Abstract
Tuberculosis (TB) is an infectious disease of epidemic proportions, fired not only by poverty and human immunodeficiency virus (HIV) infection but also by incomplete understanding of its pathogenesis and lack of access to accurate and rapid diagnosis. Mycobacterium tuberculosis is a highly successful type of bacteria because it produces two distinct disease entities, namely primary TB that mediates protective immunity to disseminated infection; and post-primary TB that causes tissue damage leading to formation of cavities necessary to bacterial transmission. Following exposure to a person with pulmonary TB, the risk of developing infection depends on the capacity of the person infected to transmit the disease and the susceptibility of the person exposed to infection. From exposure to infection to disease, there is a pathogenetic continuum. Individuals may advance or reverse states within the spectrum of infection. The dissemination of M. tuberculosis out of the lungs happens during all infections and results in secondary lesions. The microbiological diagnosis of active TB is based on acid-fast bacillus (AFB) smear microscopy, nucleic acid amplification tests (NAAT), and culture. Culture remains the gold standard for TB diagnosis. It increases the potential of diagnosing TB at early stages of the disease, allows extrapulmonary TB diagnosis, species identification, and drug susceptibility testing. However, it takes weeks before results are available. NAAT have significantly reduced this delay. The identification of individuals with latent TB infection is based on imperfect tests, namely tuberculin skin test and interferon-γ release assay.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Latent infection
- Tuberculosis
- Immunological diagnosis
- Molecular biology
- Mycobacterium tuberculosis complex
- Nucleic acid amplification tests
- Tuberculosis
- Tuberculosis bacteriology
- Tuberculosis epidemiology
- Tuberculosis microbiology
- Tuberculosis pathophysiology
1 Introduction
Tuberculosis (TB) is the leading infectious cause of mortality worldwide. The greatest burden of this disease is located in developing countries. However, developed countries are not spared from this threat. The human immunodeficiency virus (HIV) global epidemic and the emergence of multidrug-resistant TB are major obstacles to control this disease (Ankrah et al. 2018). The majority of TB cases are due to Mycobacterium tuberculosis (sensu stricto) or M. africanum. A minority of cases are due to M. bovis or M. caprae, the causal agents of bovine and caprine TB, respectively (Pai et al. 2016a, b); and exceptionally to M. canetti, M. microti, and M. pinnipedii. All these species belong to M. tuberculosis complex.
Understanding of the pathophysiology of TB, necessary for development of effective treatments and vaccines, continues to evolve. The classical model of distinct latent and active forms of TB disease has been replaced by a spectrum of TB states (Furin et al. 2019). Early diagnosis of TB including drug susceptibility testing (DST), and systematic screening of contacts and high risk groups are essential for ending the TB epidemic (Pai et al. 2016a, b).
2 Chain of Infection
2.1 Reservoir
Humans are the only known reservoir of M. tuberculosis (Pai et al. 2016a, b) and M. africanum. Unlike humans, most animals infected with M. tuberculosis die without developing pulmonary fibrosis and cavitation necessary for transmission of the infection (Ankrah et al. 2018). Almost a third of the world’s population harbor M. tuberculosis in a quiescent state (Ankrah et al. 2018). M. africanum is highly restricted to West Africa, where it causes up to 50% of all TB cases, probably due to an association with patient ethnicity (Asante-Poku et al. 2015).
Major transmitters of TB are infected individuals who test positive for acid-fast bacilli (AFB) smear microscopy or culture, who have cavitary pulmonary or laryngeal TB disease or frequent cough, or who have delayed treatment. Also, the infectiousness of the person with TB disease increases when they fail to cover the mouth and nose when coughing (Bloom et al. 2017). Young children are less of transmitters than adults, because they generally do not produce sputum when they cough (Centers for Disease Control and Prevention et al. 2021).
M. bovis and M. caprae have a broad host range and can cause TB in a wide range of domestic and wild animals (Rodríguez et al. 2009). The global burden of TB caused by these species is higher in African countries (2.8%) than in countries outside Africa (1.4%), with predominance of M. bovis. The incidence of human infections with M. bovis in developed countries has markedly decreased due to eradication campaigns in animals, and the cases that do appear are likely due to old infection reactivations (Müller et al. 2013).
