Abstract
The naming of an illness is an essential element in the care of patients and often depends on laboratory testing. Among tests to evaluate for possible autoimmunity, the antinuclear antibody (ANA) provides information that can be difficult to interpret and, thereby, can contribute to misnaming of certain signs and symptoms as an autoimmune disease, especially systemic lupus erythematosus (SLE). Although a positive ANA test is now considered necessary for the classification (diagnosis) of SLE, as many as 20% of the otherwise healthy population has a positive test. Furthermore, since ANA positivity can predate the diagnosis of SLE by many years, it is difficult to ignore a positive result even if ordered with a low pretest probability. Current technology allows for more complete and quantitative ANA profiling; such information could help determine the probability for disease, underpinning a potentially more informative approach for screening, prevention, and naming of disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
The naming of an illness occurs in two distinct dimensions: the global and the personal. In the global dimension, naming provides an essential categorization to advance the study of disease and its management. In this dimension, the naming of an illness involves a construct developed by experts to establish validated criteria for diagnosis and classification. The naming of an illness (i.e., creation of a diagnostic category) is the foundation of scientific medicine, with research over time revising and refining any proposed criteria.
In contrast to the global dimension, the personal dimension involves the individual patient, with the application of criteria determined by the individual provider. The provider who does the naming may or may not be an expert in the particular clinical situation in question, especially for conditions that are rare or uncommonly encountered. Furthermore, the data that can inform appropriate naming may not be available in the timeframe needed. Thus, the naming of an illness in the real word is often tentative and imprecise.
The number of names and diagnostic categories for illnesses has proliferated dramatically in recent years with the advent of molecular techniques to subset illnesses into ever more narrow categories [1]. Indeed, precision or personalized medicine approaches signify the inadequacy of existing names to guide effective treatment. In a world of genetic and genomic testing, the molecular mechanisms of disease (e.g., patterns of aberrant gene expression) may be more relevant than the traditional disease name in developing and prescribing new treatments for diseases that may affect different tissues and organ systems. This approach can also lead to the development of tissue-agnostic agents and the conduct of basket trials involving several different conditions [2, 3].
Despite the burgeoning number of names and diagnostic categories, many patients simply do not fit well into existing categories, leading to uncertainty. This uncertainty can impair the relationship between patients and providers, provoke extensive and unrevealing diagnostic workups, and send patients in sometimes frantic searches to find a provider willing to provide a name for their signs and symptoms. In this situation, an inability to name the illness can be both obstructive and destructive.
Among medical subspecialties, rheumatology, in particular, cares for many patients for whom the naming of illness is problematic. The most established diagnostic categories encompass a wide range of signs and symptoms (e.g., pain, fever, depression) that are common in the general population [4]. Every illness has a threshold for findings to allow diagnosis. For rheumatologic illnesses, this threshold is often vague and long periods of time can pass before the evidence for disease is decisive. As a result, an illness can be named either too early or too late. It can also be given the wrong name.
A disease of protean manifestations, systemic lupus erythematosus (SLE or lupus) is often the subject of incorrect naming. SLE primarily affects women and can range from relatively mild joint pains to devastating neurologic disease; glomerulonephritis is a common source of morbidity and mortality [5]. The pattern of disease can vary markedly among different racial and ethnic groups. As any rheumatologist can attest, many patients who carry the diagnosis of lupus probably don’t have this condition. These considerations do not diminish the severe symptoms of patients thought to have lupus; they only suggest that lupus is the wrong name.
For lupus, the designation of the wrong name often results from reliance upon laboratory tests whose characteristics are not widely appreciated. The prime example of a test that is either “misunderstood or misbegotten” is the antinuclear antibody test or ANA [6]. Antinuclear antibodies are directed toward diverse macromolecules in the cell nucleus [7, 8]. ANA positivity is now required for patient classification since studies suggest that 95–99% of patients with SLE express an ANA at some point in their illness [9,10,11]. Even though the ANA test has been used for over 60 years, its performance is subject to variability and inconsistency and its result subject to misinterpretation. The so-called lupus test is not a test for lupus. Indeed, the ANA test may not be a test for any disease.
In view of the importance of ANA testing to both the naming (and misnaming) of illness, I would like to provide a perspective on current serological testing and suggest ways it can be used to develop new nomenclature.
