Abstract
Reproductive capabilities of females in all species have been known to be affected by their nutritional status. This is true particularly of malnutrition, for example, mineral imbalance that influences almost all facets of female reproduction, right from the onset of puberty to ovarian activity, ovulation, estrus exhibition, fertility, and conception rate. Mineral deficiencies lead to disturbances along hypothalamic-pituitary-ovarian axis, causing reproductive failure. Evidence gathered from in vitro studies and laboratory animal models suggests involvement of mineral ions in regulation of upstream pathways involved in synthesis and release of gonadotropin-releasing hormone inside hypothalamic nuclei as well as downstream pathways involved in its action on pituitary gonadotrophs. In contract, mineral availability of specific reproductive tissues during various physiological states and their actions in these tissues are poorly defined in any livestock species. Declaration of mineral deficiency of individual animals based on concentration in blood is often misleading due to extreme dietary influences. Moreover, mineral deficiencies are mostly subclinical and by the time symptoms start appearing, the reproductive symptoms thereof get overlooked due to the overall deterioration of the animal’s physiology. The present review offers a summary of biochemical, enzymatic, and endocrine actions of macromineral (calcium, phosphorus, and magnesium) and micromineral (copper, zinc, and manganese) ions along the reproductive axis. We hope to encapsulate research findings to understand the role of specific minerals in female reproduction and identification of biomarkers of impairment of reproductive axis in event of mineral malnutrition, to help us in the development of new nutritional and reproductive strategies for increasing fertility of livestock.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Mineral ions are inorganic substances that serve as essential components of physicochemical processes vital to life. Minerals are distributed throughout all body tissues, having a structural role in some tissues and a regulatory role in others (Underwood and Suttle 1999). The major minerals such as calcium, magnesium, sodium, potassium, phosphorus, sulfur, and chlorine are present in the body in relatively larger amounts. Minerals like Ca and P are important components of bone and other tissues; K, Na and Cl play an important part in the maintenance of acid/base balance and membrane electric potential; Na helps to maintain osmotic pressure, whereas Ca has a role to play in nerve transmission. On the other hand, the trace minerals which include iron, copper, zinc, cobalt, molybdenum, manganese, iodine, and selenium, influence enzyme activity as metalloenzymes (Mn, Zn) or as cofactors (Co), are associated with endocrine hormones (I) and serve as stabilizers of secondary molecular structure. Productive and reproductive efficiency of the animal is the most important factor for the success of a dairy farm. Mineral deficiencies are associated with loss of reproductive performance and related production parameters of economic importance. The hypothalamic–pituitary–gonadal axis as a vital bodily system governs the production and release of gonadal steroid hormones, which further regulate species-specific patterns of sexual development and behavior. Presumably, altered hormonal milieu and ultimately disruption of reproductive functions occur due to disturbance of one or more minerals along this axis. In general, minerals especially, Ca, P and Mg (major elements), and Cu, Zn and Mn (trace elements) influence the reproductive process by their action on hypothalamo-pituitary-gonadal axis (Michaluk and Kochman 2007; Pradhan and Nakagoshi 2008; Dicken et al. 2010). Reproductive performance is, however, affected not only by the absolute concentrations of the major elements, but also by their relative proportions, most notably that of Ca and P (Steevens et al. 1971).
6.2 Hypothalamic-Pituitary-Ovarian Axis
Hypothalamic-pituitary-ovarian axis refers to endocrine coordination between the hypothalamus, pituitary, and the gonads (ovaries) responsible for initiating and regulating the cyclic changes in the female reproductive tract (Fig. 6.1). The gonadotropin-releasing hormone (GnRH), produced by the hypothalamus, regulates gonadotropin secretion by the anterior pituitary, which in turn regulates ovarian functions. Potent stimulators of GnRH include members of the kisspeptin family of peptides. It is believed that GnRH neurons express GPR54 (kisspeptin receptor, KISS1R) and kisspeptin neurons target the GnRH neuronal network to influence the release of GnRH. The hypophyseal portal system picks up GnRH at the median eminence and transports it to the anterior pituitary. The pulsatile release of follicle-stimulating and luteinizing hormones is in response to rhythmic pulses of GnRH reaching the pituitary gonadotrophs. The pulsating nature of gonadotropin release drives follicular maturation, secretion of estrogen during proestrus, and ovulation and secretion of progesterone during diestrus. At the ovaries, the granulosa cells bind follicle-stimulating hormone and theca cells bind luteinizing hormone, to produce progestins, androgens, and estradiol – a model popularly known as two-cell–two-gonadotropin theory (Schiffer et al. 2019).
The neurosecretory mechanism responsible for GnRH secretion from the mammalian hypothalamus is under the influence of a complex interaction involving several excitatory and inhibitory signals. The control of GnRH release from the hypothalamus during the estrous cycle depends mainly upon the feedback mechanism from downstream molecules such as estrogen and progesterone (Kasa-Vubu et al. 1992; Evans et al. 1997; Harris et al. 1999; Richter et al. 2001). The largest healthy follicle in the cohort releases estradiol and inhibin which through a negative feedback mechanism prevent gonadotropin secretion. The lack of gonadotropin stimulation prevents the growth of other follicles in the cohort, whereas only the dominant follicle is selected to grow and destined to be the preovulatory follicle (Hillier 1994). Towards the end of follicular phase (especially during estrous), the peak estrogen production from preovulatory follicle induces GnRH surge from the hypothalamus through a positive feedback mechanism followed by a concomitant luteinizing hormone surge (Adams et al. 2008). This luteinizing hormone surge is responsible for final growth, maturation and ovulation of preovulatory follicle, and formation of corpus luteum. Following ovulation, the granulosa and theca cells of the ovulated follicle luteinize to produce progesterone. In non-pregnant animals, during the late-luteal phase, the luminal epithelium of the endometrium releases luteolytic signal (prostaglandin F2α). However, in a pregnant animal, embryo releases enough quantities of the maternal recognition of pregnancy signal (interferon tau) between days 12 and 32 of the estrous cycle (Bazer 2013). The interferon tau prevents the formation of the luteolytic signal, thereby leading to the persistence of corpus luteum. Evidence gathered from in vitro studies and laboratory animal models suggests involvement of mineral ions in several steps along the reproductive axis.
6.3 Macrominerals
Reproductive events are cyclic in nature so the nutrient requirement of tissues also varies across different physiological stages (Hurley and Doane 1989; Robinson 1990). Macro-minerals regulate various reproductive functions including biosynthesis, secretion, and function of hormones, and their requirement may vary independently according to various physiological stages such as puberty, pregnancy, lactation, and the postpartum period (Ahmed et al. 2000; Ali et al. 2010). A perusal of the scientific literature suggests that even though several macrominerals affect the reproductive health and fertility in farm animals, their precise roles in reproduction are not clearly defined. Among the macro-minerals, because of the role they play in the hypothalamic-pituitary-ovarian axis, Ca, P and Mg have been associated with the various reproductive processes (Table 6.1).
6.3.1 Calcium
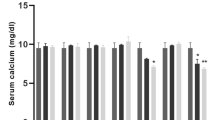
Calcium (Ca) is a major macromineral that affects reproduction. This element is involved in various reproductive processes and acts via the hypothalamic-pituitary-gonadal axis to regulate follicular development and atresia. An optimum Ca concentration ensures that reproductive organs are properly sensitized to various hormones and that the normal reproductive cycle is maintained. Blood concentration of Ca varies across the estrous cycle, being maximum at estrous in cattle (Burle et al. 1995) demonstrating the critical role it plays during the follicular phase especially in and around estrus. In contrast, subclinical hypocalcemia has been reported to cause delayed puberty and anestrus in cows (Dutta et al. 2001). The blood Ca homeostasis is under the influence of parathyroid hormone, 1,25-dihydroxyvitamin D3 and calcitonin. The parathyroid hormone and 1,25-dihydroxyvitamin D3, released in response to low Ca concentration, increase blood Ca by increasing bone resorption, decreasing renal excretion, and enhancing Ca absorption from the intestines. Calcitonin from the thyroid gland, on the other hand, decreases blood Ca by inhibiting reabsorption of Ca from bones and increasing urinary Ca excretion (Murray et al. 2008).