2.2 Modes of Transmission
M. tuberculosis and M. africanum spread by aerosol transmission (Sharma et al. 2016). Infectious droplet nuclei are produced when individuals who have pulmonary or laryngeal TB disease cough, sneeze, shout, or sing. These particles can remain suspended in the air for several hours. Transmission occurs when a person inhales infectious droplet nuclei that traverse the mouth or nasal passages to reach the alveoli of the lungs. The risk of infection among household contacts of TB patients is about 30% (Heemskerk et al. 2015). Human TB caused by M. bovis and M. caprae is due to consumption of unpasteurized dairy products and close/continuous contact with infected animals (Rodríguez et al. 2009).
2.3 Risk Factors
Risk factors influence the probability of infection, disease, or outcome. They cover physiological, genetic, environmental, and behavioral factors (Bloom et al. 2017).
2.3.1 Risk Factors of M. tuberculosis Transmission
Most risk factors reflect the social and environmental determinants of heavy exposure, namely small/enclosed spaces, inadequate local or general ventilation, recirculation of contaminated air, improper specimen handling procedures, positive air pressure in infectious patient’s room, high proximity/frequency/duration of exposure, living in densely populated spaces, being incarcerated, and working in occupations involving frequent contact with patients with TB (Bloom et al. 2017; Centers for Disease Control and Prevention et al. 2021). Some genetic loci are linked to increased risk of infection among household contacts exposed to an infectious patient (Bloom et al. 2017).
2.3.2 Risk Factors of Latent TB Infection Progressing to TB Disease
In contrast to infection, disease progression is strongly dependent on host risk factors. HIV infection is the greatest risk factor for the development of TB disease in individuals with latent tuberculosis infection (LTBI) due to immunosuppression. The risk of developing TB disease is 7% to 10% each year for individuals co-infected with HIV (and not receiving highly active anti-retroviral therapy) versus 10% over a lifetime for individuals infected only with M. tuberculosis. Other important risk factors are low body mass index, exposure to tobacco and biomass fuel, diabetes mellitus, and heavy alcohol use (Bloom et al. 2017).
Individuals are more likely to progress to active TB in the presence of drug abuse, recent infection with M. tuberculosis (within the past 2 years), history of untreated or inadequately treated TB disease, fibrotic changes on chest radiograph consistent with prior TB disease, silicosis, chronic renal failure, solid or hematological malignancies, gastrectomy or jejunoileal bypass surgery and immunosuppressive therapy (e.g., tumor necrosis factor-alpha antagonists) (Centers for Disease Control and Prevention et al. 2021). Children younger than 5 years of age are at increased risk for progression of LTBI to TB disease (Centers for Disease Control and Prevention et al. 2021) because of immune response immaturity (Newton et al. 2008).
Populations have increased risk to develop TB disease when they have LTBI, if they have an increased local incidence of TB, or if they are medically underserved or low income (Centers for Disease Control and Prevention et al. 2021). In vitro studies indicate a role for vitamin D in TB disease. Moreover, there is a correlation between seasonal variation in vitamin D and TB case numbers. The increased susceptibility of dark-skinned individuals to TB infection and more severe disease is linked to the role of melanin to absorb ultraviolet light. Nutritional factors may interact with genetic polymorphisms to increase the risk of TB (Bloom et al. 2017).
2.3.3 Risk Factors of Extrapulmonary TB
Extrapulmonary TB is overrepresented in HIV-infected patients, children, and those who are malnourished. Other risk factors of extrapulmonary TB are homelessness, incarceration, and alcohol abuse (Moule and Cirillo 2020).
2.3.4 Risk Factors Affecting TB Outcomes
Risk factors for poor treatment outcomes are HIV infection, smoking, diabetes mellitus, iron overload, renal dysfunction, and hematological malignancies (Bloom et al. 2017). Infants have a particularly high morbidity and mortality from TB (Newton et al. 2008).
3 Pathophysiology of Tuberculosis
Some individuals exposed to TB do not become infected, whereas others, with minimal exposure, rapidly succumb to infection and disease (Furin et al. 2019). Indeed, there is a complex interaction between the microorganism and the host immune response. The outcome results in a spectrum of TB states (Ankrah et al. 2018).