The Problems of ANA Determination
Box: Issues with ANA Testing in the Naming of Illness
-
Lack of standardization.
-
High frequency of false-positive results.
-
Uncertain frequency of false-negative results.
-
Lack of quantitation.
-
Uncertain interpretation of cytoplasmic staining.
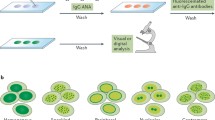
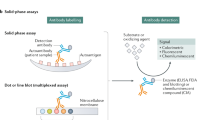
ANA testing involves a variety of assay formats that each has advantages and disadvantages. The most venerable approach is the indirect immunofluorescence assay, denoted as the IIF or IFA. In this assay, serum is incubated with a microscope slide to which is fixed an organ slice or cell line as a source of nuclei. After incubation with an immunofluorescent anti-immunoglobulin reagent, the next step is visual inspection under the microscope to assess the binding in terms of a titer as well as the pattern (e.g., homogeneous, speckled). The pattern reflects the distribution of the target antigen within the cell nucleus, with the pattern providing information concerning the specificity of the IgG ANA present [12, 13].
Current versions of the IFA utilize a long-term cell called HEp2 since most of the relevant target antigens of disease-related ANAs are abundant in this cell. The main advantage of this assay relates to the high frequency of positivity in patients with SLE and related connective tissue diseases (CTDs), also called autoantibody (or ANA)-associated rheumatic diseases (AARDs). Beyond availability of a fluorescence microscope, the assay does not require any specialized equipment and is well within the capabilities of hospital and clinical testing laboratories. Furthermore, even though the IFA is designed to detect antibodies to the cell nucleus, the assay allows identification of antibodies to cytoplasmic antigens; antibodies to cytoplasmic antigens can also be biomarkers for lupus. Of note, when a sample with cytoplasmic binding is called ANA negative, an opportunity for naming can be missed.
The IFA has two main disadvantages that impact on the naming process. The first is the very high frequency of assay positivity in the otherwise healthy population [14]. Depending on the kit used, as many as 15–20% of the healthy population can be ANA positive. The frequency of positivity is twice as high in women as men and has a peak age of around 30–40 years. Since SLE primarily affects women in this age group, confusion can result if the test is used to evaluate women with vague or non-specific symptomatology. Interestingly, the frequency of ANA positivity appears to be increasing in the population [15].
The basis of the high frequency of ANA expression in the population is unknown. To the extent that ANA positivity signifies immune disturbance, the human immune system may have an unfortunate propensity to develop autoreactivity. A less dire or worrisome explanation for the high frequency of IFA reactivity is technical. Perhaps the fixation conditions for slide preparation denature or otherwise modify proteins so that they resemble foreign proteins in immunological reactivity.
The other technologies for ANA detection utilize recombinant or purified proteins as a source of nuclear antigens. Because of advances in molecular biology, the molecular identity of most of the target antigens relevant in rheumatology is now known and specific immunoassays are available. Of these approaches, multiplex assays allow the simultaneous measurement of antibodies to a series of cloned or purified proteins by a LINE assay or an addressable laser bead immunoassay (ALBIA) [16, 17]. ALBIAs allow detection of antibodies to antigens for SLE, Sjogren’s syndrome, myositis, and progressive systemic sclerosis. Usually, results are provided as either positive or negative except for anti-DNA for which anti-DNA levels are valuable for assessing disease activity.
While, in general, the specificity of antibodies producing ANA positivity by otherwise healthy people is unknown, one exception is an antigen system called DFS70. DFS stands for dense fine speckled which is the characteristic pattern of staining associated with antibodies to a protein called DFS70 or lens-epidermal derived growth factor. The presence of these antibodies can be recognized by IFA or by a specific immunoassay. Studies have indicated that, while anti-DFS70 antibodies can appear in a variety of conditions, they are not increased in patients with CTDs, including SLE. Thus, the finding of either DFS staining or antibodies to DFS70 could suggest that the patient does not have a CTD [18, 19].
