Abstract
The gastrointestinal tract contains the enteric nervous system within its walls and a large community of microbial symbionts (microbiota) in its lumen. In recent years, studies have shown that these two systems that lie adjacent to each other interact. This review will summarize new data using mouse models demonstrating the concurrent development of the enteric nervous system and microbiota during key pre- and postnatal stages. It will also discuss the possible roles that microbiota play on influencing enteric nervous system development and implications of antibiotic exposure during developmental windows.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
The gastrointestinal tract is home to several hundred species of microbes collectively referred to as the microbiota. In humans, the colon harbours the largest microbial population (1014 bacterial cells) compared to the upper gut and the rest of the body. Although the symbiotic relationship between the host and intestinal microbiota developed over millions of years, we have only recently begun to understand its importance to the overall well-being of the host, instead of being regarded merely as pathogens. The luminal microbiota lie near the enteric nervous system (ENS) embedded in the gut wall; many studies in recent years have shown that there is crosstalk between microbiota and the ENS [4, 5, 8, 25, 26, 28, 32, 39, 45, 57]; however, when this interaction begins in life and the health implications of its disruption during critical developmental windows is a new research frontier.
15.1 The ENS and Microbiota Develop Concurrently
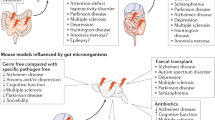
Mice have been pivotal for studying the physiological significance of ENS-microbiota interactions. Almost 99% of mouse genes are shared with humans and microbiota of mice and humans have many core similarities [11, 30]. Furthermore, the mouse is the best-studied model for the anatomical and functional development of the ENS. This enables us to create a detailed timeline for key developmental milestones which show the concurrent maturation of the ENS and microbiota within the gut (Fig. 15.1). Thus, in this chapter, I will be focusing on gut research conducted using the murine model which has revealed three key developmental windows broadly referred to as embryogenesis, early postnatal and post weaning periods.
Embryogenesis
Substantial development of the ENS begins and occurs during embryogenesis [21, 22, 50, 55]. The ENS mainly derives from the neural crest and during embryogenesis, a complex array of molecular and cellular mechanisms orchestrates the migration of neural crest cells to and within the developing gastrointestinal tract, proliferation of these precursors and differentiation into neuron and glial subtypes [24, 43]. The mature ENS comprises a large variety of enteric neuronal and glial subtypes which are differentiated by various properties, including subtype specific function, electrophysiology, neurochemical coding and morphology [16,17,18]. But, each of these identifying properties seems to have a staggered appearance during development, and when each neuronal/glial subtype has completed the collection of their unique properties remains unclear [21, 53]. Furthermore, enteric cells can express certain properties such as morphology and neurochemistry only transiently during development. It was only discovered in the last decade that young enteric neurons are electrically active early during embryogenesis long before coordinated neurally mediated gut motility has commenced. These embryonic neurons have begun to form functional connections with each other through neurotransmitters such as acetylcholine and 5-hydroxytryptamine (5-HT) and their early communication is postulated to affect the survival of later born enteric neurons [14, 19, 20, 23, 37].
Opposed to the decades of dedicated research which gave rise to a generally unified understanding of ENS development. There has been intense debate over 100 years about whether the foetal environment is sterile and whether there are microbiota transferred from mother to foetus in utero [48]. Some studies report that in normal pregnancies, the placenta, amniotic fluid and first stool of the infant (meconium) contains microorganisms that are not harmful to the foetus, thus raising the possibility that the foetus may have already encountered bacteria [1, 27, 31]. Nonetheless, the vast majority of research indicates that a foetus normally develops in a sterile environment, notably a recent heroic study examined placenta samples from 537 women to demonstrate that there are indeed no bacteria in healthy placenta [7, 48]. Yet, this was immediately challenged by another study identifying viable bacteria from murine and foetal tissues [59]. Hence, this issue remains contentious and an area of active research.
Early Postnatal Period
In an earlier book chapter [12], I discussed the emerging research on the postnatal development of the murine ENS. The first ten days of life (postnatal day, P0–10) in the mouse, which represents the early postnatal period, is a critical time for ENS development. The motility patterns of the colon and its main underlying neuronal circuitry within the myenteric plexus are still maturing [52]. Significant numbers of myenteric neurons are still being born (exiting the cell cycle), acquiring their neurochemistry and undergoing substantial maturation in their morphology and electrophysiological properties [3, 13, 14, 34, 49, 54]. Little is known about the maturation of the other division of the ENS, the submucous plexus, but, what we do know is that submucosal neurons are still developing, and that they tend to differentiate later than myenteric neurons [35, 38, 49, 54].