3.1 Bacterial Infection Determinants
Genomic comparison between M. tuberculosis and Bacillus Calmette–Guérin (BCG) has been used to search for the basis of attenuated virulence, uncovering several differences, essentially the region of difference 1. This region contains genes that encode the ESX-1 secretion system, which mediates the delivery of bacterial products into the macrophage cytoplasm. However, the presence of the ESX-1 secretion system in a few atypical mycobacteria reconsiders the primacy of ESX-1 in M. tuberculosis virulence. Therefore, ESX-1 seems to be necessary, but not solely responsible, for the full virulence of M. tuberculosis (Pai et al. 2016a, b). Whole-genome sequence analysis shows that “modern” M. tuberculosis strains induce lower-level and delayed proinflammatory cytokine production, replicate more quickly, and are more pathogenic than more “ancient” TB strains. Therefore, it seems that as human populations expand quickly, M. tuberculosis develops traits of more rapid disease progression and increased transmission (Drain et al. 2018).
3.2 Host Determinants of Infection
There is an interaction between M. tuberculosis virulence factors and host determinants of susceptibility. Indeed, unremarkable strains, according to genomic and laboratory characterization, have been linked to outbreaks in the appropriate social and epidemiological settings. Conversely, highly virulent strains in Asian populations have a normal clinical and epidemiological presentation in developed countries (Pai et al. 2016a, b). Human resistance to TB infection has a strong genetic basis, involving an evolutionary counter-response to bacterial virulence. Mendelian studies have proved that severe childhood TB could be attributable to single gene inborn errors of interferon-γ immunity (Abel 2018). Also, a chromosome 11 locus has been linked to susceptibility in multiple populations. Conversely, a chromosome 5 locus has been associated with resistance in highly susceptible HIV-positive East African populations (Bloom et al. 2017).
3.3 Infection of Host Cells
While small M. tuberculosis aerosol particles are expected to reach the distal airways, larger particles can be trapped in the upper airway or the oropharynx where they can lead to oropharyngeal or cervical lymph node TB (Bussi and Gutierrez 2019). Following inhalation, a number of pathways have been proposed regarding the dissemination of M. tuberculosis across the airway epithelia. One hypothesis is that M. tuberculosis is carried across the epithelial barrier within infected alveolar macrophages. Another hypothesis is that it directly infects the epithelial cells and translocates across the barrier, without disrupting the epithelium or causing a breach in the barrier by inducing cell death. Alternatively, specialized M cells actively translocate antigens from the alveoli to the interstitium to present them to antigen-presenting cells. Another hypothesis is that dendritic cells in the alveoli transport live mycobacteria to the lymph nodes. Finally, dissemination may involve a combination of several or all of these mechanisms (Moule and Cirillo 2020) (Fig. 1). M. tuberculosis actively delays initial T cell priming as well as T cell trafficking into the lung (Pai et al. 2016a, b).
Schematic diagram illustrates M. tuberculosis dissemination mechanisms across the airway epithelia. These comprise of dissemination within infected macrophages (a), direct epithelial cell infection (b), passage within specialized M cells (c), and dendritic cells antigen sampling in the alveoli (d) [Adapted from Moule and Cirillo 2020]
HIV infection increases the risk of progression from M. tuberculosis infection to active TB disease, as a result of CD4+ T cell depletion and T cell-independent immune response impairment (Pai et al. 2016a, b). In the neonate and infant, mycobacteria overwhelm the effects of the innate immune system because of innate pulmonary defenses impairment. Antigen presentation and the efficiency of naive T cell response to antigen seem less effective and delayed, resulting in development of active disease (Newton et al. 2008).
In patients with no previous exposure to M. tuberculosis, pattern recognition receptors expressed by macrophages, dendritic cells, and epithelial cells interact with M. tuberculosis ligands. Production of inflammatory cytokines and chemokines recruits new cells to the infection site and initiates granuloma formation by the innate immune system. The adaptive immune response occurs approximately four to six weeks following M. tuberculosis antigen presentation by dendritic cells in lymph nodes. Predominantly TH1-delayed-type response sequesters M. tuberculosis in a mature granuloma, with rapid bacillary killing power. This prevents M. tuberculosis from spreading to other tissues. The innate immune system is less efficient than the adaptive immune system in containing the infection. The early events of M. tuberculosis infection influence the ultimate outcome (Ankrah et al. 2018).
Outside the lungs, M. tuberculosis can disseminate to any organ. M cells may contribute to dissemination and disease progression. Lymphatic endothelial cells, adipose tissue, and bone marrow provide niches to M. tuberculosis, facilitating persistent infection and modulating the local tissue environment (Bussi and Gutierrez 2019).
