Abstract
The herpes simplex viruses (HSV) are responsible for acute infections of the cutaneous system. Infection presents as groups of vesicles with basal erythema. The genome of the HSV consists of linear, double-stranded DNA. Most infections are relatively mild, but occasionally HSV may produce a severe illness with the potential to harm a pregnancy. The majority of HSV infections exhibit the feature of multiple recurrences, usually at the same location each time. The most frequent manifestation is herpes labialis secondary to HSV-1 (herpes simplex type 1). Herpetic infections of the genitals are generally secondary to HSV-2. It is possible for herpes to cause other forms of illness, but this rarely occurs [1].
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The herpes simplex viruses (HSV) are responsible for acute infections of the cutaneous system. Infection presents as groups of vesicles with basal erythema. The genome of the HSV consists of linear, double-stranded DNA. Most infections are relatively mild, but occasionally HSV may produce a severe illness with the potential to harm a pregnancy. The majority of HSV infections exhibit the feature of multiple recurrences, usually at the same location each time. The most frequent manifestation is herpes labialis secondary to HSV-1 (herpes simplex type 1). Herpetic infections of the genitals are generally secondary to HSV-2. It is possible for herpes to cause other forms of illness, but this rarely occurs [1].
Shedding of virus may happen when infection first occurs, when the infection recurs or even when no symptoms are evident. For transmission to occur, mucous membranes must come into contact or skin which is damaged must be exposed. The primary herpetic infection occurs at the location where contact first occurred. The lipid and glycoprotein envelope of the virus joins to the outer plasma membrane of the epithelium (skin or mucosa), following which the viral genome becomes integrated into the nuclear DNA of the host [2]. Herpes simplex bears the C glycoprotein, which stabilizes the envelope of the virus and assists with entry into the host cell [3]. When toll-like receptors of the host recognize viral DNA, both the innate and adaptive branches of immune defence are triggered into action and interferon is synthesized [2]. There are complex interactions between the viral proteins and the host immune system, as a result of which, HSV is able to shut down the immune response and evade destruction. A key molecule which acts in this way is the virion host shutoff protein (VHS), synthesized early on in infection and acting to prevent cellular immunity responses from occurring [2]. The viral glycoprotein C attaches to the complement factor C3b, preventing a response mediated through the complement system, and this helps to suppress the action of immunoglobulins targeting HSV [3]. HSV is then free to enter nerve cells and begin replicating itself. It does the same in cells of the epidermis and dermis. The virions migrate from the location where primary infection first occurred to the dorsal root ganglion of the sensory nerves and the virus then enters a latent phase. This latency comes about because the HSV genes are not being expressed. However, when the host is subject to stress, the virus may emerge from its latency and become active again [1].
As the virus replicates itself within the dorsal ganglia, clinically this presents as a recurrent episode. There are a variety of events which may cause viral reactivation, including trauma, exposure to ultraviolet light, extreme cold or heat, stress, suppression of the immune system, a surgical operation (including laser surgery) and endocrine alterations. It appears that CD8+ T lymphocytes with specificity for HSV are key to controlling the extent to which the virus can become active again and produce infection [3]. These CD8+ T lymphocytes are brought into action by both branches of the immune response at the point when primary infection occurs. These lymphocytes express a higher than usual level of CXCR3 and CCR10, which are receptors for the chemokines. It is likely these receptors influence movement of the T lymphocytes to the area where HSV is active and help to orchestrate the inflammatory response [4].
2 Aetiology
The two variants of HSV, HSV-1 and HSV-2, are together responsible for herpetic infections of the facial lips, the genitals, mat herpes, herpetic whitlow, herpetic keratoconjunctivitis, eczema herpeticum, herpes folliculitis, lumbosacral herpes, disseminated herpes, herpetic infections in newborns and encephalitis [5]. HSVs are also sometimes implicated in patients with erythema multiforme, being found in 18% of children with this condition [6]. Pyrexia, UV irradiation, traumatic injury, and infection of the upper respiratory tract or psychological stress may all trigger a recurrence of herpes labialis due to HSV-1 [1].
3 Clinical Features
The prevalence of HSV-1 infection varies geographically, socioeconomically and with age range. For HSV-2, serological evidence of infection shows the highest prevalence in female sex workers, gay men and patients infected with HIV [1].
3.1 Orolabial Herpes
Herpes labialis presents with cold sores/fever blisters. It is usually the result of infection with HSV-1, although in some cases HSV-2 has been implicated. In such cases, transmission generally occurs through oral sex. The primary infection by HSV-1 frequently takes place while the patient is still a child and generally does not result in symptoms [1].
