaneous melanoma [5].
The majority of patients with newly diagnosed eyelid melanoma have early-stage disease. In such patients, surgery is the treatment of choice and is curative in most cases [6]. Two significant recent advances that will be highlighted in this section in relation to management of periocular melanomas are sentinel lymph node (SLN) biopsy for identifying microscopic nodal metastasis and availability of targeted therapy and immune checkpoint inhibitors for patients with metastatic or locally advanced melanoma.
1.1 Sentinel Node Biopsy for Conjunctival and Periocular Melanomas
Status of local lymph nodes is a major prognostic factor and is highly correlated with survival in patients with cutaneous and conjunctival melanoma [7,8,9]. Sentinel lymph node biopsy is a method of biopsying one or two lymph nodes that drain the lymphatics from a given anatomic region. The sentinel lymph node(s) that are biopsied are then carefully evaluated through breadloafing and careful pathologic evaluation, to find microscopic metastasis. The technique used at our center entails the injection of 0.3–0.4 mci of technetium in 0.2 cc volume around the site of the primary lesion by the ophthalmic plastic surgeon 24 h prior to surgery [8]. For conjunctival lesions, the injection is subconjunctival and for periocular skin melanoma, the injection is intradermal. Preoperative lymphoscintigraphy and more recently single-photon emission computerized tomography/computed tomography (SPECT/CT) scans (Fig. 1) are obtained prior to surgery to help with localization of the sentinel lymph node. Intraoperatively, a handheld gamma probe is used transcutaneously to confirm increased radioactivity overlying a lymph node basin. The sentinel lymph nodes are then dissected out and sent for histopathologic evaluation.
(a) SPECT/CT scan in a patient with caruncular conjunctival melanoma demonstrating the area of injection of technetium in the subconjunctival space in the right caruncular area (arrow). (b) A coronal image showing the draining sentinel lymph node in the submandibular basin (arrow). (c) An axial image showing the same draining sentinel lymph node (arrow)
In a recent American Academy of Ophthalmology Technology Transfer review article, the authors reported that there have been 127 reported cases of sentinel lymph node biopsy for periocular melanoma; 85 of these are conjunctival and 42 are eyelid cutaneous melanoma [10]. Of these patients, 19 (15%) were found to have tumor-positive sentinel node biopsy result. Included in this group, is the largest single cohort of 51 patients from our institution that had a similar positivity rate of 20%, or 10 patients [8]. The median number of SLN sampled was 2 and the most common lymph node basins sampled were the intraparotid and level 2. This publication also demonstrated that certain key histological features correlate with risk of positive SLN biopsy; tumor thickness, greater number of mitotic figures, and presence of histologic ulceration. Importantly, this study and others, examining SLN biopsy for different periocular cancers, have shown excellent safety profile with adverse events reported in 17 of 197 patients [10]. These included seven cases of transient conjunctival staining with blue dye, seven patients with transient facial nerve weakness, one case of neck hematoma, and one case of suture abscess [10].
The role of positive sentinel node biopsy in further management decisions is evolving, especially in the context of emerging therapies. Historically, of the 127 reports of SLN biopsy for periocular melanoma, treatment is reported in 7 cases and includes completion of neck dissection, parotidectomy, radiation therapy, immunotherapy, and experimental melanoma vaccine therapy [10]. Treatments are tailored to the individual patient. The recent availability of effective immune checkpoint inhibitor therapy is rapidly changing the management of patients with positive SLN biopsy results in favor of immune checkpoint inhibitor therapy rather than parotidectomy, neck dissection, or adjuvant radiation therapy [11].
1.2 Targeted and Immunotherapy for Periocular Melanomas
1.2.1 Targeted Therapies
BRAF kinase plays a key role in cell growth and proliferation and is encoded by the BRAF gene. The BRAF mutation is found in 66% of cutaneous melanomas, 90% of which is the specific V600E mutation [12, 13]. This mutated protein results in abnormal activation of the MAPK signaling cascade and results in increased survival and proliferation of affected cells. Currently, there are three U.S. Food and Drug Administration (FDA)-approved BRAF inhibitors. Vemurafenib (Zelboraf®, Genentech) was FDA-approved in 2011 for the treatment of unresectable or metastatic melanoma, in patients with confirmed V600E mutation [14]. Dabrafenib (Tafinlar®, Novartis) was FDA-approved in 2013 for the treatment of V600E-positive, unresectable or metastatic melanoma. It is also approved in combination with trametinib (Mekinist, Novartis), an MEK inhibitor, as adjuvant therapy in patients with positive lymph nodes following complete melanoma resection or as first-line therapy for V600E- or V600K-positive unresectable or metastatic melanoma [15]. The most recently approved BRAF inhibitor is encorafenib (Braftovi®, Array BioPharma Inc), which is approved in combination with binimetinib (Mektovi®, Array BioPharma), an MEK inhibitor, for the treatment of unresectable or metastatic melanoma with V600E or V600K mutation. The addition of MEK inhibitors to BRAF inhibitors improves disease-free survival in patients with metastatic melanoma compared with BRAF inhibitor therapy alone [16].
Clinical trials using BRAF inhibitors alone have shown overall response rate of 40–54%, progression-free survival of 6–9 months, and overall survival of 17–19 months [16]. Patients treated with BRAF inhibitors in combination with an MEK inhibitor show improved outcomes, with a response rate of 63–76%, progression-free survival of 9–15 months, and an overall survival of 22–34 months [16]. Due to the synergistic efficacy of BRAF and MEK inhibitors, combination therapy has largely replaced BRAF inhibitor monotherapy. There are few published case reports investigating BRAF inhibitor therapy for eyelid or conjunctival melanoma [17,18,19,20]. All reported cases demonstrated clinical response, with progression-free survival ranging from 4 to 23 months. Yet, despite these encouraging results, resistance to treatment develops in many cases with re-growth of previously responsive metastasis [21].
The most common side effects of BRAF inhibitors include arthralgia, alopecia, nausea, pruritus, photosensitivity, fatigue, and skin rash [22]. The combination of BRAF inhibitors and MEK inhibitors has been found to significantly increase certain adverse events including pyrexia, chills, hypertension, vomiting, liver toxicity, and ocular side effects such as central serous chorioretinopathy and retinal detachment [23]. BRAF inhibitor monotherapy has also been associated with an increased rate of squamous cell carcinoma (SCC) in up to 12.5% of patients versus 3% in patients treated with BRAF and MEK inhibitor combination therapy [24, 25].
1.2.2 Immune Checkpoint Inhibitors
Improved understanding of the pathophysiology of melanoma, coupled with discoveries related to the role of the immune system in tumor control, has yielded a new class of systemic medication for locally advanced and/or metastatic melanoma and for many other cancer types called immune checkpoint inhibitors. Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) are proteins found on the surface of activated T-lymphocytes. When these surface proteins are activated, they halt the proliferation of cancer fighting T-cells. It is thought that abnormal activation of these immune checkpoints (PD-1 and CTLA-4) prevents the activation of T-lymphocytes and allows evasion by host immune response and proliferation of cancer cells. Development and now commercial availability of monoclonal antibodies directed against PD-1 and CTLA-4 have revolutionized the management of patients with metastatic melanoma by improving disease-free and overall survivals in such patients. Several immune checkpoint inhibitors have been approved by the U.S. Food and Drug Administration (FDA) over the last 10 years, with expanding indications. Ipilimumab (Yervoy®, Bristol-Myers Squibb) is a CTLA-4 inhibitor approved by the FDA in 2011 [26]. It is indicated for the treatment of adult and pediatric patients with metastatic or unresectable melanoma, as well as an adjuvant therapy for patients with cutaneous melanoma and pathologically confirmed lymph node involvement of more than 1 mm who have undergone complete resection, including lymphadenectomy. Nivolumab (Opdivo®, Bristol-Myers Squibb) and pembrolizumab (Keytruda®, Merck) are two FDA-approved anti-PD-1 agents approved in 2014 and are indicated in the treatment of metastatic or unresectable melanoma, and as adjuvant therapy in patients with metastatic or lymph node involvement after complete resection [27, 28]. Pivotal multicenter, prospective, randomized trials have shown improved survival in patients with unresectable or metastatic melanoma treated with ipilimumab [29, 30], pembrolizumab [31], and nivolumab [32]. Studies show that the combination of ipilimumab with nivolumab or pembrolizumab increases efficacy but is also associated with increased toxicity [33].