3.4 Spectrum of Infection
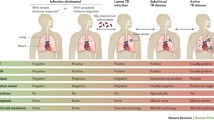
TB can present as a dynamic spectrum, from asymptomatic infection to severe or fatal disease (Pai et al. 2016a, b) (Fig. 2). Drain et al. (2018) divided the pathophysiological spectrum into five categories:
-
Eliminated TB infection refers to M. tuberculosis infection cleared by innate (in this case, immunological tests might be negative) and/or acquired immune responses (in which case immunological tests might be positive or negative) or cured with anti-TB drugs.
-
LTBI is infection with viable M. tuberculosis for which progression to TB disease is not expected to occur soon, in the absence of any significant immune deficiency. Immunological tests are typically positive.
-
Incipient TB infection is infection with viable M. tuberculosis bacteria that is likely to progress to active disease in the absence of further intervention but has not yet caused clinical symptoms, radiographical abnormalities, or microbiological evidence of active TB disease.
-
Subclinical TB disease is asymptomatic disease due to viable M. tuberculosis with radiographical abnormalities or microbiological evidence of active TB disease.
-
Active TB disease is symptomatic disease due to viable M. tuberculosis with radiographical abnormalities or microbiological evidence of active TB disease.
In most individuals with LTBI, the immune response is sufficient to maintain control of infection. However, in some cases, for unknown reasons, the infection can progress to clinical disease within weeks to decades. From a bacteriological perspective, presenting intact antigenic proteins contributes to the progression to disease. M. tuberculosis genes involved in the production of immunodominant CD4+ T cell antigens are invariable across strains and lineages. From a host perspective, three epidemiological observations inform on essential pathways in controlling infection, namely HIV, anti-TNF drugs, and inborn errors in immunity (Pai et al. 2016a, b). Moreover, immunity to TB varies over time, even within the context of an individual patient. Both local and systemic immune responses seem to be important in controlling TB infection (Furin et al. 2019).
Variations in the cellular compositions and activation levels of immune cells, epithelial cells, and extracellular matrix within granulomas expose M. tuberculosis to different microenvironments (nutrient availability, reactive intermediates, cytokine profiles, and drug penetration). In a single human host, different pulmonary and extrapulmonary infection sites induce bacterial phenotypic heterogeneity that can in turn shape the immune response and progression of infection. The types of lesions detectable in asymptomatic individuals correlate with progression to active disease (Drain et al. 2018).
3.5 Primary Versus Post-primary Tuberculosis
M. tuberculosis produces two distinct disease entities, namely primary and post-primary TB. These two entities differ in histopathology, imaging, genetic predisposition and immune status of the host, age of onset, organ distribution, clinical course, and susceptibility to BCG protection (Hunter and Actor 2019). Primary TB results from insufficient immune responses. Conversely, post-primary TB results from strong immune responses.
The early lesion is an accumulation of mycobacterial antigens in alveolar macrophages in association with highly sensitized T cells, forming a massive necrotizing hypersensitivity reaction. This is known as the Koch Phenomenon, which leads to caseous pneumonia, evolving to a pulmonary cavitation or to a focus of post-primary granulomas and fibrocaseous disease. Both granulomas, produced by primary and post-primary TB, surround and isolate infectious foci and protect against disseminated TB. Therefore, after initial sensitization to mycobacterial antigens, all subsequent infections and dissemination occur as post-primary TB. In conclusion, M. tuberculosis uses the strongest immune response to produce pulmonary cavities from which it can disseminate to new hosts, while maintaining a high degree of immunity in the body (Hunter 2020).
Progressive pulmonary TB is due to a continuous host response to mycobacterial products and not to increasing numbers of viable bacilli (Hunter 2020). Subclinical pulmonary lesions frequently develop for months before onset of symptoms. Most early infiltrates resolve completely (Hunter and Actor 2019). This is probably linked to premature development of granulomas, weak immune responses, altered macrophage polarization, and failure of bronchial obstruction (Hunter 2020). TH1 immunity reduces systemic dissemination but does not prevent pulmonary disease (Hunter and Actor 2019).