At the level of the hypothalamus, the neuronal circuitry responsible for the pulsatile secretion of GnRH from axonal terminals near median eminence is dependent on voltage-sensitive Ca2+ influx for its maintenance (Bourguignon et al. 1987; Goor et al. 2000). As GnRH concentrations increase beyond a threshold, isolated gonadotrophs have also been shown to respond by presenting dose-dependent intracellular Ca2+ signals (Leong and Thorner 1991; Stojilković et al. 1993; Tomić et al. 1996; Sánchez-Cárdenas and Hernández-Cruz 2010). The GnRH receptor (GnRHR) on the gonadotroph membrane is a member of the G-protein-coupled receptor (GPCR) family (Naor 2009). The binding of GnRH to the receptor, activates phospholipase C, which in turn hydrolyzes membrane phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) (Naor 2009). IP3 initiates Ca2+ release from the intracellular pool, the rise which is responsible for the fusion of gonadotropin-laden secretory vesicles with the plasma membrane to release hormones (Stojilković et al. 1991; Martin 2003). In addition, DAG-induced protein kinase-C activation also drives the influx of extracellular calcium into gonadotrophs through voltage-gated Ca2+ channels for sustained release of gonadotropins (Durán-Pastén and Fiordelisio 2013). Ca withdrawal either due to deficiency or due to presence of Ca blockers inhibits the release of GnRH, follicle-stimulating hormone and luteinizing hormone (Stojilković et al. 1988; Krsmanović et al. 1992; Dhanvantari and Wiebe 1994). It is believed that the detection of Ca by Ca-binding synaptotagmins is involved in both vesicle docking as well as synaptic vesicle fusion with the cell membrane by using SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) proteins as effectors (Durán-Pastén and Fiordelisio 2013).
At the ovarian level, the level of Ca in the follicular fluid increases with the increase of follicular size in cattle (Wise 1987), suggesting a possible role in the gonadotropic regulation of ovarian steroidogenesis (Veldhius and Klase 1982; Carnegie and Tsang 1984). Ca influences cholesterol delivery to and utilisation by mitochondria, and stimulates conversion of pregnenolone to progesterone, which might further explain how this mineral affects ovarian stereogenesis (Shemesh et al. 1984). As the follicle develops from early follicle to the ovulatory stage, an increase in the mineral content (including Ca) of follicular fluid osmotically drives the movement of water from the blood to antrum (Kalmath and Ravindra 2007). Moreover, an increase in Ca concentration increases plasmin activity which in turn weakens the follicular wall and initiates the process of ovulation (Espey 1970). Calcium dependent endonucleases have also been recognized for causing follicular and luteal cell apoptosis.
6.3.2 Phosphorus
The importance of Phosphorus (P) for the normal functioning of all animal tissues is underlined by its involvement in the process of energy exchange at tissue level, besides its role in growth, lactation and reproduction (Little 1970). P is an essential component of phospholipid–dependent protein kinase and cAMP-dependent protein kinase, and is, therefore, crucial in mediating hormone action at the target tissues (Hurley and Doane 1989). The deficiency of P leads to disturbances along HPO-axis, resulting in dysregulation of the normal reproductive rhythm including ovulation (Bhaskaran and Abdulla Khan 1981; Ullah et al. 2010). P-deficiency is manifested as an alteration of ovarian activity resulting in delayed puberty, irregular cyclicity and cessation of behavioral estrous (Hurley and Doane 1989; Ahmed et al. 2010). As compared to anestrous cattle and buffaloes, significantly higher serum concentrations of P (total as well as free form) have been reported in normal cyclic animals (Dutta et al. 2001; Chaurasia et al. 2010). Similarly, in comparison to blood, higher concentrations of P have been reported in follicular fluid (Abd Ellah et al. 2010; Tabatabaei and Mamoei 2011). The concentration in follicular fluid tends to increase towards (Abd Ellah et al. 2010; Eissa 1996).
The importance of dietary calcium and phosphorus ratio (Ca:P) for reproductive performance has been stressed by Pugh et al. (1985), suggesting that a disturbed dietary Ca:P ratio with a resultant improper serum ratio of the ions has a blocking action on the pituitary gland and consequently on the ovarian function. Low Ca and inorganic P levels with resultant serum Ca:P imbalance might be responsible for anestrus status in heifers under poor managemental practices (Dunn and Moss 1992). 1α-hydroxylase (key enzyme in synthesis of 1,25-dihydroxyvitamin D3) deficient [1α(OH)ase−/−] female mice have been reported to be infertile and exhibit uterine hypoplasia and absence of corpora lutea which was attributed to the resulting hypocalcemia and hypophosphatemia (Panda et al. 2001). Infertility accompanied by decreased synthesis of sex steroids, defects in follicular and luteal development, decreased expression of ovarian angiogenic factors and hypoplasia of endometrium was observed in response to hypocalcemia and hypophosphatemia in 1,25-dihydroxyvitamin D3-deficient female mice (Sun et al. 2010). The infertility and the associated defective reproductive phenotype in 1α-hydoxylase deficient female mice were reversed when serum Ca and P levels were restored by the rescue diet (Sun et al. 2010). Besides, reproductive problems such as low first service conception rates and silent heat in ewes (Mosaad and Derar 2009) and delayed puberty in buffalo heifers (Ahmed et al. 2010) have been related to abnormally wide Ca:P ratios.
6.3.3 Magnesium
Magnesium (Mg) has a role in reproduction (Stolkowski 1977), but a direct effect has not been demonstrated yet. Low Serum Mg levels have been reported during anestrous in cattle (Dutta et al. 2001) and buffaloes (Chaurasia et al. 2010). Mg on account of its association with ATP is involved in enzymatic reactions that require the transfer of a phosphate group such as protein kinases and ATPases involved in the transport of various ions (Weisinger and Bellorín-Font 1998; Romani 2007; de Baaij et al. 2015). Mg and ATP are essential substrates for adenyl cyclase, an enzyme required for the synthesis of cyclic adenosine monophosphate (cAMP) – an important second messenger for several reproductive hormones. Protein kinases and cAMP, on the other hand, are involved in hormone synthesis, storage and release at different sites in the hypothalamo-pituitary-ovarian axis (Poisner and Douglas 1968; Moriyama et al. 2000), and physiological response in target cells.
High Mg and low Ca in the perifusion medium increases activity of suprachiasmatic nucleus in brain tissue slices (Pan et al. 1992). The nucleus is involved in circadian rhythm and has receptors for estrogen and progesterone (Kruijver and Swaab 2002), and possibly be involved in the regulation of cyclicity. The release of luteinizing hormone releasing hormone (LH-RH) occurs in an Mg dependent manner (Burrows and Barnea 1982a). In an in-vitro assembly, Mg and ATP act jointly to facilitate the release of LH-RH in hypothalamic granules, though Mg alone can also release LH-RH but to a lower magnitude (Burrows and Barnea 1982a). In humans, the risk of polycystic ovarian syndrome was 19 times greater in Mg deficient females than those with normal serum concentrations (Sharifi et al. 2012).