The main disadvantage of using a multiplex assay like an ALBIA is that the assay is not really an ANA assay [6, 8, 16, 17]. Since only a small number of antibodies can be measured, many ANA specificities relevant to diagnosis are missed. Clinical testing laboratories, however, like the ALBIA because these assays are high throughput and do not require a dedicated technician skilled in reading IFA patterns. For many in the field, however, the IFA remains the gold standard since it can detect a broad range of specificities.
The positioning of the IFA as a gold standard is not as solid as often considered since variation between kits is substantial and many patients with SLE can be negative in one assay and positive in another [20,21,22]. These inconsistencies can be reduced by testing the same sample by more than one assay type (e.g., an IFA and ALBIA) but this approach is often not possible because of issues of costs or assay availability. Given the serious impact of an incorrect diagnosis of SLE (either way, missing the diagnosis of SLE or making the diagnosis in someone without the disease), the cost of seemingly redundant testing seems well justified.
Symptomatology
Autoimmune diseases often start gradually and serological findings can predate clinical findings. By using samples from a biobank repository assembled by the US military, Arbuckle et al. showed that individuals with SLE begin to express characteristic antibodies years before the diagnosis, with the number of specificities increasing over time [23]. The period of time can be termed pre-autoimmunity in distinction to autoimmunity when signs and symptoms accompany serological abnormalities and diagnostic or classification criteria are met [24,25,26]. While pre-autoimmunity is a fascinating subject, in the real world, it can lead to ambiguity and uncertainty about naming.
Consider a hypothetical case of a Ms. Jones, a 41-year-old woman with symptoms of fatigue and arthralgia. She notices headaches and does not feel like herself. She is worried about her condition since her mother had rheumatoid arthritis which started in a similar way.
Ms. Jones sees her general internist who orders a battery of tests including an ANA by immunofluorescence; the IFA is negative as is the rheumatoid factor and the anti-CCP. The provider reassures Ms. Jones and prescribes ibuprofen.
The symptoms persist, and Ms. Jones, dissatisfied with the first provider, goes to another. This provider repeats the ANA which is now positive. The provider says that he is concerned that Ms. Jones has lupus. After reading about lupus on the Internet, Ms. Jones becomes frightened.
Ms. Jones is referred to a rheumatologist who orders an ANA. This time, a multiplex assay is used. The ALBIA is positive for anti-Ro but is negative by the IFA used as part of “reflex testing” to confirm the multiplex assay. The rheumatologist says she is uncertain about the diagnosis but, because of the positive anti-Ro, suggests the diagnosis of undifferentiated connective tissue disease. Ms. Jones is now confused as well as frightened since she was first told she may have lupus and now receives another diagnosis. She is also angry and discouraged that providers cannot figure out what is wrong.
This case is hypothetical but illustrates the difficulties when the naming of illness depends upon a test that is not well standardized and is subject to variability. The case also illustrates the problems that can arise when the performance characteristics of tests are not well understood. Which is the most informative: the anti-Ro by multiplex, the one positive IFA, or the two negative IFA tests? Anti-Ro can be detected in low amounts by an ALBIA but may be missed by an IFA depending on the kit used, accounting for the negative IFA reflex assay. In reality, the actual serological profile of Ms. Jones is not clear although such information would be valuable in determining whether she has early stages of a CTD including SLE and is, thus, in a state of pre-autoimmunity.
The Issue of Nomenclature
Whether justified or not, ANA testing is very commonly performed in the evaluation of patients with a host of signs and symptoms ranging from rash to low back pain to depression. It can also be a part of the general screen for musculoskeletal disease even when the pretest probability for SLE is low. For many of these patients, the test will be false-positive, often leading to referral to a rheumatologist who may perform additional, sometimes costly, tests to understand the significance of the serology. The situation with false positivity is so extreme that some healthcare systems have considered prohibiting generalists from even ordering the ANA. On the other hand, for a very few individuals, the positive ANA is an early sign of disease, a harbinger of more serious events in the future. For these individuals, the ANA has functioned successfully as an antecedent biomarker since early treatment can perhaps attenuate disease and reduce damage.
Another approach to nomenclature (i.e., naming) could improve the use of ANA testing. For SLE as well as other CTDs, serology can be interpreted in a probabilistic way, inferring a likelihood of disease and not its presence. The likelihood increases depending on the number and kind of other serological disturbances present as well as the nature of signs and symptoms. In the future, genomic analysis as well as flow cytometric analysis of cell immune populations may provide adjunctive biomarkers but these technologies are not yet ready for widespread use [27].