There are emerging, promising results in case reports and series that support the use of immune checkpoint inhibitors in the periocular region. Sagiv et al. [34] reported on 5 cases of metastatic conjunctival melanoma treated with nivolumab or pembrolizumab, 4 of which had complete response with no evidence of disease at 1–36 months after treatment completion. Other case reports and series have demonstrated similar partial or complete response with this class of medication [35,36,37,38].
It should be noted that immune checkpoint inhibitors are associated with significant immune-related adverse events. Different from traditional chemotherapy reaction, immunotherapy side effects result from over-activation of the immune system. Common adverse events including enterocolitis, dermatitis, hepatitis, thyroiditis, arthritis, and myositis have all been reported but can be mitigated with medical treatment with steroids or similar anti-inflammatory strategies. The incidence of fatal events is between 0.3 and 1.3%, and most fatal events are due to colitis, pneumonitis, or hepatitis. Ten percent of patients will have a grade 3 or worse reaction, and the common side effects include fatigue and rash, diarrhea, and endocrinopathies [40]. Treatment of these side effects is systemic immunosuppression, most commonly steroids, in a targeted fashion, and most resolve without lasting complications [41]. Immunotherapy is also costly. Therefore, the use of immune checkpoint therapy in the periocular region should be reserved for locally advanced, surgically unresectable, and/or metastatic disease (Fig. 2).
(a, b) External photographs of a 58-year-old woman with a biopsy-proven conjunctival melanoma that involved all four quadrants and also both the upper and lower eyelid tarsal and palpebral conjunctiva and caruncle. (c, d) Slit lamp photographs of the only remaining areas of faint pigmentation (yellow circles) remaining after 12 months of treatment with nivolumab. These areas were biopsied and found to contain macrophages with pigment, and no residual melanoma. Patient has remained without evidence of disease for 2 years after end of treatment with nivolumab
2 Eyelid and Periocular Squamous Cell Carcinoma
Cutaneous squamous cell carcinoma (SCC) is the second most common eyelid malignancy accounting for 5–10% of all eyelid cancers [42,43,44]. SCC has a reported rate of local recurrence that ranges from 6.8 to 36.9% [45,46,47,48] and a propensity for perineural invasion (PNI) along cranial nerves in up to 25% of patients [45, 46]. Distant metastasis is rare (0.8–6.2%) [48,49,50,51] and regional nodal metastasis ranges from 1.3 to 24% [45, 46, 49, 52].
Surgical excision with histologic confirmation of tumor-free margins on frozen section is the standard treatment for most patients with eyelid and periorbital SCC [52,53,54]. In locally advanced cases or in the presence of PNI, postoperative adjuvant radiation therapy, concurrent chemo-radiation therapy, or immunotherapy may be considered to improve local control or to treat metastatic disease. In a recent series of 109 patients with eyelid and periorbital SCC from our center, 59 patients (54%) had eye-sparing surgery with frozen section control of margins, and 34 patients (31%) had eye-sparing surgery with frozen section control of margins followed by adjuvant radiotherapy or concurrent chemoradiation. Thirteen additional patients (12%) had orbital exenteration followed by adjuvant radiotherapy or chemoradiation. Thirty-six of the 40 patients with PNI (90%) received adjuvant radiotherapy [45]. Overall, slightly over 40% of patients had adjuvant radiation or concurrent chemo-radiation therapy.
Historically, cytotoxic chemotherapy (e.g., cisplatin, doxorubicin, bleomycin, peplomycin, methotrexate, 5-fluorouracil) was used for patients with locally advanced or metastatic squamous carcinoma. However, these older drugs have fallen to second- and third-line options in favor of the more recently available targeted therapies such as epidermal growth factor receptor (EGFR) inhibitors and immunotherapy. In this section, we will focus on advances made in the treatment of locally advanced and/or metastatic periocular SCC using EGFR-based targeted therapy and immune checkpoint inhibitor therapy as recent advances in the management of periocular cutaneous SCC.
2.1 Epidermal Growth Factor Receptor (EGFR) Inhibitors for Locally Advanced Periocular SCC
EGFR is a transmembrane receptor protein that is involved in proliferation of keratinocytes, cellular migration, and increased survival and resistance to apoptosis [55, 56]. Overexpression of EGFR has been found in cutaneous SCC [57].
Several EGFR inhibitors (cetuximab, panitumumab, gefitinib, lapatinib, erlotinib) are approved by the U.S. FDA, and are typically used either as single agent or in combination with other chemotherapy for treatment of patients with metastatic or locally advanced head and neck squamous cell carcinoma (HNSCC) [58]. Cetuximab (Erbitux, Lilly) is a monoclonal antibody EGFR inhibitor that is FDA-approved to treat locally or regionally advanced head and neck SCC (HNSCC) in combination with radiation therapy or recurrent or metastatic HNSCC progressing after treatment with platinum-based therapy. Erlotinib (Tarceva, Genentech) is an oral drug, a small-molecule tyrosine kinase EGFR inhibitor, that has been studied as an off-label potential treatment for locally advanced HNSCC [59]. Addition of EGFR inhibitors to radiation therapy as adjuvant therapy has improved locoregional control in patients with locally advanced HNSCC in several large-scale multi-institution phase III trials [59, 60].
EGFR is overexpressed in cutaneous SCC as well as in conjunctival SCC [61]. For patients with recurrent periocular SCC with orbital invasion and/or with regional metastasis secondary to periocular cutaneous SCC, both cetuximab and erlotinib have been used successfully with good responses in a few cases published by our group [62]. Cetuximab in combination with platinum-based chemotherapy can also be used in the neoadjuvant setting to chemo-reduce large periorbital SCC, such as primary lacrimal sac/duct carcinomas, to decrease surgical morbidity (Fig. 3).
(a) A 53-year-old man presented with a large squamous carcinoma of the right lacrimal sac/duct area. (b) Magnetic resonance imaging (MRI) in the same patient shows an infiltrative mass involving the lacrimal sac, nasolacrimal duct, and medial maxilla. (c) After three cycles of cetuximab plus carboplatin and paclitaxel, the mass in the medial canthal area is completely resolved; note the maculopapular rash on the face secondary to cetuximab. (d) After three cycles of neoadjuvant chemotherapy plus cetuximab, the patient had surgery; the surgical specimen showed a complete pathologic response with no tumor present
The side effects of EGFR inhibitors are relatively minor and include an acneiform rash, pruritus, nail changes, diarrhea, and secondary infections. More specifically, the following ocular side effects have also been reported: mild superficial keratopathy, and notably trichomegaly, and rarely uveitis [63, 64].