3.6 Pulmonary Versus Extrapulmonary Tuberculosis
M. tuberculosis is primarily a respiratory pathogen. However, 15% of infections occur at extrapulmonary sites, therefore complicating the disease diagnosis and treatment. The most common forms of extrapulmonary TB are cervical lymph node TB, pleural TB, and gastrointestinal TB (the latter is more commonly associated with M. bovis). A less common but potentially serious form of extrapulmonary TB is central nervous system TB, especially tuberculous meningitis. Miliary TB is the most severe form of extrapulmonary TB. It is a systemic infection caused by hematogenous spread of the bacteria, characterized by numerous small lesions predominating in highly vascularized organs (e.g., lungs, liver, spleen, bone marrow, kidneys).
Lympho-hematogenous spread is the most probable path of disease progression for both pulmonary and extrapulmonary infections. Indeed, the primary pulmonary granuloma can involve the surrounding lymph nodes, creating a Ghon complex. M. tuberculosis then spreads from infected lymph nodes into the lymphatic system, most likely entering the circulatory system through the thoracic duct and the subclavian vein. Hematogenous reseeding of the lungs leads to secondary granulomas of the lungs (in apical regions) and/or extrapulmonary organs (Fig. 3). Bacterial virulence factors may be involved in dissemination. Indeed, M. tuberculosis strains from different phylogenetic lineages are associated with different rates of extrapulmonary disease, and clinical isolates from extrapulmonary infections are responsible for an increased degree of disseminated disease in animal models (Moule and Cirillo 2020).
Schematic diagram shows progression of human M. tuberculosis infection. Inhalation of contaminated aerosols (1), followed by primary lung infection forming a Ghon complex (2). Lympho-hematogenous spread (3), with secondary granulomas in the lungs (4) and/or the extrapulmonary organs (5). [Adapted from Moule and Cirillo 2020]
4 Microbiological Diagnosis of Active Tuberculosis
Mycobacteria have several unique characteristics as compared to other genera of bacteria. This is essentially due to the higher content of complex lipids, including mycolic acids. In turn, the cell walls are extremely hydrophobic, which impacts staining with colorants and penetration by drugs. These unique bacterial characteristics imply special laboratory considerations for direct staining from specimens, culture, and DST (Caulfield and Wengenack 2016). There are three principal methods for the detection of active TB, namely microscopy, nucleic acid amplification tests (NAAT), and cultures (Pai et al. 2016a, b).
Culture is considered the gold standard for diagnosis. However, it takes weeks before results are available, because of the slow growth of TB bacilli. Furthermore, its sensitivity is only 80% (Ankrah et al. 2018). Pediatric TB diagnosis is challenging because of small amounts of sputum (usually swallowed) and scarcity of bacilli in specimens. In sputum samples, microscopy and culture are positive in around 10–15% and 30%, respectively (Newton et al. 2008).
4.1 Specimen Collection
The specimen type depends on the clinical manifestation of disease. The most common sources are respiratory specimens, including sputum (expectorate or induced sputum), bronchial aspirates, and bronchoalveolar lavage fluid. These are followed by tissues, normally sterile body fluids, blood, and urine. Specimens should be collected in sterile, leak-proof containers. Tissues may be placed in a small amount of sterile saline solution to avoid dehydration. Most of them should be refrigerated during transport and storage (Caulfield and Wengenack 2016).
4.2 Acid-Fast Bacilli Smear Microscopy
Microscopic examination of stained smears allows rapid screening of mycobacteria in clinical specimens. Mycobacteria resist decolorization by acid alcohol when stained with carbol fuchsin (during Ziehl–Neelsen staining) or auramine (during fluorescent staining). Hence, they are called “acid-fast bacilli” (or “AFB”). The sensitivity of sputum AFB smear varies between 22% and 80%, depending on the concentration of mycobacteria (reliable detection requires 103–104 CFU/ml), the type of AFB stain used, and the experience of the laboratory technician. Smear-positive predictive value for mycobacteria is >95%. However, it is not specific for M. tuberculosis complex and cannot discriminate between mycobacterial species. Positive sputum smears correlate with high infectivity for patients with pulmonary TB and high AFB concentrations correlate with infection severity (Caulfield and Wengenack 2016).
Examination of Ziehl–Neelsen-stained slides under light microscopy is the most commonly used method in low-resource settings. Fluorescent staining is relatively expensive, but it is more sensitive and allows for more rapid reading of slides. The replacement of conventional fluorescent light sources with light-emitting diodes has considerably reduced cost and maintenance requirements. It also eliminates the necessity of a darkroom. The World Health Organization (WHO) recommends replacing conventional fluorescent and light microscopy by light-emitting diode microscopy (Pai et al. 2016a, b) (Fig. 4). Additionally, it recommends performing two AFB smears (low additional sensitivity gained by performing a third smear after two negative ones). The Centers for Disease Control and Prevention (CDC) recommends reporting AFB staining laboratory results within 24 h of specimen collection (Caulfield and Wengenack 2016).