Early postnatal life is the critical period for colonization and establishment of microbiota. At birth, the intestines of infants are rapidly colonized by bacteria from their immediate environment which is the mother’s vagina or skin, depending on the mode of delivery [42]. Like humans born of natural birth, the microbiome of the newborn mouse colon has a similar composition to that of their mother’s vagina. An initial bloom of Streptococcus occurs after birth and is replaced by Lactobacillus after postnatal day (P) 3 [46]. Then, many other environmental factors contribute to the development of microbiota, including whether babies are fed breast milk or formula [42].
Post-weaning Period
The post-weaning period is defined as the period during and immediately after weaning. Mice have a shorter and more accelerated early life compared to humans, and adolescence or puberty can begin in mice as early as postnatal day (P) 18–28, when juvenile mice are weaned from the female dam [11, 36]. The vital components of the ENS would have been acquired during embryogenesis and early postnatal life. Post-weaning development is likely to involve continued formation of synapses in the enteric network that commenced in earlier stages and fine-tuning of the circuit connections that underpin maturation of gastrointestinal functions. Indeed, the electrophysiological properties and synaptic profile of enteric neurons, particularly those that are characteristic of the intrinsic sensory neurons of the circuitry, are still immature at P10 [13]. We have recently shown that between pre-weaning and post-weaning periods, there is significant maturation in the somata size of enteric neurons and numbers of synaptophysin+ varicosities closely apposed to their cell bodies. The architecture of the enteric plexi is still maturing as the ganglia containing neurons and glia are stretched further apart during development. While there is no appreciable change in neurochemistry of myenteric neurons, substantial maturation in submucosal neurochemistry still occurs during the post-weaning period [47]. Further, Schwann cell-derived enteric neurons in the distal parts of the gastrointestinal tract, and S100β + glia that are found in the intestinal mucosa are all still developing during the post-weaning period [28, 54].
In humans, major shifts in microbiota occur during and after weaning, due to the transition from mother’s milk to solid food [30]. Unsurprisingly, in mice, we observe significant increases in abundance and communities of microbiota between P10 and P42–49 (6-weeks of age) [26].
15.2 Role of Microbiota on the Developing ENS
Although the ENS and microbiota develop concurrently within the gastrointestinal tract especially during the early postnatal period, the crosstalk between the two systems and how the microbiota contributes to the development of the ENS remains unclear. A study using germfree mice showed that the ENS in the small intestine of these mice is abnormal at P3 [5]. Whether disruption to the developing ENS is due to the lack of microbiota is unclear as other systems are significantly perturbed in germ-free mice [58]. Moreover, as the germfree mice were only examined at P3, thus it may be possible that the ENS has been disrupted earlier during embryogenesis. Recent work from my group shows that oral administration of the antibiotic, vancomycin, to neonatal mice daily from birth to P10 disrupts their colonic microbiota (dysbiosis), motility, myenteric neurochemistry and activity [25]. While this supports the view that microbiota is important in supporting ENS development, the possibility that the antibiotic itself could have toxic effects directly on the ENS cannot be excluded.
There are only a couple of studies from other groups that have examined the role of microbiota in ENS development, and they studied later stages of postnatal development. Microbiota dysbiosis has been shown to impair gut neuromuscular function in 3-week-old male mice [4]. Another study showed that gut microbiota controls the influx of enteric glial cells into the lamina propria of the small intestine by comparing adult germfree mice with those raised in a specific pathogen-free environment [28]. More recently, we exposed mice to vancomycin during the post-weaning period and showed that the significant shifts in microbiota communities were accompanied by disruptions to neurochemistry and function of myenteric and submucosal neurons leading to dysmotility in mice [26]. Interestingly, compared to our work with the same antibiotic exposed in a different developmental window [25], we found that the impact of vancomycin appeared to be greater when administered during the early postnatal compared to the post-weaning period.
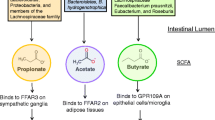
There is very little understanding of the mechanisms in which microbiota influences ENS development. Work on the adult ENS has shown that microbes signal to the ENS by modifying 5-HT metabolism and signalling from the mucosa [8, 51, 57]. Although our work on neonatal mice ENS seems to be in agreement to the adult studies by showing antibiotic-induced perturbation in the mucosal levels of the 5-HT metabolite, 5-HIAA [25], more studies investigating how the microbes signal to the developing ENS via 5-HT or other processes is warranted. Adult studies have also revealed other mechanisms by which microbiota signal to the ENS, including toll-like receptors, microvesicles, transcription and neurotrophic factors, various microbial metabolites and mediators released from enteroendocrine cells and immune system that could serve roles in the developing microbiota-ENS crosstalk [15]. Further, while collective studies so far have supported the view that microbiota plays important roles mediating ENS development, the possibility that antibiotics can have direct toxic effects on the ENS cannot be excluded [10].