2.2 Immune Checkpoint Inhibitors for Locally Advanced or Metastatic Periocular SCC
The discovery of immune checkpoints, specifically programmed cell death protein 1 (PD-1), has revolutionized the management of patients with locally advanced or metastatic squamous carcinoma. This class of drugs was first approved for use in melanoma (please see detailed discussion above in the section on periocular melanoma) but are now also approved for use in patients with locally advanced cutaneous SCC. Cemiplimab (Libtayo, Regeneron Pharmaceuticals, Tarrytown, NY, USA) and Pembrolizumab (Keytruda, Merck, Kenilworth, NJ, USA) are human monoclonal antibodies directed against programmed death 1 (PD-1) receptor, that are specifically FDA-approved in the USA for locally advanced or metastatic cutaneous SCC [65, 66]. Currently, immunotherapy is being employed as first-line treatment for cutaneous SCC that is metastatic, or locally advanced and not amenable to surgery and radiation [67]. For locally advanced cutaneous SCC, cemiplimab treatment has demonstrated a response rate of 12%, 36%, 31%, and 13% for progression, stable disease, partial response, and complete response, respectively [68]. Its role in these and other settings is the subject of 14 current prospective clinical trials at the time of the writing of this chapter [69]. Of these, two are neoadjuvant, seven are primary treatment, two are adjuvant, two are adjuvant and neoadjuvant, and one is for intralesional injection of cemiplimab. While new research supports the cost effectiveness of cemiplimab for advanced SCC when compared to traditional second-line chemotherapy [70], the cost and potential side effects of immunotherapy in the neoadjuvant setting have to be considered carefully in the treatment decisions and patient selection.
Currently, there are only case reports of the off-label use of pembrolizumab for locally advanced periocular SCC and the results have been promising [71]. At the time of this chapter’s writing, our group has submitted a cohort of seven patients treated with cemiplimab (in six patients) and pembrolizumab (in one patient) for locally advanced periorbital SCC. Our findings in this cohort suggest that anti-PD-1 immunotherapy can be highly effective at reducing the size of locally advanced periorbital cutaneous SCC (Fig. 4). In all seven patients, measurable clinical and/or radiologic response was observed. In five patients, administration of immunotherapy was followed by surgery with or without postoperative radiation therapy. In two patients with multiple recurrent periorbital SCC heavily pretreated with surgery and radiation and with massive perineural invasion involving the skull base, surgery was not done after immunotherapy as it was felt that the recurrent lesions were not surgically resectable. Both of these patients remained without evidence of disease at last follow-up, 17 months and 14 months, respectively, after completion of immunotherapy. The off-label use of immune checkpoint inhibitors in the neoadjuvant setting is an area of active study [39, 40].
(a) Axial-T1 post-contrast magnetic resonance (MR) image with fat-saturation shows abnormal enhancement at the right orbital apex and superotemporal right orbit (large arrows) as well as involving the right sphenoid bone (small arrow). (b) Axial-T1 post-contrast MR with fat-saturation demonstrates near complete resolution of the enhancement
Immune checkpoint inhibitors are generally safe, but serious immune-related adverse events are common and may require prompt management. Please see the section above under immunotherapy for periocular melanoma for a more detailed discussion of expected side effects with immunotherapy.
3 Eyelid and Periocular Basal Cell Carcinoma
Basal cell carcinoma (BCC) is the most common human cancer and comprises 90% of eyelid tumors [72]. Mortality rates from BCCs are rare and metastases rarely occur at a rate of 0.0028–0.5% [73, 74]. Typically, periocular BCC is amenable to surgical excision, with a 5-year recurrence rate of 1–5.3% [75]. Excision with intraoperative frozen sections and Mohs micrographic surgery (MMS) are the most commonly employed techniques for ensuring complete pathological excision and are associated with low local recurrence rates of 2.0% and 2.1%, respectively [76, 77]. For locally advanced periocular BCC, characterized by large tumor size or recurrent disease with extension into the orbit or paranasal sinuses, definitive surgical treatment may be impossible without significant ocular morbidity or removal of the eye and orbital contents. While surgery remains the preferred treatment modality, recent advances in the medical management of advanced periocular BCC will be the focus of the following segment.
3.1 Sonic Hedgehog Inhibitors
The discovery of mutations in the hedgehog pathway has enabled the emergence of targeted medical therapy for BCC [78]. The hedgehog pathway includes the Patched-1 transmembrane receptor (Ptch-1), which is a tumor suppressor. Ptch-1 normally inhibits the downstream receptor Smoothened (Smo) [79, 80]. A mutation in the hedgehog pathway leads to hedgehog protein binding to Ptch-1. This leads to a reversal of the normal inhibition of Smo, as well as expression of the downstream product GLI-1, which is thought to lead to the formation of BCC [80]. Medications that target this pathway can cause regression of BCC tumors.
Vismodegib (Erivedge®; Genentech) was the first sonic hedgehog inhibitor to be approved by the U.S. Food and Drug Administration (FDA) in 2012. Vismodegib is a selective hedgehog pathway inhibitor that blocks hedgehog signaling by binding to Smo, inhibiting downstream activation of hedgehog target genes [81]. Its approval was based on results from the ERIVANCE trial, which showed 43% and 30% response in patients with locally advanced BCC and metastatic BCC, respectively [82]. Adverse events were reported in 20% of patients and included muscle spasms, alopecia, dysgeusia, weight loss, fatigue, nausea, decrease in appetite, and diarrhea [82].
Since the ERIVANCE trial was published, the role for vismodegib in the periocular setting has been examined and refined. For periocular BCC treated with vismodegib, most patients show partial response, some have complete response, and a small subset will have stable disease [83]. The neoadjuvant use of vismodegib for locally advanced periocular BCC has been reported by our group at MD Anderson as an effective strategy in patients who would otherwise need an orbital exenteration or major disfiguring surgery (Fig. 5) [84]. In this cohort, patients were treated for a median of 14 months (4–36), underlining the need for long-term treatment to observe response. Also in this group, we observed that over half of patients achieved a complete response with no residual microscopic disease found at the time of surgery. Careful follow-up is needed to confirm long-term disease control.
(a) A 45-year-old man with a locally advanced recurrent basal cell carcinoma of the left medial canthus with anterior orbital soft tissue involvement and some bony erosion. (b) After 9 months of treatment with daily vismodegib, the lesion has nearly totally resolved based on clinical examination with the exception of a small residual area of ulceration noted (yellow circle). This area was excised surgically with negative margins and the scarred area in the left lower eyelid biopsied in multiple areas and found to have no tumor present
Sonidegib (ODOMZO®, Novartis) is the second sonic hedgehog inhibitor approved by FDA in 2015 based on results from the phase 2 BOLT trial [85]. Similar to vismodegib, sonidegib also binds Smo, inhibiting downstream activation of hedgehog target genes. Its efficacy in the BOLT trial was 34–36% response rate and it had a similar adverse event profile to vismodegib, including muscle spasms, dysgeusia, alopecia, nausea, elevated serum creatine kinase levels, weight loss, and fatigue [85].
Published reports on the specific use of sonidegib in the periocular region are limited to one published case showing complete response to treatment [86]. However, this is very likely due to the fact that sonidegib became available several years after vismodegib. Both drugs are very similar in their mechanism of action, expected efficacy in treatment of BCC, and their side effect profile.