4.3 Mycobacterial Culture
Culture for mycobacteria is approximately 100 folds more sensitive than AFB smear (reliable detection requires 10–102 CFU/ml). However, it requires a more developed laboratory, and needs infrastructure and maintenance to support an adequate biosafety level and uninterrupted power supply (Pai et al. 2016a, b). Mycobacteria are fastidious microorganisms. Culture is traditionally performed on solid egg-based media, such as Lowenstein-Jensen media. It offers good growth of M. tuberculosis complex, but is not as reliable for M. bovis. While some laboratories still use this media, many have chosen to use more chemically defined agar-based media optimized for faster mycobacterial growth. However, these media are less stable and more prone to deterioration.
M. tuberculosis complex species have a slow growth rate. Colonies become visible on culture plates after several weeks. Generally, cultures are carried on for six to eight weeks before being reported as negative. Plated growth allows for the detection of mixed cultures (containing multiple species) and the identification of mycobacteria (based on morphological characteristics). M. tuberculosis colonies are characteristically dry, with a rough texture and a cream/tan color (described as “rough and buff”). Differently, M. bovis colonies are flat and smooth. As all M. tuberculosis complex species are nonpigmented, the presence of any pigment favors atypical mycobacteria (Caulfield and Wengenack 2016).
Liquid culture is faster and more sensitive than solid culture for mycobacterial isolation. Indeed, M. tuberculosis complex from clinical samples is detected faster by automated broth systems (an average of 10 days) than on solid media (20–25 days). However, it must be confirmed with subculture on solid media to detect mixed cultures and to observe colony morphologies. Therefore, optimal recovery of mycobacteria from clinical specimens requires the use of both solid and liquid media (Caulfield and Wengenack 2016).
4.4 Mycobacterial Identification
Rapid discrimination of M. tuberculosis complex from other AFB isolated in culture is essential. For this purpose, molecular techniques are faster than traditional biochemical tests. They include nucleic acid hybridization probes, line probe hybridization assays, matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy, and DNA sequencing (Caulfield and Wengenack 2016).
4.5 Direct Molecular Detection from Specimens
NAAT have superior performance to AFB staining in patients with suspected TB. However, they do not differentiate between live and nonviable M. tuberculosis complex strains. Respiratory specimens must be cultured for mycobacterial growth in case of negative results by NAAT. It allows detection of false-negative NAAT and atypical mycobacteria. Furthermore, it enables monitoring the treatment response and drug susceptibility testing. The Xpert MTB/RIF tests (Cepheid) are the only U.S. Food and Drug Administration (FDA)-approved automated NAAT that can identify M. tuberculosis bacteria (MTB) and resistance to rifampicin (RIF) from respiratory specimens. It is an easy cartridge-based real-time polymerase chain reaction (PCR) method that delivers results in under 2 h. It has a closed amplification system that reduces cross contamination risk. Moreover, it does not require advanced biosafety equipment.
Compared to culture, NAAT have a higher sensitivity from smear-positive (90–99%) than smear-negative (66–74%) respiratory specimens. CDC guidelines recommend performing NAAT on at least one respiratory specimen from each patient (preferably the first) with suspected pulmonary TB (Caulfield and Wengenack 2016). WHO recommends using Xpert MTB/RIF as initial diagnostic test on adults or children suspected to have TB meningitis (on cerebrospinal fluid specimens), multidrug-resistant TB, or HIV-associated TB. If possible, it should be done for all suspected TB cases (Pai et al. 2016a, b).
4.6 Drug Susceptibility Testing
Patients contract drug-resistant TB by two modes, namely infection with a drug-resistant strain (primary resistance) or development of resistance during therapy (secondary resistance). M. tuberculosis develops drug resistance through genetic mutations. Multidrug resistance is defined as resistance to at least isoniazid and rifampicin. Extensive drug resistance is defined as multidrug resistance associated with fluoroquinolones and second-line injectable drugs resistance (Dheda et al. 2017). Rifampin resistance is a predictor of multidrug resistance (Caulfield and Wengenack 2016). The detection of resistance is impeded by limitations in both phenotypic and genotypic DST (Pai et al. 2016a, b). WHO recommends establishing laboratory capacity to detect multidrug-resistant TB by national TB control programs (Pai et al. 2016a, b).