Acute magnesium deficiency is associated with diminished activity of lecithin cholesterol acyltransferase (LCAT), an enzyme involved in reverse cholesterol transport (Gueux et al. 1984). LCAT converts cholesterol to cholesterol esters, which are taken up by follicular cells for steroidogenesis (Cigliano et al. 2002). Mg deficiency can impair estrogen metabolism and deplete brain dopamine (Sircus 2011). In addition, Mg is an essential component of sex hormone-binding globulin (SHBG), a glycoprotein that transports the sex steroids in the blood and regulates their activity in target cells (Maggio et al. 2011). Mg status affects concentrations of cytochrome P450 (CYP450) enzymes involved in both vitamin D–activating (25-hydroxylase and 1α-hydroxylase) and deactivating enzymes (24-hydroxylase) (Dai et al. 2018). It has also been demonstrated that Mg can modulate parathyroid hormone (PTH) secretion (Rodríguez-Ortiz et al. 2014). Hence, Mg influences the absorption of Ca and P, and therefore, its imbalance may also hamper reproductive efficiency indirectly.
6.4 Micro/Trace Minerals
Trace minerals are essential for the normal functioning of the body as cofactors, as activators of enzymes, or as stabilizers of secondary molecular structures, even though they constitute a miniscule of the total mass of the organism (Rabiee et al. 2010). These minerals play vital roles in vitamin synthesis, hormone production, collagen formation, oxygen transport, energy production, and other physiological processes related to growth, reproduction and health (Ceko et al. 2016). However, some processes are prioritized over others under various conditions, e.g., reproduction or immune competence may be altered during subclinical deficient states without any effect on growth and feed intake. Most micronutrient deficiencies affect reproduction through analtered enzyme activity that disrupts energy and protein metabolism, hormone synthesis, and the integrity of rapidly dividing cells within the reproductive system (Table 6.2). Besides, microminerals serve as antioxidants and scavenge free radicals to protect cells from free radical-induced oxidative damage (Leung 1998; Spears and Weiss 2008).
6.4.1 Copper
The influence of copper (Cu) on both production and reproduction renders this trace mineral of utmost importance to the livestock industry. Cu related physiological disorders may be due to Cu deficiency in diet or due to presence of molybdenum (Mo) and/or sulfur (S) in diet which interferences with Cu bioavailability (Clarkson et al. 2019).
Cu deficiency appears to play significant roles in two key areas i.e. altered reproductive performance and immune suppression (Corah 1996). The consequences of Cu deficiency (high dietary Mo and S) in grazing ruminants may vary from no alteration in estrous behavior to delayed onset of puberty and depressed estrous, but severe copper-deficient cows may show anovulation and retardation of future estrous cycles leading to low fertility (Annenkov 1981; Ingraham et al. 1987; Phillippo et al. 1987; Corah and Ives 1991). The mean serum Cu level in anestrous cattle and buffaloes is reported to be lower than in cyclic animals (Dutta et al. 2001; Akhtar et al. 2009; Ahmed et al. 2010). Neutrophils and mononuclear cells from heifers fed low Cu diet prepartum exhibited reduced phagocytic activity (Torre et al. 1995; Torre et al. 1996). Cu is essential for the activity of superoxide dismutase (SOD1) enzyme, the deficiency of which is associated with defects in ovarian folliculogenesis and dysregulation of luteal function leading to subfertility and infertility in mice (Matzuk et al. 1998; Noda et al. 2012).
Cu has specific effects on the reproductive axis at the level of the hypothalamus, pituitary and ovary. It maintains optimum fertility by affecting GnRH, FSH, LH and estrogen activity (Desai et al. 1982; Michaluk and Kochman 2007). Administration of Cu salt leads to ovulation in female rabbits (Suzuki et al. 1972; Tsou et al. 1977) and ewes (Murawski et al. 2006), through hypothalamic action. Divalent Cu ion is essential for the activity of peptidylglycine α-hydroxylating-monooxygenase (PHM), an enzyme involved in the first step of generation of C-terminal carboxamides of peptide hormones, the reaction necessary for activation of active GnRH peptide from the prohormone (Prigge et al. 2000) (Fig. 6.2). Cu complex variant of GnRH has been shown to interact with GnRH receptors with enhanced affinity, to bring about a more potent release of LH, and to modulate intracellular signaling by increasing cAMP accumulation in the gonadotrope cells, whereas the non-complexed GnRH utilizes IP3/DAG signaling pathway (Kochman et al. 2005; Michaluk and Kochman 2007; Gajewska et al. 2016).
The regulation of upstream and downstream pathways of hormone synthesis and release along hypothalamic-pituitary-ovarian axis by minetral ions and the associated hormonal dynamics. IGF1 insulin-like growth factor 1, Mn manganese, mTOR mammalian target of rapamycin complex 1, Akt serine/threonine kinase, PHM peptidylglycine α-hydroxylating-monooxygenase, PAL peptidylglycine α-amidating lyase, IP3 inositol 1,4,5-trisphosphate, DAG diacylglycerol
Cu-deficiency induced perturbation of the follicular growth and development may be due to disturbance in Cu-containing enzyme, lysyl oxidase, which promotes cross-linking of collagen and elastin, and stabilizes the extracellular matrix (Kendall et al. 2003). The basal lamina is constantly remodeled as the follicle matures (Rodgers et al. 1999), thereby requiring the expression of the lysyl oxidase in granulosa cells (Slee et al. 2001). Interestingly, a high concentration of Cu in follicular fluid is seen as an endocrine disruptor leading to polycystic ovary syndrome (Sun et al. 2019).
6.4.2 Zinc
Zinc (Zn) plays a pivotal role in reproduction as an essential component of enzymes in different biochemical pathways (Hurley and Doane 1989). It acts indirectly through the pituitary to influence the release of gonadotropic hormones (Dicken et al. 2010) and directly owing to its presence in Zn fingers (two in number) in steroid receptors (Spelsberg et al. 1989). The second step of the amidation reaction for activation of active GnRH from prohormone is carried out by peptidylglycine α-amidating lyase which requires divalent Zn as a cofactor (Michaluk and Kochman 2007) (Fig. 6.2). It has a physiological role in regulating pituitary prolactin secretion (Brandao-Neto et al. 1995; Lee and Kelleher 2016), and its deficiency is associated with hyperprolactinemia (Dicken et al. 2010), resulting in suppression of hypothalamo-pituitary-gonadal axis.
Zinc deficient animals have depressed serum vitamin A levels as it’s deficiency hampers mobilization of vitamin A from the liver. Zn and vitamin A are necessary for the normal functioning of the germinal epithelium of the ovary, and their deficiency leads to deformed or atretic follicles resulting in failure of the ovarian function (Chhabra and Arora 1985). Lower mean serum Zn levels have been reported in buffaloes suffering from anestrous (Akhtar et al. 2009; Ahmed et al. 2010) than their cyclic counterparts.
Zn deficiency is associated with suboptimal steroid hormone concentrations i.e. estrogen and progesterone (Akhtar et al. 2009) which is attributed to the involvement of the ion in the process of steroidogenesis (Hurley and Doane 1989). Ovarian (follicular fluid and granulosa cell) zinc content is reduced during follicular atresia (Kaswan and Bedwal 1995; Bhardwaj and Sharma 2011; Mahavar 2011), indicating that the ion can serve as a marker for follicular health. Higher follicular fluid concentrations of Zn and Cu positively influence the outcome of IVF in the form of greater MII oocyte retrieval, and higher fertilization and cleavage rates (Sun et al. 2017). Zn is essential for cumulus cell expansion and completion of meiosis-I, and an acute Zn deficiency blocks follicle rupture during ovulation (Tian and Diaz 2012). Additionally, Zn supplementation during in vitro maturation (IVM) significantly increased the meiotic competence of bovine oocytes (Barros et al. 2018), and supplementation during in vitro embryo culture improved the cell number of inner cell mass (Woolridge et al. 2019).