3.2 Immune Checkpoint Inhibitors for Locally Advanced BCC
Pembrolizumab (Keytruda®, Merck) is a programmed cell death protein-1 (PD-1) inhibitor that has demonstrated efficacy in multiple cancer types, including squamous cell carcinoma and melanoma as mentioned earlier in this chapter. A nonrandomized, open-label study of patients with locally advanced BCC showed a response rate of 38% (6 of 16 patients) with pembrolizumab given with or without vismodegib [87]. Efficacy has also been shown in case reports of pembrolizumab for metastatic BCC that has failed hedgehog pathway inhibitors [88]. Overall, the off-label use of pembrolizumab in patients with locally advanced BCC is reserved for surgically unresectable or metastatic disease that is not responsive to vismodegib or sonidegib.
Cemiplimab (Libtayo®, Regeneron Pharmaceuticals) is a PD-1 inhibitor originally FDA-approved for patients with metastatic or locally advanced squamous cell carcinoma who are not candidates for curative surgery or radiotherapy [89]. Recently, cemiplimab received accelerated approval to expand its indication to include surgically unresectable or metastatic BCC that has failed treatment with a hedgehog inhibitor [89]. This approval was based on interim results of a phase 2 clinical trial for advanced or metastatic BCC that has failed or is intolerant to hedgehog inhibitors. Of 84 patients enrolled in the locally advanced arm of the study, 26 showed an objective response with 5 patients showing a complete response. Of the remaining patients, 41 had stable disease and only 9 had progression at a median follow-up of 15 months [90]. In the metastatic group, of 28 patients treated, 6 patients had partial response and 13 patients had stable disease [91].
4 Ocular Adnexal Lymphoma
The term ocular adnexal lymphoma (OAL) has been used to refer to lymphomas of the conjunctiva, orbit, and eyelid [92]. The vast majority of OAL is non-Hodgkin lymphoma (NHL). NHL is further classified based on its presumed cell of origin as defined by the World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissue [93]. Greater than 98% of ocular adnexal lymphomas are of B-cell NHL variety [92, 94]. OAL can develop primarily in the ocular adnexal area as the first and only site of manifestation of lymphoma or the ocular adnexal lymphoma may represent relapsed lymphoma with systemic involvement in other extraocular sites in the past [94]. While OAL accounts for only about 2% of all lymphomas, it is the most common primary orbital malignant neoplasm in adults [95]. Of note, OAL is usually low grade with marginal zone lymphoma accounting for 60–70% of cases followed by low-grade follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL) as the next most frequent histologic varieties [96]. OAL must be distinguished from intraocular lymphoma, which are typically a high-grade diffuse large B-cell lymphoma with a distinct therapeutic strategy [96].
Treatment of OAL depends on the histologic variety and stage of OAL at the time of diagnosis. The Ann Arbor Staging and, more recently, the american joint committee on cancer (AJCC) Cancer Staging Manual are the most common methods for staging of ocular adnexal lymphoma [97]. Initial steps in management of OAL entail a fresh tissue biopsy for lymphoma studies to establish the exact histologic subtype followed by staging work-up that usually includes a total body positron emission tomography (PET)/CT scan, a bone marrow biopsy, and an MRI of orbit if the lesion is suspected to have an orbital component on clinical grounds [98, 99].
We herein will highlight two recent advances in the management of OAL that are noteworthy: the “ultra-low-dose” radiation, the so-called “boom-boom” for low-grade OAL; and the use of rituximab in combination with other chemotherapy for management of diffuse large B-cell lymphoma and for low-grade OAL that involves extraocular sites.
4.1 Ultra-Low-Dose External Beam Radiation Therapy
External beam radiation therapy (EBRT) is the most commonly used treatment modality for OAL, particularly low-grade lymphomas such as extranodal marginal zone lymphoma (EMZL), follicular lymphoma (FL), and small lymphocytic lymphoma (SLL) without systemic involvement (i.e., stage IE by Ann Arbor staging criteria) [100, 101]. Historically, OAL was treated with moderate to high radiation doses, from 25 to 54 Gy [102,103,104]. These dosages yield excellent local control from 90 to 100%; however, there is some risk of ocular toxicity [105]. Late toxic side effects, including keratitis, severe dry eye syndrome, glaucoma, retinopathy, and cataracts, have been reported in up to 47% of patients treated with the higher-dose range of EBRT for OAL [104]. More recently, in a report by Pinnix et al. from our institution, we reported complete response or significant partial response in 100% of 22 patients with low-grade OAL who were treated with only 4 Gy of radiation, and only two sessions of radiotherapy—the so-called “boom-boom” regimen. This is a significantly lower radiation dose compared with “historical standard dose,” is delivered over only 2 days (instead of 2–3 weeks), and is associated with negligible radiation-related ocular toxicity to date [106]. Complete response as defined by RECIST criteria [107] was observed in 86% of patients (Fig. 6), while 14% of patients experienced partial response. Only one of the 22 patients had very mild dry eye symptoms; no other ocular toxicity was seen in this report. We have completed a prospective trial of the same regimen of 4 Gy of radiation in 40 patients with low-grade OAL treated to date confirming essentially the same findings as was reported in our retrospective study. A report summarizing the findings in our prospective trial and long-term data on patients treated off-protocol for a total of approximately 70 patients with OAL treated with ultra-low-dose radiotherapy is currently under preparation.
(a) Computed tomography, axial image, in a patient with marginal zone lymphoma of the superolateral orbit (arrow). (b) Coronal image in the same patient further delineates the mass (arrow). (c) Axial image in the same patient 3 months after delivery of 4 Gy of radiation to the right orbit shows total resolution of the mass. (d) Coronal image again confirming complete resolution of the mass
4.2 Rituximab-Based Chemotherapy Regimens (R-CHOP, Rituximab, cyclophosphamide, vincristine sulfate, and prednisone (R-CVP), R-Bendamustine) for High-Grade OAL or Low-Grade OAL with Extraocular Sites of Involvement
Newer chemotherapy regimens add rituximab to well-established combination protocols such as CHOP—cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone—for diffuse large B-cell lymphoma (DLBCL). Prior to the addition of rituximab, the 3-year complete response rate and overall survival of patients with diffuse large B-cell lymphoma treated with CHOP was 40–50% and 35–40%, respectively [108]. The addition of rituximab increased the rate of complete response and overall survival to 60% and 76%, respectively, at 3 years and has become standard of care for DLBCL [108]. For patients with low-grade lymphomas, rituximab as single-agent monotherapy is associated with about 50% relapse rate [109]. The addition of bendamustine to rituximab as first-line therapy has yielded an overall survival of 96% at 4 years in patients with mucosal associated lymphoid tissue (MALT) lymphoma, which was significantly higher than rituximab alone or rituximab CHOP regimens [110].
5 Lacrimal Gland Carcinoma
Malignant epithelial tumors of the lacrimal gland include adenoid cystic carcinoma (ACC), carcinoma ex pleomorphic adenoma, adenocarcinoma, mucoepidermoid carcinoma, and a number of other rare variants. These cancers are quite rare with an overall incidence estimated at one per one million per year [111], ACC, which accounts for about 60% of cases of lacrimal gland carcinoma, is associated with a high rate of local recurrence and distant metastasis and is associated with death in about half of patients [112]. Carcinoma ex pleomorphic adenoma, which accounts for about 20% of lacrimal gland carcinomas, can occur either de novo or after incomplete excision of a pleomorphic adenoma [113]. Another 10% of cases of lacrimal gland carcinoma are adenocarcinoma and these can be high- or low-grade.