4.6.1 Phenotypic Tests
The proportion method is the gold standard test for DST. It counts the number of M. tuberculosis colonies that grow on agar without antibiotics, compared with agar containing a critical concentration of antibiotics. For some drugs (e.g., ethambutol), there is important overlap between critical concentrations of wild-type and resistant organisms, which limits the applicability of this method for these drugs. Commercial automated liquid culture systems use a modification of the proportion method. They have reliable results for isoniazid, rifampin, fluoroquinolones, aminoglycosides, and polypeptides, but not to other first-line (ethambutol and pyrazinamide) and second-line drugs. The gold standard for DST for second-line TB drugs is phenotypic testing (liquid or agar proportion) (Pai et al. 2016a, b).
4.6.2 Genotypic Tests
Genotypic tests detect resistance by searching for relevant genes directly by real-time PCR (Xpert MTB/RIF) or DNA sequencing. Conversely, this can be achieved indirectly through line probe assay (LPA) detecting the binding of PCR-amplified DNA to probes targeting the most prevalent mutations encoding resistance or to wild-type probes. Xpert MTB/RIF detects rpoB gene mutations responsible for approximately 96% of rifampin resistance in the M. tuberculosis complex, with a sensitivity of 94% and a specificity of 98% (Caulfield and Wengenack 2016). However, its positive predictive value is relatively low in countries where rifampin resistance is low.
LPA for drug resistance detection is faster than phenotypic tests, presents lower biosafety risk and increases throughput. LPA for first-line drug resistance detection (rifampin and isoniazid) can be done on cultured isolates or directly from smear-positive sputum samples. As LPA for second-line drug resistance detection (fluoroquinolones and second-line injectable aminoglycosides) has a good specificity (>98%) and a suboptimal sensitivity, it can be used to rule in resistance but cannot be used to completely rule them out. WHO recommends using automated liquid systems and LPA for first-line DST (on isolates or directly on smear-positive sputum) as the current gold standard. It endorses using LPA for second-line DST as the initial test. Confirmation requires conventional culture and phenotypic-based DST for detection of rifampin resistance or multidrug resistance, or resistance on smear-negative sputum (Pai et al. 2016a, b).
5 Testing Methods for Latent M. tuberculosis Infection
Testing for LTBI is generally indicated in cases with a high risk of progression to TB disease (e.g., close contact with a patient with TB, immunosuppression). It is based on indirect markers of M. tuberculosis exposure, namely tuberculin skin test (TST) and interferon-γ release assay (IGRA). Both tests have reduced sensitivity in children and immunocompromised patients. Additionally, they have low predictive value for progression to active TB. Finally, they are unable to differentiate LTBI from active TB, or to differentiate cleared infection from true infection (Pai and Behr 2016).
5.1 Tuberculin Skin Test
TST is usually performed using the Mantoux method. However, interpretation of the results is complex. It should involve the probability of prior infection and the likely risk of disease in cases of infection. TST has many advantages in low-resource settings (low reagent cost, no hardware costs, limited skill requirement, and no requirement for laboratories). However, its specificity is compromised by late or repeated BCG vaccination, exposure to atypical mycobacteria, or previous cleared infection. Moreover, TST has limited reproducibility, interreader variability, dynamic nature (boosting, conversions, and reversions), and need for patients to return for the reading (Pai and Behr 2016).
5.2 Interferon-γ Release Assays
Two commercial IGRAs are available in many countries, namely QuantiFERON-TB Gold In-Tube assay (an enzyme-linked immunosorbent assay) and T-SPOT.TB assay (an enzyme-linked immunospot assay). IGRAs measure T cell release of interferon-γ after M. tuberculosis complex specific antigens stimulation. These antigens are more specific for M. tuberculosis than purified protein derivative used for TST because they are produced by only a few atypical mycobacteria and not by BCG vaccine strains. However, IGRAs have highly dynamic natures (inconsistent results, high rates of conversions and reversions in repeated tests) probably related to transitions within the LTBI spectrum or to poor reproducibility. Compared to TST, IGRAs do not add much value, but they are more expensive (Pai and Behr 2016).