6.4.3 Manganese
Manganese (Mn) is an essential component of bovine nutrition required for carbohydrate metabolism, bone growth, normal brain function, growth, reproduction, and a variety of enzymatic systems (Aschner and Aschner 2005). Corah (1996) suggested that Mn has far more influence on reproduction than actually realized. The effects of Mn deficiency on reproduction have been reviewed over the last few decades (Hidiroglou 1979; Pugh et al. 1985; Sharma 2006; Kumar et al. 2011). Poor follicular development with delayed ovulation, delayed postpartum estrous and delayed puberty in heifers, reduced intensity of estrous, and reduced conception rates are also associated with Mn deficiency (Bentley and Philips 1951; Bourne 1967).
Although the precise mechanism of the involvement of Mn in reproduction is unknown, evidence suggests its role in the activity of certain endocrine organs. Both pituitary and ovarian tissues, particularly Graafian follicle and corpus luteum, are rich in Mn content (Hidiroglou 1979). Mn is carried by transport proteins such as transferrin and divalent metal transporter-1 across the blood-brain barrier into the hypothalamus (Garcia et al. 2006; Aschner et al. 2008). Chronic administration of Mn at low doses has been reported to increase serum levels of LH, FSH and estradiol in female rats, possibly due to the hypothalamic action of the ion (Pine et al. 2005; Lee et al. 2006; Lee et al. 2007). Mn supplementation in female rats activates upstream genes, mainly the Kiss-1 gene, which regulate GnRH release from hypothalamic nuclei (Srivastava et al. 2013). The Kiss-1 gene is responsible for the synthesis of kisspeptins (Thompson et al. 2004; Caraty et al. 2007; Keen et al. 2008; Lehman et al. 2010). The Mn-stimulated kisspeptin synthesis is mediated by the IGF-1/Akt/mTOR pathway in the prepubertal female rat (Srivastava et al. 2016; Dees et al. 2017) (Fig. 6.2).
Ovarian Mn content is particularly responsive to Mn deficiency (Wilson 1966; Hurley and Doane 1989). Mn deficiency alters the synthesis of gonadal hormones such as estrogen and progesterone in the female (Keen and Zidenberg-Cheer 1990; Pradhan and Nakagoshi 2008), possibly through inhibition of cholesterol and cholesterol precursor synthesis (Doisy 1974). Mn is an essential component of mitochondrial superoxide dismutase (SOD2). SOD2 expression in corpus luteum has been reported to increase from mid-luteal to late-luteal phase in women (Sugino et al. 2000) and sheep (Al-Gubory et al. 2005), but does not alter in pregnant ewes between days 12 and 20 (Arianmanesh et al. 2011) suggesting a role in corpus luteum regression.
Mn supplementation shortens the postpartum anestrous period and increases conception rates in dairy cows (Krolak 1968; Ahmed et al. 2010), and is effective in suspending summer acyclicity in buffaloes (Ahmed et al. 2010). The serum concentrations of Mn are lower in delayed ovulating and anovulating heifers as compared to normal ovulating heifers (Das et al. 2009). This report confirms the earlier claims that delayed ovulation can be experimentally induced by dietary withdrawal of Mn from dairy cows (Rojas et al. 1965), thus underlining the crucial role Mn plays in the normal ovulatory process.
6.5 Conclusion
Dietary mineral elements in excess or otherwise affect the normal physiological processes of an animal. Imbalances in the mineral availability also affect the reproductive process leading to subnormal fertility, anestrous and ovulatory disturbances. The hypothalamo–pituitary–gonadal axis is the primary site of action of mineral ions. Derangements of mineral availablility end up altering the hormonal milieu along the hypothalamo–pituitary–gonadal axis and cause normal reproductive functions to fail. Evidence gathered from in vitro studies and laboratory animal models suggests involvement of mineral ions in regulation of upstream pathways involved in synthesis and release of gonadotropin-releasing hormone (GnRH) inside hypothalamic nuclei as well as downstream pathways involved in its action on pituitary gonadotrophs. However, advances in the understanding of such molecular mechanisms of action of mineral ions have not found their way into ruminant nutrition research and practice, leading to a widespread mineral imbalance in prized livestock. These imbalances not only affect the health of an individual animal or that of a herd, but tend to be a major hurdle in livestock rearing from an economical dimension as well.
Future research should focus on (1) finding better ways of supplementation that will increase bioavailability of minerals ions at specific sites along reproductive axis, (2) the establishment of a panel of biomarkers of reproductive functionality to be used in farm animals for early detection of specific mineral deficiency, (3) understanding how genetics and stages of (re)production of an animal affect the dynamics of mineral requirements as well as their utilisation; (4) understanding the effect of regional as well as seasonal variations on specific and general mineral requirements of an animal instead of a one-size-fits-all approach; and (5) extension of molecular research from laboratory animal models to ruminant nutrition practice.
Abbreviations
- Akt:
-
serine/threonine kinase, also known as protein kinase B
- GnRH:
-
gonadotropin-releasing hormone
- GPCR:
-
G-protein-coupled receptors
- HPO:
-
hypothalamic-pituitary-ovarian axis
- IGF1:
-
insulin-like growth factor 1
- LCAT:
-
lecithin cholesterol acyltransferase
- mTOR:
-
mammalian target of rapamycin
- PAL:
-
peptidylglycine α-amidating lyase
- PGF2α:
-
prostaglandin F2α
- PHM:
-
peptidylglycine α-hydroxylating-monooxygenase
- PIP2:
-
phosphatidylinositol 4,5-bisphosphate
- SHBG:
-
sex hormone-binding globulin
- SNARE:
-
soluble N-ethylmaleimide-sensitive factor attachment protein receptors
- SOD:
-
superoxide dismutase
References
Abd Ellah MR, Hussein HA, Derar DR (2010) Ovarian follicular fluid constituents in relation to stage of estrus cycle and size of the follicle in Buffalo. Vet World 3(6):263–267
Adams GP, Jaiswal R, Singh J, Malhi P (2008) Progress in understanding ovarian follicular dynamics in cattle. Theriogenology 69:72–80. https://doi.org/10.1016/j.theriogenology.2007.09.026
Ahmed MM, Siham AK, Barri MES (2000) Macromineral profile in the plasma of Nubian goats as affected by the physiological state. Small Rum Res 38:249–254. https://doi.org/10.1016/S0921-4488(00)00166-8
Ahmed WM, Bashandy MM, Ibrahim AK, Shalaby SIA, El-Moez SIA, El-Moghazy FM, Ibrahim SRE (2010) Investigations on delayed puberty in Egyptian buffalo-heifers with emphasis on clinicopathological changes and treatment using GnRH (Receptal®). Glob Vet 4:78–85
Akhtar MS, Farooq AA, Mushtaq M (2009) Serum concentrations of copper, iron, zinc and selenium in cyclic and anoestrusNili-Ravi buffaloes kept under farm conditions. Pak Vet J 29:47–48