The treatments for lacrimal gland carcinoma have evolved considerably in the last decade or so and there is a shift away from orbital exenteration and toward eye-sparing multidisciplinary strategies [114,115,116,117,118,119,120]. Advances in the area of eye-sparing multimodality management of lacrimal gland carcinoma and the use of neoadjuvant intra-arterial chemotherapy specifically in patients with adenoid cystic carcinoma of lacrimal gland will be highlighted in the following section.
5.1 Eye-Sparing Surgery and Adjuvant Radiotherapy
Eye-sparing surgery, followed by adjuvant radiotherapy, is an approach that has gained in popularity in recent years [114,115,116,117,118,119,120]. Lacrimal gland carcinomas that are anatomically amenable to gross total resection are candidates for eye-sparing surgery. Outcomes of eye-preserving surgery with or without adjuvant therapy have shown favorable results in several published series [114,115,116,117,118,119,120]. One large series of 37 patients by Woo et al., combining patients from two centers including ours, concluded that multimodality eye-sparing treatments are associated with locoregional control rates similar to that of orbital exenteration with a very reasonable ocular toxicity profile and good visual and ocular function for patients who have eye-sparing surgery followed by eye-sparing adjuvant radiation therapy [115]. A visual acuity of 20/40 or better was found in 68% of patients with vision-limiting ocular toxicity most commonly occurring from dry eye and radiation retinopathy. A more recent updated series from MD Anderson reported on 55 patients with lacrimal gland carcinoma that were treated over a recent 20-year period [121]. In this series, orbital exenteration with adjuvant therapy and eye-sparing surgery with adjuvant therapy produced similar recurrence outcomes, although eye-sparing surgery was associated with better disease-specific survival. The reported rates of local recurrence, distant metastasis, and death from disease were 22%, 29%, and 20%, respectively. The reported 5-year local-recurrence-free survival rate was 0.77 (95% confidence interval [CI]: 0.59–1), and the 5- and 10-year distant-metastasis-free survival rates were 0.67 (95% CI: 0.53–0.85) and 0.49 (95% CI: 0.30–0.81), respectively. Five- and 10-year disease-specific survival rates were 0.81 (95% CI: 0.69–0.95) and 0.75 (95% CI: 0.60–0.94), respectively. Other investigators have shown similar local control and survival rates with eye-sparing surgery and adjuvant radiation therapy. For example, Wolkow et al. reported that 4 out of 18 (22%) patients with ACC of the lacrimal gland treated with eye-sparing surgery followed by adjuvant proton radiation therapy experienced local recurrence and 3 (17%) died of metastatic disease [116].
5.2 Neoadjuvant Intra-Arterial Chemotherapy for Adenoid Cystic Carcinoma
In an attempt to improve the outcomes in patients with lacrimal gland ACC, Tse et al. used intra-arterial cytoreductive chemotherapy (IACC) using cisplatin and doxorubicin as a neoadjuvant treatment followed by orbital exenteration followed by radiation therapy and additional adjuvant chemotherapy [122]. Tse et al., in a long-term study using this regimen in 19 patients with lacrimal gland ACC, divided the patients into two groups (patients with the “lacrimal artery intact” and patients with the “lacrimal artery not intact”) and concluded that all patients with an intact artery (8/8 patients), and 72% (8/11 patients) of those with the artery not intact, were alive more than 10 years after treatment. The authors did not specifically report the overall 5- and 10-year disease-specific survival for their cohort of 19 patients, but overall close to 80% of patients were still living at 10 years, some with active disease [122].
Subsequent studies from other institutions report cases of late relapse with distant metastasis and disease-specific death after IACC, and also significant morbidity and side effects associated with IACC in patients with lacrimal gland adenoid cystic carcinoma [123, 124].
As a future direction, it may be interesting to study the role of intravenous or intra-arterial chemotherapy in the neoadjuvant setting in patients with large lacrimal gland ACC in an attempt to reduce the size of tumor and make it more surgically resectable and potentially allow for a lesion that would otherwise require an orbital exenteration to be treated with eye-sparing surgery instead.
References
Esmaeli B, Wang B, Deavers M, Gillenwater A, Goepfert H, Diaz E, Eicher S. Prognostic factors for survival in malignant melanoma of the eyelid skin. Ophthalmic Plast Reconstr Surg. 2000;16(4):250–7.
Wong JR, Nanji AA, Galor A, Karp CL. Management of conjunctival malignant melanoma: a review and update. Expert Rev Ophthalmol. 2014;9(3):185–204.
Mancera N, Smalley KS, Margo CE. Melanoma of the eyelid and periocular skin: histopathologic classification and molecular pathology. Surv Ophthalmol. 2019;64(3):272–88.
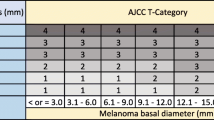
Yin VT, Warneke CL, Merritt HA, Esmaeli B. Number of excisions required to obtain clear surgical margins and prognostic value of AJCC T category for patients with eyelid melanoma. Br J Ophthalmol. 2014;98(12):1681–5.
Morton DL, Wanek L, Nizze JA, Elashoff RM, Wong JH. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes. Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Ann Surg. 1991;214(4):491.
Ross MI, Gershenwald JE. Evidence-based treatment of early-stage melanoma. J Surg Oncol. 2011;104(4):341–53.
Esmaeli B, Eicher S, Popp J, Delpassand E, Prieto VG, Gershenwald JE. Sentinel lymph node biopsy for conjunctival melanoma. Ophthalmic Plast Reconstr Surg. 2001;17(6):436–42.
Pfeiffer ML, Ozgur OK, Myers JN, Peng A, Ning J, Zafereo ME, Thakar S, Thuro B, Prieto VG, Ross MI, Esmaeli B. Sentinel lymph node biopsy for ocular adnexal melanoma. Acta Ophthalmol. 2017;95(4):e323–8.
Cohen VM, Tsimpida M, Hungerford JL, Jan H, Cerio R, Moir G. Prospective study of sentinel lymph node biopsy for conjunctival melanoma. Br J Ophthalmol. 2013;97(12):1525–9.
Freitag SK, Aakalu VK, Tao JP, Wladis EJ, Foster JA, Sobel RK, Yen MT. Sentinel lymph node biopsy for eyelid and conjunctival malignancy: a report by the American Academy of Ophthalmology. Ophthalmology. 2020;127(12):1757–65.
Herrscher H, Robert C. Immune checkpoint inhibitors in melanoma in the metastatic, neoadjuvant, and adjuvant setting. Curr Opin Oncol. 2020;32(2):106–13.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–54.
Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–16.
US-FDAFDA labeling information—ZELBORAF.2020FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202429s019lbl.pdf. Accessed 14 May 2021.
US-FDAFDA labeling information—TAFINLAR.2020FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202806s015lbl.pdf. Accessed 14 May 2021.
Greco A, Safi D, Swami U, Ginader T, Milhem M, Zakharia Y. Efficacy and adverse events in metastatic melanoma patients treated with combination BRAF plus MEK inhibitors versus BRAF inhibitors: a systematic review. Cancers. 2019;11(12):1950.
Pahlitzsch M, Bertelmann E, Mai C. Conjunctival melanoma and BRAF inhibitor therapy. J Clin Exp Ophthalmol. 2014;5(1):322.
Maleka A, Åström G, Byström P, Ullenhag GJ. A case report of a patient with metastatic ocular melanoma who experienced a response to treatment with the BRAF inhibitor vemurafenib. BMC Cancer. 2016;16(1):1–5.