3.1.1 Primary Infection
The onset of symptomatic primary infection of the mouth and lips by HSV may be preceded by prodromal pyrexia, after which pharyngitis and stomatitis develop, accompanied by submandibular or cervical lymph gland enlargement. Paediatric cases may additionally feature gingivostomatitis, and children may find swallowing painful. There is formation of labial, gingival, palatal or lingual vesicles, which are painful and often surrounded by an erythematous, swollen area. The vesicles may coalesce and become pustular, then become ulcerous. In the ulcerated form, they have a scalloped margin. Resolution occurs after 2–3 weeks [1].
3.2 Recurrences
HSV maintains latency for differing lengths of time. If HSV-1 becomes reactivated within the sensory ganglia of the fifth cranial nerve, vesicles reappear over the face, mouth, lips and mucosal surfaces of the eye. Before the appearance of vesicles, the patient may complain of pain, a burning sensation, pruritus or pins and needles. When the vesicles appear, they form an ulcer after a while or become crusted. The lesions have a predilection for the junction of the lips with the skin. Without intervention, symptomatic duration is typically around seven days. Recurrent infections of the mouth and lips by HSV-1 sometimes result in a recurrence of erythema multiforme. A study investigating the shedding of virus by HSV-1 found that the median length of time for which this occurred was from 48 to 60 h after symptoms first occurred. Once 96 h had elapsed from the first symptoms, virus shedding could no longer be detected [7].
3.3 Genital Herpes
The most frequent cause of genital herpetic infection is HSV-2. Nonetheless, primary infection of the genitalia with HSV-1 is becoming detected at ever greater rates, up to 80% in some populations [8]. These infections probably result from oral sex. Indeed, HSV-1 is implicated in a third of primary herpetic infections of the genitals. It is most frequent in individuals below the age of 30, those of Caucasian ethnicity and in men who engage in sexual activity with other men [9]. Herpetic genital infections of recurrent type, nonetheless, are virtually always the result of HSV-2 infection [1].
3.3.1 Primary Infection
The primary episode of genital herpetic infection takes place between 2 days and 2 weeks after viral transmission occurs. Primary episodes are more severe than recurrent episodes and in general have a duration of between two and three weeks.
Male patients typically develop vesicles on the penis. These lesions are surrounded by erythema and undergo ulceration. Less commonly, they are found in the anal and perineal regions. Female patients with a primary herpetic genital infection have vesicles that ulcerate on the cervix as well as on both sides of the vulva. These lesions cause pain. The occurrence of herpetic vesicles has also been noted within the vagina, on the perineum, the buttocks and, occasionally, the legs, where they follow the distribution of the sacral nerve. In both sexes, the symptomatic presentation includes pyrexia, a feeling of being unwell, swelling, enlarged inguinal lymph nodes, painful urination and a discharge from the penis or vagina.
A potential complication in women is a lumbosacral radiculopathy. Up to a quarter of female patients in whom a primary infection with HSV-2 occurs may develop aseptic meningitis [1].
3.3.2 Recurrences
Following the primary infection, viral latency may last for several months or years. Eventually, the virus undergoes reactivation. The HSV-2 resides in the lumbosacral ganglia; hence reactivation produces symptoms below the level of the waist. When recurrence occurs, the clinical picture is less severe. The initial indications of recurrence are prodromal pain, pruritus, tingling, a burning sensation or pins and needles.
There are certain patients whose initial exposure to HSV does result in infection but no symptoms are noted. The first time that symptoms occur may be several months or even years after the initial infection occurred. In such cases, the symptoms will be less severe than those in a true primary infective episode.
Above 50% of patients with serological evidence of infection with HSV-2 do not show any other evidence of infection, in other words, they are asymptomatic. Nonetheless, the virus is still shed on occasion by such patients and therefore they present a risk of transmission to individuals with whom they engage in sexual contact [1].
4 HSV During Pregnancy
Other than during a primary infective episode, the risk of transmission from a pregnant woman to her foetus is low. Nonetheless, up to 50% of foetuses may be infected if the primary infection occurs during pregnancy [10]. It is not appropriate to screen pregnant women as a matter of course for HSV, but diagnostic investigations are called for if herpes genitalis is the putative diagnosis [10, 11]. The clinician should manage herpes genitalis on the basis of a thorough history and careful physical examination. This especially applies at the delivery stage. Unless there are indications of an active herpetic infection at the time of delivery, vaginal delivery is not contraindicated [11]. In cases where there is either a primary episode of HSV infection or recurrence of latent HSV, delivery should occur by Caesarean section, since this renders vertical transmission less likely [11]. Antiviral agents are safe to administer systemically during pregnancy and may allow a normal delivery to proceed [11]. Even with the use of antivirals, transmission to the foetus remains a possibility [11].