Al-Gubory KH, Ceballos-Picot I, Nicole A, Bolifraud P, Germain G, Michaud M, Mayeur C, Blachier F (2005) Changes in activities of superoxide dismutase, nitric oxide synthase, glutathione-dependent enzymes and the incidence of apoptosis in sheep corpus luteum during the estrous cycle. Biochim. Biophys. Acta. Gen Subj 1725:348–357. https://doi.org/10.1016/j.bbagen.2005.06.018
Ali F, Lodhi LA, Qureshi ZI, Younis M (2010) Serum macromineral levels in estrual, fertile, subfertile and pregnant mares kept under two different managemental conditions. Pak Vet J 30:87–90
Annenkov BN (1981) Mineral feeding of cattle. In: Georgievskii VI, Annenkov BN, Samokbin VT (eds) Mineral nutrition of animals. Butterworths, London, p 285
Arianmanesh M, McIntosh RH, Lea RG, Fowler PA, Al-Gubory KH (2011) Ovine corpus luteum proteins, with functions including oxidative stress and lipid metabolism, show complex alterations during implantation. J Endocrinol 210:47–58. https://doi.org/10.1530/JOE-10-0336
Aschner JL, Aschner M (2005) Nutritional aspects of manganese homeostasis. Mol Asp Med 26:353–362. https://doi.org/10.1016/j.mam.2005.07.003
Aschner M, dos Santos AP, Erikson KM, Zheng W (2008) Manganese transport into the brain:putative mechanisms. In: Metal ions in biology and medicine. Proceedings of the 10th international symposium on metal ions in biology and medicine. Corsica, France, pp 695–700
Barros RG, Lodde V, Dieci C, Fraciosi F, Luciano AM (2018) Study on the effects of zinc supplementation during in vitro embryo production technologies in cattle. Int J Health Anim Sci Food Safety 5(1s). https://doi.org/10.13130/2283-3927/10021
Bazer FW (2013) Pregnancy recognition signaling mechanisms in ruminants and pigs. J Anim Sci Biotechnol 4:23. https://doi.org/10.1186/2049-1891-4-23
Bentley OG, Philips PH (1951) The effect of low manganese rations upon dairy cattle. J Dairy Sci 34:396–403. https://doi.org/10.3168/jds.S0022-0302(51)91727-4
Bhardwaj JK, Sharma RK (2011) Changes in trace elements during follicular atresia in goat (Capra hircus) ovary. Biol Trace Elem Res 140:291–298. https://doi.org/10.1007/s12011-010-8700-7
Bhaskaran R, Abdulla Khan CK (1981) Role of blood serum inorganic phosphorus in post-parturient anoestrus cows. Livest Adv 9:138–143
Bourguignon JP, Gerard A, Debougnoux G, Rose J, Franchimont P (1987) Pulsatile release of gonadotropin releasing hormone (GnRH) from the rat hypothalamus in vitro:calcium and glucose dependency and inhibition by superactive GnRH analogs. Endocrinology 121:993–999. https://doi.org/10.1210/endo-121-3-993
Bourne F (1967) Manganese:its relationship to infertility in the bovine. Feed Forum 2:33
Brandao-Neto J, Madureira G, Mendonca BB, Bloise W, Castro AV (1995) Endocrine interaction between zinc and prolactin. An interpretative review. Biol Trace Elem Res 49:139–149. https://doi.org/10.1007/BF02788963
Burle PM, Mangle NS, Kothekar MD, Kalorey DR (1995) Blood biochemical profiles during various reproductive states of Sahiwal and Jersey X Sahiwal cattle. Livest Advisor 20:13–20
Burrows GH, Barnea A (1982a) Comparison of the effects of ATP, Mg2+, and MgATP on the release of luteinizing hormone-releasing hormone from isolated hypothalamic granules. J Neurochem 38:569–573. https://doi.org/10.1111/j.1471-4159.1982.tb08664.x
Burrows GH, Barnea A (1982b) Copper stimulates the release of luteinizing hormone releasing hormone from isolated hypothalamic granules. Endocrinology 110:1456–1458. https://doi.org/10.1210/endo-110-4-1456
Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ (2007) Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology 148:5258–5267. https://doi.org/10.1210/en.2007-0554
Carnegie JA, Tsang BK (1984) The calcium-calmodulin system: participation in the regulation of steroidogenesis at different stages of granulosa cell differentiation. Biol Reprod 30:515–522. https://doi.org/10.1095/biolreprod30.2.515
Ceko MJ, O’Leary S, Harris HH, Hummitzsch K, Rodgers RJ (2016) Trace elements in ovaries:measurement and physiology. Biol Reprod 94:1–14. https://doi.org/10.1095/biolreprod.115.137240
Chaurasia R, Kushwaha HS, Chaurasia D, Gendley MK, Santra AK (2010) Comparative studies of certain macro minerals during various reproductive states in buffaloes. Buffalo Bull 29:291–298
Chhabra A, Arora SP (1985) Effect of Zn deficiency on serum vitamin A level, tissue enzymes and histological alterations in goats. Livest Prod Sci 12:69–77. https://doi.org/10.1016/0301-6226(85)90041-7
Choudhary E, Sen A, Inskeep EK, Flores JA (2005) Developmental sensitivity of the bovine corpus luteum to prostaglandin F2α (PGF2α) and endothelin-1 (ET-1):is ET-1 a mediator of the luteolytic actions of PGF2α or a tonic inhibitor of progesterone secretion? Biol Reprod 72:633–642. https://doi.org/10.1095/biolreprod.104.034736
Christian P, West KP Jr (1998) Interactions between zinc and vitamin A: an update. Am J Clin Nutr 68:435S–441S. https://doi.org/10.1093/ajcn/68.2.435S
Cigliano L, Balestrieri M, Spagnuolo MS, Dale B, Abrescia P (2002) Lecithin-cholesterol acyltransferase activity during maturation of human preovulatory follicles with different concentrations of ascorbate, a-tocopherol and nitrotyrosine. Reprod Fertil Dev 14:15–21. https://doi.org/10.1071/RD01044
Clarkson AH, Paine S, Martín-Tereso J, Kendall NR (2019) Copper physiology in ruminants: trafficking of systemic copper, adaptations to variation in nutritional supply and thiomolybdate challenge. Nutr Res Rev 33:43–49. https://doi.org/10.1017/S0954422419000180
Corah L (1996) Trace mineral requirements of grazing cattle. Anim Feed Sci Technol 59:61–70. https://doi.org/10.1016/0377-8401(95)00887-X
Corah LR, Ives S (1991) Effect of essential trace minerals on reproduction in beef cattle. Vet Clin North Am Food Anim Pract 7:41–57. https://doi.org/10.1016/S0749-0720(15)30809-4
Dai Q, Zhu X, Manson JE, Song Y, Li X, Franke AA, Costello RB, Rosanoff A, Nian H, Fan L, Murff H (2018) Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am J Clin Nutr 108:1249–1258. https://doi.org/10.1093/ajcn/nqy274
Das JM, Dutta P, Deka KC, Biswas RK, Sarmah BC, Dhali A (2009) Comparative study on serum macro and micro mineral profiles during oestrus in repeat breeding crossbred cattle with impaired and normal ovulation. Livest Res Rural Dev 21:Article 72
Davis JS, Weakland LL, Weiland DA, Farese RV, West LA (1987) Prostaglandin F2 alpha stimulates phosphatidylinositol 4, 5-bisphosphate hydrolysis and mobilizes intracellular Ca2+ in bovine luteal cells. Proc Natl Acad Sci 84:3728–3732. https://doi.org/10.1073/pnas.84.11.3728
de Baaij JH, Hoenderop JG, Bindels RJ (2015) Magnesium in man–implication for health and disease. Physiol Rev 95:1–46. https://doi.org/10.1152/physrev.00012.2014
Dees WL, Hiney JK, Srivastava VK (2017) Influences of manganese on pubertal development. J Endocrinol 235:R33–R42. https://doi.org/10.1530/JOE-17-0237
Desai MC, Thakkar TP, Dharshana RA, Janakiraman K (1982) Serum copper and iron in Surti buffalo in relation to reproduction and gonadotropins. Indian J Anim Sci 52:443–444