The existing nomenclature involves terms like undifferentiated connective tissue disease (UCTD) to encompass serological disturbances and certain signs and symptoms; while indicative of some type of disease, the findings in someone considered to have a UCTD are not decisive or specific enough to allow a diagnosis. Despite the frequent use of the term UCTD, its meaning seems nebulous. It is not clear whether UCTD denotes a final state (i.e., the differentiation has already occurred) or whether further differentiating is in the offing. I doubt that the term differentiating connective tissue disease would catch on but, perhaps, it would be more accurate.
In the past, the diagnosis of rheumatoid arthritis included stages of possible (or equivocal), probable, definite, and classical [28]. The diagnostic criteria, however, gave way to a simpler classification system in view of better serological markers (e.g., anti-citrullinated protein antibodies or ACPA, also known as anti-cyclic citrullinated peptide antibodies or anti-CCP) [29]. The importance of early aggressive therapy provided an impetus to create the new criteria to allow the use of disease modifying anti-rheumatic drugs in the earliest phases of disease. With therapy guided by treat-to-target principles, classical disease could actually disappear.
For SLE, some current disease names (e.g., preclinical lupus, incomplete lupus, non-classical lupus) indicate that diagnosis and classification can be uncertain and tentative, with the presence of ANA positivity a major determinant of these names. Given the likelihood that ANA testing will continue unabated in the future, I would argue that a categorization of serological findings based on stages of disease (possible, probable, definite, and classical) would advance scientific inquiry. Such a categorization could also facilitate communication between the patient and provider as well as underpin more effective programs of prevention and treatment.
With well-standardized assays, serology would be a valuable adjunct to help name an illness at its earliest stages in the presence of certain signs and symptoms. In terms of serology, a positive ANA is possibly lupus; a positive ANA and positive anti-DNA are probably lupus; a positive ANA with anti-DNA and anti-Sm is definitely lupus. Low C3 and C4 along with an array of ANA specificities (e.g., anti-DNA, anti-Sm, anti-RNP, anti-Ro) and complement split products would signify classical disease [30]. Rather than positing an ANA as a requirement for the diagnosis or classification of SLE, ANA testing could be used to define a risk or likelihood of disease depending on the signs and symptoms, even if non-specific or vague.
Whether insurers or professional organizations would accept such a nomenclature system is speculative. Its acceptance by patients and providers is also unknown. Nevertheless, in settings where illness has no name, immunological testing has the potential to provide unique prognostic and diagnostic information for the individual patient. Hopefully, when used rationally and wisely, ANA testing can help name illness and, thereby, relieve the distress that uncertainty can cause for so many patients.
References
Ginsburg GS, Phillips KA. Precision medicine: from science to value. Health Aff (Millwood). 2018;37(5):694–701.
Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: evolution of the treatment paradigm. Cancer Treat Rev. 2020;86:102019.
Seligson ND, Knepper TC, Ragg S, Walko CM. Developing drugs for tissue-agnostic indications: a paradigm shift in leveraging cancer biology for precision medicine. Clin Pharmacol Ther. 2021;109(2):334–42.
Pisetsky DS, Clowse MEB, Criscione-Schreiber LG, Rogers JL. A novel system to categorize the symptoms of systemic lupus erythematosus. Arthritis Care Res. 2019;71:735–41.
Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. 2016;2:16039.
Pisetsky DS. Antinuclear antibody testing: misunderstood or misbegotten? Nat Rev Rheumatol. 2017;13:495–502.
Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis. 2010;69:1420–2.
Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol. 2020;16(10):565–79.
Leuchten N, Hoyer A, Brinks R, Schoels M, Schneider M, Smolen J, et al. Performance of antinuclear antibodies for classifying systemic lupus erythematosus: a systematic literature review and meta-regression of diagnostic data. Arthritis Care Res. 2018;70:428–38.
Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019;71:1400–12.
Choi MY, Clarke AE, St Pierre Y, Hanly JG, Urowitz MB, Romero-Diaz J, et al. Antinuclear antibody-negative systemic lupus erythematosus in an international inception cohort. Arthritis Care Res. 2019;71:893–902.