Glass LR, Lawrence DP, Jakobiec FA, Freitag SK. Conjunctival melanoma responsive to combined systemic BRAF/MEK inhibitors. Ophthalmic Plast Reconstr Surg. 2017;33(5):e114–6.
Rossi E, Maiorano BA, Pagliara MM, Sammarco MG, Dosa T, Martini M, Rindi G, Bria E, Blasi MA, Tortora G, Schinzari G. Dabrafenib and trametinib in BRAF mutant metastatic conjunctival melanoma. Front Oncol. 2019;9:232.
Ascierto PA, McArthur GA, Dréno B, Atkinson V, Liszkay G, Di Giacomo AM, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L, de la Cruz-Merino L. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17(9):1248–60.
Mackin AG, Pecen PE, Dinsmore AL, Patnaik JL, Gonzalez R, Robinson WA, Palestine AG. Inflammatory side effects of BRAF and MEK inhibitors. Melanoma Res. 2019;29(5):522–6.
Liu M, Yang X, Liu J, Zhao B, Cai W, Li Y, Hu D. Efficacy and safety of BRAF inhibition alone versus combined BRAF and MEK inhibition in melanoma: a meta-analysis of randomized controlled trials. Oncotarget. 2017;8(19):32258.
Peng L, Wang Y, Hong Y, Ye X, Shi P, Zhang J, Zhao Q. Incidence and relative risk of cutaneous squamous cell carcinoma with single-agent BRAF inhibitor and dual BRAF/MEK inhibitors in cancer patients: a meta-analysis. Oncotarget. 2017;8(47):83280.
Yin VT, Wiraszka TA, Tetzlaff M, Curry JL, Esmaeli B. Cutaneous eyelid neoplasms as a toxicity of vemurafenib therapy. Ophthalmic Plast Reconstr Surg. 2015;31(4):e112–5.
US-FDAFDA labeling information—YERVOY.2020FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125377s074lbl.pdf. Accessed 10 March 2021.
US-FDAFDA labeling information—OPDIVO.2019FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125554s024lbl.pdf. Accessed 10 March 2021.
US-FDAFDA labeling information—KEYTRUDA.2021FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s015lbl.pdf. Accessed 10 March 2021.
Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.
Barone A, Hazarika M, Theoret MR, Mishra-Kalyani P, Chen H, He K, Sridhara R, Subramaniam S, Pfuma E, Wang Y, Li H. FDA approval summary: pembrolizumab for the treatment of patients with unresectable or metastatic melanoma. Clin Cancer Res. 2017;23(19):5661–5.
Beaver JA, Theoret MR, Mushti S, He K, Libeg M, Goldberg K, Sridhara R, McKee AE, Keegan P, Pazdur R. FDA approval of nivolumab for the first-line treatment of patients with BRAFV600 wild-type unresectable or metastatic melanoma. Clin Cancer Res. 2017;23(14):3479–83.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34.
Sagiv O, Thakar SD, Kandl TJ, Ford J, Sniegowski MC, Hwu WJ, Esmaeli B. Immunotherapy with programmed cell death 1 inhibitors for 5 patients with conjunctival melanoma. JAMA Ophthalmol. 2018;136(11):1236–41.
Finger PT, Pavlick AC. Checkpoint inhibition immunotherapy for advanced local and systemic conjunctival melanoma: a clinical case series. J Immunother Cancer. 2019;7(1):1–7.
Kini A, Fu R, Compton C, Miller DM, Ramasubramanian A. Pembrolizumab for recurrent conjunctival melanoma. JAMA Ophthalmol. 2017;135(8):891–2.
Chaves LJ, Huth B, Augsburger JJ, Correa ZM. Eye-sparing treatment for diffuse invasive conjunctival melanoma. Ocular Oncol Pathol. 2018;4(4):261–6.
Pinto Torres S, André T, Gouveia E, Costa L, Passos MJ. Systemic treatment of metastatic conjunctival melanoma. Case Rep Oncol Med. 2017;2017:4623964.
Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–8.
Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–80.
Martins F, Sykiotis GP, Maillard M, Fraga M, Ribi C, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019;20(1):e54–64.
Reifler DM, Hornblass A. Squamous cell carcinoma of the eyelid. Surv Ophthalmol. 1986;30(6):349–65.
Tanenbaum M, Grove AS Jr, McCord CD Jr. Eyelid tumors: diagnosis and management. Oculoplastic Surgery. 3rd ed. New York: Raven Press Ltd.; 1995. p. 145–74.
Wawrzynski J, Tudge I, Fitzgerald E, Collin R, Desai P, Emeriewen K, Saleh GM. Report on the incidence of squamous cell carcinomas affecting the eyelids in England over a 15-year period (2000–2014). Br J Ophthalmol. 2018;102(10):1358–61.
Nasser QJ, Roth KG, Warneke CL, Yin VT, El Sawy T, Esmaeli B. Impact of AJCC ‘T’designation on risk of regional lymph node metastasis in patients with squamous carcinoma of the eyelid. Br J Ophthalmol. 2014;98(4):498–501.
Sun MT, Andrew NH, O’Donnell B, McNab A, Huilgol SC, Selva D. Periocular squamous cell carcinoma: TNM staging and recurrence. Ophthalmology. 2015;122(7):1512–6.
Ford J, Thakar S, Thuro B, Esmaeli B. Prognostic value of the staging system for eyelid tumors in the 7th edition of the American Joint Committee on Cancer Staging Manual. Ophthal Plast Reconstr Surg. 2017;33(5):317–24.
Faustina M, Diba R, Ahmadi MA, Gutstein BF, Esmaeli B. Patterns of regional and distant metastasis in patients with eyelid and periocular squamous cell carcinoma. Ophthalmology. 2004;111(10):1930–2.
Sullivan TJ. Squamous cell carcinoma of eyelid, periocular, and periorbital skin. Int Ophthalmol Clin. 2009;49(4):17–24.
McCord CD Jr, Cavanagh HD. Microscopic features and biologic behavior of eyelid tumors. Ophthalmic Surg. 1980;11(10):671–81.
Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976–90.
Xu S, Sagiv O, Rubin ML, Sa HS, Tetzlaff MT, Nagarajan P, Ning J, Esmaeli B. Validation study of the AJCC cancer staging manual, staging system for eyelid and periocular squamous cell carcinoma. JAMA Ophthalmol. 2019;137(5):537–42.
Cook BE Jr, Bartley GB. Treatment options and future prospects for the management of eyelid malignancies: an evidence-based update. Ophthalmology. 2001;108(11):2088–98.
Yin VT, Merritt HA, Sniegowski M, Esmaeli B. Eyelid and ocular surface carcinoma: diagnosis and management. Clin Dermatol. 2015;33(2):159–69.
Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(2):S21–6.
Yin VT, Pfeiffer ML, Esmaeli B. Targeted therapy for orbital and periocular basal cell carcinoma and squamous cell carcinoma. Ophthal Plast Reconstr Surg. 2013;29(2):87–92.
Uribe P, Gonzalez S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: molecular bases for EGFR-targeted therapy. Pathol Res Pract. 2011;207(6):337–42.
Moreira J, Tobias A, O’Brien MP, Agulnik M. Targeted therapy in head and neck cancer: an update on current clinical developments in epidermal growth factor receptor-targeted therapy and immunotherapies. Drugs. 2017;77(8):843–57.
Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J, Youssoufian H. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21–8.
Acevedo-Henao CM, Valette G, Miglierini P, Lefur E, Pradier O. Radiotherapy combined with cetuximab for locally advanced head and neck cancer: results and toxicity. Cancer/Radiothérapie. 2012;16(7):601–3.
Shepler TR, Prieto VG, Diba R, Neuhaus RW, Shore JW, Esmaeli B. Expression of the epidermal growth factor receptor in conjunctival squamous cell carcinoma. Ophthalmic Plast Reconstr Surg. 2006;22(2):113–5.
El-Sawy T, Sabichi AL, Myers JN, Kies MS, William WN, Glisson BS, Lippman S, Esmaeli B. Epidermal growth factor receptor inhibitors for treatment of orbital squamous cell carcinoma. Arch Ophthalmol. 2012;130(12):1608–11.
Gellrich FF, Hüning S, Beissert S, Eigentler T, Stockfleth E, Gutzmer R, Meier F. Medical treatment of advanced cutaneous squamous-cell carcinoma. J Eur Acad Dermatol Venereol. 2019;33:38–43.
Bindiganavile SH, Bhat N, Lee AG, Gombos DS, Al-Zubidi N. Targeted cancer therapy and its ophthalmic side effects: a review. J Immunother Prec Oncol. 2021;4(1):6–15.
Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78(17):1841–6.
Stevenson ML, Wang CQ, Abikhair M, Roudiani N, Felsen D, Krueger JG, Pavlick AC, Carucci JA. Expression of programmed cell death ligand in cutaneous squamous cell carcinoma and treatment of locally advanced disease with pembrolizumab. JAMA Dermatol. 2017;153(4):299–303.
Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang AL, Rabinowits G. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379(4):341–51.
Migden MR, Khushalani NI, Chang AL, Lewis KD, Schmults CD, Hernandez-Aya L, Meier F, Schadendorf D, Guminski A, Hauschild A, Wong DJ. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21(2):294–305.
ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US).
Konidaris G, Paul E, Kuznik A, Keeping S, Chen CI, Sasane M, Xu Y, Atsou K, Ayers D, Ruiz ES, Khushalani NI. Assessing the value of cemiplimab for adults with advanced cutaneous squamous cell carcinoma: a cost-effectiveness analysis. Value Health. 2021;24(3):377–87.
Conger JR, Grob SR, Tao J. Massive periocular squamous cell carcinoma with response to pembrolizumab (Keytruda). Ophthalmic Plast Reconstr Surg. 2019;35(5):e127.
Cook BE Jr, Bartley GB. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in Olmsted County, Minnesota. Ophthalmology. 1999;106(4):746–50.
Margo CE, Waltz K. Basal cell carcinoma of the eyelid and periocular skin. Surv Ophthalmol. 1993;38(2):169–92.
Branson SV, McClintic E, Ozgur O, Esmaeli B, Yeatts RP. Orbitofacial metastatic basal cell carcinoma: report of 10 cases. Ophthal Plast Reconstr Surg. 2017;33(3):213–7.
Savar A, Esmaeli B. Management of primary eyelid cancers. Ophthalmic oncology. Boston, MA: Springer; 2010. p. 113–25.
Wong VA, Marshall JA, Whitehead KJ, Williamson RM, Sullivan TJ. Management of periocular basal cell carcinoma with modified en face frozen section controlled excision. Ophthalmic Plast Reconstr Surg. 2002;18(6):430–5.
Malhotra R, Huilgol SC, Huynh NT, Selva D. The Australian Mohs database, part II: periocular basal cell carcinoma outcome at 5-year follow-up. Ophthalmology. 2004;111(4):631–6.
Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, Coppola C, Chanana AM, Marji J, Bickers DR, Epstein EH Jr. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366(23):2180–8.
Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996;384(6605):176–9.
Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Mackey HM. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361(12):1164–72.
LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Chang I, Darbonne WC, Graham RA. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17(8):2502–11.
Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, Marmur E. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171–9.
Ozgur OK, Yin V, Chou E, Ball S, Kies M, William WN, Migden M, Thuro BA, Esmaeli B. Hedgehog pathway inhibition for locally advanced periocular basal cell carcinoma and basal cell nevus syndrome. Am J Ophthalmol. 2015;160(2):220–7.
Sagiv O, Nagarajan P, Ferrarotto R, Kandl TJ, Thakar SD, Glisson BS, Altan M, Esmaeli B. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol. 2019;103(6):775–80.
Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, Herd RM, Kudchadkar R, Trefzer U, Gogov S, Pallaud C. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16(6):716–28.
Hou X, Rokohl AC, Ortmann M, Heindl LM. Effective treatment of locally advanced periocular basal cell carcinoma with oral hedgehog pathway inhibitor? Graefes Arch Clin Exp Ophthalmol. 2020;258:2335–7.
Chang AL, Tran DC, Cannon JG, Li S, Jeng M, Patel R, Van der Bokke L, Pague A, Brotherton R, Rieger KE, Satpathy AT. Pembrolizumab for advanced basal cell carcinoma: an investigator-initiated, proof-of-concept study. J Am Acad Dermatol. 2019;80(2):564–6.
Fischer S, Ali OH, Jochum W, Kluckert T, Flatz L, Siano M. Anti-PD-1 therapy leads to near-complete remission in a patient with metastatic basal cell carcinoma. Oncol Res Treat. 2018;41(6):391–4.
US-FDA. FDA labeling information—LIBTAYO. FDA. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761097s007lbl.pdf. Accessed 10 May 2021.
Stratigos AJ, Sekulic A, Peris K, Bechter O, Kaatz M, Lewis KD, Basset-Seguin N, Chang AL, Dalle S, Orland AF, Licitra L. Primary analysis of phase 2 results for cemiplimab in patients (pts) with locally advanced basal cell carcinoma (laBCC) who progress on or are intolerant to hedgehog inhibitors (HHIs). Head Neck. 2021;89:75.
Lewis K, Peris K, Sekulic A, Stratigos A, Dunn L, Eroglu Z, Chang AL, Migden M, Li S, Mohan K, Coates E. 428 Interim analysis of Phase 2 results for cemiplimab in patients with metastatic basal cell carcinoma (mBCC) who progressed on or are intolerant to hedgehog inhibitors (HHIs). J Immunother Cancer. 2021;(5)1.
Olsen TG, Holm F, Mikkelsen LH, Rasmussen PK, Coupland SE, Esmaeli B, Finger PT, Graue GF, Grossniklaus HE, Honavar SG, Khong JJ. Orbital lymphoma—an international multicenter retrospective study. Am J Ophthalmol. 2019;199:44–57.
Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90.
Hindsø TG, Esmaeli B, Holm F, Mikkelsen LH, Rasmussen PK, Coupland SE, Finger PT, Graue GF, Grossniklaus HE, Honavar SG, Khong JJ. International multicentre retrospective cohort study of ocular adnexal marginal zone B-cell lymphoma. Br J Ophthalmol. 2020;104(3):357–62.
Margo CE, Mulla ZD. Malignant tumors of the orbit: analysis of the Florida Cancer Registry. Ophthalmology. 1998;105(1):185–90.
Rubenstein J, Ferreri AJ, Pittaluga S. Primary lymphoma of the central nervous system: epidemiology, pathology and current approaches to diagnosis, prognosis and treatment. Leuk Lymphoma. 2008;49(sup1):43–51.