Dhanvantari SA, Wiebe JP (1994) Suppression of follicle-stimulating hormone by the gonadal-and neurosteroid 3 alpha-hydroxy-4-pregnen-20-one involves actions at the level of the gonadotrope membrane/calcium channel. Endocrinology 134:371–376. https://doi.org/10.1210/en.134.1.371
Dicken C, Menke M, Neal-Perry G (2010) The hypothalamic-pituitary-ovarian axis. In: Santoro N, Neal-Perry G (eds) Amenorrhea, contemporary endocrinology series. Humana Press, Totowa, pp 1–19. https://doi.org/10.1007/978-1-60327-864-5_1
Doisy EA (1974) Effects of deficiency in manganese upon plasma levels of clotting proteins and cholesterol in man. In: Hoeskstra WG, Suttie JW, Ganther HE, Mertz W (eds) Trace element metabolism in animals-2. University Park Press, Baltimore, pp 668–670
Dunn TG, Moss GE (1992) Effects of nutrient deficiencies and excesses on reproductive efficiency of livestock. J Anim Sci 70:1580–1593
Durán-Pastén ML, Fiordelisio T (2013) GnRH-induced Ca2+ signaling patterns and gonadotropin secretion in pituitary gonadotrophs. Functional adaptations to both ordinary and extraordinary physiological demands. Front Endocrinol 4:127
Dutta A, Baruah B, Sharma BC, Baruah KK, Goswami RN (2001) Serum macromineral profiles in cyclic and anoestrus local heifers in lower Brahmaputra valley of Assam. Indian J Anim Res 35:44–46
Eissa HM (1996) Concentrations of steroids and biochemical constituents in follicular fluid of buffalo cows during different stages of the oestrous cycle. Br Vet J 152:573–581. https://doi.org/10.1016/S0007-1935(96)80009-1
Espey LL (1970) Effect of various substances on tensile strength of sow ovarian follicles. Am J Phys 219:230–233
Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ (1997) Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implication for neuroendocrine processing of the estradiol signal. Endocrinology 138:5408–5414. https://doi.org/10.1210/endo.138.12.5558
Gajewska A, Zielinska-Gorska M, Wolinska-Witort E, Siawrys G, Baran M, Kotarba G, Biernacka K (2016) Intracellular mechanisms involved in copper-gonadotropin-releasing hormone (Cu-GnRH) complex-induced cAMP/PKA signaling in female rat anterior pituitary cells in vitro. Brain Res Bull 120:75–82. https://doi.org/10.1016/j.brainresbull.2015.11.002
Garcia SJ, Gellein K, Syversen T, Aschner M (2006) A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol Sci 92:516–525. https://doi.org/10.1093/toxsci/kfl017
Georgievskii VI (1981) The physiological role of microelements. In: Georgievskii VI, Annenkov BN, Samokhin VT (eds) Mineral nutrition of animals. Butterworths, London, p 171
Goor FV, Krsmanovic LZ, Catt KJ, Stojilkovic SS (2000) Autocrine regulation of calcium influx and gonadotropin-releasing hormone secretion in hypothalamic neurons. Biochem Cell Biol 78:359–370. https://doi.org/10.1139/o00-058
Gueux E, Rayssiguier Y, Piot MC, Alcindor L (1984) Reduction of plasma lecithin—cholesterol acyltransferase activity by acute magnesium deficiency in the rat. J Nutr 114:1479–1483. https://doi.org/10.1093/jn/114.8.1479
Harris TG, Dye S, Robinson JE, Skinner DC, Evans NP (1999) Progesterone can block transmission of the estradiol-induced signal for luteinizing hormone surge generation during a specifi c period of time immediately after activation of the gonadotropin releasing hormone surge-generating system. Endocrinology 140:827–834. https://doi.org/10.1210/endo.140.2.6490
Hidiroglou M (1979) Trace element deficiencies and fertility in ruminants:a review. J Dairy Sci 62:1195–1206. https://doi.org/10.3168/jds.S0022-0302(79)83400-1
Hillier SG (1994) Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod 9:188–191. https://doi.org/10.1093/oxfordjournals.humrep.a138480
Hurley WL, Doane RM (1989) Recent developments in the roles of vitamins and minerals in reproduction. J Dairy Sci 72:784–804. https://doi.org/10.3168/jds.S0022-0302(89)79170-0
Ingraham RH, Kappel LC, Morgan EB, Srikandakumar A (1987) Correction of subnormal fertility with copper and magnesium supplementation. J Dairy Sci 70:167–180. https://doi.org/10.3168/jds.S0022-0302(87)79991-3
Kalmath GP, Ravindra JP (2007) Mineral profiles of ovarian antral follicular fluid in buffaloes during follicular development. Indian J Anim Res 41:87–93
Kasahara E, Lin LR, Ho YS, Reddy VN (2005) Manganese superoxide dismutase protects against oxidation-induced apoptosis in mouse retinal pigment epithelium:implications for age-related macular degeneration. Investig Ophthalmol Vis Sci 46:3426–3434. https://doi.org/10.1167/iovs.05-0344
Kasa-Vubu J, Dahl GE, Evans NP, Thrun LA, Moenter SM, Padmanabhan V, Karsch FJ (1992) Progesterone blocks the estradiol-induced gonadotropin discharge in the ewe by inhibiting the surge of gonadotropin-releasing hormone. Endocrinology 131:208–212. https://doi.org/10.1210/endo.131.1.1611998
Kaswan S, Bedwal RS (1995) Light and electron microscopic changes in the ovary of zinc deficient BALB/c mice. Indian J Exp Biol 33:469–479
Keen CL, Zidenberg-Cheer S (1990) Manganese. In: Brown ML (ed) Present knowledge in nutrition. International Life Science Institute Nutrition Foundation, Washington, DC, pp 279–268
Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E (2008) An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157. https://doi.org/10.1210/en.2008-0231
Kendall NR, Marsters P, Scaramuzzi RJ, Campbell BK (2003) Expression of lysyl oxidase and effect of copper chloride and ammonium tetrathiomolybdate on bovine ovarian follicle granulosa cells cultured in serum-free media. Reproduction 125:657–665
Kochman K, Blitek A, Kaczmarek M, Gajewska A, Siawrys G, Counis R, Ziecik AJ (2005) Different signaling in pig anterior pituitary cells by GnRH and its complexes with copper and nickel. Neuroendocrinol Lett 26:377–382
Krolak M (1968) Effect of manganese added to diet on cattle fertility and manganese content of hair. Pol Arch Wet 11:293–304
Krsmanović LZ, Stojilković SS, Merelli F, Dufour SM, Virmani MA, Catt KJ (1992) Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci 89:8462–8466. https://doi.org/10.1073/pnas.89.18.8462
Kruijver FP, Swaab DF (2002) Sex hormone receptors are present in the human suprachiasmatic nucleus. Neuroendocrinology 75:296–305. https://doi.org/10.1159/000057339
Kumar S, Pandey AK, AbdulRazzaque WA, Dwivedi DK (2011) Importance of micro minerals in reproductive performance of livestock. Vet World 4:230–233
Lebedeva IY, Denisenko VY, Lebedev VA, Kuzmina TI (1998) Prolactin in follicular fluid and intracellular store calcium in follicular cells are related to morphological signs of ovarian follicle atresia in cows:work in progress. Theriogenology 49:509–519. https://doi.org/10.1016/S0093-691X(98)00002-8
Lee S, Kelleher SL (2016) Molecular regulation of lactation: the complex and requisite roles for zinc. Arch Biochem Biophys 611:86–92. https://doi.org/10.1016/j.abb.2016.04.002
Lee B, Pine M, Johnson L, Rettori V, Hiney JK, Les Dees W (2006) Manganese acts centrally to activate reproductive hormone secretion and pubertal development in male rats. Reprod Toxicol 22:580–585. https://doi.org/10.1016/j.reprotox.2006.03.011