Wiik AS, Hoier-Madsen M, Forslid J, Charles P, Meyrowitsch J. Antinuclear antibodies: a contemporary nomenclature using HEp-2 cells. J Autoimmun. 2010;35(3):276–90.
Damoiseaux J, Andrade LEC, Carballo OG, Conrad K, Francescantonio PLC, Fritzler MJ, et al. Clinical relevance of HEp-2 indirect immunofluorescent patterns: the International Consensus on ANA patterns (ICAP) perspective. Ann Rheum Dis. 2019;78(7):879–89.
Tan EM, Feltkamp TE, Smolen JS, Butcher B, Dawkins R, Fritzler MJ, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40(9):1601–11.
Dinse GE, Parks CG, Weinberg CR, Co CA, Wilkerson J, Zeldin DC, et al. Increasing prevalence of antinuclear antibodies in the United States. Arthritis Rheumatol. 2020;72(6):1026–35.
Hanly JG, Su L, Farewell V, Fritzler MJ. Comparison between multiplex assays for autoantibody detection in systemic lupus erythematosus. J Immunol Methods. 2010;358(1–2):75–80.
Bruner BF, Guthridge JM, Lu R, Vidal G, Kelly JA, Robertson JM, et al. Comparison of autoantibody specificities between traditional and bead-based assays in a large, diverse collection of patients with systemic lupus erythematosus and family members. Arthritis Rheum. 2012;64(11):3677–86.
Carbone T, Pafundi V, Tramontano G, Gilio M, Padula MC, Padula AA, et al. Prevalence and serological profile of anti-DFS70 positive subjects from a routine ANA cohort. Sci Rep. 2019;9(1):2177.
Yumuk Z, Demir M. Clinical value of anti-DFS70 antibodies in a cohort of patients undergoing routine antinuclear antibodies testing. J Immunol Methods. 2020;480:112754.
Pisetsky DS, Rovin BH, Lipsky PE. New perspectives in rheumatology: biomarkers as entry criteria for clinical trials of new therapies for systemic lupus erythematosus: the example of antinuclear antibodies and anti-DNA. Arthritis Rheumatol. 2017;69:487–93.
Pisetsky DS, Spencer DM, Lipsky PE, Rovin BH. Assay variation in the detection of antinuclear antibodies in the sera of patients with established SLE. Ann Rheum Dis. 2018;77(6):911–3.
Pisetsky DS, Thompson DK, Wajdula J, Diehl A, Sridharan S. Variability in antinuclear antibody testing to assess patient eligibility for clinical trials of novel treatments for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1534–8.
Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–33.
Olsen NJ, Karp DR. Autoantibodies and SLE: the threshold for disease. Nat Rev Rheumatol. 2014;10(3):181–6.
Bourn R, James JA. Preclinical lupus. Curr Opin Rheumatol. 2015;27(5):433–9.
Aberle T, Bourn RL, Munroe ME, Chen H, Roberts VC, Guthridge JM, et al. Clinical and serologic features in patients with incomplete lupus classification versus systemic lupus erythematosus patients and controls. Arthritis Care Res (Hoboken). 2017;69(12):1780–8.
Slight-Webb S, Smith M, Bylinska A, Macwana S, Guthridge C, Lu R, et al. Autoantibody-positive healthy individuals with lower lupus risk display a unique immune endotype. J Allergy Clin Immunol. 2020;146(6):1419–33.
Ropes MW, Bennett GA, Cobb S, Jacox R, Jessar RA. 1958 revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958;9(4):175–6.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81.
Olsen NJ, Karp DR. Finding lupus in the ANA haystack. Lupus Sci Med. 2020;7(1):e000384.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply
About this chapter
Cite this chapter
Pisetsky, D.S. (2022). The Impact of Antinuclear Antibody Testing on the Naming and Misnaming of Disease. In: Lockshin, M.D., Crow, M.K., Barbhaiya, M. (eds) Diagnoses Without Names. Springer, Cham. https://doi.org/10.1007/978-3-031-04935-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-04935-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04934-7
Online ISBN: 978-3-031-04935-4
eBook Packages: MedicineMedicine (R0)