15.3 Implications of Antibiotic Exposure During Critical Developmental Windows
Antibiotics were developed and used as medicines around the 1940s, and they soon earned the title of “miracle cure” by effectively treating infections, thereby leading to a dramatic decrease in death rates and serious illnesses. Although antibiotics are necessary in many circumstances, they are not harmless and may have negative health consequences especially when used during critical developmental windows.
Healthy pregnancies are accompanied by increased numbers of bacteria and dramatic changes in gut microbiota composition from the first to the third trimester, which can persist till at least one month after delivery [29, 44]. Several antibiotics are considered safe for use during pregnancy, and they account for 80% of all prescribed medication to pregnant women [33]. Recent startling findings show that maternal exposure to antibiotics, even before pregnancy, is associated with an increased risk of childhood hospitalized infections, including gastroenteritis [40]. Moreover, maternal antibiotics have been linked to childhood obesity [41, 44]. More recently, maternal microbiota and their metabolites have been shown to significantly impact the development of the foetal brain [56]. However, the impact of maternal antibiotics during pregnancy on the growing foetus, especially its ENS, is massively understudied.
Infants and young children have the highest antibiotic exposures globally [2]. Exposure to antibiotics early in life has been linked to increased susceptibility to several diseases such as functional gastrointestinal disorders, obesity, metabolic dysfunction and allergies later in life [6, 9, 42]. Yet, the lasting impact of early life antibiotic exposure on host physiology, and how antibiotics given during critical developmental windows may predispose the host to gastrointestinal disorders is currently unknown.
15.4 Conclusions and Future Directions
The gastrointestinal tract is an organ where multiple systems coexist, and it is not a coincidence that the microbiota and ENS develop concurrently. Future studies should aim to provide mechanistic insights into the crosstalk between the microbiota and ENS during various developmental windows. Antibiotics are important drugs especially for their life-saving qualities. Identifying how antibiotic usage during critical developmental windows affects the host would advance antibiotic therapy by revealing preventative measures against its unwanted side effects and improve the short- and long-term health and well-being of our next generation.
References
Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J (2014) The placenta harbors a unique microbiome. Sci Transl Med 6:237ra65
Anderson H, Vuillermin P, Jachno K, Allen KJ, Tang ML, Collier F, Kemp A, Ponsonby AL, Burgner D (2017) Prevalence and determinants of antibiotic exposure in infants: a population-derived Australian birth cohort study. J Paediatr Child Health 53:942–949
Bergner AJ, Stamp LA, Gonsalvez DG, Allison MB, Olson DP, Myers MG Jr, Anderson CR, Young HM (2014) Birthdating of myenteric neuron subtypes in the small intestine of the mouse. J Comp Neurol 522:514–527
Caputi V, Marsilio I, Filpa V, Cerantola S, Orso G, Bistoletti M, Paccagnella N, de Martin S, Montopoli M, Dall’acqua S, Crema F, Di Gangi IM, Galuppini F, Lante I, Bogialli S, Rugge M, Debetto P, Giaroni C, Giron MC (2017) Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br J Pharmacol 174:3623–3639
Collins J, Borojevic R, Verdu EF, Huizinga JD, Ratcliffe EM (2014) Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil 26:98–107
Cox LM, Blaser MJ (2015) Antibiotics in early life and obesity. Nat Rev Endocrinol 11:182–190
De Goffau MC, Lager S, Sovio U, Gaccioli F, Cook E, Peacock SJ, Parkhill J, Charnock-Jones DS, Smith GCS (2019) Human placenta has no microbiome but can contain potential pathogens. Nature 572:329–334
De Vadder F, Grasset E, Manneras Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Backhed F (2018) Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A 115(25):6458–6463
De Vroey B, De Cassan C, Gower-Rousseau C, Colombel JF (2010) Editorial: antibiotics earlier, IBD later? Am J Gastroenterol 105:2693–2696
Delungahawatta T, Amin JY, Stanisz AM, Bienenstock J, Forsythe P, Kunze WA (2017) Antibiotic driven changes in gut motility suggest direct modulation of enteric nervous system. Front Neurosci 11:588