Lee B, Hiney JK, Pine MD, Srivastava VK, Dees WL (2007) Manganese stimulates luteinizing hormone releasing hormone secretion in prepubertal female rats:hypothalamic site and mechanism of action. J Physiol 578:765–772. https://doi.org/10.1113/jphysiol.2006.123083
Lehman MN, Merkley CM, Coolen LM, Goodman RL (2010) Anatomy of the kisspeptin neural network in mammals. Brain Res 1364:90–102. https://doi.org/10.1016/j.brainres.2010.09.020
Leong DA, Thorner MO (1991) A potential code of luteinizing hormone-releasing hormone-induced calcium ion responses in the regulation of luteinizing hormone secretion among individual gonadotropes. J Biol Chem 266:9016–9022. http://intl.jbc.org/cgi/content/abstract/266/14/9016
Leung FY (1998) Trace elements that act as antioxidants in parenteral micronutrition. J Nutr Biochem 9:304–307. https://doi.org/10.1016/S0955-2863(98)00018-7
Little DA (1970) Factors of importance in the phosphorus nutrition of beef cattle in northern Australia. Aust Vet J 46:241–248. https://doi.org/10.1111/j.1751-0813.1970.tb15767.x
Maggio M, Ceda GP, Lauretani F, Bandinelli S, Corsi AM, Giallauria F, Guralnik JM, Zuliani G, Cattabiani C, Parrino S, Ablondi F (2011) SHBG, sex hormones, and inflammatory markers in older women. J Clin Endocrinol Metab 96:1053–1059. https://doi.org/10.1210/jc.2010-1902
Mahavar D (2011) Changes in trace elements during follicular atresia in rat. Int Referred Res J 2:88–89. https://doi.org/10.1007/s12011-010-8700-7
Martin TF (2003) Tuning exocytosis for speed:fast and slow modes. Biochim Biophys Acta (BBA)-Mol Cell Res 1641:157–165. https://doi.org/10.1016/S0167-4889(03)00093-4
Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM (1998) Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology 139:4008–4011. https://doi.org/10.1210/endo.139.9.6289
Michaluk A, Kochman K (2007) Involvement of copper in female reproduction. Reprod Biol 7:193–205
Moriyama Y, Hayashi M, Yamada H, Yatsushiro S, Ishio S, Yamamoto A (2000) Synaptic-like microvesicles, synaptic vesicle counterparts in endocrine cells, are involved in a novel regulatory mechanism for the synthesis and secretion of hormones. J Exp Biol 203:117–125
Mosaad GM, Derar DR (2009) Effect of dietary energy and phosphorus on nutrients digestibility, blood constituents, and ovarian structures in ewes. Vet World 2:456–461
Murawski M, Bydlon G, Sawicka-Kapusta K, Wierzchos E, Zakrzewska M, Wlodarczyk S, Molik E, Zieba D (2006) The effect of long term exposure to copper on physiological condition and reproduction of sheep. Reprod Biol 6:201–206
Murray RD, Horsfield JE, McCormick WD, Williams HJ, Ward D (2008) Historical and current perspectives on the treatment, control and pathogenesis of milk fever in dairy cattle. Vet Rec 163:561–565. https://doi.org/10.1136/vr.163.19.561
Naor Z (2009) Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol 30:10–29. https://doi.org/10.1016/j.yfrne.2008.07.001
Noda Y, Ota K, Shirasawa T, Shimizu T (2012) Copper/zinc superoxide dismutase insufficiency impairs progesterone secretion and fertility in female mice. Biol Reprod 86:1–8. https://doi.org/10.1095/biolreprod.111.092999
Pan JT, Li CS, Ka-Choi T, Jing-Ying L (1992) Low calcium/high magnesium medium increases activities of hypothalamic arcuate and suprachiasmatic neurons in brain tissue slices. Neurosci Lett 144:157–160. https://doi.org/10.1016/0304-3940(92)90739-T
Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D (2001) Targeted ablation of the 25-hydroxyvitamin D 1α-hydroxylase enzyme:evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci 98:7498–7503. https://doi.org/10.1073/pnas.131029498
Phillippo M, Humphries WR, Atkinson T, Henderson GD, Garthwaite PH (1987) The effect of dietary molybdenum and iron on copper status, puberty, fertility and oestrous cycles in cattle. J Agric Sci 109:321–336. https://doi.org/10.1017/S0021859600080758
Pine M, Lee B, Dearth R, Hiney JK, Dees WL (2005) Manganese acts centrally to stimulate luteinizing hormone secretion:a potential influence on female pubertal development. Toxicol Sci 85:880–885. https://doi.org/10.1093/toxsci/kfi134
Poisner AM, Douglas WW (1968) A possible mechanism of release of posterior pituitary hormones involving adenosine triphosphate and an adenosine triphosphatase in the neurosecretory granules. Mol Pharmacol 4:531–540
Pradhan R, Nakagoshi N (2008) Reproductive disorders in cattle due to nutritional status. J Int Dev Coop 14:45–66
Prigge ST, Mains RE, Eipper BA, Amzel LM (2000) New insights into copper monooxygenases and peptide amidation:structure, mechanism and function. Cell Mol Life Sci 57:1236–1259. https://doi.org/10.1007/PL00000763
Pugh DG, Elmore RG, Hembree TR (1985) A review of the relationship between mineral nutrition and reproduction in cattle. Bovine Pract 20:10
Rabiee AR, Lean IJ, Stevenson MA, Socha MT (2010) Effects of feeding organic trace minerals on milk production and reproductive performance in lactating dairy cows:a meta-analysis. J Dairy Sci 93:4239–4251. https://doi.org/10.3168/jds.2010-3058
Richter TA, Robinson JE, Evans NP (2001) Progesterone treatment that either blocks or augments the estradiol-induced gonadotropin-releasing hormone surge is associated with different patterns of hypothalamic neural activation. Neuroendocrinology 73:378–386. https://doi.org/10.1159/000054656
Robinson JJ (1990) Nutrition in the reproduction of farm animals. Nutr Res Rev 3:253–276. https://doi.org/10.1079/NRR19900015
Rodgers, R.J., Van Wezel, I.L., Irving-Rodgers, H.F., Lavranos., T.C., Irvine, C.M., Krupa, M., 1999. Roles of extracellular matrix in follicular development. J Reprod Fertil Suppl 54, 343–352
Rodríguez-Ortiz ME, Canalejo A, Herencia C, Martínez-Moreno JM, Peralta-Ramírez A, Perez-Martinez P, Navarro-González JF, Rodríguez M, Peter M, Gundlach K, Steppan S (2014) Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentration. Nephrol Dial Transpl 29:282–289. https://doi.org/10.1093/ndt/gft400
Rojas MA, Dyer IA, Cassatt WA (1965) Manganese deficiency in the bovine. J Anim Sci 24:664–667. https://doi.org/10.2527/jas1965.243664x
Romani AM (2007) Magnesium homeostasis in mammalian cells. Front Biosci 12:308–331. https://doi.org/10.2741/2066
Sánchez-Cárdenas C, Hernández-Cruz A (2010) GnRH-induced [Ca2+] i-signalling patterns in mouse gonadotrophs recorded from acute pituitary slices in vitro. Neuroendocrinology 91:239–255. https://doi.org/10.1159/000274493
Schiffer L, Barnard L, Baranowski ES, Gilligan LC, Taylor AE, Arlt W, Shackleton CH, Storbeck KH (2019) Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: a comprehensive review. J Steroid Biochem Mol Biol 194:105439. https://doi.org/10.1016/j.jsbmb.2019.105439
Sharifi F, Mazloomi S, Hajihosseini R, Mazloomzadeh S (2012) Serum magnesium concentrations in polycystic ovary syndrome and its association with insulin resistance. Gynecol Endocrinol 28:7–11
Sharma MC (2006) Role of minerals in reproductive management with special reference to health and production,in:Compendium of national training course on reproductive health management of farm animals. Ministry of Agriculture, New Delhi, pp 14–25