6 Conclusion
The global TB elimination is hampered by the huge reservoir of individuals with LTBI. In the absence of overt immune suppression, risk factors of LTBI progression to TB disease are not completely understood. However, many advances have been made in deciphering TB epidemiology and pathophysiology. Finally, multiple laboratory methods are being developed toward a faster, affordable, and accurate diagnosis.
Abbreviations
- AFB:
-
Acid-fast bacilli
- HIV:
-
Human immunodeficiency virus
- LTBI:
-
Latent tuberculosis infection
- NAAT:
-
Nucleic acid amplification tests
- TB:
-
Tuberculosis
References
Abel L, Fellay J, Haas DW et al (2018) Genetics of human susceptibility to active and latent tuberculosis: present knowledge and future perspectives. Lancet Infect Dis 18(3):e64–e75
Ankrah AO, Glaudemans AWJM, Maes A et al (2018) Tuberculosis. Semin Nucl Med 48:108–130
Asante-Poku A, Yeboah-Manu D, Otchere ID et al (2015) Mycobacterium africanum is associated with patient ethnicity in Ghana. PLoS Negl Trop Dis 9:e3370
Bloom BR, Atun R, Cohen T et al (2017) Tuberculosis. In: Holmes KH, Bertozzi S, Bloom BR, Jha P (eds) Disease control priorities, Major infectious diseases, vol 6, 3rd edn. World Bank, Washington DC, pp 233–314
Bussi C, Gutierrez MG (2019) Mycobacterium tuberculosis infection of host cells in space and time. FEMS Microbiol Rev 43:341–361
Caulfield AJ, Wengenack NL (2016) Diagnosis of active tuberculosis disease: from microscopy to molecular techniques. J Clin Tuberc Other Mycobact Dis 4:33–43
Centers for Disease Control and Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Division of Tuberculosis Elimination (2021) Transmission and pathogenesis of tuberculosis. In: Core curriculum on tuberculosis: what the clinician should know, 7th ed. Centers for Disease Control and Prevention, Atlanta, pp. 19–44
Dheda K, Gumbo T, Maartens G et al (2017) The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med 15. S2213-2600(17)30079-6
Drain PK, Bajema KL, Dowdy D et al (2018) Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev 31:e00021–e00018
Furin J, Cox H, Pai M (2019) Tuberculosis. Lancet 393(10181):1642–1656
Heemskerk D, Caws M, Marais B, Farrar J (2015) Pathogenesis. In: Tuberculosis in adults and children. Springer, London, pp 9–16
Hunter RL (2020) The pathogenesis of tuberculosis—the Koch phenomenon reinstated. Pathogens 9:813
Hunter R, Actor J (2019) The pathogenesis of post-primary tuberculosis. A game changer for vaccine development. Tuberculosis 116S:S114–S117
Moule MG, Cirillo JD (2020) Mycobacterium tuberculosis dissemination plays a critical role in pathogenesis. Front Cell Infect Microbiol 25:65
Müller B, Dürr S, Alonso S et al (2013) Zoonotic Mycobacterium bovis–induced tuberculosis in humans. Emerg Infect Dis 19:899–908
Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B (2008) Paediatric tuberculosis. Lancet Infect Dis 8:498–510
Pai M, Behr M (2016) Latent Mycobacterium tuberculosis infection and interferon-gamma release assays. Microbiol Spectr 4(5)
Pai M, Behr MA, Dowdy D et al (2016a) Tuberculosis. Nat Rev Dis Primers 27(2):16076
Pai M, Nicol MP, Boehme CC (2016b) Tuberculosis diagnostics: state of the art and future directions. Microbiol Spectr 4(5)
Rodríguez E, Sánchez LP, Pérez S et al (2009) Human tuberculosis due to Mycobacterium bovis and M. caprae in Spain, 2004–2007. Int J Tuberc Lung Dis 13:1536–1541
Sharma A, Bloss E, Heilig CM et al (2016) Tuberculosis Caused by Mycobacterium africanum, United States, 2004–2013. Emerg Infect Dis 22(3):396–403
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Achour, W., Chebbi, Y. (2022). Pathophysiology of Tuberculosis and Microbiological Diagnosis. In: Ladeb, M.F., Peh, W.C.G. (eds) Imaging of Tuberculosis. Medical Radiology(). Springer, Cham. https://doi.org/10.1007/978-3-031-07040-2_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-07040-2_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07039-6
Online ISBN: 978-3-031-07040-2
eBook Packages: MedicineMedicine (R0)