Dutta S, Sengupta P (2016) Men and mice: relating their ages. Life Sci 152:244–248
Foong JP (2016) Postnatal development of the mouse enteric nervous system. Adv Exp Med Biol 891:135–143
Foong JP, Nguyen TV, Furness JB, Bornstein JC, Young HM (2012) Myenteric neurons of the mouse small intestine undergo significant electrophysiological and morphological changes during postnatal development. J Physiol 590:2375–2390
Foong JP, Hirst CS, Hao MM, Mckeown SJ, Boesmans W, Young HM, Bornstein JC, Vanden Berghe P (2015) Changes in nicotinic neurotransmission during enteric nervous system development. J Neurosci 35:7106–7115
Foong JPP, Hung LY, Poon S, Savidge TC, Bornstein JC (2020) Early life interaction between the microbiota and the Enteric Nervous System. Am J Physiol Gastrointest Liver Physiol 319(5):G541–G548
Fung C, Vanden Berghe P (2020) Functional circuits and signal processing in the enteric nervous system. Cell Mol Life Sci 77:4505–4522
Grundmann D, Loris E, Maas-Omlor S, Huang W, Scheller A, Kirchhoff F, Schäfer KH (2019) Enteric glia: S100, GFAP, and beyond. Anat Rec (Hoboken) 302:1333–1344
Gwynne RM, Bornstein JC (2007) Synaptic transmission at functionally identified synapses in the enteric nervous system: roles for both ionotropic and metabotropic receptors. Curr Neuropharmacol 5:1–17
Hao MM, Boesmans W, van den Abbeel V, Jennings EA, Bornstein JC, Young HM, Vanden Berghe P (2011) Early emergence of neural activity in the developing mouse enteric nervous system. J Neurosci 31:15352–15361
Hao MM, Lomax AE, Mckeown SJ, Reid CA, Young HM, Bornstein JC (2012) Early development of electrical excitability in the mouse enteric nervous system. J Neurosci 32:10949–10960
Hao MM, Bornstein JC, Vanden Berghe P, Lomax AE, Young HM, Foong JP (2013) The emergence of neural activity and its role in the development of the enteric nervous system. Dev Biol 382:365–374
Hao MM, Foong JP, Bornstein JC, Li ZL, Vanden Berghe P, Boesmans W (2016) Enteric nervous system assembly: functional integration within the developing gut. Dev Biol 417:168–181
Hao MM, Bergner AJ, Hirst CS, Stamp LA, Casagranda F, Bornstein JC, Boesmans W, vanden Berghe P, Young HM (2017) Spontaneous calcium waves in the developing enteric nervous system. Dev Biol 428:74–87
Hirst CS, Foong JP, Stamp LA, Fegan E, Dent S, Cooper EC, Lomax AE, Anderson CR, Bornstein JC, Young HM, Mckeown SJ (2015) Ion channel expression in the developing enteric nervous system. PLoS One 10:e0123436
Hung LY, Boonma P, Unterweger P, Parathan P, Haag A, Luna RA, Bornstein JC, Savidge TC, Foong JPP (2019) Neonatal antibiotics disrupt motility and enteric neural circuits in mouse colon. Cell Mol Gastroenterol Hepatol 8:298–300.e6
Hung LY, Parathan P, Boonma P, Wu Q, Wang Y, Haag A, Luna RA, Bornstein JC, Savidge TC, Foong JPP (2020) Antibiotic exposure postweaning disrupts the neurochemistry and function of enteric neurons mediating colonic motor activity. Am J Physiol Gastrointest Liver Physiol 318:G1042–g1053
Jimenez E, Marin ML, Martin R, Odriozola JM, Olivares M, Xaus J, Fernandez L, Rodriguez JM (2008) Is meconium from healthy newborns actually sterile? Res Microbiol 159:187–193
Kabouridis PS, Lasrado R, Mccallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S, Pachnis V (2015) Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 85:289–295
Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, Backhed F, Isolauri E, Salminen S, Ley RE (2012) Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150:470–480
Kostic AD, Howitt MR, Garrett WS (2013) Exploring host-microbiota interactions in animal models and humans. Genes Dev 27:701–718
Kovalovszki L, Villanyi Z, Pataki I, Veszelowvsky I, Nagy ZB (1982) Isolation of aerobic bacteria from the placenta. Acta Paediatr Acad Sci Hung 23:357–360
Kunze WA, Mao YK, Wang B, Huizinga JD, Ma X, Forsythe P, Bienenstock J (2009) Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med 13:2261–2270
Kuperman AA, Koren O (2016) Antibiotic use during pregnancy: how bad is it? BMC Med 14:91
Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P, Pachnis V (2011) Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest 121:3412–3424
Lasrado R, Boesmans W, Kleinjung J, Pin C, Bell D, Bhaw L, Mccallum S, Zong H, Luo L, Clevers H, Vanden Berghe P, Pachnis V (2017) Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science 356:722–726