Sniegowski MC, Roberts D, Bakhoum M, Mc Laughlin P, Yin VT, Turturro F, Esmaeli B. Ocular adnexal lymphoma: validation of American Joint Committee on Cancer seventh edition staging guidelines. Br J Ophthalmol. 2014;98(9):1255–60.
Thuro BA, Ning J, Peng SA, Pace ST, Dudeja G, Ozgur O, Turturro F, Samaniego F, Hagemeister FB, Fayad LE, Fowler NH. Rates of positive findings on positron emission tomography and bone marrow biopsy in patients with ocular adnexal lymphoma. Ophthal Plast Reconstr Surg. 2017;33(5):355–60.
Nasser QJ, Pfeiffer ML, Romaguera J, Fowler N, Debnam JM, Samaniego F, El-Sawy T, McLaughlin P, Bakhoum MF, Esmaeli B. Clinical value of magnetic resonance imaging and other baseline testing for conjunctival mucosa-associated lymphoid tissue lymphoma. Leuk Lymphoma. 2014;55(5):1013–7.
Kirkegaard MM, Rasmussen PK, Coupland SE, Esmaeli B, Finger PT, Graue GF, Grossniklaus HE, Honavar SG, Khong JJ, McKelvie PA, Mulay K. Conjunctival lymphoma—an international multicenter retrospective study. JAMA Ophthalmol. 2016;134(4):406–14.
Svendsen FH, Rasmussen PK, Coupland SE, Esmaeli B, Finger PT, Graue GF, Grossniklaus HE, Honavar SG, Khong JJ, McKelvie PA, Mulay K. Lymphoma of the eyelid–an international multicenter retrospective study. Am J Ophthalmol. 2017;177:58–68.
Bolek TW, Moyses HM, Marcus RB Jr, Gorden L III, Maiese RL, Almasri NM, Mendenhall NP. Radiotherapy in the management of orbital lymphoma. Int J Radiat Oncol Biol Phys. 1999;44(1):31–6.
Pelloski CE, Wilder RB, Ha CS, Hess MA, Cabanillas FF, Cox JD. Clinical stage IEA–IIEA orbital lymphomas: outcomes in the era of modern staging and treatment. Radiother Oncol. 2001;59(2):145–51.
Stafford SL, Kozelsky TF, Garrity JA, Kurtin PJ, Leavitt JA, Martenson JA, Habermann TM. Orbital lymphoma: radiotherapy outcome and complications. Radiother Oncol. 2001;59(2):139–44.
Decaudin D, de Cremoux P, Vincent-Salomon A, Dendale R, Rouic LL. Ocular adnexal lymphoma: a review of clinicopathologic features and treatment options. Blood. 2006;108(5):1451–60.
Pinnix CC, Dabaja BS, Milgrom SA, et al. Ultra-low-dose radiotherapy for definitive management of ocular adnexal B-cell lymphoma. Head Neck. 2017;39(6):1095–100.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Coiffier B, Lepage E, Brière J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–42.
Keating GM. Spotlight on rituximab in chronic lymphocytic leukemia, low-grade or follicular lymphoma, and diffuse large B-cell lymphoma. BioDrugs. 2011;25(1):55–61.
Salar A, Domingo-Domenech E, Panizo C, Nicolás C, Bargay J, Muntañola A, Canales M, Bello JL, Sancho JM, Tomás JF, Rodríguez MJ. First-line response-adapted treatment with the combination of bendamustine and rituximab in patients with mucosa-associated lymphoid tissue lymphoma (MALT2008-01): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2014;1(3):e104–11.
von Holstein SL. Tumours of the lacrimal gland. Epidemiological, clinical and genetic characteristics. Doctoral dissertation. 2013.
Shields J, Shields C. Lacrimal gland primary epithelial tumors. In: Shields J, Shields C, editors. Eyelid, conjunctival, and orbital tumors: an atlas and text. Philadelphia: Lippincott Williams and Wilkins; 2011. p. 700–25.
Tom A, Bell D, Ford JR, Debnam JM, Guo Y, Frank SJ, Esmaeli B. Malignant mixed tumor (carcinoma ex pleomorphic adenoma) of the lacrimal gland. Ophthal Plast Reconstr Surg. 2020;36(5):497–502.
Esmaeli B, Yin VT, Hanna EY, Kies MS, William WN Jr, Bell D, Frank SJ. Eye-sparing multidisciplinary approach for the management of lacrimal gland carcinoma. Head Neck. 2016;38(8):1258–62.
Woo KI, Sagiv O, Han J, Frank SJ, Kim YD, Esmaeli B. Eye-preserving surgery followed by adjuvant radiotherapy for lacrimal gland carcinoma: outcomes in 37 patients. Ophthalmic Plast Reconstr Surg. 2018;34(6):570–4.
Wolkow N, Jakobiec FA, Lee H. Long-term outcomes of globe-preserving surgery with proton beam radiation for adenoid cystic carcinoma of lacrimal gland. Am J Ophthalmol. 2019;201:84–5.
Rose GE, Gore SK, Plowman NP. Cranio-orbital resection does not appear to improve survival of patients with lacrimal gland carcinoma. Ophthalmic Plast Reconstr Surg. 2019;35(1):77–84.
Hung JY, Wei YH, Huang CH, Chen LW, Fuh CS, Liao SL. Survival outcomes of eye-sparing surgery for adenoid cystic carcinoma of lacrimal gland. Jpn J Ophthalmol. 2019;63(4):344–51.
Han J, Kim YD, Woo KI, Sobti D. Long-term outcomes of eye-sparing surgery for adenoid cystic carcinoma of lacrimal gland. Ophthal Plast Reconstr Surg. 2018;34(1):74–8.
Bonavolontà P, Esmaeli B, Donna P, Tranfa F, Iuliano A, Abbate V, Fossataro F, Attanasi F, Bonavolontà G. Outcomes after eye-sparing surgery vs orbital exenteration in patients with lacrimal gland carcinoma. Head Neck. 2020;42(5):988–93.
Ford JR, Rubin ML, Frank SJ, Ning J, Debnam JM, Bell D, El-Naggar A, Ferrarotto R, Esmaeli B. Prognostic factors for local recurrence and survival and impact of local treatments on survival in lacrimal gland carcinoma. Br J Ophthalmol. 2021;105(6):768–74.
Tse DT, Kossler AL, Feuer WJ, Benedetto PW. Long-term outcomes of neoadjuvant intra-arterial cytoreductive chemotherapy for lacrimal gland adenoid cystic carcinoma. Ophthalmology. 2013;120(7):1313–23.
Fellman M, Carter K, Call CB, Esmaeli B. Disease recurrence after intraarterial chemotherapy in 2 patients with adenoid cystic carcinoma of lacrimal gland. Can J Ophthalmol. 2013;48(2):e17–8.
Jang SY, Kim DJ, Kim CY, Wu CZ, Yoon JS, Lee SY. Neoadjuvant intra-arterial chemotherapy in patients with primary lacrimal adenoid cystic carcinoma. Cancer Imaging. 2014;14(1):1–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Goldfarb, J.A., Esmaeli, B. (2022). Recent Developments in the Management of Orbital and Periocular Neoplasms. In: Chawla, B.V., Aronow, M.E. (eds) Global Perspectives in Ocular Oncology. Springer, Cham. https://doi.org/10.1007/978-3-031-08250-4_31
Download citation
DOI: https://doi.org/10.1007/978-3-031-08250-4_31
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-08249-8
Online ISBN: 978-3-031-08250-4
eBook Packages: MedicineMedicine (R0)