Shemesh M, Hansel W, Strauss JF (1984) Calcium-dependent, cyclic nucleotide-independent steroidogenesis in the bovine placenta. Proc Natl Acad Sci 81:6403–6407. https://doi.org/10.1073/pnas.81.20.6403
Sircus M (2011) Transdermal magnesium therapy:a new modality for the maintenance of health. iUniverse, Bloomington
Slee RB, Hillier SG, Largue P, Harlow CR, Miele G, Clinton M (2001) Differentiation-dependent expression of connective tissue growth factor and lysyl oxidase messenger ribonucleic acids in rat granulosa cells. Endocrinology 142:1082–1089. https://doi.org/10.1210/endo.142.3.7990
Spears JW, Weiss WP (2008) Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet J 176:70–76. https://doi.org/10.1095/biolreprod40.1.54
Spelsberg TC, Rories C, Rejman JJ, Goldberger A, Fink K, Lau CK, Colvard DS, Wiseman G (1989) Steroid action on gene expression:possible roles of regulatory genes and nuclear acceptor sites. Biol Reprod 40:54–69. https://doi.org/10.1093/toxsci/kft195
Srivastava VK, Hiney JK, Dees WL (2013) Early life manganese exposure upregulates tumor-associated genes in the hypothalamus of female rats:relationship to manganese-induced precocious puberty. Toxicol Sci 136:373–381
Srivastava VK, Hiney JK, Dees WL (2016) Manganese-stimulated kisspeptin is mediated by the IGF-1/Akt/mammalian target of rapamycin pathway in the prepubertal female rat. Endocrinology 157:3233–3241. https://doi.org/10.1210/en.2016-1090
Steevens BJ, Bush LJ, Stout JD, Williams EI (1971) Effects of varying amounts of calcium and phosphorus in rations for dairy cows. J Dairy Sci 54:655–661. https://doi.org/10.3168/jds.S0022-0302(71)85902-7
Stojilković SS, Izumi SI, Catt KJ (1988) Participation of voltage-sensitive calcium channels in pituitary hormone release. J Biol Chem 263:13054–13061
Stojilković SS, Iida T, Merelli F, Torsello A, Krsmanović LZ, Catt KJ (1991) Interactions between calcium and protein kinase C in the control of signaling and secretion in pituitary gonadotrophs. J Biol Chem 266:10377–10384
Stojilković SS, Kukuljan M, Tomić M, Rojas E, Catt KJ (1993) Mechanism of agonist-induced [Ca2+] i oscillations in pituitary gonadotrophs. J Biol Chem 268:7713–7720
Stolkowski J (1977) Magnesium in animal and human reproduction. Rev Can Biol 36:135–177
Sugino N, Takiguchi S, Kashida S, Karube A, Nakamura Y, Kato H (2000) Superoxide dismutase expression in the human corpus luteum during the menstrual cycle and in early pregnancy. Mol Hum Reprod 6:19–25. https://doi.org/10.1093/molehr/6.1.19
Sun W, Xie H, Ji J, Zhou X, Goltzman D, Miao D (2010) Defective female reproductive function in 1, 25 (OH) 2D-deficient mice results from indirect effect mediated by extracellular calcium and/or phosphorus. Am J Physiol-Endocrinol Metab 299:E928–E935. https://doi.org/10.1152/ajpendo.00378.2010
Sun Y, Lin Y, Niu M, Kang Y, Du S, Zheng B (2017) Follicular fluid concentrations of zinc and copper are positively associated with in vitro fertilization outcomes. Int J Clin Exp Med 10:3547–3553
Sun Y, Wang W, Guo Y, Zheng B, Li H, Chen J, Zhang W (2019) High copper levels in follicular fluid affect follicle development in polycystic ovary syndrome patients:population-based and in vitro studies. Toxicol Appl Pharm 365:101–111. https://doi.org/10.1016/j.taap.2019.01.008
Suzuki M, Tanemoto Y, Takahashi K (1972) The effect of copper salts on ovulation, especially on hypothalamic ovulatory hormone releasing factor. Tohoku J Exp Med 108:9–18. https://doi.org/10.1620/tjem.108.9
Tabatabaei S, Mamoei M (2011) Biochemical composition of blood plasma and follicular fluid in relation to follicular size in buffalo. Comp Clin Pathol 20:441–445. https://doi.org/10.1007/s00580-010-1014-5
Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR (2004) Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 16:850–858. https://doi.org/10.1111/j.1365-2826.2004.01240.x
Tian X, Diaz FJ (2012) Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology 153:873–886. https://doi.org/10.1210/en.2011-1599
Tomić M, Jobin RM, Vergara LA, Stojilkovic SS (1996) Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary Gonadotrophs – integration of P2 channels in plasma membrane and endoplasmic reticulum derived calcium oscillations. J Biol Chem 271:21200–21208. https://doi.org/10.1074/jbc.271.35.21200
Torre PM, Harmon RJ, Sordillo LM, Boissonneault GA, Hemken RW, Trammell DS, Clark TW (1995) Modulation of bovine mononuclear cell proliferation and cytokine production by dietary copper insufficiency. J Nutr Immunol 3:3–20. https://doi.org/10.1300/J053v03n04_02
Torre PM, Harmon RJ, Hemken RW, Clark TW, Trammell DS, Smith BA (1996) Mild dietary copper insufficiency depresses blood neutrophil function in dairy cattle. J Nutr Immunol 4:3–24. https://doi.org/10.1300/J053v04n03_02
Tsou RC, Dailey RA, McLanahan CS, Parent AD, Tindall GT, Neill JD (1977) Luteinizing hormone releasing hormone (LHRH) levels in pituitary stalk plasma during the preovulatory gonadotropin surge of rabbits. Endocrinology 101:534–539. https://doi.org/10.1210/endo-101-2-534
Ullah N, Anwar M, Andrabi SM, Ansari MS, Murtaza S, Ali Q, Asif M (2010) Effect of mineral supplementation on post partum ovarian activity in Nili-Ravi buffaloes (Bubalus bubalis). Pak J Zool 43:195–200
Underwood EJ, Suttle NF (1999) The mineral nutrition of livestock. CABI Publishing, Wallingford
Veldhuis JD, Klase PA (1982) Mechanisms by which calcium ions regulate the steroidogenic actions of luteinizing hormone in isolated ovarian cells in vitro. Endocrinology 111:1–6. https://doi.org/10.1210/endo-111-1-1
Weisinger JR, Bellorín-Font E (1998) Magnesium and phosphorus. Lancet 352:391–396. https://doi.org/10.1016/S0140-6736(97)10535-9
Wilson JG (1966) Bovine functional infertility in Devon and Cornwall: response to manganese therapy. Vet Rec 79:562–566
Wise T (1987) Biochemical analysis of bovine follicular fluid:albumin, total protein, lysosomal enzymes, ions, steroids and ascorbic acid content in relation to follicular size, rank, atresia classification and day of estrous cycle. J Anim Sci 64:1153–1169. https://doi.org/10.2527/jas1987.6441153x
Wooldridge LK, Nardi ME, Ealy AD (2019) Zinc supplementation during in vitro embryo culture increases inner cell mass and total cell numbers in bovine blastocysts. J Anim Sci 97:4946–4950. https://doi.org/10.1093/jas/skz351
Acknowledgments
We would like to thank Dr. S.S. Dahiya (Director, ICAR-CIRB) for valuable suggestions and comments.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jan, M.H., Singh, H., Kapil, S. (2022). Mineral Ions in Regulation of Hypothalamic-Pituitary-Ovarian Axis. In: Yata, V.K., Mohanty, A.K., Lichtfouse, E. (eds) Sustainable Agriculture Reviews 57. Sustainable Agriculture Reviews, vol 57. Springer, Cham. https://doi.org/10.1007/978-3-031-07496-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-07496-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07495-0
Online ISBN: 978-3-031-07496-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)