The treatment recommendations to suppress an outbreak of herpes genitalis in a pregnant woman are listed below [1]:
-
Acyclovir 400 mg by mouth TID (beginning when the pregnancy reaches 36 weeks’ gestation)
-
Valaciclovir 500 mg by mouth BID (beginning when the pregnancy reaches 36 weeks’ gestation)
4.1 Vertical Transmission
Generally speaking, the way HSV is transmitted from the mother to the foetus is by the foetus coming directly into contact with shed virus at the time of delivery and labour. The virus may be shed from the cervical, vaginal, vulvar or perianal epithelia [12].
There are a number of facts about how HSV is transmitted to the infant that must be noted to understand the way cases present [13]:
In the majority of cases where a neonate is infected with HSV perinatally, there is no corresponding maternal history of symptoms or signs of herpetic genital infection [14].
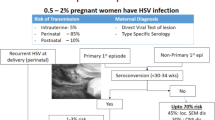
Transmission is most likely to occur if the mother had the primary episode of herpes genitalis shortly before the time of birth. In cases where the first clinically apparent episode does not coincide with the time of initial infection with HSV, there is a somewhat reduced likelihood of transmission. This risk is even lower where HSV episodes are recurrent. The authors of two case series [15, 16] performed viral culture on specimens obtained from women brought to the labour ward for delivery. From those cases where maternal viral culture identified HSV, there were different rates of neonatal infection, depending on the maternal characteristics, as follows:
-
If the mother was undergoing a primary infective episode, the rate of transmission in one study was 40% (2 out of 5 cases) [16], while in the other study the rate was 44% (4 out of 9 cases) [15].
-
In mothers where the first clinically apparent episode did not coincide with the time of initial infection with HSV, the rate of transmission was reported as 31% (4 out of 13) [16] or 24% (4 out of 17) [15].
-
Where there were recurrent episodes, the rate was either 3% (1 out of 34) [16] or 1.3% (2 out of 151) [15].
A probable explanation for the data showing that transmission is more probable when the mother is experiencing a primary or non-primary initial HSV episode is that in such cases the mother has not yet seroconverted and the shedding of virus is at its maximum, both in terms of number of particles shed and length of time over which this occurs.
The immune system typically begins expressing immunoglobulins with a specificity for HSV no later than 12 weeks following infection. Initially, IgM is produced, indicating acute infection, followed by the production of IgG, which confirms prior infection status. These immunoglobulins continue to be expressed indefinitely [17]. The fact that transmission occurs at a lower rate in mothers whose HSV infection is recurrent than in those with a primary episode most likely relates to greater protection provided by specific immunoglobulins and the fact that viral shedding from the genital tract is less intense or long-lasting when the virus is reactivated [18]. It is noteworthy that women infected with HSV who seroconvert following a primary or nonprimary initial episode at an earlier stage of pregnancy seemingly also transmit HSV at a lower rate to the foetus [19].
Even where there are non-clinically evident vesicles and no symptoms, virus may still be shed [13, 20,21,22]. Research conducted into viral shedding in patients who were not pregnant [22] found that 13% of those with a symptomatic lesion were shedding virus, whilst in asymptomatic individuals, the rate was still 9%. This study recruited individuals who were seropositive for immunoglobulins targeting HSV-2. The samples were obtained by swabbing the genitalia. Detection of viral shedding was accomplished by performing the polymerase chain reaction for viral DNA.
Shedding of virus occurs more often in herpes genitalis secondary to HSV-2 than HSV-1. This affects the rate of vertical transmission and thus the risk of complications for the newborn. However, clinical management does not differ according to whether HSV-1 or HSV-2 is the cause.
There are anecdotal reports indicating that HSV has been transmitted to the foetus before labour and delivery by crossing the placenta or ascending through the uterine cervix. Such an infection may lead to spontaneous abortion, development of congenital defects (such as ventricular enlargement or central nervous anomalies), premature delivery and/or restricted ability of the foetus to grow in utero [19, 23,24,25,26]. There appears to be no association between recurrent type HSV infection and pregnancy complications [27]. If a neonate appears infected with HSV at an early stage even though delivery occurred via caesarean section and the foetal membranes were not disturbed, there is a possibility that infection had actually already occurred in utero [15, 28].