Laviola G, Macri S, Morley-Fletcher S, Adriani W (2003) Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev 27:19–31
Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Cote F, Mallet J, Gershon MD (2011) Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 31:8998–9009
Mckeown SJ, Chow CW, Young HM (2001) Development of the submucous plexus in the large intestine of the mouse. Cell Tissue Res 303:301–305
Mcvey Neufeld KA, Perez-Burgos A, Mao YK, Bienenstock J, Kunze WA (2015) The gut microbiome restores intrinsic and extrinsic nerve function in germ-free mice accompanied by changes in calbindin. Neurogastroenterol Motil 27:627–636
Miller JE, Wu C, Pedersen LH, De Klerk N, Olsen J, Burgner DP (2018) Maternal antibiotic exposure during pregnancy and hospitalization with infection in offspring: a population-based cohort study. Int J Epidemiol 47:561–571
Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, Hassoun A, Perera F, Rundle A (2015) Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes 39:665–670
Munyaka PM, Khafipour E, Ghia JE (2014) External influence of early childhood establishment of gut microbiota and subsequent health implications. Front Pediatr 2:109
Nagy N, Goldstein AM (2017) Enteric nervous system development: a crest cell’s journey from neural tube to colon. Semin Cell Dev Biol 66:94–106
Nuriel-Ohayon M, Neuman H, Koren O (2016) Microbial changes during pregnancy, birth, and infancy. Front Microbiol 7:1031
Obata Y, Castano A, Boeing S, Bon-Frauches AC, Fung C, Fallesen T, De Aguero MG, Yilmaz B, Lopes R, Huseynova A, Horswell S, Maradana MR, Boesmans W, Vanden Berghe P, Murray AJ, Stockinger B, Macpherson AJ, Pachnis V (2020) Neuronal programming by microbiota regulates intestinal physiology. Nature 578(7794):284–289
Pantoja-Feliciano IG, Clemente JC, Costello EK, Perez ME, Blaser MJ, Knight R, Dominguez-Bello MG (2013) Biphasic assembly of the murine intestinal microbiota during early development. ISME J 7:1112–1115
Parathan P, Wang Y, Leembruggen AJ, Bornstein JC, Foong JP (2020) The enteric nervous system undergoes significant chemical and synaptic maturation during adolescence in mice. Dev Biol 458(1):75–87
Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J (2017) A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5:48
Pham TD, Gershon MD, Rothman TP (1991) Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J Comp Neurol 314:789–798
Rao M, Gershon MD (2018) Enteric nervous system development: what could possibly go wrong? Nat Rev Neurosci 19:552–565
Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC (2015) Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29:1395–1403
Roberts RR, Murphy JF, Young HM, Bornstein JC (2007) Development of colonic motility in the neonatal mouse-studies using spatiotemporal maps. Am J Physiol Gastrointest Liver Physiol 292:G930–G938
Sasselli V, Pachnis V, Burns AJ (2012) The enteric nervous system. Dev Biol 366:64–73
Uesaka T, Nagashimada M, Enomoto H (2015) Neuronal differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J Neurosci 35:9879–9888
Uesaka T, Young HM, Pachnis V, Enomoto H (2016) Development of the intrinsic and extrinsic innervation of the gut. Dev Biol 417:158–167
Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, Kazantsev M, Wilson CJ, Rendon T, Hsiao EY (2020) The maternal microbiome modulates fetal neurodevelopment in mice. Nature 586(7828):281–286
Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY (2015) Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161:264–276
Yi P, Li L (2012) The germfree murine animal: an important animal model for research on the relationship between gut microbiota and the host. Vet Microbiol 157:1–7
Younge N, Mccann JR, Ballard J, Plunkett C, Akhtar S, Araújo-Pérez F, Murtha A, Brandon D, Seed PC (2019) Fetal exposure to the maternal microbiota in humans and mice. JCI Insight 4:e127806
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Foong, J.P.P. (2022). Interaction of the Microbiota and the Enteric Nervous System During Development. In: Spencer, N.J., Costa, M., Brierley, S.M. (eds) The Enteric Nervous System II. Advances in Experimental Medicine and Biology, vol 1383. Springer, Cham. https://doi.org/10.1007/978-3-031-05843-1_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-05843-1_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-05842-4
Online ISBN: 978-3-031-05843-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)