4.2 Evidence
There are a number of studies which have played a key role in identifying the risk factors involved in vertical transmission and in quantifying the risks involved. One of these studies employed a prospective design and ran from 1982 to 1999 [15]. In this study, 58,000 pregnant women were enrolled. They were divided into cases considered primary, non-primary or recurrent infections by HSV. This division was made on the basis of serology, viral culture and amplification of viral DNA [15]. In 202 participants, representing 0.5% of the sample, HSV was detected in swabs of the vulva or cervix at the point when they entered labour. The study identified the following outcomes concerning transmission to the newborn [12]:
-
The existence of shed virus at the time of labour strongly correlated with the risk that the newborn would be infected with HSV (odds ratio: 346). Transmission to the newborn was identified in some 5% (10 patients out of 202) of those individuals where HSV was cultured, whereas this only occurred in 0.02% (6 women out of 39,821) where HSV was not cultured.
-
The maximum likelihood of vertical transmission occurred in women who had recently acquired a primary infection with herpes genitalis, as evidenced by positivity for HSV, but non-detectable specific immunoglobulins. In these cases, the rate of transmission was 54 per 100,000 live births. This is higher than where the mother produced antibodies to HSV-1 (26 in 100,000 live births) or to HSV-2 (22 in 100,000 live births).
These studies also identified other risk factors. Isolation of cervical HSV raised the risk (odds ratio:33), as did shed virus of type HSV-1 rather than HSV-2 (odds ratio:17). Some indirect factors also indicated a higher risk, namely invasive monitoring of the foetus (odds ratio:7), premature delivery (i.e. earlier than 38 weeks’ gestation; odds ratio:4) and mothers age 21 years and under (odds ratio:4).
4.3 Management During Pregnancy
There are two ways in which the risk of vertical transmission of HSV can be reduced in pregnant women. The first is to commence antiviral pharmacotherapy from 36 weeks’ gestation onwards to minimize the chance of the infection becoming reactivated at the time of giving birth. The second intervention is to offer Caesarean section to certain at-risk women. These two strategies are insufficient to entirely remove the possibility of the neonate becoming infected with HSV. One approach, which reflects the recommendations of the American College of Obstetricians and Gynecologists, involves classifying cases according to whether HSV infection of the genitalia is primary, non-primary or recurrent, how severely affected the woman is and the length of interval between symptoms and the expected due date [29].
If a woman who has not previously had an episode of herpes genitalis develops de novo ulceration of the genitalia whilst pregnant, the authors recommend an empirical trial of an antiviral agent and virological investigation. A new episode of herpes genitalis usually recovers spontaneously, but therapeutic intervention may shorten the episode, alleviate symptoms and reduce the length of time over which viruses are being shed. Acyclovir 400 mg p.o. TID is a suitable treatment. If HSV infection features complications, such as involvement of the brain and spinal cord, other organs or is disseminated around the body, this agent will need to be administered intravenously at first [17].
Treatment is usually administered for between 7 and 10 days, but may be needed for a longer period if there is no total resolution following a 10-day course. Acyclovir needs to be re-introduced at 36 weeks’ gestation to inhibit viral reactivation around the time of delivery [12].
4.3.1 Suppressive Therapy at 36 weeks
The authors’ recommendation is to administer antiviral therapy from 36 weeks’ gestation until labour to any woman who develops herpes genitalis during pregnancy. This recommendation applies whatever the timing of the infection and regardless of whether the infection is primary, non-primary or recurrent. A suitable regime involves acyclovir 400 mg TID. Note that the renal clearance of this agent is increased during pregnancy and therefore a higher dose is called for than in non-pregnant patients [12].
4.3.2 Topical Antivirals
There is also a topical preparation available, in the form of an ointment or cream containing 5% acyclovir. This preparation may be used either five or six times daily and the course lasts between 4 and 7 days. It is suitable for both herpes labialis and herpes genitalis. However, the cream is not suitable for application to the genital area. Topical acyclovir offers only a moderate degree of benefit in HSV infection, as noted in real world settings, despite clinical trial data indicating a statistically significant degree of efficacy. One trial [30] compared a cream containing 5% acyclovir with placebo to treat orolabial HSV infection and recorded a reduction in symptomatic duration of one-half day. A topical preparation containing acyclovir 5% and hydrocortisone 1% may be used 5 times daily, being initiated as soon as symptoms of a recurrence occur. This preparation demonstrated an advantage in stopping the episode from progressing when compared with acyclovir alone, or with placebo [31]. A further topical preparation that may be used for orolabial HSV infections is penciclovir 1% cream. It may be applied once every two hours and benefits from comparable efficacy to acyclovir. An over-the-counter product to consider is docosanol 10% cream, which may be administered five times daily for a maximum of ten days. A study in which docosanol competed against placebo found that cases where docosanol was used resolved on average after around four days, whereas those where placebo was used lasted a further 18 hours [32]. However, all topical preparations used as antivirals in cases of HSV infection have a considerably lower efficacy than when administered orally or parenterally. Accordingly, the US Centers for Disease Control do not support their use, citing low clinical efficacy [11].
4.4 Drug Selection, Dosage and Safety
There are three antiviral agents available which work against HSV, acyclovir, famciclovir and valaciclovir. The agent about which the most is known in pregnancy, however, is acyclovir, at a dosage of 400 mg p.o. TID. In an acute infective episode, the course lasts for between 7 and 10 days, or longer if the symptoms persist. For use as an inhibitor of viral reactivation, it is administered from the 36th week of pregnancy up to the time the child has been delivered [12].
An alternative to acyclovir in either indication is valaciclovir. However, this agent generally costs more than acyclovir, even as a generic, and the evidence base for its safety and clinical benefit is less well-established [33]. One situation in which valaciclovir may be a better choice is where patients may not be fully concordant, as this agent requires BID dosing, which may be easier to ensure than TID dosing, as needed with acyclovir.
The evidence on teratogenicity of acyclovir gathered from animal testing and in humans indicates this agent is non-teratogenic throughout pregnancy, including during organogenesis [33]. The evidence for valaciclovir is less extensive, but has not thus far highlighted any concerns. The evidence for the safety of famciclovir in pregnant women is extremely limited.
5 Postpartum and Neonatal Management
If either the parents or anyone else looking after an infant has an active herpetic infection, caution is needed when touching the child. The vesicles or ulcers must be covered and strict handwashing observed. Between 5 and 15% of HSV infections in newborns are actually transmitted by a relative after the birth [34].
There is no contraindication to breastfeeding an infant, provided the mother has no signs of infection on the breasts. Maternal treatment with acyclovir or valaciclovir is also not a reason to stop breastfeeding [35, 36].
It is vital that a paediatrician be consulted for advice in any neonate who has been exposed to HSV. If the newborn has been or is at risk, it is vital to observe the infant for clinical indications of herpetic infection.
References
McGregor SP. Dermatologic manifestations of herpes simplex treatment & management. In: James WD, editor. Medscape. Updated: 17 Mar 2020. https://emedicine.medscape.com/article/1132351-treatment#d7. Accessed online 1 April 2021.
Roizman B, Whitley RJ. An inquiry into the molecular basis of HSV latency and reactivation. Annu Rev Microbiol. 2013;67:355–74.
Komala Sari T, Gianopulos KA, Nicola AV. Glycoprotein C of herpes simplex virus 1 shields glycoprotein B from antibody neutralization. J Virol. 2020;94(5)
Hensel MT, Peng T, Cheng A, De Rosa SC, Wald A, Laing KJ, et al. Selective expression of CCR10 and CXCR3 by circulating human herpes simplex virus-specific CD8 T cells. J Virol. 2017;91(19)
Weinberg JM, Mysliwiec A, Turiansky GW, Redfield R, James WD. Viral folliculitis. Atypical presentations of herpes simplex, herpes zoster, and molluscum contagiosum. Arch Dermatol. 1997;133(8):983–6.
Zoghaib S, Kechichian E, Souaid K, Soutou B, Helou J, Tomb R. Triggers, clinical manifestations, and management of pediatric erythema multiforme: a systematic review. J Am Acad Dermatol. 2019;81(3):813–22.
Boivin G, Goyette N, Sergerie Y, Keays S, Booth T. Longitudinal evaluation of herpes simplex virus DNA load during episodes of herpes labialis. J Clin Virol. 2006;37(4):248–51.
James SH, Kimberlin DW. Neonatal herpes simplex infection: epidemiology and treatment. Clin Perinatol. 2015;42:47–59.
Dabestani N, Katz DA, Dombrowski J, Magaret A, Wald A, Johnston C. Time trends in first-episode genital herpes simplex virus infections in an urban sexually transmitted disease clinic. Sex Transm Dis. 2019;46(12):795–800.
Subramaniam A, Britt WJ. Herpesviridae infection: prevention, screening, and management. Clin Obstet Gynecol. 2018;61(1):157–76.
(Guideline) Centers for Disease Control and Prevention. 2015 Sexually transmitted diseases treatment guidelines. https://www.cdc.gov/std/tg2015/default.htm. January 25, 2017. Accessed 5 Dec 2017.
Riley LE, Wald A. Genital herpes simplex virus infection and pregnancy. In: Hirsch MS, Lockwood CJ, Mitty J, Barss VA, editors. UpTodate. Last updated: 10 Jun 2020.
Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844.
Pinninti SG, Kimberlin DW. Maternal and neonatal herpes simplex virus infections. Am J Perinatol. 2013;30:113.
Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203.
Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247.
Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1.
Johnston C, Magaret A, Selke S, et al. Herpes simplex virus viremia during primary genital infection. J Infect Dis. 2008;198:31.
Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337:509.
Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350:11.
Wald A, Corey L, Cone R, et al. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest. 1997;99(1092)
Tronstein E, Johnston C, Huang ML, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA. 2011;305:1441.
Brown ZA, Benedetti J, Selke S, et al. Asymptomatic maternal shedding of herpes simplex virus at the onset of labor: relationship to preterm labor. Obstet Gynecol. 1996;87:483.
Nahmias AJ, Josey WE, Naib ZM, et al. Perinatal risk associated with maternal genital herpes simplex virus infection. Am J Obstet Gynecol. 1971;110:825.
Brown ZA, Vontver LA, Benedetti J, et al. Effects on infants of a first episode of genital herpes during pregnancy. N Engl J Med. 1987;317:1246.
Fa F, Laup L, Mandelbrot L, et al. Fetal and neonatal abnormalities due to congenital herpes simplex virus infection: a literature review. Prenat Diagn. 2020;40:408.
Harger JH, Amortegui AJ, Meyer MP, Pazin GJ. Characteristics of recurrent genital herpes simplex infections in pregnant women. Obstet Gynecol. 1989;73:367.
Stone KM, Brooks CA, Guinan ME, Alexander ER. National surveillance for neonatal herpes simplex virus infections. Sex Transm Dis. 1989;16:152.
Management of Genital Herpes in Pregnancy. ACOG practice bulletinacog practice bulletin, number 220. Obstet Gynecol. 2020;135:e193.
Spruance SL, Nett R, Marbury T, Wolff R, Johnson J, Spaulding T. Acyclovir cream for treatment of herpes simplex labialis: results of two randomized, double-blind, vehicle-controlled, multicenter clinical trials. Antimicrob Agents Chemother. 2002;46(7):2238–43.
Hull CM, Harmenberg J, Arlander E, Aoki F, Bring J, Darpö B, et al. Early treatment of cold sores with topical ME-609 decreases the frequency of ulcerative lesions: a randomized, double-blind, placebo-controlled, patient-initiated clinical trial. J Am Acad Dermatol. 2011;64(4):696.e1–696.e11.
Sacks SL, Thisted RA, Jones TM, Barbarash RA, Mikolich DJ, Ruoff GE, et al. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J Am Acad Dermatol. 2001;45(2):222–30.
Briggs GG, Freeman RK, Yaffe, SJ. Acyclovir. In: Drugs in pregnancy and lactation, 8th ed., e-book; 2021.
Caviness AC, Demmler GJ, Selwyn BJ. Clinical and laboratory features of neonatal herpes simplex virus infection: a case-control study. Pediatr Infect Dis J. 2008;27:425.
LactMed. Acyclovir. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/f?./temp/~aVoLKr:1. Accessed 01 March 2021.
LactMed. Valacyclovir. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/f?./temp/~aVoLKr:2. Accessed 10 March 2021.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Öner, F., Cingi, C., Reisacher, W. (2022). Herpes Simplex Viral Infections in Pregnancy. In: Cingi, C., Özel, H.E., Bayar Muluk, N. (eds) ENT Diseases: Diagnosis and Treatment during Pregnancy and Lactation. Springer, Cham. https://doi.org/10.1007/978-3-031-05303-0_29
Download citation
DOI: https://doi.org/10.1007/978-3-031-05303-0_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-05302-3
Online ISBN: 978-3-031-05303-0
eBook Packages: MedicineMedicine (R0)